Abstract
Additives in fish feeds are used worldwide to provide better productivity and improve fish’s health in facing disease outbreaks. This study aimed to identify the protective effect of taurine on the immune-related parameters and antioxidant enzyme activities of Seriola rivoliana juveniles after being challenged with LPS (lipopolysaccharide). Previously, the fish were submitted to a feeding trial for 60 days with feed enriched with different doses of external taurine (0, 1%, and 2%). Juveniles fed on different doses of taurine were injected with LPS (0%, LPS+T0%; 1%, LPS+T1%; 2%, LPS+T2%), and a control group was injected with saline solution (LPS-). The immune-related mRNA expression was evaluated, as were lysozyme enzyme activity and antioxidant enzyme activities (superoxide dismutase—SOD; catalase). Regarding immune mRNA expression, all the treatments had a peak of expression at 24 h post-LPS-injection, with a sharp decrease at 72 h post-injection, reaching similar mRNA expression as at 0 h post-injection. The results showed that the LPS+T2% treatment improved the expression of il1-β, tnf-α, and tlr-3 at 24 h post-LPS injection. Antioxidant and lysozyme activities were higher in both treatments with taurine when compared to the LPS+T0% and LPS- groups after 72 h post-LPS injection. These results suggest using 2% of exogenous external taurine can improve immunocompetency and counteract the oxidative stress caused by exposure to LPS in S. rivoliana juveniles.
Key Contribution:
Taurine supplementation has the potential to improve the immunological capabilities and the antioxidant status of S. rivoliana juveniles.
1. Introduction
The aquaculture sector is faced with disease outbreaks that obstruct worldwide production; hence, prioritizing health maintenance is crucial in contemporary fish farming. It is important to develop strategies to boost the immune system and the general health of fish [1,2]. In this context, the use of feed additives as a potential solution to these issues is receiving increased attention. These supplements are frequently used to improve the general well-being of aquatic organisms as immune system boosters, thus helping to prevent or manage disease outbreaks [3]. Typically, they are incorporated into the standard diet, creating functional feeds, which are characterized as diets that satisfy the basic nutritional requirements of farmed fish and enhance their survival rates, growth, and overall health status [1].
An additive gaining attention in fish feed formulations is the amino acid (AA) taurine. Taurine has been found to be a conditionally essential amino acid in fish [4,5]. In stressful situations, an adequate amount of this β-sulphonic amino acid could prevent a debilitating status, avoiding the consequences of infectious processes [6,7]. It is worth noticing that in carnivorous fish, the presence of this amino acid is important since carnivorous fish feed is naturally rich in this amino acid. In this sense, this amino acid must be added to their diet when there is a partial or complete replacement of fish meal [8], which denotes the importance of taurine for the overall health of carnivorous fish. Taurine has been shown to have beneficial effects such as reducing oxidative damage and inflammation in organisms exposed to toxic substances and stressors [1,6,9,10,11,12,13].
In different animals, evidence has shown that lipopolysaccharides (LPSs) can induce inflammation and oxidative stress [11,14,15,16,17]. LPS, or endotoxin, is a component of the outer membrane of Gram-negative bacteria; it is also known as lipoglycans and is considered an important virulence factor [18,19,20]. The recognition of LPS by the organism activates a signaling cascade [11,21,22], since it acts as a pathogen-associated molecular pattern (PAMP), promoting the biosynthesis and release of inflammatory mediators [11,23,24,25], which makes it interesting for studies to stimulate the immune system and its response [21,23,26,27]. This molecule is highly toxic in mammals and higher vertebrates, even at low doses. However, in fish, it presents lower toxicity but still can activate the immune system similarly to a bacterial infection [21,25,26,28,29].
In the last few years, our group has studied the use of feed additives and immunomodulators in longfin yellowtail (Seriola rivoliana) juveniles on their immune response [30,31,32]. The carnivorous longfin yellowtail (Carangidae), also known as hirenaga-kanpachi, is a promising candidate for diversifying global aquaculture production due to its fast growth, high-quality meat, and circumglobal distribution [33]. As a marine carnivorous fish, taurine might be important to S. rivoliana mainly in stress conditions, such as bacterial infection, and it could improve its immune status.
This study aims to determine whether including taurine in the commercial diet of juveniles of the marine fish S. rivoliana can have a protective effect on modulating their immune and antioxidant response to a challenge with LPS. For this purpose, innate and adaptive immune-related genes (il-1β, il-10, tnf-α, myd-88, tlr-3, and marco) and an immune-related enzyme (lysozyme), as well as antioxidant enzymes (superoxide dismutase and catalase), were evaluated. This is the first report on an LPS challenge in S. rivoliana juveniles fed with different doses of taurine and their effects on immunity and antioxidant enzyme activities.
2. Materials and Methods
2.1. Ethics Statement
This study adhered to the guidelines established by the European Union Council (2010/63/EU) and the Mexican Government Normativity (NOM-062—ZOO-1999) about experimental animals’ production, care, and use. Additionally, the research protocols and procedures were meticulously reviewed and approved by an internal committee at CIBNOR, in compliance with the ARRIVE guidelines [34].
2.2. Previous Nutritional Trial
Prior to the LPS challenge, a nutritional trial was performed as described in [35] with hatchery-reared juveniles provided by Kampachi Farms, La Paz, Mexico. Briefly, three diets were formulated using AQUATEC® commercial feed (Queretaro, Mexico; protein 49%, lipids 15%, fiber 3%, ash 9.5%, humidity 12%, and N.F.E.11.5%). The basal diet was supplemented with taurine (Encapsuladoras Mexico®; 99.9% purity (Chihuahua, Mexico)) at 0, 1, and 2% concentration (Control, T1%, and T2%, respectively) for 60 days. The final taurine concentration and the formulation of each experimental diet are available in the Supplementary Materials (Table S1). Initial and final body weight and furcal length during the nutritional trial are provided in [35]. In the present trial, the fish weighed 611 ± 62 g (control), 655 ± 88 g (T1%), and 704 ± 95 g (T2%). Regarding length, control presented 33.4 ± 1.2 cm, T1% presented 34 ± 1.9 cm, and T2% presented 37 ± 1.3 cm. During the feeding trial, fish were kept in 3000 L tanks with through flow; water temperature was kept at 24.5 ± 0.5 °C, dissolved oxygen at 7.4 ± 1.2 mg L−1, salinity at 36 ± 0.7, and pH at 7.8 ± 0.2.
2.3. LPS Challenge
For LPS exposure, we followed the protocol performed in [28] with some modifications. Briefly, the exposure occurred after 60 days of the nutritional trial, during which the fish were fed with different taurine concentrations (0, 1%, and 2%). The fish were acclimatized for 48 h before the LPS challenge; then, juveniles of S. rivoliana were assigned to be challenged with LPS (Salmonella typhimurium (Sigma L—6511)—1 mg Kg−1 in 1 mL saline solution). This is the first study on the use of LPS injection for this species. This dose was chosen to trigger an immune response but to avoid immunosuppression or mortality. The experimental design was randomized and conducted in triplicate. Fish (9 fish per tank) from the experimental and control groups [LPS injected = LPS+T0%, LPS+T1%, LPS+T2%; saline solution injected = control (LPS-)] were kept in ten 3000 L indoor circular fiberglass tanks (three tanks for each treatment, i.e., LPS+T0%, LPS+T1%, and LPS+T2%, and one tank for the LPS- group) with a flow through system during 72 h after intraperitoneal injection. Dissolved oxygen was kept at 7.4 ± 1.1 mg L−1, water exchange was 100% day−1, and temperature 24 ± 1.0 °C.
2.4. Sampling
For biochemical analysis, fish were sampled (n = 3 replicates) for skin mucus (72 h post-injection), and molecular analysis was performed for the head kidney (n = 3 replicates at each sampling time) at 0, 24 h, and 72 h post-injection. Fish were first anesthetized with clove oil (eugenol), and skin mucus was collected and preserved in PBS at −80 °C until analysis. For molecular analysis, after being anesthetized, fish were euthanized by a quick and humane medullary cut using sharp scissors. Following euthanasia, samples of the head kidney were obtained and preserved in RNAlater® (Thermo-Fisher Scientific, Carlsbad, CA, USA) at −80 °C for further analysis.
2.5. Biochemical Analyses
For biochemical analyses, skin mucus preserved in PBS (1:2) was diluted at a final concentration of 1:10 (mucus: PBS). Then, the analyses described below were performed.
2.5.1. SOD Activity
SOD activity in the mucus was assayed in triplicate using the method described in [28]. Briefly, in an optical polystyrene cuvette, 10 µL of the sample, 10 µL of xanthine oxidase (0.025 IU mL−1 Sigma, X-1875, St. Louis, MO, USA), and 1.98 mL of cocktail (phosphate buffer—50 mM pH 7.8; cytochrome C—0.012 mM (Sigma, C2506, St. Louis, MO, USA); xanthine—0.1 mM; and EDTA—0.1 mM) were added. A unit of SOD activity was determined by the percentage of inhibition of the reduction rate of cytochrome c at 550 nm by 90 s kinetics. The specific activity was expressed as U mg protein −1.
2.5.2. Catalase Activity
Catalase (CAT) activity was assayed according to [36] with some modifications. Briefly, the procedure started by mixing the mucus homogenate extract (20 μL) into a 96-well microplate with 100 μL of phosphate buffer (100 mM, pH 7.0), 30 μL of absolute methanol, and 20 μL of H2O2 (30%). The mixture was incubated in a shaker (120 rpm) for 20 min at room temperature. After incubation, 30 μL of KOH (10 M) was added to stop the reaction, and 30 μL of diluted 4-Amino-3-hydrazino-5-mercapto-1,2,4-triazole (Alfa Aesar, Ward Hill, MA, USA) (46 mM in 0.5 M HCl solution) reagent was added. The plate was incubated for 10 min in a rotational shaker. Finally, 10 μL of KIO4 was added, and the plate was incubated for 5 min in a shaker at room temperature. Absorbance was read at 540 nm. The formaldehyde formation in each sample was calculated using a formaldehyde standard curve (Equation (1)). CAT activity was calculated using the equation below and was expressed as units mg protein −1 (Equation (2)):
CAT activity = (μM formaldehyde)/(20 min) × sample dilution = nmol/min/mL
2.5.3. Lysozyme Activity
Based on a modification of the method in [37], on a flat-bottomed 96-well microtiter plate, 100 µL of 0.4 mg mL−1 Micrococcus lysodeikticus (Sigma-Aldrich, St. Louis, MI, USA) in 0.05 M phosphate buffer (pH 5.2) was added to 100 µL of supernatant (1:10 dilution). A kinetics curve was performed at 450 nm with reads every 2 min for 60 min. For the positive control, the supernatant was replaced by 2 μg mL−1 Hen Egg White lysozyme (Sigma L-7001). The supernatant was replaced by a phosphate buffer for the negative control. A unit of lysozyme activity was defined as the amount of supernatant causing a decrease in absorbance of 0.001 units per minute [37].
2.5.4. Protein Determination
The soluble protein content was determined in the supernatant as described by [38]; briefly, 10 µL of homogenate was mixed with 200 µL Bio-Rad Protein assay dye reagent (BioRad 500-0205, Hercules, CA, USA). Samples were assayed in triplicate in a 96-well plate and read at 595 nm. To calculate the amount of protein in sample, a standard curve was previously performed with bovine serum albumin (BSA, A7906; Sigma-Aldrich, Madrid, Spain).
2.6. Molecular Analysis
2.6.1. RNA Extraction
To analyze the immune-related mRNA expression, total RNA was extracted from the fish head kidney. The samples were homogenized using a FastPrep™ system (Thermo-Fisher Scientific, Carlsbad, CA, USA) with silica pearls at 5 s per minute for 30 s in 1000 µL of Trizol Reagent (Invitrogen, Carlsbad, CA, USA). After homogenization, the procedure for RNA isolation was performed following the manufacturer’s instructions.
The determination of RNA quality and quantity was carried out in a Nanodrop spectrophotometer (Thermo-Fisher Scientific, Carlsbad, CA, USA) and by agarose electrophoresis (1.5%) with Sybr Safe DNA Gel Stain (Invitrogen, Paisley, UK).
2.6.2. DNase Treatment
To guarantee the elimination of genomic DNA, a DNase treatment was performed (RQ1 RNase-Free DNase; M6101; Promega, WI, USA). For each 1 μg of RNA, a mix of 1 μL of buffer 10X, 1 μL of DNase, and DEPC water was made to obtain a total of 10 μL, which was then incubated at 37 °C for 30 min, followed by adding 1 μL DNase Stop Solution and incubating at 65 °C for 10 min.
2.6.3. cDNA Synthesis
To generate complementary DNA by the Reverse Transcription System (ImProm-II™, A3800; Promega, WI, USA), 1 μL of oligo-dT was mixed with 2 μg of total RNA and then incubated at 70 °C for 10 min with a mix of 1 μL dNTP (10 mM), 2.4 μL 25 mM MgCl2, 4 μL 5X reaction buffer, 2 µL ribonuclease inhibitor (RNasin®, Madison, WI, USA), 1 μL reverse transcriptase, and 5.6 μL nuclease-free water. The protocol for reverse transcription was as follows: 25 °C for 5 min, 42 °C for 60 min, and 70 °C for 15 min. The cDNA was stored at −20 °C until further qPCR analyses.
2.6.4. RT-qPCR
Relative mRNA expression was quantified using RT-qPCR on a CFX96 Touch™ Real-Time Thermal Cycler CFX96 (Bio-Rad). Each well contained 10 μL reaction mixture comprising 5 μL SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad), 2.0 μL cDNA, and 0.1 μL primers as listed in Table 1. The run protocol was performed according to the manufacturer’s specifications: (1) 95 °C, 30 s; (2) 95 °C, 5 s; (3) 60 °C, 15 s; (4) melt curve 65 °C to 95 °C, increasing by 0.5 °C for 5 s. Steps 2 and 3 were repeated 40 times. Primer design was based on the S. rivoliana transcriptome (BioProject: PRJNA756687), with each primer sequence evaluated using OligoCalc—Oligonucleotide Properties Calculator (http://oligocalc.eu/, accesses on 12 March 2025). All primers were designed to be amplified at 60 °C, with efficiency ranging from 100 to 105.9%. Target mRNA expression was determined by the 2−ΔΔCT method, as outlined by [39], with normalization to the 18S reference gene and the control group, which was assigned an expression value of 1.

Table 1.
Oligonucleotide primers for the qPCR analysis of target genes of Seriola rivoliana juveniles.
2.7. Statistical Analysis
Molecular and biochemical analyses were performed in triplicate for each sample and are shown as mean ± standard error (SE). For biochemical analysis, as well as for the differences between treatments for each time point in the molecular analysis, a one-way analysis of variance was performed. A two-way analysis of variance (ANOVA) was performed for molecular analysis, with treatment and time as independent variables. The Shapiro–Wilk test for normality and Levene’s test for homoscedasticity were previously performed (logarithmic transformation was performed when the data did not fit normality); then, Tukey’s post hoc test was carried out to determine the differences between the groups (p < 0.05). The statistical analysis and plot were performed using the software SPSS Version 29 (Armonk, NY, USA).
3. Results
Experimental fish did not show mortality after LPS administration, nor at the end of the experiment.
3.1. Enzyme Activity
The data presented in Figure 1A show the high activity of SOD in the LPS+T1% and LPS+T2% treatments when compared with LPS+T0% and LPS-; no statistical differences were observed between LPS+T1% and LPST2%. When compared with LPS-, the LPS+T0% treatment shows a higher activity of SOD in mucus at 72 h post-injection. Figure 1B shows the highest activity of catalase in the LPS+T2% group, followed by the LPS+T1% treatment. No differences between LPS- and LPS+T0%, related to catalase activity, were detected.
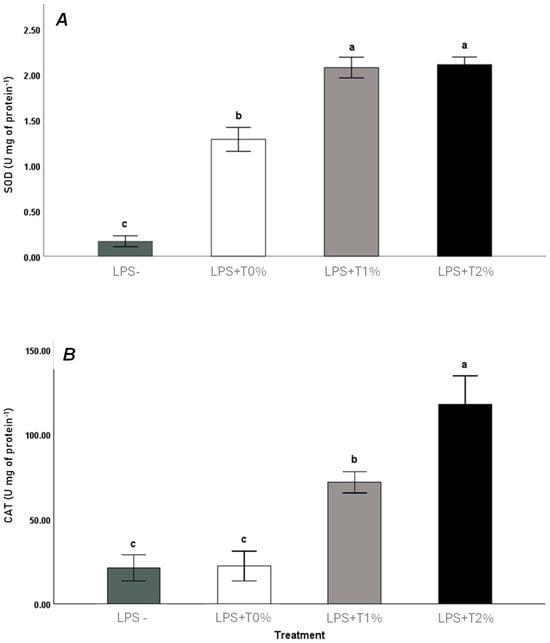
Figure 1.
Skin mucus (A) superoxide dismutase (SOD) and (B) catalase activities in Seriola rivoliana juveniles fed with different amounts of exogenous taurine (0, 1, and 2%) after 72 h LPS challenge. Data are shown as mean ± SD. Significant differences between treatments are indicated by letters (one-way ANOVA, p < 0.05). LPS- (saline solution injected without taurine feeding); LPS+T0% (LPS injected without taurine feeding); LPS+T1% (LPS injected with 1% taurine feeding); LPS+T2% (LPS injected with 2% taurine feeding).
Regarding lysozyme activity, LPS+T1% and LPS+T2% presented higher values, which were statistically different from LPS+T0% and LPS-. No differences in lysozyme activity were observed between LPS+T0% and LPS- (Figure 2).
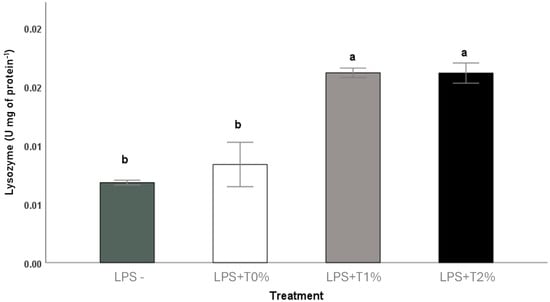
Figure 2.
Skin mucus lysozyme activity in Seriola rivoliana juveniles fed with different amounts of exogenous taurine (0, 1, and 2%) after 72 h LPS challenge. Data are shown as mean ± SD. Significant differences between treatments are indicated by letters (one-way ANOVA, p < 0.05). LPS- (saline solution injected without taurine feeding); LPS+T0% (LPS injected without taurine feeding); LPS+T1% (LPS injected with 1% taurine feeding); LPS+T2% (LPS injected with 2% taurine feeding).
3.2. Immune System Gene Expression
Significant upregulation of the time–treatment interaction of all immune-related genes evaluated in this study was observed at 24 h post-LPS injection when compared to 0 h and 72 h post-injection (p < 0.05), and no differences in the time–treatment interaction of all immune-related genes were observed between 0 and 72 h post-injection. Regarding the il-1β gene, at the onset of the trial, fish that were given a control diet (LPS-) and LPS+T1% exhibited a higher expression of this gene compared to the LPS+T2% group; at 24 h, a significant upregulation was observed in the LPS+T2% treatment when compared to LPS+T0% (p < 0.05) and LPS+T1% (p < 0.05) in the same period (Figure 3A). There were no differences between the treatments at the end of the trial, and the values did not differ from the beginning (p > 0.05). The tnf-α gene was overexpressed at the beginning of the trial in the control group when compared with LPS+T1% and LPS+T2%; however, at 24 h post-injection, it presented a significant difference between the LPS+T2% and LPS+T0%. In the former treatment, this gene was overexpressed; no differences were found between time 0 and 72 h post-injection (Figure 3B). For the tlr-3 gene, upregulation was observed in control group prior to LPS injection (0 h) when compared to the LPS+T1% and LPS+T2% groups, whereas at 24 h post-injection, upregulation was observed in LPS+T2% when compared to LPS+ and LPS+T1%. At 72 h post-injection, no differences were observed between the treatments (Figure 4A).
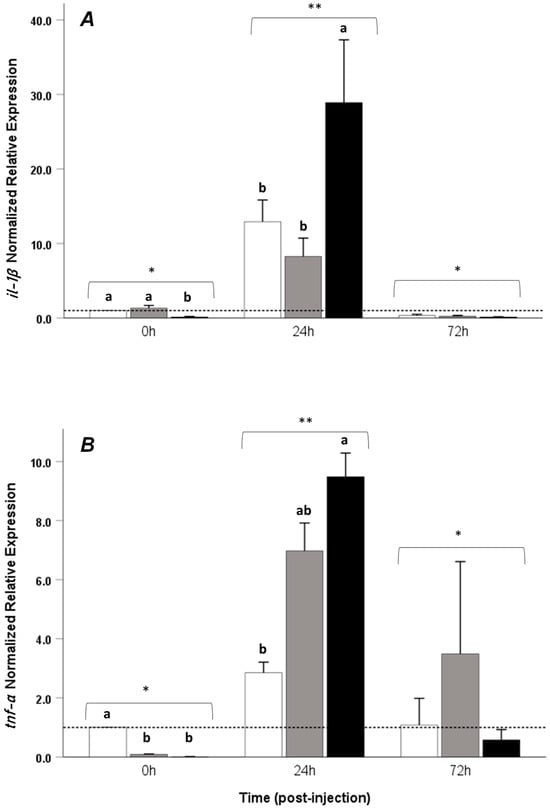
Figure 3.
Relative mRNA expression of (A) il-1β and (B) tnf-α in the head kidney of Seriola rivoliana juveniles fed with different amounts of exogenous taurine (0, 1, and 2%) at 0, 24, and 72 h post-LPS injection. LPS+0% taurine: white bars; LPS+1% taurine: gray bars; LPS+2% taurine: black bars. Data are shown as mean ± SE fold increase relative to the control (dotted line). Different lowercase letters indicate statistically significant differences between treatments at the same time point (one-way ANOVA, p < 0.05); asterisks (*) (**) denote significant differences in the interaction effects between time and treatment (two-way ANOVA, p < 0.05).
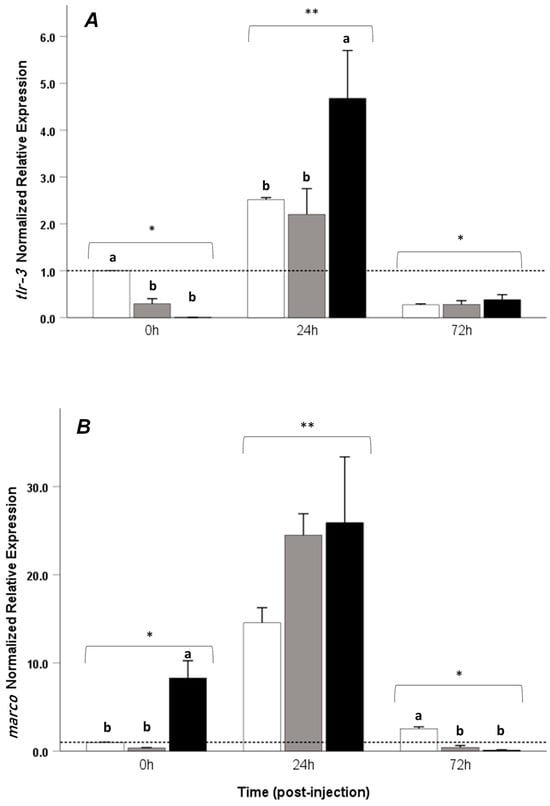
Figure 4.
Relative mRNA expression of (A) tlr-3 and (B) marco in the head kidney of Seriola rivoliana juveniles fed with different amounts of exogenous taurine (0, 1, and 2%) at 0, 24, and 72 h post-LPS injection. LPS+0% taurine: white bars; LPS+1% taurine: gray bars; LPS+2% taurine: black bars. Data are shown as mean ± SE fold increase relative to the control (dotted line). Different lowercase letters indicate statistically significant differences between treatments at the same time point (one-way ANOVA, p < 0.05); asterisks (*) (**) denote significant differences in the interaction effects between time and treatment (two-way ANOVA, p < 0.05).
Regarding the marco gene, at 0 h, LPS+T2% showed upregulation compared to the other groups in the same period. At 72 h post-injection, upregulation was observed in the LPS+T0% group when compared to the different treatments (p < 0.05). No differences were observed for this gene at 24 h (Figure 4B).
No differences between the treatments in each period were observed for the myd-88 gene (p > 0.05) (Figure 5A).
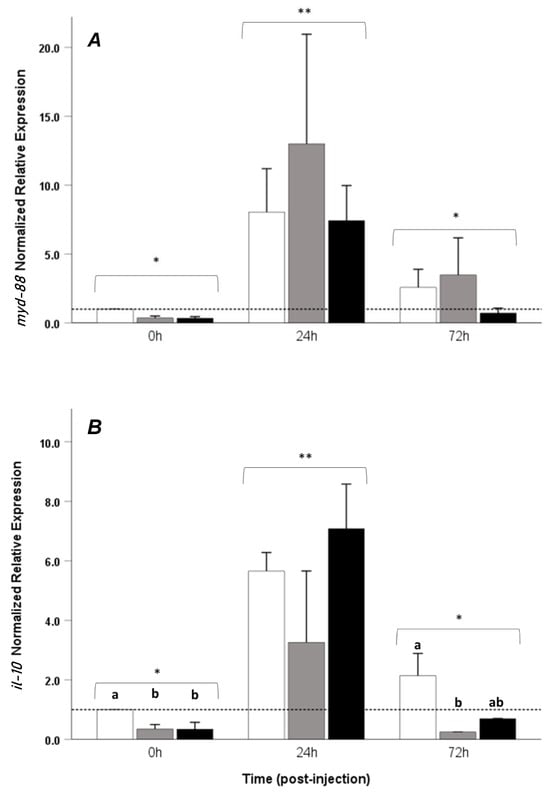
Figure 5.
Relative mRNA expression of (A) myd-88 and (B) il-10 in the head kidney of Seriola rivoliana juveniles fed with different amounts of exogenous taurine (0, 1, and 2%) at 0, 24, and 72 h post-LPS injection. LPS+0% taurine: white bars; LPS+1% taurine: gray bars; LPS+2% taurine: black bars. Data are shown as mean ± SE fold increase relative to the control (dotted line). Different lowercase letters indicate statistically significant differences between treatments at the same time point (one-way ANOVA, p < 0.05); asterisks (*) (**) denote significant differences in the interaction effects between time and treatment (two-way ANOVA, p < 0.05).
Upregulation of il-10 was observed at the beginning of the trial in the control group compared to the other groups. At 72 h post-injection, LPS+T0% presented upregulation compared to the LPS+T1% group. No differences were observed between the treatments at 24 h post-injection (Figure 5B).
During the trial, the expression of all the measured genes in the different treatments showed the same pattern. At 0 h, low gene expression was observed; then, a peak in expression was observed at 24 h, followed by a drop at 72 h, which presented no differences compared to 0 h.
4. Discussion
Due to the environment in which they are kept, cultivated fish are highly susceptible to bacterial infections, which makes producers increasingly seek solutions to these problems through the use of immunostimulants. In this sense, there is evidence that the use of taurine can provide an immunomodulatory and antioxidant effect, capable of improving animal health and welfare [40,41]. The antioxidant properties of taurine in fish have been confirmed by several studies [13,40,41,42], and its antioxidant capacity could be associated with an increase in the antioxidant enzymes activities [11,24]. S. rivoliana juveniles were fed with different amounts of exogenous taurine (0, 1, and 2%) for 8 weeks and then exposed to an LPS challenge for 72 h. Regarding the antioxidant enzymes (SOD and CAT), we observed that juveniles from the LPS+T1% and LPS+T2% groups showed a significantly enhanced activity after the challenge. Similarly to our research, ref. [13] found that Monopterus albus fed with different taurine doses during an 8-week feeding trial showed higher SOD and CAT when challenged with H2O2 when compared with the control-fed group. In other fish species, in ammonia-challenged hybrid snakehead Channa maculatus♀ × Channa argus♂ [43] and low-temperature-stressed Takifugu obscurus [44], taurine showed an antioxidant effect, improving antioxidant enzyme activity. In studies [14,15] using taurine-rich Paroctopus dofleini and mussel water extracts, the authors found a reduction in ROS in the embryos and larvae of the zebrafish Danio rerio exposed to an LPS challenge when compared to a control group (no extract). In terrestrial animals, the effects of taurine administration are similar to our findings; rats and broiler chickens fed a diet with taurine presented an improvement in their antioxidant capacity when challenged with LPS, with increased SOD and CAT activity [11,24].
LPS can induce reactive oxygen species (ROS) production in organisms due to its pro-oxidative action [24]. In the present study, it has been shown that taurine can alleviate this process by increasing antioxidant activities after the LPS challenge when compared to the LPS+T0% group. It is worth mentioning that, because of its biochemical composition, LPS mimics a Gram-negative bacterial infection, so the host immune-related system can detect it and then initiate a cascade of responses.
When a pathogen invades an organism, the PPRs (pattern recognition receptors) are activated, generating a cascade of immune responses by the host, which includes an increase in lysozyme production, which aids in the elimination of the pathogen by hydrolyzing the ß-linked glycosidic bonds in the bacterial cell wall peptidoglycans [26]. Higher doses of taurine in this study were as effective as an immunostimulant, increasing mucus lysozyme activity in juveniles challenged with LPS compared to the LPS+T0% and LPS- groups. Similarly to our findings, ref. [41] reported an increase in mucosal lysozyme when Acanthopagrus latus was fed with a taurine-rich diet (≥1.25%). The same was observed in Pelteobagrus fulvidraco fed with higher levels of taurine (1.6, 2.13, and 2.55%), with values of lysozyme activity increasing with increasing dietary taurine levels [45]. A related species from the same family, the golden pompano (Trachinotus ovatus), exhibited a similar response when given higher levels of taurine in their diet, resulting in increased lysozyme activity [46]. There seems to be a positive correlation between diet taurine level and lysozyme activity; however, further research on this topic must be performed to standardize the amounts, way of administration, or delivery of taurine. When it comes to an LPS challenge, this correlation seems to be the opposite. Some studies have demonstrated that when organisms are exposed to different doses of LPS, lysozyme activity decreases when compared to the control group (no LPS injection) [21,22,23,25,26], which could indicate an immune depression status of the organism; however, this seems to depend on the dose and tissue evaluated and to be species-specific. In our study, no differences in mucus lysozyme activity were found between LPS- and LPS+T0%, which may indicate that even though LPS promoted a host immune response, the dose of LPS was not sufficiently high to trigger a decrease in lysozyme activity. The immune system in fish is predominantly influenced by humoral factors, including lysozyme. This enzyme in bodily fluids is a reliable indicator of fish health, crucial for innate immunity by combating microbes and enhancing immune cell phagocytosis [23,25,47,48]. The improvement in lysozyme activity associated with other immune parameters suggests that taurine can positively stimulate the fish’s immune response, which could, potentially, enhance their ability to fight bacterial infections. In addition, we also observed mRNA upregulation of immune-related genes such as tlr-3, il-1β, and tnf-α 24 h post-injection.
In this study, Toll-like receptor (tlr-3) upregulation may indicate an attempt by the organism to restore proper balance in its immune system. tlr-3 can amplify the immune response by promoting the release of proinflammatory cytokines and activating other immune components. It is worth mentioning that tlr-3 is mainly associated with virus or poly I: C challenge [49,50,51,52]; however, in some fish, evidence shows that tlr-3 has a response after bacterial challenge. In this sense, ref. [51] found the upregulation of tlr-3 in Lateolabrax japonicus after bacterial challenge with Vibrio harveyi and Streptococcus agalactiae. Another study [53] demonstrated the upregulation of tlr-3 in the head kidney of yellow catfish (Pelteobagrus fulvidraco) following exposure to Aeromonas hydrophila, a Gram-negative bacterium. The authors evaluated nine tlrs genes, including tlr-3, at different times of exposure (0, 6, 12, 24, 28, and 72 h), and interestingly, similarly to what was found in our study, high fold-change was observed at 6, 12, and 24 h, decreasing drastically afterward [53]. We observed an upregulation at 24 h, with a prominent decrease at 72 h, which led us to infer that this gene participates actively in the recognition of bacteria, as well as LPS, as a PAMP and that it might induce inflammatory responses in fish.
Il-1β and tnf-α play pivotal roles as essential molecules within the innate immune response against bacterial intrusion while also serving to induce the secretion of fundamental cytokines that activate the effector functions of macrophages and lymphocytes [48]. The upregulation of both cytokines il-1β and tnf-α was observed in this study at 24 h after LPS injection, with significant differences in LPS+T2% when compared to the other treatments, demonstrating its potential immunostimulant effect. This enhanced immune response is a key feature of trained immunity, where the innate immune cells exhibit a memory-like response to subsequent challenges with pathogens or PAMPs [16]. Regarding the effect of taurine on mRNA expression after an LPS challenge, ref. [12] found that before a stress period of transition from fresh- to seawater, using an amino acid-rich diet (DL-Methionine, L-Lysine, L-Threonine, and taurine) benefits the leukocyte immune responses induced by LPS, increasing il-1β gene expression. The authors suggest that a diet containing those amino acids could increase the defense of the fish against bacterial and viral infection in this vulnerable transition period [12]. Contrary to our findings, ref. [13] found that taurine could inhibit the upregulation of il-1β and tlr-3 when Monopterus albus juveniles were challenged with H2O2; however, an upregulation of il-10 was observed in taurine treatment. In our study, the mechanism of defense after 24 h LPS injection triggered a proinflammatory response, with no difference between treatments in terms of the anti-inflammatory il-10 response, which could indicate a balance among proinflammatory and anti-inflammatory responses; in certain pathologies, the anti-inflammatory response may be insufficient to counteract inflammatory activity or, conversely, it may be excessively pronounced, leading to immunosuppression and an increased susceptibility to infection [54].
Inflammation can help to regulate the immune response, ensuring that it is properly activated to fight the infection or injury and deactivated once the problem is resolved. In the present study, this could be observed at 72 h, when the inflammatory mRNA expression decreased to the basal level, with similar values to those found at 0 h post-injection; this proinflammatory upregulation of tlr-3, il1-b, and tnf-a in LPS+T2% could indicate a better immune stimulation in the LPS+T2% group. In this sense, a study performed with LPS-challenged goats showed that the use of β-glucan as an immunostimulant increased the expression of il-1β and tnf-α when compared to the control group. The authors suggest that the increased production of il-1β and tnf-α reflects the activation of immune signaling pathways and the priming of the immune system to respond effectively to the challenge posed by the pathogen [16,55].
5. Conclusions
In conclusion, our findings suggest that supplementation with taurine has the potential to improve the immunological capabilities of juvenile S. rivoliana, thereby enabling them to initiate an efficient immune response against an immune challenge. The assessment of antioxidant enzymes and lysozyme activity, accompanied by other immune parameters, helped us to understand how taurine influenced immune function in S. rivoliana juveniles and contributed to their avoiding oxidative stress, as well as to their ability to respond to challenges such as lipopolysaccharide exposure. A higher transient immune mRNA upregulation was observed after an immune stimulus (LPS injection), mainly in the LPS+T2% treatment when compared to the LPS+T0% or LPS- groups. A similar pattern was observed at 72 h with antioxidant and lysozyme activities, leading us to infer that this acute response after LPS injection could be amplified by taurine. This could be associated with a better immune-trained status; however, further research on trained immunity needs to be carried out to validate this hypothesis.
Future research is suggested to optimize the level of taurine for this species, as well as the use of other immunostimulants against LPS or bacterial challenge, to elucidate the defense mechanisms of S. rivoliana juveniles against infection and the ways to improve their immune responses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10050225/s1, Table S1: Biochemical composition means values of longfin yellowtail diets.
Author Contributions
All authors contributed significantly to this study. Conceptualization, methodology, software, formal analysis, investigation, data curation, writing—original draft preparation, visualization, and review and editing: A.T.; conceptualization, methodology, supervision, formal analysis, investigation, data curation, writing—original draft preparation, visualization, resources, funding acquisition, review and editing, and project administration: D.T.-R.; methodology, software, visualization, review, and data curation: M.A.H.-d.D.; methodology, software, visualization, review, and data curation: L.G.-V.; methodology, software, visualization, review: D.A.C.-R.; conceptualization, methodology, validation, supervision, review, editing, funding acquisition, and project administration: M.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by Consejo Nacional de Ciencia y Tecnología (grant CONACYT-PRONACES 321279 FOP07) and Kampachi Farms México Project n° 20464.
Institutional Review Board Statement
The experiment complied with the “Guidelines of the European Union Council” (2010/63/EU) and the Mexican Government laws (NOM-062-ZOO-1999) on the production, care, and use of experimental animals. All the experimental protocols were carefully revised and approved by an internal council at CIBNOR (Ethics Committee). In terms of procedures to minimize the harm to animals, sampled fish were euthanized with an overdose of clove oil (eugenol) and then dissected on frozen glass to avoid enzyme denaturation and to separate and weight the organs of our interest. As such, space allocations and general conditions were in accordance with the “Guide for the Care and use of Laboratory Animals” (Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council, National Academy Press, Washington D.C. 1996).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors thank Kampachi Farms for providing the juveniles. We thank Patricia Hinojosa Baltazar (in memoriam) and Pablo Monsalvo Spencer for technical support. A.T. is a recipient of a postdoctoral fellowship (CONAHCYT) project n° 319865.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ramos-Pinto, L.; Machado, M.; Calduch-Giner, J.; Pérez-Sánchez, J.; Dias, J.; Conceição, L.E.C.; Silva, T.S.; Costas, B. Dietary Histidine, Threonine, or Taurine Supplementation Affects Gilthead Seabream (Sparus aurata) Immune Status. Animals 2021, 11, 1193. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Choubey, A.K.; Srivastava, P.K. The Effects of Dietary Immunostimulants on the Innate Immune Response of Indian Major Carp: A Review. Fish Shellfish Immunol. 2022, 123, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Contreras, Á.; Teles, A.; Salas-Leiva, J.S.; Chaves-Pozo, E.; Tovar-Ramírez, D. Feed Additives in Aquaculture. In Sustainable Use of Feed Additives in Livestock; Arsenos, G., Giannenas, I., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 811–846. ISBN 978-3-031-42854-8. [Google Scholar]
- Salze, G.P.; Davis, D.A. Taurine: A Critical Nutrient for Future Fish Feeds. Aquaculture 2015, 437, 215–229. [Google Scholar] [CrossRef]
- Sampath, W.W.H.A.; Rathnayake, R.M.D.S.; Yang, M.; Zhang, W.; Mai, K. Roles of Dietary Taurine in Fish Nutrition. Mar. Life Sci. Technol. 2020, 2, 360–375. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Li, R.; Deng, S.; Qin, Q.; Ran, C.; Hao, Y.; Zhang, J.; Zhu, L. Mechanism of Taurine Reducing Inflammation and Organ Injury in Sepsis Mice. Cell. Immunol. 2022, 375, 104503. [Google Scholar] [CrossRef]
- Peng, L.; Li, D.; Yang, D.; Peng, B. Taurine Promotes Oreochromis niloticus Survival against Edwardsiella Tarda Infection. Fish Shellfish Immunol. 2022, 129, 137–144. [Google Scholar] [CrossRef]
- Aragão, C.; Gonçalves, A.T.; Costas, B.; Azeredo, R.; Xavier, M.J.; Engrola, S. Alternative Proteins for Fish Diets: Implications beyond Growth. Animals 2022, 12, 1211. [Google Scholar] [CrossRef]
- Yan, L.; Feng, L.; Jiang, W.; Wu, P.; Liu, Y.; Jiang, J.; Tang, L.; Tang, W.; Zhang, Y.; Yang, J.; et al. Dietary Taurine Supplementation to a Plant Protein Source-based Diet Improved the Growth and Intestinal Immune Function of Young Grass Carp (Ctenopharyngodon idella). Aquac. Nutr. 2019, 25, 873–896. [Google Scholar] [CrossRef]
- Gunathilaka, G.L.B.E.; Kim, M.-G.; Lee, C.; Shin, J.; Lee, B.-J.; Lee, K.-J. Effects of Taurine Supplementation in Low Fish Meal Diets for Red Seabream (Pagrus major) in Low Water Temperature Season. Fish Aquatic Sci. 2019, 22, 23. [Google Scholar] [CrossRef]
- Han, H.; Zhang, J.; Chen, Y.; Shen, M.; Yan, E.; Wei, C.; Yu, C.; Zhang, L.; Wang, T. Dietary Taurine Supplementation Attenuates Lipopolysaccharide-Induced Inflammatory Responses and Oxidative Stress of Broiler Chickens at an Early Age. J. Anim. Sci. 2020, 98, skaa311. [Google Scholar] [CrossRef]
- Holen, E.; Chen, M.; Fjelldal, P.G.; Skjærven, K.; Sissener, N.H.; Remø, S.; Prabhu, A.J.; Hamre, K.; Vikeså, V.; Subramanian, S.; et al. Tailoring Freshwater Diets towards Boosted Immunity and Pancreas Disease Infection Robustness in Atlantic Salmon Post Smolts. Fish Shellfish Immunol. 2022, 120, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhong, L.; Fan, Y.; Zhang, J.; Dai, J.; Zhong, H.; Fu, G.; Hu, Y. Taurine Inhibits Hydrogen Peroxide-Induced Oxidative Stress, Inflammatory Response and Apoptosis in Liver of Monopterus albus. Fish Shellfish Immunol. 2022, 128, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.H.; Lee, S.-H.; Jeon, Y.-J.; Lee, D.-S. Mussel (Mytilus coruscus) Water Extract Containing Taurine Prevents LPS-Induced Inflammatory Responses in Zebrafish Model. In Taurine 10; Advances in Experimental Medicine and Biology; Lee, D.-H., Schaffer, S.W., Park, E., Kim, H.W., Eds.; Springer: Dordrecht, The Netherlands, 2017; Volume 975, pp. 931–942. ISBN 978-94-024-1077-8. [Google Scholar]
- Kim, Y.-S.; Kim, E.-K.; Jeon, N.-J.; Ryu, B.-I.; Hwang, J.-W.; Choi, E.-J.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Antioxidant Effect of Taurine-Rich Paroctopus Dofleini Extracts Through Inhibiting ROS Production Against LPS-Induced Oxidative Stress In Vitro and In Vivo Model. In Taurine 10; Advances in Experimental Medicine and Biology; Lee, D.-H., Schaffer, S.W., Park, E., Kim, H.W., Eds.; Springer: Dordrecht, The Netherlands, 2017; Volume 975, pp. 1165–1177. ISBN 978-94-024-1077-8. [Google Scholar]
- Angulo, M.; Reyes-Becerril, M.; Cepeda-Palacios, R.; Angulo, C. Oral Administration of Debaryomyces hansenii CBS8339-β-Glucan Induces Trained Immunity in Newborn Goats. Dev. Comp. Immunol. 2020, 105, 103597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, X.; Zhu, H.; Wang, Y.; Hou, Y.; Liu, Y. Dietary Fish Oil Supplementation Alters Liver Gene Expressions to Protect against LPS-Induced Liver Injury in Weanling Piglets. Innate Immun. 2019, 25, 60–72. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.-O. Recognition of Lipopolysaccharide Pattern by TLR4 Complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Farhana, A.; Khan, Y.S. Biochemistry, Lipopolysaccharide; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Swain, P.; Nayak, S.; Nanda, P.; Dash, S. Biological Effects of Bacterial Lipopolysaccharide (Endotoxin) in Fish: A Review. Fish Shellfish Immunol. 2008, 25, 191–201. [Google Scholar] [CrossRef]
- Alves, A.P.D.C.; Paulino, R.R.; Pereira, R.T.; Costa, D.V.; Rosa, P.V. Nile Tilapia Fed Insect Meal: Growth and Innate Immune Response in Different Times under Lipopolysaccharide Challenge. Aquac. Res. 2021, 52, 529–540. [Google Scholar] [CrossRef]
- Li, Q. Polyunsaturated Fatty Acids Influence LPS-Induced Inflammation of Fish Macrophages Through Differential Modulation of Pathogen. Front. Immunol. 2020, 11, 559332. [Google Scholar]
- Giri, S.S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Kwon, J.; Lee, S.B.; Sukumaran, V.; Chang Park, S. Effectiveness of the Guava Leaf Extracts against Lipopolysaccharide-Induced Oxidative Stress and Immune Responses in Cyprinus carpio. Fish Shellfish Immunol. 2020, 105, 164–176. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Zhang, L.; Wu, J.; Wang, Y.; Yu, H. Taurine Alleviates Lipopolysaccharide-Induced Liver Injury by Anti-Inflammation and Antioxidants in Rats. Mol. Med. Rep. 2017, 16, 6512–6517. [Google Scholar] [CrossRef]
- Zhu, X.; Li, M.; Liu, X.; Xia, C.; Niu, X.; Wang, G.; Zhang, D. Effects of Dietary Astaxanthin on Growth, Blood Biochemistry, Antioxidant, Immune and Inflammatory Response in Lipopolysaccharide-challenged Channa argus. Aquac. Res. 2020, 51, 1980–1991. [Google Scholar] [CrossRef]
- Biller, J.D.; Polycarpo, G.D.V.; Moromizato, B.S.; Sidekerskis, A.P.D.; Silva, T.D.D.; Reis, I.C.D.; Fierro-Castro, C. Lysozyme Activity as an Indicator of Innate Immunity of Tilapia (Oreochromis niloticus) When Challenged with LPS and Streptococcus agalactiae. Rev. Bras. Zootec. 2021, 50, e20210053. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Mandiki, S.N.M.; Salomon, J.M.A.J.; Baruti, J.B.; Thi, N.T.T.; Nguyen, T.H.; Nhu, T.Q.; Kestemont, P. Pro- and Anti-Inflammatory Responses of Common Carp Cyprinus carpio Head Kidney Leukocytes to E. coli LPS as Modified by Different Dietary Plant Oils. Dev. Comp. Immunol. 2021, 114, 103828. [Google Scholar] [CrossRef]
- Guzmán-Villanueva, L.T.; Ascencio-Valle, F.; Macías-Rodríguez, M.E.; Tovar-Ramírez, D. Effects of Dietary β-1,3/1,6-Glucan on the Antioxidant and Digestive Enzyme Activities of Pacific Red Snapper (Lutjanus peru) after Exposure to Lipopolysaccharides. Fish Physiol. Biochem. 2014, 40, 827–837. [Google Scholar] [CrossRef]
- Saravia, J.; Paschke, K.; Pontigo, J.P.; Nualart, D.; Navarro, J.M.; Vargas-Chacoff, L. Effects of Temperature on the Innate Immune Response on Antarctic and Sub-Antarctic Fish Harpagifer antarcticus and Harpagifer bispinis Challenged with Two Immunostimulants, LPS and Poly I:C: In Vivo and In Vitro Approach. Fish Shellfish Immunol. 2022, 130, 391–408. [Google Scholar] [CrossRef]
- Mazón-Suástegui, J.; Salas-Leiva, J.; Teles, A.; Tovar-Ramírez, D. Immune and Antioxidant Enzyme Response of Longfin Yellowtail (Seriola rivoliana) Juveniles to Ultra-Diluted Substances Derived from Phosphorus, Silica and Pathogenic Vibrio. Homeopathy 2019, 108, 43–53. [Google Scholar] [CrossRef]
- Hernández-López, I.A.; Tovar-Ramírez, D.; De La Rosa-García, S.; Álvarez-Villagómez, C.S.; Asencio-Alcudia, G.G.; Martínez-Burguete, T.; Galaviz, M.A.; Guerrero-Zárate, R.; Martínez-García, R.; Peña-Marín, E.S.; et al. Dietary Live Yeast (Debaryomyces hansenii) Provides No Advantages in Tropical Gar, Atractosteus tropicus (Actinopterygii: Lepisosteiformes: Lepisosteidae), Juvenile Aquaculture. Acta Ichthyol. Piscat. 2021, 51, 311–320. [Google Scholar] [CrossRef]
- Asencio-Alcudia, G.G.; Sepúlveda-Quiroz, C.A.; Pérez-Urbiola, J.C.; Rodríguez-Jaramillo, M.D.C.; Teles, A.; Salas-Leiva, J.S.; Martínez-García, R.; Jiménez-Martínez, L.D.; Galaviz, M.; Tovar-Ramírez, D.; et al. Stress-Protective Role of Dietary α-Tocopherol Supplementation in Longfin Yellowtail (Seriola rivoliana) Juveniles. Fishes 2023, 8, 526. [Google Scholar] [CrossRef]
- Teles, A.; Alvarez-González, C.A.; Llera-Herrera, R.; Gisbert, E.; Salas-Leiva, J.; Del Carmen Rodríguez-Jaramillo, M.; Tovar-Ramírez, D. Debaryomyces hansenii CBS 8339 Promotes Larval Development in Seriola rivoliana. Aquaculture 2022, 560, 738587. [Google Scholar] [CrossRef]
- Percie Du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Hernández-de Dios, M.A.; Tovar-Ramírez, D.; Maldonado-García, D.; Galaviz- Espinoza, M.A.; Barreto-Curiel, F.; Spanopoulos-Zarco, M.; Maldonado-García, M. Taurine Improves Juvenile Seriola rivoliana Growth Performance and Biochemical Profiles in Blood Serum and Skeletal Muscle. Lat. Am. J. Aquat. Res. 2024, 52, 443–458. [Google Scholar] [CrossRef]
- Johansson, L.H.; Håkan Borg, L.A. A Spectrophotometric Method for Determination of Catalase Activity in Small Tissue Samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Guđmundsdottir, B.K.; Magnadottir, B. Humoral Immune Parameters of Cultured Atlantic Halibut (Hippoglossus hippoglossus L.). Fish Shellfish Immunol. 2001, 11, 523–535. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bañuelos-Vargas, I.; López, L.M.; Pérez-Jiménez, A.; Peres, H. Effect of Fishmeal Replacement by Soy Protein Concentrate with Taurine Supplementation on Hepatic Intermediary Metabolism and Antioxidant Status of Totoaba Juveniles (Totoaba macdonaldi). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 170, 18–25. [Google Scholar] [CrossRef]
- Dehghani, R.; Oujifard, A.; Mozanzadeh, M.T.; Morshedi, V.; Bagheri, D. Effects of Dietary Taurine on Growth Performance, Antioxidant Status, Digestive Enzymes Activities and Skin Mucosal Immune Responses in Yellowfin Seabream, Acanthopagrus latus. Aquaculture 2020, 517, 734795. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Wang, Z.; Zhou, J.; Zhang, J.; Zhong, H.; Fu, G.; Zhong, L. The Protective Effect of Taurine on Oxidized Fish-Oil-Induced Liver Oxidative Stress and Intestinal Barrier-Function Impairment in Juvenile Ictalurus punctatus. Antioxidants 2021, 10, 1690. [Google Scholar] [CrossRef]
- Tan, X.; Sun, Z.; Zhu, X.; Ye, C. Dietary Supplementation with Taurine Improves Ability to Resist Ammonia Stress in Hybrid Snakehead (Channa maculatus ♀ × Channa argus ♂). Aquac. Res. 2018, 49, 3400–3410. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Guo, Z.-X.; Wang, A.-L. The Protective Effects of Taurine on Oxidative Stress, Cytoplasmic Free-Ca2+ and Apoptosis of Pufferfish (Takifugu obscurus) under Low Temperature Stress. Fish Shellfish Immunol. 2018, 77, 457–464. [Google Scholar] [CrossRef]
- Li, M.; Lai, H.; Li, Q.; Gong, S.; Wang, R. Effects of Dietary Taurine on Growth, Immunity and Hyperammonemia in Juvenile Yellow Catfish Pelteobagrus fulvidraco Fed All-Plant Protein Diets. Aquaculture 2016, 450, 349–355. [Google Scholar] [CrossRef]
- Liu, J.-X.; Guo, H.-Y.; Zhu, K.-C.; Liu, B.-S.; Zhang, N.; Zhang, D.-C. Effects of Exogenous Taurine Supplementation on the Growth, Antioxidant Capacity, Intestine Immunity, and Resistance against Streptococcus agalactiae in Juvenile Golden Pompano (Trachinotus ovatus) Fed with a Low-Fishmeal Diet. Front. Immunol. 2022, 13, 1036821. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B. Innate Immunity of Fish (Overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Becerril, M.; Guluarte, C.; Ceballos-Francisco, D.; Angulo, C.; Esteban, M.Á. Dietary Yeast Sterigmatomyces halophilus Enhances Mucosal Immunity of Gilthead Seabream (Sparus aurata L.). Fish Shellfish Immunol. 2017, 64, 165–175. [Google Scholar] [CrossRef]
- Rodriguez, M.F.; Wiens, G.D.; Purcell, M.K.; Palti, Y. Characterization of Toll-like Receptor 3 Gene in Rainbow Trout (Oncorhynchus mykiss). Immunogenetics 2005, 57, 510–519. [Google Scholar] [CrossRef]
- Herrero, M.J. ABC de los «Toll-like receptors»: Relación con el desarrollo y progresión de enfermedades autoinmunes. Semin. Fund. Española Reumatol. 2010, 11, 135–143. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, C.; Wang, C.; Fan, S.; Yan, L.; Qiu, L. TLR3 Gene in Japanese Sea Perch (Lateolabrax japonicus): Molecular Cloning, Characterization and Expression Analysis after Bacterial Infection. Fish Shellfish Immunol. 2018, 76, 347–354. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Zhao, Y.; Ma, X.; Yi, H. Toll-like Receptor 3 (TLR3) Regulation Mechanisms and Roles in Antiviral Innate Immune Responses. J. Zhejiang Univ. Sci. B 2021, 22, 609–632. [Google Scholar] [CrossRef]
- Zhang, X.-T.; Zhang, G.-R.; Shi, Z.-C.; Yuan, Y.-J.; Zheng, H.; Lin, L.; Wei, K.-J.; Ji, W. Expression Analysis of Nine Toll-like Receptors in Yellow Catfish (Pelteobagrus fulvidraco) Responding to Aeromonas hydrophila Challenge. Fish Shellfish Immunol. 2017, 63, 384–393. [Google Scholar] [CrossRef]
- De Pablo Sánchez, R.; Monserrat Sanz, J.; Prieto Martín, A.; Reyes Martín, E.; Álvarez De Mon Soto, M.; Sánchez García, M. Balance entre citocinas pro y antiinflamatorias en estados sépticos. Med. Intensiv. 2005, 29, 151–158. [Google Scholar] [CrossRef]
- Angulo, M.; Reyes-Becerril, M.; Tovar-Ramírez, D.; Ascencio, F.; Angulo, C. Debaryomyces hansenii CBS 8339 β-Glucan Enhances Immune Responses and down-Stream Gene Signaling Pathways in Goat Peripheral Blood Leukocytes. Dev. Comp. Immunol. 2018, 88, 173–182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).