Microstructural Surface Properties of Drifting Seeds—A Model for Non-Toxic Antifouling Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Surface Analysis of Drifting Seeds with Scanning Electron Microscopy (SEM)
2.3. Quantification of the Three Dimensional Surface Roughness of Drifting Seeds
2.4. Reproduction of the Surface Structure of the Drifting Seeds on Technical Surfaces
2.5. Fouling Experiments with Drifting Seeds
2.6. Fouling Experiments with Technical Surface According to the Model of the Drifting Seeds
3. Results
3.1. Size and Shape of the Investigated Drifting Seeds
3.2. Results of Fouling Experiments with Drifting Seeds
3.3. Results of Surface Analysis of Drifting Seeds with Scanning Electron Microscopy (SEM)
3.4. Results of the Quantification of the Three Dimensional Surface Roughness of Drifting Seeds
3.5. Reproduction of the Surface Structures on Technical Surfaces
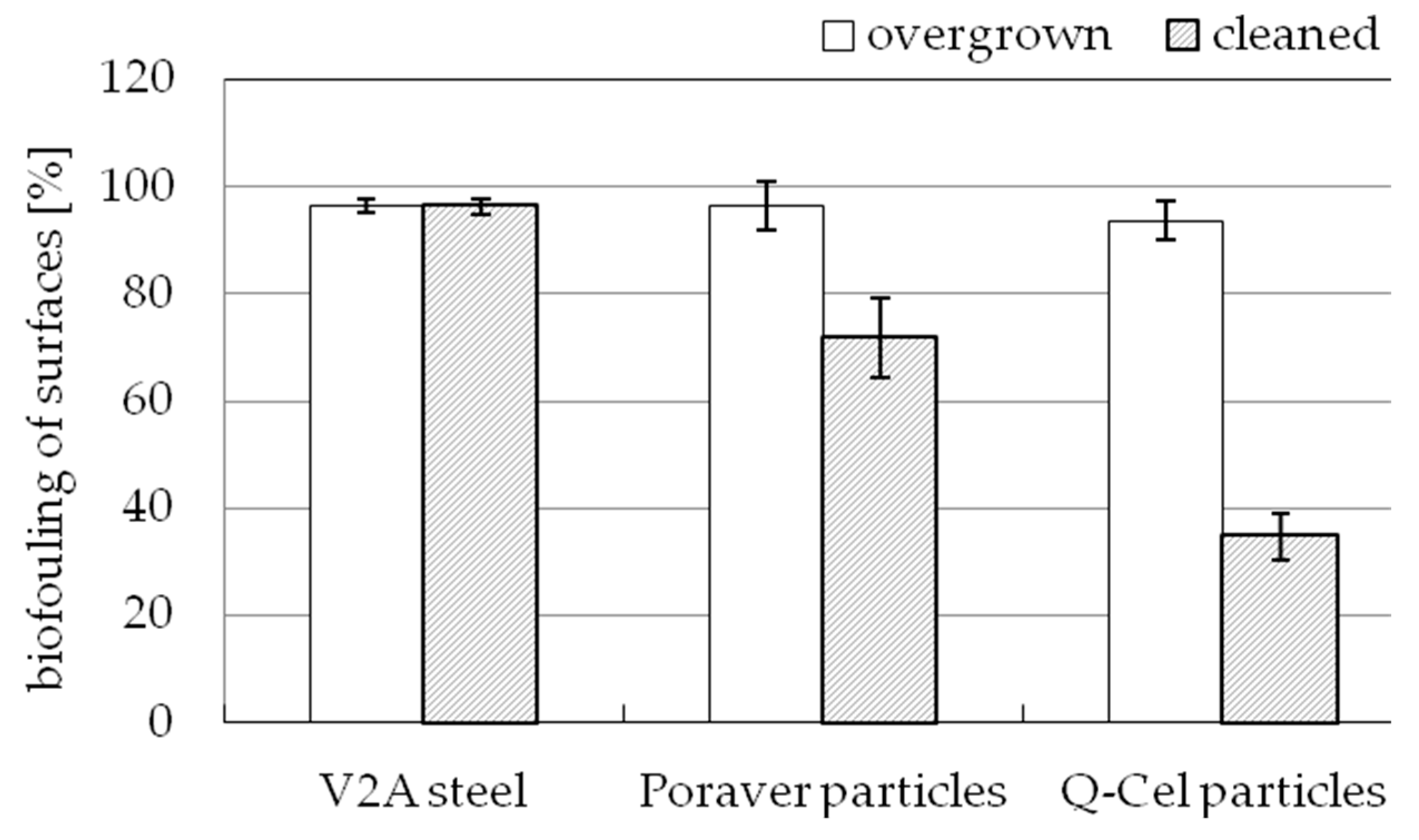
3.6. Results of the Fouling Experiments on Technical Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, Y.W.; Fragkopoulos, A.A.; Marquez, S.; Kim, H.F.; Angelini, T.E.; Fernándesz-Nieves, A. Biofilm formation in geometries with different surface curvature and oxygen availability. New J. Phys. 2015, 17, 033017. [Google Scholar] [CrossRef]
- Costerton, J.W.; Chen, K.J.; Geesey, G.G.; Timothy, I.L.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coating. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef]
- Kargar, M.; Wang, J.; Nain, A.S.M.; Behkam, B. Controlling bacterial adhesion to surface using topographical cues: A study of the interaction of Pseudomonas aeruginosa with nanofiber-textured surfaces. Soft Matter 2012, 8, 10254–10259. [Google Scholar] [CrossRef]
- Graham, M.V.; Cady, N.C. Nano and microscale topographies for prevention of bacterial surface fouling. Coating 2014, 4, 37–59. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.E.; Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Finlay, J.A.; Brennan, A.B. Engineered antifouling microtopographies-effect of feature size, geometry, and roughness on settlement of zoospores of the green alga Ulva. Biofouling 2007, 1, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Rosenhahn, A.; Schilp, S.; Kreuzer, H.J.; Grunze, M. The role of “inert” chemistry in marina biofouling prevention. Phys. Chem. Chem. Phys. 2010, 12, 4275–4286. [Google Scholar] [CrossRef]
- Chapman, J.; Hellio, C.; Sullivan, T.; Brown, R.; Russell, S.; Kiterringham, E.; Le Nor, L.; Regan, F. Bioinspired synthethic macroalgae: Example nature for antifouling applications. Int. Biodeterior. Biodegrad. 2014, 86, 6–13. [Google Scholar] [CrossRef]
- Ranke, J. Vorstudie zum Bewuchsschutz für Seeschiffe: Stand und Perspektiven des Chemischen Bewuchsschutzes; University of Bremen: Bremen, Germany, 1999. [Google Scholar]
- Brügmann, L. Meeresverunreinigungen: Ursachen, Zustand, Trends und Effekte; Akademie Verlag: Berlin, Germany, 1993; ISBN 3055013816. [Google Scholar]
- Fent, K. Ecotoxicology of organotin compounds. Crit. Rev. Toxicol. 1996, 26, 1–117. [Google Scholar] [CrossRef]
- Sousa, A.C.A.; Pastorinho, M.R.; Takahashi, S.T.; Tanabe, S. History on organotin compounds, from snails to humas. Environ. Chem. Lett. 2014, 12, 117–137. [Google Scholar] [CrossRef]
- Plouguerné, E.; Ioannou, E.; Georgantea, P.; Vagias, C.; Roussis, V.; Hellio, C.; Kraffe, E.; Stiger-Pouvreau, V. Anti-microfouling activity of lipidic metabolites from the invasive brown alga Sargassum muticum (Yendo) Fensholt. Mar. Biotechnol. 2010, 12, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Feng, D.Q.; Ke, C.H. Coumarins from the herb Cnidium monnieri and chemically modified derivatives as antifoulants against Balanus albicostatus and Bugula neritina larvae. Int. J. Mol. Sci. 2013, 14, 1197–1602. [Google Scholar] [CrossRef] [PubMed]
- Bao, V.W.W.; Leung, K.M.Y.; Qiu, J.W.; Lam, M.H.W. Acute toxicities of five commonly used antifouling booster biocides to selected subtropical and cosmopolitan marine species. Mar. Pollut. Bull. 2011, 62, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- da Gama, B.A.P.; Carvalho, A.G.V.; Weidner, K.; Soares, A.R.; Coutinho, R.; Fleury, B.G.; Teixeira, V.L.; Pereira, R.C. Antifouling activity of natural products from Brazilian Seaweeds. Bot. Mar. 2008, 51, 191–201. [Google Scholar] [CrossRef]
- Salta, M.; Whartin, J.A.; Stoodley, P.; Dennington, S.P.; Goodes, L.R.; Werwinski, S.; Mart, U.; Wood, R.J.K.; Stokes, K.R. Designing biomimetic antifouling surfaces. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 4729–4754. [Google Scholar] [CrossRef] [PubMed]
- Decker, J.T.; Kirschner, C.M.; Long, C.J.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Brennan, A.B. Engineered antifouling microtopographies: An energetic model that predict cell attachment. Langmuir 2013, 29, 13023–13030. [Google Scholar] [CrossRef]
- Barthlott, W.; Erdelen, W.R.; Rafiqpoor, M.D. Biodiversity and technical innovations: Bionics. In Concepts and Values in Biodiversity; Lanzerath, D., Friele, M., Eds.; Routledge: London, UK; New York, NY, USA, 2014; pp. 300–315. [Google Scholar]
- Armstrong, W. Charles Darwin and driftseeds. Drift. Seed 2005, 11, 2–4. [Google Scholar]
- Nelson, E.C.; Oswald, P.H. Sea Beans and Nickar Nuts: A Handbook of Exotic Seeds and Fruits Stranded on Beaches in North-Western Europe; BSBI Publications: Broompark, UK, 2000; No. 102000. [Google Scholar]
- Bers, A.V.; Wahl, M. The influence of natural surface microtopographies on fouling. Biofouling 2004, 20, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.; Regan, F. Marine diatom settlement on microtextured materials in static field trials. J. Mater. Sci. 2017, 52, 5846–5856. [Google Scholar] [CrossRef]
- Magin, C.M.; Cooper, S.P.; Brennan, A.B. Non-toxic antifouling strategies. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Scardino, A.J.; Hudleston, D.; Peng, Z.; Paul, N.A.; de Nys, R. Biomimetic characterisation of key surface parameters for the development of fouling resistant materials. Biofouling 2010, 25, 83–93. [Google Scholar] [CrossRef]
- Pirisinu, M.; Mazzarello, V. 3D profilometric characterization of the aged skin surface using a skin replica and alicona Mex software. Scanning 2016, 38, 213–220. [Google Scholar] [CrossRef]
- Strickstrock, M.; Rothe, H.; Grohmann, S.; Hildebrand, G.; Zylla, I.M.; Leifeith, K. Influence of surface roughness of dental zirconia implants on their mechanical stability, cell behavior and osseointegration. BioNanoMat 2017, 18, 20160013. [Google Scholar] [CrossRef]
- Afeltowicz, C. Wirtschaftlichkeit und Wirkungsweise von silikonbasierten Antifoulings bei Seeschiffen; Salzwasser-Verlag: Paderborn, Germany, 2007; ISBN 978393768698. [Google Scholar]
- Singh, R.A.; Yoon, E.-S.; Kim, H.J.; Kim, J.; Jeong, H.E.; Suh, K.Y. Replication of surface of natural leaves for enhanced micro-scale tribiological property. Mater. Sci. Eng. C 2007, 27, 875–879. [Google Scholar] [CrossRef]
- Koch, K.; Schulte, A.J.; Fischer, A.; Gorb, S.N.; Barthlott, W. A fast, precise and low-cost replication technique for nano- and high-aspect-ratio structures of biological and artificial surfaces. Bioinspir. Biomim. 2009, 3, 046002. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.; Regan, F. The characterization, replication and testing of dermal denticles of Scyliorhinus canicula for physical mechanisms of biofouling prevention. Bioinspir. Biomim. 2011, 6, 046001. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.G.; Ke, C.H.; Lu, C.Y.; Li, S.J. Herbal plants as a promising source of natural antifoulants: Evidence from barnacle settlement inhibition. Biofouling 2009, 25, 181–190. [Google Scholar] [CrossRef] [PubMed]
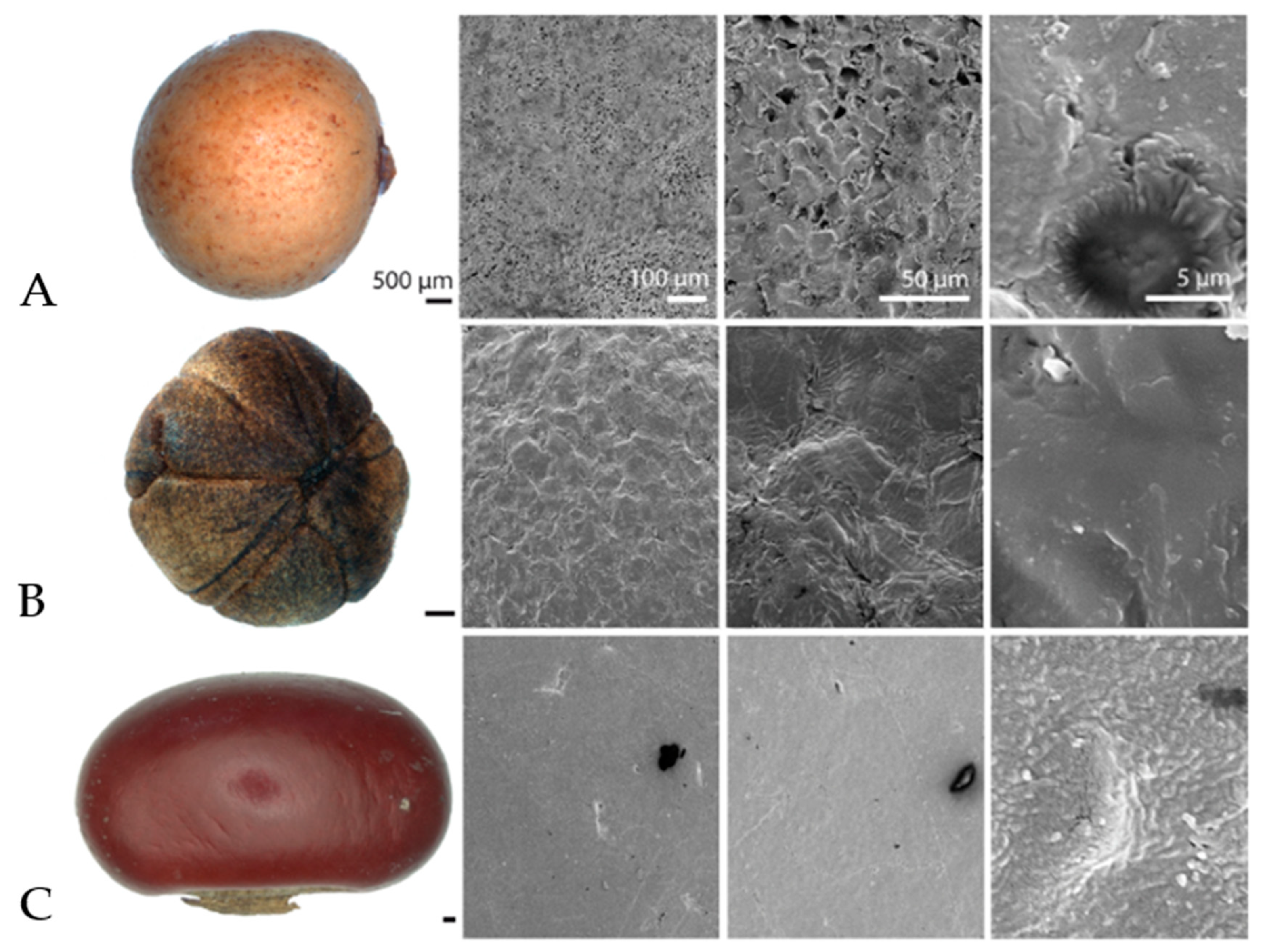
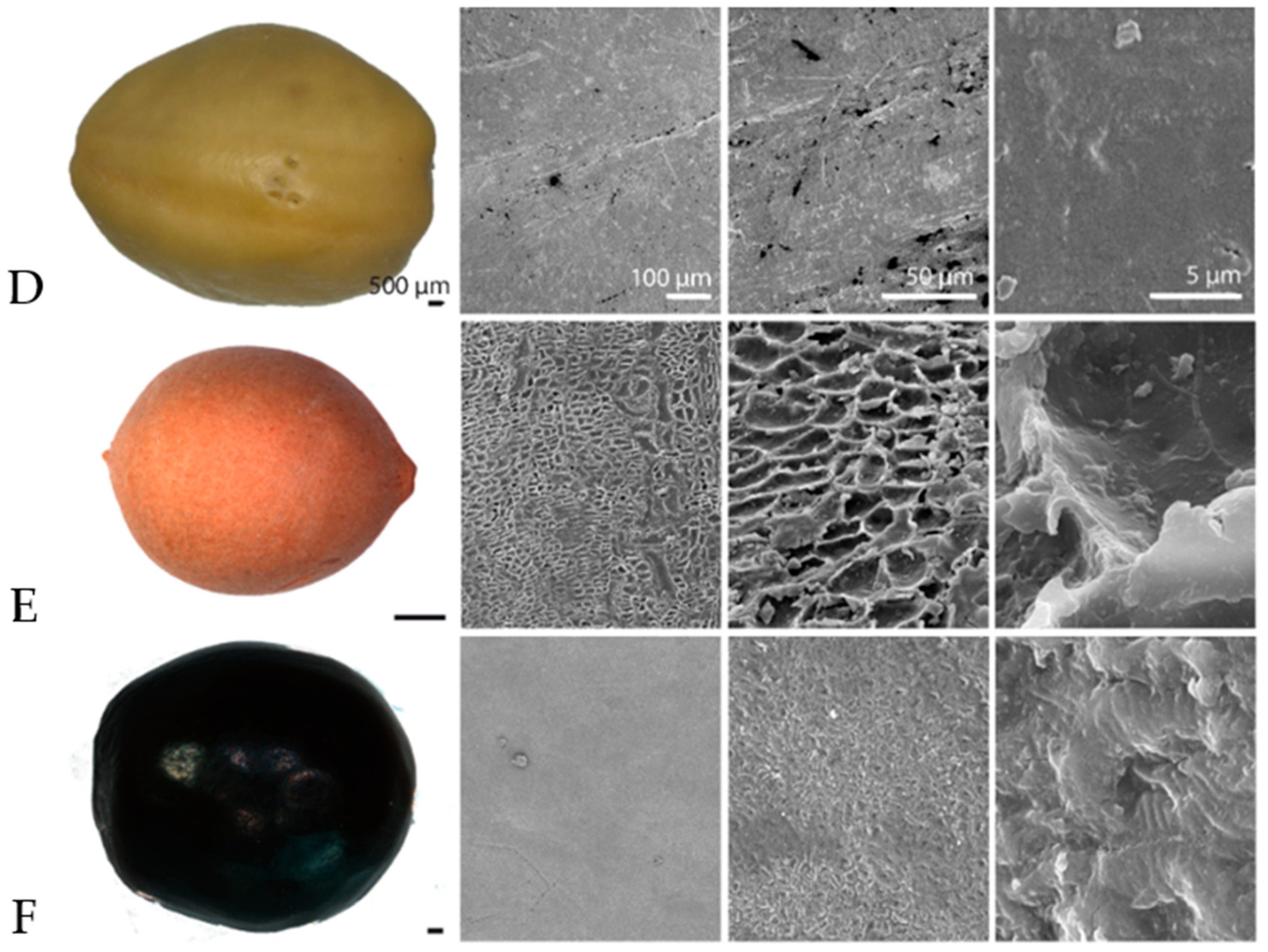
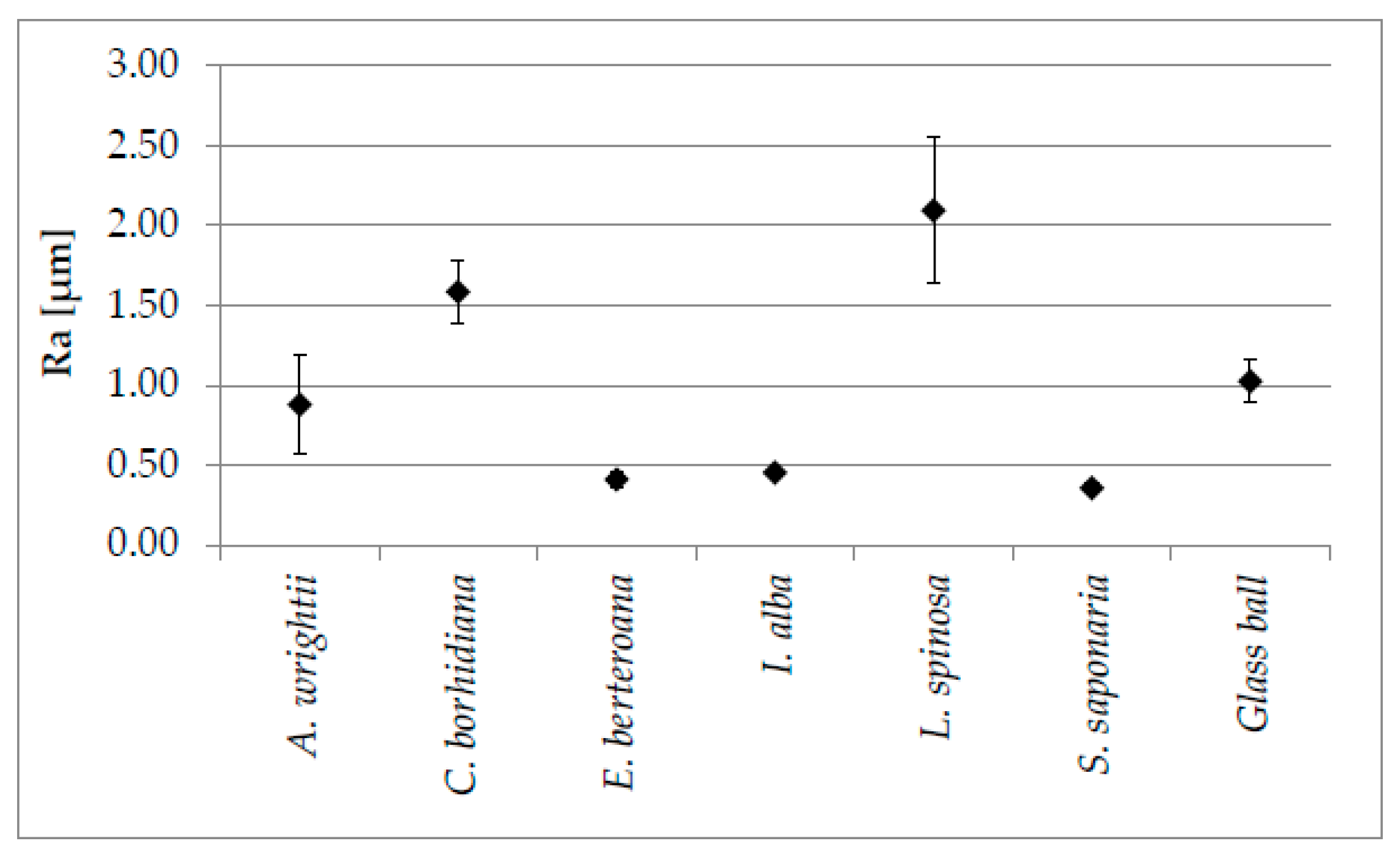

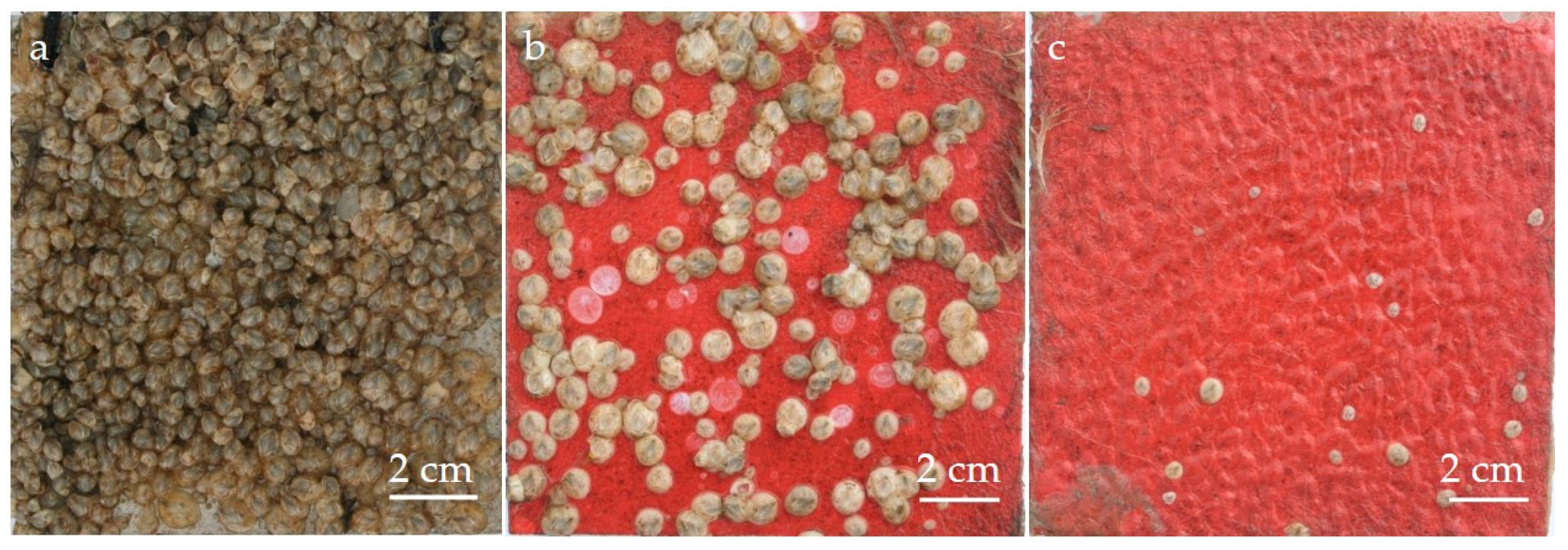
| Family | Species | Regions and Countries of Origin |
|---|---|---|
| Anacardiaceae | Spondias mombin | Peru, Brazil, Venezuela, Bolivia, Colombia, Mexico, Belize, Costa Rica, West Indies |
| Annonaceae | Annona glabra | Florida, Caribbean, Central America, South America, West Africa |
| Apocynaceae | Thevetia peruviana yellow | Mexico, Central America, South America |
| Arecaceae | Acoelorrhaphe wrightii | West Indies, Cuba, Central America |
| Acrocomia totai | Brazil, Bolivia, Argentina | |
| Allagoptera arenaria | Brazil | |
| Archontophoenix myolensis | Queensland | |
| Astrocaryum standleyanum | South America, Central America, Ecuador to Panama, Colombia to Ecuador | |
| Astrocaryum vulgare | Brazil, Peru, Venezuela | |
| Calamus erinaceus | Southeast Asian, Southern Thailand, Philippines, Malaysia, Brunei, Singapore, Indonesia | |
| Coccothrinax borhidiana | Cuba | |
| Coccothrinax boschiana | Dominican Republic | |
| Dypsis paludosa | Madagascar | |
| Dypsis rivularis | Madagascar | |
| Elaeis guineensis | Africa, tropical America, Southeast Asia | |
| Licuala paludosa | Southeast Asia | |
| Licuala spinosa | Southeast Asia | |
| Manicaria saccifera | Central America, South America | |
| Orbignya cohune | Central America, Mexico, Costa Rica | |
| Phoenix roebelenii | Southeast Asia | |
| Pritchardia minor | Hawaii | |
| Pseudophoenix sargentii | Mexico, Belize, Northern Caribbean | |
| Raphia australis | Tropics, Africa, Madagascar | |
| Bignoniaceae | Crescentia cujete | West Indies, South-Mexico to Peru, Brazil, Caribbean |
| Bombacaceae | Adansonia digitata | Africa, Madagascar, Sri Lanka |
| Combretaceae | Laguncularia racemosa | West Africa, North America, South America, Mexico to Brazil, Ecuador |
| Terminalia catappa | Southeast Asia, West Africa | |
| Convolvulaceae | Ipomoea alba | Argentina, Mexico, Florida |
| Ipomoea pes-caprae | Tropics | |
| Merremia tuberosa | Central America, South America, Mexico, Tropics, Subtropics | |
| Euphorbiaceae | Ricinus communis | Northeast Africa, Tropics |
| Fabaceae | Canavalia rosea | Tropics, Subtropics, Florida, California, Texas, Mexico |
| Entada rheedii | Africa, Southeast Asia, India to China, Philippines, Northern Australia | |
| Erythrina berteroana | Central America, South America | |
| Mucuna nigricans | Asia, Florida | |
| Mucuna sempervirens | Bhutan, China, Japan | |
| Mucuna urens | Tropics | |
| Sophora tometosa | South Florida, Cuba, Caribbean | |
| Juglandaceae | Carya illionensis | Mexico |
| Nelumbonaceae | Nelumbo nucifera | Asia, China, Japan, India |
| Pandanaceae | Pandanus conicus | Australia |
| Pandanus monotheca | Thailand | |
| Pandanus pulcher | Madagascar | |
| Pandanus utilis | Tropics, Madagascar | |
| Polygonaceae | Coccoloba uvifera | Tropics, tropical America, Caribbean, South America |
| Rhizophoraceae | Rhizophora mangle | Tropics, West Africa, North America, South America |
| Sapindaceae | Sapindus saponaria | South Carolina, Caribbean, Central America, South America |
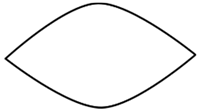
| Geometric Shape | Species of Drifting Seeds | n | Arithmetic Mean and Standard Deviation of Length (mm) | Arithmetic Mean and Standard Deviation of Width (mm) |
|---|---|---|---|---|
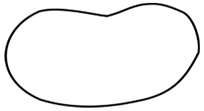 bean | Adansonia digitata | 20 | 12.13 ± 0.49 | 9.58 ± 0.55 |
| Canavalia rosea | 20 | 13.72 ± 0.80 | 9.20 ± 0.99 | |
| Entada rheedii | 04 | 46.50 ± 3.83 | 42.71 ± 2.35 | |
| Erythrina berteroana | 20 | 11.35 ± 0.52 | 7.63 ± 0.34 | |
| Mucuna nigricans | 20 | 23.33 ± 0.90 | 19.11 ± 2.33 | |
| Mucuna semperviren | 20 | 30.27 ± 1.53 | 19.50 ± 1.60 | |
| Mucuna urens | 10 | 27.77 ± 2.40 | 22.87 ± 2.48 | |
 nut | Allagoptera arenaria | 22 | 14.09 ± 4.42 | 10.02 ± 1.15 |
| Archontophoenix myolensis | 44 | 12.76 ± 0.87 | 8.40 ± 0.28 | |
| Astrocaryum standleyum | 11 | 31.00 ± 3.77 | 23.37 ± 1.38 | |
| Astrocaryum vulgare | 12 | 27.50 ± 1.65 | 23.09 ± 1.00 | |
| Carya illinoensis | 20 | 31.24 ± 3.99 | 15.24 ± 0.87 | |
| Coccoloba uvifera | 20 | 11.61 ± 0.95 | 8.40 ± 0.47 | |
| Dypsis paludosa | 38 | 8.71 ± 0.71 | 3.53 ± 0.18 | |
| Dypsis rivularis | 44 | 6.77 ± 0.69 | 4.28 ± 0.35 | |
| Elais guineensis | 11 | 22.15 ± 5.37 | 18.27 ± 2.36 | |
| Laguncularia racemosa | 20 | 14.88 ± 2.11 | 6.35 ± 0.75 | |
| Licuala paludosa | 23 | 6.79 ± 0.49 | 5.99 ± 0.19 | |
| Licuala spinosa | 20 | 6.84 ± 0.67 | 5.38 ± 0.27 | |
| Orbignya cohune | 8 | 60.00 ± 2.62 | 34.58 ± 1.51 | |
| Pritchardia minor (P. eriophora) | 25 | 16.33 ± 0.85 | 12.09 ± 0.74 | |
| Raphia australis | 21 | 67.00 ± 5.20 | 37.02 ± 2.20 | |
| Ricinus communis | 30 | 10.93 ± 0.52 | 7.07 ± 0.32 | |
| Terminalia catappa | 20 | 56.45 ± 6.03 | 35.08 ± 2.86 | |
 boat | Ipomoea alba | 30 | 9.95 ± 0.60 | 7.53 ± 0.57 |
| Ipomoea pes-caprae | 30 | 9.38 ± 0.56 | 7.05 ± 0.41 | |
| Merremia tuberosa | 20 | 17.00 ± 1.54 | 14.24 ± 0.91 | |
 spherical | Acoelorrhaphe wrightii | 46 | 6.87 ± 0.49 | 6.69 ± 0.45 |
| Acrocomia totai | 10 | 21.91 ± 2.13 | 20.73 ± 1.39 | |
| Coccothrinax borhidiana | 20 | 5.81 ± 0.55 | 5.73 ± 0.59 | |
| Coccothrinax boschiana | 23 | 11.61 ± 0.95 | 8.40 ± 0.47 | |
| Manicaria saccifera | 21 | 37.37 ± 2.26 | 37.23 ± 2.20 | |
| Nelumbo nucifera | 20 | 14.21 ± 0.53 | 11.26 ± 0.45 | |
| Pseudophoenix sargentii | 34 | 9.72 ± 0.84 | 9.19 ± 0.07 | |
| Sapindus saponaria | 20 | 11.48 ± 0.55 | 10.63 ± 0.57 | |
| other shapes | Annona glabra | 20 | 13.97 ± 1.02 | 8.71 ± 0.75 |
| Calamus erinaceus | 45 | 5.47 ± 0.30 | 5.30 ± 0.38 | |
| Crescentia cujete | 20 | 7.36 ± 0.68 | 6.68 ± 0.69 | |
| Pandanus conicus | 10 | 51.33 ± 5.70 | 40.00 ± 5.86 | |
| Pandanus monotheca | 10 | 32.26 ± 2.85 | 10.21 ± 1.57 | |
| Pandanus pulcher | 10 | 21.02 ± 2.10 | 5.27 ± 1.06 | |
| Pandanus utilis | 10 | 34.46 ± 4.62 | 26.10 ± 10.00 | |
| Phoenix roebelenii | 79 | 8.55 ± 1.18 | 4.35 ± 0.44 | |
| Rhizophora mangle | 6 | 250.33 ± 8.76 | 14.01 ± 0.64 | |
| Spondias mombin | 10 | 22.44 ± 2.01 | 14.17 ± 0.92 | |
| Thevetia peruviana yellow | 20 | 33.91 ± 3.40 | 18.41 ± 1.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clasen, A.; Kesel, A.B. Microstructural Surface Properties of Drifting Seeds—A Model for Non-Toxic Antifouling Solutions. Biomimetics 2019, 4, 37. https://doi.org/10.3390/biomimetics4020037
Clasen A, Kesel AB. Microstructural Surface Properties of Drifting Seeds—A Model for Non-Toxic Antifouling Solutions. Biomimetics. 2019; 4(2):37. https://doi.org/10.3390/biomimetics4020037
Chicago/Turabian StyleClasen, Antje, and Antonia B. Kesel. 2019. "Microstructural Surface Properties of Drifting Seeds—A Model for Non-Toxic Antifouling Solutions" Biomimetics 4, no. 2: 37. https://doi.org/10.3390/biomimetics4020037
APA StyleClasen, A., & Kesel, A. B. (2019). Microstructural Surface Properties of Drifting Seeds—A Model for Non-Toxic Antifouling Solutions. Biomimetics, 4(2), 37. https://doi.org/10.3390/biomimetics4020037




