Sustainable Aquaculture Through Enzymatic Hydrolysis of Raw Chitin from Crab By-Products: Functional Fish Feeds Targeting Fish Health with Implications for Human Health
Abstract
1. Introduction
2. Methodology
3. Chitin Extraction Technologies from Crustacean By-Products
| Method | Chitin Yield | Energy Consumption | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| EH | Satisfactory | Low | Environmentally friendly, mild process | Time-consuming, lower yield | [14,26] |
| UAE | Moderate to High | Moderate | Increased yield, reduced solvent usage | Specialized equipment required | [29,30] |
| MAE | Low | Very low | Fast, lower energy requirement | May affect chitin structure | [14,31] |
| scCO2 | High | Moderate | Environmentally friendly, high yield | Expensive equipment | [36,37,38,39] |
| ILs | High | Moderate | Solvent recycling, high yield | Expensive production | [32,33] |
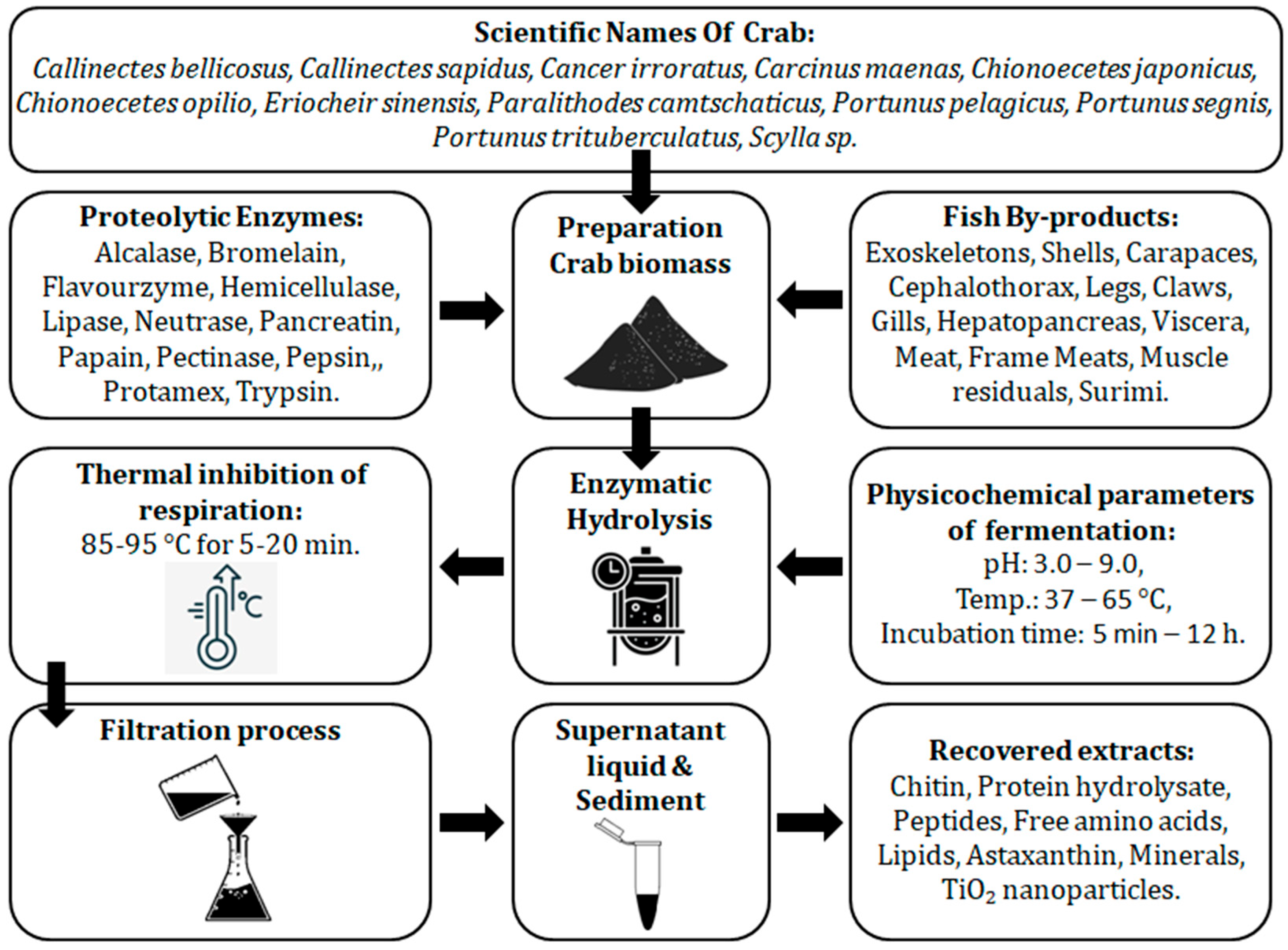
3.1. Preparation of Crab By-Product Samples
| Scientific Names of Species | A: Country B: Company | Crab Biomass | Required Pre-Treatment of Crab Raw Material for the Application of Enzymatic Hydrolysis | References |
|---|---|---|---|---|
| Callinectes sapidus | A: Brazil B: Small processing crab meat industries | Carapaces, Legs | Biomass pre-treatment procedure:
| [40] |
| Portunus trituberculatus | A: China B: Seafood Company | Processing by-products | Biomass pre-treatment procedure:
| [48] |
| Portunus trituberculatus | A: Not mentioned B: pilot plant transformation line | Legs, Claw, Cephalothorax, Shells | Biomass pre-treatment procedure:
| [59] |
| Chionoecetes opilio | A: Canada B: pilot plant transformation line | By-products | Biomass pre-treatment procedure:
| [41] |
| Not mentioned | A: Not mentioned B: Local supermarkets | Surimi | Biomass pre-treatment procedure:
| [46] |
| Paralithodes camtschaticus | A: Not mentioned B: ship-derived | Processing waste | Biomass pre-treatment procedure:
| [42] |
| Chionoecetes japonicus | A: Republic of Korea B: Crab processing plant | Shell | Biomass pre-treatment procedure:
| [50] |
| Carcinus maenas | A: USA B: harvested at the back river | Carapace | Biomass pre-treatment procedure:
| [58] |
| Cancer irroratus | A: Canada B: Factory | Legs, Claws, Cephalothorax, | Biomass pre-treatment procedure:
| [44] |
| Scylla sp. | A: Malaysia B: Supplier | Meat | Biomass pre-treatment procedure:
| [53] |
| Portunus pelagicus | A: Malaysia B: Fish market | Meat | Biomass pre-treatment procedure:
| [52] |
| Callinectes bellicosus | A: Mexico B: Processing company | Exoskeletons | Biomass pre-treatment procedure:
| [54] |
| Chionoecetes japonicus | A: Republic of Korea B: Crab processing factory | Shells, Frame Meats | Biomass pre-treatment procedure:
| [51] |
| Portunus segnis | A: Tunisia B: Processing plant and Fishery market | Viscera, Shells | Biomass pre-treatment procedure:
| [57] |
| Paralithodes camtschaticus | A: Russia B: industrial processing | Gills, Carapace | Biomass pre-treatment procedure:
| [56] |
| Chionoecetes opilio | A: Canada B: Company | By-products | Biomass pre-treatment procedure:
| [47] |
| Not mentioned | A: China B: Biological Products Factory | Shell | Biomass pre-treatment procedure:
| [43] |
| Paralithodes camtschaticus | A: Russia B: Caught in the Barents Sea | Hepatopancreas | Biomass pre-treatment procedure:
| [55] |
| Eriocheir sinensis | A: China B: Crab products factory | Muscle residuals | Biomass pre-treatment procedure:
| [49] |
3.2. Description of the Enzymatic Hydrolysis Process
| Enzymes | Incubation (pH) | Incubation Temperature | Incubation Time | Enzyme Inhibition Temp./Time | Recovered Extracts | References |
|---|---|---|---|---|---|---|
| Alcalase Βromelain | Single pH 9.0 6.0 | Single temp 53 °C 53 °C | Different periods: 5, 15, 30, 45, 60, 90, 120, 180, 240 min. | 90 °C for 5 min. | Protein hydrolysate, Carotenoid | [40] |
| Neutrase Flavorase Papain | Single pH 6.0 6.0 6.0 | Single temp 55 °C 55 °C 55 °C | Single time 7 h 7 h 7 h | 95 °C for 10 min. | Protein hydrolysate | [48] |
| Trypsin Neutrase Bromelin Protamex Pepsin | Single pH 7.0 7.0 8.0 7.0 3.0 | Single temp 55 °C 55 °C 45 °C 50 °C 35 °C | Single time 1 h 1 h 1 h 1 h 1 h | 95 °C for 15 min. | Proteins | [59] |
| Protamex | Single pH 8.0 | Single temp 40 °C | Single time 60 min | 85 °C for 10 min. | Proteins, Lipids, Chitin, Minerals | [41] |
| Pancreatin Lipase | Single pH 7.4 7.4 | Single temp 37 °C 37 °C | Single time 12 h | - | TiO2 nanoparticle | [46] |
| Proteinase preparation | Single pH 6.0–9.5 | Single temp 50.0 ± 0.5 °C | - | - | Free Amino Acids, Protein hydrolysates, Chitin, Antioxidant compounds | [42] |
| Alcalase | Single pH 7.0 | Single temp 50 °C | Single time 25 h | 100 °C | Protein hydrolysates | [50] |
| Alcalase Protamex Flavourzyme Papain | Single pH pH 8.0 pH 7.0 pH 7.0 pH 6.0 | Single temp 50 °C 50 °C 50 °C 65 °C | Single time 1 h 1 h 1 h 1 h | 85–90 °C for 10 min. | Protein hydrolysates | [58] |
| Protamex | Single pH pH 9.0 | Single temp 40 °C | Single time 90 min. | 85 °C for 10 min. | Protein hydrolysates | [44] |
| Alcalase Protamex Neutrase Papain | Single pH 8.5 6.5 7.0 6.0 | Single temp 55 °C 50 °C 55 °C 50 °C | Different periods: 1, 2, 3, 4 h | 85 °C | Protein hydrolysates, Bioactive peptides, free amino acids | [53] |
| Alcalase Protamex Neutrase Papain | Single pH 8.0 6.5 6.5 6.0 | Single temp 55 °C 55 °C 50 °C 60 °C | Different periods: 2–4 h | 85 °C for 20 min. | Bioactive peptides, Protein hydrolysates | [52] |
| Pectinase Lipase Hemicellulase | Single pH 7.0 | Single temp 40 °C ± 5 °C | Single time 1 h | - | Chitin, Lipids | [54] |
| Flavourzyme Neutrase alcalase protamex | Single pH 7.0 7.0 7.0 7.0 | Single temp 60 °C 60 °C 60 °C 60 °C | Single time 5 h 5 h 5 h 5 h | 95 °C for 5 min | Protein, Protein Hydrolysate, Free Amino Acids | [51] |
| Neutrase | Single pH 7.0 | Single temp 50 °C | Single time 3 h | 90 °C, 20 min | Chitin | [57] |
| Neutrase | Single pH 7.0 | Single temp 50 °C | - | - | Protein hydrolysates, free amino acids | [56] |
| Enzyme | Single pH 6.5–7.0 | Single temp 50 °C | Single time 6 h | 95 °C | Chitin | [55] |
| Flavourzyme | Single pH 6.5 | Single temp 65.3 °C | - | - | Crab flavoring rich in sweet-taste free amino acids | [49] |
| Alcalase Bromelain | Single pH 8.0 6.0–7.0 | Single temp | 120 min | Protein hydrolysates, chitin, Astaxanthin-enriched | [40] |
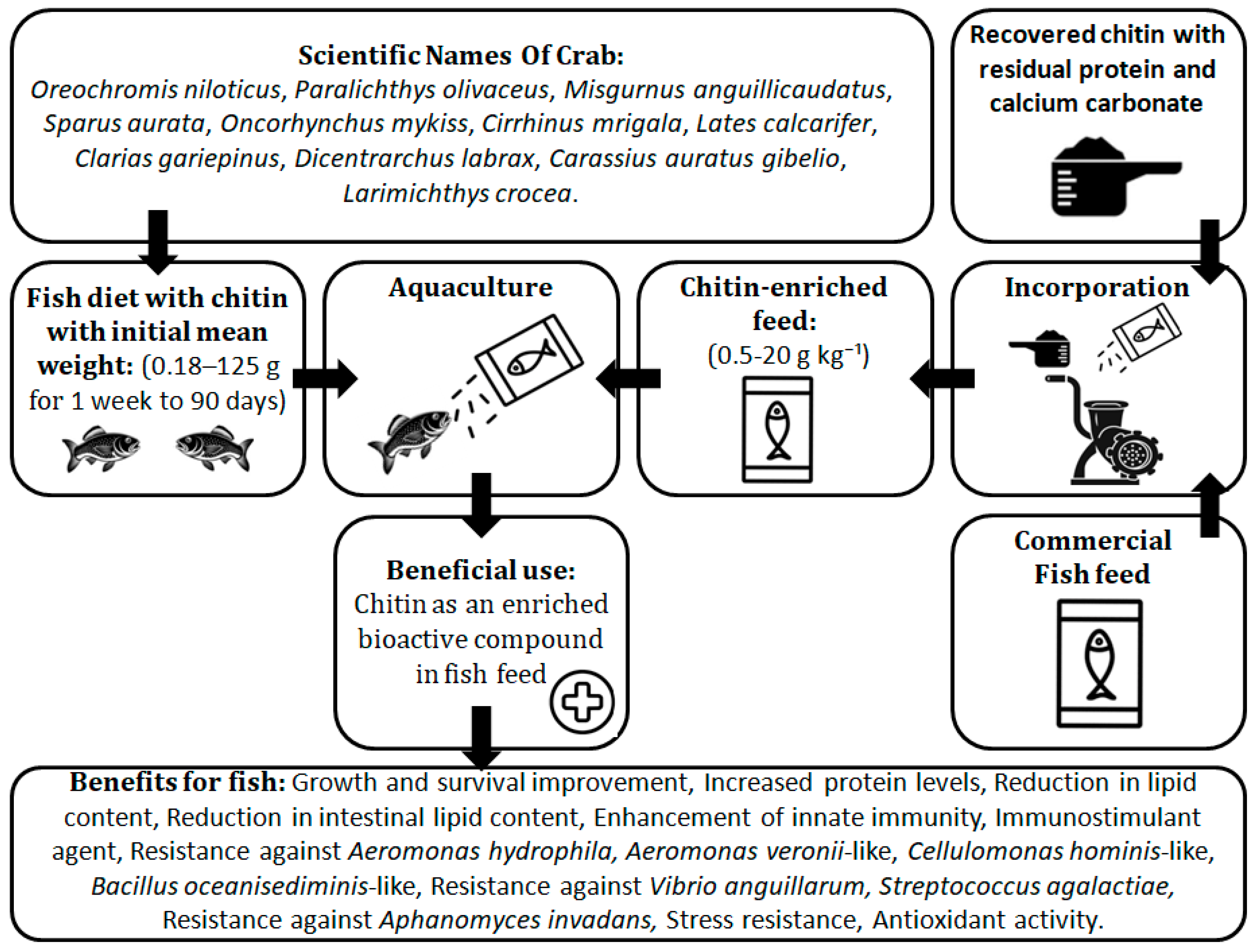
4. Description of the Production of Aquafeed with Chitin and Its Benefit to Farmed Fish
| Scientific Name | Chitosan Addition (g/kg) | Feeding Trial at Initial Weight (g) | Feeding Duration | Benefits | References |
|---|---|---|---|---|---|
| Carassius auratus gibelio | Different chitosan levels: 1.8, 4, 7.5, 10, 20 | Average Initial weight: 4.80 ± 0.01 g | 75 days | Resistance against Aeromonas veronii-like, improved Cellulomonas hominis-like, Bacillus oceanisediminis-like | [67] |
| Cirrhinus mrigala | Recovered chitin and chitosan from exoskeleton of Giant freshwater prawn: 0.05, 0.5, 5 | Average Initial weight: 25.6 ± 1.7 g | 1–4 weeks | Enhancement of innate immunity. Resistance against Aphanomyces invadans | [69] |
| Clarias gariepinus | Recovered chitosan nanoparticles from crab shell: 5 | Average Initial weight: 2.79 ± 0.05 g | 90 Days | Growth and survival improvement | [74] |
| Dicentrarchus labrax | Different chitosan levels: 5, 10, 20, 30, 40 | Average Initial weight: 0.21 ± 0.01 g | 75 days | Growth improvement | [75] |
| Larimichthys crocea | Different chitosan levels: 3, 6, 9 | Average Initial weight: 3.81 ± 0.20 mg | 30 days | Growth and survival improvement. Enhancement of digestive enzyme activity and intestinal development. Antioxidant activity. Immunostimulant agent. | [76] |
| Lates calcarifer | Different chitosan levels: 5, 10, 20 | Average Initial weight: 15 ± 2 g | 15–60 Days | Enhancement of innate immunity. Resistance against Vibrio anguillarum | [21] |
| Misgurnus anguillicadatus | Different chitosan levels: 1, 5, 10 | Average Initial weight: 3.14 ± 0.05 g | 10 weeks | Growth and survival improvement. Resistance against Aeromonas hydrophila | [60] |
| Misgurnus anguillicaudatus | Different chitosan levels: 5, 10, 20, 50 | Average Initial weight: 0.18 g | 50 days | Antioxidant activity. Reduction in intestinal lipid content. Stress resistance | [71] |
| Oncorhynchus mykiss | Different nano-chitosan levels: 0.05, 0.5, 5 | Average Initial weight: 27.75 ± 0.34 g | 70 Days | Growth improvement. Resistance against Aphanomyces invadans | [17] |
| Oncorhynchus mykiss | Different chitosan levels: 2.5, 5, 10 | Average Initial weight: 25 ± 0.1 g | 8 weeks | Immunostimulant agent. Stress resistance | [66] |
| Oreochromis nilotica | Chitosan nanoparticles: 2.5, 5, 10, 20 | Average Initial weight: 19.8 ± 0.59 g | 45 Days | Growth improvement. Antioxidant activity. Enhancement of innate immunity. | [18] |
| Oreochromis nilotica | Recovered chitin from shrimp shells: 5, 10, 20 | Average Initial weight: 40.12 ± 4.25 g | 4 weeks | Immunostimulant agent. Resistance against Aeromonas hydrophila. | [19] |
| Oreochromis nilotica | Recovered chitin from shrimp shells: 5 | Average Initial weight: 23.56 ± 1.23 g | 60 days | Growth and survival improvement. | [77] |
| Oreochromis nilotica | Recovered chitosan from shrimp shells: 20, 40, 60, 80 | Average Initial weight: 50.13 ± 4.13 g | 56 days | Growth improvement. Increased protein levels. Stress resistance. | [70] |
| Oreochromis nilotica | Different chitosan nanoparticle levels: 1, 3, 5 | Average Initial weight: 5.66 ± 0.02 g | 70 days | Growth improvement. Enhancement of innate immunity. | [73] |
| Oreochromis nilotica | Different chitin and chitosan levels: 20, 50, 100 | Average Initial weight: 0.99 ± 0.01 g | 8 weeks | Reduction in lipid content | [22] |
| Oreochromis nilotica | Different chitosan levels: 30, 50 | Average Initial weight: 39.3 ± 0.3 g | - | Resistance against Streptococcus agalactiae | [68] |
| Oreochromis nilotica | Different chitosan levels: 50, 100 | Average Initial weight: 17.32 ± 2.2 g | 60 days | Growth improvement. Antioxidant activity. Stress resistance. Reduction in lipid content. | [72] |
| Paralichthys olivaceus | Chitosan-coating solution: 10 | Average Initial weight: 80 g | 12 weeks | Immunostimulant agent. Reduction of COD and suspended solids. | [20] |
| Sparus aurata L. | Different chitin levels: 0.025, 0.05, 0.1 | Average Initial weight: 125 ± 13 g | 2–6 weeks | Immunostimulant agent. Enhancement of innate immunity. | [65] |
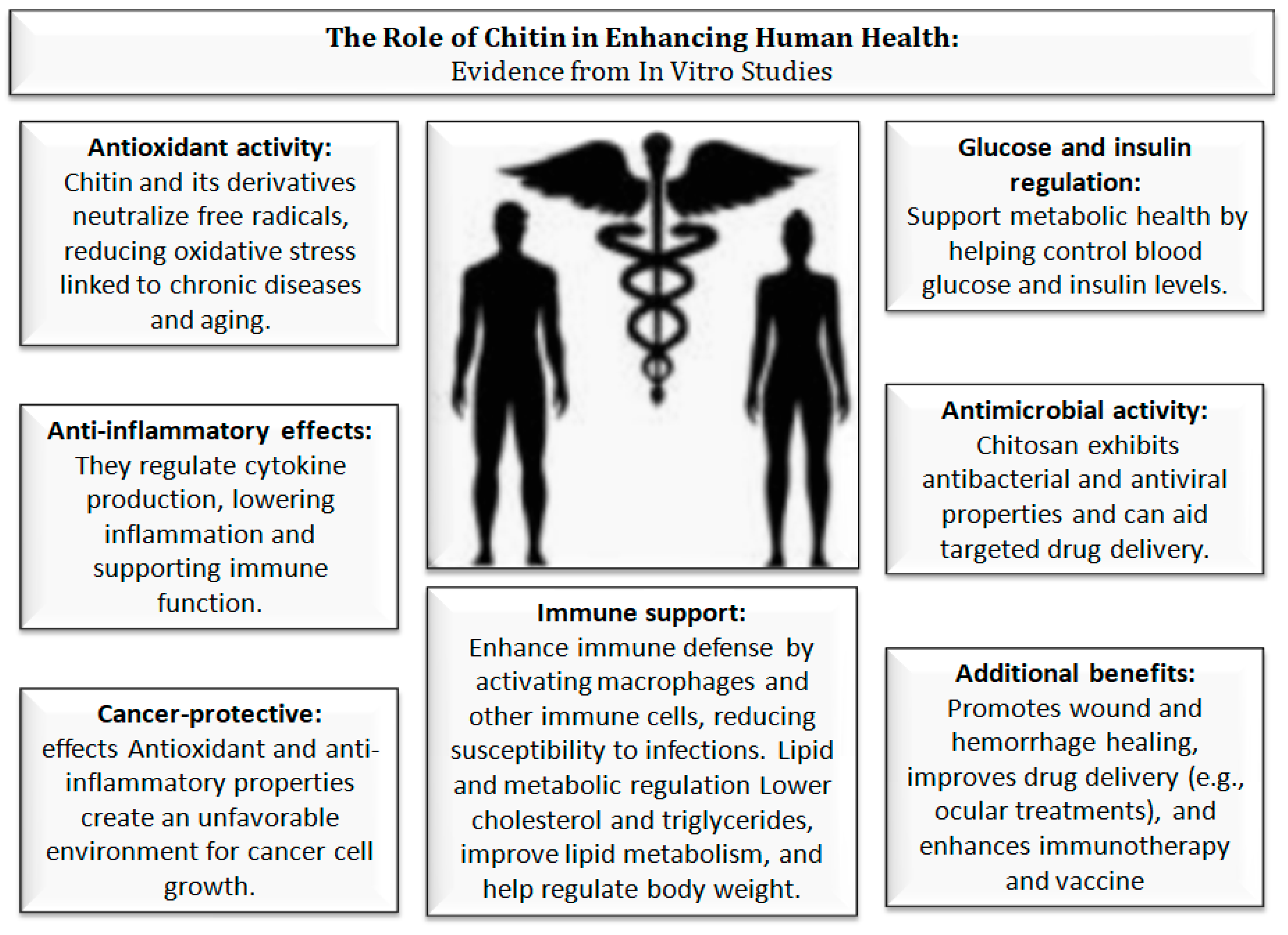
5. Chitin and Their Impact on Human Health
| Biological Effect | Compound | Mechanism | Evidence (Study Type) | References |
|---|---|---|---|---|
| Regulation of glucose & insulin | Chitin, Chitosan | Improves glucose metabolism | Mainly in vitro studies, limited small-scale human clinical trials | [79,80,81] |
| Lipid metabolism (reduction of cholesterol and triglycerides, enhancement of fatty acid oxidation) | Chitin, Chitosan | Binds fats & bile acids, regulates lipid-related genes | In vitro and animal studies, limited human clinical trials | [80,81,82,83,84] |
| Antioxidant activity | Chitin, COS | Neutralizes free radicals, reduces oxidative stress | In vitro and animal studies, limited human trials; COS shows higher bioavailability | [80,81,83,86,89,90,92] |
| Anti-inflammatory | Chitin, COS | Cytokine regulation | In vitro and animal studies, limited human evidence | [86,87] |
| Antimicrobial & Antiviral | Chitosan | Inhibits bacteria, carrier for antiviral drugs | Mainly in vitro and preclinical studies, limited human applications | [87] |
| Cancer prevention & therapy | Chitin, Chitosan | Immunostimulation, targeted drug delivery | In vitro and animal studies, few human data | [88,89] |
| Wound healing | Chitosan | Hemostatic, antibacterial | Strong preclinical support, applied in clinical wound healing | [87,90] |
| Ocular disease treatment | Chitosan nanoparticles | Local drug delivery | Preclinical studies, limited human applications | [87] |
| Blood pressure regulation (ACE inhibition) | Chitin, Chitosan | ACE inhibition | In vitro studies only | [90] |
6. Trends and Future Challenges of Crab By-Product Utilization
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, C.; Chen, X.; Chen, Z.; Zhao, Y.; Yang, R.; Xia, Y.; Zeng, Q.; He, Y.; Lan, H. Source-dependent variations in chitin: A comparative study on Antarctic krill, white shrimp and crayfish. Front. Mar. Sci. 2025, 12, 1592331. [Google Scholar] [CrossRef]
- Tavakoli, S.; Li, Q.; Han, W.; Zhang, H.; Hui, M.; Deng, L.; Kouhdasht, A.M.; Tan, Y.; Luo, Y.; Hong, H.; et al. Valorization of marine crustacean shells waste via fermentation technology: A comprehensive review on derived value-added compounds and enhancing their industrial applications. Waste Manag. 2025, 202, 114831. [Google Scholar] [CrossRef]
- Sagar, B.M.; Islam, M.M.; Habib, M.L.; Ahmed, S.; Hossain, M.S. Utilization of natural and waste sources for synthesis of cellulose, chitin, and chitosan for a suitable environment. RSC Adv. 2025, 15, 26276–26301. [Google Scholar] [CrossRef]
- Majengbasan, O.S.; Unuofin, J.O.; Daramola, M.O.; Iwarere, S.A.; Semenya, K.; Odeniyi, O.A. Chitinous waste depolymerization and biocontrol potential of Stenotrophomonas maltophilia 3E chitinase against mycophytopathogens and Anopheles gambiae instar 3. Bioresour. Technol. Rep. 2025, 30, 102095. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, L.; Yang, K.; Zhang, H.; Jiang, M.; Liao, S.; Yang, D.; Shen, N. Enhanced chitinase production by Bacillus paralicheniformis GXMU-J23.1: Optimization, genomic insights, and chitin degradation mechanism. Bioresour. Technol. 2024, 418, 131911. [Google Scholar] [CrossRef]
- Sumaila, A.; Ibrahim, J.; Yahaya, M.K.; Sumaila, A.O.; Aniki, S.A. Investigation into the influence of treatment parameters using central composite design (CCD) and characterization of chitosan extracted from marine crab shell wastes. J. Mar. Stud. 2025, 2, 2302. [Google Scholar] [CrossRef]
- Rosalina, D.; Wijayanti, W. Optimization of chitosan production from mangrove crab shell waste using response surface methodology (RSM). J. Teknol. Pengolah. Pertan. 2025, 7, 19–31. [Google Scholar] [CrossRef]
- Song, X.; Wei, H.; Zhou, Y.; Song, W.; Shi, C.; Mu, C.; Wang, C.; Wang, X. Utilization of crab shell waste for value-added bioplastics by Pseudomonas-based microbial cell factories. Int. J. Mol. Sci. 2025, 26, 2543. [Google Scholar] [CrossRef]
- Hajji, S.; Ghorbel-Bellaaj, O.; Younes, I.; Jellouli, K.; Nasri, M. Chitin extraction from crab shells by Bacillus bacteria. Biological activities of fermented crab supernatants. Int. J. Biol. Macromol. 2015, 79, 167–173. [Google Scholar] [CrossRef]
- Lei, J.; Zhang, J.; Li, K.; Qin, H.; Liu, H.; Li, P.; Liu, S.; Xu, J. Pretreatment of shrimp shells with an acidic deep eutectic solvent system for chitin extraction and its enhanced performance as a carrier for immobilized lipase. Int. J. Biol. Macromol. 2024, 264, 130774. [Google Scholar] [CrossRef]
- Rai, S.; Pokhrel, P.; Udash, P.; Chemjong, M.; Bhattarai, N.; Thuanthong, A.; Nalinanon, S.; Nirmal, N. Chitin and chitosan from shellfish waste and their applications in agriculture and biotechnology industries. Crit. Rev. Biotechnol. 2025, 1–19. [Google Scholar] [CrossRef]
- Pedrazzani, C.; Rinaldi, M.; Assaf, N.; Caligiani, A. Evaluating physical pre-treatment methods for improving insect chitin hydrolysis using Streptomyces griseus chitinase. Carbohydr. Polym. Technol. Appl. 2025, 10, 100803. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, R.; Zhou, X.; Liu, Q.; Zhang, X.; Chen, F.; Chen, K. Genome sequence and chitinases repertoire of the Chitinibacter sp. strain GC72: Heterologous expression and characterization of the chitinase (Chi1) for degradation of chitinous waste. Int. J. Biol. Macromol. 2025, 320, 145867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, Z.; Song, L.; Farag, M.A. Maximizing crustaceans (shrimp, crab, and lobster) by-products value for optimum valorization practices: A comparative review of their active ingredients, extraction, bioprocesses and applications. J. Adv. Res. 2023, 57, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Frenchibai, P.B.B.; Vinayagam, R.; Ambigapathi, D.; Anbazhagan, G.K.; Elumalai, L.; Palaniyandi, S.; Siddan, N.; Thangam, G.S.; Dharmalingam, K. Chitosan from shells of crustaceans and its application in the synthesis of biodegradable polymers. Biomed. Mater. Devices, 2025; in press. [Google Scholar] [CrossRef]
- Eggink, K.M.; Aalto, S.L.; Pedersen, P.B.; Lund, I.; Dalsgaard, J. Effects of dietary chitin on nutrient digestibility, cholesterol metabolism, digestive enzyme activity, and gut microbiome in rainbow trout. Anim. Feed Sci. Technol. 2025, 328, 116447. [Google Scholar] [CrossRef]
- Oushani, A.K.; Soltani, M.; Sheikhzadeh, N.; Mehrgan, M.S.; Islami, H.R. Effects of dietary chitosan and nano-chitosan loaded clinoptilolite on growth and immune responses of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2020, 98, 210–217. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Razek, N.A.; Abdel-Rahman, A.M. Immunostimulatory effect of dietary chitosan nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.). Fish Shellfish Immunol. 2019, 88, 254–258. [Google Scholar] [CrossRef]
- Abu-Elala, N.M.; Mohamed, S.H.; Zaki, M.M.; Eissa, A.E. Assessment of the immune-modulatory and antimicrobial effects of dietary chitosan on Nile tilapia (Oreochrmis niloticus) with special emphasis to its bio-remediating impacts. Fish Shellfish Immunol. 2015, 46, 678–685. [Google Scholar] [CrossRef]
- Cha, S.; Lee, J.; Song, C.; Lee, K.; Jeon, Y. Effects of chitosan-coated diet on improving water quality and innate immunity in the olive flounder, Paralichthys olivaceus. Aquaculture 2008, 278, 110–118. [Google Scholar] [CrossRef]
- Ranjan, R.; Prasad, K.P.; Vani, T.; Kumar, R. Effect of dietary chitosan on haematology, innate immunity and disease resistance of Asian seabass Lates calcarifer (Bloch). Aquac. Res. 2012, 45, 983–993. [Google Scholar] [CrossRef]
- Shiau, S.Y.; Yu, Y.P. Dietary supplementation of chitin and chitosan depresses growth in Tilapia, Oreochromis niloticus × O. aureus. Aquaculture 1999, 179, 439–446. [Google Scholar] [CrossRef]
- Wijesekara, T.; Xu, B. New Insights into Sources, Bioavailability, Health-Promoting Effects, and Applications of Chitin and Chitosan. J. Agric. Food Chem. 2024, 72, 17138–17152. [Google Scholar] [CrossRef] [PubMed]
- Khiari, Z. Enzymes from Fishery and Aquaculture Waste: Research Trends in the Era of Artificial Intelligence and Circular Bio-Economy. Mar. Drugs 2024, 22, 411. [Google Scholar] [CrossRef] [PubMed]
- Sufian, A.S.; O’Donnell, C.; Geever, L. Extraction and Characterisation of Chitin and Chitosan from Irish Brown Crab Shell Waste. Adv. Sci. Technol. 2025, 168, 71–90. [Google Scholar] [CrossRef]
- Pohling, J.; Ramakrishnan, V.V.; Hossain, A.; Trenholm, S.; Dave, D. Optimization of Enzymatic Deproteination of Northern Shrimp (Pandalus borealis) Shell Chitin Using Commercial Proteases. Mar. Drugs 2024, 22, 445. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of Chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Pingret, D.; Fabiano-Tixier, A.; Chemat, F. Ultrasound-Assisted Extraction. In RSC Green Chemistry Series; Royal Society of Chemistry: London, UK, 2013; pp. 89–112. [Google Scholar] [CrossRef]
- Vardanega, R.; Santos, D.; De Almeida, M. Intensification of bioactive compounds extraction from medicinal plants using ultrasonic irradiation. Pharmacogn. Rev. 2014, 8, 88. [Google Scholar] [CrossRef]
- Yang, H.; Gözaydın, G.; Nasaruddin, R.R.; Har, J.R.G.; Chen, X.; Wang, X.; Yan, N. Toward the shell biorefinery: Processing crustacean shell waste using hot water and carbonic acid. ACS Sustain. Chem. Eng. 2019, 7, 5532–5542. [Google Scholar] [CrossRef]
- Setoguchi, T.; Kato, T.; Yamamoto, K.; Kadokawa, J.I. Facile production of chitin from crab shells using ionic liquid and citric acid. Int. J. Biol. Macromol. 2012, 50, 861–864. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.J. Chitin and chitosan production from shrimp shells using ammonium-based ionic liquids. Int. J. Biol. Macromol. 2019, 130, 818–826. [Google Scholar] [CrossRef]
- Nowacki, K.; Stępniak, I.; Machałowski, T.; Wysokowski, M.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Langer, E.; Richter, A.; Ziętek, J.; et al. Electrochemical method for isolation of chitinous 3D scaffolds from cultivated Aplysina aerophoba marine demosponge and its biomimetic application. Appl. Phys. A 2020, 126, 368. [Google Scholar] [CrossRef]
- Han, X.M.; Wu, X.H.; Li, W.Y.; Sun, Y.D.; Sang, Y.X.; Sun, J.L. Application of electrolyzed water in extracting chitin from shrimp shell. Food Res. Dev. 2019, 40, 51–57. [Google Scholar]
- Ahmadkelayeh, S.; Hawboldt, K. Extraction of lipids and astaxanthin from crustacean by-products: A review on supercritical CO2 extraction. Trends Food Sci. Technol. 2020, 103, 94–108. [Google Scholar] [CrossRef]
- Maróstica, M.R.; Leite, A.V., Jr.; Dragano, N.R.V. Supercritical fluid extraction and stabilization of phenolic compounds from natural sources—Review (Supercritical extraction and stabilization of phenolic compounds). Open Chem. Eng. J. 2010, 4, 51–60. [Google Scholar] [CrossRef][Green Version]
- Nguyen, T.T.; Zhang, W.; Barber, A.R.; Su, P.; He, S. Significant enrichment of polyunsaturated fatty acids (PUFAs) in the lipids extracted by supercritical CO2 from the livers of Australian rock lobsters (Jasus edwardsii). J. Agric. Food Chem. 2015, 63, 4621–4628. [Google Scholar] [CrossRef]
- Amiguet, V.T.; Kramp, K.L.; Mao, J.; McRae, C.; Goulah, A.; Kimpe, L.E.; Blais, J.M.; Arnason, J.T. Supercritical carbon dioxide extraction of polyunsaturated fatty acids from Northern shrimp (Pandalus borealis Kreyer) processing by-products. Food Chem. 2011, 130, 853–858. [Google Scholar] [CrossRef]
- Albino Antunes-Valcareggi, S.; Ferreira, S.R.S.; Hense, H. Enzymatic hydrolysis of blue crab (Callinectes sapidus) waste processing to obtain chitin, protein, and astaxanthin-enriched extract. Int. J. Environ. Agric. Res. 2017, 3, 81–92. [Google Scholar]
- Beaulieu, L.; Thibodeau, J.; Bryl, P.; Carbonneau, M. Characterization of enzymatic hydrolyzed snow crab (Chionoecetes opilio) by-product fractions: A source of high-valued biomolecules. Bioresour. Technol. 2009, 100, 3332–3342. [Google Scholar] [CrossRef] [PubMed]
- Mukhin, V.A.; Novikov, V.Y. Enzymatic hydrolysis of proteins from crustaceans of the Barents Sea. Appl. Biochem. Microbiol. 2001, 37, 538–542. [Google Scholar] [CrossRef]
- Xu, P.; Wu, X.; Guo, X.; Tang, J.; Zong, M.; Lou, W. Double-Chitinase hydrolysis of crab shell chitin pretreated by ionic liquid to generate Chito-Oligosaccharide. ACS Sustain. Chem. Eng. 2018, 7, 1683–1691. [Google Scholar] [CrossRef]
- Beaulieu, L.; Thibodeau, J.; Bonnet, C.; Bryl, P.; Carbonneau, M. Detection of antibacterial activity in an enzymatic hydrolysate fraction obtained from processing of Atlantic rock crab (Cancer irroratus) by-products. PharmaNutrition 2013, 1, 149–157. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, Y.; Liu, Y.; Pan, D.; Zhang, Y. Continuous Production of Chitin Oligosaccharides Utilizing an Optimized Enzyme Production-Adsorption-Enzymolysis-Product Separation (EAES) System. Fermentation 2024, 10, 634. [Google Scholar] [CrossRef]
- Taboada López, M.V.; Herbello Hermelo, P.; Domínguez González, R.; Bermejo Barrera, P.; Moreda Piñeiro, A. Enzymatic hydrolysis as a sample pre treatment for titanium dioxide nanoparticles assessment in surimi (crab sticks) by single particle ICP MS. Talanta 2018, 195, 23–32. [Google Scholar] [CrossRef]
- Souchet, N.; Laplante, S. Recovery and characterization of a serine collagenolytic extract from snow crab (Chionoecetes opilio) by-products. Appl. Biochem. Biotechnol. 2010, 163, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cao, M.; Yu, H.; Hu, Y.; Zhang, L.; Su, W. Optimisation of enzymatic hydrolysis of the by-products of marine crab processing using mixed enzymes. Int. J. Food Sci. Technol. 2010, 45, 1198–1204. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.C.; Liu, Y. Optimization of crab flavoring extraction by means of enzyme processing aids from Chinese mitten crab (Eriocheir sinensis) by-products using response surface methodology (RSM). Appl. Mech. Mater. 2013, 333–335, 1959–1966. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Shim, K.; Lim, C.; Kim, S. Antioxidant and angiotensin I converting enzyme inhibitory activities of red snow crab (Chionoecetes japonicas) shell hydrolysate by enzymatic hydrolysis. Fish. Aquat. Sci. 2013, 16, 237–242. [Google Scholar] [CrossRef]
- Lee, G.; Jung, M.; Nam, J.; Han, A.; Kim, B.; Jun, J. Preparation and taste profiling of the enzymatic protein hydrolysate from a by-product of red snow crab processing as a natural seasoning compound. Foods 2022, 11, 3911. [Google Scholar] [CrossRef]
- Amiza, M.A.; Zaliha, H.; Intan Liyana, M.R. Optimization of enzymatic protein hydrolysis conditions to obtain maximum angiotensin-I converting enzyme (ACE) inhibitory activity from flower crab (Portunus pelagicus) meat. Asian J. Agric. Biol. 2019, 7, 146–155. [Google Scholar]
- Harun, Z.; Amin, A.M.; Sarbon, N.M.; Zainol, M.K.M. Optimisation of enzymatic protein hydrolysis of mud crab (Scylla sp.) to obtain maximum angiotensin converting enzyme inhibitory (ACEI) activity using response surface methodology. Malays. Appl. Biol. 2017, 46, 33–40. [Google Scholar]
- Montiel-Montoya, J.; Valdez-Morales, M.; Reyes, C.; Barrales-Cureño, H.J. Sustainable production with obtaining glucosamine from crab exoskeletons. Ciência Rural 2019, 49, e20190021. [Google Scholar] [CrossRef]
- Novikov, V.Y.; Shumskaya, N.V.; Mukhin, V.A.; Zolotarev, K.V.; Mikhailov, A.N.; Nakhod, V.I.; Mikhailova, M.V. Chemical characterization of Atlantic cod (Gadus morhua) collagen hydrolyzed using enzyme preparation derived from red king crab (Paralithodes camtschaticus) and its potential as a core component of bacterial culture medium. Mar. Drugs 2021, 19, 472. [Google Scholar] [CrossRef] [PubMed]
- Novikov, V.; Rysakova, K.; Shumskaya, N.; Mukhortova, A.; Kesarev, K. King crab gills as a new source of chitin/chitosan and protein hydrolysates. Int. J. Biol. Macromol. 2023, 232, 123346. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Hammami, A.; Hajji, S.; Jridi, M.; Nasri, M.; Nasri, R. Chitin extraction from blue crab (Portunus segnis) and shrimp (Penaeus kerathurus) shells using digestive alkaline proteases from P. segnis viscera. Int. J. Biol. Macromol. 2017, 101, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Skonberg, D.I.; Myracle, A.D. Anti-hyperglycemic effects of green crab hydrolysates derived by commercially available enzymes. Foods 2020, 9, 258. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, S.; Li, S.; Liu, Y. Biochemical and antioxidant properties of peptidic fraction generated from crab (Portunus trituberculatus) shells by enzymatic hydrolysis. Int. J. Food Sci. Technol. 2017, 52, 2479–2488. [Google Scholar] [CrossRef]
- Chen, J.; Chen, L. Effects of chitosan-supplemented diets on the growth performance, nonspecific immunity and health of loach fish (Misgurnus anguillicadatus). Carbohydr. Polym. 2019, 225, 115227. [Google Scholar] [CrossRef]
- Mao, X.; Feng, D.; Chen, Y.; Ren, J. Enzymatic hydrolysis of shrimp shell for chitin extraction: Optimization and characterization. J. Agric. Food Chem. 2016, 64, 4604–4611. [Google Scholar]
- Wang, S.; Chen, H.; Yang, S. Enzymatic processing of crustacean shell waste: Production of protein hydrolysates and chitin recovery. Bioresour. Technol. 2020, 295, 122258. [Google Scholar]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Gopalakannan, A.; Arul, V. Immunomodulatory effects of dietary intake of chitin, chitosan and levamisole on the immune system of Cyprinus carpio and control of Aeromonas hydrophila infection in ponds. Aquaculture 2006, 255, 179–187. [Google Scholar] [CrossRef]
- Esteban, M.; Cuesta, A.; Ortuño, J.; Meseguer, J. Immunomodulatory effects of dietary intake of chitin on gilthead seabream (Sparus aurata L.) innate immune system. Fish Shellfish Immunol. 2001, 11, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Meshkini, S.; Tafy, A.; Tukmechi, A.; Farhang-Pajuh, F. Effects of chitosan on hematological parameters and stress resistance in rainbow trout (Oncorhynchus mykiss). Vet. Res. Forum 2012, 3, 49–54. [Google Scholar] [PubMed]
- Chen, Y.; Zhu, X.; Yang, Y.; Han, D.; Jin, J.; Xie, S. Effect of dietary chitosan on growth performance, haematology, immune response, intestine morphology, intestine microbiota and disease resistance in gibel carp (Carassius auratus gibelio). Aquac. Nutr. 2014, 20, 532–546. [Google Scholar] [CrossRef]
- Fadl, S.E.; El-Gammal, G.A.; Abdo, W.S.; Barakat, M.; Sakr, O.A.; Nassef, E.; Gad, D.M.; El-Sheshtawy, H.S. Evaluation of dietary chitosan effects on growth performance, immunity, body composition and histopathology of Nile tilapia (Oreochromis niloticus) as well as the resistance to Streptococcus agalactiae infection. Aquac. Res. 2019, 51, 1120–1132. [Google Scholar] [CrossRef]
- Mari, L.S.S.; Jagruthi, C.; Anbazahan, S.M.; Yogeshwari, G.; Thirumurugan, R.; Arockiaraj, J.; Mariappan, P.; Balasundaram, C.; Harikrishnan, R. Protective effect of chitin and chitosan enriched diets on immunity and disease resistance in Cirrhina mrigala against Aphanomyces invadans. Fish Shellfish Immunol. 2014, 39, 378–385. [Google Scholar] [CrossRef]
- Wu, S. The growth performance, body composition and nonspecific immunity of Tilapia (Oreochromis niloticus) affected by chitosan. Int. J. Biol. Macromol. 2019, 145, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, C.; Dawood, M.; Gao, J. Effects of dietary chitosan on growth, lipid metabolism, immune response and antioxidant-related gene expression in Misgurnus anguillicaudatus. Benef. Microbes 2017, 8, 439–450. [Google Scholar] [CrossRef]
- Rashwan, A.G.; Assar, D.H.; Salah, A.S.; Liu, X.; Al-Hawary, I.I.; Abu-Alghayth, M.H.; Salem, S.M.R.; Khalil, K.; Hanafy, N.A.N.; Abdelatty, A.; et al. Dietary Chitosan Attenuates High-Fat Diet-Induced Oxidative Stress, Apoptosis, and Inflammation in Nile Tilapia (Oreochromis niloticus) through Regulation of Nrf2/Kaep1 and Bcl-2/Bax Pathways. Biology 2024, 13, 486. [Google Scholar] [CrossRef]
- El-Naby, F.S.A.; Naiel, M.A.; Al-Sagheer, A.A.; Negm, S.S. Dietary chitosan nanoparticles enhance the growth, production performance, and immunity in Oreochromis niloticus. Aquaculture 2018, 501, 82–89. [Google Scholar] [CrossRef]
- Udo, I.U. Effects of Chitosan and Chitosan Nanoparticles on Water Quality, Growth Performance, Survival Rate and Meat Quality of the African Catfish, Clarias gariepinus. Nanoscience 2018, 1, 12. [Google Scholar] [CrossRef]
- Zaki, M.A.; Salem, M.E.; Gaber, M.M.; Nour, A.M. Effect of Chitosan Supplemented Diet on Survival, Growth, Feed Utilization, Body Composition & Histology of Sea Bass (Dicentrarchus labrax). World J. Eng. Technol. 2015, 3, 38–47. [Google Scholar] [CrossRef]
- Liu, J.; Xu, W.; Liu, Y.; Wang, Y.; Zhang, J.; Wang, Z.; Mai, K.; Ai, Q. Effects of Chitosan-Coated Microdiet on Dietary Physical Properties, Growth Performance, Digestive Enzyme Activities, Antioxidant Capacity, and Inflammation Response of Large Yellow Croaker (Larimichthys crocea) Larvae. Aquac. Nutr. 2022, 2022, 4355182. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J. Effects of chitosan nanoparticles on survival, growth and meat quality of tilapia, Oreochromis nilotica. Nanotoxicology 2010, 5, 425–431. [Google Scholar] [CrossRef]
- Fotodimas, I.; Ioannou, Z.; Kanlis, G. A review of the benefits of the sustainable utilization of shrimp waste to produce novel foods and the impact on human health. Sustainability 2024, 16, 6909. [Google Scholar] [CrossRef]
- Inanli, A.G.; Tümerkan, E.T.A.; Abed, N.E.; Regenstein, J.M.; Özogul, F. The impact of chitosan on seafood quality and human health: A review. Trends Food Sci. Technol. 2020, 97, 404–416. [Google Scholar] [CrossRef]
- Rameshthangam, P.; Solairaj, D.; Arunachalam, G.; Ramasamy, P. Chitin and Chitinases: Biomedical And Environmental Applications of Chitin and its Derivatives. J. Enzym. 2020, 1, 20–43. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Koide, S. Chitin-Chitosan: Properties, Benefits and Risks. Nutr. Res. 1998, 18, 1091–1101. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Morganti, A. Transforming Nanostructured Chitin from Crustacean Waste into Beneficial Health Products: A Must for Our Society. Nanotechnol. Sci. Appl. 2011, 4, 123–129. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Human Enzymatic Activities Related to the Therapeutic Administration of Chitin Derivatives. Cell. Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A Review on the Preparation of Chitosan Oligosaccharides and Application to Human Health, Animal Husbandry and Agricultural Production. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Ahmad, R.; Khan, M.S.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, M.I. Chitin and Its Derivatives: Structural Properties and Biomedical Applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef]
- Hayes, M.; Carney, B.; Slater, J.; Brück, W. Mining marine shellfish wastes for bioactive molecules: Chitin and chitosan—Part B: Applications. Biotechnol. J. 2008, 3, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Park, R. Bioproduction of Chitooligosaccharides: Present and Perspectives. Mar. Drugs 2014, 12, 5328–5356. [Google Scholar] [CrossRef]
- Karagozlu, M.Z.; Kim, S. Anti-Cancer Effects of Chitin and Chitosan Derivatives; Springer: Cham, Switzerland, 2014; pp. 413–421. [Google Scholar] [CrossRef]
- Espinales, C.; Romero-Peña, M.; Calderón, G.; Vergara, K.; Cáceres, P.J.; Castillo, P. Collagen, Protein Hydrolysates and Chitin from By-Products of Fish and Shellfish: An Overview. Heliyon 2023, 9, e14937. [Google Scholar] [CrossRef]
- Verardi, A.; Sangiorgio, P.; Moliterni, S.; Errico, S.; Spagnoletta, A.; Dimatteo, S. Advanced Technologies for Chitin Recovery from Crustacean Waste. Clean Technol. Recycl. 2023, 3, 4–43. [Google Scholar] [CrossRef]
- Kim, Y.; Park, R. Progress in Bioextraction Processes of Chitin from Crustacean Biowastes. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 545–554. [Google Scholar] [CrossRef]
- De Holanda, H.D.; Netto, F.M. Recovery of Components from Shrimp (Xiphopenaeus kroyeri) Processing Waste by Enzymatic Hydrolysis. J. Food Sci. 2006, 71, C298–C303. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotodimas, I.; Vidalis, K.L.; Theodorou, J.A.; Logothetis, P.; Kanlis, G. Sustainable Aquaculture Through Enzymatic Hydrolysis of Raw Chitin from Crab By-Products: Functional Fish Feeds Targeting Fish Health with Implications for Human Health. Fishes 2025, 10, 514. https://doi.org/10.3390/fishes10100514
Fotodimas I, Vidalis KL, Theodorou JA, Logothetis P, Kanlis G. Sustainable Aquaculture Through Enzymatic Hydrolysis of Raw Chitin from Crab By-Products: Functional Fish Feeds Targeting Fish Health with Implications for Human Health. Fishes. 2025; 10(10):514. https://doi.org/10.3390/fishes10100514
Chicago/Turabian StyleFotodimas, Ioannis, Kosmas L. Vidalis, John A. Theodorou, Panagiotis Logothetis, and Grigorios Kanlis. 2025. "Sustainable Aquaculture Through Enzymatic Hydrolysis of Raw Chitin from Crab By-Products: Functional Fish Feeds Targeting Fish Health with Implications for Human Health" Fishes 10, no. 10: 514. https://doi.org/10.3390/fishes10100514
APA StyleFotodimas, I., Vidalis, K. L., Theodorou, J. A., Logothetis, P., & Kanlis, G. (2025). Sustainable Aquaculture Through Enzymatic Hydrolysis of Raw Chitin from Crab By-Products: Functional Fish Feeds Targeting Fish Health with Implications for Human Health. Fishes, 10(10), 514. https://doi.org/10.3390/fishes10100514






