Indicator Tubes: A Novel Solution for Monitoring Temperature Excursions in Biobank Storage
Abstract
1. Introduction
2. Experimental Design
2.1. Materials
- Thermo Scientific™, Nunc™, Biobanking and Cell Culture Cryogenic Tubes, 1.8 mL (Thermo Fisher Scientific, Waltham, MA, USA; Cat. no.: 375418).
- Thermo Scientific™, Cryo Vial Closure Color Coders, red (Thermo Fisher Scientific, Waltham, MA, USA; Cat. no.: 375515).
- Food-grade nonpareil sprinkles (various commercial suppliers).
- Quinaldine Red (Thermo Fisher Scientific, Waltham, MA, USA; Cat. no.: AC197410010).
- Distilled water.
- Ethanol (Warner-Graham Company, Cockeysville, MD, USA; Cat. no.: 64-17-5).
- KimTech Science™, Precision Wipes (Kimberly-Clark, Irving, TX, USA; Cat. no.: 34155).
- Pencil, scissors, and forceps.
- Thermosafe multi-purpose insulated shipper: 355 EPS with corrugated carton, 1.5″ Wall Thickness, 36″ × 36″ × 37″ (Sonoco, Hartsville, SC, USA; Cat. no.: 03-530-17).
- Dry Ice.
2.2. Equipment
- A −20 °C freezer (LabRepco, Horsham, PA, USA), a −80 °C ultra-low-temperature freezer (Panasonic PHC, Tokyo, Japan), a vapor-phase liquid nitrogen storage unit (MVE), and a controlled-rate freezer CBS 2100 (Custom Biogenics, Bruce Township, MI, USA), all electronically monitored for temperature excursions and mapped for consistent temperature throughout the unit.
- A temperature monitoring probe (Rees Scientific, Trenton, NJ, USA).
- A liquid nitrogen dewar.
- A laboratory balance (0.1 mg precision).
3. Procedure
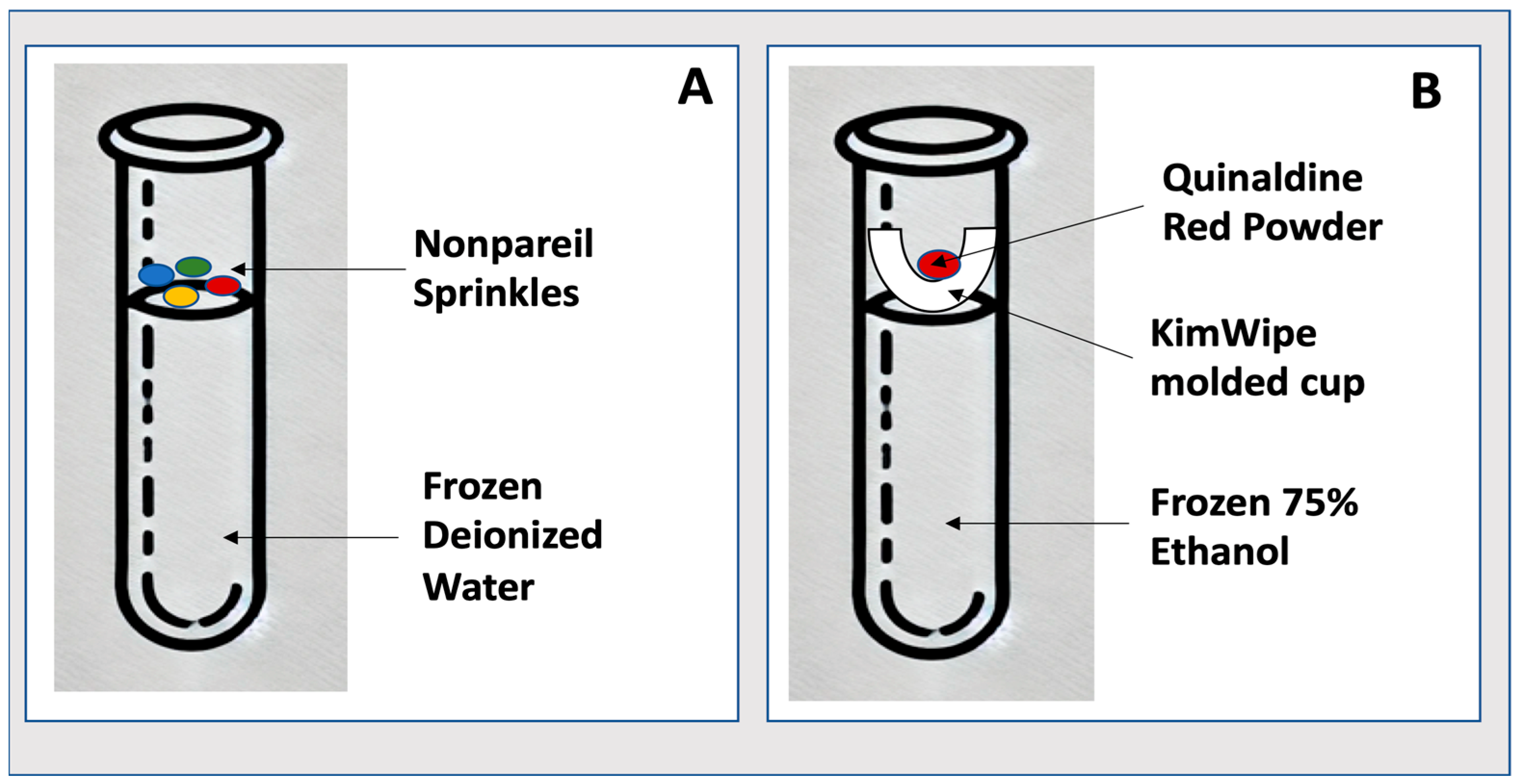
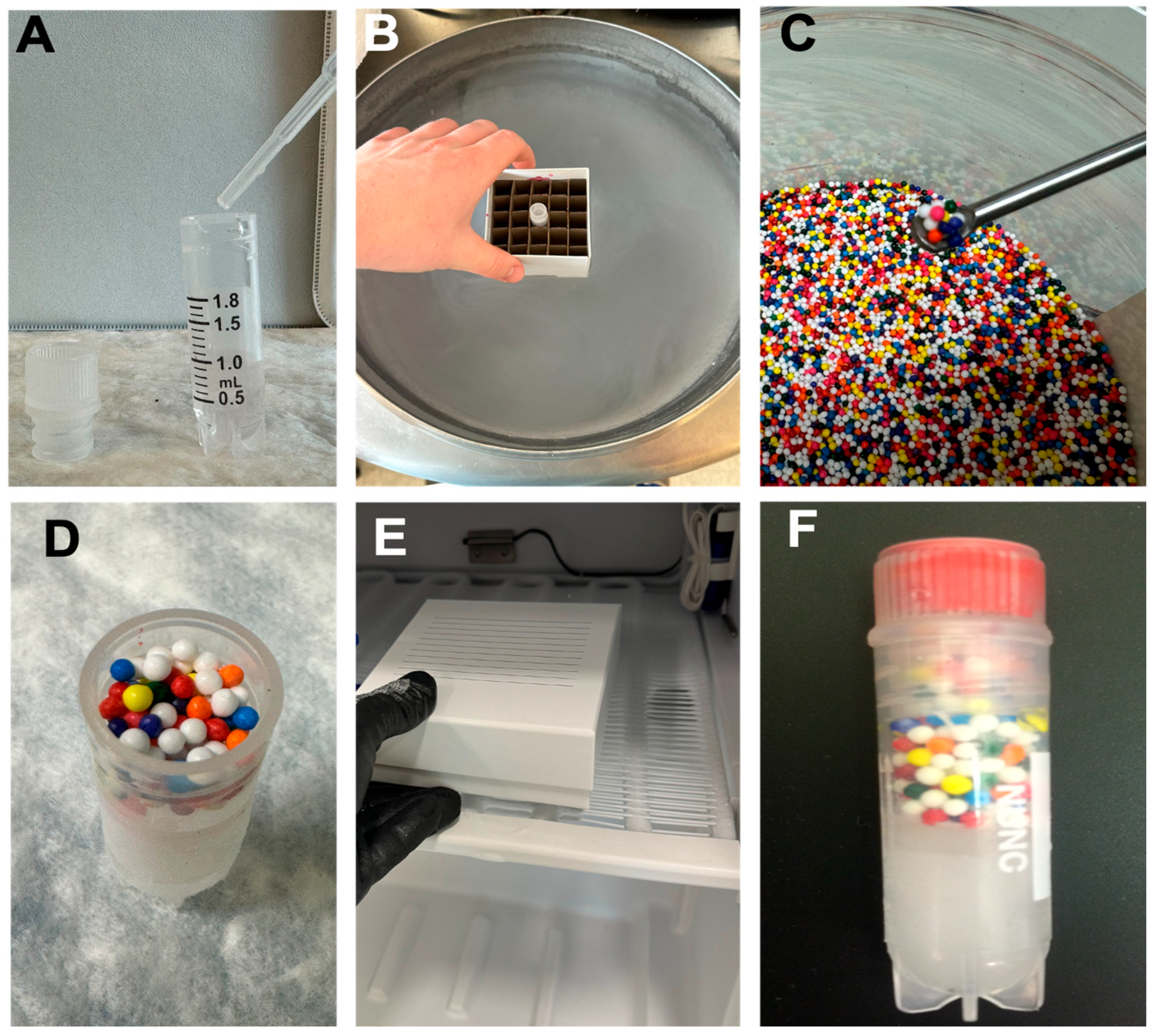
3.1. Making the Frozen Storage Indicator (−20 °C)
- Fill each cryogenic vial with 1 mL of deionized water.
- Place vials in a −20 °C freezer until completely frozen (minimally overnight).
- Once frozen, open the vial and gently add ~0.8 g of food-grade sprinkles to the ice surface. CRITICAL STEP: Ensure sprinkles rest on the surface without embedding into the ice.
- Cap the vials and return to their designated position in frozen storage.
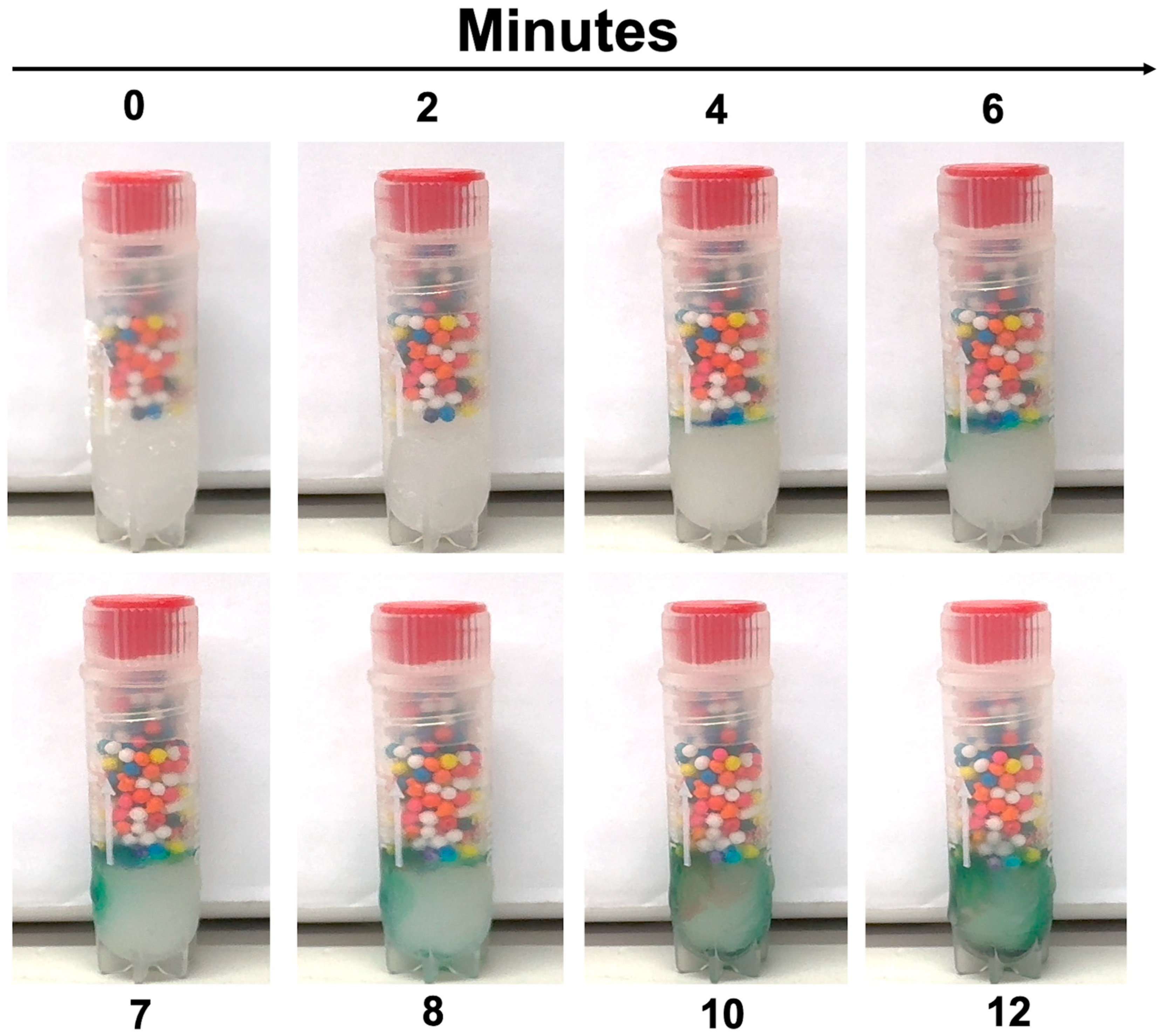
- Inspect regularly. If sprinkles remain intact, no thaw has occurred. If dispersed, thaw has occurred.
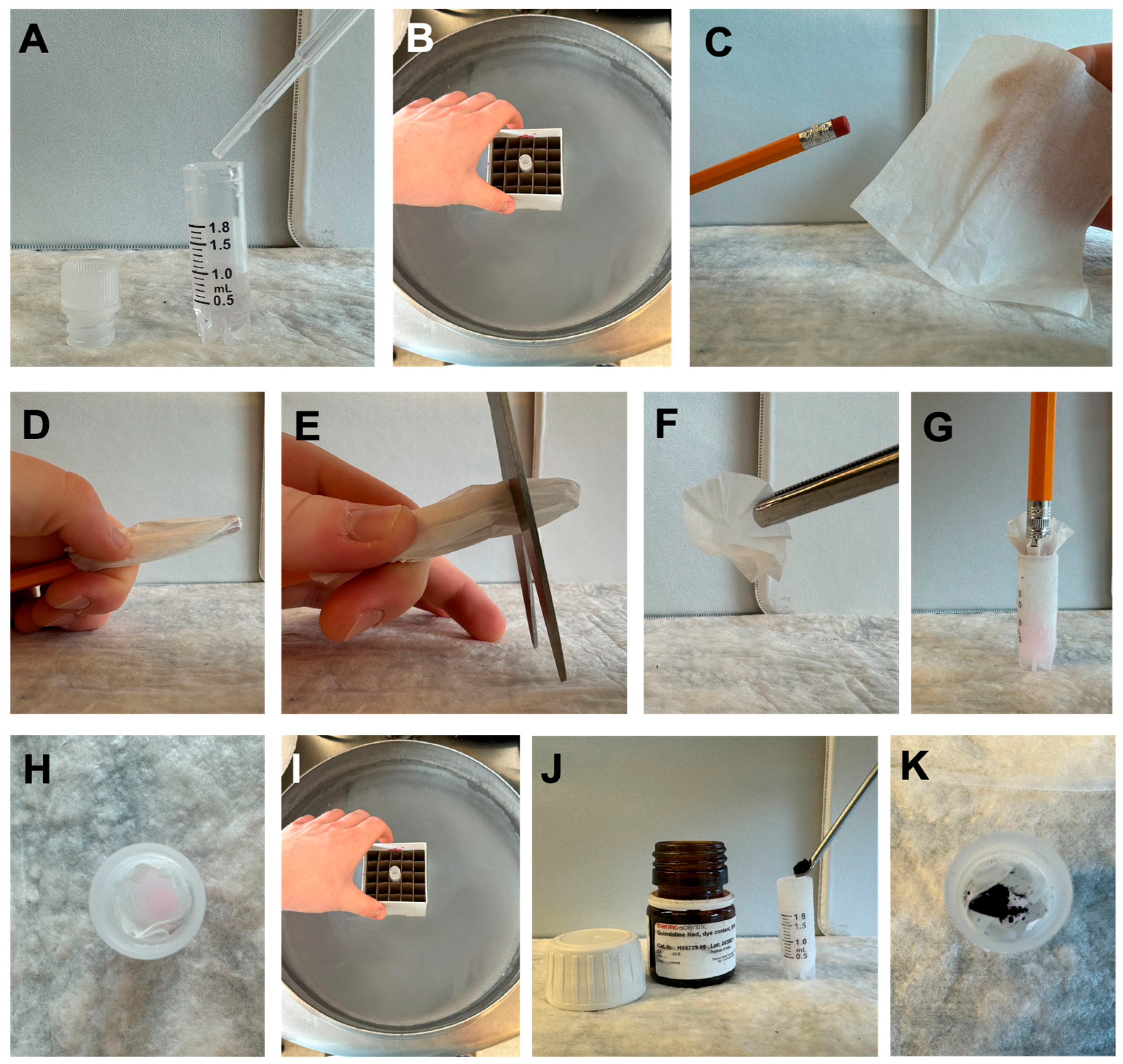
3.2. Making the Ultra-Low-Temperature Storage Indicator (−80 °C)
- Add 1 mL of 75% ethanol (deionized water and ethanol) to a cryogenic vial.
- Freeze the vial in a liquid nitrogen vapor-phase freezer to solidify the ethanol (minimally one hour).
- Cut a small 1-inch square from a KimTech wipe and form a cone using a pencil.
- Form a cone shape with the cut square around a pencil. CRITICAL STEP: Handle gently to avoid tearing, which could compromise the indicator’s functionality.
- Slide the cone approximately two centimeters off the pencil and trim the end to create a ‘cup’.
- Insert the cup into the vial, resting on top of the frozen solution.
- Chill the entire assembly under a liquid nitrogen vapor phase for 5 min.
- Add ~35 mg of Quinaldine Red powder into the cup and cap the vial.
- Place the vial in −80 °C storage.
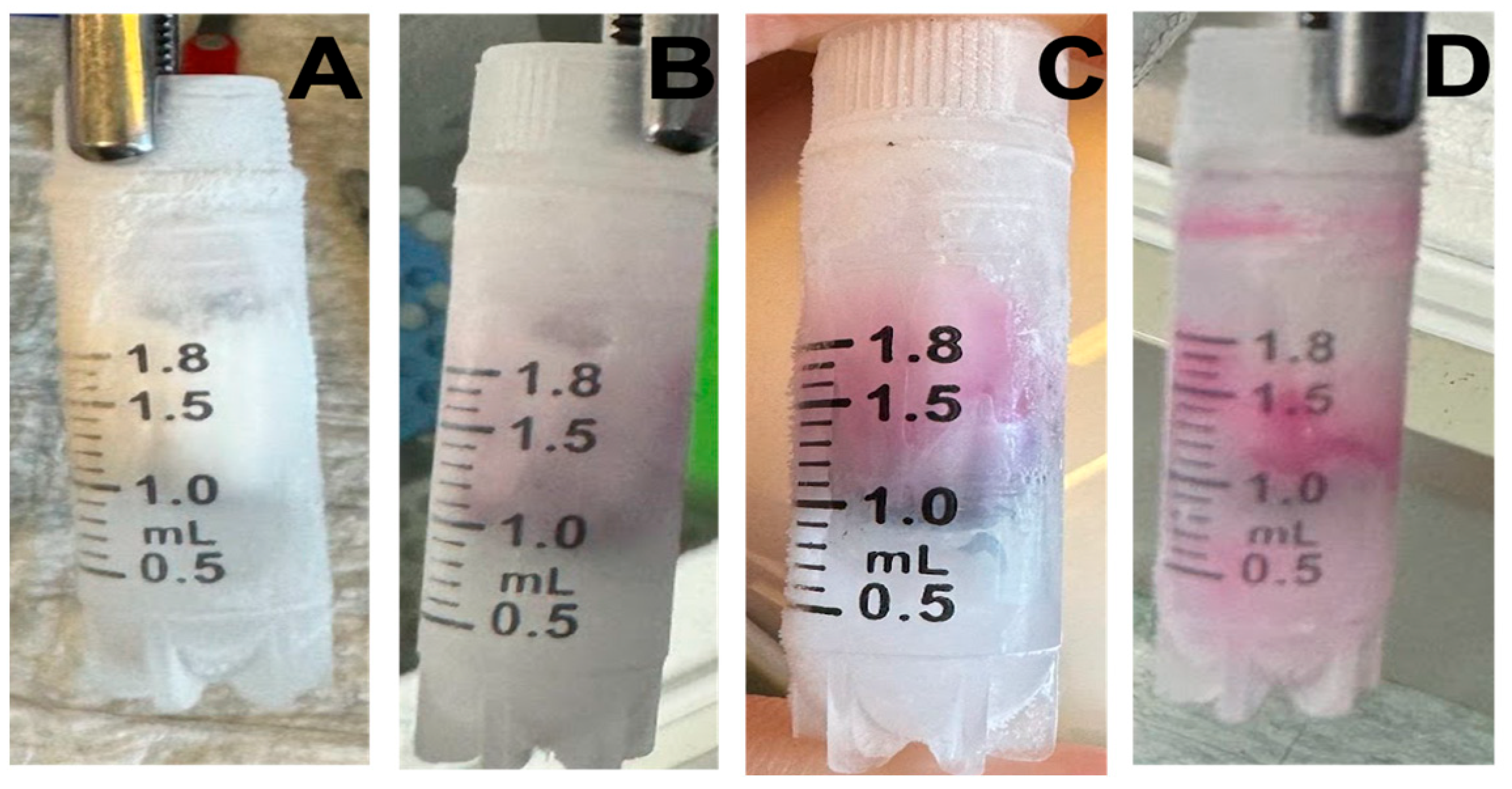
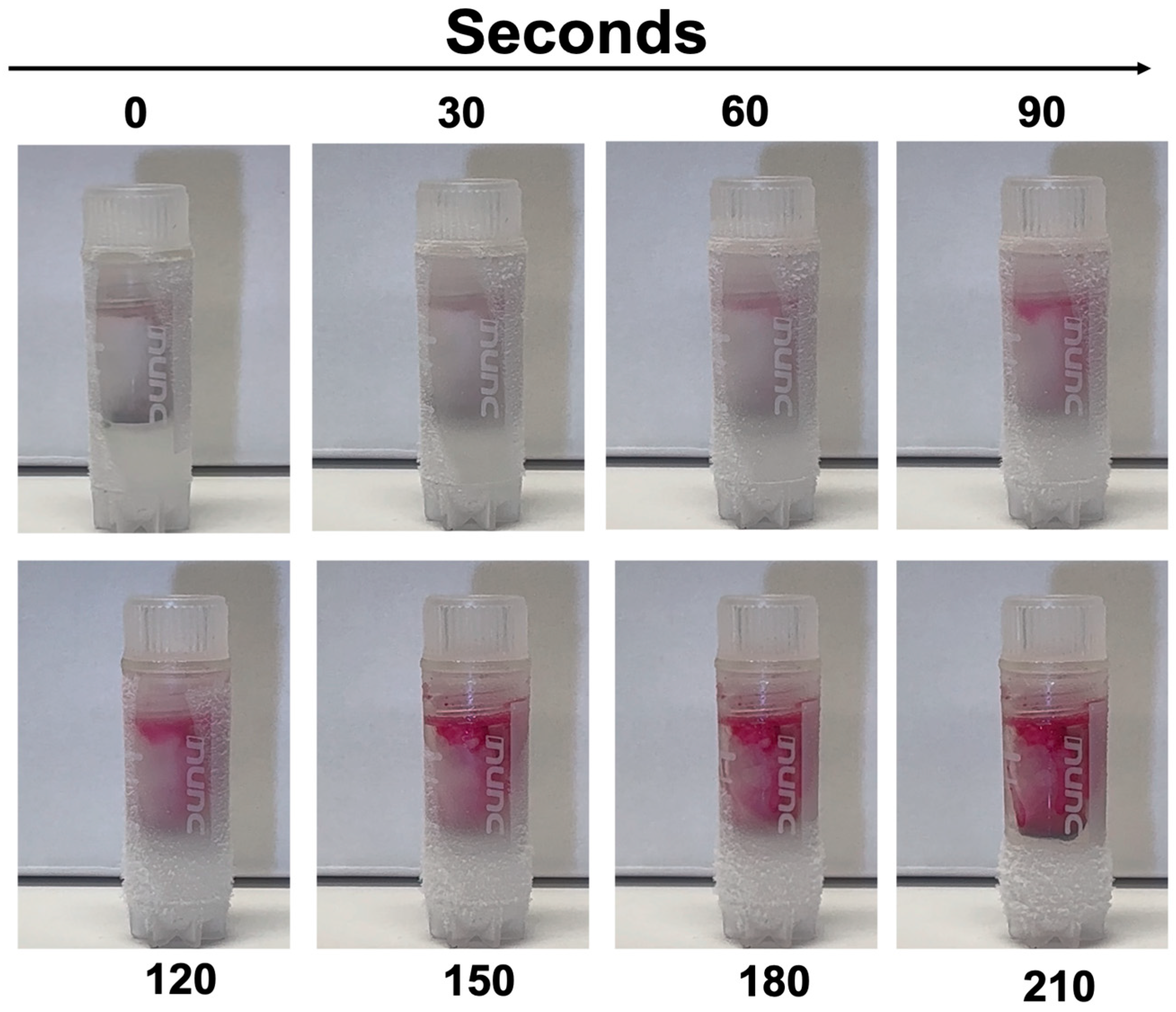
- Inspect regularly. Dye dispersion (pink in ethanol) signals partial thawing.
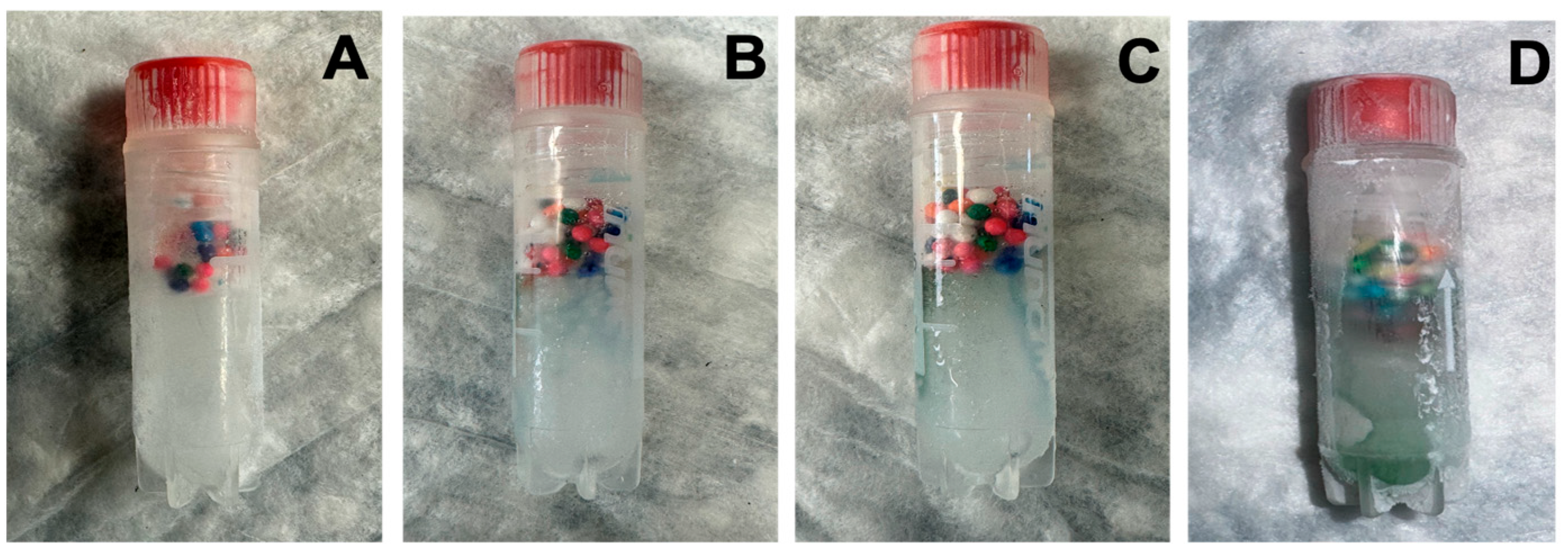
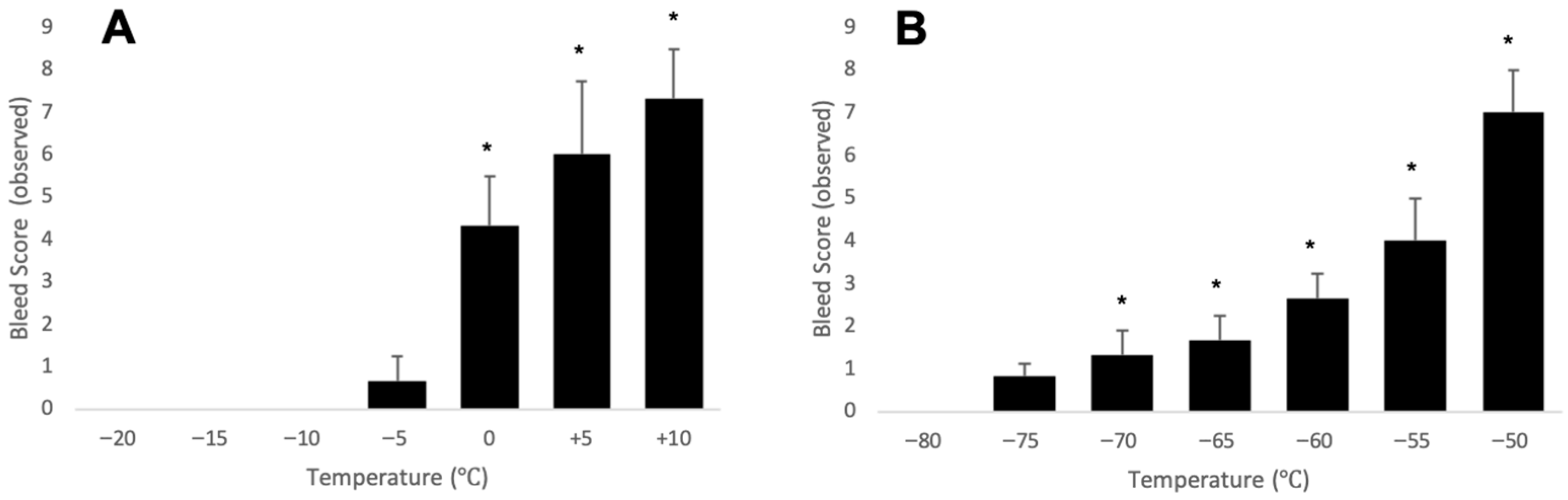
3.3. Validation Tests
3.4. Practical Application Testing
4. Expected Results
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Liu, X.; Hu, Y.; Chen, X.; Tan, S. Cryopreservation of tissues and organs: Present, bottlenecks, and future. Front. Veter. Sci. 2023, 10, 1201794. [Google Scholar] [CrossRef] [PubMed]
- Khaydukova, I.V.; Ivannikova, V.M.; Zhidkov, D.A.; Belikov, N.V.; Peshkova, M.A.; Timashev, P.S.; Tsiganov, D.I.; Pushkarev, A.V. Current State and Challenges of Tissue and Organ Cryopreservation in Biobanking. Int. J. Mol. Sci. 2024, 25, 11124. [Google Scholar] [CrossRef]
- Hunt, C.J. Cryopreservation of Human Stem Cells for Clinical Application: A Review. Transfus. Med. Hemother. 2011, 38, 107–123. [Google Scholar] [CrossRef]
- Du, Y.; Xie, W.; Liu, C. Strategies and considerations for distributing and recovering mouse lines. Methods Enzym. 2010, 476, 37–52. [Google Scholar]
- He, B.; Su, S.; Yuan, G.; Duan, J.; Zhu, Z.; Wang, Z. Clinical guideline for vascularized composite tissue cryopreservation. J. Tissue Eng. Regen. Med. 2021, 15, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Johnson, R.; Yu, G.; Mckenna, D.H.; Hubel, A. Preservation of cell-based immunotherapies for clinical trials. Cytotherapy 2019, 21, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Gil-Chinchilla, J.I.; Bueno, C.; Martínez, C.M.; Ferrández-Múrtula, A.; García-Hernández, A.M.; Blanquer, M.; Molina-Molina, M.; Zapata, A.G.; Sackstein, R.; Moraleda, J.M.; et al. Optimizing cryopreservation conditions for use of fucosylated human mesenchymal stromal cells in anti-inflammatory/immunomodulatory therapeutics. Front. Immunol. 2024, 15, 1385691. [Google Scholar] [CrossRef] [PubMed]
- Meneghel, J.; Kilbride, P.; Morris, G.J. Cryopreservation as a Key Element in the Successful Delivery of Cell-Based Therapies—A Review. Front. Med. 2020, 7, 592242. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.G.; Snyder, K.K.; Van Buskirk, R.; Baust, J.M. Integrating Molecular Control to Improve Cryopreservation Outcome. Biopreserv. Biobank. 2017, 15, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Benson, E.E.; Betson, F.; Fuller, B.J.; Harding, K.; Kofanova, O. Translating cryobiology principles into trans-disciplinary storage guidelines for biorepositories and biobanks: A concept paper. CryoLetters 2013, 34, 277–312. [Google Scholar] [PubMed]
- Kilbride, P.; Meneghel, J.; Fonseca, F.; Morris, J. The transfer temperature from slow cooling to cryogenic storage is critical for optimal recovery of cryopreserved mammalian cells. PLoS ONE 2021, 16, e0259571. [Google Scholar] [CrossRef] [PubMed]
- Whaley, D.; Damyar, K.; Witek, R.P.; Mendoza, A.; Alexander, M.; Lakey, J.R. Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transpl. 2021, 30, 963689721999617. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Weegman, B.P.; Baicu, S.C.; Giwa, S.E. New Approaches to Cryopreservation of Cells, Tissues, and Organs. Transfus. Med. Hemother. 2019, 46, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Hubel, A.; Spindler, R.; Skubitz, A.P. Storage of human biospecimens: Selection of the optimal storage temperature. Biopreserv. Biobank. 2014, 12, 165–175. [Google Scholar] [CrossRef] [PubMed]
- International Society for Biological and Environmental Repositories. Biopreservation and Biobanking Best Practices; Mary Ann Liebert: New Rochelle, NY, USA, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catterson, P.J.; Olson, T.T.; Penno, M.B.; Callahan, S.P.; Olson, M.V. Indicator Tubes: A Novel Solution for Monitoring Temperature Excursions in Biobank Storage. Methods Protoc. 2025, 8, 120. https://doi.org/10.3390/mps8050120
Catterson PJ, Olson TT, Penno MB, Callahan SP, Olson MV. Indicator Tubes: A Novel Solution for Monitoring Temperature Excursions in Biobank Storage. Methods and Protocols. 2025; 8(5):120. https://doi.org/10.3390/mps8050120
Chicago/Turabian StyleCatterson, Patrick J., Tyler T. Olson, Margaret B. Penno, Steven P. Callahan, and Melissa V. Olson. 2025. "Indicator Tubes: A Novel Solution for Monitoring Temperature Excursions in Biobank Storage" Methods and Protocols 8, no. 5: 120. https://doi.org/10.3390/mps8050120
APA StyleCatterson, P. J., Olson, T. T., Penno, M. B., Callahan, S. P., & Olson, M. V. (2025). Indicator Tubes: A Novel Solution for Monitoring Temperature Excursions in Biobank Storage. Methods and Protocols, 8(5), 120. https://doi.org/10.3390/mps8050120





