Phosphorus Pools Associated with Fish in the Archipelago Sea
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Phosphorus and Water Quality Analytics
2.3. Phosphorus Budget of the Archipelago Sea
2.4. Catch Statistics
2.5. Herring Stock Assessment
2.6. Perch Stock Assessment
2.7. Bioenergetic Models
2.7.1. Herring Growth and Energy
2.7.2. Perch Growth and Energy
2.8. Phosphorus Content of Other Fish Species
2.9. Statistical Analysis
3. Results
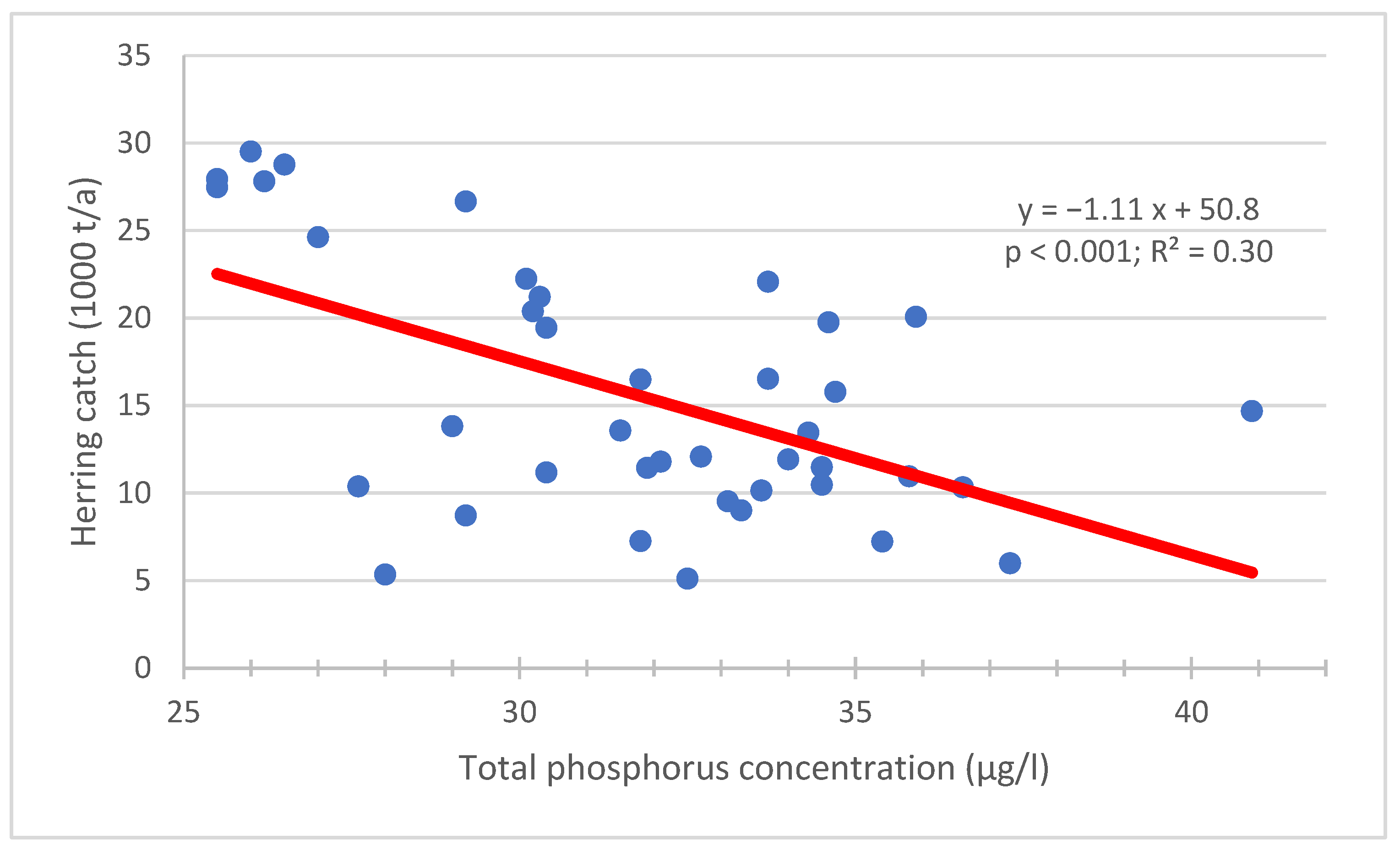
3.1. The Phosphorus Pool in the Water Column of the Archipelago Sea
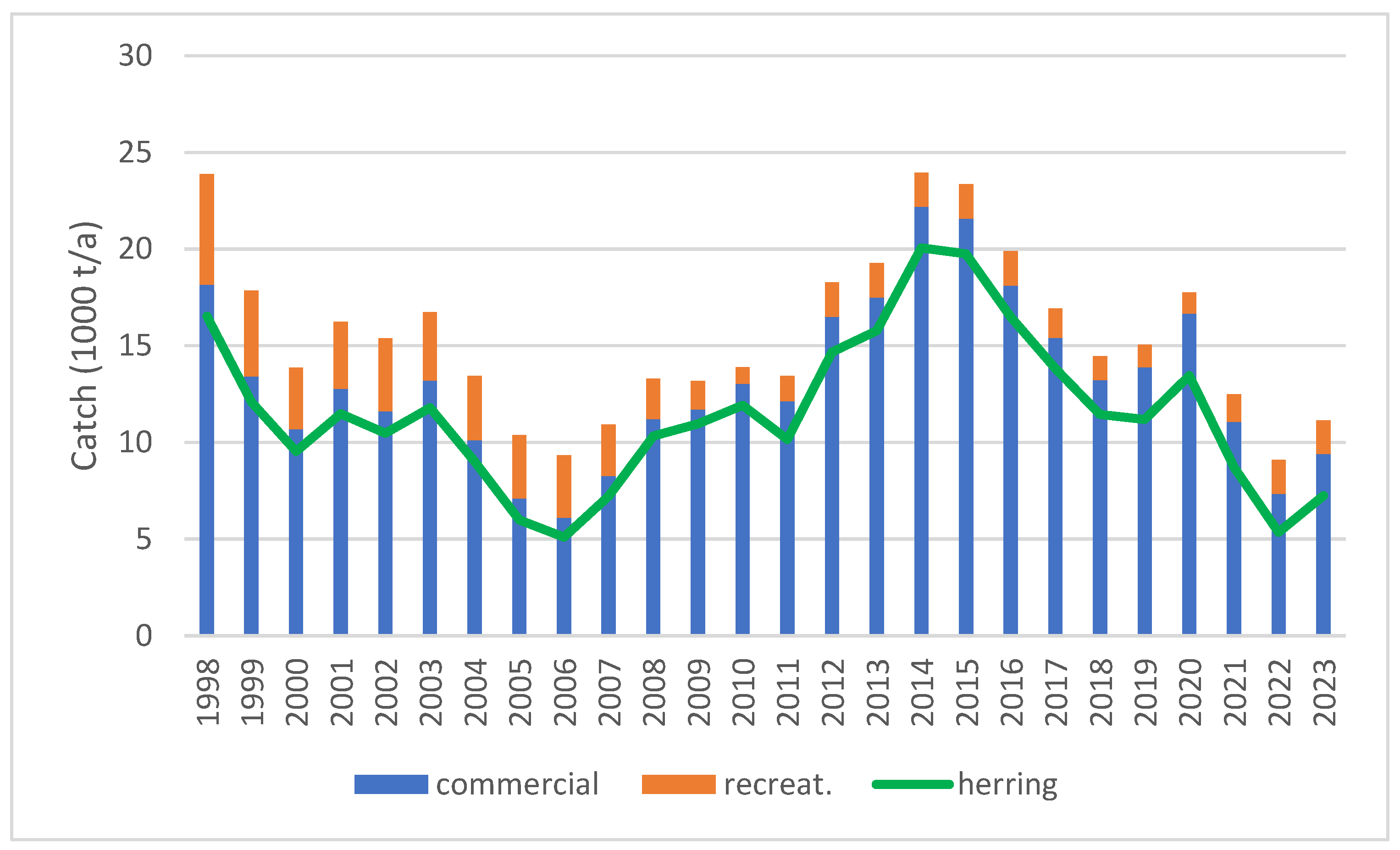
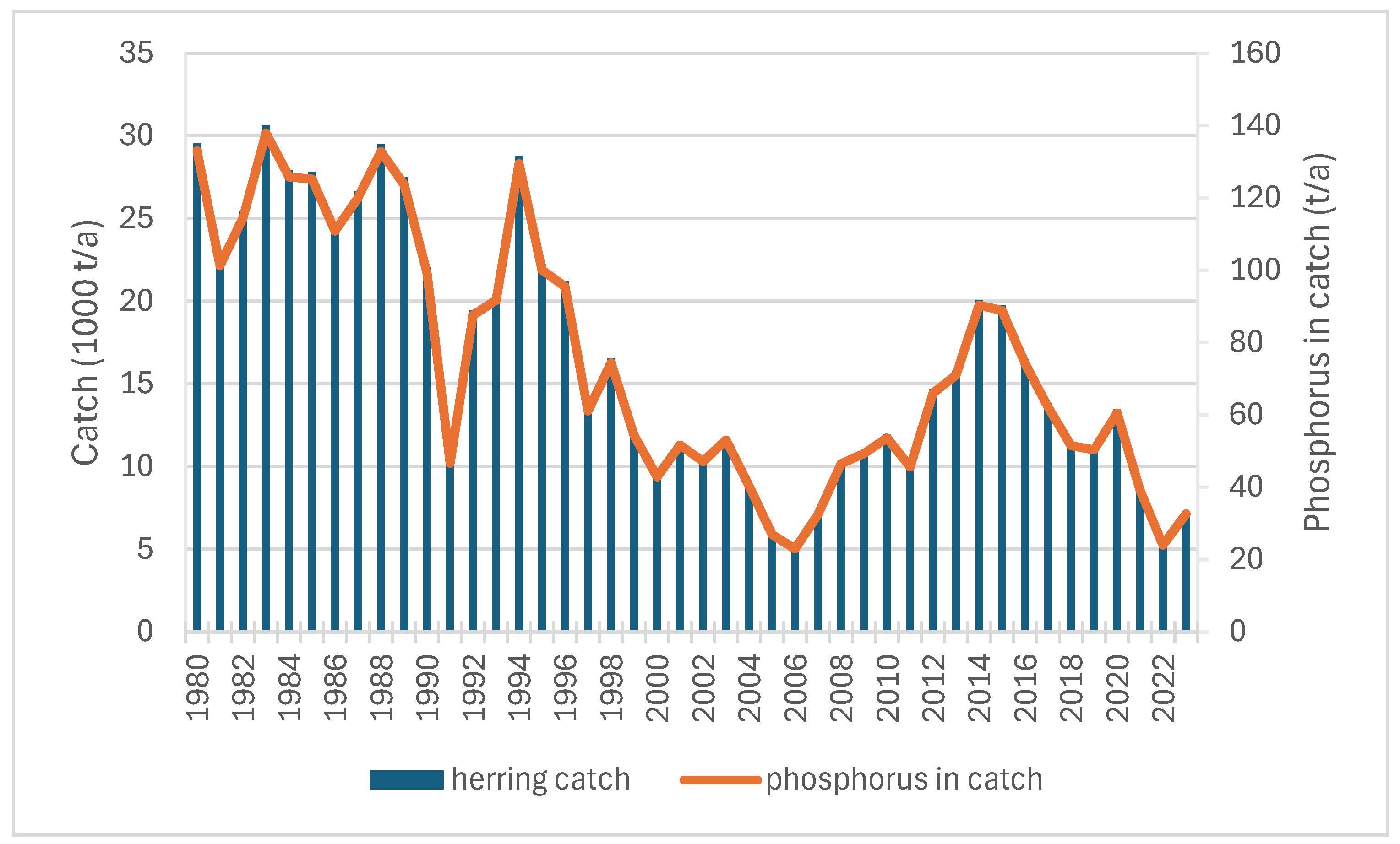
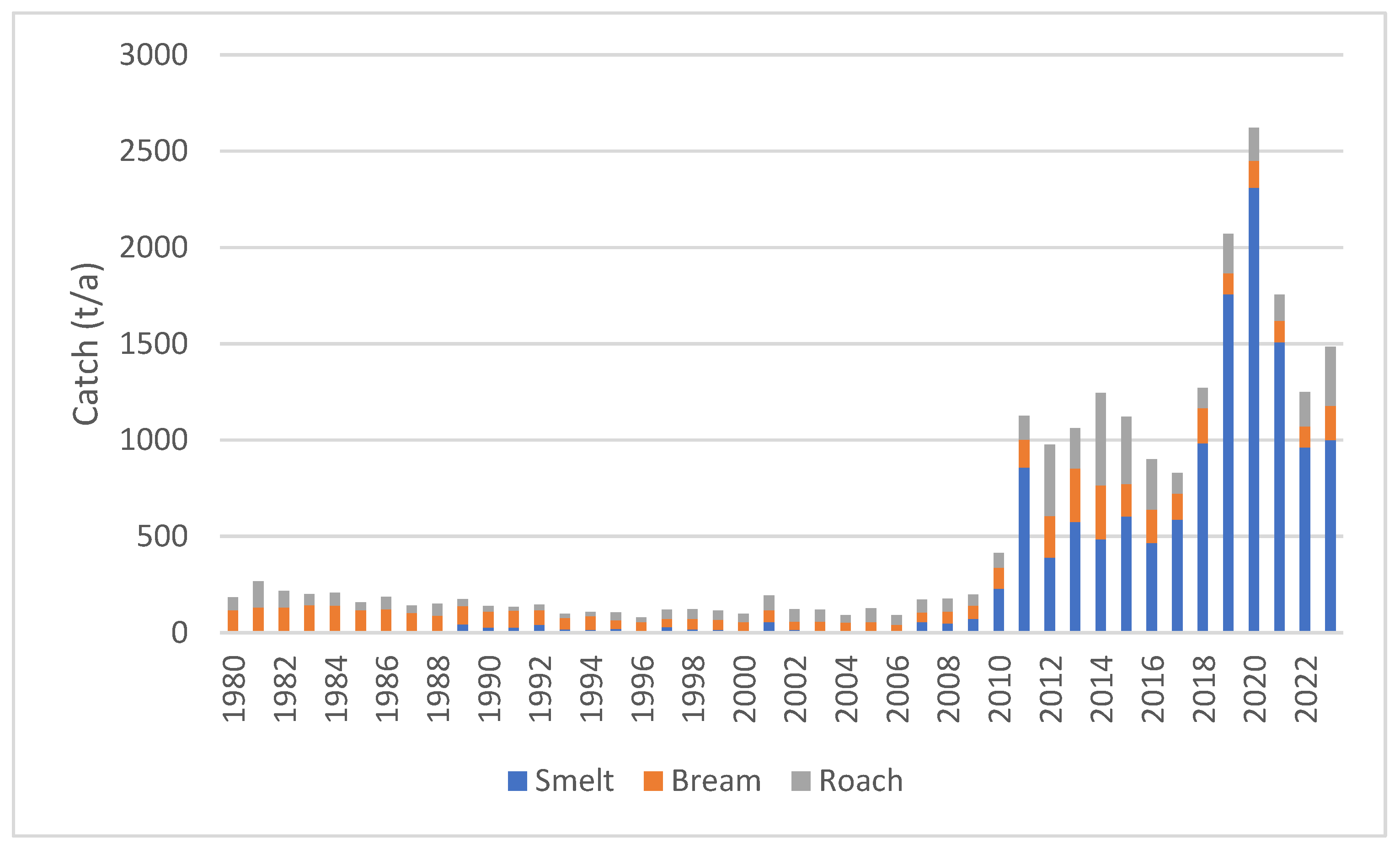
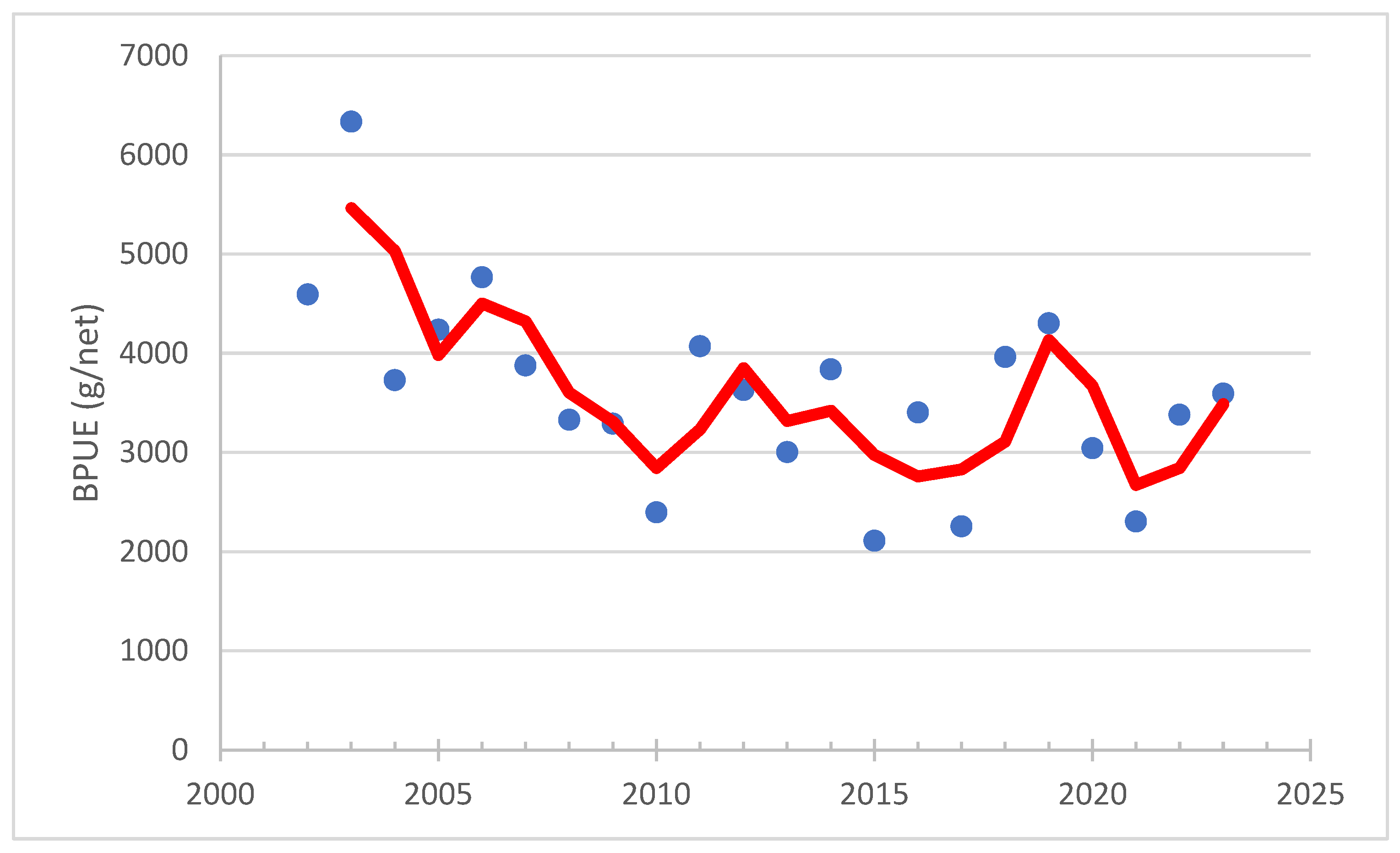
3.2. Fish Catches in the Archipelago Sea and Their Associated Phosphorus Contents
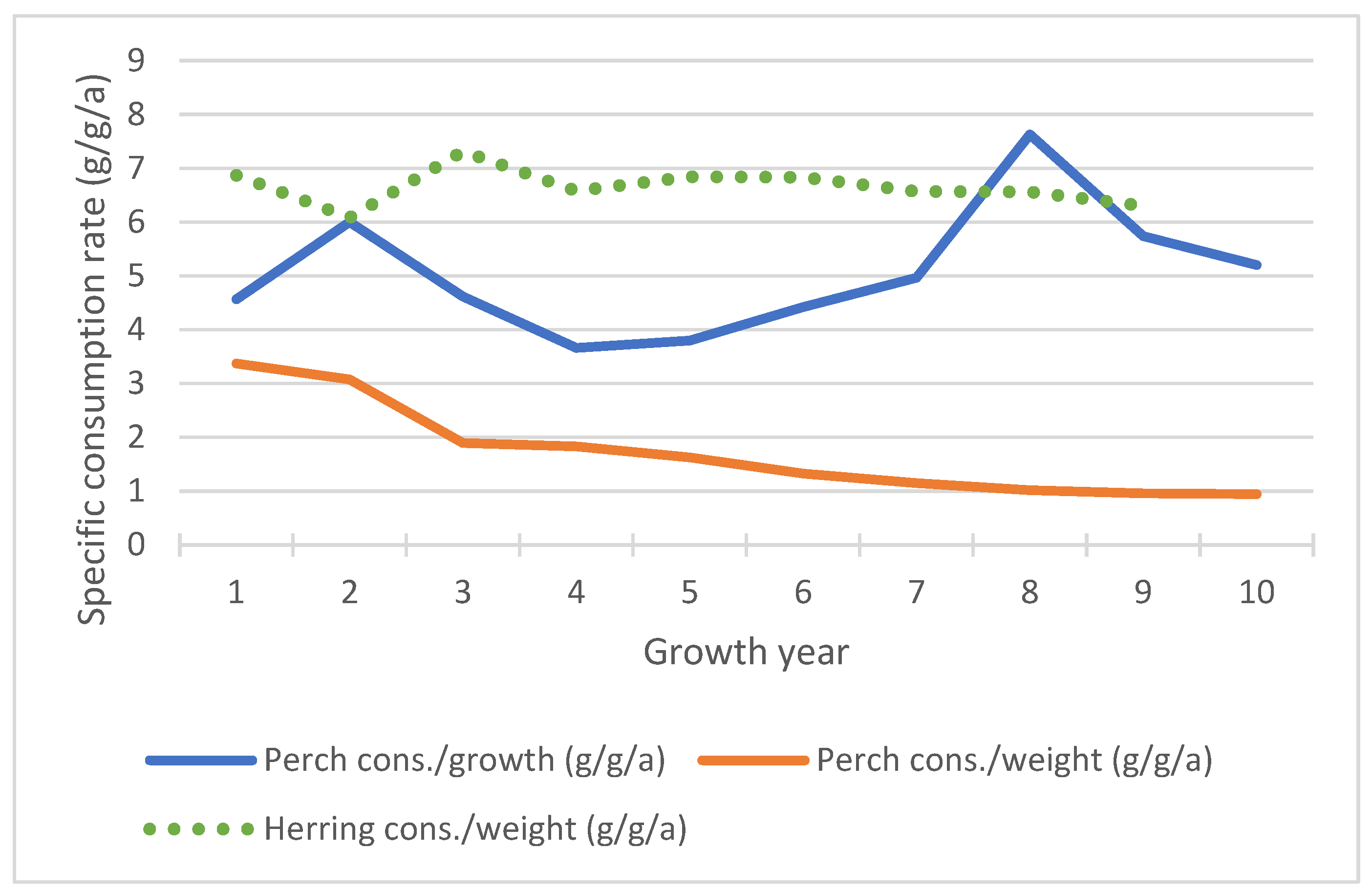
3.3. Internal Phosphorus Recycling of Herring Population
3.4. Internal Phosphorus Recycling/Regeneration of Perch Population
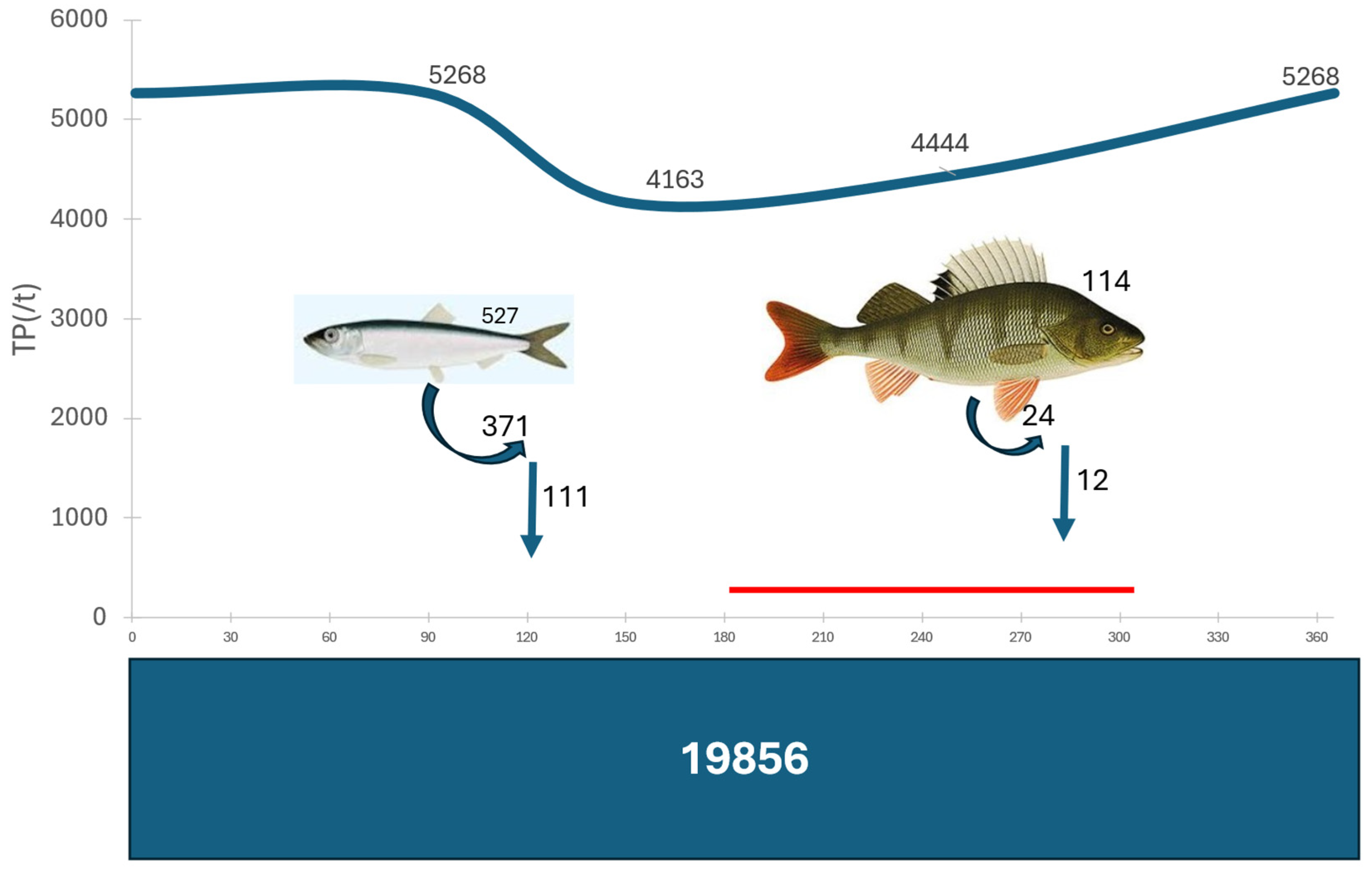
3.5. Generalized Phosphorus Budget of the Archipelago Sea
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, Y.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Laamanen, M.; Suomela, J.; Ekebom, J.; Korpinen, S.; Paavilainen, P.; Lahtinen, T.; Nieminen, S.; Hernberg, A. Suomen merenhoitosuunnitelman toimenpideohjelma vuosille 2022–2027. Ympäristöministeriön Julk. 2021, 30. (In Finnish) [Google Scholar]
- Helminen, H. The Role of Internal Phosphorus Loading in the Archipelago Sea Ecological Status. Water 2025, 17, 248. [Google Scholar] [CrossRef]
- Berninger, K.; Fleming, V.; Huttunen, M.; Iho, A.; Niskanen, L.; Kuosa, H.; Piiparinen, J.; Räike, A.; Salo, M.; Sarkkola, S.; et al. Rannikkovesille Tuntuvia Kuormitusvähennyksiä; Policy Brief No. 26; Valtioneuvoston Selvitys-ja Tutkimustoiminta: Helsinki, Finland, 2023. (In Finnish) [Google Scholar]
- Available online: https://www.ely-keskus.fi/saaristomeri/saaristomeri-ohjelma (accessed on 1 June 2025). (In Finnish).
- Reynolds, C.S. The development of perceptions of aquatic eutrophication and its control. Int. J. Ecohydrol. Hydrobiol. 2003, 3, 149–163. [Google Scholar]
- Griffits, D. The direct contribution of fish to lake phosphorus cycles. Ecol. Freshw. Fish 2006, 15, 86–95. [Google Scholar] [CrossRef]
- Hart, R.C.; Harding, W.R. Impacts of fish on phosphorus budget dynamics of some SA reservoirs: Evaluating prospects of ‘bottom up’ phosphorus reduction in eutrophic systems through fish removal (biomanipulation). Water SA 2015, 41, 4. [Google Scholar] [CrossRef]
- Vanni, M.J.; Boros, G.; McIntyre, P.B. When are fish sources vs. sinks of nutrients in lake ecosystems? Ecology 2013, 94, 2195–2206. [Google Scholar] [CrossRef]
- Available online: https://balticwaters.org/en/can-you-fish-nutrients-out-of-the-baltic-sea/ (accessed on 1 June 2025).
- Helminen, H.; Inkala, A. Modelled Water and Phosphorus Transports in the Archipelago Sea and through the Åland Sea and Northern Baltic Sea and Their Links to Water Quality. J. Mar. Sci. Eng. 2024, 12, 1252. [Google Scholar] [CrossRef]
- Helminen, H. The Effects of Reduced Wastewater Load in the Marine Area off Turku in the Archipelago Sea During the Period 1965–2025. Water 2025, 17, 1251. [Google Scholar] [CrossRef]
- Deslauriers, D.; Chipps, S.R.; Breck, J.E.; Rice, J.A.; Madenjian, C.P. Fish Bioenergetics 4.0: An R-based modeling application. Fisheries 2017, 42, 586–596. [Google Scholar] [CrossRef]
- Hansen, M.J.; Boisclair, D.; Brandt, S.B.; Hewett, S.W.; Kitchell, J.F.; Lucas, M.C.; Ney, J.J. Applications of bioenergetics models to fish ecology and management: Where do we go from here? Trans. Am. Fish. Soc. 1993, 122, 1019–1030. [Google Scholar] [CrossRef]
- Rolff, C.; Elfwing, T. Increasing nitrogen limitation in the Bothnian Sea, potentially caused by inflow of phosphate-rich water from the Baltic Proper. Ambio 2015, 44, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Eilola, K.; Meier, H.E.M.; Almroth, E. On the dynamics of oxygen, phosphorus and cyanobacteria in the Baltic Sea; A model study. J. Mar. Syst. 2009, 75, 163–184. [Google Scholar] [CrossRef]
- Nausch, M.; Achterberg, E.P.; Bach, L.T.; Brussaard, C.P.D.; Crawfurd, K.J.; Fabian, J.; Riebesell, U.; Stuhr, A.; Unger, J.; Wannicke, N. Concentrations and Uptake of Dissolved Organic Phosphorus Compounds in the Baltic Sea. Front. Mar. Sci. 2018, 5, 386. [Google Scholar] [CrossRef]
- Andersson, A.; Höglander, H.; Karlsson, C.; Huseby, S. Key role of phosphorus and nitrogen in regulating cyanobacterial community composition in the northern Baltic Sea. Estuar. Coast Shelf Sci. 2015, 164, 161–171. [Google Scholar] [CrossRef]
- Becker, S.; Aoyama, M.; Woodward, E.M.S.; Bakker, K.; Coverly, S.; Mahaffey, C.; Tanhua, T. GO-SHIP Repeat Hydrography Nutrient Manual: The Precise and Accurate Determination of Dissolved Inorganic Nutrients in Seawater, Using Continuous Flow Analysis Methods. Front. Mar. Sci. 2020, 7, 581790. [Google Scholar] [CrossRef]
- SFS-EN ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. ISO: Geneva, Switzerland, 2017.
- SFS 3026; Determination of Phosphorus in Water. Finnish Standards Association SFS: Helsinki, Finland, 1986; 11p.
- Lignell, R.; Miettunen, E.; Tuomi, L.; Ropponen, J.; Kuosa, H.; Attila, J.; Puttonen, I.; Lukkari, K.; Peltonen, H.; Lehtoranta, J.; et al. Rannikon Kokonaiskuormitusmalli: Ravinnepäästöjen Vaikutus Veden Tilaan—Kehityshankkeen Loppuraportti (XI 2015–VI 2018); Finnish Environment Institute: Helsinki, Finland, 2019. (In Finnish) [Google Scholar]
- Lignell, R.; Miettunen, E.; Kuosa, H.; Ropponen, J.; Tuomi, L.; Puttonen, I.; Lukkari, K.; Korppoo, M.; Huttunen, M.; Kaurila, K.; et al. Modeling how eutrophication in northern Baltic coastal zone is driven by new nutrient inputs, internal loading, and 3D hydrodynamics. J. Mar. Syst. 2025, 249, 104049. [Google Scholar] [CrossRef]
- Helminen, H.; Juntura, E.; Koponen, J.; Laihonen, P.; Ylinen, H. Assessing of long-distance background nutrient loading to the Archipelago Sea, northern Baltic with a hydrodynamic model. Environ. Model. Softw. 1998, 13, 511–518. [Google Scholar] [CrossRef]
- Suomela, J. Kirkkaasta Sameaan. Meren Kuormitus ja tila Saaristomerellä ja Ahvenanmaalla; Varsinais-Suomen Elinkeino-, Liikenne- ja Ympäristökeskuksen Julkaisuja: Turku, Finland, 2011; Volume 6. (In Finnish) [Google Scholar]
- Puttonen, I.; Mattila, J.; Jonsson, P.; Karlsson, O.M.; Kohonen, T.; Kotilainen, A.; Lukkari, K.; Malmaeus, J.M.; Rydin, E. Distribution and estimated release of sediment phosphorus in the northern Baltic Sea archipelagos. Estuar. Coast. Shelf Sci. 2014, 145, 9–21. [Google Scholar] [CrossRef]
- Puttonen, I. Phosphorus in the Sediments of the Northern Baltic Sea Archipelagos—Internal P Loading and Its Impact on Eutrophication. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 2017. Available online: https://www.doria.fi/handle/10024/131068 (accessed on 31 May 2025).
- Official Statistics of Finland (OSF). Commercial Marine Fishing [Online Publication]; Natural Resources Institute Finland: Helsinki, Finland, 2025; Available online: https://www.luke.fi/en/statistics/commercial-marine-fishery (accessed on 31 May 2025).
- Official Statistics of Finland (OSF). Recreational Fishing [Online Publication]; Natural Resources Institute Finland: Helsinki, Finland, 2025; Available online: https://www.luke.fi/en/statistics/recreational-fishing (accessed on 31 May 2025).
- ICES. Baltic Fisheries Assessment Working Group (WGBFAS). ICES Scientific Reports; ICES: Toronto, ON, Canada, 2024; 628p. [Google Scholar] [CrossRef]
- Available online: https://helcom.fi/wp-content/uploads/2019/08/Guidelines-for-Coastal-fish-Monitoring-of-HELCOM.pdf (accessed on 1 June 2025).
- ICES. Baltic Fisheries Assessment Working Group (WGBFAS). ICES Scientific Reports; ICES: Toronto, ON, Canada, 2022; 659p. [Google Scholar] [CrossRef]
- Rajasilta, M. Relationship between food, fat, sexual maturation and spawning time of Baltic herring (Clupea harengus membras) in the Archipelago Sea. Can. J. Fish. Aquat. Sci. 1992, 49, 644–654. [Google Scholar] [CrossRef]
- Rajasilta, M.; Eklund, J.; Hänninen, J.; Kurkilahti, M.; Kääriä, J.; Rannikko, P.; Soikkeli, M. Spawning of herring (Clupea harengus membras L.) in the Archipelago Sea. ICES J. Mar. Sci. 1993, 50, 233–246. [Google Scholar] [CrossRef]
- Kokkonen, E.; Heikinheimo, O.; Pekcan-Hekim, Z.; Vainikka, A. Effects of water temperature and pikeperch (Sander lucioperca) abundance on the stock–recruitment relationship of Eurasian perch (Perca fluviatilis) in the northern Baltic Sea. Hydrobiologia 2019, 841, 79–94. [Google Scholar] [CrossRef]
- Setälä, J.; Heikinheimo, O.; Raitaniemi, J.; Saarni, K. Verkon solmuvälin suurentamisen vaikutus Saaristomeren ammattikalastuksen kuha-ja ahvensaaliin arvoon. Kala-Ja Riistaraportteja 2003, 297. (In Finnish) [Google Scholar]
- Chang, W.; Cheng, J.; Allaire, J.; Xie, Y.; McPherson, J. Shiny: Web Application Framework for R, R package version 0.12.0; Shiny: Wellington, New Zealand, 2015; Available online: http://CRAN.R-project.org/package=shiny (accessed on 1 June 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Rudstam, L.G. Exploring the dynamics of herring consumption in the Baltic: Applications of an energetic model of fish growth. Kiel. Meeresforsch Sonderh. 1988, 6, 312–322. [Google Scholar]
- Karås, P.; Thoresson, G. An application of a bioenergetics model to Eurasian Perch (Perca fluviatilis L.). J. Fish Biol. 1992, 41, 217–230. [Google Scholar] [CrossRef]
- Mäkinen, T. (Ed.) Voidaanko Kalastuksella Vähentää Kalankasvatuksen Ravinnekuormaa? Kalankasvatuksen Nettokuormitusjärjestelmän Esiselvitys; RKTL Selvityksiä; Riista-ja Kalatalouden Tutkimuslaitos: Helsinki, Finland, 2008; Volume 2. (In Finnish) [Google Scholar]
- Rajasilta, M.; Hänninen, J.; Laaksonen, L.; Suomela, J.-P.; Vuorinen, I.; Mäkinen, K. Influence of environmental conditions, population density, and prey type on the lipid content in Baltic herring (Clupea harengus membras) from the northern Baltic Sea. Can. J. Fish. Aquat. Sci. 2018, 49, 644–654. [Google Scholar] [CrossRef]
- Rajasilta, M.; Hänninen, J.; Karpela, T.; Mäkinen, K.; Ollennu-Chuasam, P.; Sahlstén, J.; Suomela, J.-P. Silakan rasvapitoisuuden ja rasvojen laadun ajallinen ja alueellinen vaihtelu Saaristomerellä. SEILI Archipel. Res. Inst. Publ. 2022, 9. (In Finnish) [Google Scholar]
- Available online: https://fineli.fi/fineli/fi/elintarvikkeet/800 (accessed on 1 June 2025).
- Hjerne, O.; Hansson, S. The role of fish and fisheries in Baltic Sea nutrient dynamics. Limnol. Oceanogr. 2002, 47, 1023–1032. [Google Scholar] [CrossRef]
- Ruohonen, K.; Vielma, J.; Grove, D.J. Comparison of nutrient losses into the water from rainbow trout culture based on fresh Baltic herring, moist and dry diets. Aquac. Int. 1998, 6, 441–450. [Google Scholar] [CrossRef]
- Möllmann, C.; Kornilovs, G.; Fetter, M.; Köster, F.W. Feeding ecology of central Baltic Sea herring and sprat. J. Fish Biol. 2004, 65, 1563–1581. [Google Scholar] [CrossRef]
- Flinkman, J.; Vuorinen, I.; Aro, E. Planktivorous Baltic herring (Clupea harengus) prey selectively on reproducing copepods and cladocerans. Can. J. Fish. Aquat. Sci. 1992, 49, 73–77. [Google Scholar] [CrossRef]
- Bachiller, E.; Utne, K.R.; Jansen, T.; Huse, G. Bioenergetics modeling of the annual consumption of zooplankton by pelagic fish feeding in the Northeast Atlantic. PLoS ONE 2018, 13, e0190345. [Google Scholar] [CrossRef] [PubMed]
- Walve, J.; Larsson, U. Carbon, nitrogen and phosphorus stoichiometry of crustacean zooplankton in the Baltic Sea: Implications for nutrient recycling. J. Plankton Res. 1999, 21, 2309–2321. [Google Scholar] [CrossRef]
- Helminen, H.; Sarvala, J.; Hirvonen, A. Growth and food consumption of vendace (Coregonus albula (L.)) in Lake Pyhäjärvi, SW Finland: A bioenergetics modeling analysis. Hydrobiologia 1990, 200, 511–522. [Google Scholar] [CrossRef]
- Tarvainen, M.; Sarvala, J.; Helminen, H. The role of phosphorus release by roach (Rutilus rutilus (L.)) in the water quality changes of a biomanipulated lake. Freshw. Biol. 2002, 47, 2325–2336. [Google Scholar] [CrossRef]
- Olin, M.; Veneranta, L. Merenkurkun Ahvenkantojen Rakenne ja Kalastuksen Vaikutukset; Luonnonvara-ja biotalouden tutkimus, Luonnonvarakeskus: Helsinki, Finland, 2020; Volume 94, 42p. (In Finnish) [Google Scholar]
- Schreckenbach, K.; Knösche, R.; Ebert, K. Nutrient and energy content of freshwater fishes. J. Appl. Ichthyol. 2001, 17, 142–144. [Google Scholar] [CrossRef]
- Jacobson, P.; Bergström, U.; Eklöf, J. Size-dependent diet composition and feeding of Eurasian perch (Perca fluviatilis) and northern pike (Esox lucius) in the Baltic Sea. Boreal Environ. Res. 2019, 24, 137–153. [Google Scholar]
- Chipps, S.R.; Bennett, D.H. Evaluation of Mysis bioenergetic model. J. Plankton Res. 2002, 24, 77–82. [Google Scholar] [CrossRef]
- Lappalainen, A.; Rask, M.; Koponen, H.; Vesala, S. Relative abundance, diet and growth of perch (Perca fluviatilis) and roach (Rutilus rutilus) at Tvärminne, northern Baltic Sea, in 1975 and 1997: Responses to eutrophication? Boreal Environ. Res. 2001, 6, 107–118. [Google Scholar]
- Hovel, R.A.; Beauchamp, D.A.; Hansen, A.G.; Sorel, M.H. Development of a Bioenergetics Model for the Threespine Stickleback. Trans. Am. Fish. Soc. 2015, 144, 1311–1321. [Google Scholar] [CrossRef]
- Lee, V.A.; Johnson, T.B. Development of a Bioenergetics Model for the Round Goby (Neogobius melanostomus). J. Great Lakes Res. 2005, 31, 125–134. [Google Scholar] [CrossRef]
- Karjalainen, J.; Miserque, D.; Huuskonen, H. The estimation of food consumption in larval and juvenile fish: Experimental evaluation of bioenergetics models. J. Fish Biol. 1997, 51 (Suppl. A), 39–51. [Google Scholar] [CrossRef]
- Pennuto, C.M.; Mehler, K.; Weidel, B.; Lantry, B.F.; Bruestle, E. Dynamics of the seasonal migration of Round Goby (Neogobius melanostomus, Pallas 1814) and implications for the Lake Ontario food web. Ecol. Freshw. Fish 2021, 30, 151–161. [Google Scholar] [CrossRef]
- Vanni, M. Nutrient Cycling by Animals in Freshwater Ecosystems. Annu. Rev. Ecol. Syst. 2002, 33, 341–370. [Google Scholar] [CrossRef]
- Mézo, P.M.; Guiet, J.; Scherrer, K.; Bianchi, D.; Galbrait, E. Global nutrient cycling by commercially targeted marine fish. Biogeosci. Discuss. 2022, 19, 2537–2555. [Google Scholar] [CrossRef]
- Rajasilta, M.; Mäkinen, K.; Ruuskanen, S.; Hänninen, J.; Laine, P. Long-Term Data Reveal the Associations of the Egg Quality with Abiotic Factors and Female Traits in the Baltic Herring Under Variable Environmental Conditions. Front. Mar. Sci. 2021, 8, 698480. [Google Scholar] [CrossRef]
- Casini, M.; Cardinale, M.; Hjelm, J. Inter-annual variation in herring, Clupea harengus, and sprat, Sprattus sprattus, condition in the central Baltic Sea: What gives the tune? OIKOS 2006, 112, 638–650. [Google Scholar] [CrossRef]
- Casini, M.; Kornilovs, G.; Cardinale, M.; Möllmann, C.; Grygiel, W.; Jonsson, P.; Raid, T.; Flinkman, J.; Feldman, V. Spatial and temporal density dependence regulates the condition of central Baltic Sea clupeids: Compelling evidence using an extensive international acoustic survey. Popul. Ecol. 2011, 53, 511–523. [Google Scholar] [CrossRef]
- Laine, P.; Rajasilta, M. Changes in the reproductive properties of Baltic herring females during the spawning season. Fish. Res. 1998, 36, 67–73. [Google Scholar] [CrossRef]
- Thurow, F. Estimation of the total fish biomass in the Baltic Sea during the 20th century. ICES J. Mar. Sci. 1997, 54, 444–461. [Google Scholar] [CrossRef]
- Axenrot, T.; Hansson, S. Predicting herring recruitment from young-of-the-year densities, spawning stock biomass, and climate. Limnol. Oceanogr. 2003, 48, 1716–1720. [Google Scholar] [CrossRef]
- Wotton, R.S.; Malmqvist, B. Feces in Aquatic Ecosystems: Feeding animals transform organic matter into fecal pellets, which sink or are transported horizontally by currents; these fluxes relocate organic matter in aquatic ecosystems. BioScience 2001, 51, 537–544. [Google Scholar] [CrossRef]
- Turner, J.T. Zooplankton fecal pellets, marine snow, phytodetritus and the ocean’s biological pump. Prog. Oceanogr. 2015, 130, 205–248. [Google Scholar] [CrossRef]
- Rousi, H.; Fält-Nardman, J.; Niemelä, P.; Hänninen, J. Changes in Atlantic climatic regulation mechanisms that underlie mesozooplankton biomass loss in the northern Baltic Sea. Heliyon 2024, 10, e31268. [Google Scholar] [CrossRef]
- Steinkopf, M.; Krumme, U.; Schulz-Bull, D.; Wodarg, D.; Loick-Wilde, N. Trophic lengthening triggered by filamentous, N2-fixing cyanobacteria disrupts pelagic but not benthic food webs in a large estuarine ecosystem. Ecol. Evol. 2024, 14, e11048. [Google Scholar] [CrossRef]
- Elmgren, R.; Blenckner, T.; Andersson, A. Baltic Sea management: Successes and failures. Ambio 2015, 44 (Suppl. S3), 335–344. [Google Scholar] [CrossRef]
- Blenckner, T.; Möllmann, C.; Stewart Lowndes, J.; Griffiths, J.R.; Campbell, E.; De Cervo, A.; Belgrano, A.; Boström, C.; Fleming, V.; Frazier, M.; et al. The Baltic Health Index (BHI): Assessing the social–Ecological status of the Baltic Sea. People Nat. 2021, 3, 359–375. [Google Scholar] [CrossRef]





| Herring | Perch | |
|---|---|---|
| Consumption equation | 3 | 2 |
| CA | 0.642 | 0.4 |
| CB | −0.256 | −0.27 |
| CQ | 1 | 3.9 |
| CTO | 13 | 23 |
| CTM | 15 | 28 |
| CTL | 23 | |
| CK1 | 0.1 | |
| CK4 | 0.01 | |
| Respiration equation | 1 | 2 |
| RA | 0.0033 | 0.0108 |
| RB | −0.227 | −0.2 |
| RQ | 0.0548 | 2.8 |
| RTO | 0.03 | 28 |
| RTM | 0 | 33 |
| RTL | 9 | 0 |
| RK1 | 15 | 0 |
| RK4 | 0.13 | 0 |
| ACT | 3.9 | 1 |
| BACT | 0.149 | 0 |
| SDA | 0.175 | 0.15 |
| Egestion equation | 1 | 3 |
| FA | 0.16 | 0.158 |
| FB | 0 | −0.222 |
| FG | 0 | 0.631 |
| Excretion equation | 1 | 3 |
| UA | 0.1 | 0.0292 |
| UB | 0 | 0.58 |
| UG | 0 | −0.299 |
| Predator equation | 1 | 1 |
| Growth | a | c |
| Temperature | b | d |
| Recycled P in AS (t) | Recycled P in AS (mg/m2/day) | Recycled P Total (t/v) | |
|---|---|---|---|
| 1988 | |||
| catch | 93.4 | 0.040 | 274.1 |
| SSB | 370.6 | 0.159 | 1087.5 |
| TotB | 552.7 | 0.237 | 1621.7 |
| 2023 | |||
| catch | 33.3 | 0.014 | 76.4 |
| SSB | 80.2 | 0.034 | 184.1 |
| TotB | 130.1 | 0.056 | 298.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helminen, H. Phosphorus Pools Associated with Fish in the Archipelago Sea. Fishes 2025, 10, 328. https://doi.org/10.3390/fishes10070328
Helminen H. Phosphorus Pools Associated with Fish in the Archipelago Sea. Fishes. 2025; 10(7):328. https://doi.org/10.3390/fishes10070328
Chicago/Turabian StyleHelminen, Harri. 2025. "Phosphorus Pools Associated with Fish in the Archipelago Sea" Fishes 10, no. 7: 328. https://doi.org/10.3390/fishes10070328
APA StyleHelminen, H. (2025). Phosphorus Pools Associated with Fish in the Archipelago Sea. Fishes, 10(7), 328. https://doi.org/10.3390/fishes10070328




