Abstract
Recycling spent lithium-ion batteries (LIBs) is crucial to prevent environmental pollution and recover valuable metals. Traditional methods for recycling spent LIBs include hydrometallurgy and pyrometallurgy. Among these methods, solvent extraction can selectively extract valuable metals in spent LIB leachate. Meanwhile, spent LIBs that underwent pyrometallurgical treatment generate a so-called ‘black alloy’ of Ni, Co, Cu, and so on. These elements in the black alloy need to be separated by solvent extraction and there have been few studies on extracting valuable metals from black alloy. Therefore, it is necessary to examine the extraction behavior of elements in black alloy and optimize the solvent extraction process to recover valuable metals. In this paper, four types of organic extractants are used to extract metals from simulated black alloy leachate: di-(2ethylhexyl) phosphoric acid (D2EHPA), bis-(2,4,4-trimethylpentyl) phosphinic acid (Cyanex272), 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (PC88A), and neodecanoic acid (Versatic acid 10). Based on the pH isotherms, D2EHPA would be the most reasonable for Mn extraction and impurity removal. Cyanex 272 would be more suitable for Co separation than PC88A, and Versatic acid 10 is preferred for Cu extraction over other metals. In conclusion, the optimal combination of extractants is suggested for the recovery of valuable metals.
1. Introduction
A growing demand for lithium-ion batteries (LIBs) would lead to a significant increase in spent batteries in the near future. According to the International Energy Agency Stated Policy and Net Zero Emissions by 2050 Scenarios [1], electricity will become the dominant fuel in the global transport sector by the early 2040s, with electric vehicles increasing from around 5% of global car sales to more than 60% by 2030. Additionally, the demand for lithium used in the batteries is expected to grow 30-fold by 2030 and is expected to be more than 100 times higher in 2050 compared to 2020. Consequently, recycling spent LIBs is crucial to prevent environmental pollution and recover valuable metals [2]. Conventional methods for recycling spent LIBs include hydrometallurgy and pyrometallurgy [3]. The hydrometallurgical recycling process typically involves leaching, purification, and separation methods to acquire final products [4,5]. Furthermore, this process necessitates a pretreatment step aiming at the safe and effective separation of different components in LIBs [6,7]. As an essential procedure in the hydrometallurgical recycling process, leaching is used to dissolve metals from spent LIBs into leachate using acid solutions such as sulfuric acid (H2SO4) and hydrochloric acid (HCl) for subsequent processing [8,9,10,11]. Metals are recovered through a sequence of chemical methods such as precipitation, solvent extraction, and electrolytic deposition. The hydrometallurgical recycling offers advantages including high metal selectivity, high recycling efficiency, and reduced waste emission [12]. In contrast, the pyrometallurgical recycling process was firstly industrialized and can treat spent LIBs in large quantities [13,14]. This is achieved by using a high-temperature furnace to reduce metal oxides to a so-called ‘black alloy’ of Ni, Co, Cu, and other elements [15,16]. Contrary to the hydrometallurgical process, this pyrometallurgical process can be completed simply without the necessity of the pretreatment step. However, the pyrometallurgical process loses a portion of valuable metals in a form of slag or vapor [17,18,19]. Economic efficiency is also a crucial aspect of the recycling industry, with the economic efficiency of the pyrometallurgical process largely depending on the kinds of recovered metals [19,20]. Therefore, it is necessary to separate elements in black alloy to recover valuable metals including Ni and Co. According to a couple of previous studies, combining pyrometallurgical and hydrometallurgical processes can offer a more practical approach to effectively retrieve valuable metals and reduce operation costs [20,21,22].
Among separation methods employed in the LIB recycling, solvent extraction has been popular due to its high recovery efficiency, mild experimental conditions, and simple operation [23,24,25,26]. Metallic ions are separated based on their different solubility in liquid phases that are immiscible with each other. Generally, valuable metals from LIB leachate are extracted into organic extractants, as expressed below [27]:
where R is an organic extractant and Me is a metal in leachate. The metal ion reacts with the extractant to generate protons, and the pH of the aqueous solution significantly influences the extraction of metal ions. Currently used organic extractants are commonly divided into neutral extractants and acid extractants. The acidic extractants can be further divided into organic phosphoric acid extractants and carboxylic acid extractants. Specifically, di(2-ethylhexyl) phosphoric acid (D2EHPA), bis(2,4,4-trimethylpentyl) phosphinic acid (Cyanex 272), and 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (PC88A) are types of the organic phosphoric acid extractant, while the carboxylic acid extractant includes 2-ethyl-2-methylheptanoic acid (Versatic acid 10).
nRH(org) + Men+ (aq) → RnMe(org) + nH+(aq),
However, there have been few studies on extracting valuable metals from the leachate of black alloy [28,29]. The previous studies focused on impurity removal or the extraction of certain metals using a particular type of organic extractant without comparing the performance of various types of solvent extractants. Due to the lack of comprehensive solvent extraction data on black alloy, it is necessary to verify the extraction behavior of all elements that might exist during the recycling process of spent LIBs. It is expected that black alloy and black mass, which undergoes a physical comminution step, have different elemental compositions, and each element would exhibit different extraction behaviors depending on the organic extractants. Therefore, it is important to examine the extraction behavior of elements in black alloy and optimize the solvent extraction process to circulate battery raw materials from spent LIBs. In this study, we examine the extraction efficiency of metals based on the pH isotherms of simulated black alloy leachate and suggest an optimum combination of extractants for the recovery of valuable metals.
2. Materials and Methods
As can be seen from Table 1, the composition of black mass provided by an LIB recycling company (SungEel Hi-Tech, Gunsan, Republic of Korea) includes the constituent elements of LiNixCoyMnzO2 (NCM) cathode materials such as Ni, Co, and Mn with the metallic impurity elements of Al, Fe, Cu, Zn, Ca, and Mg. These impurity elements are known to originate from LIB current collectors, casings, and the hydrometallurgical process of LIB recycling [30]. Meanwhile, black alloys 1 and 2 from the two available relevant references did not show exactly the same compositions, even considering the difference in units for displaying the composition of black alloys in each reference [28,29]. However, it is evident that black mass and black alloy have distinct compositions from each other. Specifically, the concentration of Al decreases in black alloy compared to black mass, while Cu is relatively more abundant in black alloy. Additionally, trace amounts of Zn, Ca, and Mg are also present in both black mass and black alloy. Consequently, the elemental concentrations in this study were designed based on the compositions of black mass and black alloy as shown in Table 1. Al and Fe were excluded from the experimental leachate under investigation because they are usually removed prior to solvent extraction by using a precipitation method, where the pH of the leachate is regulated by adding an alkaline solution, such as sodium hydroxide (NaOH), due to their distinct precipitation behavior [31].

Table 1.
Elemental concentrations in black mass, black alloy, and the leachate in this study.
Both aqueous and organic solutions were prepared to examine the extraction behavior of black alloy leachate as follows. In the case of organic solution, four types of organic extractants were used to extract the metals from the black alloy leachate: D2EHPA, Cyanex 272, PC88A, and Versatic acid 10. Kerosene (Exxsol D80), which prevents the formation of a third phase during the extraction process, was used as a diluent to adjust the concentration of organic extractants. Regarding the aqueous solution, we utilized actual purified leachate provided by the LIB recycling company (SungEel Hi-Tech, Gunsan, Korea). The industrial leachate originating from black mass was diluted because the Co concentration is higher in the industrial leachate than that in the black alloy-based leachate in Table 1. Accordingly, the composition of the diluted industrial leachate was adjusted by adding certain elements to meet a target composition reflecting the black alloy composition.
The extraction efficiency of metals was evaluated, and their extraction behaviors were compared by analyzing extraction isotherms. The concentrations of organic extractants (D2EHPA, Cyanex 272, PC88A, and Versatic acid 10) were diluted to 1.0 M with Exxsol D80. Initially, a mixture of equal volumes (250 mL) of aqueous and organic phases was stirred in a glass vessel for 30 min at room temperature. The pH of the mixture was continuously monitored with a pH meter and adjusted by adding either H2SO4 or NaOH. A portion of the aqueous phase was sampled in an equilibrium pH range from 1.0 to 6.5. The concentrations of elements in the aqueous phase were diluted to an appropriate concentration and analyzed using inductively coupled plasma optical emission spectroscopy (ICP-OES). The extraction efficiency of a specific metal can be calculated by using the following equation after the specific metal is extracted from the aqueous phase to the organic phase:
where Maq is the initial mass in the aqueous phase before extraction and Morg is the mass in the organic phase after extraction. Morg is estimated from a difference in the mass of each element before and after extraction [29].
Extraction efficiency = Morg/Maq × 100%,
3. Results
3.1. Extraction pH Isotherms for D2EHPA
Figure 1 shows the extraction pH isotherms of the elements in the case of D2EHPA. Ca was extracted from a low pH of 2.0 and extracted completely after pH 3.0. Cu and Mn exhibited a similar extraction behavior, where their extraction started at pH 2.0 and was completed after pH 5.0. Also, Mg displayed a similar extraction behavior to Cu and Mn, where the extraction started at pH 1.0 and nearly 100% extraction efficiency was achieved at pH 6.5. While the starting pH of extraction was different for Co (3.0) and Zn (1.0), their final extraction yield at pH 6.5 was similarly about 60%. Lastly, the extraction efficiency of Ni reached approximately 33% at pH 6.5 with pH 3.0 being the starting pH. Overall, D2EHPA would be the most suitable extractant for Mn extraction due to its selective separation of Mn from the leachate, while some impurities such as Cu, Ca, and Zn could also be extracted simultaneously.
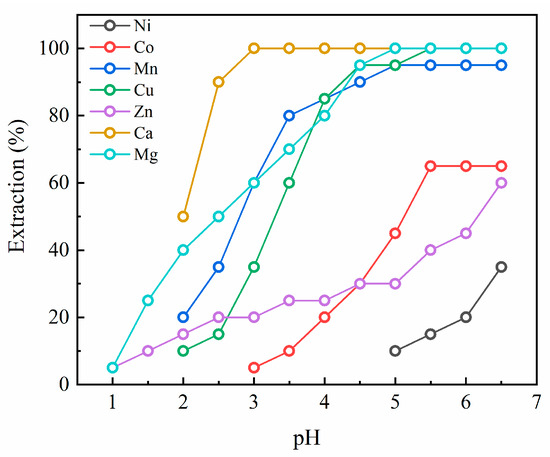
Figure 1.
Extraction pH isotherms for D2EHPA.
3.2. Extraction pH Isotherms for Cyanex 272
Extraction pH isotherms for Cyanex 272 are presented in Figure 2. Most elements displayed 100% extraction yield at the endpoint of pH 6.5, except for Ni and Zn. The 100% extraction efficiency of Cu was achieved at pH 5.5 with its extraction initiating at a low pH of 2.0. Ca and Mn exhibited a similar extraction behavior, where their extraction finished around pH 6.0 and started at pH 3.5 and 4.0, respectively. The extraction efficiency of Co and Mg reached nearly 100% at pH 6.5, with Co being extracted at pH 2.5 and Mg extracted at 1.0. The extraction yield of Zn increased slowly with increasing pH and was saturated to approximately 30% by the endpoint. The Ni extraction was negligible from pH 1.0 to 5.5 and about 5% of Ni started to be extracted from pH 5.5 with no further increase in its extraction efficiency.
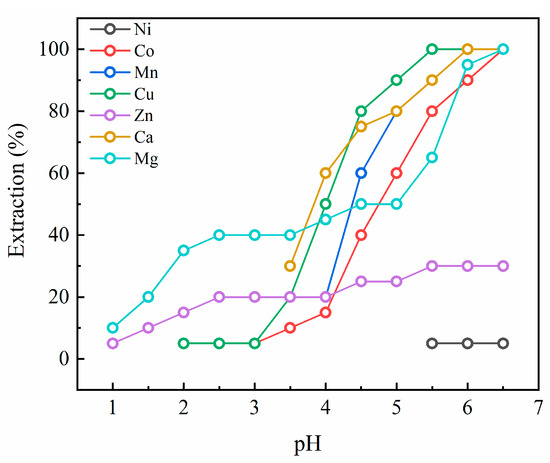
Figure 2.
Extraction pH isotherms for Cyanex 272.
3.3. Extraction pH Isotherms for PC88A
Figure 3 presents the extraction pH isotherms of the elements in the case of PC88A. Mn and Cu showed an identical extraction behavior where their extraction started at pH ~3 and reached 100% at pH 5.5. Similarly, Mg and Co started to be extracted at pH 3.5 and their extraction was saturated to 100% in a pH range of 5.5 and 6. Although Ca and Zn started to be extracted from pH 2.5~3.0, their extraction efficiency increased gradually and failed to reach 100% at the endpoint. Similar to the cases of D2EHPA and Cyanex 272, the extraction efficiency of Ni was the lowest throughout the whole pH range in PC88A.
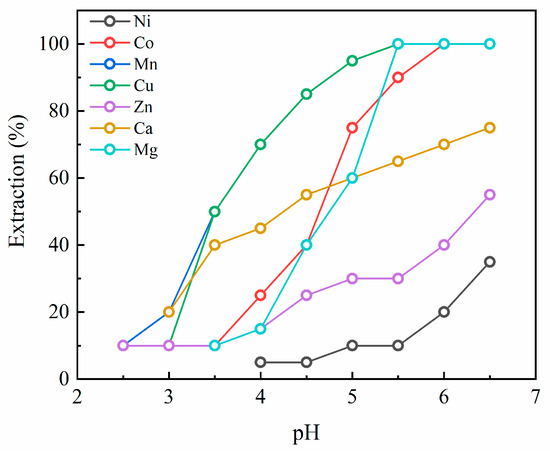
Figure 3.
Extraction pH isotherms for PC88A.
3.4. Extraction pH Isotherms for Versatic Acid 10
Figure 4 indicates the extraction pH isotherms of the elements in the case of Versatic acid 10. Because most elements except Cu are extracted beyond pH 6.5, we extended the maximum pH from pH 6.5 to 8.0 for the Versatic acid 10 solvent extraction experiments. Even though Cu, Zn, Ni, and Co showed 100% extraction efficiency at pH 8.0, their extraction behaviors were quite different between Cu and the other metals. Whereas the extraction of Cu initiated at pH 3.5 and nearly finished around pH 4.0, the other metals displayed almost similar extraction behavior from pH 5.0 to 8.0 except that Zn started its extraction at a slightly lower pH of 2.5. The extraction of Mn began at pH 5.0 with its efficiency reaching about 80% at pH 8.0, while Ca was extracted at pH 6.0 and showed 45% extraction efficiency at pH 8.0. The extraction of Mg started from a low pH of 2.0, and its extraction efficiency reached only about 50% at pH 8.0. Versatic acid 10 displayed distinct features in contrast to the other extractants. Initially, it demonstrated a propensity for Cu extraction to occur predominantly before pH 5, suggesting its effectiveness in separating Cu from the black alloy leachate. However, Versatic acid 10 is not preferred for the extraction of Mn because Mn is expected to be co-extracted with the other metals except Cu, unlike the other extractants of D2EHPA, Cyanex 272, and PC88A. Therefore, Versatic acid 10 could be excluded in the selection of extractants for leachate containing the significant amounts of Ni, Co, and Mn.
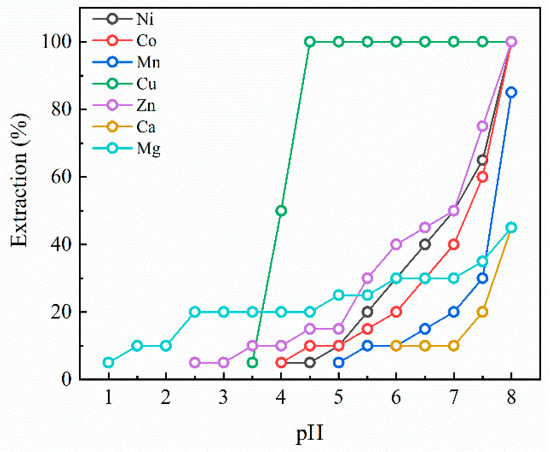
Figure 4.
Extraction pH isotherms for Versatic acid 10.
3.5. Extraction Behaviors of the Metals
The extraction behaviors of the elements depending on the kinds of organic extractants except for Versatic acid 10 are summarized in Figure 5. Ni was not practically extracted in Cyanex 272 while its extraction efficiency was generally low in D2EHPA and PC88A. Whereas the extraction efficiency of Co showed similar values at low pH among the three extractants, it reached 100% in Cyanex 272 and PC88A and was saturated to ~60% at high pH in D2EHPA. Mn and Cu showed a similar extraction behavior for all the extractants, where the extraction of Mn and Cu started in a low pH range and their extraction efficiency reached nearly 100% at the endpoint. While the extraction yield of Mg was 100% for all the extractants, that of Ca did not reach 100% at the endpoint only with PC88A.
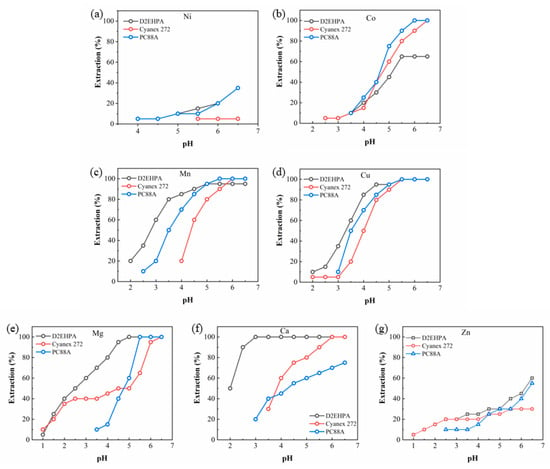
Figure 5.
Extraction behaviors of (a) Ni, (b) Co, (c) Mn, (d) Cu, (e) Zn, (f) Ca, and (g) Mg in D2EHPA, Cyanex 272, and PC88A.
However, the extraction efficiency of Zn was below 50% with all the extractants while the other elements were extracted at over 50% efficiency. Contrary to previous studies that Zn was extracted completely for all extractants including Versatic acid 10 [32], the extraction efficiency of Zn failed to reach 100% in this study although the extraction of Zn initiated in a low pH range. Therefore, additional extraction experiments were conducted to examine the possibility of extraction interference among the elements. For this purpose, the Zn extraction was carried out in a ZnSO4 solution in the absence of the other elements with identical experimental conditions including the kinds and volume ratio of organic extractants and reaction time. Figure 6 shows the extraction pH isotherms of Zn with four kinds of extractants. Zn was completely extracted by all the extractants, similar to the previous studies where it was solely present. Judging from the fact that the extraction yield of Zn was lower in the leachate from black alloy than in the ZnSO4 solution, it was concluded that Zn would be susceptible to interference from the other elements, resulting in a decrease in its extraction efficiency.
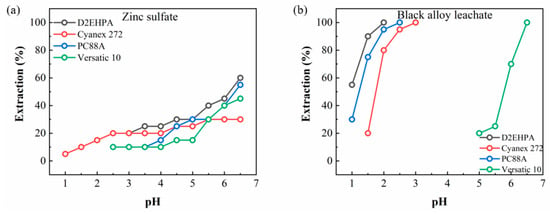
Figure 6.
Extraction behaviors of Zn in two different solutions: (a) zinc sulfate and (b) black alloy leachate.
4. Discussion
To select an optimum combination of extractants for the separation of Ni, Co, and Mn, which are the NCM cathode constituent elements of LIBs and often target elements for recovery through solvent extraction, from the minor impurity elements (Cu, Zn, Ca, and Mg), their extraction behaviors depending on the four kinds of extractants were summarized as follows. D2EHPA proved to be the most appropriate extractant for Mn since its extraction efficiency was more than 80% while Ni and Co were extracted at approximately 10% in a pH range of 3–4. Cyanex 272 was found to be more suitable than PC88A for the separation of Co from Ni, as the difference in extraction yield between Co and Ni was larger in the case of Cyanex 272. Versatic acid 10 is not considered further for the recovery of Ni, Co, and Mn owing to their co-extraction behavior in this extractant. We verified our results by calculating the distribution ratio and separation factor. The distribution ratio (D) was calculated by using the following equation:
where [M]org and [M]aq are the concentrations of the organic and aqueous solutions, respectively. Additionally, separation factor (β) can be represented by the distribution ratio of two metals such as D1/D2. A high distribution ratio indicates that a specific metal is efficiently extracted, while a high separation factor means that a metal is extracted more efficiently than another. As can be seen in Table 2, the comparison of β values in the case of D2EHPA indicates that the separation of Mn is more effective than that of Co and Ni. Similarly, the comparison between the β values of Cyanex 272 and PC88A implies that the Co separation is facilitated in Cyanex 272. Table 3 also represents that the extraction of Ni, Co, and Mn is expected to be insignificant unlike Cu.
Distribution ratio = [M]org/[M]aq,

Table 2.
Separation factor of two adjacent NCMs by D2EHPA, Cyanex 272, and PC88A.

Table 3.
Distribution ratio of Ni, Co, Mn, and Cu by Versatic acid 10.
The minor impurity elements were predominantly extracted in a low pH range with D2EHPA, while they were also removed in a relatively higher pH range with the other extractants. Taking the abovementioned extraction behaviors in the extractants into account, it would be reasonable to apply D2EHPA first to the black alloy leachate for the Mn removal. Then, Co needs to be removed in the Mn-removed solution by Cyanex 272, which is preferred to PC88A. In the meantime, multi-stage extraction is often attempted to achieve high selectivity in conventional solvent extraction processes [33]. Accordingly, an optimum process design is sought by simulating solvent extraction procedures based on single or two-stage extraction with D2EHPA followed by Cyanex 272. The extraction efficiency and purity of Ni, Co, and Mn were compared in each simulated extraction procedure. The purity of Mn and Co was calculated based on their concentration in extracted D2EHPA and Cyanex 272, respectively. The purity of Ni was determined from its concentration in the Mn/Co-removed aqueous phase.
As mentioned, an optimum pH range of D2EHPA for effectively separating Mn from Ni and Co would be between 3 and 4. Therefore, we examined four scenarios by combining the extraction pH levels of 3 and 4 with the number (1 and 2) of extraction stages. Figure 7 represents the extraction efficiency and concentrations of Ni, Co, and Mn in the Mn-extracted solutions for each scenario. In all four cases, Ni was not extracted and only a small amount of Co was extracted, whereas Mn showed comparatively higher extraction efficiency as shown in Figure 7a. It was observed that the extraction efficiency of Mn reached 100% at pH 4.0 with a two-stage extraction and increased as both pH and the number of extraction stage increased. Considering the extraction yield of Mn, a pH of 4 with the two-stage extraction appeared most reasonable, but the purity of recovered Mn was expected to be low owing to the significant amount of co-extracted Co. In addition to the case of two-stage Mn extraction at a pH of 4, the two-stage Mn extraction at a pH of 3 could deserve consideration due to its fairly high Mn extraction efficiency and its high Mn selectivity over Co. Therefore, subsequent extraction processes were simulated with the two types of Mn-removed solutions, which were a pH level of 3.0 with two stages (Mn3-2) and a pH level of 4.0 with two stages (Mn4-2). Figure 7b illustrates the concentrations of Ni, Co, and Mn in the Mn-extracted solution, with each elemental concentration showing a similar trend to the extraction efficiency.
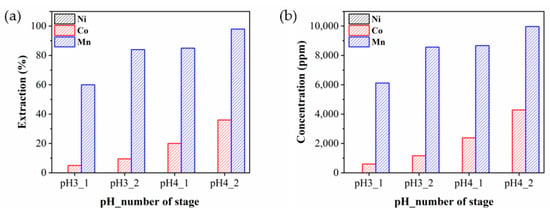
Figure 7.
(a) Extraction efficiency and (b) concentration of Ni, Co, and Mn in the Mn-extracted solutions.
Figure 8 displays the extraction efficiency and concentrations of Ni, Co, and Mn in the Co-extracted solutions by using Cyanex 272 at a pH level of 5.0 in a configuration of single or two stages after the Mn extraction. The pH level of 5.0 was determined because the separation of Co and Ni was most effective as seen in Figure 2, where the extraction efficiency of Co reached 60% with Ni remaining unextracted. However, extracting Co above pH 5.0 would be deemed inappropriate despite an increase in its extraction yield, since Ni was the most abundant element in the Mn-extracted solutions and a slight increase in the extraction efficiency of Ni would lead to a significant decrease in the resulting Co purity. Ni was not extracted in all four cases in Figure 8a while the extraction efficiency of Co was 60% to 80%. On the other hand, Mn also exhibited a high extraction efficiency, which was attributed to the extraction behavior of Mn with Cyanex 272 at pH 5.0. However, the actual Mn concentration in the Co-extracted solutions was very low because most of the Mn had been previously extracted with D2EHPA, as displayed in Figure 8b. The purity of Co was estimated to be 95%, 94%, 77%, and 74% in Mn4-2_2, Mn4-2_1, Mn3-2_2, and Mn3-2_1, respectively.
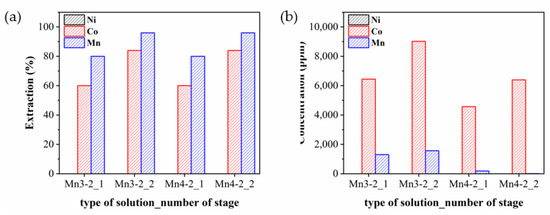
Figure 8.
(a) Extraction efficiency and (b) concentration of Ni, Co, and Mn in the Co-extracted solutions.
Figure 9 displays the concentrations of Ni, Co, and Mn in the Mn/Co-extracted solutions after the Co extraction. The concentration of Ni remained constant at ~36,000 ppm in all the cases, but a difference in purity occurred due to the residual presence of Co and Mn. Specifically, the purity of Ni was highest at 97% with two-stage extraction for the Mn4-2 solution, while the Ni purity was estimated to be 92%, 95%, and 88% in Mn4-2_1, Mn3-2_2, and Mn3-2_1, respectively. In conclusion, the optimal combination for the separation of Ni, Co, and Mn should involve more than single-stage extraction, with Mn effectively separated at pH 3.0 or 4.0 by D2EHPA and Co extracted at pH 5.0 with Cyanex 272. While Mn4-2_2 is the most reasonable combination for recovering Ni in terms of both Ni recovery rate and purity, the concentration of Co was ~6400 ppm in the Co-extracted Mn4-2_2 solution, representing a 55% recovery rate compared to the initial concentration in the black alloy leachate. Therefore, the case of Mn3-2_2 would also be a considerable combination for recovering Co, as it can recover 90% of Co.
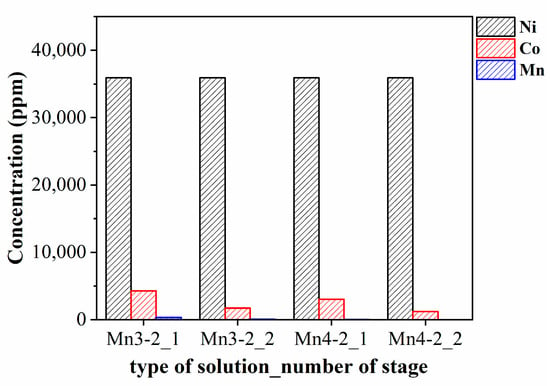
Figure 9.
Concentration of Ni, Co, and Mn in the Mn/Co-extracted solutions.
5. Conclusions
Triggered by the lack of comprehensive solvent extraction data on black alloy, we verified the extraction behavior of all elements that might exist during the recycling process of spent LIBs. To examine the extraction behaviors of the metals in black alloy composition, four types of organic extractants (D2EHPA, Cyanex 272, PC88A, and Versatic acid 10) were employed to extract the metals from the black alloy leachate. D2EHPA would be the most suitable extractant for Mn extraction due to its selective separation of Mn and the removal of impurities such as Cu, Ca, and Zn in a low pH range. Cyanex 272 was found to be reasonable for the separation of Co from the leachate. Also, most elements displayed 100% extraction efficiency at the endpoint of pH 6.5, except for Ni and Zn. The extraction yield of Ni was the lowest among the metals throughout the whole pH range with PC88A, while it slightly increased compared to D2EHPA and Cyanex 272. Versatic acid 10 demonstrated a propensity for Cu extraction predominantly before pH 5, suggesting its effectiveness in separating Cu from the black alloy leachate. However, Versatic acid 10 is not preferred for the extraction of the other metals due to the co-extraction of these metals unlike the other extractants. However, the extraction yield of Zn was below 50% with all the extractants contrary to previous studies where Zn was extracted completely for all extractants. Because Zn was completely extracted in the absence of the other elements by all the extractants, Zn would be susceptible to interference from the other elements, resulting in a decreased extraction efficiency in the black alloy leachate. Furthermore, we deduced the optimum combination of extractants for the separation of Ni, Co, and Mn depending on their extraction behaviors with the four kinds of extractants. D2EHPA could be the most appropriate extractant for Mn since its extraction efficiency was more than 80% while approximately 10% of Ni and Co were extracted. Cyanex 272 was found to be more suitable than PC88A for the separation of Co from Ni. Also, it would be reasonable to apply D2EHPA first to the black alloy leachate for the Mn removal. Then, Co would need to be removed in the Mn-removed solution by Cyanex 272, which is preferred to PC88A. In sum, the optimal combination of extractants for the separation of Ni, Co, and Mn could involve a two-stage extraction process, with Mn effectively separated at pH 3.0 or 4.0 by D2EHPA, and Co extracted at pH 5.0 with Cyanex 272. Future works are planned to verify the optimum solvent extraction strategy by using the actual industrial leachate of black alloy.
Author Contributions
Conceptualization, H.-I.K. and K.K.; methodology, N.K.; validation, B.K.; formal analysis, N.K.; data curation, B.K.; writing—original draft preparation, N.K.; writing—review and editing, K.K.; supervision, H.-I.K.; project administration, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT (RS-2023-00254424) and Ministry of Education (2020R1A6A1A03038540)). H.-I.K. acknowledges the support from the Technology development project to improve secondary battery circulation suability (Development of pollutants reduction technology generated in the lithium ion batteries recycling process) through the Korea Environmental Industry & Technology Institute funded by the Ministry of Environment (RS-2024-00345911).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kim, H.C.; Lee, S.; Wallington, T.J. Cradle-to-Gate and Use-Phase Carbon Footprint of a Commercial Plug-in Hybrid Electric Vehicle Lithium-Ion Battery. Environ. Sci. Technol. 2023, 57, 11834–11842. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Kim, S.; Bang, J.; Yoo, J.; Shin, Y.; Bae, J.; Jeong, J.; Kim, K.; Dong, P.; Kwon, K. A comprehensive review on the pretreatment process in lithium-ion battery recycling. J. Clean. Prod. 2021, 294, 126329. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Jung, J.C.-Y.; Sui, P.-C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Nakamura, T.; Shibata, E.; Iizuka, A. Adaptation of minerals processing operations for lithium-ion (LiBs) and nickel metal hydride (NiMH) batteries recycling: Critical review. Miner. Eng. 2013, 45, 4–17. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Jiang, L.; Wen, J.; Wang, H.; Guan, R.; Zhang, J.; Zeng, G. Regeneration and reutilization of cathode materials from spent lithium-ion batteries. Chem. Eng. J. 2020, 383, 123089. [Google Scholar] [CrossRef]
- Wang, J.; Chen, M.; Chen, H.; Luo, T.; Xu, Z. Leaching Study of Spent Li-ion Batteries. Procedia Environ. Sci. 2012, 16, 443–450. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.-I. Reductive leaching of cathodic active materials from lithium ion battery wastes. Hydrometallurgy 2003, 68, 5–10. [Google Scholar] [CrossRef]
- Joulié, M.; Laucournet, R.; Billy, E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J. Power Sources 2014, 247, 551–555. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Chen, R.; Wu, F.; Chen, S.; Zhang, X. Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries. Waste Manag. 2010, 30, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Vieceli, N.; Nogueira, C.A.; Guimarães, C.; Pereira, M.F.C.; Durão, F.O.; Margarido, F. Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite. Waste Manag. 2018, 71, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Impact of Recycling on Cradle-to-Gate Energy Consumption and Greenhouse Gas Emissions of Automotive Lithium-Ion Batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef] [PubMed]
- Barik, S.P.; Prabaharan, G.; Kumar, B. An innovative approach to recover the metal values from spent lithium-ion batteries. Waste Manag. 2016, 51, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Ruismäki, R.; Rinne, T.; Dańczak, A.; Taskinen, P.; Serna-Guerrero, R.; Jokilaakso, A. Integrating Flotation and Pyrometallurgy for Recovering Graphite and Valuable Metals from Battery Scrap. Metals 2020, 10, 680. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, L.; Du, J.; Zhang, G.; Cao, Z.; Wu, S. Improving Extraction Performance of D2EHPA for Impurities Removal from Spent Lithium-Ion Batteries Leaching Solution by TPC[4]. ACS Sustain. Chem. Eng. 2022, 10, 4312–4322. [Google Scholar] [CrossRef]
- Gaines, L. The future of automotive lithium-ion battery recycling: Charting a sustainable course. Sustain. Mater. Technol. 2014, 1–2, 2–7. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Lei, S.; Sun, W.; Yang, Y. Solvent extraction for recycling of spent lithium-ion batteries. J. Hazard. Mater. 2022, 424, 127654. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, J.; Li, H.; Li, K. An integrated process for the separation and recovery of valuable metals from the spent LiNi0.5Co0.2Mn0.3O2 cathode materials. Sep. Purif. Technol. 2020, 245, 116869. [Google Scholar] [CrossRef]
- Tang, Y.-C.; Wang, J.-Z.; Shen, Y.-H. Separation of Valuable Metals in The Recycling of Lithium Batteries via Solvent Extraction. Minerals 2023, 13, 285. [Google Scholar] [CrossRef]
- Swain, B.; Jeong, J.; Lee, J.-C.; Lee, G.-H.; Sohn, J.-S. Hydrometallurgical process for recovery of cobalt from waste cathodic active material generated during manufacturing of lithium ion batteries. J. Power Sources 2007, 167, 536–544. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Kucuker, M.A.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes—A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, B.; Zou, J.; Li, P.; Liu, Z.; Cheng, L.; Ou, X.; Zhang, J. Regeneration of high-capacity Ni-rich layered cathode material from spent lithium-ion batteries. J. Energy Storage 2022, 45, 103512. [Google Scholar] [CrossRef]
- Tran, T.T.; Moon, H.S.; Lee, M.S. Recovery of valuable metals from the hydrochloric leaching solution of reduction smelted metallic alloys from spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2022, 97, 1247–1258. [Google Scholar] [CrossRef]
- Beak, M.; Park, J.; Park, S.; Jeong, S.; Kang, J.; Choi, W.; Yoon, W.-S.; Kwon, K. Understanding the effect of nonmetallic impurities in regenerated cathode materials for lithium-ion battery recycling by tracking down impurity elements. J. Hazard. Mater. 2022, 425, 127907. [Google Scholar] [CrossRef]
- Kim, W.; Park, S.; Ko, G.; Lee, J.; Kwon, K. Optimizing pH conditions for impurity removal in closed-loop Li-ion battery recycling. Chem. Eng. J. 2023, 475, 146121. [Google Scholar] [CrossRef]
- Sole, K. Solvent extraction in the hydrometallurgical processing and purification of metals: Process design and selected applications. In Solvent Extraction and Liquid Membranes: Fundamentals and Applications in New Materials; CRC Press: Boca Raton, FL, USA; Taylor &Francis: Boca Raton, FL, USA, 2008; pp. 141–200. [Google Scholar]
- Keller, A.; Hlawitschka, M.W.; Bart, H.J. Application of saponified D2EHPA for the selective extraction of manganese from spend lithium-ion batteries. Chem. Eng. Process.–Process. Intensif. 2022, 171, 108552. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).