Effect of Yeast Selenium on Growth Performance, Muscle Selenium Deposition, and Antioxidant Capacity of Juvenile Cherax quadricarinatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Nutrient Composition Content of the Basic Feed
2.3. Experimental Diet Preparation
2.4. Feeding and Management
2.5. Sample Collection and Data Acquisition
2.6. Growth Performance Evaluation
2.7. Determination of Feed and Muscle Selenium Content
2.8. Biochemical Assays
2.9. Statistical Analysis of the Data
3. Results and Analysis
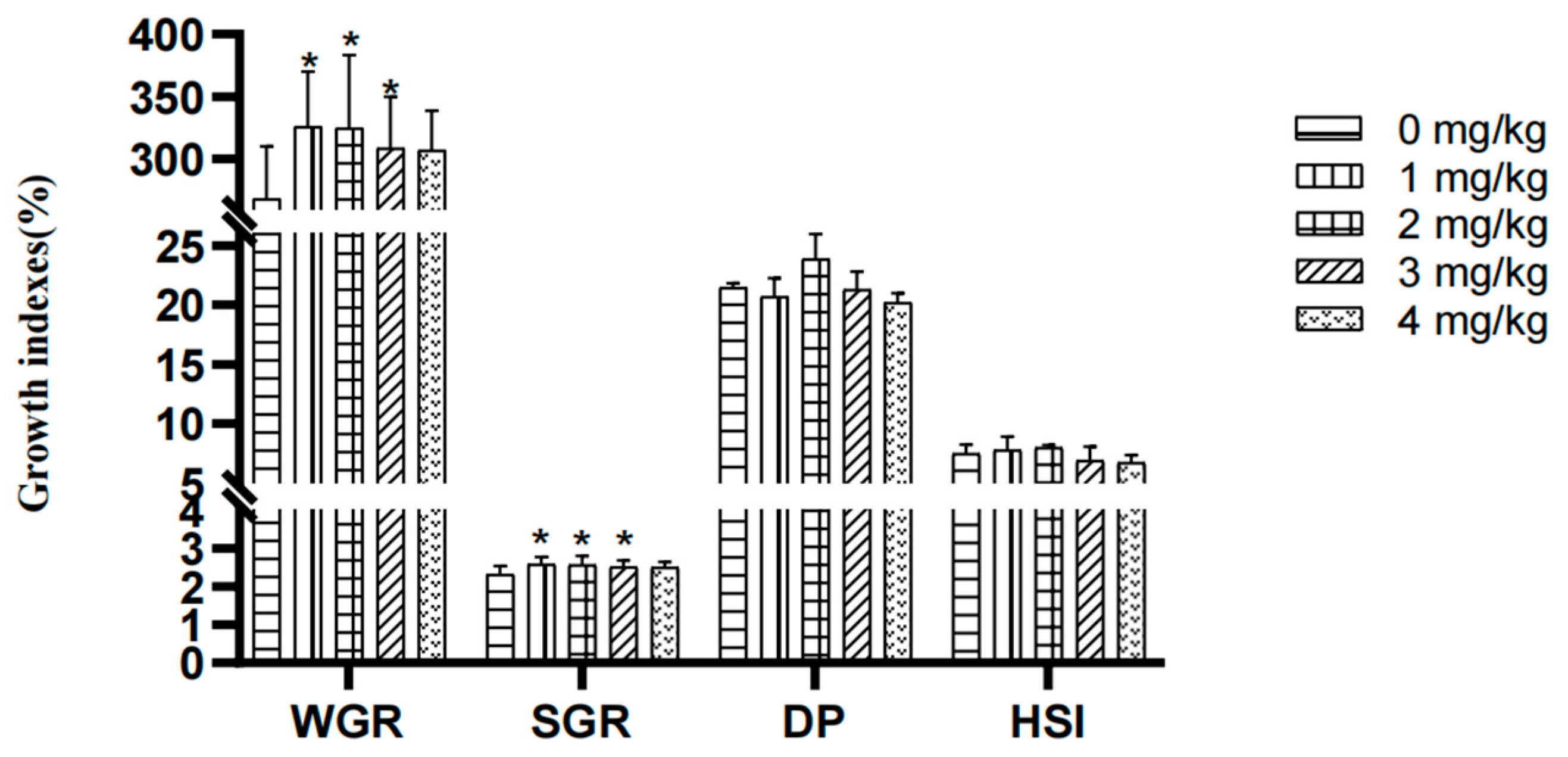
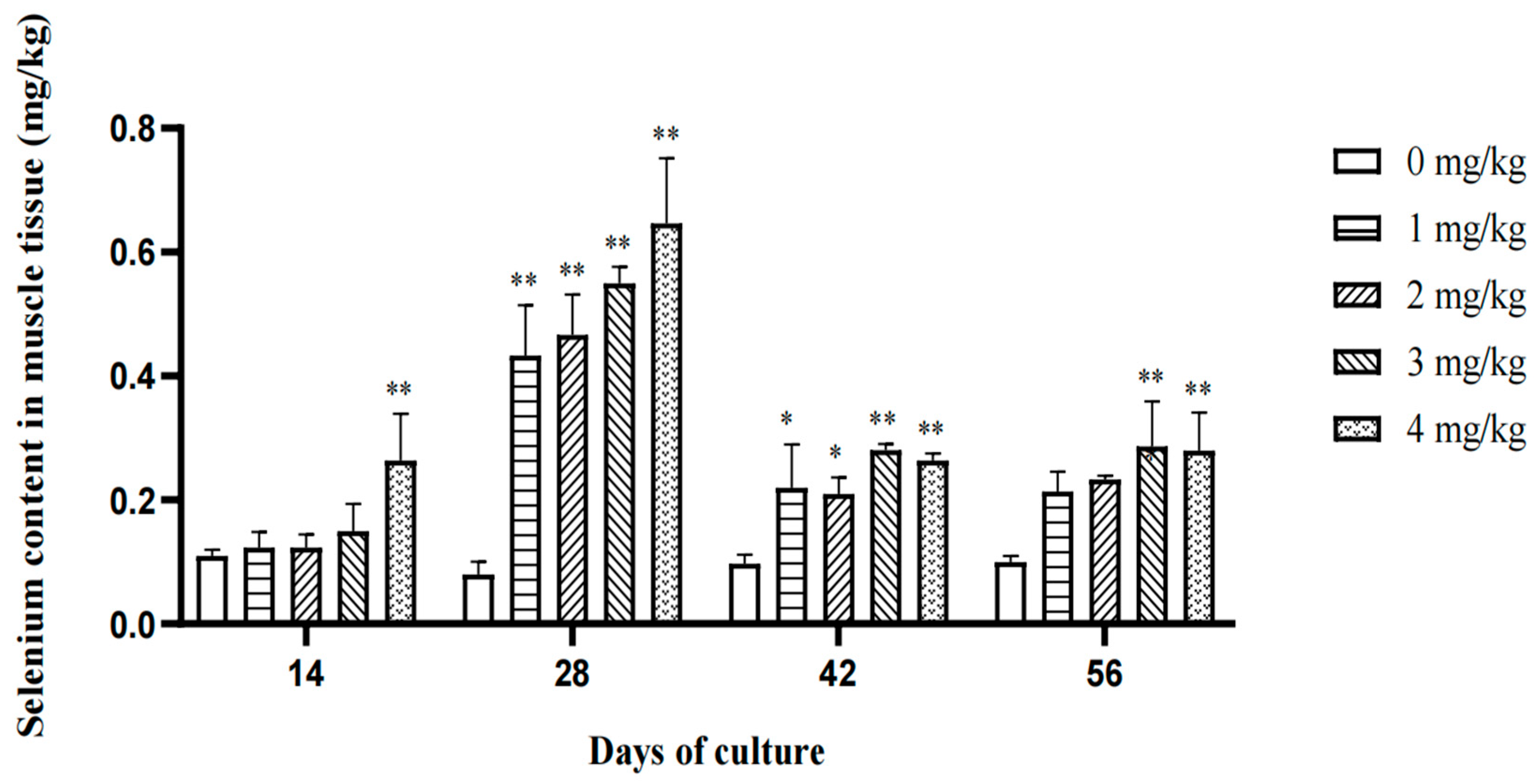
3.1. Effect of Yeast Selenium on Growth, Muscle Deposition, and Digestive Capacity
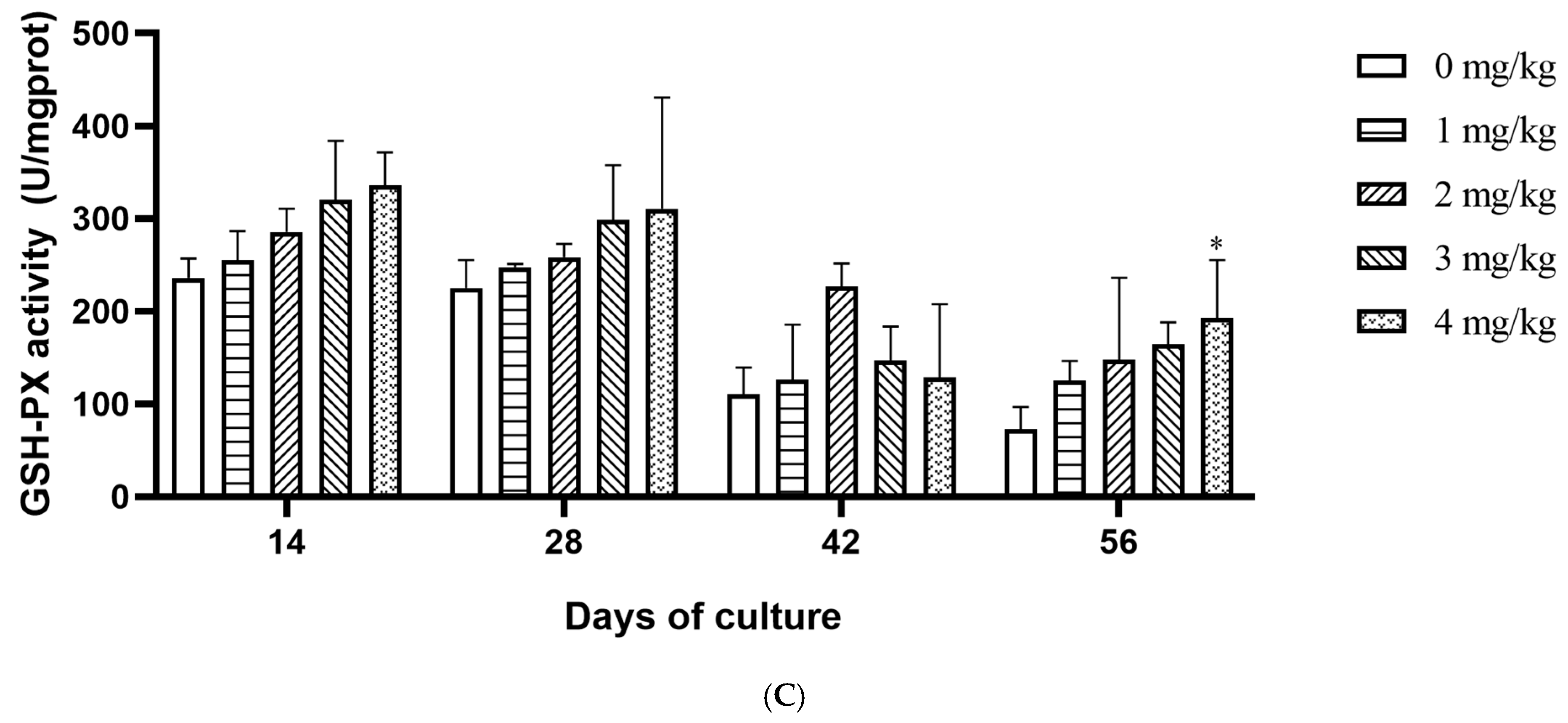
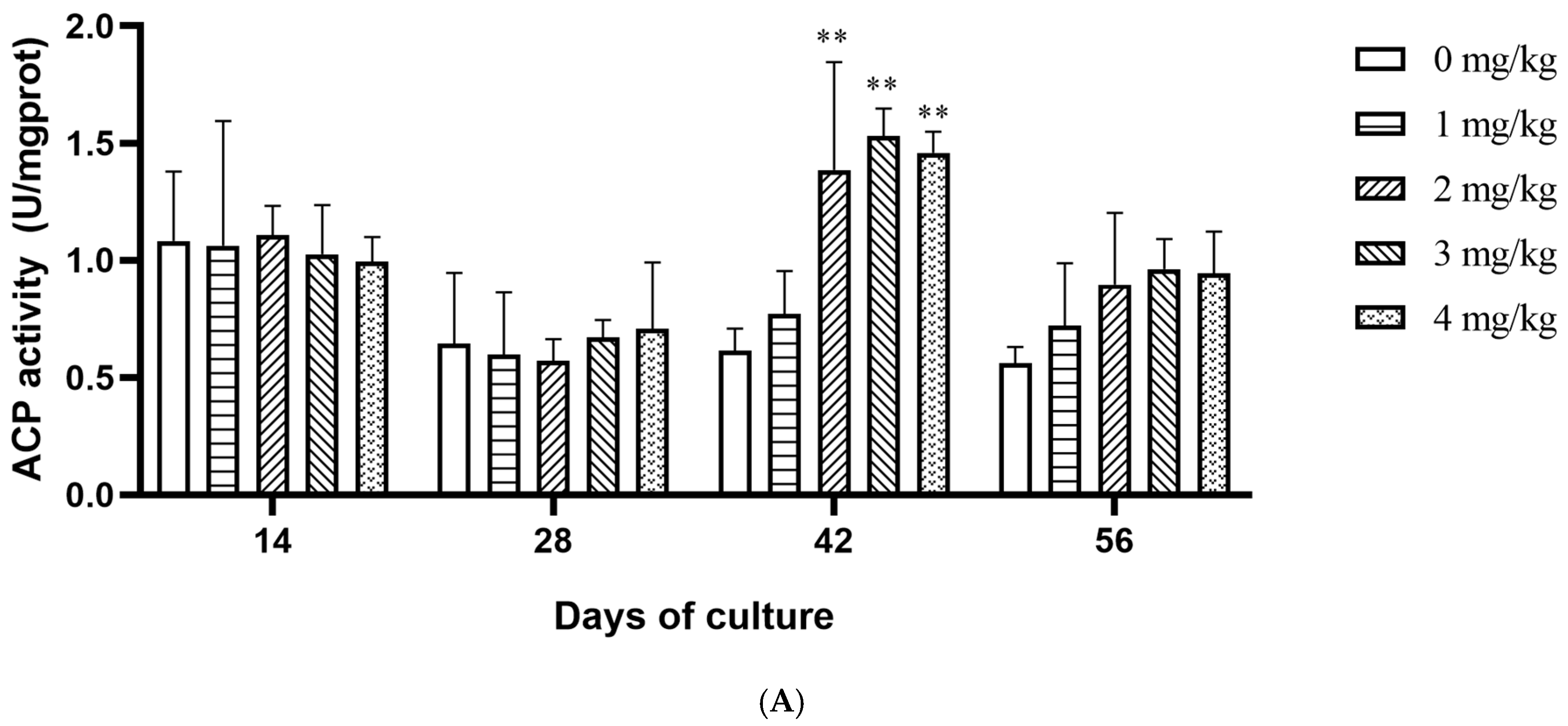
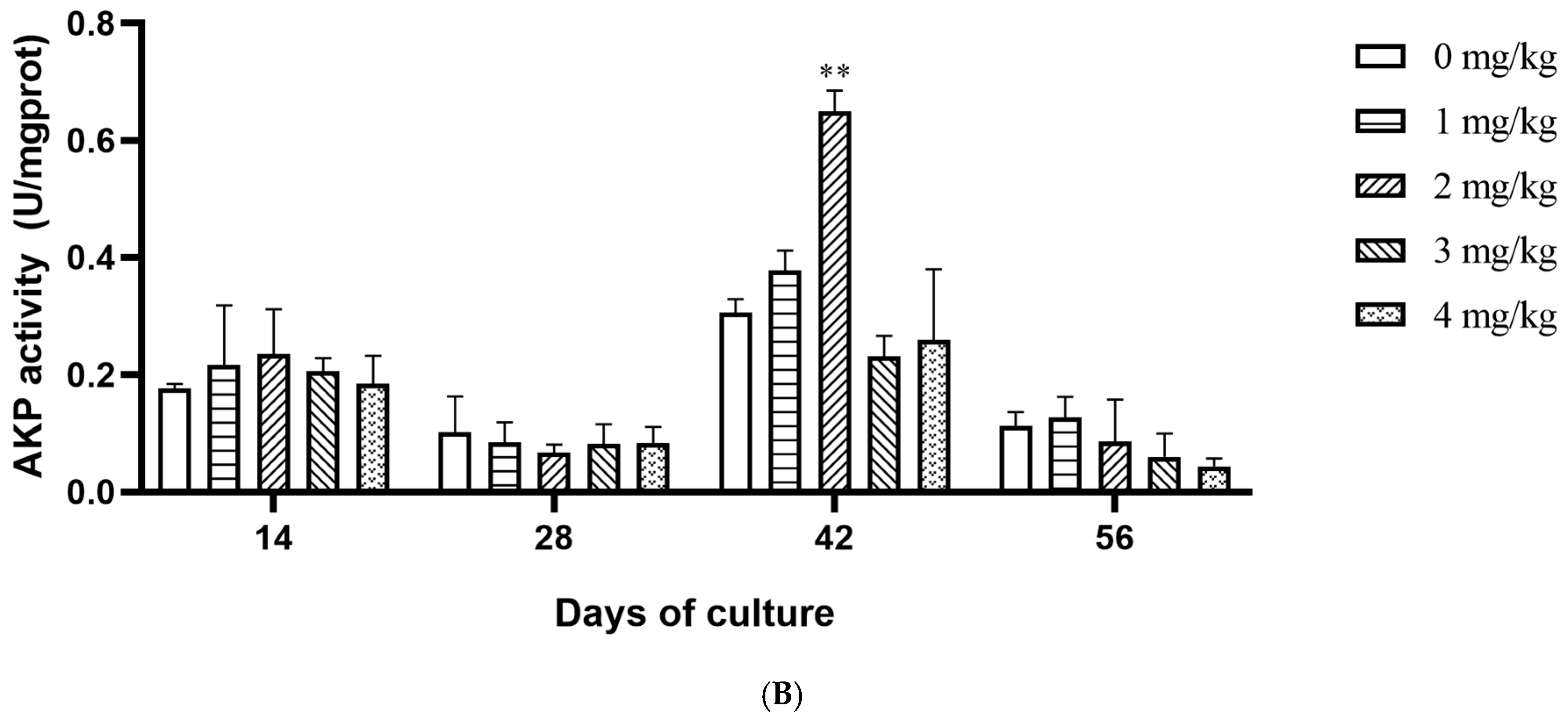
3.2. Effect of Yeast Selenium on the Vitality of Digestive Enzymes and Antioxidant Enzymes in Cherax quadricarinatus
3.3. Effect of Yeast Selenium on Immunoenzyme Activity of Cherax quadricarinatus
4. Discussion
4.1. Effect of Yeast Selenium on the Growth Performance of Cherax quadricarinatus
4.2. Effect of Yeast Selenium on the Muscle Selenium Content of Cherax quadricarinatus
4.3. Effect of Yeast Selenium on Antioxidant and Immune Enzymes of Cherax quadricarinatus
4.4. Effects of Excessive Selenium Intake Have Potential Negative on Organisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Chen, B.; Huang, Y.; Li, J.; Cao, D.; Chen, Z.; Li, J.; Ran, B.; Yang, J.; Wang, R.; et al. Selenium intake and multiple health-related outcomes: An umbrella review of meta-analyses. Front. Nutr. 2023, 10, 1263853. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.T.E.; Goda, A.A.; Omar, E.A.; Khalil, H.S.; Esteban, M.Á. Dietary supplementation of organic selenium improves growth, survival, antioxidant and immune status of meagre, Argyrosomus regius, juveniles. Fish Shellfish Immunol. 2017, 68, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M. Selenium–fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Dang, H.; Zhang, Y.; Yang, M.; Zhang, W.; Zhao, Y.; Zhang, H.; Ji, H.; Zhang, B. Inorganic Selenium Transformation into Organic Selenium by Monascus purpureus. Foods 2023, 12, 3375. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Yan, L.; Cao, J.; Li, D.; Huang, G.Y.; Shi, W.J.; Dong, W.; Zha, J.; Ying, G.G.; et al. Subchronic effects of dietary selenium yeast and selenite on growth performance and the immune and antioxidant systems in Nile tilapia Oreochromis niloticus. Fish Shellfish Immunol. 2020, 97, 283–293. [Google Scholar] [CrossRef]
- Mohamed, D.A.; Sazili, A.Q.; Teck Chwen, L.; Samsudin, A.A. Effect of microbiota-selenoprotein on meat selenium content and meat quality of broiler chickens. Animals 2020, 10, 981. [Google Scholar] [CrossRef]
- Liu, L.; Wu, C.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; et al. Selenium-enriched yeast alleviates oxidative stress-induced intestinal mucosa disruption in weaned pigs. Oxidative Med. Cell. Longev. 2020, 2020, 5490743. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Z.; Wang, C.; Li, E.; Qin, J.G.; Chen, L. Dietary supplementation of selenium yeast enhances the antioxidant capacity and immune response of juvenile Eriocheir sinensis under nitrite stress. Fish Shellfish Immunol. 2019, 87, 22–31. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Li, S.; Li, C.; Hu, X.; Li, Z.; Yue, T.; Hu, Z. Effect of the selenized yeast added in feed on selenium-containing proteins of albumins in egg yolk. Food Chem. 2023, 402, 134435. [Google Scholar] [CrossRef]
- Chen, H.; Li, P.; Shen, Z.; Wang, J.; Diao, L. Protective effects of selenium yeast against cadmium-induced necroptosis through mir-26a-5p/pten/pi3k/akt signaling pathway in chicken kidney. Ecotoxicol. Environ. Saf. 2021, 220, 112387. [Google Scholar] [CrossRef]
- Sun, H.; Chen, J.; Xiong, D.; Long, M. Detoxification of Selenium Yeast on Mycotoxins and Heavy Metals: A Review. Biol. Trace Elem. Res. 2023, 201, 5441–5454. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Zhang, D.G.; Zhao, T.; Xu, Y.H.; Luo, Z. Dietary selenium sources differentially regulate selenium concentration, mRNA and protein expression of representative selenoproteins in various tissues of yellow catfish Pelteobagrus fulvidraco. Br. J. Nutr. 2022, 127, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, R.A.; Fotedar, R. Dietary organic selenium improves growth, survival and resistance to Vibrio mimicus in cultured marron, Cherax cainii (Austin, 2002). Fish Shellfish Immunol. 2013, 35, 79–85. [Google Scholar] [CrossRef]
- GB 5009.5-2016; National Food Safety Standard—Determination of Protein in Foods. Standards Press of China: Beijing, China, 2016.
- GB 5009.6-2016; National Food Safety Standard—Determination of Fat in Foods. Standards Press of China: Beijing, China, 2016.
- GB 5009.4-2016; National food Safety Standard—Determination of Ash in Foods. Standards Press of China: Beijing, China, 2016.
- GB/T 6436-2018; Determination of Calcium in Feeds. Standards Press of China: Beijing, China, 2018.
- GB/T 6437-2018; Determination of Phosphorus in Feeds—Spectrophotometry. Standards Press of China: Beijing, China, 2018.
- Liu, J.X.; Tao, L.Z.; Yang, M.; Chen, J.; Li, Y.L.; Wei, W.Y.; Chen, X.; Tang, H.; Zhang, X.L.; Yang, Z.Z. Effects of Selenium Yeast on growth performance, muscle selenium deposition and disease resistance of Common carp. China Feed 2021, 19, 43–48. [Google Scholar]
- GB 5009.268-2016; National Food Safety Standard—Determination of Multi-Elements in Foods. Standards Press of China: Beijing, China, 2016.
- Xie, M.; Xiang, J.G.; Li, T.Y. Effects of selenium yeast supplementation on growth and body composition of juvenile Elopichthys elopichthys. Feed Ind. 2013, 34, 57–59. [Google Scholar]
- Cao, H. Effects of selenium yeast on growth performance of Grass carp. Jiangxi Aquat. Sci. Technol. 2022, 2, 30–32. (In Chinese) [Google Scholar]
- Wang, L.; Wu, L.; Liu, Q.; Zhang, D.F.; Yin, J.J.; Xu, Z.; Zhang, X.Z. Improvement of flesh quality in rainbow trout (Oncorhynchus mykiss) fed supranutritional dietary selenium yeast is associated with the inhibited muscle protein degradation. Aquac. Nutr. 2018, 24, 1351–1360. [Google Scholar] [CrossRef]
- Pacitti, D.; Lawan, M.M.; Sweetman, J.; Martin, S.A.; Feldmann, J.; Secombes, C.J. Selenium supplementation in fish: A combined chemical and biomolecular study to understand Sel-Plex assimilation and impact on selenoproteome expression in rainbow trout (Oncorhynchus mykiss). PLoS ONE 2015, 10, e0127041. [Google Scholar] [CrossRef]
- Takahashi, L.S.; Biller-Takahashi, J.D.; Mansano, C.F.M.; Urbinati, E.C.; Gimbo, R.Y.; Saita, M.V. Long-term organic selenium supplementation overcomes the trade-off between immune and antioxidant systems in pacu (Piaractus mesopotamicus). Fish Shellfish Immunol. 2017, 60, 311–317. [Google Scholar] [CrossRef]
- Hong, Y.; Jiang, W.; Kuang, S.; Hu, K.; Tang, L.; Liu, Y.; Jiang, J.; Zhang, Y.; Zhou, X.; Feng, L. Growth, digestive and absorptive capacity and antioxidant status in intestine and hepatopancreas of sub-adult grass carp Ctenopharyngodonidella fed graded levels of dietary threonine. J. Anim. Sci. Biotechnol. 2015, 6, 34. [Google Scholar] [CrossRef][Green Version]
- Luo, L.; Wen, H.; Wang, L.; Li, Q.; Long, Y.; Guo, J.L.; Yang, X. Effects of taurine on growth, quality, digestive and metabolic enzyme activities of Grass carp. Chin. J. Anim. Nutr. 2006, 18, 166–171. [Google Scholar]
- Douglas, S.E.; Mandla, S.; Gallant, J.W. Molecular analysis of the amylase gene and its expression during development in the winter flounder, Pleuronectes americanus. Aquaculture 2000, 190, 247–260. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; Benjakul, S. Lipase from liver of seabass (Lates calcarifer): Characteristics and the use for defatting of fish skin. Food Chem. 2018, 240, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Oku, H.; Koizumi, N.; Okumura, T.; Kobayashi, T.; Umino, T. Molecular characterization of lipoprotein lipase, hepatic lipase and pancreatic lipase genes: Effects of fasting and refeeding on their gene expression in red sea bream Pagrus major. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 145, 168–178. [Google Scholar] [CrossRef]
- Marcuschi, M.; Espósito, T.S.; Machado, M.F.; Hirata, I.Y.; Machado, M.F.; Silva, M.V.; Carvalho, L.B., Jr.; Oliveira, V.; Bezerra, R.S. Purification, characterization and substrate specificity of a trypsin from the Amazonian fish tambaqui (Colossoma macropomum). Biochem. Biophys. Res. Commun. 2010, 396, 667–673. [Google Scholar] [CrossRef]
- Poonsin, T.; Simpson, B.K.; Visessanguan, W.; Yoshida, A.; Klomklao, S. Optimal immobilization of trypsin from the spleen of albacore tuna (Thunnus alalunga) and its characterization. Int. J. Biol. Macromol. 2020, 143, 462–471. [Google Scholar] [CrossRef]
- Klomklao, S.; Benjakul, S. Two trypsin isoforms from albacore tuna (Thunnus alalunga) liver: Purification and physicochemical and biochemical characterization. Int. J. Biol. Macromol. 2018, 107, 1864–1870. [Google Scholar] [CrossRef]
- Ma, S.X. Effects of Nano-Selenium on Growth Performance and Muscle Quality of Largemouth Bass. Master’s Thesis, Beibu Gulf University, Qinzhou, China, 2022. [Google Scholar]
- Wang, J.L.; Ye, L.H.; Zhou, M. Effects of selenium source and selenium level on meat quality of Crayfish. Hunan Feed 2010, 03, 24–26. [Google Scholar]
- Yang, Y.Z.; Nie, J.Q.; Tan, B.P.; Dong, X.H.; Yang, Q.H.; Chi, S.Y. Effects of Selenium Source and Selenium Level on Growth Performance, Liver and Serum antioxidant Indexes and Tissue Selenium Content of juvenile Cobia. Chin. J. Anim. Nutr. 2016, 28, 3894–3904. [Google Scholar]
- Ashouri, S.; Keyvanshokooh, S.; Salati, A.P.; Johari, J.A.; Pasha-Zanoosi, H. Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 2015, 446, 25–29. [Google Scholar] [CrossRef]
- Lorentzen, M.; Maage, A.; Julshamn, K. Effects of dietary selenite or selenomethionine on tissue selenium levels of Atlantic salmon (Salmo salar). Aquaculture 1994, 121, 359–367. [Google Scholar] [CrossRef]
- Pedrosa, L.F.C.; Motley, A.K.; Stevenson, T.D.; Stevenson, T.D.; Hill, K.E.; Burk, R.F. Fecal selenium excretion is regulated by dietary selenium intake. Biol. Trace Elem. Res. 2012, 149, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Juniper, D.T.; Bertin, G. Effects of dietary selenium supplementation on tissue selenium distribution and glutathione peroxidase activity in Chinese Ring necked Pheasants. Animal 2013, 7, 562–570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borilova, G.; Fasiangova, M.; Harustiakova, D.; Kumprechtova, D.; Illek, J.; Auclair, E.; Raspoet, R. Effects of selenium feed supplements on functional properties of eggs. J. Food Sci. Technol. 2020, 57, 32–40. [Google Scholar] [CrossRef]
- Zhou, S.Y. Physiological Study on Copper, Selenium and Cobalt Nutrition in Juvenile Shrimp (Litopenaeus vannamei). Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2014. [Google Scholar]
- Wu, L.; Zang, X.Z.; Wang, L.; Liu, Q.; Li, J.; Zhang, D.F.; Tian, D.C. Effects of Dietary Selenium Yeast on Growth performance, muscle quality and antioxidant Capacity of juvenile Sturgeon Sturgeon. Freshw. Fish. 2019, 53, 3–11. [Google Scholar]
- Wu, X.Y.; Sheng, W.B.; Nie, Q.; Jing, C.C.; Ning, Y.C.; Chang, Y.Q.; Song, J.; Zuo, R.T. Effects of Dietary Selenium Yeast on growth, antioxidant capacity and Selenium content of Stichopus japonicum before and after aestivation. Chin. J. Anim. Nutr. 2019, 32, 2791–2798. [Google Scholar]
- Li, X.X.; Chen, F.; Pan, Q.; Chen, C.Y.; Tian, Y.W.; Lei, X.T.; Cao, J.M. Effects of Selenium yeast on growth and antioxidant properties of Litopenaeus vannamei. J. South China Agric. Univ. 2014, 35, 108–112. [Google Scholar]
- Wang, M.X.; Lu, J.J.; Guo, R.; Li, P.; Xia, H.; Li, Y.Y.; Xie, W.; Yang, P.X. Effects of selenium yeast on Litopenaeus vannamei in diets with different levels of soybean meal instead of fish meal. Feed Ind. 2017, 38, 30–34. [Google Scholar]
- Cheng, X.M.; Huang, Q.C.; Wang, F.H.; Li, G.H. Effects of myimaggot protein on serum biochemical indexes and non-specific immune function of Chinese trionyx sinensis. Fish. Sci. 2017, 36, 768–772. [Google Scholar]
- Jaramillo, F., Jr.; Peng, L.I.; Gatlin Iii, D.M. Selenium nutrition of hybrid striped bass (Morone chrysops × M. saxatilis) bioavailability, toxicity and interaction with vitamin, E. Aquac. Nutr. 2009, 15, 160–165. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Chen, Q.X.; Zheng, W.Z.; Lin, J.Y.; Zhuang, Z.L.; Zhou, H.M. Inhibition kinetics of green crab (Scylla serrata) alkaline phosphatase activity by dithiothreitol or 2-mercaptoethanol. Int. J. Biochem. Cell Biol. 2000, 32, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Nawsherwan; Khan, S.; Zeb, F.; Shoaib, M.; Nabi, G.; Ul Haq, I.; Xu, K.; Li, H. Selected Micronutrients: An Option to Boost Immunity against COVID-19 and Prevent Adverse Pregnancy Outcomes in Pregnant Women: A Narrative Review. Iran. J. Public Health 2020, 49, 2032–2043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pérez-Valenzuela, J.; Mejías, M.; Ortiz, D.; Salgado, P.; Montt, L.; Chávez-Báez, I.; Vera-Tamargo, F.; Mandakovic, D.; Wacyk, J.; Pulgar, R. Increased dietary availability of selenium in rainbow trout (Oncorhynchus mykiss) improves its plasma antioxidant capacity and resistance to infection with Piscirickettsia salmonis. Vet. Res. 2021, 52, 64. [Google Scholar] [CrossRef] [PubMed]
- Mal, J.; Veneman, W.J.; van Hullebusch, E.D.; Peijnenburg, W.J.; Vijver, M.G.; Lens, P.N. A comparison of fate and toxicity of selenite, biogenically, and chemically synthesized selenium nanoparticles to zebrafish (Danio rerio) embryogenesis. Nanotoxicology 2017, 11, 87–97. [Google Scholar] [CrossRef]
- Seyedi, J.; Kalbassi, M.R.; Tayemeh, M.B.; Amiri Moghadam, J. Toxicity and deleterious impacts of selenium nanoparticles at supranutritional and imbalance levels on male goldfish (Carassius auratus) sperm. J. Trace Elem. Med. Biol. 2021, 66, 126758. [Google Scholar] [CrossRef]
- Graves, S.D.; Liber, K.; Palace, V.; Hecker, M.; Doig, L.E.; Janz, D.M. Effects of selenium on benthic macroinvertebrates and fathead minnow (Pimephales promelas) in a boreal lake ecosystem. Ecotoxicol. Environ. Saf. 2019, 182, 109354. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Wang, C.; Deng, J.; Zhong, L.; Huang, X.; Sun, Y.; Cui, X. Effect of Yeast Selenium on Growth Performance, Muscle Selenium Deposition, and Antioxidant Capacity of Juvenile Cherax quadricarinatus. Fishes 2025, 10, 226. https://doi.org/10.3390/fishes10050226
Han Y, Wang C, Deng J, Zhong L, Huang X, Sun Y, Cui X. Effect of Yeast Selenium on Growth Performance, Muscle Selenium Deposition, and Antioxidant Capacity of Juvenile Cherax quadricarinatus. Fishes. 2025; 10(5):226. https://doi.org/10.3390/fishes10050226
Chicago/Turabian StyleHan, Ying, Chenchen Wang, Jimin Deng, Lizhen Zhong, Xiao Huang, Yuandong Sun, and Xiaojuan Cui. 2025. "Effect of Yeast Selenium on Growth Performance, Muscle Selenium Deposition, and Antioxidant Capacity of Juvenile Cherax quadricarinatus" Fishes 10, no. 5: 226. https://doi.org/10.3390/fishes10050226
APA StyleHan, Y., Wang, C., Deng, J., Zhong, L., Huang, X., Sun, Y., & Cui, X. (2025). Effect of Yeast Selenium on Growth Performance, Muscle Selenium Deposition, and Antioxidant Capacity of Juvenile Cherax quadricarinatus. Fishes, 10(5), 226. https://doi.org/10.3390/fishes10050226




