Accuracy of Patient Setup Using Surface Guided Radiotherapy (SGRT) for Abdominal Malignancies
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Group
2.1.1. Patient Cohort and Clinical Characteristics
Group A
Group B
2.2. Planning CT Scanning and Radiation Therapy
- -
- Linac 1 Infinity (Elekta, SW): 6 MV single photon energy and a CBCT system XVI R5.0.4 (Elekta, SW). All patients of Group B were treated on this machine.
- -
- Linac 2 Infinity (Elekta, SW): 6, 10, 15 MV photon energies and a CBCT system XVI R5.0.4 (Elekta, SW). All patients of Group A were treated on this machine.
2.3. Patient Setup Method
2.4. CBCT Verification and Position Correction
2.5. Statistical Analysis
3. Results
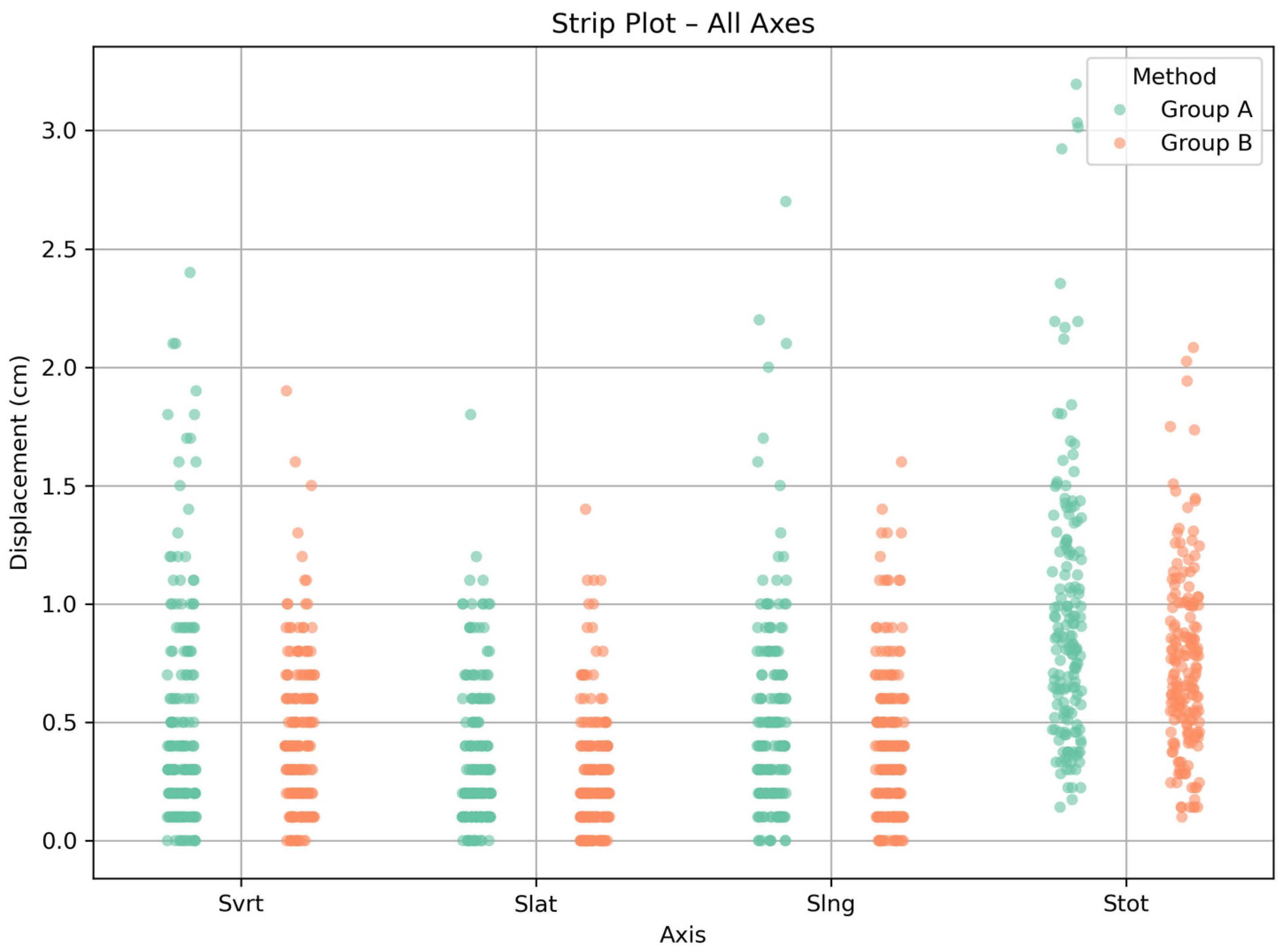
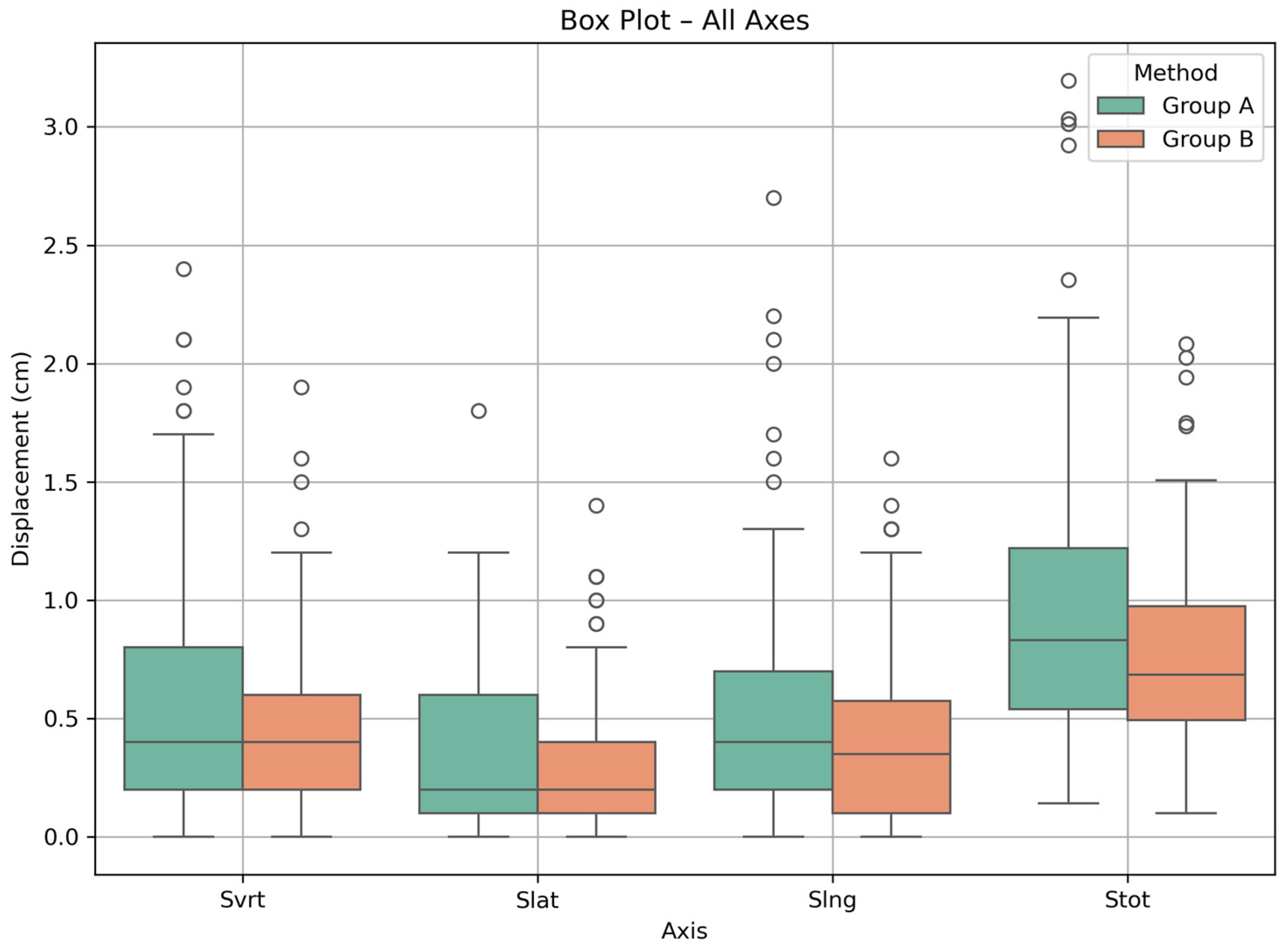
3.1. Patient Positioning Corrections
3.2. Statistical Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazonakis, M.; Tzanis, E.; Lyraraki, E.; Damilakis, J. Automatic Radiobiological Comparison of Radiation Therapy Plans: An Application to Gastric Cancer. Cancers 2022, 14, 6098. [Google Scholar] [CrossRef]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Ten Haken, R.K.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. The Use of Normal Tissue Complication Probability (NTCP) Models in the Clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, V.; Tsironi, F.; Tolia, M.; Mazonakis, M. Comparison between the SGRT and the conventional setup method for patients undergoing VMAT for pelvic malignancies. Appl. Radiat. Isot. 2025, 217, 111659. [Google Scholar] [CrossRef]
- Naidoo, W.; Leech, M. Feasibility of surface guided radiotherapy for patient positioning in breast radiotherapy versus conventional tattoo-based setups: A systematic review. Tech. Innov. Patient Support Radiat. Oncol. 2022, 22, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Lawler, G. A review of surface guidance in extracranial stereotactic body radiotherapy (SBRT/SABR) for set-up and intra-fraction motion management. Tech. Innov. Patient Support Radiat. Oncol. 2022, 21, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.; Munshi, A.; Agarwal, J. Skin markings methods and guidelines: A reality in image guidance radiotherapy era. South Asian J. Cancer 2012, 1, 27–29. [Google Scholar] [CrossRef]
- Freislederer, P.; Batista, V.; Öllers, M.; Sutter, G.; Belka, C.; Roeder, F.; Fontenot, J.D.; Gordon, J.; Grosu, A.L.; Lütgendorf-Cullmann, N.; et al. ESTRO-ACROP guideline on surface guided radiation therapy. Radiother. Oncol. 2022, 173, 188–196. [Google Scholar] [CrossRef]
- Mannerberg, A.; Kügele, M.; Hamid, S.; Shinde, A.; Ågren, N.; Beck, D.; Matuschek, C. Faster and more accurate patient positioning with surface guided radiotherapy for ultra-hypofractionated prostate cancer patients. Tech. Innov. Patient Support Radiat Oncol. 2021, 19, 41–45. [Google Scholar] [CrossRef]
- Zhou, S.; Li, J.; Zhu, X.; Yin, W.; Lin, Y.; Yuan, H.; Chen, Y.; Huang, Y.; Xu, Y.; Li, W.; et al. Initial clinical experience of surface guided stereotactic radiation therapy with open-face mask immobilization for improving setup accuracy: A retrospective study. Radiat. Oncol. 2022, 17, 104. [Google Scholar] [CrossRef]
- Hattel, S.H.; Andersen, P.A.; Wahlstedt, I.H.; Damkjær, S.; Saini, A.; Thomsen, J.B. Evaluation of setup and intrafraction motion for surface guided whole-breast cancer radiotherapy. J. Appl. Clin. Med. Phys. 2019, 20, 39–44. [Google Scholar] [CrossRef]
- Bartoncini, S.; Fiandra, C.; Ruo Redda, M.G.; Allis, S.; Muñoz, F.; Ricardi, U. Target registration errors with surface imaging system in conformal radiotherapy for prostate cancer: Study on 19 patients. Radiol. Med. 2012, 117, 1419–1428. [Google Scholar] [CrossRef]
- Kügele, M.; Mannerberg, A.; Nørring Bekke, S.; Rath, F.; Palumbo, I.; Yazici, D.; Dalichau, H.; Rivera, M.; Beck, D.; Matuschek, C.; et al. Surface guided radiotherapy (SGRT) improves breast cancer patient setup accuracy. J. Appl. Clin. Med. Phys. 2019, 20, 61–68. [Google Scholar] [CrossRef]
- Al-Hallaq, H.A.; Cerviño, L.; Gutiérrez, A.N.; Havnen-Smith, A.; Higgins, S.A.; Kügele, M.; Padilla, L.; Pawlicki, T.; Remmes, N.; Smith, K.; et al. AAPM Task Group Report 302: Surface-Guided Radiotherapy. Med. Phys. 2022, 49, e82–e112. [Google Scholar] [CrossRef]
- Rudat, V.; Shi, Y.; Zhao, R.; Xu, S.; Yu, W. Setup accuracy and margins for surface-guided radiotherapy (SGRT) of head, thorax, abdomen, and pelvic target volumes. Sci. Rep. 2023, 13, 17018. [Google Scholar] [CrossRef]
- Psarras, M.; Stasinou, D.; Stroubinis, T.; Protopapa, M.; Zygogianni, A.; Kouloulias, V.; Platoni, K. Surface-Guided Radiotherapy: Can We Move on from the Era of Three-Point Markers to the New Era of Thousands of Points? Bioengineering 2023, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Cellini, F.; Riddell, A.; Brunner, T.B.; Roeder, F.; Giuliante, F.; Alfieri, S.; Manfredi, R.; Ardito, F.; Fiorillo, C.; et al. ESTRO ACROP Guidelines for the Delineation of Lymph Nodal Areas in Upper Gastrointestinal Malignancies. Radiother. Oncol. 2021, 164, 92–97. [Google Scholar] [CrossRef]
- Mir, R.; Kelly, S.M.; Xiao, Y.; Moore, A.; Clark, C.H.; Clementel, E.; Corning, C.; Ebert, M.; Hoskin, P.; Hurkmans, C.W.; et al. Organ at Risk Delineation for Radiation Therapy Clinical Trials: Global Harmonization Group Consensus Guidelines. Radiother. Oncol. 2020, 150, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Brunner, T.B.; Haustermans, K.; Huguet, F.; Morganti, A.G.; Mukherjee, S.; Belka, C.; Krempien, R.; Hawkins, M.A.; Valentini, V.; Roeder, F. ESTRO ACROP Guidelines for Target Volume Definition in Pancreatic Cancer. Radiother. Oncol. 2021, 154, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.E.; Hanley, J.; Bayouth, J.; Yin, F.F.; Simon, W.; Dresser, S.; Serago, C.; Aguirre, F.; Ma, L.; Arjomandy, B.; et al. Task Group 142 Report: Quality Assurance of Medical Accelerators. Med. Phys. 2009, 36, 4197–4212. [Google Scholar] [CrossRef]
- Li, G. Advances and Potential of Optical Surface Imaging in Radiotherapy. Phys. Med. Biol. 2022, 67, 16TR02. [Google Scholar] [CrossRef]
- Paxton, A.B.; Manger, R.P.; Pawlicki, T.; Kim, G.Y. Evaluation of a Surface Imaging System’s Isocenter Calibration Methods. J. Appl. Clin. Med. Phys. 2017, 18, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, G.; Baccaglione, G.; Clauser, T.; Scaini, R.; Grassi, R.; Testarelli, L.; Reda, R.; Testori, T.; Del Fabbro, M. Total Face Approach (TFA) 3D Cephalometry and Superimposition in Orthognathic Surgery: Evaluation of the Vertical Dimensions in a Consecutive Series. Methods Protoc. 2021, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Bonifazi, L.; Karaban, M.; Berti, C.; Pellegrino, G.; Barausse, C. Dynamic Navigated “Sandwich” Technique: A Novel Surgical Approach for Safe Osteotomies in the Rehabilitation of an Atrophic Posterior Mandible: A Case Report. Methods Protoc. 2021, 4, 34. [Google Scholar] [CrossRef]
- Ferrari, C.H.; Steffany de Carvalho, L.; Rocha, C.T.; Abu Hasna, A. Correlation between Tooth Position Parameters and Apical Fenestration: A Cone-Beam Computed Tomography Study. Methods Protoc. 2024, 7, 14. [Google Scholar] [CrossRef]
- Patil, D.J.; More, C.B.; Venkatesh, R.; Shah, P. Insight into the Awareness of CBCT as an Imaging Modality in the Diagnosis and Management of ENT Disorders: A Cross-Sectional Study. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, S5283–S5293. [Google Scholar] [CrossRef]
- Baccher, S.; Gowdar, I.M.; Guruprasad, Y.; Solanki, R.N.; Medhi, R.; Shah, M.J.; Mehta, D.N. CBCT: A Comprehensive Overview of Its Applications and Clinical Significance in Dentistry. J. Pharm. Bioallied Sci. 2024, 16, S1923–S1925. [Google Scholar] [CrossRef]
- Srinivasan, K.; Mohammadi, M.; Shepherd, J. Applications of Linac-Mounted Kilovoltage Cone-Beam Computed Tomography in Modern Radiation Therapy: A Review. Pol. J. Radiol. 2014, 79, 181–193. [Google Scholar]
- Walter, F.; Freislederer, P.; Belka, C.; Heinz, C.; Söhn, M.; Roeder, F. Evaluation of daily patient positioning for radiotherapy with a commercial 3D surface-imaging system (Catalyst™). Radiat. Oncol. 2016, 11, 154. [Google Scholar] [CrossRef]
- Carl, G.; Reitz, D.; Schönecker, S.; Pazos, M.; Freislederer, P.; Reiner, M.; Alongi, F.; Niyazi, M.; Ganswindt, U.; Belka, C.; et al. Optical surface scanning for patient positioning in radiation therapy: A prospective analysis of 1902 fractions. Technol. Cancer Res. Treat. 2018, 17, 1533033818806002. [Google Scholar] [CrossRef]
- Stanley, D.N.; McConnell, K.A.; Kirby, N.; Gutiérrez, A.N.; Papanikolaou, N.; Rasmussen, K. Comparison of initial patient setup accuracy between surface imaging and three-point localization: A retrospective analysis. J. Appl. Clin. Med. Phys. 2017, 18, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Flores-Martinez, E.; Cerviño, L.I.; Pawlicki, T.; Kim, G.Y. Assessment of the Use of Different Imaging and Delivery Techniques for Cranial Treatments on the Halcyon Linac. J. Appl. Clin. Med. Phys. 2020, 21, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ioannides, P.J.; Sehgal, V.; Daroui, P. Quantifying the Impact of Optical Surface Guidance in the Treatment of Cancers of the Head and Neck. J. Appl. Clin. Med. Phys. 2020, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- 3Qubala, A.; Schwahofer, A.; Jersemann, S.; Eskandarian, S.; Harrabi, S.; Naumann, P.; Winter, M.; Ellerbrock, M.; Shafee, J.; Abtehi, S.; et al. Optimizing the Patient Positioning Workflow of Patients with Pelvis, Limb, and Chest/Spine Tumors at an Ion-Beam Gantry Based on Optical Surface Guidance. Adv. Radiat. Oncol. 2023, 8, 101105. [Google Scholar] [CrossRef] [PubMed]
| Disease Category | Subtype | Group A (n = 19) | Group B (n = 24) |
|---|---|---|---|
| Hematologic Malignancies | Non-Hodgkin Lymphoma (NHL) | 6 | 9 |
| Hodgkin Disease/Splenomegaly | 2 | 1 | |
| Para-aortic Metastases | 4 | 0 | |
| Upper GI Cancers | Gastric Cancer | 3 | 9 |
| Pancreatic Adenocarcinoma | 1 | 2 | |
| Esophagogastric Junction (EGJ) | 1 | 0 | |
| Sarcomas | Intra-abdominal/Retroperitoneal | 2 | 2 |
| Setup Technique | Displacement | Median (cm) | Range (cm) |
|---|---|---|---|
| Group A | Svrt | 0.4 | 0.0–2.4 |
| Slat | 0.2 | 0.0–1.8 | |
| Slng | 0.4 | 0.0–2.7 | |
| Stot | 0.8 | 0.1–3.2 | |
| Group B | Svrt | 0.4 | 0.0–1.9 |
| Slat | 0.2 | 0.0–1.4 | |
| Slng | 0.4 | 0.0–1.6 | |
| Stot | 0.7 | 0.1–2.1 |
| Setup Technique | Displacement | IQR (Q1, Q3) (cm) | Q1 (cm) | Q3 (cm) |
|---|---|---|---|---|
| Group A | Svrt | 0.6 | 0.2 | 0.8 |
| Slat | 0.5 | 0.1 | 0.6 | |
| Slng | 0.5 | 0.2 | 0.7 | |
| Stot | 0.7 | 0.5 | 1.2 | |
| Group B | Svrt | 0.4 | 0.2 | 0.6 |
| Slat | 0.3 | 0.1 | 0.4 | |
| Slng | 0.5 | 0.1 | 0.6 | |
| Stot | 0.5 | 0.5 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotiropoulou, V.; Kachris, S.; Mazonakis, M. Accuracy of Patient Setup Using Surface Guided Radiotherapy (SGRT) for Abdominal Malignancies. Methods Protoc. 2025, 8, 119. https://doi.org/10.3390/mps8050119
Sotiropoulou V, Kachris S, Mazonakis M. Accuracy of Patient Setup Using Surface Guided Radiotherapy (SGRT) for Abdominal Malignancies. Methods and Protocols. 2025; 8(5):119. https://doi.org/10.3390/mps8050119
Chicago/Turabian StyleSotiropoulou, Varvara, Stefanos Kachris, and Michalis Mazonakis. 2025. "Accuracy of Patient Setup Using Surface Guided Radiotherapy (SGRT) for Abdominal Malignancies" Methods and Protocols 8, no. 5: 119. https://doi.org/10.3390/mps8050119
APA StyleSotiropoulou, V., Kachris, S., & Mazonakis, M. (2025). Accuracy of Patient Setup Using Surface Guided Radiotherapy (SGRT) for Abdominal Malignancies. Methods and Protocols, 8(5), 119. https://doi.org/10.3390/mps8050119





