Abstract
Neon flying squid Ommastrephes bartramii and jumbo flying squid Dosidicus gigas are two important commercial ommastrephid species in the Pacific Ocean. As short-lived marine species, squids are highly susceptible to changes in climate and marine environments. According to samples collected from the northwest and southeast Pacific Ocean in different years, we explored the growth characteristics of these two squids in terms of their mantle length (ML) distribution and the relationship between mantle length and body weight (LWR), also considering the relative condition factors (Kn), and explored the effects of the El Niño and Southern Oscillation (ENSO) on their growth. The results showed that the ML for O. bartramii and D. gigas had significant differences among different years and different sexes (p < 0.01), and the size of females was larger than that of males. LWR showed that both squids demonstrated a positive allometric growth pattern (b > 3), and parameters a and b were influenced by year and sex. Furthermore, there were significant differences in Kn in both squids for different years and different sexes (p < 0.01), and their interannual fluctuations were quite significant. In conclusion, the alterations in the marine environment caused by climate change had a significant impact on the growth of O. bartramii and D. gigas in this study. ENSO events had opposite effects on the growth of both squid species.
1. Introduction
Cephalopods are considered one of the most valuable potential fishery resources in the world’s oceans [1]. At present, the commercially developed cephalopods mainly include species of the families Ommastrephidae, Loliginidae, Sepiidae, and Octopodidae, with Ommastrephidae accounting for 51–62% [1,2,3]. Ommastrephid squids are typical ecological opportunists with a short life cycle, fast growth, complex population structure, and long-distance migration [4,5,6]. The squid is an important component in the marine ecosystem and occupies an intermediate position in the food web [7]. The squid plays an important role as prey for large fish and marine mammals, and also as a predator that preys on crustaceans, small pelagic fish, and other cephalopods [1,8]. A change in its biomass directly affects the stability of the whole marine ecosystem [1,8,9]. In order to sustainably develop and utilize oceanic squid resources, it is necessary to strengthen basic research on its fishery biology [1,10,11]. At present, two large squid species, neon flying squid Ommastrephes bartramii and jumbo flying squid Dosidicus gigas, are the primary targets for Chinese squid-jigging fisheries in the Pacific Ocean [6,12].
Ommastrephes bartramii is a highly migratory oceanic species with distribution mainly in the North Pacific Ocean [13,14,15]. This species has become an important fishing target since 1974 [14]. Currently, the highest catch country of this species is Mainland China, which began to commercially exploit this species in 1994 [14]. O. bartramii can be divided into two segregated populations based on the different spawning peak and geographical location: a winter–spring population (Western population) that is mainly located west of 170° E and an autumn population (Eastern population) occurring east of 170° E, both of which have only about a one-year lifespan [16,17,18,19]. The winter–spring population, distributed in the western waters of the North Pacific Ocean, is the main fishing target population for Mainland China, with an annual catch of 37,000–132,000 tons, accounting for 80% of the global total catch of squid in the North Pacific Ocean [20,21].
The largest ommastrephid, D. gigas, is widely distributed in the eastern Pacific Ocean [22,23,24]. The population structure of D. gigas is complex [25]. Three groups were defined based on the size of adult males and females: the small group (male: 130–260 mm; female: 140–340 mm), the medium-sized group (male: 240–420 mm; female: 280–600 mm), and the large group (male: >400–500 mm; female: 550–650 mm to 1000–1200 mm) [25,26,27]. The main fishing grounds are located in the Gulf of California, the waters off the coast of Costa Rica, equatorial waters (5° N–5° S, 95–120° W), and the coastal and oceanic regions off Peru and Chile [6,25,28]. China conducted its first fishery survey of D. gigas in the high seas off Peru and Costa Rica in 2001, followed by commercial exploration [14]. In recent years, the most abundant fishing ground is located off the Peru exclusive economic zone waters, and the catch of D. gigas accounted for 60–90% of its global total catch [29].
As short-lived marine species, squids are highly susceptible to external factors. Among these factors, changes in marine environment are considered to be the main factor affecting the growth of squid [30,31,32]. Previous studies found that a warm water temperature results in faster growth, smaller body size, and smaller gonads, while a cold temperature results in slower growth, larger body size, longer lifespan, and larger gonads [31,32,33]. At the same time, the frequent extreme weather events (such as El Niño and La Niña) in recent years have not only had a great impact on squid resources but have also directly affected squid growth, resulting in a large difference in individual size compared with previous years [15,34,35]. In addition, previous studies have discussed how O. bartramii and D. gigas might experience concurrent fluctuations in catch, abundance, and/or distribution on various temporal scales, and found that they undergo synchronous changes due to climate or environmental changes [6,12,29]. During the warm Pacific decadal oscillation (PDO) phase, the environmental conditions in the western Pacific and eastern Pacific changed in opposite directions, resulting in an increase in suitable habitat for O. bartramii in the western Pacific and a decrease in suitable habitat for D. gigas in the eastern Pacific [12,36]. Yu et al. (2021) found that, from September to November, 2006 to 2015, there were interannual variations and synchronous fluctuation, with significantly negative associations in the abundance and distribution of O. bartramii and D. gigas, and they believed that the proportion of favorable sea surface temperature (SST) area conversely shifted due to SST anomaly changes at Niño 3.4 (5° N–5° S and 120–170° W) and Niño 1 + 2 regions (0–10° S and 90–80° W) [6]. In terms of individual growth, however, it is still unclear whether there is a link between the growth changes in these two squid species.
Individual growth patterns are most commonly described by the length–weight relationship (LWR), which is also one of the most important biological parameters in statistical modeling [37,38]. It provides important information for assessing individual population characteristics, understanding life cycles, ontogeny changes, growth, and fishery resource conservation [39,40]. Meanwhile, the relative condition factor, another important biological parameter derived from the LWR, measures the deviation of an individual from the average weight in a given sample in order to assess the suitability of environmental changes for individual growth [38,41,42,43].
The purpose of this study is to analyze the interannual growth changes in O. bartramii and D. gigas in the Pacific Ocean. Specifically, the growth characteristics of O. bartramii and D. gigas were studied according to samples collected in the Northwestern and Eastern Pacific Ocean in different years. We analyzed their sex and annual growth differences using a linear mixed-effects model (LMEM), and also explored the effects of climate change and the marine environment on the growth of two species under a decadal time series. This study provides basic information and a scientific basis for a comprehensive understanding of individual growth changes and the responses of the biological characteristics to climate change for two ommastrephid squids in the Pacific Ocean.
2. Materials and Methods
2.1. Sampling and Measurement
All samples in this study were collected in the Pacific Ocean by Chinese jigging fishing vessels. A total of 8560 O. bartramii samples were collected from 2009 to 2018 in the Northwest Pacific Ocean (38–46° N, 149–164° E), and a total of 5520 D. gigas samples were collected from 2008 to 2017 outside the exclusive economic zone waters of Peru (8–19° S, 78–87° W) (Table 1, Figure 1). All samples were immediately frozen at −20 °C on the vessel and transported to the laboratory for subsequent biological analysis in each consecutive year.

Table 1.
Sample size (total number) of Ommastrephes bartramii and Dosidicus gigas among years and sexes.
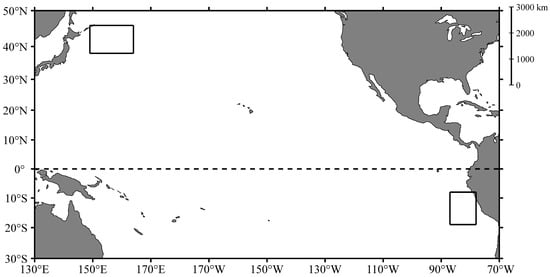
Figure 1.
Sampling area of Ommastrephes bartramii and Dosidicus gigas in the Pacific Ocean.
The thawed samples were subjected to biological determination, including mantle length (ML, ±1 mm) and body weight (BW, ±1 g). According to the approach proposed by Lipinski and Underhill (1995) [44], the sex and maturity stage were visually assessed.
2.2. The Relationship between Mantle Length and Body Weight (LWR)
The relationship between mantle length and body weight can be expressed as follows [41]:
where BW is the body weight of samples (g), ML is the mantle length of samples (mm), a is the scaling parameter, and b is the allometric growth parameter. Since the variance of BW increases with the increase in ML, the above equation was logarithmically transformed, and the equation becomes:
Simple LWR models are often constructed as single-factor regression models, which have poor ability to model the compound impact of multiple factors (such as geographic, interannual, and sex) [45]. A linear mixed-effect model (LMEM) is a powerful tool that allows for analyzing a wide variety of data structures [46,47]. In addition to the overall average and random error, the models also contain both fixed effects and random effects [47]. Due to its unique advantages, it has been widely used in many fields [46,48,49].
In this paper, a generalized linear model (GLM) and nine linear mixed-effect models were used to describe the relationship between mantle length and body weight in these two squids. Nine LMEMs take the influence of year (Y.I, Y.S) and/or sex (S.I, S.S) as the random effect of coefficient a and/or parameter b to explain the relationship between mantle length and body weight [49] (Table 2). The lme4 and nlme packages of R language software were used to complete the construction process of all models [50].

Table 2.
Models for length–weight relationship of two ommastrephid species and its fitting effects.
2.3. Model Comparison
Akaike information criterion (AIC) and root mean square error (RMSE) were used to compare the fit of the 10 models. AIC values are widely used to compare the quality of models [51]. We selected the best model with the lowest AIC value. RMSE is a value that measures how close a predicted value is to the final result [52]. The closer the value is to 0, the smaller the difference between the predicted value of the model and the observed value of the sample—that is, the better the prediction performance.
The AIC was calculated using the following equation:
where p is the number of parameters in the model, N is the number of samples, and M is the likelihood function.
The formula for calculating RMSE is as follows:
where represents the predicted value and represents the observed value.
2.4. Relative Condition Factor
The relative condition factor (Kn) was calculated to evaluate the condition factors of two squids. Kn was determined from the following equation [41,53]:
where Kn is the relative condition factor, W is the observed body weight, and Ws is the calculated weight.
2.5. Climatic Index Data
El Niño and La Niña are major climatic events that have a great impact on the growth of marine life in the Pacific Ocean [20,54]. The Niño index (NI) is an indicator that reflects the intensity of El Niño and La Niña events. The monthly SST anomalies (SSTA) in the Niño 3.4 region (5° N–5° S and 120–170° W, close to the O. bartramii fishing ground) and Niño 1 + 2 region (0–10° S and 90–80° W, close to the D. gigas fishing ground) were used as indicators to represent the climate variability in the Northwest and the Southeast Pacific Ocean, respectively [6]. The Oceanic NI data were obtained from the IRI/LDEO Climate Data Library (http://iridl.ldeo.columbia.edu/SOURCES/.Indices/ (accessed on 15 June 2022)).
According to the time of sample collection and the definition of El Niño and La Niña events, El Niño and La Niña events during 2008–2017 are classified (Table 3, Figure 2).

Table 3.
The classification of the El Niño and La Niña event from 2008 to 2018 according to the time of sample collection and the definition of El Niño and La Niña. All other unclassified years were normal.
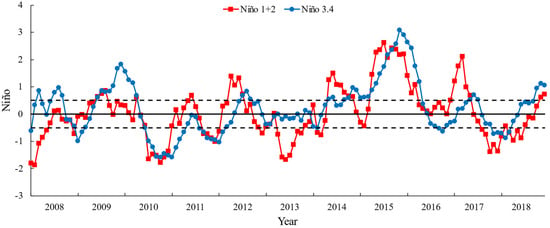
Figure 2.
The time series of Niño 3.4 (between 5° N and 5° S and 120° and 170° W) index and Niño 1 + 2 (between 0° and 10° S and 90° and 80° W) index from 2008 to 2018. Dashed lines of ± 0.5 are the definition of the El Niño/La Niña event: the NI over 0.5 represents the El Niño event and below −0.5 represents the La Niña event.
3. Results
3.1. Mantle Length Distribution
The mantle length of O. bartramii and D. gigas showed changing trends over the years. The distribution of O. bartramii was generally unimodal. Its ML was larger in 2009 and smaller in 2010, then gradually decreased from 2011 to 2014, and increased after 2015 (Figure 3). The distribution of D. gigas was scattered. Its ML was larger in 2009–2010, dominated by small and medium-size groups. Then, the ML gradually decreased from 2012 to 2016 and slightly increased in 2017, dominated by small groups (Figure 4). The ML of females and males was also different in each year. The size of females’ was larger than that of males’; the same trend was seen for both species. Two-way analysis of variance (ANOVA) results showed that the ML of both squids showed significant differences by year and sex (p < 0.01).
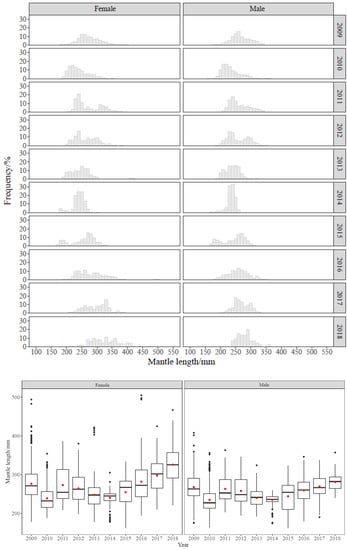
Figure 3.
Distribution of mantle length of O. bartramii in different years. The boxes show the median and the interquartile range. The black dots are indications of outliers in the data. The red dots show the mean.
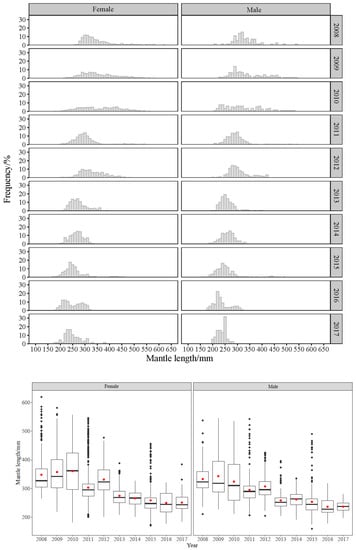
Figure 4.
Distribution of mantle length of D. gigas in different years. The boxes show the median and the interquartile range. The black dots are indications of outliers in the data. The red dots show the mean.
3.2. LWR
Based on the pooled data, a GLM was used to establish the LWR for both O. bartramii and D. gigas (Figure 5), and the following equations were obtained:
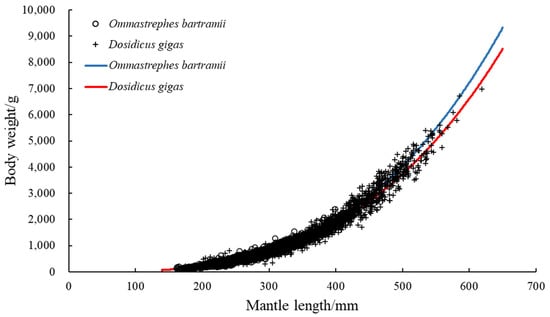
Figure 5.
The mantle length–weight relationships of O. bartramii and D. gigas based on the generalized linear model.
O. bartramii: BW = 1.0298 × 10−5 ML3.1843 (a: 95%CI = 9.5959 × 10−6, 1.1052 × 10−5; b: 95%CI = 3.1718, 3.1971).
D. gigas: BW = 1.2425 × 10−5 ML3.1412 (a: 95%CI = 1.1504 × 10−5, 1.3420 × 10−5; b: 95%CI = 3.1277, 3.1547).
The estimated allometric growth parameter b for O. bartramii was slightly larger than the estimated value for D. gigas, and the value was greater than 3 for both species.
3.3. Recommended LWR and Temporal and Sexual Variations in LWR
In this study, a generalized linear model and nine linear mixed-effects models were established to describe the relationship between mantle length and weight for the two ommastrephid species. The results showed that the LMEM (Y and S.I and S) had the smallest AIC and RMSE values (–17,216 and 0.08794, respectively), indicating that a random effect on both intercept and slope of year and sex was the best fit for LWR of O. bartramii. The same was true for D. gigas, where the AIC was –9748 and the RMSE was 0.09893 (Table 2). According to this selected model, a and b with fixed effects of O. bartramii were estimated at 1.3295 × 10−5 and 3.1395, respectively; and a and b with fixed effects of D. gigas were estimated at 1.8982 × 10−5 and 3.0629, respectively (Table 4, Figure S1). There were significant differences in the LWR of both squids by year and sex (p < 0.01).

Table 4.
Estimates values for parameters a and b in the LMEM (R and Y.I and S).
In terms of different years, the maximum estimated b value occurred in 2011 (3.2725) for O. bartramii, followed by the estimate (3.2515) in 2015, and it had its minimum value (2.9794) in 2018 (Table 4, Figure S1). For D. gigas, the largest estimate of the b value was 3.2418 in 2010, followed by the estimate (3.2204) in 2012, and the smallest estimate was 2.8727 in 2014 (Table 4, Figure S1). In terms of sex, the b value of females was larger than that of males, whether O. bartramii or D. gigas (Table 4, Figure S1).
3.4. The Relative Condition Factor (Kn)
The fluctuations of Kn values are represented in Figure 6. There were significant differences in Kn by year and sex (p < 0.01). For O. bartramii, The Kn value of the female ranged from 0.9668 to 1.0276, and the Kn value of the male ranged from 0.9844 to 1.0375. The Kn value of females was lower than that of males. The annual variation trend for females and males was roughly the same, showing an upward trend from 2010 to 2012 and a downward trend from 2012 to 2016. In 2010–2011 and 2014–2016, the Kn value was less than 1. A cross-correlation analysis revealed significantly positive relationships between the Niño 3.4 index and Kn, and the highest correlation coefficients were 0.5108 for females and 0.4323 for males.
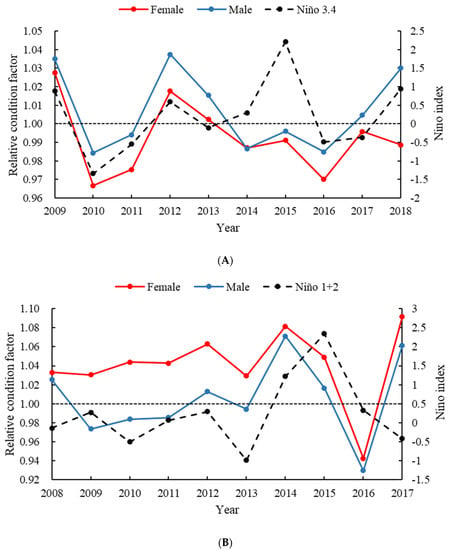
Figure 6.
Variation in relative condition factors and Niño index of O. bartramii (A) and D. gigas (B). The black dot lines show the relative condition factor equal to 1.0.
As far as D. gigas was concerned, the Kn value of females ranged from 0.9425 to 1.0913, and that of males ranged from 0.9295 to 1.0713. The Kn value of females was greater than that of males. The annual variation trend in female and male Kn values was also roughly the same. From 2009 to 2013, Kn values showed an upward trend, sharply decreased from 2014 to 2016, and were lowest in 2016. With the exception of 2016, the Kn value of females was greater than 1, while the Kn value of males was less than 1 in half of the years. A significantly negative cross-correlation was found between Niño 1+2 index and Kn at a time lag of one year, and the highest correlation coefficients were –0.7134 for females and –0.5613 for males.
4. Discussion
4.1. Body Size of the Two Squids
Short-lived cephalopods are always affected by large-scale climate change and marine environmental changes, resulting in changes in habitat, fishery center of gravity, resource abundance, and spatial distribution [55,56]. As typical phenotypic plastic species, squids tend to make their morphology variable to adapt to the changing environmental conditions they experience, especially as oceanic species [15,32,57]. In this study, the ML group of O. bartramii and D. gigas was analyzed, and it was found that there were significant differences in ML by year and sex (p < 0.01).
In this study, squid had a larger body size in El Niño years (2009), followed by normal years (2012, 2013, and 2014), and the smallest body size in La Niña years (2010 and 2011). A similar conclusion was also suggested for Todarodes pacificus in the East China Sea [58]. Previous studies have suggested that individual body size is closely related to water temperature, with higher water temperatures causing body size to shrink, and lower water temperatures increasing body size [32,33]. In El Niño years, the water temperature in the fishing ground for O. bartramii was lower, which made the individual body size increase, while in La Niña years, the water temperature was higher, leading to a decrease in body size [35,54]. From 2015 to 2016, a strong El Niño event occurred in the Pacific Ocean. The water temperature decreased abnormally and the growth rate was slow, which made the body size larger during this period than in the last several years [35,54]. The impact of this phenomenon continued until 2018.
With regard to D. gigas, previous studies indicated that the dominant population of this squid off Peru changed dramatically between the 1990s (the small size group) and the early 2000s (mainly large individuals) [27]. In this study, the population structure of D. gigas from 2008 to 2017 had small and medium-sized groups dominating in 2008–2010, while small groups dominated in the remaining years. The year 2010 was a La Niña year. At this time, the water temperature off Peru decreased and upwelling increased, which promoted the entry of nutrients into the upper and middle waters, promoting the massive reproduction of phytoplankton, and provided more primary productivity for the squid, resulting in a large body size [59,60]. However, El Niño events occurred for two consecutive years in 2014 and 2015. Previous studies showed that, during the El Niño event, the water temperature in the eastern Pacific Ocean increased abnormally and productivity decreased [59,60]. The higher temperature and lack of food may inhibit the growth of D. gigas, making the body size smaller during this period, and the influence of this phenomenon continued until 2017. In addition, previous studies noted that climate change leads to changes in the morphology of cephalopod beaks, which in turn affect individual food intake and ultimately reflect individual body size changes [24,61].
In general, the change in individual size of O. bartramii showed an opposite trend to that of D. gigas, especially in 2009–2011 and 2014–2016, which may be related to its geographical location. O. bartramii and D. gigas are located in the northern and southern hemispheres, respectively. ENSO events in the northwest Pacific and the southeast Pacific would show opposite environmental changes, i.e., the water temperature in the Northwest Pacific Ocean would decrease and the water temperature in the Southeast Pacific Ocean would increase during an El Niño year. This phenomenon presented an opposite pattern in La Niña years [6,54].
In addition, the average ML of female individuals was larger than that of male individuals in each year, and the annual difference in body size of females was greater than that of males. This indicated that female body morphology tends to be more plastic than males’, which is consistent with previous studies [15,18,62]. Generally, a female squid grows faster than a male squid due to its high feeding rate, resulting in faster metabolism and gonad growth, as also proved in Illex species (Illex illecebrous and Illex argentinus) [63]. This may be a survival strategy for squid to maintain a certain population scale [15,63].
4.2. LWR of Two Squids and Their Sexual and Interannual Variations
The intraspecific variation in LWR is a very important indicator, depending on geographic region, the year or season, population size range, and/or annual variation in environmental conditions [42,53]. Ten models were used to estimate the LWR of two important squid species in the Pacific Ocean. The results indicated that mixed-effects models performed better than the generalized linear model. Substantial sexual and annual variations in growth were revealed for these two squids on different aspects of scales. The allometric growth parameter b can be used to explore the growth form in the body proportion, and its interannual change can be used as an indicator of the adaptability of marine organisms to changes in the marine environment [42,46]. In addition, in LWR, parameter b can represent the growth pattern of squid (negative allometric, isometric, and positive allometric growth patterns) [64]. In addition, Hile (1936) pointed out that there were obvious differences in the intraspecific growth patterns of squid, with growth parameter b ranging from 2.5 to 4.0 [65]. According to the results of LMEM (Y and S.I and S), the b values of O. bartramii (2.9636–3.2338) and D. gigas (2.8488–3.2657) in this study were both within this range.
For O. bartramii, the effect of ENSO events on its growth pattern was not obvious. When ENSO events occurred, the growth of O. bartramii was in positive allometric growth mode with little change. However, we found that the b value of O. bartramii in very strong El Niño years (2015) was greater than that in moderate El Niño years (2009). On the contrary, the b value in a moderate La Niña year (2011) was higher than that in a strong La Niña year (2010), which suggested that the b value might be negatively correlated with temperature. Higher temperatures with higher productivity lead to an increase in the metabolic rate of squid; the digestion process will speed up and the body will tend to become slender [66]. In addition, studies showed that the Kuroshio Current advected warmer and food-rich waters into the fishing ground of O. bartramii in La Niña years, while in El Niño years, the fishing ground had colder water and lower productivity [35,67]. Abundant food often increased the thickness of the body, which resulted in the b value in La Niña years (2010, 2011) being higher than that in moderate El Niño years (2009). So, temperature and food supply are both important factors affecting the interannual variation in squid growth [15,66]. In D. gigas, the ENSO event caused significant differences in the growth relationship in different years. The growth parameter b of D. gigas in La Niña years (2010) was much larger than that in El Niño years (2014 and 2015). The b value of the individual in a normal year was somewhere in between. High temperature and low nutrient intake may inhibit the growth and development of squid, while low temperature and high nutrient intake are conducive to growth and development [59,60]. This explains why the b value of D. gigas was low/high during the El Niño/La Niña event in this study. Moreover, the interannual variation of the growth parameter b of D. gigas was greater than that of O. bartramii, and O. bartramii generally exhibited positive allometric growth, while D. gigas exhibited negative allometric growth for several years, which may be due to the difference in response of these two squids to ENSO events [35,60]. As mentioned above, when ENSO events occurred, the influence of the changes in temperature and bait abundance in the fishing ground of D. gigas on their b values was consistent, while the influence of the changes in temperature and bait abundance in the fishing ground of D. gigas on their b values was inconsistent.
In addition, this study found that the growth parameter b of females was greater than that of males in the two squid species, indicating that females had thicker bodies than males, a phenomenon also seen in Sepia officinalis [68] and Uroteuthis edulis [69]. Males convert most of the energy ingested into mantle growth, while females convert more of it into the reproductive system, which is reflected in the increase in body weight [70,71]. It can be inferred that the growth differences in cephalopods between different sexes may be related to the development of reproductive organs [15,71].
4.3. Relative Condition Factors of Two Squids
A change in growth pattern reflects changes in the squid body, which can be analyzed by conditional factors [41]. The relative condition factor (Kn) is an effective method to compare the relationship between individual weight and length at different stages [41,42,52]. The deviation between the Kn value and 1 reveals individual feeding condition or the fitness/well-being [41,72]. When Kn ≥ 1, the growth condition of the individual is good, while when Kn < 1, the growth of the individual is poor compared to the average individual of the same length [43,72].
In this study, the growth status of O. bartramii fluctuated dramatically. The Kn value was relatively low, indicating that the growth condition of O. bartramii was poor. The growth condition of females was worse than that of males. We also found that in La Niña years (2010 and 2011) and the super El Niño year (2015), the Kn value was less than 1, indicating that its growth condition was poor, while in the moderate El Niño year of 2009, its growth condition was good.
For D. gigas, the growth condition was relatively good in these years, and the growth condition of females was greater than that of males. Its growth condition fluctuated slightly from 2009 to 2012 and showed an upward trend, but there was a large difference between males and females. Pecl et al. (2004) believed that the female individual had better mantle condition and higher reproductive growth in colder years [73]. Thus, the low water temperature caused by the La Niña event in 2010 may have contributed to this phenomenon. In addition, the Kn value fell sharply from 2014 to 2015, which might be due to the adverse environmental conditions caused by the El Niño in 2014–2015. Previous studies have shown that the poor growth condition of D. gigas in the Gulf of California and Mexican waters in 1998 and 1999 was considered to be caused by the 1997–1998 El Niño event [53]. The Kn value increased sharply in 2017, which may be related to the weakening of El Niño in 2016 and the La Niña event in the second half of 2017. Overall, the growth condition of O. bartramii was positively correlated with the Niño index, while the growth condition of D. gigas was negatively correlated with the Niño index. We also found that the Kn value of D. gigas showed a low peak and a high peak in 2013 (La Niña year) and 2014 (El Niño year), respectively, and the Kn of O. bartramii was also at a high peak in some normal years. Therefore, we believe that the growth of squid might not only be regulated by climatic factors but also affected by some other factors. Studies had also shown that many factors affect the individual’s growth, including the reproductive cycle, level of gonadal maturity, number and density of predators, food availability, and habitat [42,53,74]. In addition, the relative condition factor is considered as the nutrient conditions of spawners; the survival rate of eggs is also lower when the growth/nutrient condition is poor [71,75,76,77]. Therefore, we can appropriately adjust the management strategy according to the actual situation. For example, with decreasing trends of Kn, fishermen can reduce resource development in the next few years to protect resources.
5. Conclusions
In this study, we explored the growth characteristics of these two squids by mantle length composition, LWR, and relative condition factors, and a series of life history parameters were obtained, which provided a certain reference value for subsequent study of population dynamics and related fishery management. In addition, the research showed that the growth of O. bartramii and D. gigas underwent obvious interannual changes, and their change trends were almost opposite, especially during the period of ENSO events. Thus, when ENSO events occur, how the growth of the two squids change with the environmental conditions can be deduced based on the present study, and we can provide different management schemes for the two squids. In addition, we could combine statolith microchemistry and stable isotopes (δ13C and δ15N) in future research to more accurately determine the habitats they experience at each stage of their life history by speculating on their migration route, so as to carry out profound research into the impact of climate change on their individual growth and migration paths.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes7050280/s1, Figure S1. Variations of a and b among years and sexes from the LMEM (Y&S.I&S). (A,B): estimates of parameter a and b with random effects of both years and sexes. (C): temporal variations of ln(a) and b. (D): Sexual variations of ln(a) and b.
Author Contributions
Conceptualization, Z.F. and J.L.; methodology, P.H. and Y.D.; software, P.H. and Y.D.; formal analysis, P.H.; writing—original draft preparation, P.H.; writing—review and editing, Z.F., B.L. and X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Program of China (Ministry of Science and Technology of China, 2019YFD0901404), the National Nature Foundation of China on the project (National Nature Science Foundation of China, NSFC41876141), and Funding for the Opening of Key Laboratories for Offshore Fishery Development by the Ministry of Agriculture (South China Sea Fisheries Research Institute, LOF 2021-01).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Code of Ethics of the University Department of Marine Studies; all specimens obtained from the surveys were already dead.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The support from the captains and crews of commercial jigging vessels in scientific surveys is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, X.J.; Lu, H.J.; Liu, B.L.; Tian, S.Q. Current exploitation and some scientific issues in the sustainable utilization of Ommastrephidae. J. Shanghai Ocean. Univ. 2012, 21, 831–840. [Google Scholar]
- Arkhipkin, A.I.; Rodhouse, P.G.K.; Pierce, G.J.; Sauer, W.; Sakai, M.; Allcock, L.; Arguelles, J.; Bower, J.R.; Castillo, G.; Ceriola, L.; et al. World squid fisheries. Rev. Fish. Sci. Aquac. 2015, 23, 92–252. [Google Scholar] [CrossRef]
- Wang, J.T.; Chen, X.J.; Tanaka, K.; Cao, J.; Chen, Y. Environmental influences on commercial oceanic ommastrephid squids: A stock assessment perspective. Sci. Mar. 2017, 81, 37–47. [Google Scholar] [CrossRef]
- Boyle, P.R. Cephalopod biology in the fisheries context. Fish. Res. 1990, 8, 303–321. [Google Scholar] [CrossRef]
- Rodhouse, P.G. Large-scale range expansion and variability in ommastrephid squid populations: A review of environmental links. CalCOFI Rep. 2008, 49, 83–89. Available online: https://oceanrep.geomar.de/id/eprint/53855 (accessed on 5 September 2022).
- Yu, W.; Chen, X.J.; Liu, L.W. Synchronous Variations in Abundance and Distribution of Ommastrephes bartramii and Dosidicus gigas in the Pacific Ocean. J. Ocean. Univ. China 2021, 20, 695–705. [Google Scholar] [CrossRef]
- Parry, M. Feeding behavior of two ommastrephid squids Ommastrephes bartramii and Sthenoteuthis oualaniensis off Hawaii. Mar. Ecol. Prog. Ser. 2006, 318, 229–235. [Google Scholar] [CrossRef]
- Cao, J.; Chen, X.J.; Liu, B.L.; Tian, S.Q.; Qian, W.G. Review on the relationship between stock recruitment of squid and oceanographic environment. J. Shanghai Ocean Univ. 2010, 19, 232–239. [Google Scholar]
- Caccavo, J.A.; Christiansen, H.; Constable, A.J.; Ghigliotti, L.; Trebilco, R.; Brooks, C.M.; Cotte, C.; Desvignes, T.; Dornan, T.; Jones, C.D.; et al. Productivity and Change in Fish and Squid in the Southern Ocean. Front. Ecol. Evol. 2021, 9, 624918. [Google Scholar] [CrossRef]
- Chemshirova, I.; Hoving, H.J.; Arkhipkin, A. Temperature effects on size, maturity, and abundance of the squid Illex argentinus (Cephalopoda, Ommastrephidae) on the Patagonian Shelf. Estuar. Coast. Shelf Sci. 2021, 255, 107343. [Google Scholar] [CrossRef]
- Wang, Y.; Han, P.W.; Fang, Z.; Chen, X.J. Climate-induced life cycle and growth variations of neon flying squid (Ommastrephes bartramii) in the North Pacific Ocean. Aquac. Fish. 2021, 8, 211–220. [Google Scholar] [CrossRef]
- Yu, W.; Wen, J.; Chen, X.J.; Gong, Y.; Liu, B.L. Trans-Pacific multidecadal changes of habitat patterns of two squid species. Fish. Res. 2021, 233, 105762. [Google Scholar] [CrossRef]
- Murata, M. Oceanic resources of squids. Mar. Freshw. Behav. Phy 1990, 18, 19–71. [Google Scholar] [CrossRef]
- Chen, X.J.; Liu, B.L.; Chen, Y. A review of the development of Chinese distant-water squid jigging fisheries. Fish. Res. 2008, 89, 211–221. [Google Scholar] [CrossRef]
- Fang, Z.; Han, P.W.; Wang, Y.; Chen, Y.Y.; Chen, X.J. Interannual variability of body size and beak morphology of the squid Ommastrephes bartramii in the North Pacific Ocean in the context of climate change. Hydrobiologia 2021, 848, 1295–1309. [Google Scholar] [CrossRef]
- Bower, J.R.; Ichii, T. The red flying squid (Ommastrephes bartramii): A review of recent research and the fishery in Japan. Fish. Res. 2005, 76, 39–55. [Google Scholar] [CrossRef]
- Fang, Z.; Thompson, K.; Jin, Y.; Chen, X.J.; Chen, Y. Preliminary analysis of beak stable isotopes (δ13C and δ15N) stock variation of neon flying squid, Ommastrephes bartramii, in the North Pacific Ocean. Fish. Res. 2016, 177, 153–163. [Google Scholar] [CrossRef]
- Fang, Z.; Li, J.H.; Thompson, K.; Hu, F.F.; Chen, X.J.; Liu, B.L.; Chen, Y. Age, growth, and population structure of the red flying squid (Ommastrephes bartramii) in the North Pacific Ocean, determined from beak microstructure. Fish. Bull. 2016, 114, 34–44. [Google Scholar] [CrossRef]
- Han, P.W.; Fang, Z.; Li, N.; Chen, X.J. Migration Route Reconstruction of Different Cohorts of Ommastrephes bartramii in the North Pacific Based on Statolith Microchemistry. Front. Mar. Sci. 2022, 9, 832639. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X.J.; Yi, Q.; Gao, G.; Chen, Y. Impacts of climatic and marine environmental variations on the spatial distribution of Ommastrephes bartramii in the Northwest Pacific Ocean. Acta Oceanol. Sin. 2016, 35, 108–116. [Google Scholar] [CrossRef]
- Wang, Y.; Han, P.W.; Chen, X.J.; Fang, Z. Interannual and ontogenetic stage differences on trophic ecology of neon flying squid Ommastrephes bartramii inferred from stable isotope analyses in beaks. Fish. Res. 2022, 249, 106252. [Google Scholar] [CrossRef]
- Argüelles, J.; Rodhouse, P.G.; Villegas, P.; Castillo, G. Age, growth and population structure of the jumbo flying squid Dosidicus gigas in Peruvian waters. Fish. Res. 2001, 54, 51–61. [Google Scholar] [CrossRef]
- Taipe, A.; Yamashiro, C.; Mariategui, L.; Rojas, P.; Roque, C. Distribution and concentrations of jumbo flying squid (Dosidicus gigas) off the Peruvian coast between 1991 and 1999. Fish. Res. 2001, 54, 21–32. [Google Scholar] [CrossRef]
- Hu, G.Y.; Yu, W.; Li, B.L.; Chen, X.J.; Chen, Y.; Li, J.H. Impacts of El Niño on the somatic condition of Humboldt squid based on the beak morphology. J. Ocean. Limnol. 2019, 37, 1440–1448. [Google Scholar] [CrossRef]
- Liu, B.L.; Chen, X.J.; Yi, Q. A comparison of fishery biology of jumbo flying squid, Dosidicus gigas outside three exclusive economic zones in the Eastern Pacific Ocean. Chin. J. Oceanol. Limnol. 2013, 31, 523–533. [Google Scholar] [CrossRef]
- Nigmatullin, C.M.; Nesis, K.N.; Arkhipkin, A.I. A review of the biology of the jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae). Fish. Res. 2001, 54, 9–19. [Google Scholar] [CrossRef]
- Argüelles, J.; Tafur, R.; Taipe, A.; Villegas, P.; Keyl, F.; Dominguez, N.; Salazar, M. Size increment of jumbo flying squid Dosidicus gigas mature females in Peruvian waters, 1989–2004. Prog. Oceanogr. 2008, 79, 308–312. [Google Scholar] [CrossRef]
- Ibáñez, C.M.; Sepúlveda, R.D.; Ulloa, P.; Keyl, F.; Pardo-Gandarillas, M.C. The biology and ecology of the jumbo squid Dosidicus gigas (Cephalopoda) in Chilean waters: A review. Lat. Am. J. Aquat. Res. 2015, 43, 402–414. [Google Scholar] [CrossRef]
- Chen, P.; Chen, X.J.; Yu, W.; Lin, D.M. Interannual Abundance Fluctuations of Two Oceanic Squids in the Pacific Ocean Can Be Evaluated Through Their Habitat Temperature Variabilities. Front. Mar. Sci. 2021, 8, 770224. [Google Scholar] [CrossRef]
- Jackson, G.; Moltschaniwskyj, N. Spatial and temporal variation in growth rates and maturity in the Indo-Pacific squid Sepioteuthis lessoniana (Cephalopoda: Loliginidae). Mar. Biol. 2022, 140, 747–754. [Google Scholar] [CrossRef]
- Forsythe, J.W. Accounting for the effect of temperature on squid growth in nature: From hypothesis to practice. Mar. Freshw. Res. 2004, 55, 331–339. [Google Scholar] [CrossRef]
- Arkhipkin, A.; Argüelles, J.; Shcherbich, Z.; Yamashiro, C. Ambient temperature influences adult size and life span in jumbo squid (Dosidicus gigas). Can. J. Fish. Aquat. Sci. 2015, 72, 400–409. [Google Scholar] [CrossRef]
- Takahara, H.; Kidokoro, H.; Sakurai, Y. High temperatures may halve the lifespan of the Japanese flying squid, Todarodes pacificus. J. Nat. Hist. 2017, 51, 2607–2614. [Google Scholar] [CrossRef]
- Hoving, H.J.T.; Gilly, W.F.; Markaida, U.; Benoit-Bird, K.; Brown, Z.W.; Daniel, P.; Field, W.F.; Parassenti, L.; Liu, B.L.; Campos, B. Extreme plasticity in life-history strategy allows a migratory predator (jumbo squid) to cope with a changing climate. Glob. Chang. Biol. 2013, 19, 2089–2103. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Chen, X.J.; Chen, C.S.; Zhang, Y. Impacts of oceanographic factors on interannual variability of the winter-spring cohort of neon flying squid abundance in the Northwest Pacific Ocean. Acta Oceanol. Sin. 2017, 36, 48–59. [Google Scholar] [CrossRef]
- Feng, Z.P.; Yu, W.; Chen, X.J. Concurrent habitat fluctuations of two economically important marine species in the Southeast Pacific Ocean off Chile in relation to ENSO perturbations. Fish. Oceanogr. 2021, 31, 123–134. [Google Scholar] [CrossRef]
- Zepeda-Benitez, V.Y.; Morales-Bojórquez, E.; Díaz-Uribe, J.G.; Nevárez-Martínez, M.O.; Hernández-Herrera, A.; López-Martínez, J. Implementation of catch-at-age model for the jumbo squid Dosidicus gigas. Ecol. Model. 2017, 344, 6–16. [Google Scholar] [CrossRef]
- Granados-Amores, J.; Salinas-Zavala, C.A.; Flores-ortega, J.R.; Díaz-Santana-Iturrios, M. Length-weight relationship and condition factor for 7 loliginid squid species in Mexican waters. Cienc. Mar. 2019, 45, 175–180. [Google Scholar] [CrossRef]
- Seçer, B.; Sungur, S.; Çiçek, E.; Mouludi-Saleh, A.; Eagderi, S. Length-weight relationship and condition factor of endemic genus Seminemacheilus (Teloestei = Nemacheilidae) for Turkey. Limnol. Freshw. Biol. 2021, 4, 1152–1155. [Google Scholar] [CrossRef]
- Acarli, D.; Kale, S.; Çakır, K. Length–Weight Relationships of Eighteen Fishes and a Cephalopod from Gökçeada Island, Northern Aegean Sea, Turkey. Thalassas 2022, 38, 479–486. [Google Scholar] [CrossRef]
- Le Cren, E.D. The length-weight relationship and seasonal cycle in gonad weight and condition in the Perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Jisr, N.; Younes, G.; Sukhn, C.; El-Dakdouki, M.H. Length-weight relationships and relative condition factor of fish inhabiting the marine area of the Eastern Mediterranean city, Tripoli-Lebanon. Egypt. J. Aquatic. Res. 2018, 44, 299–305. [Google Scholar] [CrossRef]
- Lipinski, M.R.; Underhill, L.G. Sexual maturation in squid: Quantum or continuum. S. Afr. J. Mar. Sci. 1995, 15, 207–223. [Google Scholar] [CrossRef]
- Han, P.W.; Li, N.; Fang, Z.; Chen, X.J. Heterogeneity of mantle length-body mass relationship in different Ommastrephes bartramii populations based on linear mixed model. S. China Fish. Sci. 2020, 16, 12–20. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Tian, S.Q.; Han, D.Y.; Richard, K.; Gao, C.H.; Liu, W.C. Growth and maturity heterogeneity of three croaker species in the East China Sea. Reg Stud. Mar. Sci. 2021, 41, 101483. [Google Scholar] [CrossRef]
- Schielzeth, H.; Dingemanse, N.J.; Nakagawa, S.; Westneat, D.F.; Allegue, H.; Teplitsky, C.; Réale, D.; Dochtermann, N.A.; Garamszegi, L.Z.; Araya-Ajoy, Y.G. Robustness of linear mixed-effects models to violations of distributional assumptions. Methods Ecol. Evol. 2020, 11, 1141–1152. [Google Scholar] [CrossRef]
- Qian, S.S.; Cuffney, T.F.; Alameddine, I.; McMahon, G.; Reckhow, K.H. On the application of multilevel modeling in environmental and ecological studies. Ecology 2010, 91, 355–361. [Google Scholar] [CrossRef]
- Xu, H.; Sun, Y.J.; Wang, X.J.; Wang, J.; Fu, Y. Linear mixed-effects models to describe individual tree crown width for China-fir in Fujian province, southeast China. PLoS ONE 2015, 10, e0122257. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Automat. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Cai, K.; Richard, K.D.; Ma, Q.Y.; Han, X.B.; Qin, S. Growth Heterogeneity of Chub Mackerel (Scomber japonicus) in the Northwest Pacific Ocean. J. Mar. Sci. Eng. 2022, 10, 301. [Google Scholar] [CrossRef]
- Ferreri, G.A.B. Length-Weight Relationships and Condition Factors of the Humboldt Squid (Dosidicus Gigas) from the Gulf of california and the Pacific Ocean. J. Shellfish Res. 2014, 33, 769–780. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.J.; Fang, Z. Effects of marine environment variation on the growth of neon flying squid (Ommastrephes bartramii) in the north Pacific Ocean. J. Fish. China 2022, 46, 569–582. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhao, X.H.; Chen, Y. Influence of El Niño/La Niña on the western winter-spring cohort of neon flying squid (Ommastrephes bartramii) in the northwestern Pacific Ocean. ICES J. Mar. Sci. 2007, 64, 1152–1160. [Google Scholar] [CrossRef]
- Nishikawa, H.; Toyoda, T.; Masuda, S.; Ishikawa, Y.; Sasaki, Y.; Igarashi, H.; Sakai, M.; Seito, M.; Awaji, T. Wind-induced stock variation of the neon flying squid (Ommastrephes bartramii) winter-spring cohort in the subtropical North Pacific Ocean. Fish. Oceanogr. 2015, 24, 229–241. [Google Scholar] [CrossRef]
- Jones, J.B.; Pierce, G.J.; Saborido-Rey, F.; Brickle, P.; Kuepper, F.C.; Shcherbich, Z.N.; Arkhipkin, A.I. Size-dependent change in body shape and its possible ecological role in the Patagonian squid (Doryteuthis gahi) in the Southwest Atlantic. Mar. Biol. 2019, 166, 54. [Google Scholar] [CrossRef]
- Ning, X.; Lu, H.J.; Liu, K.; Chen, Z.Y.; Chen, X.J. Fisheries biological characteristics of Japanese common squid (Todarodes pacificus) in spring in the La Niña a year of 2018 in the East China Sea. J. Fish. China 2020, 44, 1676–1684. [Google Scholar] [CrossRef]
- Waluda, C.M.; Rodhouse, P.G.K. Remotely sensed mesoscale oceanography of the Central Eastern Pacific and recruitment variability in Dosidicus gigas. Mar. Ecol. Prog. Ser. 2006, 310, 25–32. [Google Scholar] [CrossRef]
- Robinson, C.J.; Gómez-Gutiérrez, J.; Markaida, U.; Gilly, W.F. Prolonged decline of jumbo squid (Dosidicus gigas) landings in the Gulf of California is associated with chronically low wind stress and decreased chlorophyll a after El Niño 2009–2010. Fish. Res. 2016, 173, 128–138. [Google Scholar] [CrossRef]
- Hu, G.Y.; Chen, X.J.; Fang, Z. Effect of individual growth on beak morphometry of jumbo flying squid, Dosidicus gigas off the Peruvian Exclusive Economic Zone. J. Fish. China 2016, 40, 36–44. [Google Scholar] [CrossRef]
- Markaida, U.; Quiñónez-Velázquez, C.; Sosa-Nishizaki, O. Age, growth and maturation of jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae) from the Gulf of California, Mexico. Fish. Res. 2004, 66, 31–47. [Google Scholar] [CrossRef]
- O’Dor, R.K. Chapter 11. Squid life-history strategies. In Squid Recruitment Dynamic; Rodhouse, P.D., Dawe, E.G., O’Dor, R.K., Eds.; FAO: Rome, Italy, 1998; pp. 233–254. [Google Scholar]
- Froese, R.; Tsikliras, A.C.; Stergiou, K.I. Editorial Note on Weight–Length Relations of Fishes. Acta Ichthyol. Piscat. 2011, 41, 261–263. [Google Scholar] [CrossRef]
- Hile, R. Age and growth of the cisco, Leucichthys artedi (le Sueur), in the lakes of the Northeastern highlands, Wisconsin. Bull. Bur. Fish. 1936, 48, 211–317. [Google Scholar]
- Keyl, F.; Argüelles, J.; Mariategui, L.; Tafur, R.; Wolff, M.; Yamashiro, C. A hypothesis on range expansion and spatio-temporal shifts in size-at-maturity of jumbo squid (Dosidicus gigas) in the Eastern Pacific Ocean. CCOFI. Rep. 2008, 49, 119–128. [Google Scholar]
- Qian, M.T.; Gong, J.W.; Fan, J.T.; Yu, W.; Chen, X.J.; Qian, W.G. Variations in the abundance and spatial distribution of Ommastrephes bartramii in the Northwest Pacific Ocean based on photosynthetic active radiation. Haiyang Xuebao 2020, 42, 44–53. [Google Scholar] [CrossRef]
- Vasconcelos, P.; Pereira, F.; Carvalho, A.N.; Gaspar, M.B. Weight-length relationships and relative growth of the cuttlefish (Sepia officinalis): Causes and effects of hypoallometry. Thalassas 2018, 34, 323–331. [Google Scholar] [CrossRef]
- Chen, J.Y.; Ching, T.Y.; Chen, C.S. Inter-annual variability in growth and maturation of the swordtip squid Uroteuthis edulis in Yilan Bay off northeastern Taiwan. J. Mar. Sci. Technol. 2022, 30, 158–171. [Google Scholar] [CrossRef]
- Dawe, E.G.; Beck, P.C. Population structure, growth, and sexual maturation of short-finned squid (Illex illecebrosus) at Newfoundland. Can. J. Fish. Aquat. Sci. 1997, 54, 137–146. [Google Scholar] [CrossRef]
- Chen, X.J.; Han, F.; Zhu, K.; Punt, A.E.; Lin, D.M. The breeding strategy of female jumbo squid Dosidicus gigas: Energy acquisition and allocation. Sci. Rep. 2020, 10, 9639. [Google Scholar] [CrossRef] [PubMed]
- Fauziyah.; Purwiyanto, A.I.S.; Agustriani, F.; Putri, W.A.E. Growth aspect of squid (Loligo chinensis) from the Banyuasin Coastal Waters, South Sumatra, Indonesia. Ecol. Montenegrina 2020, 27, 1–10. [Google Scholar] [CrossRef]
- Pecl, G.T.; Moltschaniwskyj, N.A.; Tracey, S.R.; Jordan, A.R. Inter-annual plasticity of squid life history and population structure: Ecological and management implications. Oecologia 2004, 139, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Morato, T.; Afonso, P.; Loirinho, P.; Barreiros, J.P.; Sanstos, R.S.; Nash, R.D.M. Length-weight relationships for 21 costal fish species of the Azores, Northeastern Atlantic. Fish. Res. 2001, 50, 297–302. [Google Scholar] [CrossRef]
- Yatsu, A.; Midorikawa, S.; Shimada, T.; Uozumi, Y. Age and growth of the neon flying squid, Ommastrephes bartrami, in the North Pacific Ocean. Fish. Res. 1997, 29, 257–270. [Google Scholar] [CrossRef]
- Nigmatullin, C.M.; Markaida, U. Oocyte development, fecundity and spawning strategy of large sized jumbo squid Dosidicus gigas (Oegopsida: Ommastrephinae). J. Mar. Biol. Assoc. UK 2009, 89, 789–801. [Google Scholar] [CrossRef]
- Nishikawa, H.; Igarashi, H.; Ishikawa, Y.; Sakai, M.; Kato, Y.; Ebina, M.; Usui, N.; Kamachi, M.; Awaji, T. Impact of paralarvae and juveniles feeding environment on the neon flying squid (Ommastrephes bartramii) winter-spring cohort stock. Fish. Oceanogr. 2014, 23, 289–303. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).