Rapid, Refined, and Robust Method for Expression, Purification, and Characterization of Recombinant Human Amyloid beta 1-42
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- pET-Sac-Abeta(M1-42) plasmid (Addgene, Watertown, MA, USA; Cat. no.: 71875)
- Tryptone (Fisher Scientific, Waltham, MA, USA; Cat. no.: BP1421-500)
- Yeast Extract (Fisher Scientific, Waltham, MA, USA; Cat. no.: BP9727-500)
- LB Agar (Miller, Granulated) (Fisher Scientific, Waltham, MA, USA; Cat. no.: BP9724-500)
- Ampicillin (VWR International, Radnor, PA, USA, Cat. no.: 80055-786)
- Chloramphenicol (Fisher Scientific, Waltham, MA, USA; Cat. no.: AAJ67273AB)
- Plasmid Miniprep System (Promega, Madison, WI, USA; Cat. no.: A12222)
- Rosetta™(DE3)pLysS Competent Cells (MilliporeSigma, Burlington, MA, USA; Cat. no.: 70956-3)
- Isopropyl β-D-1- thiogalactopyranoside (IPTG) (Fisher Scientific, Waltham, MA, USA; Cat. no.: 15-529-019)
- Tris hydrochloride (Tris-HCl) (Fisher Scientific, Waltham, MA, USA; Cat. no.: BP153-500)
- Urea (Invitrogen, Carlsbad, CA, USA; Cat. no.: 15505-035)
- 0.22 μm non-sterile hydrophilic PVDF syringe filter (Fisher Scientific, Waltham, MA, USA; Cat. no.: 09-719-000)
- HPLC grade Acetonitrile (Fisher Scientific, Waltham, MA, USA; Cat. no.: A998-4)
- Purified anti-β-Amyloid, 1-16 Antibody (BioLegend, San Diego, CA, USA; Cat. no.: 803001)
- HRP Goat anti-mouse IgG (minimal x-reactivity) Antibody (BioLegend, San Diego, CA, USA; Cat. no.: 405306)
- SuperSignal West Pico Chemiluminescent Substrate (Fisher Scientific, Waltham, MA, USA; Cat. no.: 34080)
- 1,1,1,3,3,3-Hexafluoroisopropyl alcohol (Chem-Impex International, Wood Dale, IL, USA; Cat. no.: 00080)
- Hamilton syringe with a Teflon plunger and a sharp needle [Hamilton Company, Reno, NV, USA; Part. no.: 81343]
- Mica sheet (Ted Pella, Redding, CA, USA; Cat. no.: 50)
- Aluminum coated silicon probes with resonant frequency ~300 kHz and 40 N/m force constant (Ted Pella, Redding, CA, USA; Cat. no.: TAP300AL-G-10)
- Phosphate-buffered saline (PBS), 10x at pH 7.4 (Alfa Aesar, Haverhill, MA, USA; Cat. no.: J62036-K7)
2.2. Equipment
- Benchtop incubator shaker (New Brunswick™ Excella® E24) (Eppendorf, Hamburg, Germany; Cat. no.: M1352-0000)
- Sonicator Ultrasonic Homogenizer (125W) with 1/4" Probe (Qsonica, Newton, CT, USA; Cat. no.: Q700-110 and 4435)
- CO2 Incubator (New Brunswick™ Galaxy® 48S) (Eppendorf, Hamburg, Germany; Cat. no.: CO48S-120-0000)
- Centrifuge (Sorvall LYNX 6000) with a Swinging-Bucket Rotor (BIOFlex™ HC) (Thermo Fisher Scientific, Waltham, MA USA; Cat. no.: 75006591 and 75003000)
- UV-Visible Spectrophotometer (JASCO, Easton, MD, USA; Cat. no.: V-730)
- Microplate reader for nucleic acid quantification (Take3™ Micro-Volume plate) with Gen5 Software (BioTek, Winooski, VT, USA; Cat. no.: TAKE3)
- Combiflash EZ prep UV/ELSD (Teledyne ISCO, Lincoln, NE, USA; Cat. no.: 218J00936)
- RediSep Prep 10 × 250 mm C18 100A, 5 µm column (Teledyne ISCO, Lincoln, NE, USA; Cat. no.: 692203809)
- RediSep Prep Guard 20 × 30 mm, C18Aq, 100A, 5 µm (Teledyne ISCO, Lincoln, NE, USA; Cat. no.: 692203805)
- GenPure UV/UF × CAD plus Ultrapure Water Purification System (Thermo Fisher Scientific, Waltham, MA USA; Cat. no.: 41956240)
- Rotary evaporator and water bath (EYELA, Keyland Court Bohemia, NY, USA; Cat. no.: N-1110 and SB-1200)
- Dry Bath with heating block (Thermo Fisher Scientific, Waltham, MA USA; Cat. no.: 88870002)
- Labconco Freezone 12 Liter Console Freeze Dry System (Lyophilizer) (Labconco, Kansas City, MO, USA; Cat. no.: 710612000)
- Voyager-DE PRO (MALDI-TOF mass spectrometer) (Applied Biosystems, Foster City, CA, USA;)
- For AFM: Veeco Multimode instrument with NanoScope V controller
3. Procedure
3.1. Preparation of Solutions
3.2. Expression of Aβ(M1-42) Peptide. Time for Completion: 55:30 h
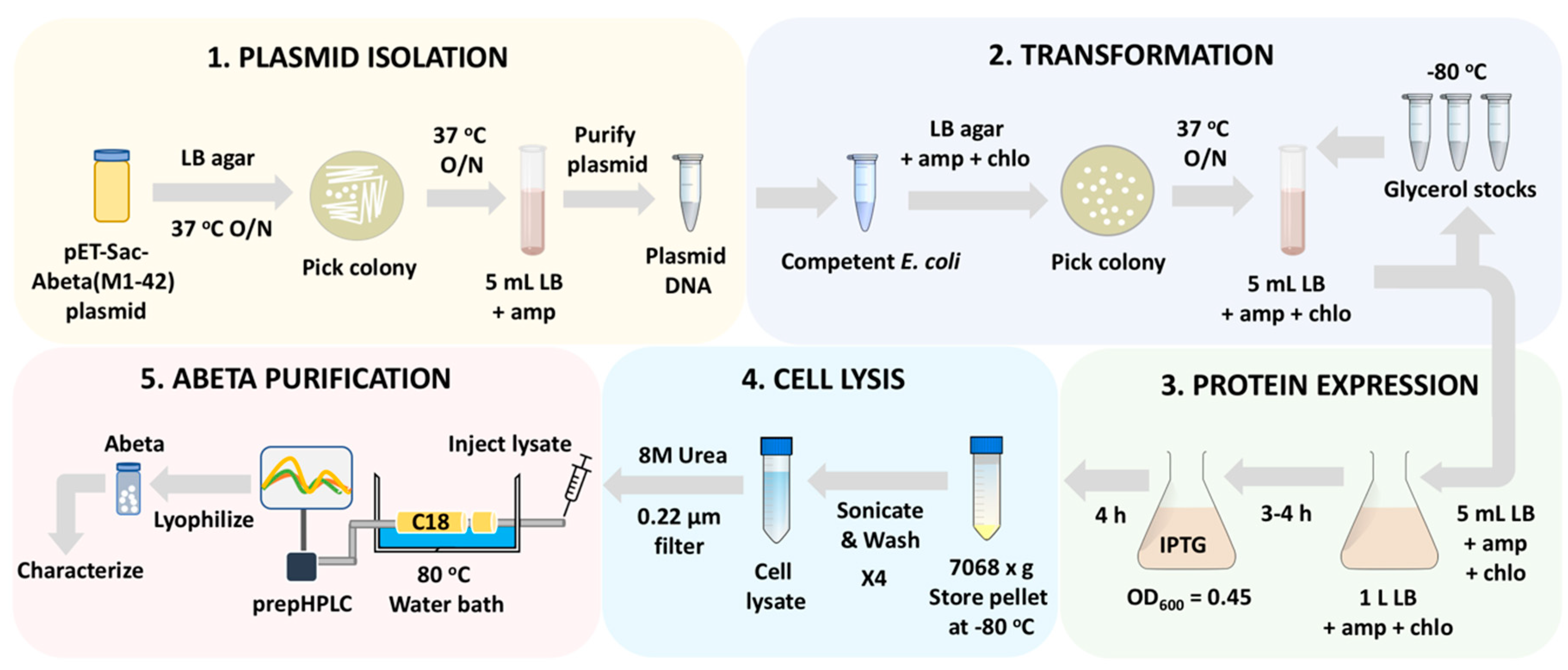
3.2.1. Isolation of the pET-Sac-Aβ(M1-42) Plasmid. Time for Completion: 31:00 h
- Streak the bacteria onto a solid LB agar plate containing 100 mg/L ampicillin using a sterile loop.Note: The pET-Sac-Aβ(M1-42) plasmid arrives as a bacterial agar stab culture.
- Keep the plate overnight for ~ 16 h in the incubator at 37 °C for the colonies to grow.
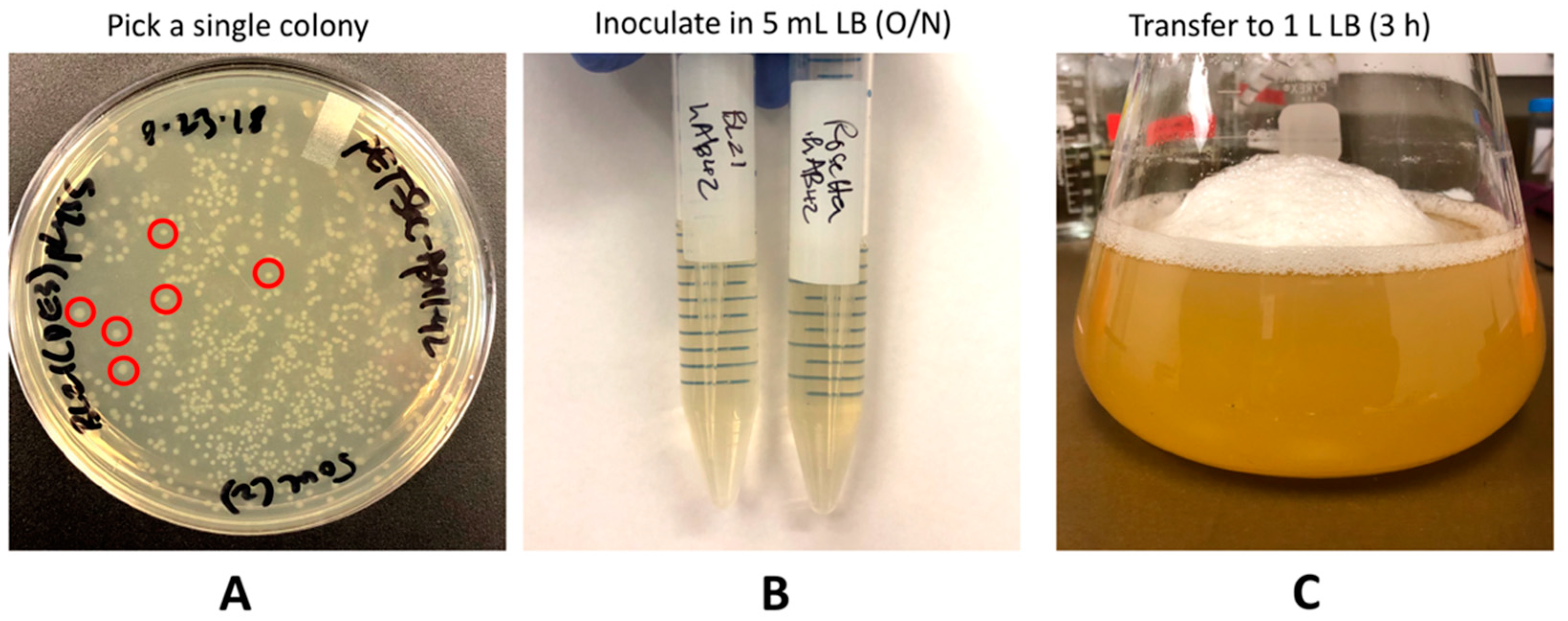
- The next day, pick a single colony from the plate (Figure 2A) using a sterile loop or a sterile 10 μL pipet tip and inoculate into 5 mL of liquid LB containing 100 mg/L ampicillin.
- Keep the culture in a shaking incubator at 220 rpm and 37 °C overnight for ~ 16 h.
- The next day, isolate the plasmid (Figure 1, Part 1) from the culture using the plasmid isolation miniprep kit following instructions per the user manual.
- Measure the concentration of the plasmid at 260 nm absorbance using a spectrophotometer for nucleic acid quantification. Note: Typical plasmid yield is around 45-55 ng/µL from 5 mL liquid LB culture.
3.2.2. Transformation of pET-Sac-Aβ(M1-42) Plasmid into Competent E. Coli by Heat Shock Method. Time for Completion: 01:30 h
- 1.
- To transform the Aβ(M1-42) plasmid into E. coli (Figure 1, Part 2), thaw the frozen vials of Rosetta(DE3)pLysS cells or BL21(DE3)pLysS competent cells (20–50 μL) on ice.
- 2.
- Once thawed, add around 1–2 μL of the isolated plasmid (to have 50–100 ng total DNA) to the cells and gently flick the tube a few times to mix the plasmid with the cells.
- 3.
- Incubate the cells and the plasmid mixture on ice for 20 min and then place the tube in a 42 °C water bath for 45 s to facilitate the transformation of plasmid into the cells via heat shock method.
- 4.
- After heat shock, immediately place the tubes on ice for 2 mins.Inoculate the transformed bacteria into 500 μL of liquid LB media without any antibiotics and keep the tube in the 37 °C shaker for 1 h at 220 rpm.
-
- CRITICAL STEP: The transformed bacteria is first inoculated in liquid LB media without any antibiotics for 1 h to allow the bacteria to express the antibiotic resistance proteins necessary for future steps.
3.2.3. Expression of Aβ(M1-42) Peptide by the Transformed E. Coli. Time for Completion: 23:00 h
- 1.
- After 1 h, spread around 25–30 μL (out of 500 μL) of the transformed cells onto solid LB agar plates containing 100 mg/L ampicillin and 34 mg/L chloramphenicol using a sterile glass spreader.
- 2.
- Let the plates sit in the biosafety cabinet for 5 min to allow the cells to absorb on the solid LB.
- 3.
- Next, keep the plates overnight in the incubator at 37 °C for the transformed colonies to grow.
-
- CRITICAL STEP: It is best to use a lower volume of the transformed cells (25–30 μL) as this results in more single colonies that are easier to pick and prevents overcrowding on the plate.
- 4.
- The next day, pick a single colony from the plate using a sterile loop or a sterile 10 μL pipet tip and inoculate into 5 mL of liquid LB containing 100 mg/L ampicillin and 34 mg/L chloramphenicol (first culture).
- 5.
- OPTIONAL STEP: At the same time, pick another single colony of the transformed bacteria and inoculate into a second 5 mL liquid LB media containing 100 mg/L ampicillin and 34 mg/L chloramphenicol for overnight growth (second culture). This culture will be used to make frozen glycerol stocks for future use.
- 6.
- Place both the cultures in the shaking incubator at 220 rpm at 37 °C overnight (16 h) (Figure 2B).
- 7.
- The following day, inoculate the first 5 mL culture into a large 2 L Erlenmeyer flask containing 1 L liquid LB media with 100 mg/L ampicillin and 34 mg/L chloramphenicol (Figure 2C).
- 8.
- Keep this 1 L culture at 220 rpm and 37 °C until the cell density reaches an optical density (OD) value between 0.40 to 0.45 at 600 nm (OD600).
-
- CRITICAL STEP: The BL21(DE3)pLysS cells reach an OD600 between 0.40 to 0.45 in 3 h time, whereas the Rosetta(DE3)pLysS cells requires around 3.5–4.0 h to reach an OD600 between 0.40 to 0.45. It is best to measure the OD of the culture at regular 20 min intervals starting from the 3-h time point before proceeding to the next step.
- 9.
- OPTIONAL STEP: The second 5 mL culture of transformed bacteria is used to make 25% glycerol stocks by adding 500 μL of 50% glycerol to 500 μL bacterial culture and is frozen at −80 °C for future use. A −80 °C frozen glycerol stock of the transformed bacteria is thawed for use in the future for inoculating 5 mL liquid LB containing 100 mg/L ampicillin and 34 mg/L chloramphenicol to grow additional cultures of the transformed bacteria containing the Aβ(M1-42) plasmid. Note: Frozen aliquots of 250–500 µL glycerol stocks may be stored for future use. It is recommended that the stored aliquots of glycerol stock be thawed 1–2 times only. Additionally, some of the frozen stock may be scrapped and thawed for inoculation.
-
- CRITICAL STEP: This step serves as a starting point for all future experiments performed for the isolation of the Aβ(M1-42) peptide (Figure 1, Part 3). It is important to note that this step is critical for reducing the time taken for the entire protocol along with saving the reagents used for peptide expression.
- 10.
- Once the OD600 of the 1 L culture reaches 0.40 to 0.45, induce protein expression by adding isopropyl β-D-1- thiogalactopyranoside (IPTG) to obtain a final concentration of 0.1 mM in 1 L of the liquid LB media. Note: Add 1 mL of 0.1 M IPTG stock solution prepared in water to obtain a final concentration of 0.1 mM in 1 L LB media.Note: IPTG induction must occur early in the exponential growth phase to form insoluble inclusion bodies, which are essential for the isolation procedure.
- 11.
- Keep the culture again on the shaking incubator at 220 rpm and 37 °C for additional an 4 h in the presence of IPTG to allow the cells to express the Aβ(M1-42) peptide.
- 12.
- After 4 h, centrifuge the 1 L culture at 7068× g at 4 °C for 25 min.Note: The cultures are centrifuged at 4 °C temperature to arrest cell growth and metabolism.Note: Our centrifuge allowed for a maximum speed of 7068× g with the corresponding swinging bucket rotor (see Section 2.2. Equipment). Hence, this speed was used to collect the transformed cells from the 1 L cultures. The cells may also be pelleted by centrifuging the cultures at 2800× g if using a JA-10 rotor [14].
- 13.
- Discard the supernatant liquid LB and resuspend the pelleted cells in 25 mL of 1× PBS and transfer the thick cell suspension to a 50 mL falcon tube using a 10 mL pipet.
- 14.
- Centrifuge the cells at 7068× g at 4 °C for 25 min and discard the 1× PBS supernatant.
- 15.
PAUSE STEP: Store the pelleted cells at −80 °C until the next day or when ready for cell lysis.
3.3. Aβ(M1-42) Peptide Purification Using Reverse-Phase HPLC. Time for Completion: 05:40 h
3.3.1. Cell Lysis and Resuspension. Time for Completion: 02:24 h
- 1.
- To lyse the cells (Figure 1, Part 4), resuspend the cell pellet in 25 mL Buffer A. Cut the tip off a 1 mL pipette tip to efficiently dissociate the thick pellet in the buffer.
- 2.
- Disrupt the cell pellet mechanically by mixing the cells with Buffer A. Place the tube in an ice bucket containing ice and water and introduce the sonicator probe into the cell mixture. Note: Ensure that the cell mixture remains cold throughout the sonication.
- 3.
- Sonicate the cells at 30 s pulse with an amplitude of 60% for 2 min until the lysate appears homogenous. Note: Four 30 s on/off cycles for a total of 4 min.
-
- CRITICAL STEP: To increase the lysis efficiency, sonicate the cell pellet in the original 50 mL frozen falcon tube since transferring the cell mixture to containers with a large surface area reduces the lysis efficiency.
- 4.
- Centrifuge the sonicated mixture at 7068× g for 25 min at 4 °C and discard the supernatant.Note: The sonicated mixture was centrifuged at 7068× g based on the maximum speed allowed on our centrifuge and swinging bucket rotor (see Section 2.2 Equipment). This speed was sufficient to collect the pellet from the cell lysis at the bottom of the tube. The sonicated mixture may also be centrifuged at higher speeds of up to 38,000× g if using the JA-18 rotor to collect the pellet [14].
- 5.
- Repeat the sonication and centrifugation steps (steps 1 to 4) three more times.
- 6.
- Resuspend the pellet in 20 mL of freshly prepared Buffer B and sonicate as above until the solution appears clear.
-
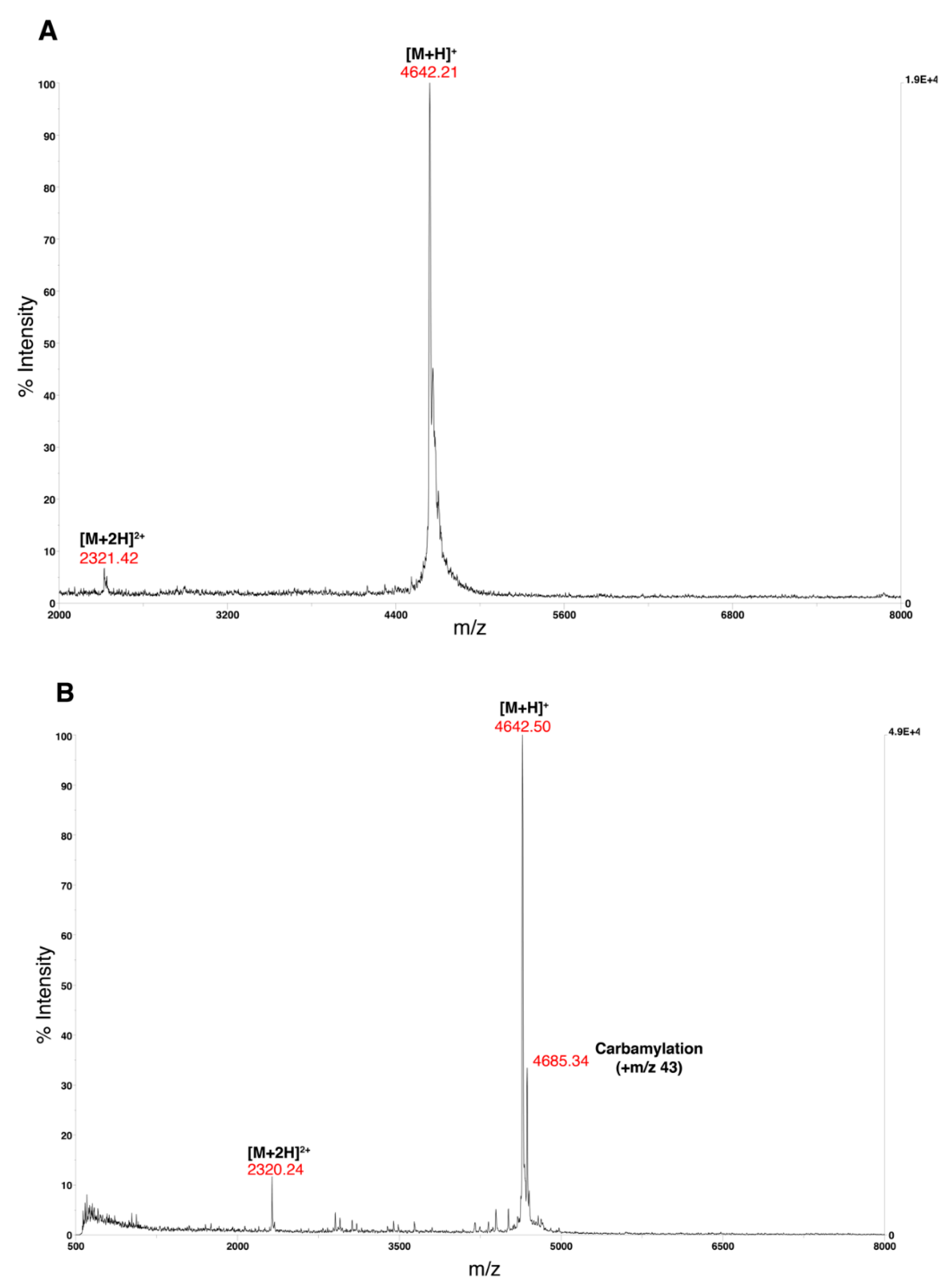
- CRITICAL STEP: Purification should be performed immediately after the peptide is dissolved in 8 M urea solution (Buffer B). Extended exposure of the peptide to urea is known to cause carbamylation of lysine residues.
-
- CRITICAL STEP: Due to the inconsistencies of mechanical lysis using the sonicator, the solution may appear to be cloudy. Note: Prepare fresh Buffer B every time. The pH of the buffers is critical for the complete dissolution of the peptide fraction.
- 7.
- Centrifuge the solution at 7068× g for 25 min at 4 °C to remove any foam that may have appeared during the sonication step and to pellet unwanted insoluble cell debris, if any.Finally, filter the supernatant through a 0.22 μm non-sterile hydrophilic PVDF syringe filter using a 30 mL syringe to obtain a clear solution.
- 8.
- OPTIONAL STEP: Prior to purification, MALDI-TOF MS may be performed on the urea-solubilized recombinant peptide to confirm the presence of Aβ(M1-42) in the solution (data not shown). Since the solution contains additional salts, the solution must be passed through a C18 Zip Tip resin to remove the salts in order to obtain a clear spectrum.
3.3.2. Peptide Purification Using Reverse-Phase HPLC. Time for Completion: 03:16 h
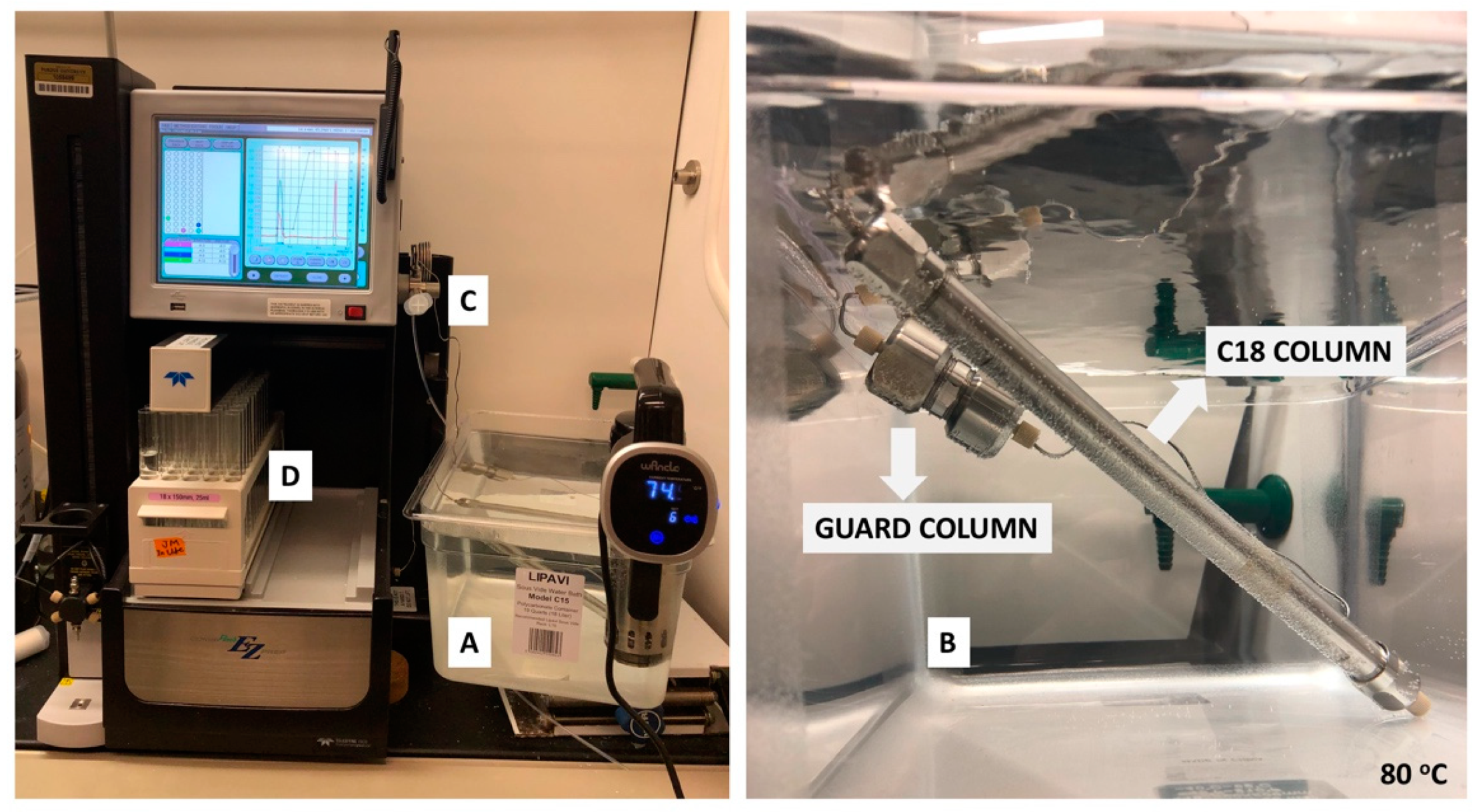
- Place a rack of clean and dry 18 × 150 mm glass test tubes (Figure 3D) in the instrument.
- Run the solvent gradient as per the cleaning protocol in Table 1 at a flow rate of 5 mL/min.
- Equilibrate the column with the starting solvent system in Table 2 at a flow rate of 5 mL/min. And inject 4 mL of the filtered solution obtained from Section 3.2.1 step 8 into the HPLC injection loop for separation (Figure 3C).
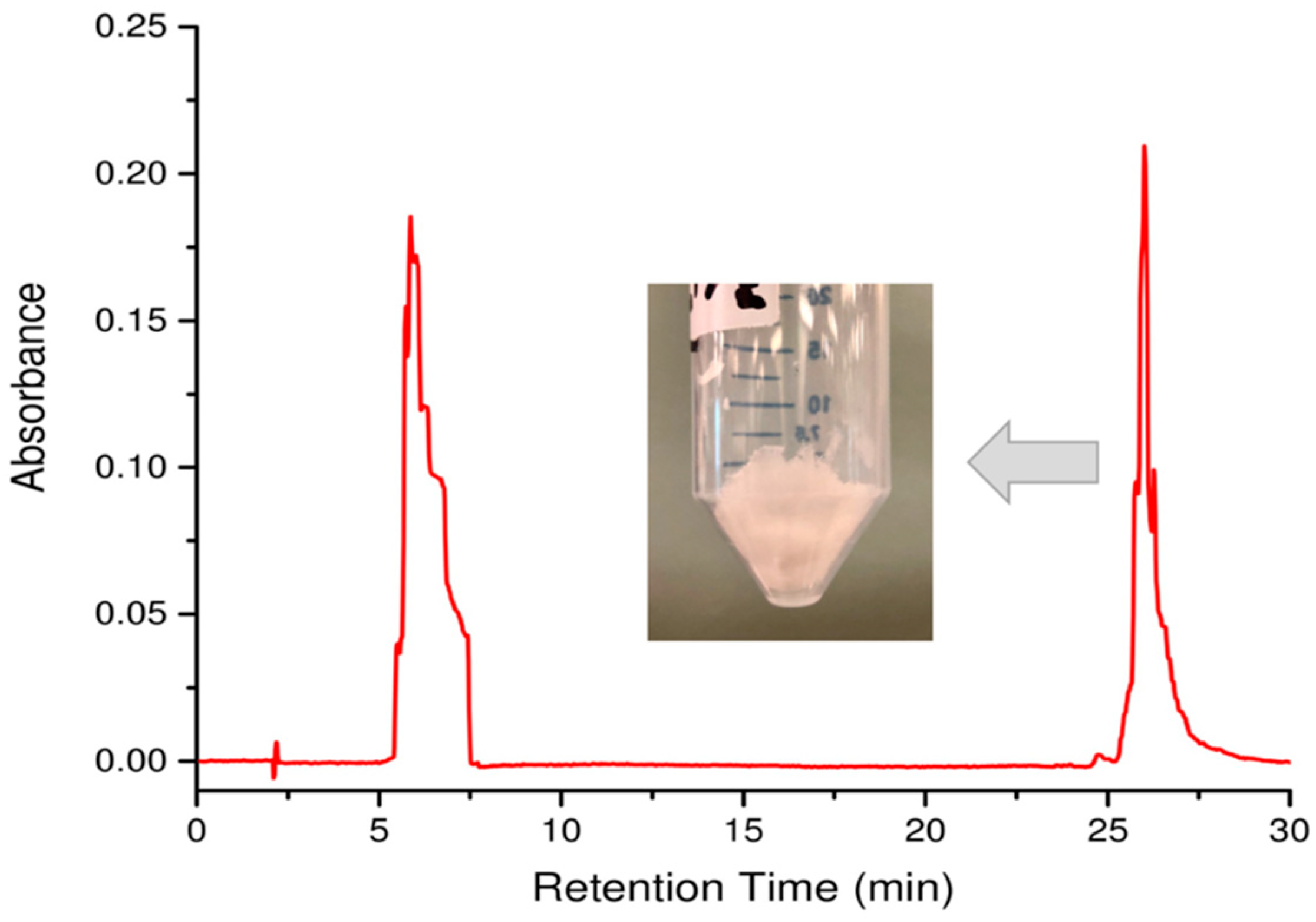
- Run the solvent gradient described in Table 2 and collect peaks detected at 214 nm. Note: Aβ(M1-42) typically elutes at 26 min.
- Upon completion of the purification protocol, clean the column with the solvent gradient described in Table 1.
- Repeat the cleaning and purification steps three more times or until all the solution is used.
- Combine the collected Aβ(M1-42) fractions eluted at 26 min (Figure 4).
- Evaporate off the acetonitrile under reduced pressure at 65 °C using a rotary evaporator until a cloudy aqueous solution remains.
- Freeze the solution at −80 °C and then submerge in liquid nitrogen for 5 min.
- Perform overnight lyophilization at −90 °C at 0.003 mbar pressure to obtain the white Aβ(M1-42) powder (Figure 4).
PAUSE STEP Store the lyophilized peptide at −80 °C until further characterization.
3.4. Characterization of Aβ(M1-42)
3.4.1. Characterization of Aβ(M1-42) Using MALDI-TOF MS and High-Resolution LC-MS. Time for Completion: 00:45 h
- Dissolve a small quantity of Aβ(M1-42) in water and dilute in water until the sample is approximately 100 μg/mL.
- Thoroughly mix 1 μL of the Aβ(M1-42) solution (analyte) with 1 μL of α-cyano-4-hydroxycinnamic acid (CHCA) matrix solution (10 mg/mL CHCA in 0.1% TFA).
- Spot the analyte/matrix mixture on a MALDI target plate and allow to dry.
- Obtain the MALDI-TOF mass spectra on a Voyager-DE PRO from 1000–22000 Da in the positive ion mode with an accelerating voltage of 25,000 V (Figure 5).
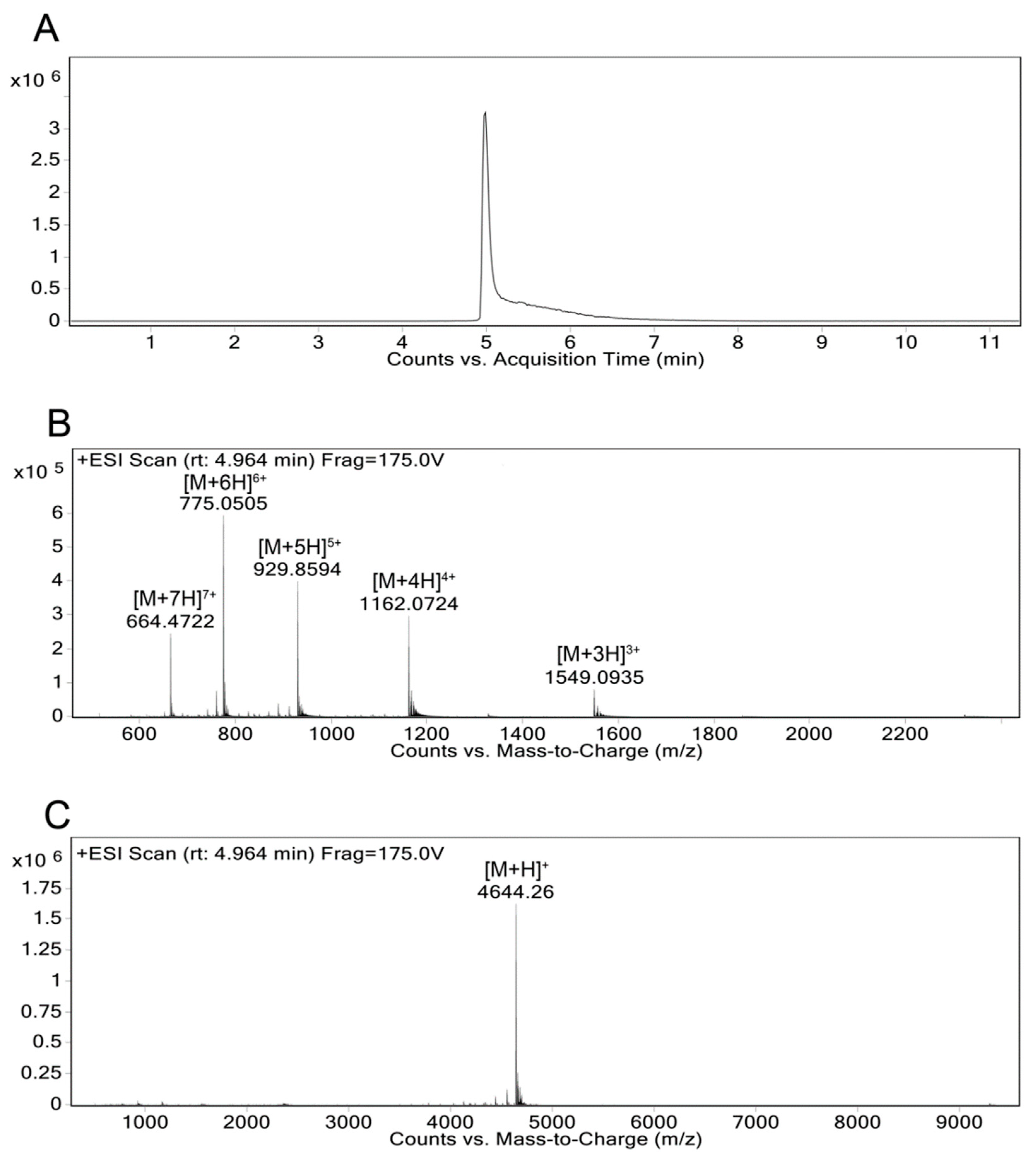
- Further dilute the analyte solution prepared for the MALDI-TOF MS and inject into the Agilent 6550 iFunnel Q-TOF LC-MS in positive mode and fragment using electrospray ionization (ESI) with a fragmentor voltage of 175V (Figure 6).
3.4.2. Western Blot Characterization of the Purified Aβ(M1-42) Peptide. Time for Completion: Two Days
- When the peptide is dried overnight in the chemical hood, dissolve 1 mg of the peptide in 221 μL DMSO to obtain a final concentration of 1.0 mM.
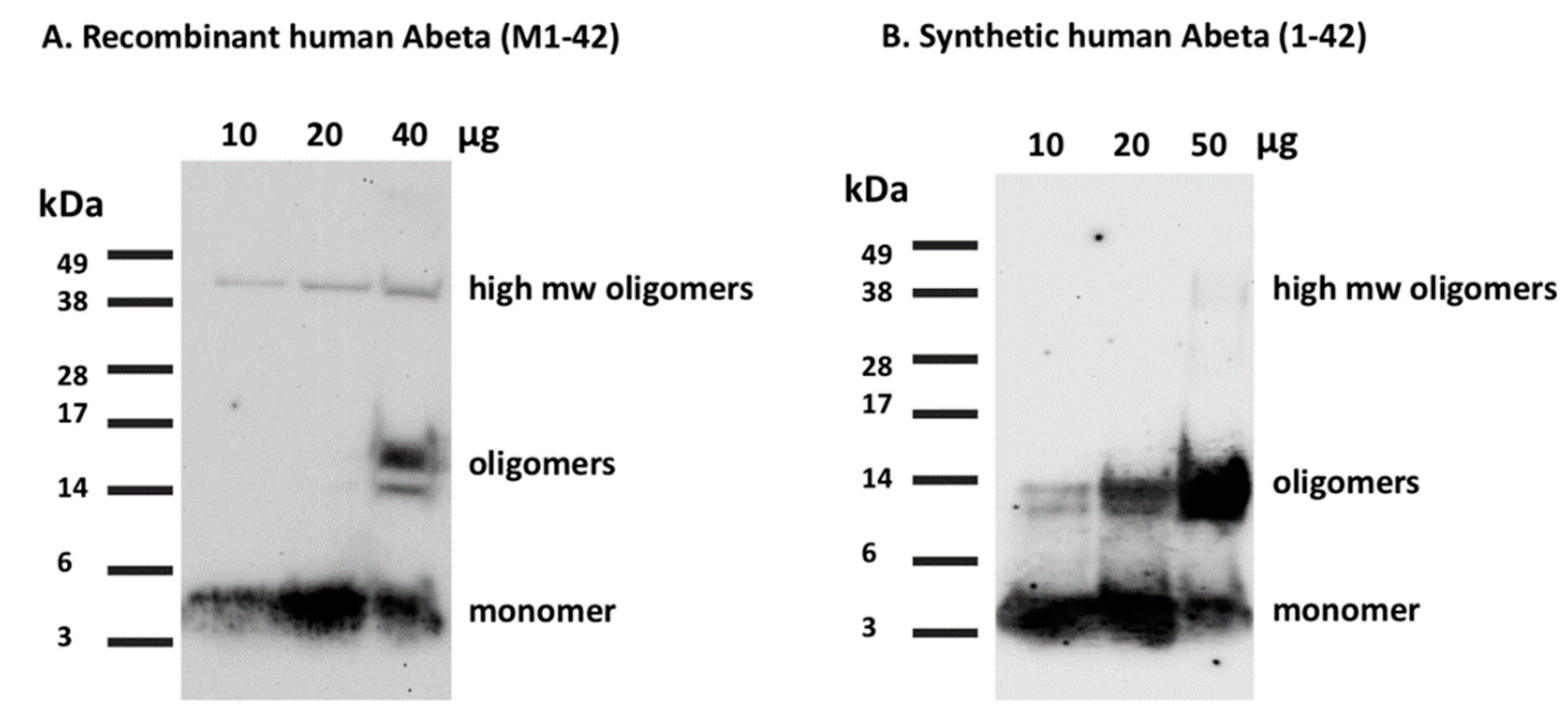
- Load the peptide on 12% Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) at different concentrations (10, 20, and 40 μg per 40 µL total well volume) and run the gel at 115 V for 1 h and 20 min or until the loading buffer line reaches the bottom of the gel.
- Transfer the gel to a nitrocellulose membrane at 10 V for 35 min.
- After transfer, boil the membrane in PBS for 5 min, then incubate on a rocker with blocking buffer (5% milk in Tris-buffered saline, 0.1% Tween 20 (TBST)) for 1 h.
- After blocking, incubate the membrane in the blocking buffer containing the 6E10 monoclonal antibody (with target specificity to the human Aβ peptide) overnight on a rocker at 4 °C.
- The next morning, wash the membrane 3 times for 10 min each with TBST and incubate with the secondary antibody (HRP-conjugated goat anti-mouse antibody) on the rocker for 1 h.
- Washed again three times for 10 min each with TBST and develop in the dark room using chemiluminescence reagents as per the manufacturer’s protocol.
- Run the synthetic Aβ(1-42) in the same gel with the same protocol for comparison (Figure 7).
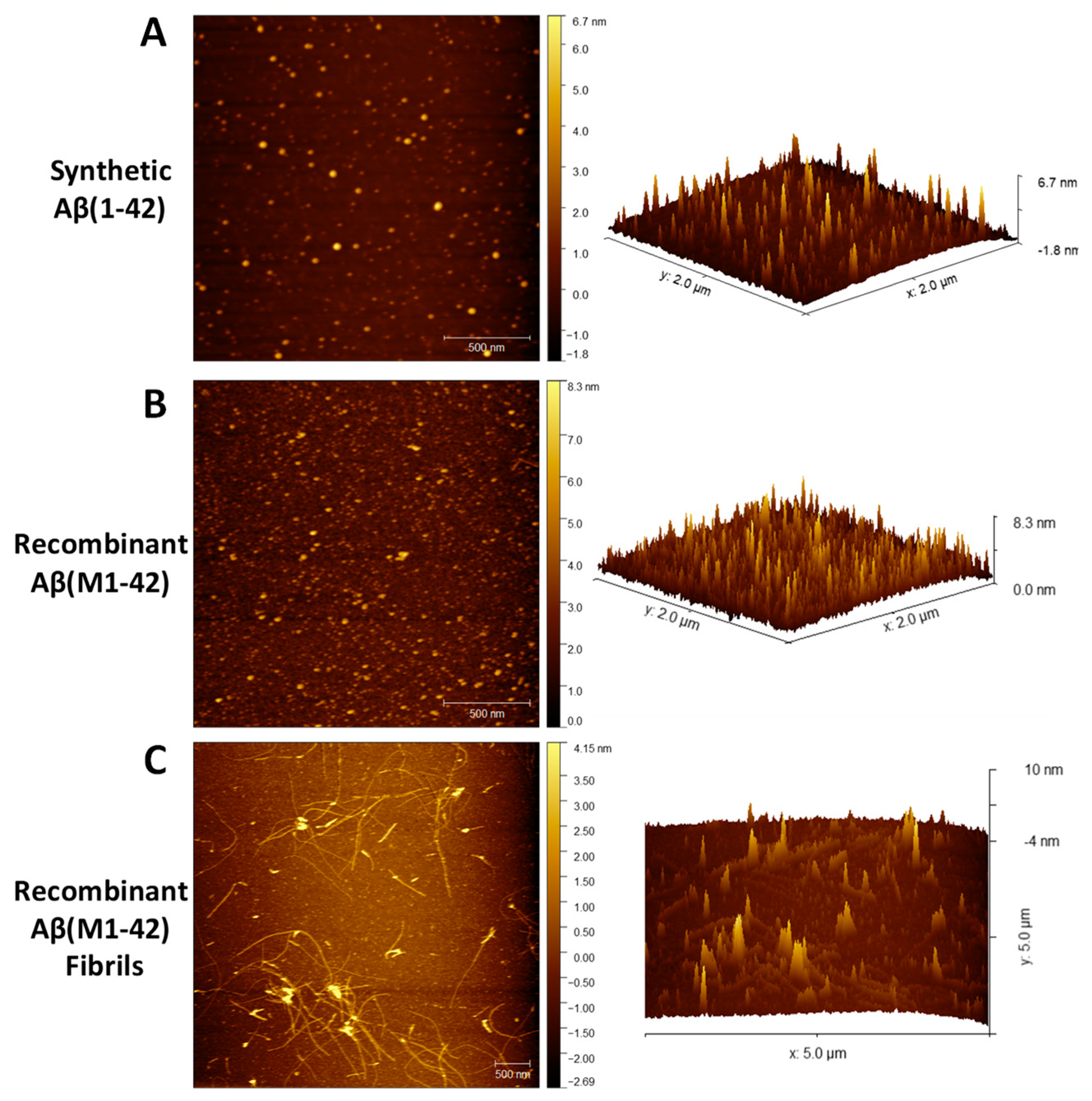
3.4.3. Characterization of Aβ(M1-42) Oligomers by Atomic Force Microscopy. Time for Completion: Two days
- 1.
- 2.
- In brief, allow the tubes containing monomeric peptide films to equilibrate at room temperature for a few minutes and prepare 5 mM of synthetic Aβ(1-42) and recombinant Aβ(M1-42) DMSO stocks by adding 20 μL of cell-grade DMSO to each tube containing ~0.45 mg of the peptide.
- 3.
- Pipet the solution thoroughly by scraping down the sides of the tube and vortexed for ~30 s followed by bath sonication for 10 min to ensure complete resuspension of the peptide film.
-
- CRITICAL STEP: This stock solution is used immediately as the starting material for oligomeric Aβ preparation.
- 4.
- To prepare Aβ oligomers, mix 2 μL of the freshly resuspended 5 mM of synthetic Aβ(1-42) and recombinant Aβ(M1-42) in DMSO with 98 μL of 1× PBS (filtered) to make 100 μM solution. To prepare Aβ fibrils, mix 98 μL of 10 mM HCl (filtered) to 2 μL of freshly resuspended 5 mM of recombinant Aβ(M1-42) in DMSO.
- 5.
- Vortex the solution thoroughly for 15 s and incubate at 4 °C (for oligomers) and at 37 °C (for fibrils) for 24 h.
- 6.
- After 24 h, prepare the samples for AFM with proper sterile technique in the hood as follows: Dilute the 100 μM samples to a concentration of 30 μM in filtered water.
- 7.
- Mount the mica sheet on 15 mm stainless steel pucks.
- 8.
- Immediately before sample plating, remove a few layers of the mica sheet using adhesive tape to reveal a featureless surface for the absorption of the peptide.
- 9.
- Next, pretreat the mica surface with ~5–8 μL of filtered 1M HCl for 30 s and rinse with 2–3 drops of ultrapure water (filtered) using the 1 mL syringe.
- 10.
CRITICAL STEP: Hold the mica plate at a 45° angle to wash with drops of water. Immediately after cleaning, spot the peptide sample onto mica and incubate for 3 min.
- 11.
- Gently rinse the mica with 2–3 drops of water using the 1 mL syringe and dry with several gentle pulses of clean compressed air or nitrogen gas.
- 12.
- Incubate the samples at room temperature for a few hours until imaging.
- 13.
- Perform the AFM imaging using a multimode AFM equipped with aluminum-coated silicon probes with ~300 kHz resonant frequency and 40 N/m force constant under the tapping mode.
- 14.
- Finally, perform image analysis using the NanoScope Analysis software.
4. Expected Results and Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamley, I.W. The Amyloid Beta Peptide: A Chemist’s Perspective. Role in Alzheimer’s and Fibrillization. Chem. Rev. 2012, 112, 5147–5192. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, J.T.; Berger, E.P.; Lansbury, P.T. The carboxy terminus of the beta. amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer’s disease. Biochem. 1993, 32, 4693–4697. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Levine, H. Alzheimer’s Disease and the β-Amyloid Peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s Disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Doig, A.J.; Del Castillo-Frias, M.P.; Berthoumieu, O.; Tarus, B.; Nasica-Labouze, J.; Sterpone, F.; Nguyen, P.H.; Hooper, N.M.; Faller, P.; Derreumaux, P. Why Is Research on Amyloid-β Failing to Give New Drugs for Alzheimer’s Disease? ACS Chem. Neurosci. 2017, 8, 1435–1437. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Ritter, A.; Zhong, K. Alzheimer’s Disease Drug Development Pipeline: 2018. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018. [Google Scholar] [CrossRef]
- Zuroff, L.; Daley, D.; Black, K.L.; Koronyo-Hamaoui, M. Clearance of cerebral Aβ in Alzheimer’s disease: Reassessing the role of microglia and monocytes. Cell. Mol. Life Sci. 2017, 74, 2167–2201. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.R.; Li, Y.-M. The role of astrocytes in amyloid production and Alzheimer’s disease. Open Boil. 2017, 7, 170228. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arboledas, A.; Davila, J.C.; Sanchez-Mejias, E.; Navarro, V.; Nuñez-Diaz, C.; Sanchez-Varo, R.; Sanchez-Mico, M.V.; Trujillo-Estrada, L.; Fernandez-Valenzuela, J.J.; Vizuete, M.; et al. Phagocytic Clearance of Presynaptic Dystrophies by Reactive Astrocytes in Alzheimer’s Disease. Glia 2018. [Google Scholar] [CrossRef] [PubMed]
- Tickler, A.K.; Clippingdale, A.B.; Wade, J.D. Amyloid-β as a “Difficult Sequence” in Solid Phase Peptide Synthesis. Protein Pept. Lett. 2004. [Google Scholar] [CrossRef]
- Finder, V.H.; Vodopivec, I.; Nitsch, R.M.; Glockshuber, R. The Recombinant Amyloid-β Peptide Aβ1–42 Aggregates Faster and Is More Neurotoxic than Synthetic Aβ1–42. J. Mol. Boil. 2010, 396, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Thulin, E.; Minogue, A.M.; Gustavsson, N.; Pang, E.; Teplow, D.B.; Linse, S. A facile method for expression and purification of the Alzheimer’s disease-associated amyloid β-peptide. FEBS J. 2009, 276, 1266–1281. [Google Scholar] [CrossRef] [PubMed]
- Szczepankiewicz, O.; Linse, B.; Meisl, G.; Thulin, E.; Frohm, B.; Frigerio, C.S.; Colvin, M.T.; Jacavone, A.C.; Griffin, R.G.; Knowles, T.; et al. N-Terminal Extensions Retard Aβ42 Fibril Formation but Allow Cross-Seeding and Coaggregation with Aβ42. J. Am. Chem. Soc. 2015, 137, 14673–14685. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Zhang, S.; Kreutzer, A.G.; Nowick, J.S. An Efficient Method for the Expression and Purification of Aβ(M1-42). Biochemistry 2018. [Google Scholar] [CrossRef] [PubMed]
- Teplow, D.B. Preparation of Amyloid β-Protein for Structural and Functional Studies. Methods in Enzymology 2006, 413, 20–33. [Google Scholar]
- Stine, W.B.; Dahlgren, K.N.; Krafft, G.A.; LaDu, M.J. In Vitro Characterization of Conditions for Amyloid-β Peptide Oligomerization and Fibrillogenesis. J. Biol. Chem. 2003. [Google Scholar] [CrossRef] [PubMed]
- Stine, W.B.; Jungbauer, L.; Yu, C.; Ladu, M.J. Preparing Synthetic Aβ in Different Aggregation States. Methods Mol. Biol. 2011. [Google Scholar] [CrossRef]
- Chhetri, G.; Pandey, T.; Chinta, R.; Kumar, A.; Tripathi, T. An improved method for high-level soluble expression and purification of recombinant amyloid-beta peptide for in vitro studies. Protein Expr. Purif. 2015, 114, 71–76. [Google Scholar] [CrossRef]
- Lee, E.K.; Hwang, J.H.; Shin, D.Y.; Kim, D.I.; Yoo, Y.J. Production of recombinant amyloid-β peptide 42 as an ubiquitin extension. Protein Expr. Purif. 2005, 40, 183–189. [Google Scholar] [CrossRef]
- Hoarau, M.; Malbert, Y.; Irague, R.; Hureau, C.; Faller, P.; Gras, E.; André, I.; Remaud-Siméon, M. A Robust and Efficient Production and Purification Procedure of Recombinant Alzheimers Disease Methionine-Modified Amyloid-β Peptides. PLoS ONE 2016. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, J.Y.; Yang, W.; Zhang, H. Inhibition of Protein Carbamylation in Urea Solution Using Ammonium-Containing Buffers. Anal. Biochem. 2014. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Tokuda, T.; Kasai, T.; Ishigami, N.; Hidaka, H.; Kondo, M.; Allsop, D.; Nakagawa, M. High-molecular-weight β-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J. 2010, 24, 2716–2726. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.L.; Dupuis, N.F.; Lazo, N.D.; Wyttenbach, T.; Condron, M.M.; Bitan, G.; Teplow, D.B.; Shea, J.-E.; Ruotolo, B.T.; Robinson, C.V.; et al. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat. Chem. 2009, 1, 326–331. [Google Scholar] [CrossRef] [PubMed]
| % Solvent A 1 | % Solvent B 2 | Elapsed Time (min) |
|---|---|---|
| 90 | 10 | 0 |
| 90 | 10 | 5 |
| 10 | 90 | 10 |
| 10 | 90 | 20 |
| % Solvent A 1 | % Solvent B 2 | Elapsed Time (min) |
|---|---|---|
| 90 | 10 | 0 |
| 90 | 10 | 9 |
| 5 | 95 | 19 |
| 5 | 95 | 27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakash, P.; Lantz, T.C.; Jethava, K.P.; Chopra, G. Rapid, Refined, and Robust Method for Expression, Purification, and Characterization of Recombinant Human Amyloid beta 1-42. Methods Protoc. 2019, 2, 48. https://doi.org/10.3390/mps2020048
Prakash P, Lantz TC, Jethava KP, Chopra G. Rapid, Refined, and Robust Method for Expression, Purification, and Characterization of Recombinant Human Amyloid beta 1-42. Methods and Protocols. 2019; 2(2):48. https://doi.org/10.3390/mps2020048
Chicago/Turabian StylePrakash, Priya, Travis C. Lantz, Krupal P. Jethava, and Gaurav Chopra. 2019. "Rapid, Refined, and Robust Method for Expression, Purification, and Characterization of Recombinant Human Amyloid beta 1-42" Methods and Protocols 2, no. 2: 48. https://doi.org/10.3390/mps2020048
APA StylePrakash, P., Lantz, T. C., Jethava, K. P., & Chopra, G. (2019). Rapid, Refined, and Robust Method for Expression, Purification, and Characterization of Recombinant Human Amyloid beta 1-42. Methods and Protocols, 2(2), 48. https://doi.org/10.3390/mps2020048