Tobacco and Pituri Use in Pregnancy: A Protocol for Measuring Maternal and Perinatal Exposure and Outcomes in Central Australian Aboriginal Women
Abstract
:1. Introduction
1.1. Tobacco and Nicotine Pharmacodynamics and Pharmacokinetics
1.1.1. Nicotine Maternal, Placental, Fetal, and Breast Milk Distribution
1.1.2. Nicotine General Actions
1.1.3. Nicotine Metabolism and Excretion
1.1.4. Tobacco and Nicotine Biochemical Analysis
1.2. Tobacco Exposure and Adverse Pregnancy Outcomes
1.2.1. Maternal Smoked Tobacco Exposure and Adverse Pregnancy Outcomes
1.2.2. Maternal Smokeless Tobacco Exposure and Adverse Pregnancy Outcomes
1.2.3. Maternal Smoking and Australian Birth Outcomes
1.3. Chewing Tobacco Use in Central Australia
1.4. Study Aim and Research Questions
- What are the biochemical concentrations of tobacco, nicotine, and their metabolites in a range of maternal and neonatal biological samples from mothers with differing levels of self-reported tobacco use?
- Does maternal pituri chewing impact maternal and perinatal outcomes, and if so, what is the significance of that impact in comparison to smoking or non-tobacco use in pregnancy?
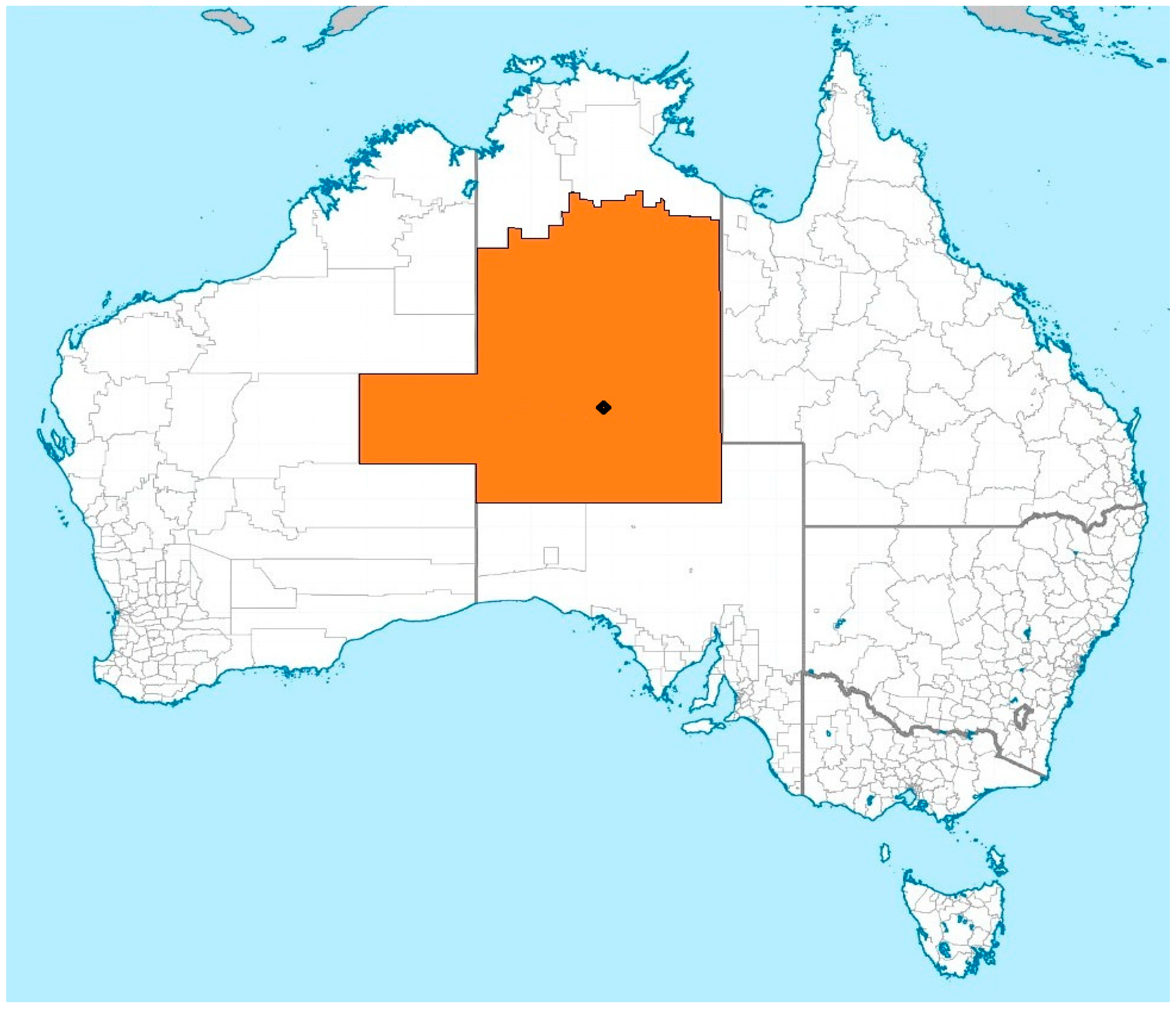
1.5. Research Setting and Considerations
2. Methods and Design
2.1. Study Design
Sampling, Recruitment, and Consent
3. Procedure
3.1. Data Collection
- (1)
- A maternal face-to-face interview was conducted at the time of enrolment and consisted of 23 semi-structured questions recording information not collected elsewhere, for example: Language group, skin name, employment and income, housing situation, community of origin and school leaving level, in addition to lifestyle factors including alcohol and tobacco use. The participants were clearly informed that the research was exploring the effect of tobacco and pituri use in pregnancy. The interviewer read each question to the maternal participant, and their responses were documented verbatim on the interview tool. “Yarning” [90,91] was used to navigate through the data elements of the maternal interview. Yarning was conducted in a conversational style which enabled the participant to elaborate on responses as they chose. The tool was designed to enable the participant to share their ethnobotanical knowledge of pituri preparation and use as chewing tobacco. This aspect of the data collection informed the development of a derivative project which examined Aboriginal pharmacological knowledge of pituri and its use [57].
- (2)
- The second data collection tool obtained maternal and perinatal data from each participant’s electronic medical record. In Australia, the capture of mandatory information pertaining to each Australian pregnancy and birth is directed by the Australian National Minimum Perinatal Data Set [92]. In the NT, this information is collected and electronically stored as part of the NT Perinatal Data report within CARESYS®, the electronic medical record [93], hereafter referred to as the CARESYS® data. The CARESYS® data records 80 primary and 40 secondary maternal and perinatal variables. The CARESYS® information is progressively entered by attending health care professionals throughout the pregnancy. For this research, a complete Perinatal Data record was printed from CARESYS® following the participant’s hospital discharge after birthing. Table 2 lists the maternal interview and the CARESYS® data variables and their operational definitions collected and used in this research.
- (3)
- The third data collection involved the collection of biological samples from the participant and their neonate. The objective of this was to quantify the level of tobacco and nicotine and metabolite concentrations in maternal and perinatal samples from different self-reported tobacco exposures as a question of possible causality in adverse maternal and perinatal outcomes. As such, epidemiological research [30,94,95] demonstrates that the transit of biochemical tobacco and nicotine from maternal exposure through to fetal exposure, and then to maternal and neonatal excretion [4,12,29,31,32,33,34,35] can be measured in maternal, placental and neonatal biological samples. Informed by the literature, Table 3 details the biological samples and their rationale for collection in response to this question. The biological samples and their collection procedures are standard clinical procedures and required no additional staff training and minimal clinical resources. The samples were collected at the time of enrolment (maternal venous blood, urine, and hair) and as they became available during the study and were identified with a unique participant identifier before being transferred to the hospital pathology for storage in a −80 °C freezer.
3.2. Data Processing
3.3. Biological Sample Processing
3.3.1. Reagent Setup
3.3.2. Sample Preparation
3.3.3. LC-MS/MS Analysis
3.3.4. Method Validation
3.4. Data Analysis
- Demographic variables (age, residential address, education level)
- Lifestyle factors (alcohol and tobacco use)
- Past and current medical history (cardiac, hypertension, diabetes and renal disease)
- Pregnancy-related factors (parity, gravida, elevated glucose, hypertension, STI, UTI, anemia, number of antenatal visits, placental weight and size)
- Labor and birthing factors (LUSCS, meconium staining, post-partum hemorrhage)
- Birth outcome variables (gestational length, birth weight, gender, admission to SCN and APGAR score)
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akehurst, B.C. Tobacco; Longnes, Green and Company: London, UK, 1968. [Google Scholar]
- Australia’s Virtual Herbarium. Nicotiana. Available online: https://avh.ala.org.au/occurrences/search?taxa=nicotiana#tab_mapView (accessed on 20 April 2019).
- Moghbel, N.; Ryu, B.; Ratsch, A.; Steadman, K.J. Nicotine alkaloid levels, and nicotine to nornicotine conversion, in Australian Nicotiana species used as chewing tobacco. Heliyon 2017, 3, e00469. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P., III. Nicotine chemistry, metabolism, kinetics and biomarkers. In Nicotine Psychopharmacology, Handbook of Experimental Pharmacology; Henningfield, J.E., London, E.D., Pogun, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 192, pp. 29–60. [Google Scholar]
- Moghbel, N.; Ryu, B.; Cabot, P.J.; Ratsch, A.; Steadman, K.J. In Vitro Cytotoxicity of Nicotiana Gossei Leaves, Used in the Australian Aboriginal Smokeless Tobacco Known as Pituri or Mingkulpa. Toxicol. Lett. 2016, 254, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Shafey, O.; Eriksen, M.; Ross, H.; Mackey, J. The Tobacco Atlas. Available online: http://www.afro.who.int/en/clusters-a-programmes/hpr/health-risk-factors/tobacco/tobacco-country-profiles.html (accessed on 20 June 2018).
- Fant, R.V.; Owen, L.L.; Henningfield, J.E. Nicotine replacement therapy. Prim. Care 1999, 26, 633–652. [Google Scholar] [CrossRef]
- Grana, R.; Benowitz, N.; Glantz, S.A. E-cigarettes: A scientific review. Circulation 2014, 129, 1972–1986. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, T.A. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol. Teratol. 2008, 30, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Luck, W.; Nau, H.; Hansen, R.; Steldinger, R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev. Pharmacol. Ther. 1985, 8, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Ansell, C.; Moore, A.; Barrie, H. Electrolyte pH changes in human milk. Pediatr. Res. 1977, 11, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Dahlstrom, A.; Ebersjo, C.; Lundell, B. Nicotine exposure in breastfed infants. Acta Paediatr. 2004, 93, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Luck, W.; Nau, H. Nicotine and cotinine concentrations in serum and milk of nursing smokers. Br. J. Clin. Pharmacol. 1984, 18, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.M. Tobacco and pregnancy. Reprod. Toxicol. 2009, 28, 152–160. [Google Scholar] [CrossRef]
- Wong, M.K.; Barra, N.G.; Alfaidy, N.; Hardy, D.B.; Holloway, A.C. Adverse effects of perinatal nicotine exposure on reproductive outcomes. Reproduction 2015, 150, R185–R193. [Google Scholar] [CrossRef] [PubMed]
- Grunberg, N.; Starosciak, A. Nicotine. In Encyclopedia of Behavioral Neuroscience; Knoob, G., Le Moal, M., Thomson, R.F., Eds.; Elsevier Science & Technology: Amsterdam, The Netherlands, 2010; pp. 464–470. [Google Scholar] [CrossRef]
- Mao, C.; Lv, J.; Li, H.; Chen, Y.; Wu, J.; Xu, Z. Development of fetal nicotine and muscarinic receptors in utero. Braz. J. Med. Biol. Res. 2007, 40, 735–741. [Google Scholar] [CrossRef] [PubMed]
- England, L.J.; Aagaard, K.; Bloch, M.; Conway, K.; Cosgrove, K.; Grana, R.; Gould, T.J.; Hatsukami, D.; Jensen, F.; Kandel, D.; et al. Developmental toxicity of nicotine: A transdisciplinary synthesis and implications for emerging tobacco products. Neurosci. Biobehav. Rev. 2017, 72, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Lambers, D.S.; Clark, K.E. The maternal and fetal physiologic effects of nicotine. Semin. Perinatol. 1996, 20, 115–126. [Google Scholar] [CrossRef]
- Dwyer, J.B.; Broide, R.S.; Leslie, F.M. Nicotine and brain development. Birth Defects Res. C Embryo Today Rev. 2008, 84, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-Y.; Chen, Y.-J.; Lee, J.-F.; Lu, C.-L.; Chen, C.-H. Cigarettes and the developing brain: Picturing nicotine as a neuroteratogen using clinical and preclinical studies. Tzu Chi Med. J. 2012, 24, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Slotkin, T.A.; Lappi, S.E.; McCook, E.C.; Lorber, B.A.; Seidler, F.J. Loss of neonatal hypoxia tolerance after prenatal nicotine exposure: Implications for sudden infant death syndrome. Brain Res. Bull. 1995, 38, 69–75. [Google Scholar] [CrossRef]
- Cornelius, M.D.; De Genna, N.M.; Leech, S.L.; Willford, J.A.; Goldschmidt, L.; Day, N.L. Effects of prenatal cigarette smoke exposure on neurobehavioral outcomes in 10-year-old children of adolescent mothers. Neurotoxicol. Teratol. 2011, 33, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Cecil, C.A.M.; Walton, E.; Smith, R.G.; Viding, E.; McCrory, E.J.; Relton, C.L.; Suderman, M.; Pingault, J.B.; McArdle, W.; Gaunt, T.R.; et al. DNA methylation and substance-use risk: A prospective, genome-wide study spanning gestation to adolescence. Transl. Psychiat. 2016, 6, e976. [Google Scholar] [CrossRef]
- Lindblad, F.; Hjern, A. ADHD after fetal exposure to maternal smoking. Nicotine Tob. Res. 2010, 12, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Ivorra, C.; Fraga, M.; Bayon, G.; Fernandez, A.; Garcia-Vicent, C.; Chaves, F.; Redon, J.; Lurbe, E. DNA methylation patterns in newborns exposed to tobacco in utero. J. Transl. Med. 2015, 13, 25. [Google Scholar] [CrossRef]
- Benowitz, N.L. Clinical pharmacology of nicotine. Annu. Rev. Med. 1986, 37, 21–32. [Google Scholar] [CrossRef]
- Grenhoff, J.; Svensson, T.H. Pharmacology of nicotine. Br. J. Addict. 1989, 84, 477–492. [Google Scholar] [CrossRef]
- Hukkanen, J.; Jacob, P.; Benowitz, N.L. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 2005, 57, 79–115. [Google Scholar] [CrossRef]
- Dempsey, D.; Iii, P.J.; Benowitz, N.L. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J. Pharmacol. Exp. Ther. 2002, 301, 594–598. [Google Scholar] [CrossRef]
- Dempsey, D.; Jacob, P.; Benowitz, N.L. Nicotine metabolism and elimination kinetics in newborns. Clin. Pharmacol. Ther. 2000, 67, 458–465. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Partridge, J.C.; Jones, R.T.; Rowbotham, M.C. Cocaine, nicotine, caffeine, and metabolite plasma concentrations in neonates. J. Anal. Toxicol. 1998, 22, 220–224. [Google Scholar] [CrossRef]
- Todd, S.-J. Biomarkers for Tobacco Exposure in Utero and Adverse Birth Outcomes; Omaye, S.T., Ed.; ProQuest Dissertations Publishing; University of Nevada: Reno, NV, USA, 2003. [Google Scholar]
- Tsinisizeli, N.; Sotiroudis, G.; Xenakis, A.; Lykeridou, K.E. Determination of nicotine and cotinine in meconium from Greek neonates and correlation with birth weight and gestational age at birth. Chemosphere 2015, 119, 1200–1207. [Google Scholar] [CrossRef]
- Wickstrom, R. Effects of nicotine during pregnancy: Human and experimental evidence. Curr. Neuropharmacol. 2007, 5, 213–222. [Google Scholar] [CrossRef]
- Benowitz, N.; Kuyt, F.; Jacob, P.; Jones, R.; Osman, A.-L. Cotinine disposition and effects. Clin. Pharmacol. Ther. 1983, 34, 604–611. [Google Scholar] [CrossRef]
- Pichini, S.; Basagaña, X.B.; Pacifici, R.; Garcia, O.; Puig, C.; Vall, O.; Harris, J.; Zuccaro, P.; Segura, J.; Sunyer, J. Cord serum cotinine as a biomarker of fetal exposure to cigarette smoke at the end of pregnancy. Environ. Health Perspect. 2000, 108, 1079–1083. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Dains, K.M.; Dempsey, D.; Yu, L.; Jacob, P. Estimation of nicotine dose after low level exposure using plasma and urine nicotine metabolites. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1160–1166. [Google Scholar] [CrossRef]
- Jacob, P.; Hatsukami, D.; Severson, H.; Hall, S.; Yu, L.; Benowitz, N.L. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1668–1673. [Google Scholar]
- Simpson, W.J. A preliminary report on cigarette smoking and the incidence of prematurity. Am. J. Obstet. Gynecol. 1957, 73, 808–815. [Google Scholar] [CrossRef]
- Behl, M.; Rao, D.; Aagaard, K.; Davidson Terry, L.; Levin Edward, D.; Slotkin Theodore, A.; Srinivasan, S.; Wallinga, D.; White Morris, F.; Walker Vickie, R.; et al. Evaluation of the association between maternal smoking, childhood obesity, and metabolic disorders: A national toxicology program workshop review. Environ. Health Perspect. 2013, 121, 170–180. [Google Scholar] [CrossRef]
- Knopik, V.S. Maternal smoking during pregnancy and child outcomes: Real or spurious effect? Dev. Neuropsychol. 2009, 34, 1–36. [Google Scholar] [CrossRef]
- Cnattingius, S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob. Res. 2004, 6, S125–S140. [Google Scholar] [CrossRef]
- Mohsenzadeh, Y.; Rahmani, A.; Cheraghi, J.; Pyrani, M.; Asadollahi, K.; Mohsenzadeh, Y.; Rahmani, A.; Cheraghi, J.; Pyrani, M.; Asadollahi, K. Prenatal exposure to nicotine in pregnant rat increased inflammatory marker in newborn rat. Mediat. Inflamm. 2014, 2014, 274048. [Google Scholar] [CrossRef]
- Orellana, J.; Busso, D.; Ramirez, G.; Campos, M.; Rigotti, A.; Eugenin, J.; von Bernhardi, R.; Orellana, J.; Busso, D.; Ramirez, G.; et al. Prenatal nicotine exposure enhances C×43 and Panx1 unopposed channel activity in brain cells of adult offspring mice fed a high-fat/cholesterol diet. Front. Cell. Neurosci. 2014, 8, 403. [Google Scholar] [CrossRef]
- Chan, W.S. Venous thromboembolism in pregnancy. Expert Rev. Cardiovasc. Ther. 2010, 8, 1731–1740. [Google Scholar] [CrossRef]
- Ekblad, M.; Lehtonen, L.; Korkeila, J.; Gissler, M. Maternal smoking during pregnancy and the risk of psychiatric morbidity in singleton sibling pairs. Nicotine Tob. Res. 2017, 19, 597–604. [Google Scholar] [CrossRef]
- Bauld, L.; Oncken, C. Smoking in pregnancy: An ongoing challenge. Nicotine Tob. Res. 2017, 19, 495–496. [Google Scholar] [CrossRef]
- Berlin, I.; Oncken, C. Maternal smoking during pregnancy and negative health outcomes in the offspring. Nicotine Tob. Res. 2018, 20, 663–664. [Google Scholar] [CrossRef]
- Haustein, K.-O.; Groneberg, D. Smoking and pregnancy. In Tobacco or Health? Springer: Berlin, Germany, 2010; pp. 221–246. [Google Scholar] [CrossRef]
- Asma, S.; Mackay, J.; Song, S.Y.; Zhao, L.; Morton, J.; Palipudi, K.M. The GATS Atlas: Global Adult Tobacco Survey; CDC Foundation: Atlanta, GA, USA, 2015. [Google Scholar]
- Caleyachetty, R.; Tait, C.A.; Kengne, A.P.; Corvalan, C.; Uauy, R.; Echouffo-Tcheugui, J.B. Tobacco use in pregnant women: Analysis of data from Demographic and Health Surveys from 54 low-income and middle-income countries. Lancet Glob. Health 2014, 2, e513–e520. [Google Scholar] [CrossRef]
- England, L.J.; Kim, S.Y.; Tomar, S.L.; Ray, C.S.; Gupta, P.C.; Eissenberg, T.; Cnattingius, S.; Bernert, J.T.; Tita, A.T.; Winn, D.M.; et al. Non-cigarette tobacco use among women and adverse pregnancy outcomes. Acta Obstet. Gynecol. Scand. 2010, 89, 454–464. [Google Scholar] [CrossRef]
- Ratsch, A.; Bogossian, F. Smokeless tobacco use in pregnancy: An integrative review of the literature. Int. J. Publ. Health 2014, 59, 599–608. [Google Scholar] [CrossRef]
- University of New South Wales. National Perinatal Epidemiology and Statistics Unit (NPESU). Available online: http://npesu.unsw.edu.au/ (accessed on 16 January 2019).
- Li, L.; O’Neil, L. Mothers and Babies 2015: Northern Territory Midwives’ Collection; Department of Health: Darwin, Australia, 2018.
- Ratsch, A.; Mason, A.; Rive, L.; Bogossian, F.; Steadman, K. The Pituri Learning Story: Central Australian Aboriginal women’s knowledge and practices around the use of Nicotiana spp. as a chewing tobacco. Rural Remote Health 2017, 17, 4044. [Google Scholar] [CrossRef]
- Richter, P.; Spierto, F.W. Surveillance of smokeless tobacco nicotine, pH, moisture, and unprotonated nicotine content. Nicotine Tob. Res. 2003, 5, 885–889. [Google Scholar] [CrossRef]
- Watson, C.; Fleming, J.; Alexander, K. A Survey of Drug Use Patterns in Northern Territory Aboriginal Communities: 1986–1987; NT Department of Health and Community Services, NT Drug and Alcohol Bureau: Darwin, Australia, 1988.
- Guo, H.; Tian, L.; Zhang, J.Z.; Kitani, T.; Paik, D.T.; Lee, W.H.; Wu, J.C. Single-cell RNA sequencing of human embryonic stem cell differentiation delineates adverse effects of nicotine on embryonic development. Stem Cell Rep. 2019, 12, 772–786. [Google Scholar] [CrossRef]
- Kawakami, T.; Yoshimi, M.; Kadota, Y.; Inoue, M.; Sato, M.; Suzuki, S.; Kawakami, T.; Yoshimi, M.; Kadota, Y.; Inoue, M.; et al. Prolonged endoplasmic reticulum stress alters placental morphology and causes low birth weight. Toxicol. Appl. Pharmacol. 2014, 275, 134–144. [Google Scholar] [CrossRef]
- Holloway, A.; Salomon, A.; Soares, M.; Garnier, V.; Raha, S.; Sergent, F.; Nicholson, C.; Feige, J.; Benharouga, M.; Alfaidy, N.; et al. Characterization of the adverse effects of nicotine on placental development: In vivo and in vitro studies. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E443–E456. [Google Scholar] [CrossRef]
- De Long, N.; Barra, N.; Hardy, D.; Holloway, A.; De Long, N.; Barra, N.; Hardy, D.; Holloway, A. Is it safe to use smoking cessation therapeutics during pregnancy? Expert Opin. Drug Saf. 2014, 13, 1721–1731. [Google Scholar] [CrossRef]
- Kreyberg, I.; Bains, K.E.S.; Carlsen, K.-H.; Granum, B.; Gudmundsdóttir, H.K.; Haugen, G.; Hedlin, G.; Hilde, K.; Jonassen, C.M.; Nordhagen, L.S.; et al. Stopping when knowing: Use of snus and nicotine during pregnancy in Scandinavia. ERJ Open Res. 2019, 5, 00197–02018. [Google Scholar] [CrossRef]
- Gunnerbeck, A. Prenatal Nicotine Exposure and Effects on the Health of the Newborn; Karolinska Institutet: Solna, Sweden, 2017. [Google Scholar]
- Inamdar, A.S.; Croucher, R.E.; Chokhandre, M.K.; Mashyakhy, M.H.; Marinho, V.C.C. Maternal smokeless tobacco use in pregnancy and adverse health outcomes in newborns: A systematic review. Nicotine Tob. Res. 2014, 17, 1058–1066. [Google Scholar] [CrossRef]
- Rygh, E.; Gallefoss, F.; Reiso, H. Use of snus and smoking tobacco among pregnant women in the Agder counties. Tidsskr. Nor. Legeforen. Nr. 2016, 16, 1351–1355. [Google Scholar] [CrossRef]
- Health Gains Planning Unit. Central Australian Regional Plan (2010-2012); Department of Health, Alice Springs: Northern Territory, Australia, 2012.
- Ngaanyatjarra Pitjantjatjara Yankunytjatjara Women’s Council Aboriginal Corporation. History and Map. Available online: https://www.npywc.org.au/about/ (accessed on 19 January 2019).
- Cassowary. Local Government Areas of Australia. Available online: https://commons.wikimedia.org/wiki/File:Australian_local_government_areas.png (accessed on 8 January 2019).
- National Health and Medical Research Council. Keeping Research on Track: A Guide for Aboriginal and Torres Strait Islander Peoples about Health Research Ethics; E65; National Health and Medical Research Council: Canberra, Australia, 2006.
- National Health and Medical Research Council. Values and Ethics: Guidelines for Ethical Conduct in Aboriginal and Torres Strait Islander Health Research; National Health and Medical Research Council: Canberra, Australia, 2003.
- Hardon, A.; Hodgkin, C.; Fresle, D. How to Investigate the Use of Medicines by Consumers; World Health Organization: Geneva, Switzerland; University of Amsterdam: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Blagg, H. A just measure of shame? Aboriginal youth and conferencing in Australia. Br. J. Criminol. 1997, 37, 481–501. [Google Scholar] [CrossRef]
- Li, Z.; Zeki, R.; Hilder, L.; Sullivan, E. Australia’s Mothers and Babies 2011; Australian Institute of Health and Welfare: Canberra, Australia, 2013.
- Thompson, F. Northern Territory Midwives’ Collection. Mothers and Babies 2011; Department of Health: Darwin, Australia, 2014.
- Benowitz, N.; Porchet, H.; Sheiner, L.; Jacob, P. Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clin. Pharmacol. Ther. 1988, 44, 23–28. [Google Scholar] [CrossRef]
- Hertzog, M. Considerations in determining sample size for pilot studies. Res. Nurs. Health 2008, 31, 180–191. [Google Scholar] [CrossRef]
- Lawn, J.E.; Yakoob, M.Y.; Haws, R.A.; Soomro, T.; Darmstadt, G.L.; Bhutta, Z.A. 3.2 million stillbirths: Epidemiology and overview of the evidence review. BMC Pregnancy Childbirth 2009, 9. [Google Scholar] [CrossRef]
- Pallotto, E.K.; Kilbride, H.W. Perinatal outcome and later implications of intrauterine growth restriction. Clin. Obstet. Gynecol. 2006, 49, 257–269. [Google Scholar] [CrossRef]
- Vasudevan, C.; Renfrew, M.; McGuire, W. Fetal and perinatal consequences of maternal obesity. Arch. Dis. Child Fetal Neonatal Ed. 2011, 96, F378–F382. [Google Scholar] [CrossRef]
- Hutcheon, J.A.; Lisonkova, S.; Joseph, K. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 391–403. [Google Scholar] [CrossRef]
- Salihu, H.M.; Wilson, R.E. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum. Dev. 2007, 83, 713–720. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Mumford, S.L.; Schisterman, E.F. Conditioning on intermediates in perinatal epidemiology. Epidemiology 2012, 23, 1–9. [Google Scholar] [CrossRef]
- Beck, S.; Wojdyla, D.; Say, L.; Betran, A.P.; Merialdi, M.; Requejo, J.H.; Rubens, C.; Menon, R.; Van Look, P.F. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull. World Health Organ. 2010, 88, 31–38. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Australia’s Mothers and Babies 2013 in Brief; AIHW: Canberra, Australia, 2015.
- AIHW; Humphrey, M.; Bonello, M.; Chughtai, A.; Macaldowie, A.; Harris, K.; Chambers, G. Maternal Deaths in Australia 2008-2012; Maternal deaths series no. 5. Cat. No. Per 70; Australian Institute of Health and Welfare: Canberra, Australia, 2015.
- Hall, J.; Case, A.; O’Neil, L. Northern Territory Midwives’ Collection. Mothers and Babies 2013; Health Gains Planning Branch: Darwin, Australia, 2015.
- World Health Organization. Maternal, Newborn, Child and Adolescent Health. Data, Statistics and Epidemiology. Available online: http://www.who.int/maternal_child_adolescent/epidemiology/en/ (accessed on 4 January 2019).
- Bessarab, D.; Ng’andu, B. Yarning about yarning as a legitimate method in Indigenous research. Int. J. Crit. Indig. Stud. 2010, 3, 37–50. [Google Scholar] [CrossRef]
- Walker, M.; Fredericks, B.; Mills, K.; Anderson, D. “Yarning” as a method for community-based health research with Indigenous women: The Indigenous women’s wellness research program. Health Care Women Int. 2014, 35, 1216–1226. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Perinatal NMDS 2018-19. Available online: https://meteor.aihw.gov.au/content/index.phtml/itemId/668811 (accessed on 2 January 2019).
- Tew, K.; You, J.; Pircher, S. Validation of Patient Demographic Data: Northern Territory Hospitals, 2008; Northern Territory Department of Health and Families: Darwin, Australia, 2008.
- George, L.; Granath, F.; Johansson, A.L.V.; Cnattingius, S. Self-reported nicotine exposure and plasma levels of cotinine in early and late pregnancy. Acta Obstet. Gynecol. Scand. 2006, 85, 1331–1337. [Google Scholar] [CrossRef]
- Hebert, R. What’s new in nicotine & tobacco research? Nicotine Tob. Res. 2009, 11, 1127–1130. [Google Scholar] [CrossRef]
- Luck, W.; Nau, H. Exposure of the fetus, neonates and nursed infant to nicotine and cotinine from maternal smokers. N. Engl. J. Med. 1984, 311, 672. [Google Scholar] [CrossRef]
- Mercelina-Roumans, P.; Schouten, H.; Ubachs, J.M.H.; van Wersch, J.W.J. Cotinine concentrations in plasma of smoking pregnant women and their infants. Clin. Chem. Lab. Med. 1996, 34, 525–528. [Google Scholar] [CrossRef]
- Hurt, R.D.; Renner, C.C.; Patten, C.A.; Ebbert, J.O.; Offord, K.P.; Schroeder, D.R.; Enoch, C.C.; Gill, L.; Angstman, S.E.; Moyer, T.P. Iqmik—A form of smokeless tobacco used by pregnant Alaska natives: Nicotine exposure in their neonates. J. Matern. Fetal Neonatal. Med. 2005, 17, 281–289. [Google Scholar] [CrossRef]
- World Health Organization. Smokeless Tobacco and Some Tobacco-Specific N-Nitrosamines; WHO: Lyon, France, 2007. [Google Scholar]
- Habek, D.; Habek, J.Č.; Ivanišević, M.; Djelmiš, J. Fetal tobacco syndrome and perinatal outcome. Fetal Diagn. Ther. 2002, 17, 367–371. [Google Scholar] [CrossRef]
- Gray, T.R.; Shakleya, D.M.; Huestis, M.A. Quantification of Nicotine, Cotinine, trans-3′-Hydroxycotinine, Nornicotine and Norcotinine in Human Meconium by Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 863, 107–114. [Google Scholar] [CrossRef]
- Ostrea, E.; Brady, M.; Gause, S.; Raymundo, A.; Stevens, M. Drug screening of newborns by meconium analysis: A large-scale, prospective, epidemiologic study. Pediatrics 1992, 89, 107–113. [Google Scholar]
- Steldinger, R.; Luck, W. Half lives of nicotine in milk of smoking mothers: Implications for nursing. J. Perinat. Med. 1988, 18, 261–262. [Google Scholar]
- Vaz, L.R.; Coleman, T.; Aveyard, P.; Cooper, S.; Leonardi-Bee, J.; on behalf of the SNAP trial team. The nicotine metabolite ratio in pregnancy measured by trans-3′-hydroxycotinine to cotinine ratio: Characteristics and relationship with smoking cessation. Nicotine Tob. Res. 2015, 17, 1318–1323. [Google Scholar] [CrossRef]
- Jacqz-Aigrain, E.; Zhang, D.; Maillard, G.; Luton, D.; Andre, J.; Oury, J.F. Maternal smoking during pregnancy and nicotine and cotinine concentrations in maternal and neonatal hair. Br. J. Obstet. Gynaecol. 2002, 109, 909–911. [Google Scholar] [CrossRef]
- Al-Delaimy, W.; Crane, J.; Woodward, A. Is the hair nicotine level a more accurate biomarker of environmental tobacco smoke exposure than urine cotinine? J. Epidemiol. Community Health 2002, 56, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Florescu, A.; Ferrence, R.; Einarson, T.; Selby, P.; Kramer, M.; Woodruff, S.; Grossman, L.; Rankin, A.; Jacqz-Aigrain, E.; Koren, G. Reference values for hair cotinine as a biomarker of active and passive smoking in women of reproductive age, pregnant women, children, and neonates: Systematic review and meta-analysis. Ther. Drug Monit. 2007, 29, 437–446. [Google Scholar] [CrossRef]
- Koren, G. Measurement of drugs in neonatal hair: A window to fetal exposure. Forensic Sci. Int. 1995, 70, 77–82. [Google Scholar] [CrossRef]
- Kintz, P.; Kieffer, I.; Messer, J.; Mangin, P. Nicotine analysis in neonates’ hair for measuring gestational exposure to tobacco. J. Forensic Sci. 1993, 38, 119–123. [Google Scholar] [CrossRef]
- Eliopoulos, C.; Klein, J.; Phan, M.K.; Knie, B.; Greewald, M.; Chitayat, D.; Koren, G. Hair concentrations of nicotine and cotinine in women and their newborn infants. J. Am. Med. Assoc. 1994, 271, 621–623. [Google Scholar] [CrossRef]
- Al-Ebaisat, H. Determination of some benzimidazole fungicides in tomato puree by high performance liquid chromatography with sampliQ polymer SCX solid phase extraction. Arab. J. Chem. 2011, 4, 115–117. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Br. Med. J. 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Hoaglin, D.C.; Iglewicz, B. Fine-tuning some resistant rules for outlier labeling. J. Am. Stat. Assoc. 1987, 82, 1147–1149. [Google Scholar] [CrossRef]
- England, L.J.; Kim, S.Y.; Shapiro-Mendoza, C.K.; Wilson, H.G.; Kendrick, J.S.; Satten, G.A.; Lewis, C.A.; Tucker, M.J.; Callaghan, W.M. Effects of maternal smokeless tobacco use on selected pregnancy outcomes in alaska native women: A case-control study. Acta Obstet. Gynecol. Scand. 2013, 92, 648–655. [Google Scholar] [CrossRef]
- Agrawal, A.; Scherrer, J.F.; Grant, J.D.; Sartor, C.E.; Pergadia, M.L.; Duncan, A.E.; Madden, P.A.; Haber, J.R.; Jacob, T.; Bucholz, K.K.; et al. The effects of maternal smoking during pregnancy on offspring outcomes. Prev. Med. 2010, 50, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Reeves, S.; Bernstein, I. Effects of maternal tobacco-smoke exposure on fetal growth and neonatal size. Expert Rev. Obstet. Gynecol. 2008, 3, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Scanlon, K.S.; Yip, R.; Schieve, L.A.; Cogswell, M.E. High and low hemoglobin levels during pregnancy: Differential risks for preterm birth and small for gestational age. Obstet. Gynecol. 2000, 96, 741–748. [Google Scholar] [CrossRef]
- Karumanchi, S.A.; Levine, R.J. How does smoking reduce the risk of preeclampsia? Hypertension 2010, 55, 1100–1101. [Google Scholar] [CrossRef]
- Bruin, J.E.; Gerstein, H.C.; Holloway, A.C. Long-term consequences of fetal and neonatal nicotine exposure: A critical review. Toxicol. Sci. 2010, 116, 364–374. [Google Scholar] [CrossRef]
| Maternal Outcomes | Fetal/Neonatal Outcomes | Childhood/Adolescent Outcomes |
|---|---|---|
| Antepartum hemorrhage Placental previa Placental abruption | Low birthweight Small for Gestational Age (SGA) Intrauterine Growth Retardation (IUGR) | Obesity Metabolic disorders |
| Miscarriage Ectopic pregnancy | Preterm labor and delivery | Type 2 diabetes |
| Placental changes, hypertrophy, calcification | Stillbirth, neonatal death, SIDS | Hypertension |
| Venous thrombosis, Pulmonary embolism | Microcephalus Cleft defects Clubfoot deformities | Neurobehavioural changes |
| Variable | Operational Definition of Variable | Measurement Scale |
|---|---|---|
| Aboriginal language group | Central Australian Aboriginal language group | Nominal |
| Access to secondary health care | Maternal residential distance from Alice Springs | Continuous: km |
| Admission to Special Care Nursery (SCN) | Any admission of the neonate to any special care nursery following birth | Dichotomous: yes/no |
| Age | Maternal age at last birthday | Continuous: years |
| Alcohol use | Maternal interview, self-report of any alcohol use in this pregnancy | Dichotomous: yes/no |
| Anemia | <110 g/L hemoglobin in venous blood | Dichotomous: yes/no |
| Antepartum hemorrhage (APH) | Any antepartum haemorrhage | Dichotomous: yes/no |
| Apgar 1, 5, 10 min | Neonatal score of 0, 1 or 2 for: Heart rate, breathing, color, muscle tone and reflex irritability. Total 0–10. | Ordinal |
| Augmentation | The stimulation of ineffective uterine contractions after the onset of labor, to manage labor dystocia. | Dichotomous: yes/no |
| Birthing method | Method of birth | Nominal: SVB, LUSCS, forceps, ventouse |
| Body mass index (BMI) | Ratio between weight (kg) and height (cm) as measured by weight divided by height squared = kg/m2 | Ordinal: underweight, obese normal, overweight |
| Born before arrival (BBA) | Birth that occurs before arrival at the Alice Springs Hospital | Dichotomous: yes/no |
| Cardiac disease | Health practitioner diagnosis of any maternal cardiac disease | Dichotomous: yes/no |
| Cigarette use | Maternal interview, self-reported use of cigarettes in this pregnancy | Dichotomous: yes/no |
| Diabetes Mellitus | Health practitioner diagnosis of pre-gestational diabetes | Dichotomous: yes/no |
| Duration of labor | Duration from onset of established labor to complete birth of neonate | Continuous: hours |
| Education level | Self-reported school leaving grade | Continuous: grade |
| Variable | Operational definition of variable | Measurement scale |
| Elevated glucose | Maternal elevated glucose where inadequate identification of pre-gestational diabetes or gestational diabetes status exists | Dichotomous: yes/no |
| Episiotomy | Perineal incision to facilitate birth of neonate | Dichotomous: yes/no |
| Forceps | Instrumental delivery of the neonate via the vagina | Dichotomous: yes/no |
| Gender | Gender of the neonate | Dichotomous: male/female |
| Gestation at 1st antenatal visit | Time since last menstrual period and attendance at 1st antenatal visit | Continuous: completed weeks |
| Gestation at 1st ultrasound | Time since last menstrual period and attendance at 1st ultrasound | Continuous: completed weeks |
| Gestational diabetes mellitus (GDM) | Health practitioner diagnosis of diabetes that develops during pregnancy | Dichotomous: yes/no |
| Gestational length | Time since last menstrual period and birth of neonate | Continuous: completed weeks |
| Gravida | Number of times a woman has been pregnant regardless of whether the pregnancies result in a live birth | Discrete: number |
| Head circumference | Neonatal head circumference at birth | Continuous: cm |
| Housing situation | Self-report of number of residents that live with participant | Discrete: number |
| Hypertension (pre-gestational) | Health practitioner diagnosis of pre-gestational hypertension | Dichotomous: yes/no |
| Hypertension | Maternal elevated blood pressure where inadequate identification of pre-gestational hypertension or pregnancy-induced hypertension status exists | Dichotomous: yes/no |
| Income | Self-report of weekly income | Continuous: Australian dollars |
| Induction and indicator | The purposeful stimulation of uterine contractions for the purpose of accomplishing delivery, prior to the natural onset of labor | Dichotomous: yes/no |
| Labor complications | Health practitioner diagnosis of labor complications | Dichotomous: yes/no |
| Livebirth | Neonatal outcome following the complete expulsion or extraction from its mother which after separation, shows signs of life | Dichotomous: yes/no |
| Lower Uterine Segment Caesarean Section (LUSCS) | Operative delivery of the neonate from the uterus via the abdomen | Dichotomous: yes/no |
| Meconium stained liquor | Presence of meconium in liquor | Dichotomous: yes/no |
| Membranes complete | Presence of complete membranes | Dichotomous: yes/no |
| Neonatal abnormalities | Presence of any neonatal abnormalities | Dichotomous: yes/no |
| Neonatal body length | Neonatal body length at birth | Continuous: cm |
| Number of antenatal visits | Number of antenatal visits recorded in the perinatal record following birth | Discrete: number |
| Number of cord vessels | Visual inspection of cord after separation from neonate | Discrete: number |
| Parity | Number of previous pregnancies resulting in live births or stillbirths, excluding the current pregnancy | Discrete: number |
| Pituri use | Maternal interview, self-reported use of pituri in this pregnancy | Dichotomous: yes/no |
| Placenta complete | Presence of complete placenta | Dichotomous: yes/no |
| Placental abruption | Placental separation prior to birth of the neonate | Dichotomous: yes/no |
| Placental lie | Relationship of the maternal axis to the fetal axis | Nominal: longitudinal, transverse, oblique |
| Placental previa | Placental lie across the cervical os | Dichotomous: yes/no |
| Placental size | Diameter of placenta at the two widest points in cm. Result multiplied together to find area (cm2) | Continuous: cm2 |
| Placental weight | Weight of the placenta following drainage of blood | Continuous: grams |
| Post-partum hemorrhage | >500 mL blood loss in first 24 h post birth | Dichotomous: yes/no |
| Pre-eclampsia – eclampsia | Hypertension, oedema and proteinuria during pregnancy | Dichotomous: yes/no |
| Pregnancy complications | Health practitioner diagnosis of pregnancy complications | Dichotomous: yes/no |
| Pregnancy-induced hypertension | Health practitioner diagnosis of hypertension that develops during pregnancy | Dichotomous: yes/no |
| Premature rupture of membrane | Rupture of membranes <37 weeks gestation | Dichotomous: yes/no |
| Presentation | Part of the neonate presenting at the superior aperture of the maternal pelvis | Nominal: cephalic, breech, shoulder |
| Previous adverse obstetric history | Health practitioner diagnosis of adverse obstetric history | Dichotomous: yes/no |
| Race | Self-report of race | Nominal |
| Rubella immune status | Rubella IgG antibody level >10 IU/mL | Dichotomous: yes/no |
| Sexually transmitted infection | Health practitioner diagnosis of sexually transmitted infection | Dichotomous: yes/no |
| Significant adverse medical history | Health practitioner diagnosis of any significant adverse medical history | Dichotomous: yes/no |
| Spontaneous vaginal birth (SVB) | Unassisted vaginal birth | Dichotomous: yes/no |
| Stillbirth | Neonate with no signs of life following the complete expulsion or extraction from its mother | Dichotomous: yes/no |
| Third stage method (active) | Method of delivery of placenta and membranes | Dichotomous: yes/no |
| Urinary tract infection | Health practitioner diagnosis of any urinary tract infection | Dichotomous: yes/no |
| Ventouse | Assisted birth using a suction cap applied to the neonate’s head. | Dichotomous: yes/no |
| Biological Sample | Rationale for Collection | Collection Process | Collection Method |
|---|---|---|---|
| Maternal venous blood | Indicates recency of maternal nicotine exposure [94]. | Maternal plasma collected concurrently with other plasma collections in order to minimize participant discomfort. | FBC for Hb. If recently collected do not repeat. Standard pink top tube. U&E for standard biochemical measures. If recently collected do not repeat. Standard green top tube. Tobacco, nicotine and its metabolites: 2 × 2 mL lithium heparin light green non-gel tubes. |
| Venous cord blood and Arterial cord blood | Indicates nicotine placental transfer and fetal exposure. Nicotine rapidly crosses the placental barrier with considerable amounts of nicotine occurring in the fetal blood of maternal smokers [96,97] and ST users [98]. By analyzing venous and arterial cord blood, a determination can be made of: (a) Fetal nicotine exposure (venous cord), and (b) fetal nicotine circulating levels (arterial cord). | Cord blood will be collected as per standard arterial and venous cord blood collection procedures following the complete expulsion of the placenta from the uterus and the complete separation of the placenta from the neonate. | Arterial: 2 × 2 mL lithium heparin light green non-gel tubes. Venous: 2 × 2 mL lithium heparin light green non-gel tubes. |
| Amniotic fluid | Amniotic fluid demonstrates fetal exposure to nicotine that penetrates through the amniotic membrane and fetal excretion (via the fetal kidneys and lungs) of nicotine and its metabolites into the amniotic fluid. Amniotic fluid concentrations are expected to be significantly higher than the umbilical arterial and venous concentrations as the foetus ingests, metabolizes and excretes and then re-ingests the amniotic fluid through the pregnancy [10,19,99,100]. | A clean sample of amniotic fluid (visibly uncontaminated with maternal blood or meconium) will be obtained at lower uterine segment cesarean section (LUSCS). | 10 mL, sterile yellow top collection jar. |
| Neonatal urine Day 1 Day 3 | Fetal urinary concentrations of nicotine fluctuate dependent on recency of exposure, level of exposure, and metabolism rate and therefore are less indicative of long-term exposure than meconium. However, comparative analysis of Day 1 and Day 3 urine and to venous and arterial cord blood concentrations may demonstrate neonatal metabolism and excretion capacity following separation from the nicotine supply through the placenta [31]. If the neonate is breastfed and the mother is using tobacco in the early post-delivery period, colostrum may contain nicotine concentration at least equivalent to that of the breast milk therefore Day 3 urine testing will not provide an absolute indication of neonatal metabolism of nicotine following placental separation as they will have been exposed to nicotine through the colostrum and/or breast milk. | Neonatal urine collection bags will be placed on the neonate after birth and again on Day 3 and collected when available and uncontaminated with meconium, i.e., a clean sample. | Day 1: 2–3 mL, sterile yellow top collection jar Day 3: 2–3 mL, sterile yellow top collection jar |
| Meconium | Meconium is a fetal gut excretion product that begins to develop at about 12 weeks gestation and is generally not eliminated during the pregnancy. Meconium nicotine concentrations reflect fetal exposure throughout the second and third trimesters (i.e., longevity of exposure is demonstrated). Drug metabolite testing in meconium demonstrates high concentrations are detected in the meconium (100%) compared to urinary screens for the same drugs (37%) and that meconium testing has both high sensitivity and specificity [31,101,102]. | Mothers will be encouraged to collect the neonatal meconium when it becomes available. | One scoop: Brown top sterile fecal collection jar, within first three days of birth |
| Colostrum and/or breast milk | Colostrum and breast milk is an excretion process and a possible route of post-birth nicotine exposure. The acidic milk compartments of the breast concentrate nicotine [12], with nicotine levels in breast milk reaching considerably higher levels than in the serum [103]. | Maternal colostrum and/or breast milk will be concurrently collected with colostrum and/or breast milk expression in order to minimize participant discomfort. | 2–3 mL, sterile yellow top collection jar |
| Maternal hair | Nicotine and its metabolites are deposited in hair from the time of exposure [104,105,106,107,108,109,110] indicating duration of exposure, changes in exposure during pregnancy are also indicated. | The maternal and neonatal hair samples will be collected from the nape of the neck, with mothers encouraged to obtain their neonates’ hair. | Several strands, sterile yellow top collection jar |
| Neonatal hair | Fetal hair begins to grow in the last three months of pregnancy, accumulating and concentrating cotinine and reflecting third-trimester exposure to nicotine [102,107] | As above | As above |
| Placenta | Placental size and weight indicative of neonatal perfusion. | ________cm x ________cm __________grams | Measured across from the edge across the broadest sides and weight placenta in grams. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratsch, A.; Steadman, K.; Ryu, B.; Bogossian, F. Tobacco and Pituri Use in Pregnancy: A Protocol for Measuring Maternal and Perinatal Exposure and Outcomes in Central Australian Aboriginal Women. Methods Protoc. 2019, 2, 47. https://doi.org/10.3390/mps2020047
Ratsch A, Steadman K, Ryu B, Bogossian F. Tobacco and Pituri Use in Pregnancy: A Protocol for Measuring Maternal and Perinatal Exposure and Outcomes in Central Australian Aboriginal Women. Methods and Protocols. 2019; 2(2):47. https://doi.org/10.3390/mps2020047
Chicago/Turabian StyleRatsch, Angela, Kathryn Steadman, BoMi Ryu, and Fiona Bogossian. 2019. "Tobacco and Pituri Use in Pregnancy: A Protocol for Measuring Maternal and Perinatal Exposure and Outcomes in Central Australian Aboriginal Women" Methods and Protocols 2, no. 2: 47. https://doi.org/10.3390/mps2020047
APA StyleRatsch, A., Steadman, K., Ryu, B., & Bogossian, F. (2019). Tobacco and Pituri Use in Pregnancy: A Protocol for Measuring Maternal and Perinatal Exposure and Outcomes in Central Australian Aboriginal Women. Methods and Protocols, 2(2), 47. https://doi.org/10.3390/mps2020047






