Abstract
The mixed sodium-iron ortho-pyrophosphate Na4Fe3(PO4)2P2O7 (NFPP) is a promising Na-containing cathode material with the highest operating voltage among sodium framework structured materials. It operates both in Na and Li electrochemical cells. When cycled in a hybrid Li/Na cell, a competitive co-intercalation of the Li+ and Na+ ions occurs at the cathode side. The present study shows that this process can be tuned by changing the concentration of the Na+ ions in the mixed Li+/Na+-ion electrolyte and current density. It is shown that if the Na concentration in the electrolyte increases, the specific capacity of NFPP also increases and its high-rate capability is significantly improved.
1. Introduction
Sodium-ion batteries (SIBs) have attracted more and more attention in the past decades owing to high abundance of sodium on the Earth, high voltage characteristics, as well as low price and safety [1]. However, a number of sodium intercalation electrodes is much less due to a higher ionic radius of the Na+ ions. Special attention is paid to the polyanionic-type cathode materials because of good thermal stability due to strong covalent bonds in polyanions, which imply improved safety characteristics of the batteries [2]. Despite a larger ionic radius of the Na+ ions (1.02 Å) compared to the Li+ ions (0.76 Å), fast kinetics with Na+ as a charge carrier could nevertheless be expected, since (i) sodium forms longer Na-O bonds, providing more open framework structure; (ii) it is characterized by less polarizing ability than lithium; (iii) weaker Lewis acidity of the Na+ cations reduces solvation energy, hence facilitating their transfer through the electrode/electrolyte interface [3]. Since the operation principles of SIBs are very similar to those of lithium-ion batteries (LIBs), many of the sodium insertion compounds can be considered as lithium hosts and therefore as electrode materials for LIBs. Besides, many attempts have been undertaken last years to achieve high energy and power density, low price, long cycle life, and high safety using hybrid-ion batteries (HIBs) [4]. In HIBs, several types of metal ions are engaged simultaneously in the energy storage process, which provides the synergetic effect. The difference between the migration barriers for the Na and Li motion highly depends on the structure of the materials. Therefore, investigation of the effect of various parameters (including the ability of Na/Li ion electrochemical exchange, the stability of the Li ions in the framework of the Na-containing materials, electrolyte composition, and current density) on the electrochemical processes in HIBs is necessary to control their cycling performance. It is a promising choice to design new mixed Na-Li cathode materials, which are able to intercalate/deintercalate the Na+ and Li+ ions consistently or concurrently.
To date, there are many studies devoted to hybrid Na/Li electrochemical cells containing Na-based polyanionic framework-structured cathode materials and Li-intercalating anodes. Barker et al. [5,6] first constructed a hybrid battery and demonstrated its electrochemical behavior using the NaVPO4F cathode with different anodes, such as Li metal, graphite or Li4Ti5O12 and a Li or mixed Na-Li electrolyte. It was concluded that in all cases the anode reaction was based on the Li insertion mechanism, while the predominant Na insertion mechanism was realized at the cathode side. Later on, HIBs based on Na3V2(PO4)2F coupled with a Li metal anode and a Li electrolyte were studied [7,8]. The authors suggested that a competitive insertion of the Li+ and Na+ ions was realized in Na3V2(PO4)2F which was caused by the impact of the Na+/Li+ exchange rate, as well as the difference in the diffusion rate of the Li+ and Na+ ions in a hybrid electrolyte with certain concentrations of these two ions. It was shown that Na3V2(PO4)2F exhibited a negligible Na+/Li+ electrochemical exchange in HIB due to instability of the Li ions in its structure. The degree of the Na+/Li+-ion exchange in Na3V2(PO4)2F is significantly lower than in some other Na-containing cathodes, such as NaFePO4F [9,10] and Na2FeP2O7 [11], demonstrating that the properties of HIB can be changed using cathode materials with different alkali-ion selectivity. All studied materials show different Na+/Li+ selectivity as a result of differences in crystal structure, alkaline-ion diffusion coefficient, and Na+/Li+ exchange rate; however all of them have excellent cycling performance and improved high-rate capability. Recently, it was shown that a small addition of sodium to a Li electrolyte can inhibit the Li dendrite growth, which improves cycle life and safety of HIB [12].
According to the literature, Na4Fe3(PO4)2P2O7 (NFPP) has the 3D intercalation channels for the Na diffusion and a theoretical capacity of 129 mAh·g−1. The operating potential of the Fe2+/Fe3+ redox reaction is 3.0 V. Kim et al. [13,14] found that three of the four Na+ ions per f.u. participate in the charge/discharge processes. The authors estimated the activation energy for the Na+ transition from one position to another and showed that it has the lowest value in the case of the Na1-Na1 transition. Thus, the diffusion proceeds mainly in the b direction. In References [13,15], it was shown that NFPP is able to cycle both in Na and Li cells. During cycling in a hybrid Na/Li cell, a partial Na+/Li+ electrochemical exchange occurs. As a result, the mixed Na-Li ortho-pyrophosphates are formed. A part of the Na+ ions does not exchange for the Li+ ions, indicating the instability of the Li+ ions in the open framework of NFPP. The remaining Na+ ions stabilize the structure of NFPP.
In the present paper, we studied the effect of the mixed Li+/Na+-ion electrolyte on the electrochemical performance of NFPP in HIB, as well as the participation of the Li+ and Na+ ions in charge-discharge cathode reactions. Three types of electrochemical cells were assembled and studied: (1) the pristine NFPP in a Li cell with the Li electrolyte (NFPP/Li), (2) the de-sodiated NFPP with the Li electrolyte (ed-NFPP/Li), and (3) the de-sodiated NFPP with the mixed 0.9Li-0.1Na electrolyte (ed-NFPP/Li-Na).
2. Result and Discussion
2.1. Crystal Structure
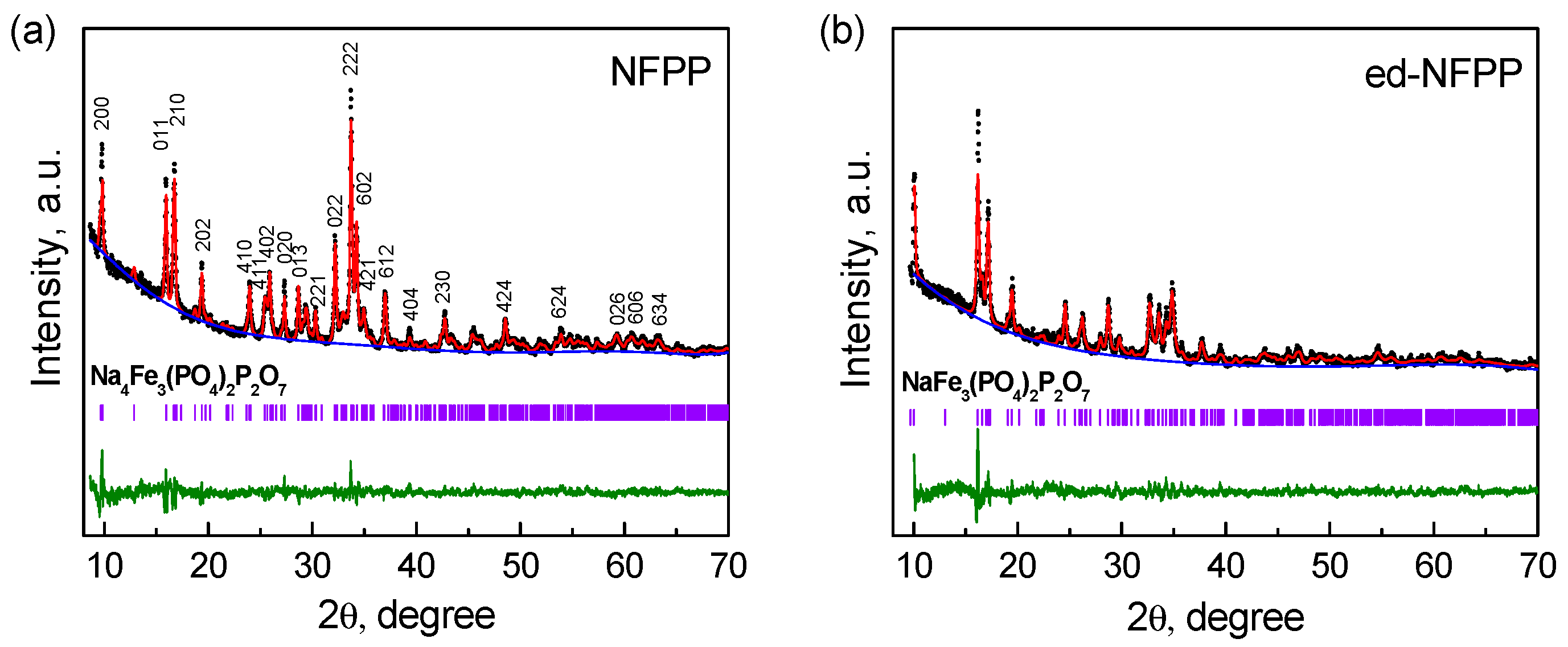
Figure 1 shows the Rietveld refined XRD patterns of the pristine NFPP and of the product of its electrochemical de-sodiation. The XRD patterns of both samples comprise a set of reflections, which were assigned to the NFPP orthorhombic structure with the Pn21a space group [13]. Besides, additional reflections with low intensity are present, which can be attributed to the NaFePO4 (maricite) impurity. As it was previously noted, it is very difficult to avoid the formation of such impurity during the synthesis of NFPP. The framework of NFPP consists of a 3D network of the [Fe3P2O13] infinite layers parallel to the b-c plane. The [Fe3P2O13] infinite layers are connected along the a axis by the P2O7 groups and this connection produces large tunnels providing diffusion of the Na+ ions [14]. There are four symmetrically distinguishable Na sites and three Fe sites in the NFPP crystal structure.
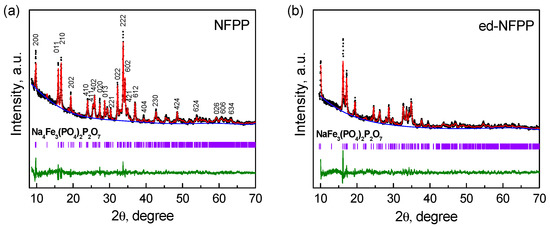
Figure 1.
XRD patterns of the pristine Na4Fe3(PO4)2P2O7 (NFPP) (a) and of the electrochemically de-sodiated NFPP (ed-NFPP) sample (b).
It was evident that the structure of NFPP was retained after de-sodiation, however the reflections became broader and less intensive. The major peaks, such as (200), (011), and (210), move to higher 2θ, indicating that de-intercalation of sodium is accompanied by oxidation of Fe2+ to Fe3+; the intensity ratio of the (011)/(210) peaks changed. Accordingly, the lattice parameters a and b of ed-NFPP decrease, while the parameter c increases (see Table 1), which correlates with the results of other authors [13,15]. It is found that the volume change after de-sodiation is only 4.3%. Based on the values of the lattice parameters and specific capacity of ed-NFPP at the de-sodiation process, its expected composition is close to Na1.2Fe3(PO4)2P2O7. This means that a part of the Na+ ions does not exchange for the Li+ ions. The remaining Na+ ions stabilize the structure of NFPP.

Table 1.
Lattice parameters of the pristine sample (NFPP) and the electrochemically de-sodiated sample (ed-NFPP) compared to the literature data.
2.2. Electrochemical Cycling
Three types of the electrochemical cells were assembled and studied: (1) NFPP/Li, (2) ed-NFPP/Li, and (3) ed-NFPP/Li-Na. The anode was metallic Li in all cases. It should be noted that the cycling of pristine NFPP in a Li cell started with the charge stage, i.e., sodium was extracted from NFPP and entered the Li electrolyte to create mixed Li-Na electrolyte. The first cycling stage of the preliminary de-sodiated sample ed-NFPP was discharge. It was associated with the insertion of the Li+ ions only in the case of ed-NFPP/Li and presumably both Li+ and Na+ ions in the case of ed-NFPP/Li-Na, since mixed Li-Na electrolyte was originally used in the latter case. Though two of these cells—NFPP/Li and ed-NFPP/Li-Na—operated with a mixed Li-Na electrolyte, the concentration of the Na+ ions was different. It was equal to about 0.05 mol·L−1 for the NFPP/Li cell assuming the amount of the de-intercalated Na+ ions on charge (de-sodiation) in the presence of a definite amount of the Li+ ions in the initial electrolyte in the cell. The amount of sodium in electrolyte was noticeably larger for the ed-NFPP/Li-Na cell, in which a preliminary prepared mixed Li-Na electrolyte with the 0.9/0.1 mol·L−1 concentration was used.
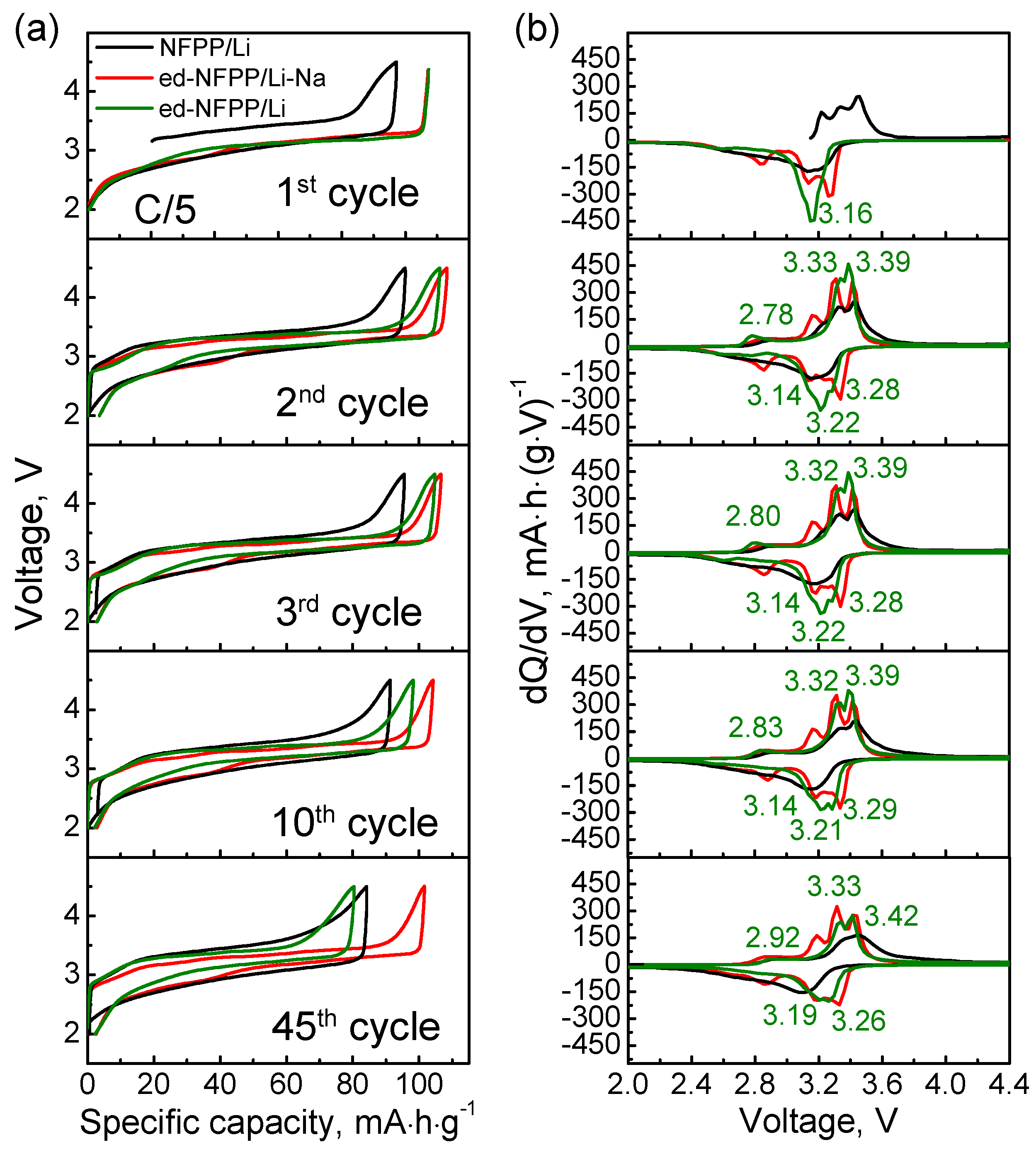
Figure 2 shows the charge-discharge profiles and differential capacity curves for three types of the electrochemical cells at the C/5 cycling rate for the 1st, 2nd, 3rd, 10th, and 45th cycles. It was seen that all the samples had sloping charge-discharge profiles differing by the length of plateaus, capacity, and polarization. These profiles are very similar to those observed for NFPP cycled in a Na cell [15,16], but the cell voltage increased by ~0.15 V. On the differential capacity curves of ed-NFPP/Li, there was only one reduction peak at 3.16 V at the first cycle, associated with the insertion of the Li+ ions in the available Na vacancies of the de-sodiated NFPP structure. On the contrary, there were three oxidation peaks at 2.78 V, 3.33 V, and 3.39 V on the subsequent differential charge curve of ed-NFPP/Li, pointing to a possible structural rearrangement, which occurs when free Na sites are occupied by the Li+ ions upon initial intercalation. During the following cycling, the differentiation of the redox peaks increased, evidencing the occurrence of ongoing structural rearrangement from charge to discharge. When some Na+ salt was added to the electrolyte, four distinct redox peaks were observed for ed-NFPP/Li-Na that keep unchanged till the 45th cycle. This indicates that no significant structural rearrangements occur during prolong cycling in this case. For the NFPP/Li cell, the situation is intermediate.
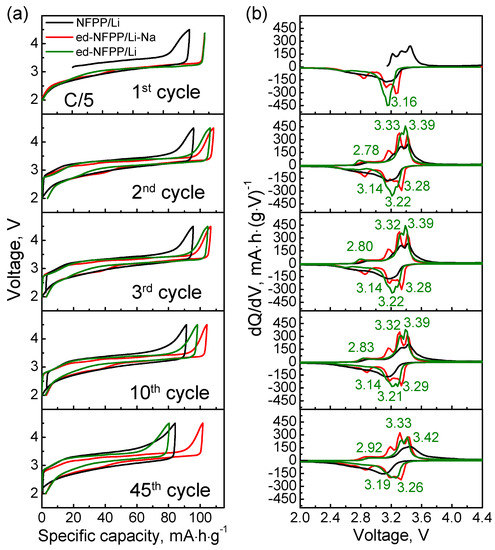
Figure 2.
Charge-discharge profiles (a) and dQ/dV vs. voltage plots (b) at different cycles for three electrochemical cells: A pristine NFPP in a Li cell with the Li electrolyte (NFPP/Li), a de-sodiated NFPP with the mixed 0.9Li-0.1Na electrolyte (ed-NFPP/Li-Na), and a de-sodiated NFPP with the Li electrolyte (ed-NFPP/Li).
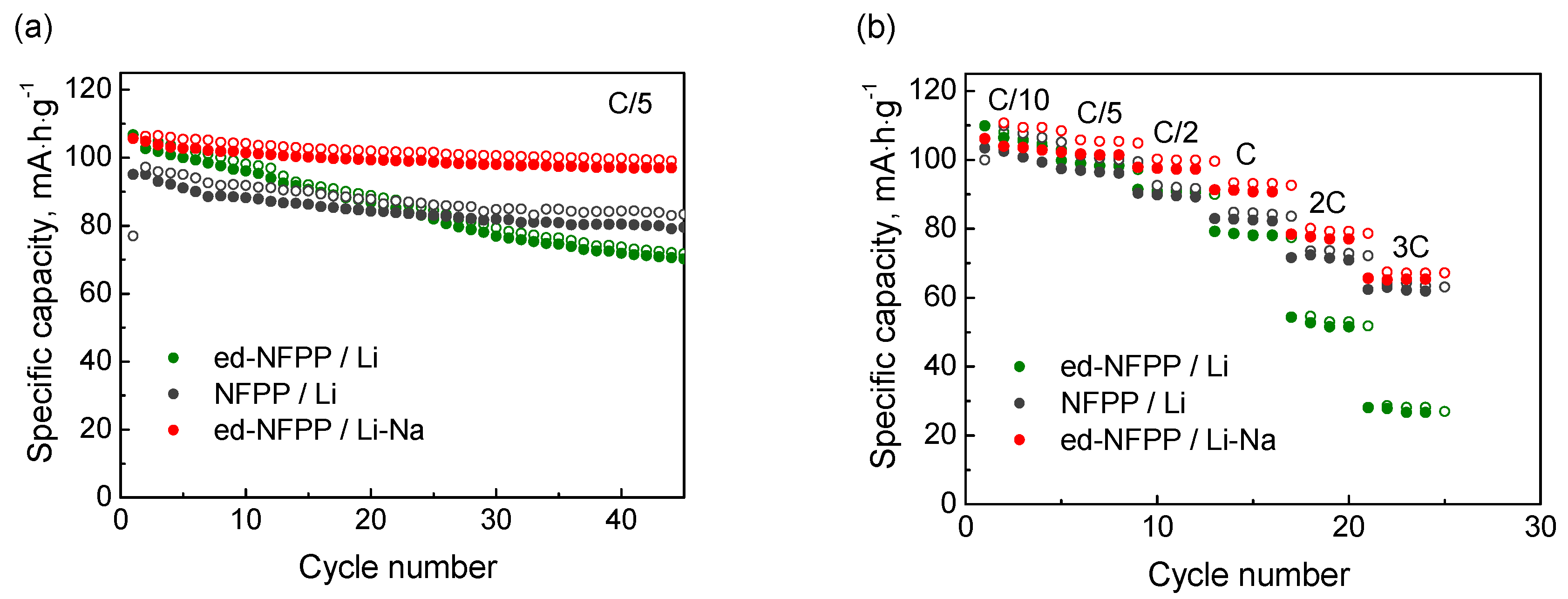
The dependence of the charge-discharge capacity vs. the cycle number is shown in Figure 3a. The initial specific discharge capacities of ed-NFPP/Li and ed-NFPP/Li-Na exceeded that of NFPP/Li and were equal to 106.5 mAh·g−1 and 105.6 mAh·g−1, respectively, against 95.1 mAh·g−1 for NFPP/Li. However, at the 45th cycle the discharge capacity decreased to 70.3 mAh·g−1, 79.5 mAh·g−1, and 97.0 mAh·g−1 for ed-NFPP/Li, NFPP/Li, and ed-NFPP/Li-Na, respectively, indicating that it was influenced by the concentration of sodium in electrolyte, because the hosting sites, suitable for sodium intercalation, are not completely suitable for lithium insertion. Indeed, ed-NFPP/Li with Li electrolyte showed the lowest discharge capacity, thus evidencing that the Li+ ions are unstable in the NFPP structure. The charge-discharge capacities vs. the cycling rate (C/10–3C) for these three cells are shown in Figure 3b (the charge rate was kept constant C/10). The specific discharge capacity was equal to 78 mAh·g−1, 83 mAh·g−1, and 91 mAh·g−1 at the 1C rate and to 28 mAh·g−1, 62 mAh·g−1, and 65 mAh·g−1 at the 3C rate for ed-NFPP/Li, NFPP/Li and ed-NFPP/Li-Na, respectively. Thus, ed-NFPP/Li-Na cycled in the mixed Li-Na electrolyte with a higher Na concentration showed the highest capacity, the best capacity retention, and the lowest polarization.
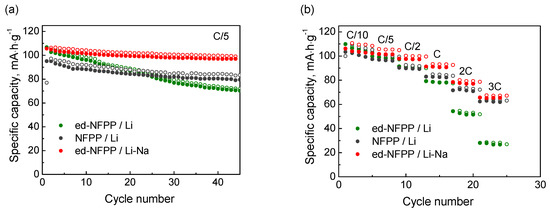
Figure 3.
Charge-discharge capacity vs. cycle number (at the C/5 cycling rate) (a) and cycling discharge rate (b) for three electrochemical cells: NFPP/Li, ed-NFPP/Li-Na and ed-NFPP/Li.
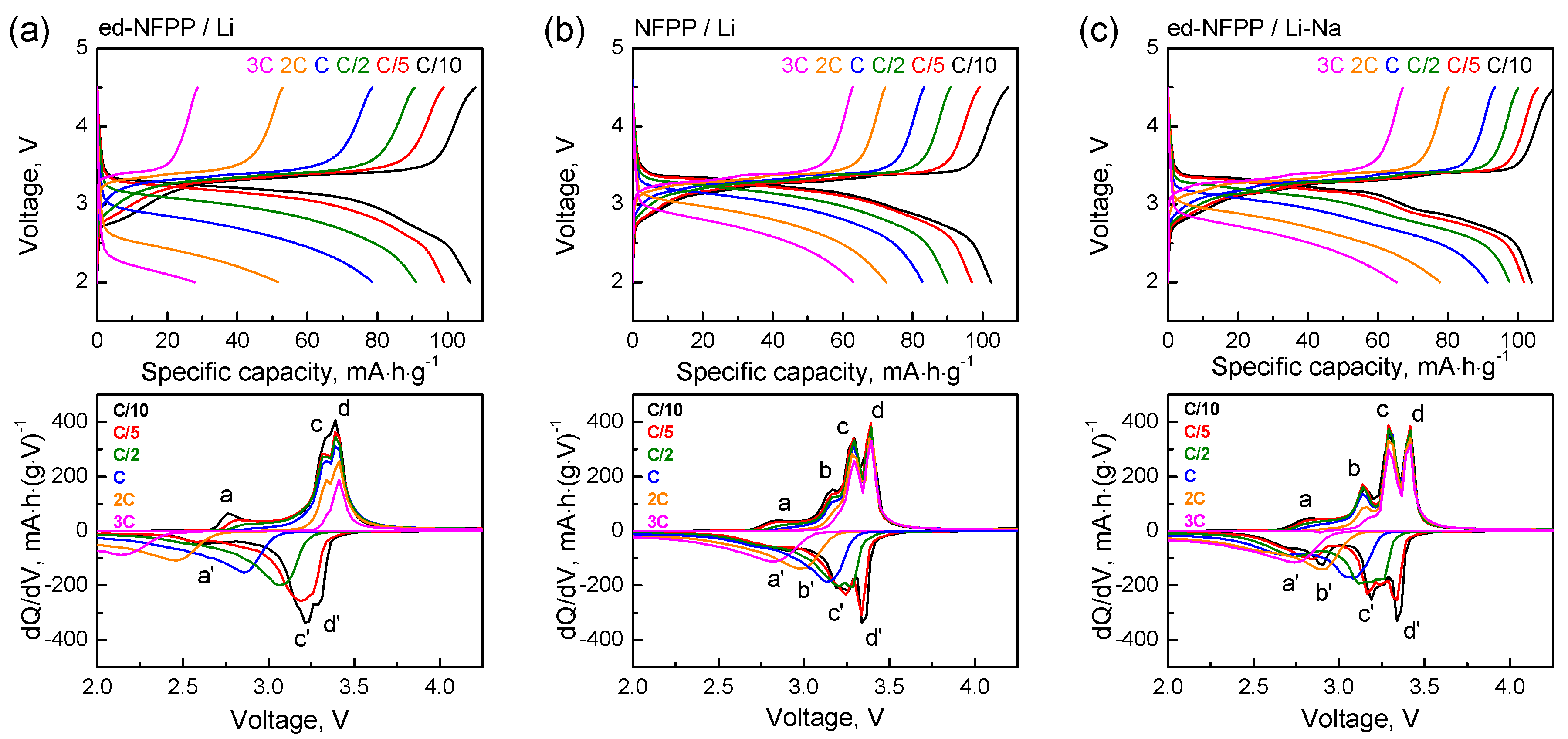
The corresponding cycling profiles and the differential capacity vs. voltage plots as a function of the discharge cycling rate were further analyzed in detail (Figure 4). Three oxidation peaks, namely, the peak with low intensity at 2.76 V and two overlapped peaks at 3.33 V and 3.39 V (labeled as a, c, and d), as well as the corresponding reduction peaks at 2.60 V, 3.21 V, and 3.29 V (labeled as a′, c′, and d′) were observed on the differential curves of the ed-NFPP/Li at C/10. With increasing the cycling rate, the redox peaks merged and their intensity decreased. Kim et al. [14] and Wu et al. [16] suggested the existence of preferences in the order of the Na extraction from the structure of NFPP, which led to the appearance of three plateaus on the charge curves. However, there is some discrepancy between the results of the first principle calculations [14] and the experimental evidence using the solid NMR spectroscopy [16]. According to Reference [14], among four symmetrically different Na sites in the NFPP structure, the Na+ ions from the Na2 site in the five-coordinated polyhedral are extracted first. Once they are fully extracted, half of the Na+ ions in the Na1 sites and half of the Na+ ions in the Na4 sites are extracted simultaneously. Finally, half of the Na+ ions in the Na3 sites and the remaining Na+ ions in the Na1 sites are extracted, leaving half of the Na3 and Na4 sites in NFPP occupied. According to reference [16], the extraction sequence of sodium can be described as follows: Na3 (five coordinated) and Na1 (six coordinated) are extracted first followed by Na4 (six coordinated), while Na2 (seven coordinated) barely participate in the reaction, thus emphasizing that the Na+ ions with fewer coordination numbers are extracted earlier. Our previous results of Mӧssbauer spectroscopy on the changes in the electronic state of the Fe ions upon cycling also confirm that the Na2 ions remain almost intact in the NFPP structure after de-sodiation [15].
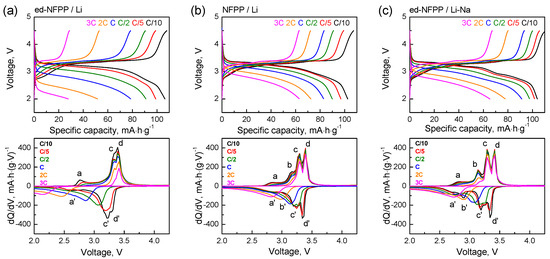
Figure 4.
Charge-discharge profiles and dQ/dV vs. voltage plots for ed-NFPP/Li (a), NFPP/Li (b) and ed-NFPP/Li-Na (c) at different cycling rates.
In the presence of the Na+ ions in the electrolyte (samples NFPP/Li and ed-NFPP/Li-Na), the oxidation peaks c and d (at 3.3 V and 3.4 V) became more distinct and pronounced; they do not change their positions and intensities with increasing the cycling rate, thus pointing to preservation of the composition and the structure of NFPP upon cycling in these two cells. Moreover, new redox peaks were present on the differential curves at 3.14 V/2.90 V (labeled as b and b′) with greater intensity for ed-NFPP/Li-Na, in which the sodium concentration in the electrolyte was higher. These peaks gradually disappeared at high cycling rates, and evidently correspond to intercalation/de-intercalation of the Na+ ions, since they were absent for ed-NFPP/Li with the Li electrolyte; however the peaks d/d′ and c/c′ were associated with the concurrent insertion/de-insertion of the Na+ and Li+ ions in the cathode reaction with the Na+ ions being predominant. Meanwhile, at high rates, the Li intercalation/de-intercalation became predominant, and the crystal structure of NFPP was stabilized due to sufficient amount of the Na+ ions.
In our previous paper [15], it was found that the composition of NFPP cycled in a Li cell, determined by ex situ energy-dispersive X-ray spectroscopy (EDS), changes from ~Na1.2Fe3(PO4)2P2O7 after the 1st charge (de-intercalation) to ~Na2.4Li1.2Fe3(PO4)2P2O7 after the 1st discharge due to the concurrent Na+/Li+-ion intercalation. This demonstrates that almost equal amounts of the de-intercalated Na+ ions that move from cathode to electrolyte on charge, and of the Li+ ions, which are available in the initial electrolyte, participate in the cathode intercalation reaction. It can be suggested that the sequence of filling the Na sites in the de-sodiated NFPP structure by the alkali ions on discharge is opposite to that on charge and can be presented as follows: Na4 to Na1 to Na3; each step must correspond to one third of the theoretical capacity (129 mAh·g−1), i.e., to 43 mAh·g−1.
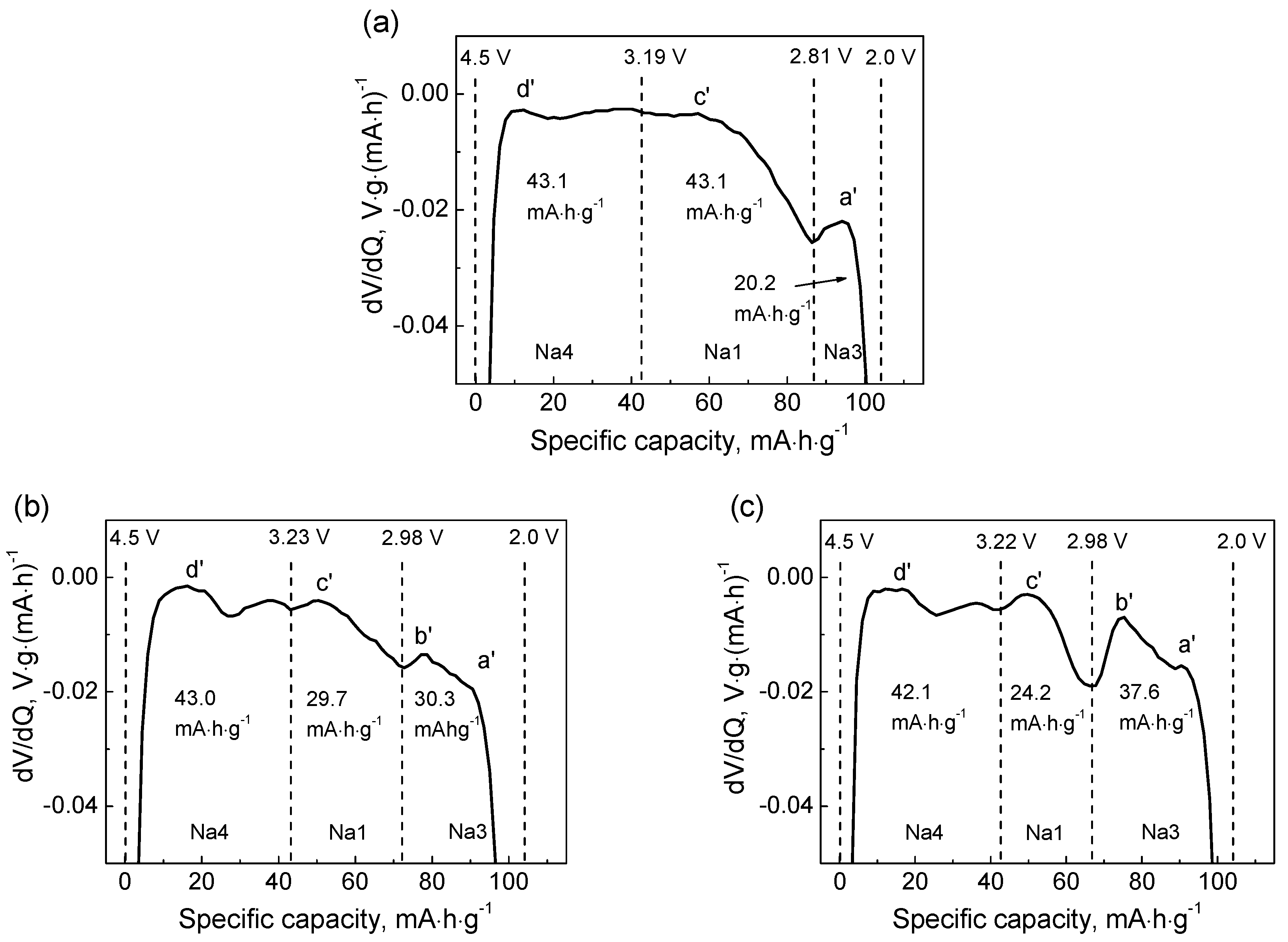
To evaluate the proportion of the intercalated alkali metal ion at different plateaus, the dV/dQ vs. Q plots for the cells with Li and mixed Li-Na electrolytes upon discharge have been plotted (Figure 5). The as-observed curves can be divided into three parts, relating to the reduction peaks d′, c′ and a′. For ed-NFPP/Li, these capacities were equal to 43.1 mAh·g−1, 43.1 mAh·g−1, and 20.2 mAh·g−1, respectively, and correspond to the three sequential steps mentioned above. It was seen that the Li+ ions first insert the Na4 and Na1 sites, and then the Na3 sites. For ed-NFPP/Li-Na, the total capacity of the second step decreases, but the additional capacity arose due to the new peak b′, which indicates that the intercalation degree increases at a lower voltage. Thus, it can be suggested that the insertion of the alkali ions in ed-NFPP/Li-Na starts with the concurrent Li+/Na+ insertion at a high voltage and is followed by the predominant Na+-ion insertion at a lower voltage (peak b′), since it is absent for ed-NFPP cycled with the Li electrolyte. The situation is similar for NFPP/Li, though the low-voltage capacity is a little lower that corresponds to the lower amount of sodium in the electrolyte. These two samples show good capacity retention on cycling along with high Coulomb efficiency due to an increased amount of Na in the solid phase, which stabilize the NFPP crystal structure. A similar positive effect of mixed Li-Na electrolyte has been recently observed by Xiong et al. [17], who investigated the electrochemical performance of Na3V2(PO4)2F3 in a Li-Na dual ion hybrid cell.
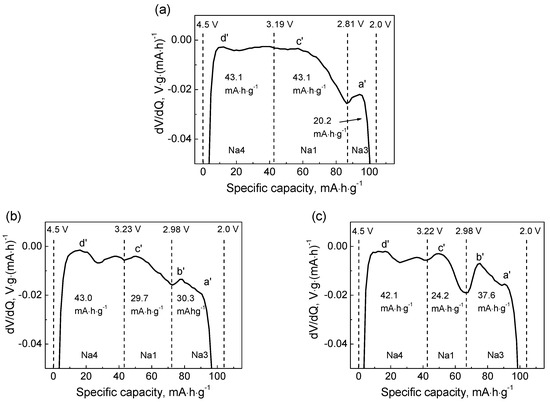
Figure 5.
dV/dQ vs. voltage plots for ed-NFPP/Li (a), NFPP/Li (b) and ed-NFPP/Li-Na (c).
When considering the effect of the mixed Li-Na electrolyte on the high-rate performance of NFPP cathode, the competitive processes with participation of the Na+ and Li+ ions in hybrid cells should be also taken into account; they include (i) diffusion in the electrolyte; (ii) diffusion through the solid-electrolyte interface (SEI); (iii) diffusion in the solid phase; iv) Na+/Li+ electrochemical exchange. According to [18,19], ionic conductivity of LiPF6 in the EC-DMC based electrolyte twice exceeds that of NaPF6. On the other hand, slow Li+ diffusion through the solid-electrolyte interface may impede the Li+ intercalation/de-intercalation processes and lead to deterioration of the electrochemical performance of ed-NFPP/Li, while diffusion of the Na+ ions through SEI is superior because of its lower polarizing ability than that of Li [20]. On the contrary, the diffusion coefficient of the Li+ ions in the solid state is slightly higher than that of the Na+ ions due to smaller ionic radius of Li+ [15]. However, the Li+ ions are unstable in the NFPP structure. Even after prolong cycling of NFPP in the Li electrolyte, the Li+ ions, incorporated into the structure of NFPP, readily exchange for the Na+ ions from electrolyte [15].
3. Materials and Methods
Na4Fe3(PO4)2P2O4/C (NFPP/C) was synthesized by a mechanochemically assisted solid-state rout, as detailed in the previous report [15]. Stoichiometric amounts of Na4P2O7 (95%), Fe2C2O4·2H2O (99%), and (NH4)2HPO4 (98%) were used as the reagents. An appropriate amount of soot (3 wt% to the final product) was added to the reagent mixture as a carbon coating agent. The reagent mixture underwent short-term (5 min) mechanical activation (MA) in an Ar atmosphere using a high-energy planetary mill AGO-2 (900 rpm). The activated mixture was annealed at a temperature of 450 °C for 4 h under an Ar flow. The annealed mixture was then cooled to room temperature at a naturally cooling rate.
Electrochemically de-sodiated NFPP (ed-NFPP) was prepared as follows. The NFPP electrode was first charged in a Li cell to extract three Na+ ions per f.u. from its structure. Then the cell was disassembled in a glovebox; the cathode material was thoroughly washed with dimethyl carbonate, and ed-NFPP electrode was reassembled in a new cell with the fresh Li or mixed Li-Na electrolyte.
Crystal structure of the as-prepared samples was analyzed by X-ray diffraction (XRD). The XRD patterns were obtained by a D8 Advance Bruker diffractometer (Bruker AXS Gmbl, Karlsruhe, Germany) with Cu Kα irradiation (λ = 1.54184 Å) within the 2θ = 5–70° range with the step of 0.02° s−1 and the uptake time 0.2 s. The structural refinement of the XRD data was carried out by the Rietveld method using the GSAS software package.
For the electrochemical testing, the composite cathodes were fabricated using 75 wt% active material, 20 wt% Super P as a conductive carbon, and 5 wt% PVDF/NMP as a binder. The mixed slurry was then pasted on the aluminum foil to obtain the working electrodes. The loading density of the prepared samples was 2–3 mg·cm−2, and an electrode diameter of 10 mm was used throughout. The working electrodes were dried at 100 °C in vacuum before cell assembly. The Swagelok-type cells were assembled in an argon-filled glovebox with Li metal as an anode and 1M LiPF6 (Sigma Aldrich, 99.99%) solution in a mixture of ethylene carbonate (Alfa Aeser, 99%) and dimethyl carbonate (Alfa Aesar, 99%) 1:1 by weight as a Li electrolyte. Hybrid solutions consist of 0.9 mol·L−1 LiPF6 and 0.1 mol·L−1 NaPF6 in ethylene carbonate and dimethyl carbonate (1:1 by weight). A glass fiber filter (Whatman, Grade GF/C GE Healthcare UK Ltd., Little Chalfont, UK) was used as a separator. The cycling was performed in a galvanostatic mode at cycling rates of C/10–3C in the range of 2.0–4.5 V vs. Li/Li+, keeping the charging rate constant (C/10).
4. Conclusions
In the present work, the effect of the mixed Li-Na electrolyte on the electrochemical performance of NFPP in HIB and the participation of the Li+ and Na+ ions in charge-discharge cathode reactions have been studied. Three types of electrochemical cells were analyzed: the de-sodiated NFPP with Li electrolyte, and the pristine NFPP and the de-sodiated NFPP with the mixed Li-Na electrolyte. It was shown that in all cases, full Na/Li electrochemical exchange was not achieved, because the hosting sites, suitable for the Na intercalation, are not completely suitable for the Li+ ions. The insertion of the Li+ ions does not cause noticeable structural changes in NFPP. A concurrent insertion mechanism of the Li+ and Na+ ions in the NFPP structure was proposed at high voltage, followed by the predominant Na+-ion insertion in the Na sites with fewer coordination numbers at lower voltage. It has been shown that if the Na concentration in the mixed Li-Na electrolyte increases to 0.9/0.1 mol·L−1, the electrochemical performance of NFPP is significantly improved. Meanwhile, at high rates, the Li intercalation/de-intercalation becomes predominant at high voltage, and the crystal structure of NFPP is stabilized due to sufficient amount of the Na+ ions. Thus, the electrochemical performance of NFPP can be tuned by changing the concentration of the Na+ ions in the mixed Li+/Na+-ion electrolyte and current density. The preliminarily de-sodiated ed-NFPP/Li-Na cycled in the mixed Li-Na electrolyte shows the highest specific capacity, the best capacity retention and high-rate capability.
Author Contributions
N.V.K. conceived and designed the experiments; A.A.S. performed the experiments; both authors participated in the analysis of the experimental data and in writing the paper.
Funding
This research was carried out within the State Assignment to ISSCM SB RAS (project 0301-2018-0001).
Acknowledgments
The authors are thankful to Valery A. Belotserkovsky for his help in the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, B.; Murugesan, C.; Sharma, L.; Lochab, S.; Barpanda, P. An overview of mixed polyanionic cathode materials for sodium-ion batteries. Small Methods 2018, 1800253. [Google Scholar] [CrossRef]
- Sagane, F.; Abe, T.; Iriyama, Y.; Ogumi, Z. Li+ and Na+ transfer through interfaces between inorganic solid electrolytes and polymer or liquid electrolytes. J. Power Sources 2005, 146, 749–752. [Google Scholar] [CrossRef]
- Yao, H.-R.; You, Y.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Rechargeable dual-metal-ion batteries for advanced energy storage. Phys. Chem. Chem. Phys. 2016, 18, 9326–9333. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Gover, R.K.B.; Burns, P.; Bryan, A.J. Hybrid-ion a lithium-ion cell based on a sodium insertion material. Electrochem. Solid-State Lett. 2006, 9, A190–A192. [Google Scholar] [CrossRef]
- Barker, J.; Gover, R.K.B.; Burns, P.; Bryan, A.J. Li4/3Ti5/3O4 || Na3V2(PO4)2F3: An example of a hybrid-ion cell using a non-graphitic anode. J. Electrochem. Soc. 2007, 154, A882–A887. [Google Scholar] [CrossRef]
- Song, W.; Ji, X.; Pan, C.; Zhu, Y.; Chen, Q.; Banks, C.E. A Na3V2(PO4)3 cathode material for use in hybrid lithium ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 14357–14363. [Google Scholar] [CrossRef] [PubMed]
- Kosova, N.V.; Rezepova, D.O. Na1+yVPO4F1+y (0 ≤ y ≤ 0.5) as cathode materials for hybrid Na/Li batteries. Inorganics 2017, 5, 19. [Google Scholar] [CrossRef]
- Song, W.; Ji, X.; Wu, Z.; Zhu, Y.; Yao, Y.; Huangfu, K.; Chena, Q.; Banks, C.E. Na2FePO4F cathode utilized in hybrid-ion batteries: A mechanistic exploration of ion migration and diffusion capability. J. Mater. Chem. A 2014, 2, 2571–2577. [Google Scholar] [CrossRef]
- Kosova, N.V.; Podugolnikov, V.R.; Devyatkina, E.T.; Slobodyuk, A.B. Structure and electrochemistry of NaFePO4 and Na2FePO4F cathode materials prepared via mechanochemical route. Mater. Res. Bull. 2014, 60, 849–857. [Google Scholar] [CrossRef]
- Kosova, N.V.; Rezepova, D.O.; Petrov, S.A.; Slobodyuk, A.B. Electrochemical and chemical Na+/Li+ ion exchange in Na-based cathode materials: Na1.56Fe1.22P2O7 and Na3V2(PO4)2F3. J. Electrochem. Soc. 2017, 164, A6192–A6200. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, Y.; Shao, H. Enhanced cycleability and dendrite-free lithium deposition by addition of sodium ion in electrolyte for lithium metal batteries. Electrochim. Acta 2018, 271, 617–623. [Google Scholar] [CrossRef]
- Kim, H.; Park, I.; Seo, D.-H.; Lee, S.; Kim, S.-W.; Kwon, W.J.; Park, Y.-U.; Kim, C.S.; Jeon, S.; Kang, K. New iron-based mixed-polyanion cathodes for lithium and sodium rechargeable batteries: Combined first principles calculations and experimental study. J. Am. Chem. Soc. 2012, 134, 10369–10372. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, I.; Lee, S.; Kim, H.; Park, K.-Y.; Park, Y.-U.; Kim, H.; Kim, J.; Lim, H.-D.; Yoon, W.-S.; et al. Understanding the electrochemical mechanism of the new iron-based mixed-phosphate Na4Fe3(PO4)2(P2O7) in a Na rechargeable battery. Chem. Mater. 2013, 25, 3614–3622. [Google Scholar] [CrossRef]
- Kosova, N.V.; Belotserkovsky, V.A. Sodium and mixed sodium/lithium iron ortho-pyrophosphates: Synthesis, structure and electrochemical properties. Electrochim. Acta 2018, 278, 182–195. [Google Scholar] [CrossRef]
- Wu, X.; Zhong, G.; Yang, Y. Sol-gel synthesis of Na4Fe3(PO4)2(P2O7)/C nanocomposite for sodium ion batteries and new insights into microstructural evolution during sodium extraction. J. Power Sources 2016, 327, 666–674. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, Y.; Shao, H.; Yang, Y. Understanding the electrochemical mechanism of high sodium selective material Na3V2(PO4)2F3 in Li+/Na+ dual-ion batteries. Electrochim. Acta 2018, 292, 234–246. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Guyomard, D. New electrolyte compositions stable over the 0 to 5 V voltage range and compatible with the Li1+xMn2O4/carbon Li-ion cells. Solid State Ionics 1994, 69, 293–305. [Google Scholar] [CrossRef]
- Bhide, A.; Hofmann, J.; Durr, A.K.; Janek, J.; Adelhelm, P. Electrochemical stability of non-aqueous electrolytes for sodium-ion batteries and their compatibility with Na0.7CoO2. Phys. Chem. Chem. Phys. 2014, 161987. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, V.A.; Fedotov, S.S.; Vassiliev, S.Y.; Samarin, A.S.; Khasanova, N.R.; Antipov, E.V. Transport and kinetic aspects of alkali metal ions intercalation into AVPO4F framework. J. Electrochem. Soc. 2017, 164, A6373–A6380. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).