Practical Considerations in Abdominal MRI: Sequences, Patient Preparation, and Clinical Applications
Abstract
1. Introduction
- Single-Shot Fast Spin Echo (Canon: FASE) is a spin echo sequence, which uses the single-shot technique to acquire the data after a single 90˚ radio frequency (RF) excitation pulse, followed by a series of 180˚ RF pulses. All the data (e.g., 256) are acquired in a single TR (Scheme 3—right); therefore, all the lines from the k space are completed at the same time. In the case of Fast Spin Echo (FSE), a multi-shot technique, the number of shots depends on the value of echo train length (if echo train length (ETL) = 16 and Phase Encode Matrix Size = 256, then the number of shots is equals to 256 divided by 16) (Scheme 3—left). Using FASE, sequences with a shorter duration are obtained, which, in the case of the abominable MRI, leads to the reduction or even elimination of movement artifacts (Scheme 3). A total of 45 abdominal examinations were evaluated to assess the feasibility of performing FASE versus FSE sequences. These data were used to provide a statistical overview of sequence applicability. Detailed morphological analysis is illustrated through representative cases, rather than for all patients individually. Out of the 45 patients from the Smeeni Chronic Disease Hospital, only 19 (42.2%) were able to adequately hold their breath during the FSE sequence. For the 26 patients (57.8%), the faster FASE sequence was required, as it allowed for a shorter acquisition time, reducing the challenges associated with breath-holding (Scheme 3).
- Steady-State Free Precession is a gradient-echo sequence used in different applications like cardiac imaging, great vessels, abdominal imaging, cervical spine, and internal auditory meatus (IAM), as well as in other investigations because the cerebrospinal fluid (CSF) flow is reduced [15]. This technique is especially useful for depicting tissue and blood vessels with relatively long T2 during breath-holding, and it presents a great interest due to inherently high SNR and a very short acquisition time. The contrast obtained is more T1/T2 than T2 or T1, which is useful for a good contrast between fluids and surrounding tissue, allowing visualization of vascular structures and the distribution of body fluids with great contrast-to-noise ratio (CNR). An important aspect is that this sequence is not used to visualize diverse lesions because the difference between normal tissue T2/T1 ratio and lesion T2/T1 ratio might be quite small [16]; it is also susceptible to artifacts and have loud gradient noise.
- In Spin Echo–Echo Planer Imaging (SE-EPI), the RF excitation pulse is followed by a single refocusing pulse, similar to the Spin Echo sequence [17], but then multiple echoes are generated using the reversed polarity of the readout gradient and acquired using phase-encode gradient pulses of varying sizes. This technique uses Half-Fourier Acquisition, or Asymmetric Fourier Imaging (AFI), in the phase-encode direction in order to reduce TE (and increase the echo factor).
2. Materials and Methods
2.1. Patient Preparation
2.2. MRI Equipment
2.3. Abdominal Protocol–Sequences and Parameters
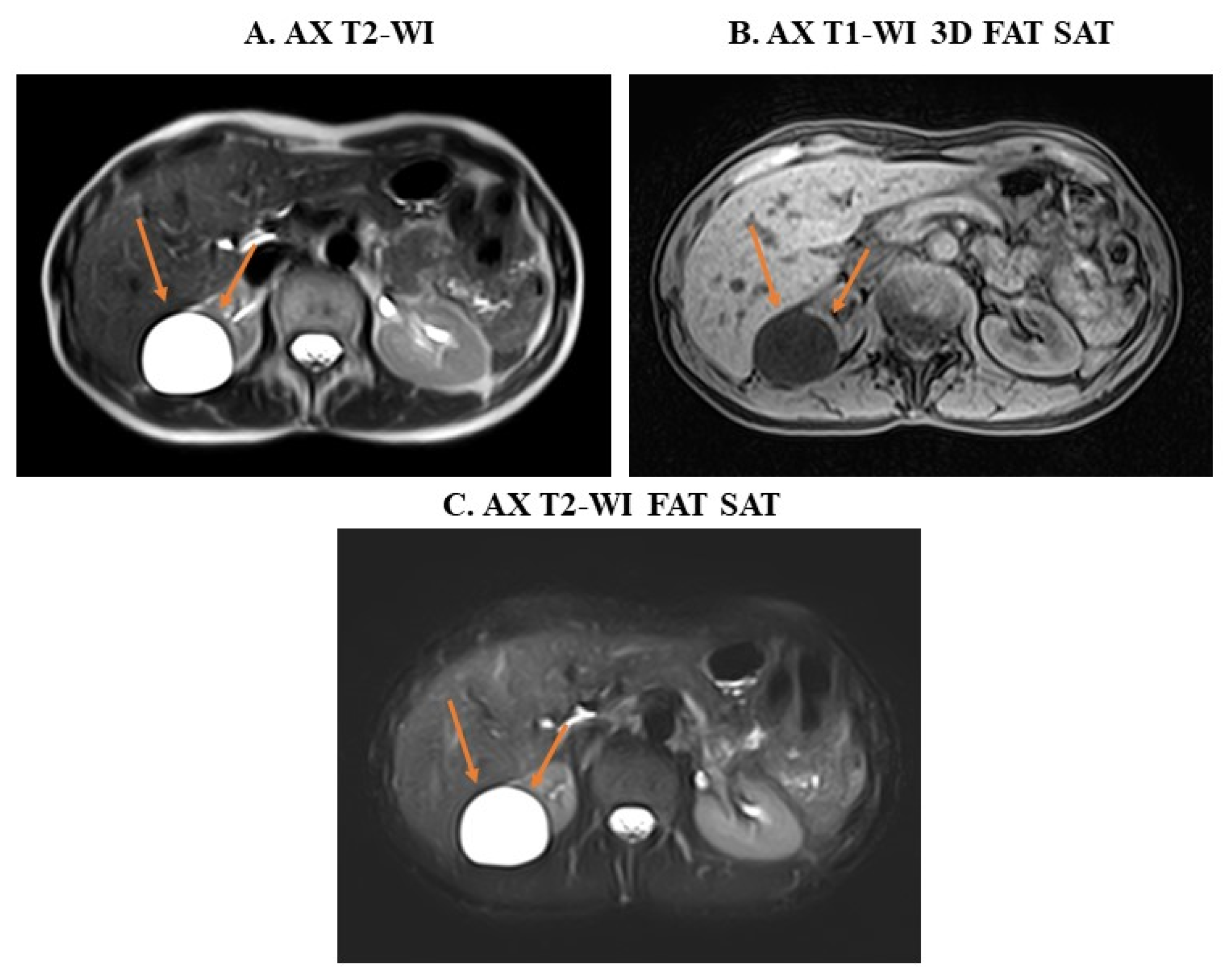
3. Examples of Applications of MRI Sequences in Upper Abdominal Analysis
4. Advancing Upper Abdominal Imaging—The Role of MRI Beyond Other Images Techniques
4.1. The Use of MRI to Reduce Radiation Exposure in Oncology Patients
4.2. Monitoring of Abdominal Lesions from CT to MRI
4.3. Confirm Upper Abdomen Ultrasound Diagnosis Through MRI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| LI-RADS | Liver Imaging Reporting and Data System |
| SNR | Signal-to-Noise Ratio |
| NSA/NEX | Number of Averages |
| DL | Deep learning |
| TR | Repetition Time |
| FOV | Field of View |
| TE | Echo Time |
| B/W | Receiver Bandwidth |
| ETL | Echo Train Length |
| SE | Spin Echo |
| GE | Gradient Echo |
| FASE | Fast Advanced Spin-Echo |
| HASTE | Half-Fourier Acquisition Single-Shot Turbo Spin |
| SSFSE | Single-Shot Fast Spin Echo |
| SSFP | Steady-State Free Precession |
| PSIF | Reverse Fast Imaging with Steady-State Free Precession |
| T2 FLASH | T2- Fast Low Angle Shot |
| FE | Field Echo |
| RF | Radio Frequency |
| FSE | Fast Spin Echo |
| IAM | internal auditory meatus |
| CSF | cerebrospinal fluid |
| CNR | Contrast-to-Noise Ratio |
| SE-EPI | Spin Echo—Echo Planer Imaging |
| AFI | Asymmetric Fourier Imaging |
| GRASP | Golden-angle RAdial Sparse Parallel |
| DISCO | DIfferential Subsampling with Cartesian Ordering |
| T2-WI | T2-weighted imaging |
| DWI | Diffusion weighted images |
| ADC | apparent diffusion coefficient |
| T1-WI | T1-weighted imaging |
| T1-WI in-phase and out-of-phase | T1-WI IP and OOP |
| WFS | Water-Fat Separation |
| Fat Sat | Fat Saturation |
| CT | Computed Tomography |
| PET-CT | Positron Emission Tomography—Computed Tomography |
References
- Yoon, J.H.; Nickel, M.D.; Peeters, J.M.; Lee, J.M. Rapid Imaging: Recent Advances in Abdominal MRI for Reducing Acquisition Time and Its Clinical Applications. Korean J. Radiol. 2019, 20, 1597–1615. [Google Scholar] [CrossRef]
- Noone, T.C.; Semelka, R.C.; Chaney, D.M.; Reinhold, C. Abdominal imaging studies: Comparison of diagnostic accuracies resulting from ultrasound, computed tomography, and magnetic resonance imaging in the same individual. Magn. Reson. Imaging 2004, 22, 19–24. [Google Scholar] [CrossRef]
- Raheem, A. Effects of Artifacts on the Diagnosis of Ultrasound Image. Medico-Legal Updat. 2021, 21, 327–336. [Google Scholar] [CrossRef]
- Ji, X.; Zhou, S.; Yang, P.; Liu, F.; Li, Y.; Li, H. Value of ultrasound combined with MRI in the diagnosis of primary and recurrent hepatocellular carcinoma. Oncol. Lett. 2019, 18, 6180–6186. [Google Scholar] [CrossRef]
- Lee, V.S.; Lavelle, M.T.; Rofsky, N.M.; Laub, G.; Thomasson, D.M.; Krinsky, G.A.; Weinreb, J.C. Hepatic MR Imaging with a Dynamic Contrast-enhanced Isotropic Volumetric Interpolated Breath-hold Examination: Feasibility, Reproducibility, and Technical Quality. Radiology 2000, 215, 365–372. [Google Scholar] [CrossRef]
- Krinsky, G.A.; Lee, V.S.; Theise, N.D.; Weinreb, J.C.; Rofsky, N.M.; Diflo, T.; Teperman, L.W. Hepatocellular Carcinoma and Dysplastic Nodules in Patients with Cirrhosis: Prospective Diagnosis with MR Imaging and Explantation Correlation. Radiology 2001, 219, 445–454. [Google Scholar] [CrossRef]
- Azevedo, R.M.; de Campos, R.O.P.; Ramalho, M.; Herédia, V.; Dale, B.M.; Semelka, R.C. Free-Breathing 3D T1-Weighted Gradient-Echo Sequence With Radial Data Sampling in Abdominal MRI: Preliminary Observations. Am. J. Roentgenol. 2011, 197, 650–657. [Google Scholar] [CrossRef]
- Pietryga, J.A.; Burke, L.M.B.; Marin, D.; Jaffe, T.A.; Bashir, M.R. Respiratory Motion Artifact Affecting Hepatic Arterial Phase Imaging with Gadoxetate Disodium: Examination Recovery with a Multiple Arterial Phase Acquisition. Radiology 2014, 271, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.R.; Castelli, P.; Davenport, M.S.; Larson, D.; Marin, D.; Hussain, H.K.; Jaffe, T.A. Respiratory Motion Artifact Affecting Hepatic Arterial Phase MR Imaging with Gadoxetate Disodium Is More Common in Patients with a Prior Episode of Arterial Phase Motion Associated with Gadoxetate Disodium. Radiology 2015, 274, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Takahashi, N.; Yamaguchi, M.; Lou, D.; Saida, Y.; Itai, Y. Double arterial phase dynamic MRI with sensitivity encoding (SENSE) for hypervascular hepatocellular carcinomas. J. Magn. Reson. Imaging 2002, 16, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Ikram, N.S.; Yee, J.; Weinstein, S.; Yeh, B.M.; Corvera, C.U.; Monto, A.; Hope, T.A. Multiple arterial phase MRI of arterial hypervascular hepatic lesions: Improved arterial phase capture and lesion enhancement. Abdom. Imaging 2016, 42, 870–876. [Google Scholar] [CrossRef]
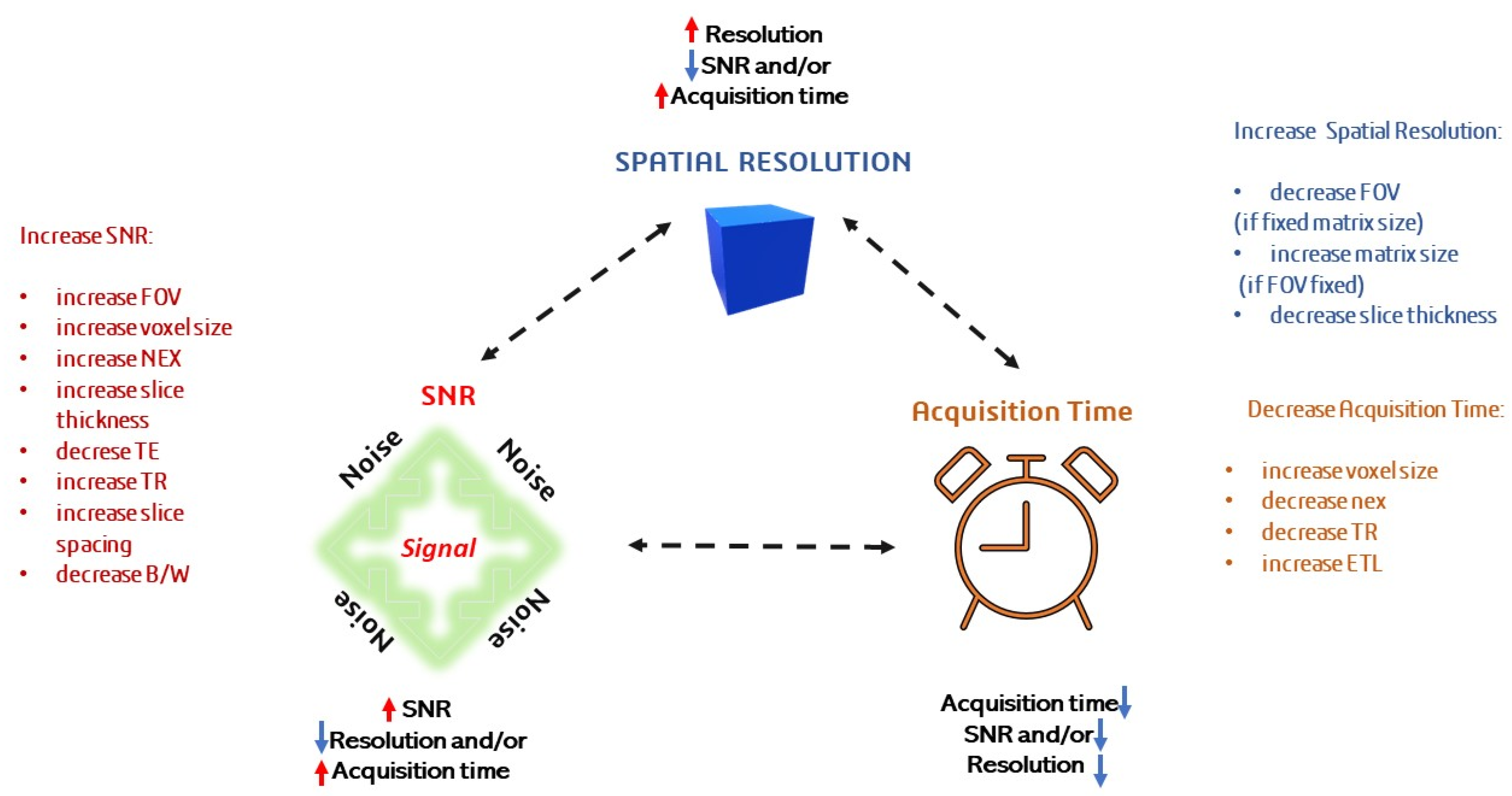
- Zafar, W. Resolution, SNR, Signal Averaging and Scan Time in MRI for Metastatic Lesion in Spine: A Case Report in a 74 Years Old Patient. Clin. Radiol. Imaging J. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Abdala-Junior, R.; No-Cortes, J.; Arita, E.S.; Ackerman, J.L.; da Silva, R.L.B.; Kim, J.H.; Cortes, A.R.G. Influence of receiver bandwidth on MRI artifacts caused by orthodontic brackets composed of different alloys. Imaging Sci. Dent. 2021, 51, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.S.; Elseady, B.A.; Elabd, O.L.; Kohla, M.S.; Samea, M.E.S.A. Comparison of magnetic resonance elastography and diffusion-weighted imaging for differentiating benign and malignant liver lesions. Egypt. J. Radiol. Nucl. Med. 2024, 55, 1–10. [Google Scholar] [CrossRef]
- Westbrook, C.; Roth, C.K.; Talbot, J. MRI in Practice, 4th ed.; Wiley-Blackwell: Chichester, UK, 2011. [Google Scholar]
- Elmaoğlu, M.; Çelik, A. MRI Handbook: MR Physics, Patient Positioning, and Protocols, 1st ed.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Cazacu, N.; Chilom, C. MAGNETIC RESONANCE IMAGING OF THE LUMBAR SPINE. Rom. J. Biophys. 2024, 34, 117–135. [Google Scholar] [CrossRef]
- Kabasawa, H. MR Imaging in the 21st Century: Technical Innovation over the First Two Decades. Magn. Reson. Med Sci. 2022, 21, 71–82. [Google Scholar] [CrossRef]
- Gassenmaier, S.; Küstner, T.; Nickel, D.; Herrmann, J.; Hoffmann, R.; Almansour, H.; Afat, S.; Nikolaou, K.; Othman, A.E. Deep Learning Applications in Magnetic Resonance Imaging: Has the Future Become Present? Diagnostics 2021, 11, 2181. [Google Scholar] [CrossRef] [PubMed]
- Haaga, J.R.; Boll, D. CT and MRI of the Whole Body, 6th ed.Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Morani, A.C.; Elsayes, K.M.; Liu, P.S.; Weadock, W.J.; Szklaruk, J.; Dillman, J.R.; Khan, A.; Chenevert, T.L.; Hussain, H.K. Abdominal applications of diffusion-weighted magnetic resonance imaging: Where do we stand. World J. Radiol. 2013, 5, 68–80. [Google Scholar] [CrossRef]
- Filipe, J.P.; Curvo-Semedo, L.; Casalta-Lopes, J.; Marques, M.C.; Caseiro-Alves, F. Diffusion-weighted imaging of the liver: Usefulness of ADC values in the differential diagnosis of focal lesions and effect of ROI methods on ADC measurements. Magn. Reson. Mater. Physics, Biol. Med. 2012, 26, 303–312. [Google Scholar] [CrossRef]
- Jain, T.P.; Ter Kan, W.; Edward, S.; Fernon, H.; Naider, R.K. Evaluation of ADCratio on liver MRI diffusion to discriminate benign versus malignant solid liver lesions. Eur. J. Radiol. Open 2018, 5, 209–214. [Google Scholar] [CrossRef]
- Syrkashev, E.M.; Kadyrberdieva, F.Z.; Abuladze, L.R.; Semenov, D.S.; Privalova, E.G. Basic pulse sequences in the diagnosis of abdominal pathology. Digit. Diagn. 2023, 4, 39–50. [Google Scholar] [CrossRef]
- Ramalho, M.; Herédia, V.; de Campos, R.O.; Dale, B.M.; Azevedo, R.M.; Semelka, R.C. In-phase and out-of-phase gradient-echo imaging in abdominal studies: Intra-individual comparison of three different techniques. Acta Radiol. 2012, 53, 441–449. [Google Scholar] [CrossRef]
- Anta, J.A.; Moreno-Vedia, J.; López, J.G.; Rios-Vives, M.A.; Munuera, J.; Rodríguez-Comas, J. Artificial intelligence for detection and characterization of focal hepatic lesions: A review. Abdom. Imaging. 2024, 50, 1564–1583. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.; Albadawy, M. AI in diagnostic imaging: Revolutionising accuracy and efficiency. Comput. Methods Programs Biomed. Updat. 2024, 5. [Google Scholar] [CrossRef]
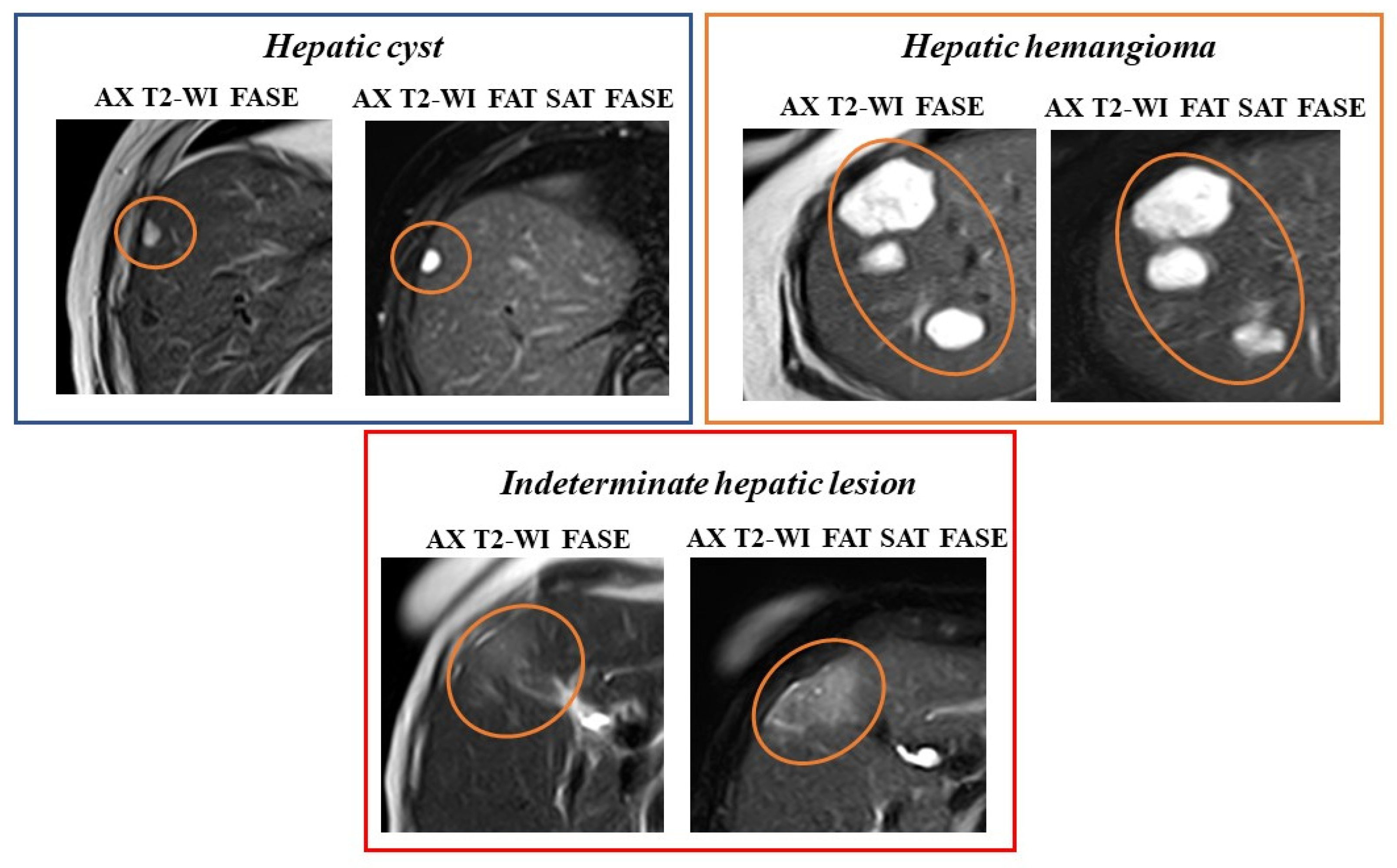
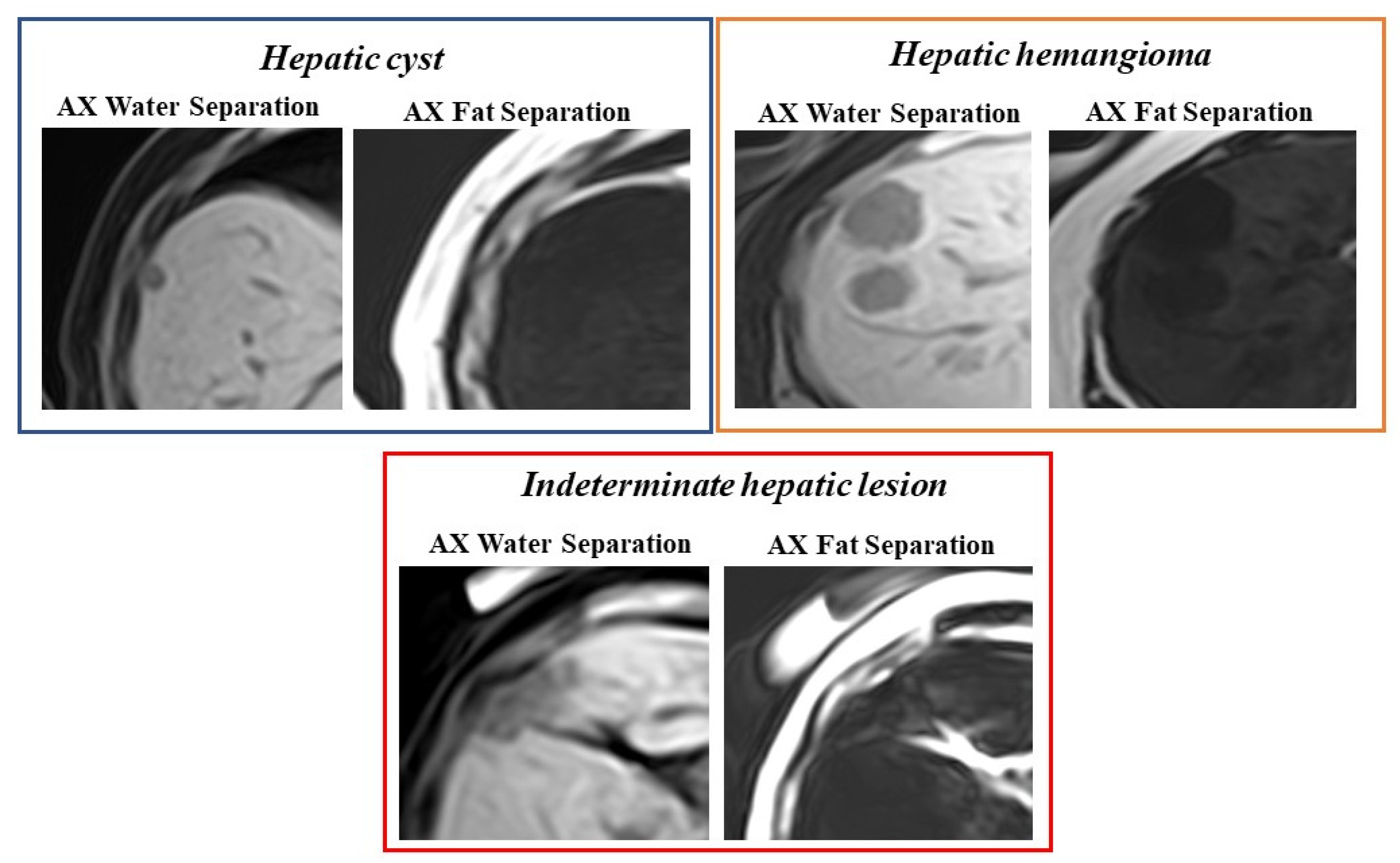
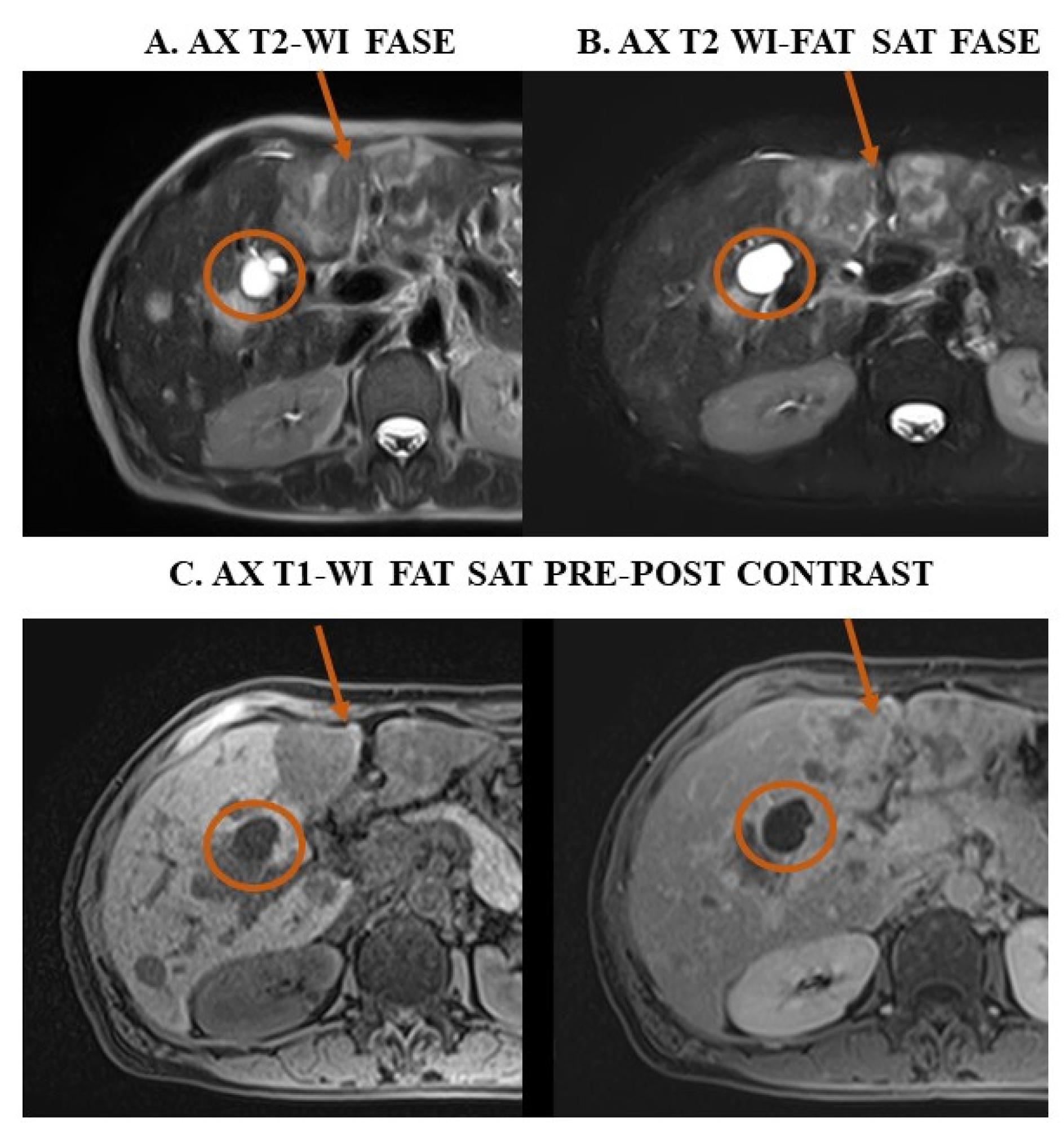
- Chenin, M.; Paisant, A.; Lebigot, J.; Bazeries, P.; Debbi, K.; Ronot, M.; Laurent, V.; Aubé, C. Cystic liver lesions: A pictorial review. Insights into Imaging 2022, 13, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ishak, K.G.; Rabin, L. Benign Tumors of the Liver. Med Clin. North Am. 1975, 59, 995–1013. [Google Scholar] [CrossRef]
- Bajenaru, N.; Balaban, V.; Săvulescu, F.; Campeanu, I.; Patrascu, T. Hepatic hemangioma—review. J. Med. Life 2015, 8, 4–11. [Google Scholar]
- Silverman, S.G.; Pedrosa, I.; Ellis, J.H.; Hindman, N.M.; Schieda, N.; Smith, A.D.; Remer, E.M.; Shinagare, A.B.; Curci, N.E.; Raman, S.S.; et al. Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology 2019, 292, 475–488. [Google Scholar] [CrossRef]
- Tse, J.R.; Shen, J.; Shen, L.; Yoon, L.; Kamaya, A. Bosniak Classification of Cystic Renal Masses Version 2019: Comparison of Categorization Using CT and MRI. Am. J. Roentgenol. 2021, 216, 412–420. [Google Scholar] [CrossRef]
- Esfeh, J.M.; Hajifathalian, K.; Ansari-Gilani, K. Sensitivity of ultrasound in detecting hepatocellular carcinoma in obese patients compared to explant pathology as the gold standard. Clin. Mol. Hepatol. 2020, 26, 54–59. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazacu, N.; Chilom, C.G.; Adrian, C.; Minoiu, C.A. Practical Considerations in Abdominal MRI: Sequences, Patient Preparation, and Clinical Applications. Methods Protoc. 2025, 8, 129. https://doi.org/10.3390/mps8060129
Cazacu N, Chilom CG, Adrian C, Minoiu CA. Practical Considerations in Abdominal MRI: Sequences, Patient Preparation, and Clinical Applications. Methods and Protocols. 2025; 8(6):129. https://doi.org/10.3390/mps8060129
Chicago/Turabian StyleCazacu, Nicoleta, Claudia G. Chilom, Cosmin Adrian, and Costin A. Minoiu. 2025. "Practical Considerations in Abdominal MRI: Sequences, Patient Preparation, and Clinical Applications" Methods and Protocols 8, no. 6: 129. https://doi.org/10.3390/mps8060129
APA StyleCazacu, N., Chilom, C. G., Adrian, C., & Minoiu, C. A. (2025). Practical Considerations in Abdominal MRI: Sequences, Patient Preparation, and Clinical Applications. Methods and Protocols, 8(6), 129. https://doi.org/10.3390/mps8060129






