Study Protocol for the Japan Pregnancy, Eating, Activity, Cohort (J-PEACH) Study: Investigating Perinatal Maternal Lifestyle and Infant Health
Abstract
1. Introduction
2. Methods and Design
2.1. Study Design and Setting
2.2. Inclusion and Exclusion Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Recruitment and Consent
2.4. Study Protocol
2.5. Primary Outcomes and Potential Determinant Factors (Figure 3)
- (1)
- Current status of maternal lifestyle during pregnancy and its impact on GWG;
- (2)
- Potential determinant factors of infant LBW;
- (3)
- Potential determinant factors of maternal and neonatal health outcomes.
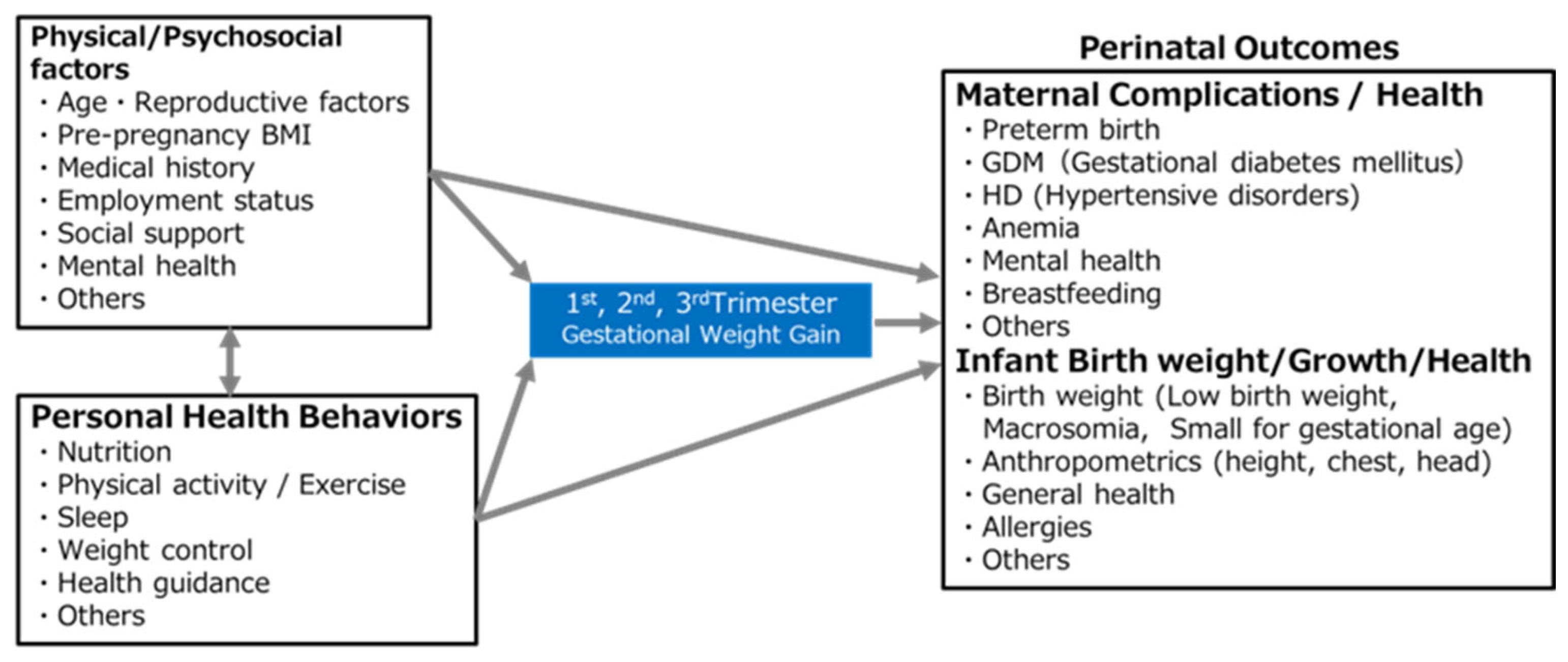
2.6. Secondary Outcomes
2.7. Measures (Scales)
2.7.1. Anthropometric Measurements
2.7.2. Dietary Intakes and Behaviors
2.7.3. Physical Activity
2.7.4. Mental Health
2.7.5. Severity of Nausea and Vomiting
2.7.6. Woman Abuse Screening
2.7.7. Sense of Coherence
2.7.8. Subjective Sleep Quality
2.7.9. Sleepiness Scale
2.8. Measures (Devices)
2.8.1. Accelerometer
2.8.2. Sleep Monitor
2.9. Sample Size
2.10. Statistical Analysis
2.11. Dissemination of Study Findings
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- eStat: The Portal Site of Official Statistics of Japan. Annual Report on Vital Statistics 2023. Available online: https://www.e-stat.go.jp/dbview?sid=0003411616 (accessed on 13 August 2025). (In Japanese)
- Takemoto, Y.; Ota, E.; Yoneoka, D.; Mori, R.; Takeda, S. Japanese secular trends in birthweight and the prevalence of low birthweight infants during the last three decades: A population-based study. Sci. Rep. 2016, 6, 31396. [Google Scholar] [CrossRef]
- Mine, T.; Tsuboi, S.; Fukushima, F. Twenty-year trends of low birth weight in Japan: A joinpoint regression analysis of data from 2000 to 2019. Front. Reprod. Health 2021, 3, 772575. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. OECD Family Database. Available online: https://web-archive.oecd.org/2020-09-22/129022-CO_1_3_Low_birth_weight.pdf (accessed on 13 August 2025).
- Agarwal, P.; Morriseau, T.S.; Kereliuk, S.M.; Doucette, C.A.; Wicklow, B.A.; Dolinsky, V.W. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit. Rev. Clin. Lab. Sci. 2018, 55, 71–101. [Google Scholar] [CrossRef] [PubMed]
- Calkins, K.; Devaskar, S.U. Fetal origins of adult disease. Curr. Probl. Pediatr. Adolesc. Health Care 2011, 41, 158–176. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef]
- Murai, U.; Nomura, K.; Kido, M.; Takeuchi, T.; Sugimoto, M.; Rahman, M. Pre-pregnancy body mass index as a predictor of low birth weight infants in Japan. Asia Pac. J. Clin. Nutr. 2017, 26, 434–437. [Google Scholar] [CrossRef]
- Han, Z.; Lutsiv, O.; Mulla, S.; Rosen, A.; Beyene, J.; McDonald, S.D.; Knowledge Synthesis Group. Low gestational weight gain and the risk of preterm birth and low birthweight: A systematic review and meta-analyses. Acta Obstet. Gynecol. Scand. 2011, 90, 935–954. [Google Scholar] [CrossRef] [PubMed]
- Japan Ministry of Health, Labour and Welfare. National Health and Nutrition Survey. Available online: https://www.mhlw.go.jp/content/000711005.pdf (accessed on 13 August 2025). (In Japanese)
- eStat: The Portal Site of Official Statistics of Japan. Annual Report on Vital Statistics 2021-Confirmed Number of Births. Available online: https://www.e-stat.go.jp/dbview?sid=0003411599 (accessed on 13 August 2025). (In Japanese)
- Itoh, H.; Itakura, A.; Kanayama, N.; Ikeda, T. Withdrawal of the 1999 JSOG recommendation of weight gain restriction during pregnancy (commentary of the JSOG Perinatal Committee). J. Obstet. Gynaecol. Res. 2019, 45, 2302. [Google Scholar] [CrossRef] [PubMed]
- Healthy Parent and Child 21. Japanese Ministry of Health, Labor and Welfare: Dietary Guidelines for Pregnant Women. Available online: https://www.mhlw.go.jp/houdou/2006/02/h0201-3a.html (accessed on 13 August 2025). (In Japanese)
- Institute of Medicine (IOM) and National Research Council (NRC). Weight Gain During Pregnancy: Reexamining the Guidelines; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Japan Society of Obstetrics and Gynecology. The guidelines on gestational weight gain. Acta. Obstet. Gynaecol. Jpn. 2021, 73, 678–679. (In Japanese) [Google Scholar]
- Japan Ministry of Health, Labour and Welfare, Promotion Council for Healthy Parents and Children. “Ninsanpu Notameno Shokuseikatu Sisin” 2006. Available online: https://www.mhlw.go.jp/houdou/2006/02/dl/h0201-3a4.pdf (accessed on 13 August 2025). (In Japanese)
- J-P, N.A.; Minami, M.; Eitoku, M.; Maeda, N.; Fujieda, M.; Suganuma, N.; Japan Environment and Children’s Study (JECS) Group. Lack of concern about body image and health during pregnancy linked to excessive gestational weight gain and small-for-gestational-age deliveries: The Japan Environment and Children’s Study. BMC Pregnancy Childbirth 2021, 21, 396. [Google Scholar] [CrossRef]
- Herrera, E.; Desoye, G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm. Mol. Biol. Clin. Investig. 2016, 26, 109–127. [Google Scholar] [CrossRef]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Uchinuma, H.; Tsuchiya, K.; Sekine, T.; Horiuchi, S.; Kushima, M.; Otawa, S.; Yokomichi, H.; Miyake, K.; Akiyama, Y.; Ooka, T.; et al. Gestational body weight gain and risk of low birth weight or macrosomia in women of Japan: A nationwide cohort study. Int. J. Obes. 2021, 45, 2666–2674. [Google Scholar] [CrossRef]
- Morisaki, N.; Piedvache, A.; Morokuma, S.; Nakahara, K.; Ogawa, M.; Kato, K.; Sanefuji, M.; Shibata, E.; Tsuji, M.; Shimono, M.; et al. Gestational weight gain growth charts adapted to Japanese pregnancies using a Bayesian approach in a longitudinal study: The Japan Environment and Children’s Study. J. Epidemiol. 2023, 33, 217–226. [Google Scholar] [CrossRef]
- Linné, Y.; Dye, L.; Barkeling, B.; Rössner, S. Long-term weight development in women: A 15-year follow-up of the effects of pregnancy. Obes. Res. 2004, 12, 1166–1178. [Google Scholar] [CrossRef] [PubMed]
- Crossley, N.P.; Jones, E.J. Relationships among postpartum weight retention, stress, and disinhibited eating: A scoping review. West. J. Nurs. Res. 2023, 45, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.H.; Hwang, L.C.; Huang, J.P.; Hsu, H.Y. Postpartum weight retention risk factors in a Taiwanese cohort study. Obes. Facts 2018, 11, 37–45. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef]
- Shiraishi, M.; Haruna, M.; Matsuzaki, M.; Murayama, R.; Sasaki, S. The biomarker-based validity of a brief-type diet history questionnaire for estimating eicosapentaenoic acid and docosahexaenoic acid intakes in pregnant Japanese women. Asia Pac. J. Clin. Nutr. 2015, 24, 316–322. [Google Scholar] [CrossRef]
- Chasan-Taber, L.; Schmidt, M.D.; Roberts, D.E.; Hosmer, D.; Markenson, G.; Freedson, P.S. Development and validation of a Pregnancy Physical Activity Questionnaire. Med. Sci. Sports Exerc. 2004, 36, 1750–1760. [Google Scholar] [CrossRef]
- Chasan-Taber, L.; Park, S.; Marcotte, R.T.; Staudenmayer, J.; Strath, S.; Freedson, P. Update and novel validation of a pregnancy physical activity questionnaire. Am. J. Epidemiol. 2023, 192, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Haruna, M.; Nakayama, K.; Shiraishi, M.; Ota, E.; Murayama, R.; Murashima, S.; Yeo, S. Adapting the pregnancy physical activity questionnaire for Japanese pregnant women. J. Obstet. Gynecol. Neonatal. Nurs. 2014, 43, 107–116. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Levis, B.; Negeri, Z.; Sun, Y.; Benedetti, A.; Thombs, B.D.; DEPRESsion Screening Data (DEPRESSD) EPDS Group. Accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for screening to detect major depression among pregnant and postpartum women: Systematic review and meta-analysis of individual participant data. BMJ 2020, 371, m4022. [Google Scholar] [CrossRef]
- Okano, T.; Murata, M.; Masuji, S.; Tamaki, R.; Nomura, J.; Miyaoka, H.; Kitamura, T. Validation and reliability of a Japanese version of the EPDS. Arch. Psychiatr. Diagn. Clin. Eval. 1996, 7, 525–533. [Google Scholar]
- Usuda, K.; Nishi, D.; Okazaki, E.; Makino, M.; Sano, Y. Optimal cut-off score of the Edinburgh Postnatal Depression Scale for major depressive episode during pregnancy in Japan. Psychiatry Clin. Neurosci. 2017, 71, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Kubota, C.; Inada, T.; Nakamura, Y.; Shiino, T.; Ando, M.; Aleksic, B.; Yamauchi, A.; Morikawa, M.; Okada, T.; Ohara, M.S.; et al. Stable factor structure of the Edinburgh Postnatal Depression Scale during the whole peripartum period: Results from a Japanese prospective cohort study. Sci. Rep. 2018, 8, 17659. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, A.; Rey, E.; Ferreira, E.; Morin, C.; Bérard, A. Validity of a modified Pregnancy-Unique Quantification of Emesis and Nausea (PUQE) scoring index to assess severity of nausea and vomiting of pregnancy. Am. J. Obstet. Gynecol. 2008, 198, 71.e1–71.e7. [Google Scholar] [CrossRef]
- Koren, G.; Piwko, C.; Ahn, E.; Boskovic, R.; Maltepe, C.; Einarson, A.; Navioz, Y.; Ungar, W.J. Validation studies of the Pregnancy Unique-Quantification of Emesis (PUQE) scores. J. Obstet. Gynaecol. 2005, 25, 241–244. [Google Scholar] [CrossRef]
- Hada, A.; Minatani, M.; Wakamatsu, M.; Koren, G.; Kitamura, T. The pregnancy-unique quantification of emesis and nausea (PUQE-24): Configural, measurement, and structural invariance between nulliparas and multiparas and across two measurement time points. Healthcare 2021, 9, 1553. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.C.; Schei, B.; Myhr, T.L.; Du Mont, J. Abuse: A risk factor for low birth weight? A systematic review and meta-analysis. CMAJ 2001, 164, 1567–1572. [Google Scholar]
- Brown, J.B.; Lent, B.; Schmidt, G.; Sas, G. Application of the Woman Abuse Screening Tool (WAST) and WAST-short in the family practice setting. J. Fam. Pract. 2000, 49, 896–903. [Google Scholar]
- Brown, J.B.; Lent, B.; Brett, P.J.; Sas, G.; Pederson, L.L. Development of the Woman Abuse Screening Tool for use in family practice. Fam. Med. 1996, 28, 422–428. [Google Scholar] [PubMed]
- Kita, S.; Haruna, M.; Hikita, N.; Matsuzaki, M.; Kamibeppu, K. Development of the Japanese version of the Woman Abuse Screening Tool-Short. Nurs. Health. Sci. 2017, 19, 35–43. [Google Scholar] [CrossRef]
- Bladh, M.; Sydsjö, G.; Ekselius, L.; Vingård, E.; Agnafors, S. Sense of coherence and health in women: A 25-year follow-up study. BMC Women’s Health 2023, 23, 670. [Google Scholar] [CrossRef]
- Antonovsky, A. Unraveling the Mystery of Health: How People Manage Stress and Stay Well, 1st ed.; Jossey-Bass: San Francisco, CA, USA, 1987; pp. 15–32. [Google Scholar]
- Togari, T.; Yamazaki, Y.; Nakayama, K.; Shimizu, J. Development of a short version of the sense of coherence scale for population survey. J. Epidemiol. Commun. Health 2007, 61, 921–922. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Doi, Y.; Minowa, M.; Uchiyama, M.; Okawa, M.; Kim, K.; Shibui, K.; Kamei, Y. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000, 97, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Skouteris, H.; Wertheim, E.H.; Germano, C.; Paxton, S.J.; Milgrom, J. Assessing sleep during pregnancy: A study across two time points examining the Pittsburgh Sleep Quality Index and associations with depressive symptoms. Women’s Health Issues 2009, 19, 45–51. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Takegami, M.; Suzukamo, Y.; Wakita, T.; Noguchi, H.; Chin, K.; Kadotani, H.; Inoue, Y.; Oka, Y.; Nakamura, T.; Green, J.; et al. Development of a Japanese version of the Epworth Sleepiness Scale (JESS) based on item response theory. Sleep Med. 2009, 10, 556–565. [Google Scholar] [CrossRef]
- Japan Physical Activity Research Platform. (n.d.). About Physical Activity Monitors. Available online: https://paplatform.umin.jp/device.html (accessed on 13 August 2025).
- ACCELStars, Inc. Unraveling Sleep to Create New Medical Care. 2025. Available online: https://www.accelstars.com/ (accessed on 13 August 2025).
- Wainberg, M.; Jones, S.E.; Beaupre, L.M.; Hill, S.L.; Felsky, D.; Rivas, M.A.; Lim, A.S.P.; Ollila, H.M.; Tripathy, S.J. Association of accelerometer-derived sleep measures with lifetime psychiatric diagnoses: A cross-sectional study of 89,205 participants from the UK Biobank. PLoS Med. 2021, 18, e1003782. [Google Scholar] [CrossRef]
- Katori, M.; Shi, S.; Ode, K.L.; Tomita, Y.; Ueda, H.R. The 103,200-arm acceleration dataset in the UK Biobank revealed a landscape of human sleep phenotypes. Proc. Natl. Acad. Sci. USA 2022, 119, e2116729119. [Google Scholar] [CrossRef]
- Ode, K.L.; Shi, S.; Katori, M.; Mitsui, K.; Takanashi, S.; Oguchi, R.; Aoki, D.; Ueda, H.R. A jerk-based algorithm ACCEL for the accurate classification of sleep-wake states from arm acceleration. iScience 2022, 25, 103727. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Mulla, S.; Beyene, J.; Liao, G.; McDonald, S.D. Maternal underweight and the risk of preterm birth and low birth weight: A systematic review and meta-analyses. Int. J. Epidemiol. 2010, 40, 65–101. [Google Scholar] [CrossRef] [PubMed]
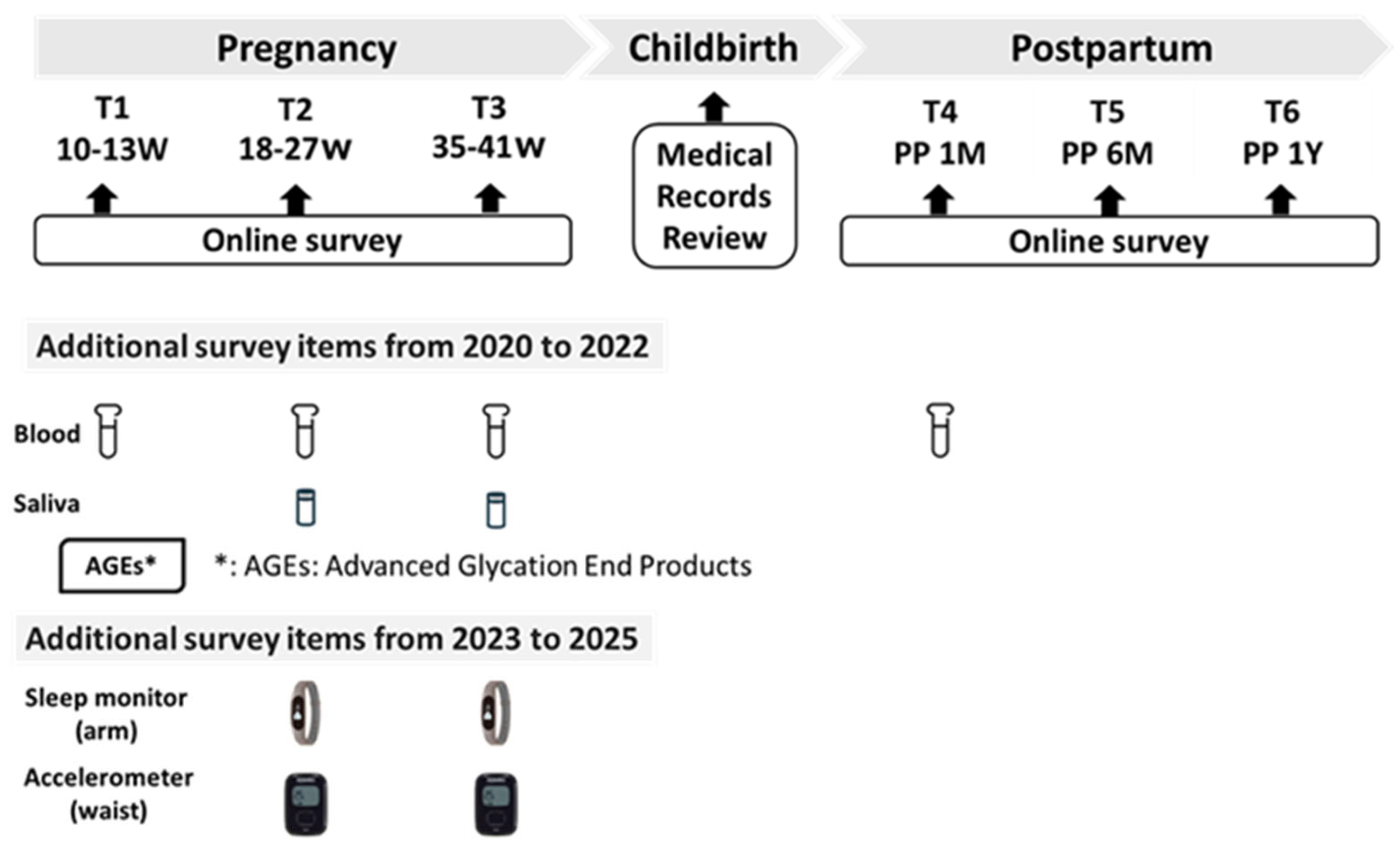
| Pregnancy | Childbirth | Postpartum | |||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | ||
| 10–13 Weeks | 18–27 Weeks | 35–41 Weeks | 1 Month | 6 Months | 12 Months | ||
| Questionnaire items | |||||||
| m-PUQE | ✓ | ✓ | ✓ | ||||
| BDHQ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| PPAQ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| EPDS | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| WAST-short | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| SOC-3 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| PSQI | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| ESS | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Demographicsand lifestyle factors | |||||||
| <Mother> | |||||||
| Age | ✓ | ✓ | ✓ | ||||
| Educational attainment | ✓ | ||||||
| Employment | ✓ | ||||||
| Marital status | ✓ | ✓ | |||||
| Alcohol consumption | ✓ | ✓ | |||||
| Smoking status | ✓ | ✓ | |||||
| Family member | ✓ | ✓ | |||||
| Medication | ✓ | ✓ | |||||
| Supplement intake | ✓ | ✓ | |||||
| Lifetime habits (sleep, meals, sedentary) | ✓ | ✓ | |||||
| Meal skipping | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Night-time fasting period | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Meal frequency | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Subjective general health | ✓ | ✓ | |||||
| Weight control, self-efficacy | ✓ | ✓ | |||||
| Body image | ✓ | ✓ | |||||
| Health guidance, target weight | ✓ | ✓ | |||||
| Maternal feelings toward pregnancy and fetus | ✓ | ✓ | |||||
| Family support | ✓ | ✓ | |||||
| Maternal skin trouble | ✓ | ✓ | |||||
| Self-reporting maternal weight | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| <Infant> | |||||||
| Weight, Height, Chest, Head | ✓ | ✓ | ✓ | ✓ | |||
| Feeding type | ✓ | ✓ | ✓ | ||||
| Breastfeeding status | ✓ | ✓ | ✓ | ||||
| Complementary feeding | ✓ | ||||||
| General health, Allergies | ✓ | ✓ | ✓ | ||||
| Hospital medical records | |||||||
| Medical history | ✓ | ✓ | ✓ | ||||
| Infertility treatment history | ✓ | ||||||
| Pregnancy complications | ✓ | ✓ | ✓ | ||||
| Glucose tolerance tests | ✓ | ✓ | |||||
| Maternal height | ✓ | ||||||
| Maternal weight | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Gestational weight gain | ✓ | ✓ | ✓ | ||||
| Blood pressure | ✓ | ✓ | ✓ | ✓ | |||
| Mode of delivery | ✓ | ||||||
| Measurements/samples | |||||||
| Sleep monitor | ✓ | ✓ | ✓ | ||||
| Accelerometer | ✓ | ✓ | ✓ | ||||
| AGEs * | ✓ | ||||||
| Blood sample * | ✓ | ✓ | ✓ | ✓ | |||
| Saliva sample ** | ✓ | ✓ | |||||
| Variable | n (%) or Mean ± SD |
|---|---|
| Total number of participants | |
| Number of individuals recruited | 2290 |
| Participants who provided consent | 2108 |
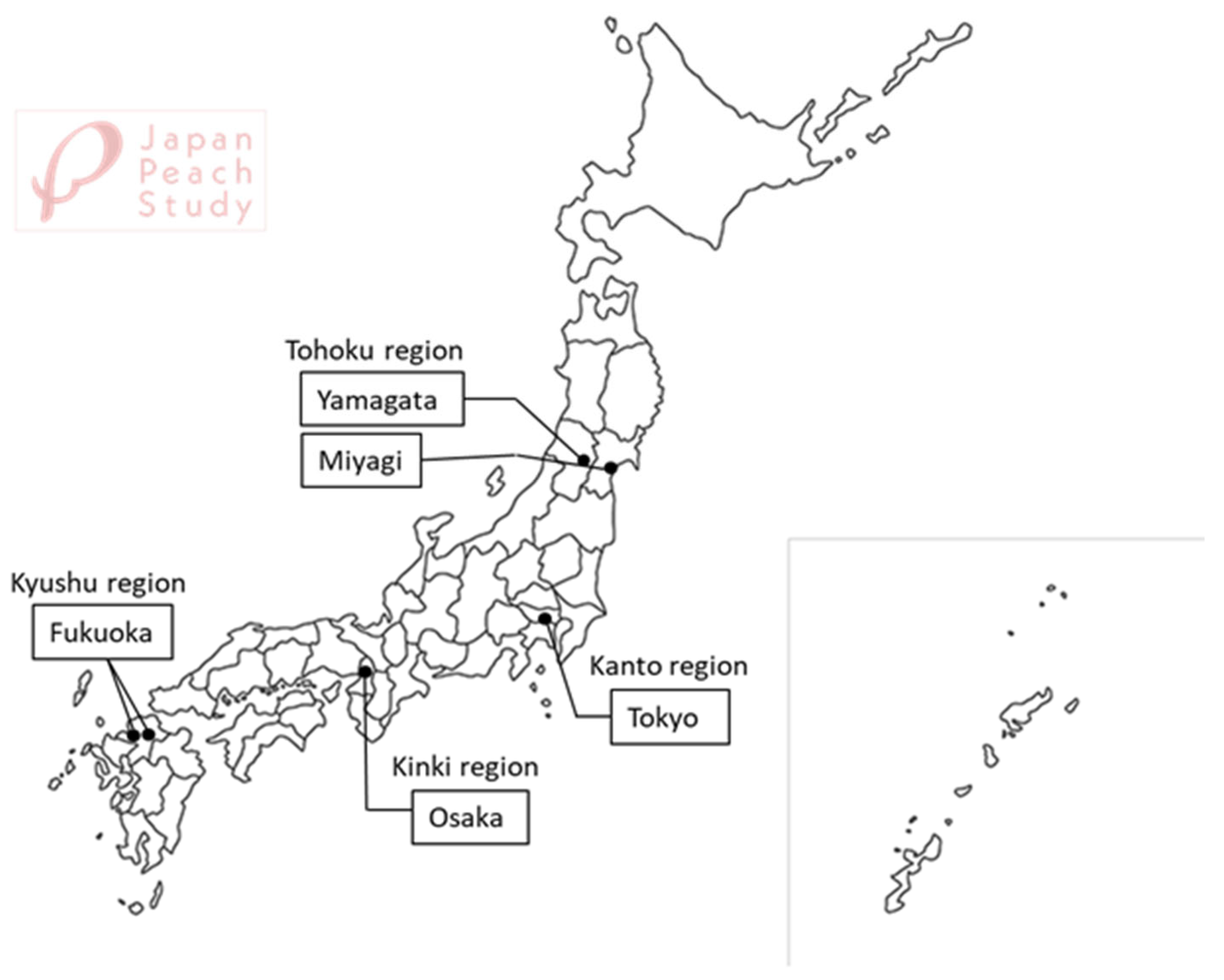
| Study regions | |
| Yamagata/Miyagi | 334 (15.8) |
| Tokyo | 1027 (48.7) |
| Osaka | 309 (14.7) |
| Fukuoka | 438 (20.8) |
| Maternal characteristics | |
| Age at recruitment (years) (n = 2102) | 33.9 ± 4.8 |
| Height (cm) (n = 2087) | 159.0 ± 5.5 |
| Weight (kg) (n = 2085) | 54.5 ± 9.6 |
| Pre-pregnancy BMI (kg/m2) (n = 2085) | 21.5 ± 3.6 |
| BMI categories | |
| <18.5 | 312 (14.8) |
| ≥18.5–<25 | 1502 (71.3) |
| ≥25 | 271 (12.9) |
| Missing data | 23 (1.1) |
| Parity status | |
| Primiparous women | 1089 (51.7) |
| Multiparous women | 1000 (47.4) |
| Missing data | 19 (0.01) |
| Mode of delivery | |
| Vaginal birth | 1339 (63.5) |
| Cesarean section | 622 (29.5) |
| Missing data | 147 (7.0) |
| Gestational age at birth | |
| Term birth | 1948 (92.4) |
| Preterm birth | 160 (7.6) |
| Infant characteristics | |
| Singleton vs. Multiple births (n = 2108) | |
| Singleton births | 2026 (96.1) |
| Male infants | 1010 (49.9) |
| Female infants | 1016 (50.1) |
| Multiple births | 79 (3.7) |
| Missing data | 3 (0.1) |
| Birth weight (g) (n = 2031) | 2943 ± 508 |
| Low birth weight (<2500 g) (n = 2031) | 281 (13.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haruna, M.; Fujita, M.; Matsuzaki, M.; Shiraishi, M.; Hikita, N.; Suetsugu, Y.; Sato, Y.; Yonezawa, K.; Tanaka, M.; Ohori, R.; et al. Study Protocol for the Japan Pregnancy, Eating, Activity, Cohort (J-PEACH) Study: Investigating Perinatal Maternal Lifestyle and Infant Health. Methods Protoc. 2025, 8, 128. https://doi.org/10.3390/mps8060128
Haruna M, Fujita M, Matsuzaki M, Shiraishi M, Hikita N, Suetsugu Y, Sato Y, Yonezawa K, Tanaka M, Ohori R, et al. Study Protocol for the Japan Pregnancy, Eating, Activity, Cohort (J-PEACH) Study: Investigating Perinatal Maternal Lifestyle and Infant Health. Methods and Protocols. 2025; 8(6):128. https://doi.org/10.3390/mps8060128
Chicago/Turabian StyleHaruna, Megumi, Megumi Fujita, Masayo Matsuzaki, Mie Shiraishi, Naoko Hikita, Yoshiko Suetsugu, Yoko Sato, Kaori Yonezawa, Moeko Tanaka, Riko Ohori, and et al. 2025. "Study Protocol for the Japan Pregnancy, Eating, Activity, Cohort (J-PEACH) Study: Investigating Perinatal Maternal Lifestyle and Infant Health" Methods and Protocols 8, no. 6: 128. https://doi.org/10.3390/mps8060128
APA StyleHaruna, M., Fujita, M., Matsuzaki, M., Shiraishi, M., Hikita, N., Suetsugu, Y., Sato, Y., Yonezawa, K., Tanaka, M., Ohori, R., Aoyama, S., Yokoyama, M., Takeuchi, A., Nagamatsu, T., & Sasaki, S. (2025). Study Protocol for the Japan Pregnancy, Eating, Activity, Cohort (J-PEACH) Study: Investigating Perinatal Maternal Lifestyle and Infant Health. Methods and Protocols, 8(6), 128. https://doi.org/10.3390/mps8060128








