Abstract
Batteries play a crucial role in the domain of energy storage systems and electric vehicles by enabling energy resilience, promoting renewable integration, and driving the advancement of eco-friendly mobility. However, the degradation of batteries over time remains a significant challenge. This paper presents a comprehensive review aimed at investigating the intricate phenomenon of battery degradation within the realm of sustainable energy storage systems and electric vehicles (EVs). This review consolidates current knowledge on the diverse array of factors influencing battery degradation mechanisms, encompassing thermal stresses, cycling patterns, chemical reactions, and environmental conditions. The key degradation factors of lithium-ion batteries such as electrolyte breakdown, cycling, temperature, calendar aging, and depth of discharge are thoroughly discussed. Along with the key degradation factor, the impacts of these factors on lithium-ion batteries including capacity fade, reduction in energy density, increase in internal resistance, and reduction in overall efficiency have also been highlighted throughout the paper. Additionally, the data-driven approaches of battery degradation estimation have taken into consideration. Furthermore, this paper delves into the multifaceted impacts of battery degradation on the performance, longevity, and overall sustainability of energy storage systems and EVs. Finally, the main drawbacks, issues and challenges related to the lifespan of batteries are addressed. Recommendations, best practices, and future directions are also provided to overcome the battery degradation issues towards sustainable energy storage system.
1. Introduction
Batteries serve as indispensable energy storage devices, converting chemical energy into electrical energy, thereby powering numerous aspects of modern life [1]. These batteries enable the consistent supply of electricity, overcoming the intermittency of renewable energy production, and ensuring a reliable power source even during periods of low generation [2]. Moreover, batteries are the heart of electric vehicles, powering their engines and enabling them to function without reliance on traditional fossil fuels. The development of high-capacity, durable, and fast-charging batteries has been instrumental in driving the adoption of electric vehicles, significantly reducing greenhouse gas emissions and paving the way for a more sustainable future in transportation [3]. As technological advancements continue to improve battery efficiency and capacity, their role in both energy storage systems and electric vehicles becomes increasingly pivotal in the global shift towards cleaner and more efficient energy utilization [2]. Battery technologies, despite their widespread use and critical role in modern society, grapple with several significant drawbacks [4]. One of the primary limitations is the degradation of batteries over time. This constraint impacts their efficiency, notably in applications like electric vehicles and portable electronics, where battery life and increased extended range are paramount. Additionally, the capacity degradation of batteries presents a crucial challenge, leading to reduced performance and the eventual need for replacement or recycling.
Battery degradation refers to the progressive loss of a battery’s capacity and performance over time, presenting a significant challenge in various applications relying on stored energy [5]. Figure 1 shows the battery degradation mechanism. Several factors contribute to battery degradation. One primary cause is cycling, where the repeated charging and discharging of a battery causes chemical and physical changes within the battery cells. This leads to the gradual breakdown of electrode materials, diminishing the ability of the battery to hold a charge. Elevated temperature, another factor that accelerates chemical reactions within the battery, hastens degradation [6]. Overcharging or deep discharging a battery beyond its recommended voltage limits can also accelerate degradation by causing physical stress on the electrodes or electrolyte [3]. Additionally, storage conditions and the type of battery chemistry play crucial roles; some chemistries are more prone to degradation than others. As batteries degrade, their overall capacity diminishes, reducing their ability to provide power, ultimately leading to decreased performance and the need for replacement or refurbishment [7]. Strategies such as optimal charging practices, temperature management, and advancements in battery chemistry aim to mitigate degradation and extend battery lifespan.
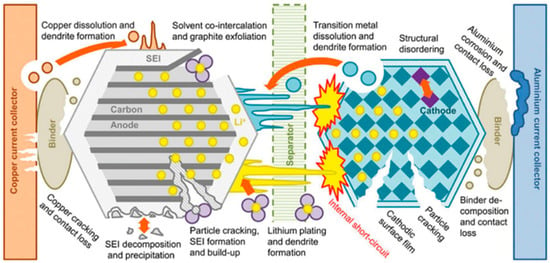
Figure 1.
Degradation mechanism of lithium-ion battery [8].
Battery degradation significantly impacts energy storage systems, compromising their efficiency and reliability over time [9]. As batteries degrade, their capacity to store and deliver energy diminishes, resulting in reduced overall energy storage capabilities. This degradation translates into shorter operational lifespans for energy storage systems, requiring more frequent replacements or refurbishments, which escalates operational costs. Moreover, decreased storage capacity limits the amount of renewable energy that can be stored, affecting the system’s ability to provide consistent power during peak demand or when renewable sources are not actively generating energy [10]. This fluctuation in storage capacity due to degradation can create challenges in maintaining a stable and reliable power supply from renewable sources, impacting grid stability and the integration of renewable energy into the electrical grid.
The performance, range, and general usability of electric vehicles (EV) are all significantly impacted by battery deterioration [11]. The driving range of an electric vehicle (EV) decreases with battery deterioration, affecting the amount of distance it can drive between charges. Degradation-related reduction in battery capacity also results in a drop in power output, which impacts the vehicle’s acceleration and general performance. In addition, EV drivers must charge their cars more frequently to go the same distance as their battery’s capacity decreases, which raises operating costs and annoyance [12]. Furthermore, a deteriorating battery that has to be replaced may turn off potential buyers, which lowers the resale value of electric vehicles. Addressing battery degradation through technological advancements, efficient battery management systems, and improvements in battery chemistry remains crucial to prolonging the lifespan of EV batteries and ensuring the long-term viability and attractiveness of electric vehicles in the transportation sector [13]. The lithium-ion (Li-ion) battery is a form of intercalation-type battery that is mostly utilized in electric vehicles (EVs). Intercalation-type batteries are a specific category of rechargeable batteries where the energy storage mechanism involves the insertion and extraction of ions or molecules into the crystal structure of the electrode material during charging and discharging cycles. Consequently, the most-often utilized batteries in EVs are intercalation-type batteries, most especially Li-ion batteries. So, this study will mainly focus on the intercalation types of batteries. In recent years, data-driven approaches have emerged as powerful tools for estimating battery degradation. Leveraging vast amounts of historical and real-time data, these techniques offer a holistic understanding of battery health and degradation patterns [14]. By harnessing advanced analytics, machine learning algorithms, and statistical modeling, data-driven methods enable precise predictions of degradation rates, allowing stakeholders to proactively address issues and optimize operational parameters [15].
Recently, battery degradation became an issue of great concern among the researchers around the world [16]. Xiong et al. presented a review about the aging mechanism of lithium-ion batteries [17]. Authors have claimed that the degradation mechanism of lithium-ion batteries affected anode, cathode and other battery structures, which are influenced by some external factors such as temperature. However, the effect of battery degradation on EV and energy storage system has not been taken into consideration. Jialong et al. investigated battery degradation behavior at a low temperature using destructive and nano-destructive methods, where it has been found that a high charging rate accelerates the battery aging at a low temperature [18]. The rate of aging of a battery charging at 0.6 °C is higher than a battery charging at 0.8 °C. Although the aging rate at a low temperature has been investigated, what the situation will be at a high temperature has not been explained. Further study is still required. Guo et al. presented a review about the aging mechanism of lithium-ion batteries in EV where the aging behavior of lithium-ion batteries in charging, stand-by, and driving modes are reviewed [19]. Xu et al. proposed a data-driven battery degradation estimation model. The authors explained the estimation method with accuracy; however, the battery degradation effect on energy storage systems and electric vehicles still needs to be investigated [14]. Wankmüller et al. presented a case study about the impact of battery degradation on energy storage systems; however, the authors did not investigate the case of electric vehicles [20]. In another work, Ou explained in detail the impact of battery aging on EV [21]. The author claimed that battery degradation can be delayed by around 0.5% with the help of a battery thermal management system. Higher outside temperatures enhance the use of BEV batteries. For example, compared to the New England area, the Los Angeles area has 6% higher battery capacity; however, this paper does not provide any information about the degradation mechanism of EV batteries [21]. Kaliaperumal et al. presented a review about the mitigation techniques of battery degradation where the authors claimed that the use of extra protective mechanisms, fire suppression, ventilation, and intrinsic safety as mitigation techniques in LiB can help them overcome the failure modes; however, the issues and challenges of these techniques have not been taken into consideration [22]. Further study is still required in this regard. Table 1 summarizes the current research status of battery degradation.

Table 1.
Current research status of battery degradation for EVs and energy storage systems.
To bridge the gaps in the field of battery degradation, this paper will provide a comprehensive review for the degradation factors, aging mechanisms, and the data-driven approaches to the modeling of battery degradation, considering both electric vehicles and energy storage systems. Future research directions in this field will also be a part of this work.
This paper is divided into six sections. Section 2 presents the battery degradation mechanism in brief. Key degradation factors are shown in Section 3. In Section 4, the effects of battery degradation in EVs and energy storage systems are reported. Data-driven algorithms, strategies, and approaches regarding battery degradation are highlighted in Section 5. Lastly, the conclusion and future directions are suggested in Section 6.
2. Battery Degradation Mechanism
The degradation of lithium-ion battery can be mainly seen in the anode and the cathode. In the anode, the formation of a solid electrolyte interphase (SEI) increases the impendence which degrades the battery capacity. Mechanical stress results in a crack in the surface layer, and lithium plating makes the formation of dendrite on the surface of anode layer. On the other hand, cathode degradation occurs due to a film on the cathode surface layer, stress, and dissolution of transition material.
2.1. Degradation in Anode
To create improved anodes for rechargeable lithium batteries, a great deal of research has been conducted during the last three decades. Negative electrodes, or battery anodes, are typically composed of silicon, lithium metal, titanate, graphite, or various composite materials [22]. Commercial batteries use graphite-based anodes, including graphite anodes and graphite–silicon composite anodes [23]. Carbon materials have dominated the market for negative electrode materials due to their high electronic/ionic conductivity, affordability, availability of raw materials, and suitable mechanical and thermal qualities [24]. This is particularly true of graphite with consistent performance. Degradation in the battery anode mainly occurs due to SEI, lithium plating, and stress.
2.1.1. Degradation in the Layer of Solid Electrolyte Interphase
Graphite is used as the main anode material in most lithium-ion batteries. Graphite anodes operate in the 0.05–1 V range, which is beyond the 1–4.5 V window for organic electrolyte stability [25]. Because of this, graphite-based LIBs produce a reductive electrolyte breakdown process due to their thermodynamic instability. Li+ ions are consumed in this process, which also creates a surface layer on the anode that leads to loss of active material (LAM) [26] and loss of lithium inventory (LLI) [27]. The rapid reaction rate, particularly in the initial few cycles, produces a surface layer that is permeable to Li ions but less permeable to electrolyte components. As a result, there is less degradation and subsequent electrode corrosion [28,29]. Nonetheless, throughout the life of the battery, solvated lithium and other electrolyte components are still transported through this semipermeable layer even while the reaction rate decreases. This reaction mostly takes place in the solid electrolyte interphase (SEI) layer, which is the interphase between the electrolyte and the anode. The primary cause of graphite anode aging is frequently the expansion of the SEI layer. It lowers the capacity of the battery to hold energy and its ability to handle power. Another kind of anode surface layer, known as the non-SEI layer, is known to form on the basal plane surface and is impervious to Li ions [30]. It is common to refer to both the SEI and non-SEI layers as the SEI layer [31]. Thus, the same custom is also adhered to here. The SEI keeps growing once it first forms, both when it is cycling and when it is not. The operational circumstances of the cell determine how quickly the SEI layer develops [32]. Agubra et al. presented the main factor of SEI formation which are electrolyte flow rate, electrolyte composition, charging current, voltage, and pressure [33]. Figure 2 shows the formation of SEI on a lithium-ion battery. Temperature and SOC are the primary drivers of SEI development under idle circumstances. More Li ions are intercalated into the anode at a greater SOC, which lowers the anode potential and speeds up the reductive reaction rate [34]. Agubra et al. claimed that SEI formation undergoes a very critical evolution of pre-formation, self-improvement, and gradual decay in succession at 25 °C [33]. At high temperature, a film of LiF and Li2CO2 forms which increases the impedance and triggers the anode towards degradation. The ionic conductivity of the SEI layer is further reduced by rising temperatures, which can speed up the reaction rate and may convert less stable organic SEI components into more stable inorganic compounds. Not even thermal runaway can prevent the cell from exploding or catching fire at severe temperatures exceeding 60 °C. The volume variations brought on by the (de)intercalation of Li ions during cycling, in addition to SOC and temperature, put mechanical stress on the electrodes. These variations in volume have the ability to breach the SEI and initiate new reactions. This fracture and repair cause the SEI to keep expanding, which leads to more LLI [26] and LAM [27].
In addition, cycling produces a more porous SEI layer than idling, especially at high C-rates [35]. While a denser SEI layer lowers the reaction rate, this greater porosity permits more reductive processes to occur. Electrolyte composition and electrode balance are other parameters that impact the pace of reaction [36]. There are two competing hypotheses on how SEI form: (1) According to the first hypothesis, formation occurs at the electrode–electrolyte interface, and the SEI’s electrical conductivity ought to be the formation’s limiting factor [17]. (2) According to the second hypothesis, solvent diffusion limits SEI production, which occurs at the anode–SEI contact [37]. Nonetheless, the aging behavior resulting from both hypotheses follows a time connection, which is consistent with the passivation character of the SEI layer and is frequently shown in tests.
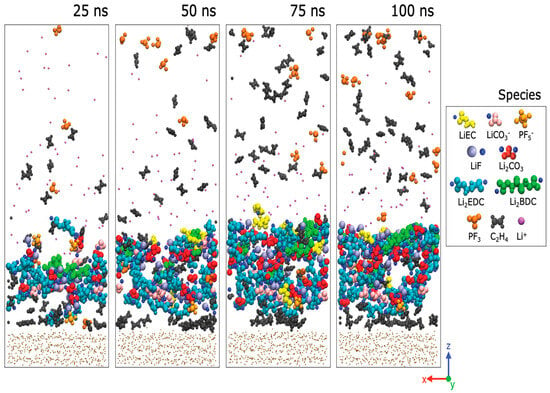
Figure 2.
Process of SEI film production in the electrolyte based on EC. Snapshots of the reaction products taken every 25 ns throughout a 100 ns period. Bulk EC and PF6-anions are not displayed for viewing reasons. Li+ that have not participated in any reactions are magenta in color [38].
At the electrode–SEI interface, the solvent and lithium ions pass through the SEI layer and react with the surface electrons to produce an insoluble product. Different SEI modeling techniques have been presented in [39,40,41]. According to Fick’s second law, solvent diffusion through the SEI layer will be as follows [39]:
where is the concentration of the solvent in pores and is the effective diffusivity of the solvents. The liquid electrolyte fills the holes in the SEI and reaches the electrode surface because the SEI layer has a porous structure. Thus, the first boundary condition is determined by the effective solvent concentration at the electrolyte/SEI, which is as follows [39]:
where ε represents the porosity of the layer, and is the concentration of the bulk solution. The kinetics of the irreversible formation process of the product are expressed using a Tafel expression as follows [39]:
where is the reaction of solvent reduction equilibrium potential and is its rate constant. Moreover, which depends on SOC, is the anode’s open circuit potential. The rate of the solvent reduction process,, and the growth rate of the SEI layer brought on by the creation of the insoluble product, determine the solvent flow at the SEI/electrode interface [39].
where is the thickness of the SEI layer and is the molar density of the reaction product. The ratio of the number of lost lithium ions in the SEI to the number of starting lithium ions is known as the capacity loss, or x(t) [40].
where is the starting number of moles of lithium ions in the cell, is the anode surface area, and is the number of Li atoms in the product.
2.1.2. Lithium Plating
Lithium plating, which happens during charging when lithium ions unevenly deposit on the anode, generating metallic lithium, is one major cause for concern in battery degradation [41]. The mechanism of lithium plating is shown in Figure 3. Exposure to low temperatures or rapid charging frequently intensifies this effect. For example, charging a 7.5 Ah cell at 1 C rate at 0 °C would result in a significant 3.6% capacity loss [42]. The three following main variables cause the power and energy densities of a lithium-ion battery to decrease at low temperatures, especially when charging: 1. inadequate charge-transfer rate; 2. low solid diffusivity of lithium ions in the electrode; and 3. reduced ionic conductivity in the electrolyte [43,44,45]. Ionic conductivity in the electrolyte diminishes, which causes an increase in cell internal resistance; nevertheless, this is not the primary issue with low-temperature charging. Lithium plating at low temperatures, where lithium ions collect at the interface between carbon particles and electrolytes, may be primarily caused by poor Li+ diffusivity inside the electrodes [46,47]. Dendrite development, which can result in internal short circuits, overheating, or even explosions, increases internal resistance, capacity loss, and safety issues associated with lithium plating [48,49]. Lower temperatures, often below 20 °C, cause the intercalation potential of graphite material to approach that of metallic lithium and decrease the diffusion rate of lithium into the anode or electrolyte. As a result, metallic lithium plating could happen. High C-rates, low temperatures, and low SOC are particularly conducive to lithium plating [43]. During fast charging, there is a greater chance that the charging rate will surpass the intercalation rate. The quantity of Li+ ions transferred during the charge-transfer process from the cathode to the anode per unit of time rises at a high C-rate [44]. High SOC has a great impact on the lithium plating. The impact of high SOC on graphite/LCO 5 Ah pouch cells at different cutoff voltages ranging from 4.2 V to 4.8 V was investigated by Juarez-Robles et al. [45]. Significant capacity decline, lithium plating, electrolyte breakdown, and volume expansion were seen in cells charged over 4.5 V. The cells that were charged to 4.6 V, 4.7 V, and 4.8 V showed dendritic structures [45]. Moreover, the anode resistance may approach a critical limit beyond a particular age. Lithium plating begins at this limit, where the anode potential falls below 0 V against Li/Li+ [46]. Lithium plating, in contrast to SEI layer development, is a positive reinforcing event whereby the deposits on the anode reduce the active surface area, which raises the current density at the remaining accessible pores and increases the metal plating. This leads to the observation of a knee point in the deterioration behavior at the age of lithium plating. Lithium plating results in LLI and may make the cell less safe since it can induce internal short circuits by allowing lithium dendrites to form [17]. Various modeling techniques of lithium plating have been presented in [50,51,52,53]. Lithium plating can be estimated using the Butler–Volmer equation [47]. Zhao et al. utilized a reduced order model to consider the lithium plating evolution as shown in the following equations [54]:
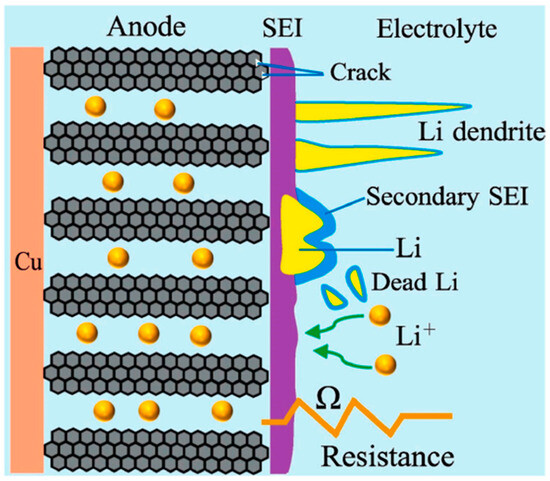
Figure 3.
Degradation mechanism in anode due to lithium plating [55].
2.1.3. Stress
One of the main factors contributing to lithium-ion battery degradation is mechanical stress at the anode [56]. There is a number of negative consequences that might arise from mechanical stress on the anode. Initially, the anode material may experience physical damage from the stress, such as deformation or cracking, which would compromise its structural integrity and lessen its capacity to store and release lithium ions during cycles of charging and discharging [57]. Wang et al. investigated the mechanical stress on the anode and cathode and found cracks in both surface and cross-sectional areas [58], as shown in Figure 4. This damage may cause the internal resistance to rise, which would lower its overall performance and efficiency. Furthermore, mechanical stress could permit the electrolyte to penetrate the anode material and start chemical reactions that eventually weaken the anode and reduce battery capacity [50]. Moreover, internal short circuits brought on by stress-induced dendritic formation—a process in which microscopic lithium metal fibers protrude from the anode surface—can enter the separator and result in safety hazards like thermal runaway, overheating, or even explosions [51,52,53,59]. As the particles go through a phase transition, the intercalation of lithium ions into the anode may cause sudden variations in volume [60,61]. As more lithium is added, the orientation of the molecules shifts during a phase transition, producing distinct geometrical and electrical features. This results in mechanical stress brought on by a volume shift, which can cause contact loss with the composite electrode (CL), surface layer cracking (LLI and LAM), and anode structural damage (LAM) [62]. Electrode porosity, which is necessary for the electrolyte to reach the electrode’s bulk, may also decrease as a result of the active material’s volume change [21,63]. Different modeling techniques of stress factors of lithium-ion batteries have been presented in [64,65,66,67]. The hydrostatic stress of an anode can be evaluated using the following equations [59,68]:
where the volume fluctuation of the intercalated lithium and solution host material is described by the partial molar volume, or Ω. The particle’s lithium concentration field is denoted by C. The Young modulus (E) and Poisson ratio (ν) are used, respectively.
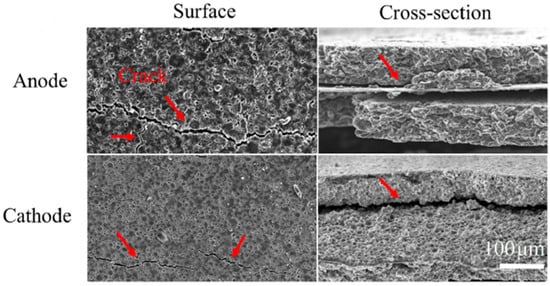
Figure 4.
Impact of stress in both anode and cathode of lithium-ion battery [58].
2.2. Degradation in Cathode
Battery degradation in the cathode of lithium-ion batteries involves mechanisms such as transition metal dissolution, formation of surface layer film, stress, and particle cracking. These processes contribute to capacity loss, reduced cycling stability, decreased energy density, and decreased battery performance over time.
2.2.1. Film on Cathode Surface Layer
Surface layer films, sometimes called cathode electrolyte interphase (CEI) layers, can develop on the cathode owing to electrolyte oxidation and salt deposition, much like they can on the anode [69]. Using direct STEM (EELS) measurements, Roy et al. investigated structural and compositional changes at the surfaces of LixMn2O4y epitaxial films at the atomic level [70]. The findings unequivocally demonstrate that O deficiency near the pure LiMn2O4 surface is linked to Li deficiency and a mean Mn oxidation state that is lower than predicted by the classical intercalation mechanism. A thin surface layer with an approximate LiMn2O4 composition that fluctuates progressively down to a depth of around 15 nm is produced by this non-stoichiometry. When Li is removed, Jahn–Teller Mn3+ ions oxidize to smaller Mn4+ ions, causing a decrease in lattice volume inside the film and a significant rise in strain on the surficial layer during the first charge. In this state, the highly strained surficial layer is unstable and reconstructs to create Mn3O4 at a depth of 2–3 nm with additional oxygen loss; during the first cycle, Mn2+ dissolution and/or spallation of the strained selvedge cause a significant reduction in specific capacity. The cathode surface layer has poor lithium-ion conductivity, which raises the impedance, in contrast to the SEI layer. Additionally, gas evolution is conceivable [71]. Several modeling techniques have been introduced by [72,73,74,75]. SEI film layer formation rate can be described by Tafel equation [76]. The formation rate of the particle on site can be described by the following equation:
where
Here, is the potential site of I, and is the local equilibrium of SEI formation. Kemeny et al. state that organic compounds produced at the anode by reduction may also be carried to the cathode, where they undergo oxidation and deposit on the cathode surface to produce LAM and LLI [60]. Similar conclusions were reached in [61]. Unfortunately, it is more challenging to detect this surface film layer because of the greater cathode voltage.
2.2.2. Stress
Similar to the anode, the cathode may experience phase transitions that lead to LAM and CL during the intercalation and deintercalation of Li ions. Depending on the chemistry, there are different amounts of volume changes. For instance, LFP cells only have a two-phase regime with FePO4 and LiFePO4 [64]. Different chemistries exhibit various transitions of phase in the anode and cathode voltage profiles, such as NMC or NCA [65]. As a result, the shape transitions from cubic to tetragonal. This can raise the cathode volume in the case of LMO by around 16% [66], producing more LAM [67]. Low SOC is when the Jahn–Teller distortion happens. Capacity fading brought on the volume variation can be lessened by adding dopants and adjusting the end-of-discharge voltage (EODV). Kim and Huang performed an experimental study where it has been shown that under high C-rate, the electrode is fully saturated in a shorter period of time and mechanical stress dramatically increased in lithium intercalation areas which significantly increased the capacity fade [77]. The stress of the lithium-ion battery can be modeled by the following equations [78]:
where
Here, is the hydrostatic stress.
2.2.3. Dissolution of Transition Metals
Transition metal oxides, such those based on manganese, nickel, and cobalt, are frequently used as cathode materials in LIB [79]. These transition metal ions can dissolve into the electrolyte during the battery’s repeated cycles of charging and discharging, especially in extreme weather or at high temperatures. The development of soluble metal species at the cathode contact is the result of side reactions that cause this dissolution [72]. The transition metal ions that have been dissolved then go to the anode, where they may plate and form metallic deposits known as “dendrites”. These dendrites have the ability to pierce the separator, resulting in internal short circuits, lowering the battery’s total capacity and jeopardizing the lithium-ion battery’s functionality and safety.LiPF6 salt is included in most battery electrolytes [73]. These cells can produce hydrogen fluoride (HF) from impurities within the electrolyte during cycling and storage, especially at high cell voltages and temperatures [74]. Liao et al. presented the oxidization carbonates at the LixNi0.5Mn0.5O2 where the formation of HF usually includes the hydrolysis of LiPF6 from the incidental electrolyte water, as shown in Equation (16) [79].
The synthesis of and the presence of HF have been coupled by Mikheenkova et al. to explain Mn migration to the negative electrode, as demonstrated by Equation (17) [75].
may migrate from the electrolyte to the negative electrode via this hypothesized pathway. The mechanism has been expanded to incorporate high cell voltage operation. This raises the electrolyte’s acidity. The resultant HF raises the impedance and causes capacity fading by dissolving the transition metals and corroding the cathode. It also contributes to various degradation processes in the anode and cathode. First, structural deterioration of the cathode (LAM) due to metal dissolution might lower the cathode’s capacity to insert lithium [79]. Moreover, upon dissolution, the metals may migrate through the electrolyte and settle on the anode, where they will serve as catalysts for the reductive breakdown event that produces the formation of the SEI layer [80]. Lastly, lithium metal dendrites (LLI and LAM) may develop on the deposited metals as a result of the transition metals that are formed on the anode during this reductive process. A serious safety issue arises from the possibility of internal short circuits caused by these dendrites. Transition metal dissolution can be particularly harmful to spinel-containing chemistries like LiMn2O4, since the degree of dissolution can be significantly higher and cause more structural damage of the cathode (LAM) [81]. Transition metal dissolution during the charging process is inevitable, but it may be minimized by lowering the electrolyte’s impurity level, employing dopants, and utilizing protective coatings to lessen direct contact between the cathode and the electrolyte [82]. Krupp et al. claim that at potentials higher than 4.6 V, a significant increase in the rate of manganese dissolution was observed. Regardless of the charge level, manganese deposits on the graphite electrode were always in the oxidation state +2 while the system was in operation [83]. Transition metal ion dissolution often occurs in the presence of acidic species in the electrolytes. It is commonly known that LiPF6 may include traces of hydrogen fluoride (HF), and that HF can be produced when LiPF6 combines with traces of water or alcohol in carbonate solvents [84,85]. Degradation in cathode due to dissolution of transition materials has been shown in Figure 5.
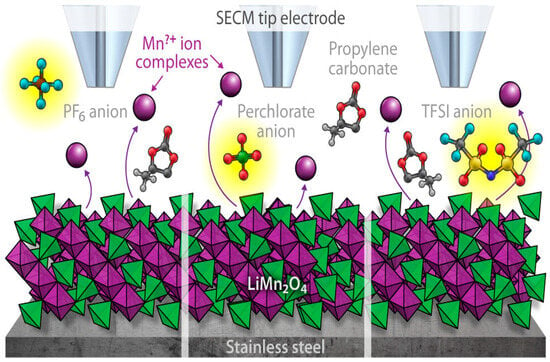
Figure 5.
Degradation mechanism in cathode due to transition material dissolution [82].
3. Key Degradation Factor
Despite being popular and effective, lithium-ion batteries deteriorate over time for a number of reasons. Cycling, or the charge–discharge cycle that a battery experiences throughout its lifespan, is one important component. Every cycle alters the composition of a battery physically and chemically, breaking down the electrode materials and forming undesirable compounds that lower the battery performance and capacity [86]. Another important degrading element is temperature. Higher temperatures hasten chemical processes in the battery, which speed up the deterioration of the electrolytes and electrode materials. In the same way, low temperature, SOC, DOD, and calendar aging also play a vital role in battery degradation. Reduced energy efficiency, capacity loss, and even safety risks like thermal runaway might result from this.
3.1. Cycling Degradation
Cycling degradation in lithium-ion batteries refers to the progressive deterioration in performance that occurs as the battery undergoes repeated charge and discharge cycles during its operational life [87]. With each cycle, various physical and chemical processes contribute to the gradual degradation of the battery components [88]. Mechanical stress resulting from the expansion and contraction of electrode materials, particularly in the anode, can lead to structural damage and decreased capacity [85]. Xu et al. presented an empirical model of degradation prediction of lithium-ion batteries and the authors also claim that five stress factors (temperature, DOD, charging C rate, discharging C rate, and middle SOC) have a great influence on the cycling aging [89]. Additionally, chemical reactions at the electrode–electrolyte interface can form a solid–electrolyte interface (SEI) layer, hindering ion transport and increasing internal resistance [90]. Wang et al. presents a post mortem study of a lithium-ion battery using neutron depth profiling (NDP) where it has been shown that SEI grow logarithmically with cycle number starting with the main formation of the initial cycle [71]. Transition metal dissolution from the cathode, electrolyte decomposition, and thermal effects further contribute to capacity fade and reduced overall efficiency [72]. Lee presented the impact of transition metal dissolution on cycling aging where LiMn2O4 is used as transition metal [91]. In this study, 5, 50, and 500 ppm concentrations of Mn are dissolved in the electrolyte which is used as the input of the experiment and the results show that the higher dissolution rate caused severe fraction and volume losses during the cycling.
Lithium-ion battery cycling deterioration results from a combination of chemical and physical reactions that take place during repeated cycles of charging and discharging. The mechanical stress that the electrode materials, particularly in the anode, endure during the volume changes that occur during charging and discharging, is one of the main contributing factors. The cycle deterioration of lithium-ion batteries is caused by the combined impacts of mechanical, electrochemical, and thermal stressors [92]. This degradation affects the batteries’ capacity, energy retention, and overall performance throughout the course of their operational lives. There are several models for estimating the cycling aging estimation [93,94,95,96]. Cycling degradation can be estimated from the following equations:
where N is the number of cycles, mentioned that is a full cycle or half, is the depth of discharge, is the average SOC of the cycle, and is the average cell temperature. The cycling aging problem of lithium-ion batteries can be mitigated by adopting different techniques. Wood et al. present an aging level-based charging strategy which may slow down the capacity fade by 3.84% and increase the life time by 40 cycles to 50 cycles [97].
3.2. Electrolyte Breakdown
Electrolyte breakdown is the chemical reaction and deterioration of the electrolyte. Repeated cycles, high temperatures, or overcharging are some of the reasons that might cause this breakdown and result in the development of undesirable byproducts [98]. Increased internal resistance, capacity decline, and decreased overall performance in lithium-ion batteries are caused by electrolyte degradation [99]. The breakdown of the electrolyte, an essential aspect that facilitates ion transport between the positive and negative electrodes in lithium-ion batteries is a complicated process involving a variety of chemical and electrochemical events that affect the electrolyte’s usefulness and stability. The creation of a solid–electrolyte interface during the battery’s repeated cycles of charge and discharge is one important factor [100]. Even while the SEI is necessary for a battery to function normally, its constant expansion can obstruct ion transport and cause electrolyte degradation. Furthermore, high voltages can cause electrolyte breakdown, producing heat, gas, and unwanted chemical species that exacerbate breakdown, particularly when overcharging or at high temperatures.
Chemical reactions inside the electrolyte can be accelerated by thermal stress from internal and external heat sources in combination with undesirable side reactions at the electrode–electrolyte contact. Investigating low- and high-Ni content NMC using single-solvent LiPF6-based electrolytes by Zhang et al. has revealed new information regarding the higher interfacial reactivity for charged Ni-rich NMC cathodes and the crucial impact the electrolyte solvent plays [101]. This study reveals that there is an inherent connection between the deterioration of the cathode surface and sub-surfaces and the amount of lattice oxygen loss from NMC, which was shown to be dependent on both the Ni-content and the electrolyte solvent. The number of charge–discharge cycles that occur once a battery’s usable capacity drops to 80% of its initial value is known as the cycle life. LIBs typically display two-phase capacity degradation behavior, which denotes that the capacity first deteriorates slowly before experiencing an accelerated degradation that begins at a specific beginning point. This phase, which is sometimes referred to as the knee point, is crucial to the cell cycle as a whole. The combination of internal chemical reaction, external charging/discharging circumstances, and battery environment affects the development of knee point [102]. Following this, the capacity will quickly drop very significantly.
3.3. Temperature
Lithium-ion battery performance and deterioration are significantly influenced by temperature. Temperature variations can have an effect on the electrochemical processes taking place inside the battery, hence compromising its general health.
3.3.1. High Temperature
High temperatures have a significant impact on lithium-ion battery performance and safety in a number of different ways. The battery’s chemical processes are accelerated by elevated temperatures, which can be caused by external factors or internal heat produced during cycles of charging and discharging. This involves the electrolyte breaking down and an increase in electrode–electrolyte interface reactions, which increases the possibility of thermal runaway. Furthermore, on electrode surfaces, high temperatures hasten the creation and expansion of the solid–electrolyte interface. While the SEI is necessary for battery operation, too much development can increase internal resistance and reduce the efficiency of ion transport. Furthermore, the elevated temperature raises the possibility of dendrite development, particularly in lithium metal anodes. LiCoO2 and LiMn2O4 cathode capacity variation was investigated by Gao et al. [103]. Thermal aging was applied to the LiCoO2 and LiMn2O4 cathodes for 10 and 6 days, respectively, at 75 °C. Following age treatment, discharge patterns of these two cathodes demonstrated a significant loss of capacity. Almeida et al. [104] examined the aging process of LIBs based on Li (Ni, Mn, Co)O2 (NMC). The tested battery’s capacity lost 7.5% when it was cycled at 85 °C and 22% when it was cycled at 120 °C. Two potential explanations for the aging deterioration were presented by means of characterization techniques that allowed for the assessment of the binder and SEI changes during the aging process. The anode surface had been moved to by the polyvinylidene fluoride (PVDF) binder, which prevented the intercalation of lithium ions. Conversely, at high temperatures, the carbonates species vanished, and the inorganic species multiplied at the SEI layers, increasing the internal resistance of the batteries. Kalaga et al. [105] examined the impact of temperature on the rate of capacity deterioration of a Sony Prismatic LIB during aging from 25 °C to 55 °C using the electrochemistry-based electrical (ECBE) model. Increasing the temperature within the measured range partly boosted the battery’s capacity but also sped up the rate at which the capacity degraded during cycling [106].
3.3.2. Low Temperature
Lithium-ion battery performance is significantly impacted by cold temperatures, primarily impacting internal resistance and ion mobility. The mobility of lithium ions in the battery is reduced in cold environments, which slows down electrochemical processes. At a temperature of 0 or below that, lithium-ion batteries started to degrade [107]. According to Naga Subramanian, Panasonic 18,650 LIBs had power and energy densities of about 800 W/L and 100 Wh/L at 25 °C. At −40 °C, these values decreased by 98.75% and 95% to less than 10 W/L and ~5 Wh/L. When the operating temperature dropped from 25 °C to −15 °C [103], it was also discovered that the state of charge (SOC) of a LIB, which is defined as the ratio of the current residual capacity to the overall available capacity, reduced by almost 23% [104]. An improved electrolyte formulation of 1.0 M LiPF6 in ethylene carbonate (EC)−propylene carbonate (PC)−ethyl methyl carbonate (EMC) (1:1:8 by wt) with 0.05 M CsPF6 was also reported by Tang et al. [108]. For the batteries tested at −40 °C, this formulation allowed for a capacity retention of 68%, whereas the batteries with the standard formulation only demonstrated a 20% capacity retention. Lithium difluoro phosphate (LiPO2F2) is one particular electrolyte addition that has been shown to be useful in enhancing LIB performance at low temperatures [109]. Another significant element that plays a role in the performance reduction in LIB at low temperatures is the rise in charge-transfer resistance. It has been observed that LiFePO4-based cathodes exhibit a three-fold increase in charge-transfer resistance at −20 °C compared to ambient temperature [105]. Reduced capacity and power production result from slow ion movement, which is especially noticeable during discharge cycles. Furthermore, the battery’s internal resistance increases in a cold environment, obstructing the free passage of ions [110]. Particularly in scenarios with high demand, the higher internal resistance lowers overall efficiency and causes voltage dips. Moreover, the electrolyte may partially solidify in extremely low temperatures, which would impair its capacity to efficiently promote ion mobility [93,94]. The battery’s performance is further hindered by this solidification, which eventually causes capacity reduction.
Several temperature modeling techniques has been shown in [98,111,112]. The temperature of the lithium-ion battery could be assumed from the simplified energy balance which is shown in the following equation [93]:
where, I is the cell current, V is the cell voltage, is the open circuit voltage, T is the absolute temperature, is the cell over potential, is the polarization heat, and is the reversible entropic heat.
3.4. Calendar Aging
The slow deterioration of a lithium-ion battery performance over time, even when it is not in use, is known as calendar aging [25]. Calendar aging is related to time passing, as opposed to cycle aging, which is caused by charging and discharging cycles [95]. Calendar aging is greatly influenced by variables including temperature, charge level, and storage circumstances. Keli et al. shows that calendar aging strongly depends on the graphite electrode which is the main driver of capacity fade [25]. This study also revealed that if the graphite anode lithiated more than 50%, the low anode potential will accelerate the loss of cyclable lithium. As the battery ages, slow-moving chemical processes take place inside it, forming solid–electrolyte interphase (SEI) and other degradation products. Alsagri et al. demonstrated that the loss of cycleable lithium as a result of SEI layer formation is the primary cause of capacity decline during calendar aging [96]. When compared to a fresh cell, this might explain the thermal behavior of the electrodes in their older form. Because the previous state’s SEI layer is more prominent and undergoes exothermic breakdown, the onset temperature is lowered. Simultaneously, the aged anode releases a lot less total energy. This is because there is less intercalated lithium in the aged anode’s charged state, which might react with the electrolyte during the thermal tests. This procedure may cause the battery’s capacity to decrease, its internal resistance to rise, and its overall performance to decline [113]. However, because the old cathode’s potential in the charged state remains unchanged, the aged cathode/electrolyte process does not exhibit any appreciable divergence from its thermal behavior. The aged lithium-ion cell’s somewhat higher heating rate over 200 C can be attributed to the cathode’s larger surface area. Increased internal resistance is a result of the solid–electrolyte interphase (SEI) layer on the electrode surfaces forming and rebuilding over time.
The degradation of active materials in batteries is caused by unwanted side reactions, which have an adverse effect on the battery’s overall performance. The effective surface area for electrochemical reactions can be decreased and the battery’s capacity affected by the agglomeration and development of active material particles as well as lithium plating on the anode under specific circumstances [114,115]. Since these aging processes are accelerated by elevated temperatures, temperature regulation is essential for reducing calendar aging. Manufacturers concentrate on developing efficient electrolytes, optimizing battery materials, and putting in place cutting-edge battery management systems in order to overcome these obstacles and increase the longevity and functionality of lithium-ion batteries [116]. Several calendar aging modeling techniques have been shown in [117,118,119,120]. Suri et al. presented a brief review about the electrochemical, semi-empirical, and data-driven models [121]. Calendar aging of lithium-ion battery can be explained by the Arrhenius equation [121].
where, both and are the SOC dependent terms, is the gas constant, and z is the power law parameter used to denote the dependence of time parameters.
3.5. State of Charge
In lithium-ion batteries, battery degradation due to SOC is the result of keeping the battery at a certain charge level for lengthy periods of time, either high or low. This causes the general health of battery to gradually deteriorate. Long-term full-charge times (high SOC) can lead to the production of unwanted byproducts such the solid–electrolyte interphase (SEI) layer on the electrode surfaces. The ongoing development of this layer may raise internal resistance, restricting the movement of ions and electrons within the battery and eventually lowering its capacity. However, persistently using the battery at low SOC levels may cause lithium plating, or the accumulation of lithium metal on the anode. Bui et al. performed an experimental investigation on five cells subjected to calendar aging for 12 months and the results indicate that the worst calendar aging conditions are 45 °C and 50% SOC [122]. The SOC gradually increases with continuous voltage charging until the battery is almost at capacity. When the voltage is maintained during charging, the SOC rises until it reaches almost full charge, at which point the voltage is kept constant while the SOC moves closer to saturation. Guena et al. found that 90% of the cycling degradation occurs due to the constant voltage [123]. There is a strong relationship between SOC and battery degradation. Many studies find that increasing degradation can be seen in the lower SOC [124,125,126]. This can be explained by the fact that when the cell discharges the impedance of the cell will be increased, which results in more self-heating and reduces power capabilities. Lin et al. presented the effect of minimum SOC on a battery at 30 °C with 25–45% minimum SOC and the results show an exponential increase in degradation at higher SOC [127]. However, in [128], a negligible degradation is found with higher SOC cycled with a C rate of 10 C. The authors used the Arrhenius equation for modeling which is given below.
The safety and capacity of the battery are adversely affected by lithium plating. There are several charging strategies which may mitigate this degradation. Wright et al. proposed a state-of-charge pre-conditioning model and this method does not accelerate the battery degradation and was also able to mitigate battery degradation by 7.3% to 26.7% for the first 100 days of operation [111].
3.6. Depth of Discharge
The depth of discharge in a lithium-ion battery is the proportion of its total capacity that has been utilized during a single discharge cycle, expressed as a percentage. For instance, if a battery with a capacity of 100 ampere-hours has been discharged by 50 ampere-hours, the DOD is 50%. The monitoring and managing of DOD are critical for optimizing the performance and lifespan of lithium-ion batteries. Generally, shallower discharges (lower DOD) contribute to extended battery life, and battery management systems often regulate DOD to strike a balance between maximizing energy storage capacity and preserving the longevity of the battery. Many studies found that an increasing DOD reduces the cycle life of a lithium-ion battery, which triggers the fast degradation of the lithium-ion battery [129,130,131]. Guana and Lebnac presented an experimental investigation on the effect of DOD on the battery cycle life of a lithium-ion battery and the results indicate that that increased DOD reduces the cycle life and increases the capacity fade, which is closely related to battery degradation [112]. This study also found that over a four-month period, microcycling at a capacity of 0.6% did not result in any performance impairment. Thirty-two full cycles were completed at this time. Furthermore, about sixteen thousand 0.6% microdischarges were carried out.
Effective DOD management is essential in various applications, including electric vehicles and portable electronics. Deep discharge cycles increase the risk of lithium plating forming on the anode of a battery, which can result in dendrite formation and risk its capacity and safety. Deeper discharge cycles also put more stress on the active materials in the electrode, which can lead to chemical and physical changes such as cracking and electrode structural degradation. Youksek and Alkaya illustrated a complex relationship between DOD and battery degradation [112]. The authors claim that battery degradation and lifetime is directly impacted by DOD. Higher DoD levels, which are indicative of deeper discharges, put the battery under more stress throughout each charge–discharge cycle. Increased mechanical stress, heat production, chemical reactions, capacity fading, and shorter cycle life are all caused by this increased stress. Many studies claimed that DOD can be modeled based on the Wohler curve and according to their findings, model degradation is exponentially dependent on the DOD. The equations are as follows [112]:
where, is the cycle life as a function of DOD and are the curve fit parameters.
Increased internal resistance is a result of the solid–electrolyte interphase (SEI) layer on the electrode surfaces repeatedly forming and reconstructing during discharge and recharge cycles, especially at high DOD [32]. This eventually results in decreased performance as the battery’s ability to transport ions is diminished [132]. Deeper discharges also increase the battery’s temperature, which speeds up chemical reactions and encourages degradation processes. Table 2, Table 3 and Table 4 shows the impact of DOD on battery life cycle, comparison of performance parameter of different types of lithium ion battery and Cycle life of different kinds of lithium-ion batteries respectively.

Table 2.
Impact of DOD on the cycle and the lifetime of lithium-ion battery [112].

Table 3.
Comparison of the performance parameters of the different types of batteries [133].

Table 4.
Cycle life of different kinds of lithium-ion batteries.
4. Key Effect of Battery Degradation on EVs and Energy Storage Systems
Battery degradation poses significant challenges for energy storage systems, impacting their overall efficiency and performance. Over time, the gradual loss of capacity in batteries reduces the system’s ability to store and deliver the expected amount of energy. This capacity loss, coupled with increased internal resistance and voltage fade, leads to decreased energy density and efficiency. As a result, energy storage systems experience a shortened cycle life, reduced power output, and increased maintenance costs.
4.1. Capacity Fade
The steady decline in a battery’s capacity to store and release energy over time is referred to as capacity fade in battery energy storage systems (BESS). This phenomenon is especially important for rechargeable batteries used in energy storage systems, grid storage, and electric vehicles, among other applications. Numerous reasons contribute to degradation, including the impact of high operating temperatures, structural changes in electrodes, unintentional side reactions producing byproducts that interfere with normal functioning, and chemical and electrochemical processes during charge and discharge cycles [31,137]. The amount of regular charge and discharge cycles, or cycling depth, in addition to the charge level, might affect how quickly capacity fades.
Battery degradation affects each battery cell in the battery energy storage system (BESS), which in turn causes capacity fading throughout the system. Waldmann et al. estimated an 18% capacity fade in lithium Li0.89NiCoO2 during the first charge discharge cycle [44]. Generally, BESS is made up of multiple interconnected batteries, and the system’s total performance and storage capacity are impacted by the individual cells’ deterioration combined. The interior components of a battery cell gradually change as a result of the chemical and electrochemical processes that occur during charge and discharge cycles. Within individual cells, accumulation of byproducts takes place, such as the development of solid–electrolyte interfaces (SEI) or dendritic growth [119]. Li et al. said that the SEI layer growth is the major contributor of the capacity fade of a lithium-ion battery [120]. This study also found that after 1000 cycles, 4.45% and 4.46% capacity fade has been observed for the regenerative and non-regenerative cases, respectively. The ability of each cell to store energy decreases as a result of this degeneration. Temperature has a great impact on the capacity fading of a lithium-ion battery. Lam and Bauer investigated capacity fade at different temperatures such as −20 °C, 0 °C, 25 °C, and 40 °C and the results of this temperature stress factor study excluded the cell that cycled at −20 °C due to extreme capacity fading, which made it impossible to define the rate of fading. The total effect of several cells experiencing capacity fade is a decrease in the BESS’s capacity to store and distribute energy. Degraded capacity cells may not contribute as efficiently to the system’s overall energy production, which might result in decreased system dependability and overall performance [124,138]. Various models used to estimate capacity fade have been found in different studies [25,139,140]. Wood et al. used the following equation to estimate the capacity fade of the lithium-ion battery [97]:
where Q is the battery capacity, K is the number of cycles, a and b is the constant related to the internal impedance and c and d indicate the aging rate. Figure 6 illustrates a cross-sectional view of a lithium-ion battery.
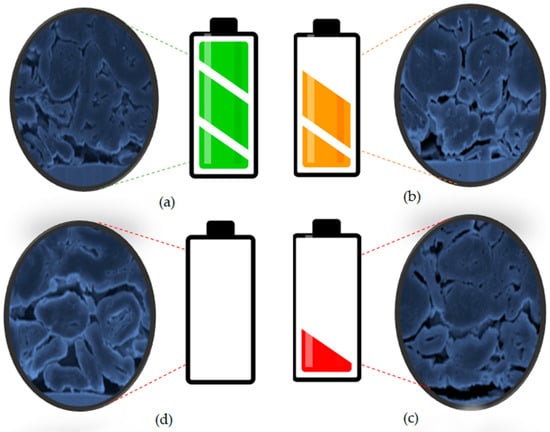
Figure 6.
Cross-sectional image of capacity fade of lithium-ion battery, (a) fresh, (b) 1000, (c) 2000, and (d) 3000 cycles [119].
4.2. Reduction in Energy Density
A battery may only go through a certain number of charge and discharge cycles before its capacity decreases to a certain point [125,126]. This is known as the limited cycle life of a battery energy storage system, or BESS. As it establishes the maximum number of times a battery may be charged and discharged without compromising performance, cycle life is an important aspect of energy storage systems’ efficacy and lifespan [124]. The cycle life of a battery is frequently shortened by internal processes that take place with each cycle, including mechanical deterioration, heat stress, and chemical and electrochemical reactions [141]. The ability of a BESS to supply energy storage consistently and steadily over an extended length of time is impacted as its overall efficacy and reliability are undermined as its cycle life shortens. Tröltzsch, et al. investigated the efficiency degradation of two types of lithium cell: NMC and LFP, which are affected by the calendar aging, [142]. Comparison of efficiency degradation of NMC and LFP have shown in Figure 7. In this study, the cells are experimented in 60 °C and 100% SOC, where it has been observed that after 190 days, the overall efficiency of the cell is reduced by 9% for the NMC and 1% for the LFP cell, and the capacity fade reached 37%. The composition and arrangement of the batteries’ active components vary as a result of chemical processes that occur throughout each cycle of charging and discharging. The slow deterioration of the battery cells can be accelerated by this aging process, which can frequently be accelerated by factors like temperature changes and cycling depth [143]. Gradually, the accumulation of aging effects in individual cells reduces the BESS’s overall capacity to store energy. The aging process of the overall energy storage system is accelerated by the growth of dendrites, the formation of solid–electrolyte interfaces, and thermal stress.
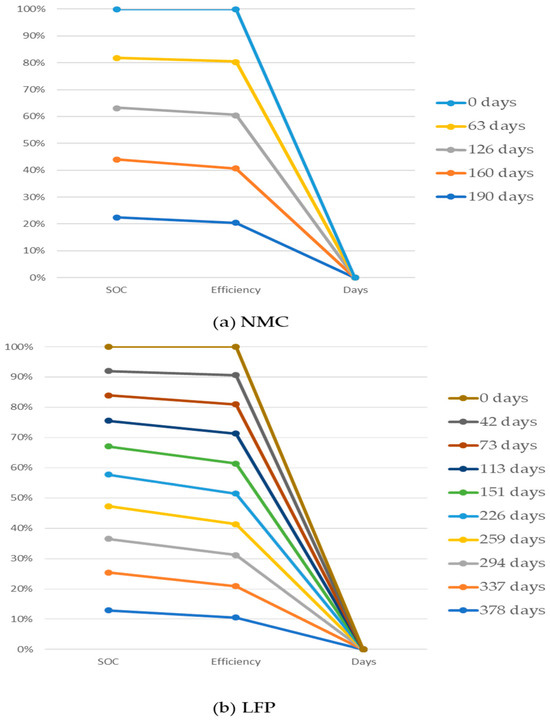
Figure 7.
Efficiency degradation of lithium-ion cell due to calendar aging of (a) NMC and (b) LFP [124].
4.3. Increase in Internal Resistance
The performance of a battery energy storage system (BESS) can be greatly impacted by increased internal resistance, which can result from a number of different causes. This increase in resistance is frequently the result of the battery aging and degrading, a process that is sped up by frequent cycles of charge and discharge. Battery degradation also affects EV batteries. Fanoro et al. finds great fluctuations in voltage and increasing internal resistance of EV batteries for lithium polymer batteries which are comparatively higher them any other batteries [129]. The observed rise in internal resistance is attributed to a number of factors, including the creation of solid–electrolyte interfaces (SEI), high operating temperatures, cycling depth, and dendritic growth in some chemistries, such as lithium-ion batteries. Increased internal resistance has a variety of effects. Yang et al. claimed that internal resistance increased mostly at 82% of SOH for an EV battery [130]. Because more energy is lost in the battery during operation, the BESS’s overall efficiency is decreased. Voltage stability is also impacted by a voltage drop across the battery terminals during high-current applications. The heat produced by the resistance presents problems for thermal control and may hasten the deterioration process.
An increasing amount of internal resistance frequently results in capacity fade, which is characterized by a progressive decrease in the BESS’s ability to store energy [131]. The internal resistance of the lithium ion can be estimated by the following equations [144]:
When the extended state estimation is multiplied by the input , and it might make fine-tuning the feedback gain more challenging, represents the extended state’s dynamics.
4.4. Reduction in Overall Efficiency
Through several interconnected factors, battery degradation poses a serious threat to a battery energy storage system’s overall efficiency. In an experimental study Kassem et al. showed a complex relationship between degradation and efficiency [145]. Authors experimented with two different types of lithium-ion batteries; NMC and LFP batteries where it has been shown that NMC and LFP cells age differently from one another. While the degradation curves of NMC cells show a two-phase concave degradation tendency, those of LFP cells exhibit a typical convex form. However, in both cases, loss of active material not only reduces lithium inventory but also quickens battery deterioration and lowers columbic efficiency (CE) values which leads to an overall efficiency reduction. The increase in internal resistance that occurs because of aging, chemical changes, and thermal stress during charge and discharge cycles is one important component. Because more energy is lost as heat as a result of this increased internal resistance, the overall efficiency of the battery is decreased.
Battery degradation also impacts on the overall efficiency of EVs. Table 3 presents a summary of the performance parameters of different types of lithium-ion battery. Darma et al. claimed that battery degradation decreases the travel range of EVs which leads to a decrease in the overall efficiency of EVs [146]. Another aspect of deterioration is capacity fade, which over time reduces the battery’s capacity to store and distribute energy and has an immediate effect on the BESS’s energy efficiency [9,147]. Older batteries may also have reduced energy retention, faster rates of self-discharge, and less stored energy accessible for use when needed [139,140]. Voltage instabilities, stemming from degradation-induced fluctuations, further undermine the efficiency of power conversion systems within the BESS [148,149]. Moreover, increased heat generation during degradation processes contributes to thermal stress, exacerbating efficiency losses. An analysis applies the state-level operation condition to the EV energy operation model by considering the battery degradation effect on mid-size EVs with a 24 kWh lithium-ion manganese oxide (LMO) battery pack in order to investigate the impacts of battery degradation on the energy consumption and GHG emissions of EVs in the USA. According to the study, due to a 10-year battery deterioration, the unit energy consumption and GHG emission increases vary from 29.2 Wh/km in Alaska to 127.4 Wh/km in Mississippi, and 0.2 g CO2/km in Vermont to 56.9 g CO2/km in Indiana, respectively [137,150,151]. A summary of the impact of degradation factors on lithium-ion batteries has been presented in Table 5 [152].

Table 5.
Impact of degradation factors on battery anode and cathode.
5. Data-Driven Approaches for Estimation of Battery Degradation
5.1. Support Vector Machines (SVM)
Battery deterioration is predicted using a machine learning approach called support vector machines (SVM). SVM models anticipate the degree of battery degradation or estimate the battery’s remaining usable life by using historical data and battery performance characteristics, including voltage, current, temperature, and cycle count [165]. SVMs function by determining the best hyperplane with the largest margin that divides data points from several classes or regression objectives. With kernel functions, they provide robustness against overfitting, efficiency in high-dimensional areas, and the capacity to manage nonlinear interactions [166]. Li et al. presented a novel SVM-based technique for battery degradation prediction by RUL and the results indicate that the proposed model is 99.89% accurate and RMSE is 0.11% [153]. Chen et al. proposed a capacity degradation prediction model for a lithium-ion battery based on SVMs and the authors claimed that the artificial bee colony and support vector regression model can predict more accurately compared to other state-of-the-art algorithms [165].
5.2. Relevance Vector Machines (RVM)
Battery degradation prediction using relevance vector machines (RVMs) is a machine learning technique that leverages historical data and pertinent characteristics taken from battery performance indicators to estimate battery deterioration and remaining usable life. Support vector machines (SVM) and Relevance vector machine (RVMs) function similarly; however, RVMs’ probabilistic structure and their sparsity of model parameters are different. With benefits including automated feature selection and prediction uncertainty calculation, RVMs seek to identify the most pertinent data points (vectors) to forecast battery deterioration [154]. Cong et al. proposed a hybrid model (Broad Leaning–Relevance Vector Machine) for RUL prediction of lithium-ion batteries and the results indicate that the proposed model has higher accuracy and strong long-term predictive capability [167]. This study also revealed that the RMSE of the model is 0.01. In another study, Nash et al. proposed another hybrid model to predict the RUL of a battery, where the authors showed the model has a relative prediction error of 3.3% and 3.21%, respectively, for two unseen datasets [168].
5.3. Gaussian Process Regression (GPR)
Modeling the link between input parameters and degradation levels, Gaussian Process Regression (GPR) is a machine learning approach used to forecast battery deterioration. GPR is capable of estimating the output’s mean and variance at every given input point since it is predicated on the idea that the output data have a Gaussian distribution [169]. GPR forecasts the degree of deterioration and calculates the battery’s remaining usable life by utilizing past data on battery performance characteristics, such as voltage, current, temperature, and cycle count. Chinomona et al. presented a GPR-based battery degradation diagnosis model for lithium-ion battery [170]. This study reveals that the use of an aging dataset, C rates variation data, temperature, and user profiles leads to higher prediction accuracy. This model had a mean absolute error of less than 0.1 and a coefficient of determinant greater than 0.9. In another study, Sengupta et al. proposed a GPR-based hybrid model of battery capacity degradation [171]. The mean absolute percentage errors of predicted battery capacity degradation were less than 0.4%.
5.4. Deep Learning
By using neural network architectures, most often deep neural networks (DNN), deep learning is used to estimate battery deterioration and remaining usable life based on past data as well as several performance characteristics. Depending on the unique features of the battery degradation data, these models might be convolutional neural networks (CNN), recurrent neural networks (RNNs), or mixtures of the two. Nutakki et al. presented a review of the different deep learning techniques of different degradation prediction models [172]. Compared to the other degradation models, the deep learning method showed higher prediction accuracy which is 90%. Using a typical long short-term memory (LSTM) model, May et al. [156] created a technique for estimating the remaining useful life (RUL) of lithium-ion batteries. The study used a systematic sampling strategy to efficiently gather battery data features from many metrics and provide a full 31-dimensional dataset. Pang et al. [157] trained the LSTM model with the 18 characteristics that were obtained using a statistical sampling technique. Almuhaylan et al. proposed a model for battery degradation trajectory prediction and the model shows that with normal data, the lifetime median prediction error is 1.1%, while with noisy data, it is 1.3% [158].
5.4.1. Artificial Neural Network (ANN)
The modeling of battery deterioration made possible by artificial neural networks (ANN) is a strong foundation for forecasting battery health and remaining usability. Artificial neural networks can efficiently learn complicated correlations between several parameters, including voltage, current, temperature, and cycle count, by gathering and preprocessing data from battery-monitoring systems or experiments [159,160]. The main components of a simple artificial neural network model are usually one input layer, one hidden layer, and one output layer. The input layer receives input data initially, processes them, forwards them to the hidden layer, and then the output layer generates results [161]. Jafari et al. presented a new ANN-based model to predict the degradation of lithium-ion battery [162]. The results indicate that the new model showed robustness against varying temperature and varying loads [163]. Lu et al. proposed a novel ANN-based battery degradation prediction model for lithium-ion batteries and the authors claimed that the proposed model is able predict battery degradation with 5.9% error [173].
5.4.2. Long Short-Term Memory Neural Network
Recurrent neural networks (RNN) with long short-term memory (LSTM) architecture are particularly good at modeling sequential input and capturing long-term relationships. Based on past performance data, LSTM networks are used in the context of battery degradation prediction to anticipate the deterioration and remaining usable life of batteries. Because LSTM networks are capable of capturing temporal patterns and dependencies in the behavior of the battery over time, including voltage profiles, current fluctuations, temperature changes, and cycle counts, they are a good fit for this purpose. LSTM networks may learn intricate correlations and accurately estimate future deterioration levels or remaining lifespan by examining sequential data. Ren et al. presents a CNN-LSTM model and the model achieved 99.6% accuracy in partial discharge voltage and its prediction error is greater than 99% for capacity estimation prediction [174].
5.4.3. Gated Recurrent Unit Neural Network
Sequential data modeling is a strong fit for the gated recurrent unit (GRU) kind of recurrent neural network (RNN) architecture. GRU networks are used in the context of battery degradation prediction in order to anticipate the deterioration process and calculate the batteries’ remaining usable life based on past performance data [175]. Because they can record long-term dependencies and temporal patterns in the battery’s activity over time, such as voltage swings, current profiles, temperature changes, and cycle counts, GRU networks are especially well suited for this purpose. GRU networks may discover complex correlations and forecast future battery degradation levels or remaining lifespan by examining sequential data [176]. Haris et al. presented a case study about a battery degradation prediction model based on recurrent neural network, where 77 commercial batteries are used as test sample [177]. The experimental results showed that the median root means square error of this model were 2.4% for the NMC/graphite batteries and 2.3% for LFP/graphite batteries, using only 3.8% of data regarding the whole battery life.
5.4.4. Convolution Neural Network
The capabilities of CNN architectures—which were first created for image processing tasks—are utilized by convolutional neural networks (CNN) for battery deterioration prediction, which analyzes sequential battery performance data. CNNs are used in this context to extract pertinent characteristics from the input data, which usually consist of time-series data like voltage, current, temperature, and cycle count [52]. CNNs are able to extract both spatial and temporal patterns from the battery performance data by utilizing convolutional layers. These patterns could be an indication of usage patterns, deterioration tendencies, or other elements affecting battery health. CNNs can forecast future deterioration levels or calculate the amount of battery life left by using existing data. A convolution neural network-based model has been presented in [175] for battery degradation diagnosis and prediction, and the results illustrate that the root mean square error is around 2% for 100 duty cycles, which is 2.64% to 7.27% better than the other algorithms. In another study, a degradation curve prediction model for lithium-ion batteries has been presented [174]. This study shows that the proposed model is successfully able to predict the degradation of a lithium-ion battery, with the root mean square error being 0.005 and the mean absolute percentage error being 0.416. Table 3 shows the summary of recent progress on data-driven techniques of battery degradation estimation. Table 6 summarizes the different data-driven approaches.

Table 6.
Various kinds of data-driven approaches for battery degradation prediction.
6. Conclusions and Future Directions
Battery degradation can significantly impact BMSs and EVs. This review illuminates the complex factors influencing lithium-ion battery degradation, stressing its crucial implications for sustainable energy storage and EVs. This paper offers insights into the multifaceted nature of battery degradation, examining its impacts on performance metrics like capacity fade, increased internal resistance, and reduced energy density. Various degradation mechanisms, including electrolyte breakdown, SEI formation, temperature, SOC, and DOD variation, cycling, and calendar aging, are thoroughly analyzed. SEI formation increases battery impedance, leading to capacity fade, while factors such as high temperature and depth of discharge contribute to electrolyte breakdown, increasing internal resistance and reducing capacity. SOC saturation accounts for 90% of cycling degradation. Acknowledging the importance of addressing degradation challenges, this review underscores the need for innovative, data-driven strategies to model degradation effects and improve battery system longevity and sustainability. Based on critical discussion, information, and analysis, this study proposes the following suggestions and future directions:
- ▪
- Electrolyte breakdown and the formation of SEI are key factors contributing to battery degradation. Research into novel electrode materials, electrolytes, and coatings can potentially result in batteries with enhanced durability, stability, and resistance to degradation mechanisms such as electrode dissolution, electrolyte decomposition, and SEI formation. Integration of advanced nanomaterials, solid-state electrolytes, and multifunctional coatings has the potential to improve battery performance and longevity.
- ▪
- The detrimental effects of heat stress and overheating in EV batteries can be mitigated through enhanced thermal management systems, which incorporate active cooling, thermal insulation, and temperature regulation. Longer battery life spans and optimal operating temperatures can be attained by integrating advanced cooling technologies such as liquid immersion cooling, thermoelectric cooling, and phase change materials.
- ▪
- Energy consumption in EV batteries can be maximized, and degradation effects reduced by implementing dynamic load-balancing strategies, adaptive energy management algorithms, and intelligent charging profiles. BMS can decrease losses caused by deterioration and enhance overall battery performance by adjusting charging parameters in response to environmental conditions and battery status.
- ▪
- The integration of AI and ML algorithms holds great potential for predictive modeling and optimization of battery degradation under diverse operating conditions. Leveraging data-driven approaches can enable proactive maintenance strategies, predictive failure analysis, and adaptive control algorithms, thereby enhancing the efficiency and reliability of the battery systems.
- ▪
- Real-time monitoring of battery degradation and health can be facilitated by implementing advanced diagnostic techniques such as electrochemical impedance spectroscopy (EIS), voltammetry, and impedance spectroscopy. Lithium-ion battery systems can benefit from proactive maintenance and management enabled by the integration of sensor technologies and data analytics platforms.
Author Contributions
Conceptualization, T.R. and T.A.; methodology, T.A.; formal analysis, T.R. and T.A.; investigation, T.R. and T.A.; resources, T.A.; data curation, T.R.; writing—original draft preparation, T.R. and T.A.; writing—review and editing, T.A.; visualization, T.R.; supervision, T.A.; funding acquisition, T.A. All authors have read and agreed to the published version of the manuscript.
Funding
The Researchers would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2024-9/1).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Huang, W.C.; Zhang, Q.; You, F. Impacts of battery energy storage technologies and renewable integration on the energy transition in the New York State. Adv. Appl. Energy 2022, 9, 100126. [Google Scholar] [CrossRef]
- Lipu, M.S.H.; Mamun, A.A.; Ansari, S.; Miah, M.S.; Hasan, K.; Meraj, S.T.; Abdolrasol, M.G.M.; Rahman, T.; Maruf, M.H.; Sarker, M.R.; et al. Battery Management, Key Technologies, Methods, Issues, and Future Trends of Electric Vehicles: A Pathway toward Achieving Sustainable Development Goals. Batteries 2022, 8, 119. [Google Scholar] [CrossRef]
- Huang, P.; Zeng, G.; He, Y.; Liu, S.; Li, E.; Bai, Z. Damage evolution mechanism and early warning using long short-term memory networks for battery slight overcharge cycles. Renew. Energy 2023, 127, 119171. [Google Scholar] [CrossRef]
- Habib, A.K.M.A.; Hasan, M.K.; Issa, G.F.; Singh, D.; Islam, S.; Ghazal, T.M. Lithium-Ion Battery Management System for Electric Vehicles: Constraints, Challenges, and Recommendations. Batteries 2023, 9, 152. [Google Scholar] [CrossRef]
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J.; et al. Lithium ion battery degradation: What you need to know. Phys. Chem. Chem. Phys. 2021, 23, 8200–8221. [Google Scholar] [CrossRef]
- Spitthoff, L.; Wahl, M.S.; Lamb, J.J.; Shearing, P.R.; Vie, P.J.S.; Burheim, O.S. On the Relations between Lithium-Ion Battery Reaction Entropy, Surface Temperatures and Degradation. Batteries 2023, 9, 249. [Google Scholar] [CrossRef]
- Sarkar, J.; Bhattacharyya, S. Application of graphene and graphene-based materials in clean energy-related devices Minghui. Arch. Thermodyn. 2012, 33, 23–40. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Perez, A.; Moreno, R.; Moreira, R.; Orchard, M.E.; Strbac, G. Effect of Battery Degradation—Policy invest by imperial. IEEE 2018, 1–11. [Google Scholar]
- Amini, M.; Nazari, M.H.; Hosseinian, S.H. Predictive energy management strategy for battery energy storage considering battery degradation cost. IET Renew. Power Gener. 2023, 17, 1119–1138. [Google Scholar] [CrossRef]
- Timilsina, L.; Badr, P.R.; Hoang, P.H.; Ozkan, G.; Papari, B.; Edrington, C.S. Battery Degradation in Electric and Hybrid Electric Vehicles: A Survey Study. IEEE Access 2023, 11, 42431–42462. [Google Scholar] [CrossRef]
- Cui, J.; Tan, Q.; Liu, L.; Li, J. Environmental Benefit Assessment of Second-Life Use of Electric Vehicle Lithium-Ion Batteries in Multiple Scenarios Considering Performance Degradation and Economic Value. Environ. Sci. Technol. 2023, 57, 8559–8567. [Google Scholar] [CrossRef] [PubMed]
- Pasini, G.; Lutzemberger, G.; Ferrari, L. Renewable Electricity for Decarbonisation of Road Transport: Batteries or E-Fuels? Batteries 2023, 9, 135. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Y.; Chen, Z. Data-Driven Battery Aging Mechanism Analysis and Degradation Pathway Prediction. Batteries 2023, 9, 129. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, Q.; Zheng, B.; Wu, S.; Pecht, M. Quantitative analysis of lithium-ion battery capacity prediction via adaptive bathtub-shaped function. Energies 2013, 6, 3082–3096. [Google Scholar] [CrossRef]
- Lipu, M.S.H.; Miah, M.S.; Jamal, T.; Rahman, T.; Ansari, S.; Rahman, M.S.; Ashique, R.H.; Shihavuddin, A.S.M.; Shakib, M.N. Artificial Intelligence Approaches for Advanced Battery Management System in Electric Vehicle Applications: A Statistical Analysis towards Future Research Opportunities. Vehicles 2023, 6, 22–70. [Google Scholar] [CrossRef]
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-ion battery aging mechanisms and diagnosis method for automotive applications: Recent advances and perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, L.; Zhang, Y.; Wang, J.; Wang, Z. Aging behavior and mechanisms of lithium-ion battery under multi-aging path. J. Clean. Prod. 2023, 423, 138678. [Google Scholar] [CrossRef]
- Liu, D.; Song, Y.; Li, L.; Liao, H.; Peng, Y.; Guo, J.; Li, Y.; Pedersen, K.; Stroe, D.; Sarkar, J.; et al. Lithium-Ion Battery Operation, Degradation, and Aging Mechanism in Electric Vehicles: An Overview. Energies 2021, 14, 5220. [Google Scholar] [CrossRef]
- Wankmüller, F.; Thimmapuram, P.R.; Gallagher, K.G.; Botterud, A. Impact of battery degradation on energy arbitrage revenue of grid-level energy storage. J. Energy Storage 2017, 10, 56–66. [Google Scholar] [CrossRef]
- Ou, S. Estimate long-term impact on battery degradation by considering electric vehicle real-world end-use factors. J. Power Sources 2023, 573, 233133. [Google Scholar] [CrossRef]
- Kaliaperumal, M.; Dharanendrakumar, M.S.; Prasanna, S.; Abhishek, K.V.; Chidambaram, R.K.; Adams, S.; Zaghib, K.; Reddy, M.V. Cause and mitigation of lithium-ion battery failure—A review. Materials 2021, 14, 5676. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Bhutto, J.K.; Alrobaian, A.; Kaladgi, A.R.; Khan, S.A. Modelling and computational experiment to obtain optimized neural network for battery thermal management data. Energies 2021, 14, 7370. [Google Scholar] [CrossRef]
- Sun, J.; Ye, L.; Zhao, X.; Zhang, P.; Yang, J. Electronic Modulation and Structural Engineering of Carbon-Based Anodes for Low-Temperature Lithium-Ion Batteries: A Review. Molecules 2023, 28, 2108. [Google Scholar] [CrossRef]
- Keil, P.; Schuster, S.F.; Wilhelm, J.; Travi, J.; Hauser, A.; Karl, R.C.; Jossen, A. Calendar Aging of Lithium-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1872–A1880. [Google Scholar] [CrossRef]
- Ohzuku, T.; Iwakoshi, Y.; Sawai, K. Formation of Lithium-Graphite Intercalation Compounds in Nonaqueous Electrolytes and Their Application as a Negative Electrode for a Lithium Ion (Shuttlecock) Cell. J. Electrochem. Soc. 1993, 140, 2490–2498. [Google Scholar] [CrossRef]
- Barré, A.; Deguilhem, B.; Grolleau, S.; Gérard, M.; Suard, F.; Riu, D. A review on lithium-ion battery ageing mechanisms and estimations for automotive applications. J. Power Sources 2013, 241, 680–689. [Google Scholar] [CrossRef]
- Atalay, S.; Sheikh, M.; Mariani, A.; Merla, Y.; Bower, E.; Widanage, W.D. Theory of battery ageing in a lithium-ion battery: Capacity fade, nonlinear ageing and lifetime prediction. J. Power Sources 2020, 478, 229026. [Google Scholar] [CrossRef]
- Carnovale, A.; Li, X. A modeling and experimental study of capacity fade for lithium-ion batteries. Energy AI 2020, 2, 100032. [Google Scholar] [CrossRef]
- Pantenburg, I.; Cronau, M.; Boll, T.; Duncker, A.; Roling, B. Challenging Prevalent Solid Electrolyte Interphase (SEI) Models: An Atom Probe Tomography Study on a Commercial Graphite Electrode. ACS Nano 2023, 17, 21531–21538. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, Y.; Tang, A. Capacity Degradation and Aging Mechanisms Evolution of Lithium-Ion Batteries under Different Operation Conditions. Energies 2023, 16, 4232. [Google Scholar] [CrossRef]
- Maheshwari, A. Modelling, Aging and Optimal Operation of Lithium-Ion Batteries; Eindhoven University of Technology: Eindhoven, The Netherlands, 2018; ISBN 9789038646077. [Google Scholar]
- Agubra, V.A.; Fergus, J.W. The formation and stability of the solid electrolyte interface on the graphite anode. J. Power Sources 2014, 268, 153–162. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, D.; Schaltz, E.; Stroe, D.I.; Gismero, A.; Yang, B. Lithium-ion battery calendar aging mechanism analysis and impedance-based State-of-Health estimation method. J. Energy Storage 2023, 64, 107029. [Google Scholar] [CrossRef]
- Deckenbach, D.; Schneider, J.J. A Long-Overlooked Pitfall in Rechargeable Zinc–Air Batteries: Proper Electrode Balancing. Adv. Mater. Interfaces 2023, 10, 2202494. [Google Scholar] [CrossRef]
- Cheng, L.; Wan, Y.; Zhou, Y.; Gao, D.W. Operational Reliability Modeling and Assessment of Battery Energy Storage Based on Lithium-ion Battery Lifetime Degradation. J. Mod. Power Syst. Clean Energy 2022, 10, 1738–1749. [Google Scholar] [CrossRef]
- Köbbing, L.; Latz, A.; Horstmann, B. Growth of the solid-electrolyte interphase: Electron diffusion versus solvent diffusion. J. Power Sources 2023, 561, 232651. [Google Scholar] [CrossRef]
- Alzate-Vargas, L.; Blau, S.M.; Spotte-Smith, E.W.C.; Allu, S.; Persson, K.A.; Fattebert, J.L. Insight into SEI Growth in Li-Ion Batteries using Molecular Dynamics and Accelerated Chemical Reactions. J. Phys. Chem. C 2021, 125, 18588–18596. [Google Scholar] [CrossRef]
- Kamyab, N.; Weidner, J.W.; White, R.E. Mixed Mode Growth Model for the Solid Electrolyte Interface (SEI). J. Electrochem. Soc. 2019, 166, A334–A341. [Google Scholar] [CrossRef]
- Ploehn, H.J.; Ramadass, P.; White, R.E. Solvent Diffusion Model for Aging of Lithium-Ion Battery Cells. J. Electrochem. Soc. 2004, 151, A456. [Google Scholar] [CrossRef]
- Smith, A.J.; Fang, Y.; Mikheenkova, A.; Ekström, H.; Svens, P.; Ahmed, I.; Lacey, M.J.; Lindbergh, G.; Furó, I.; Lindström, R.W. Localized lithium plating under mild cycling conditions in high-energy lithium-ion batteries. J. Power Sources 2023, 573, 233118. [Google Scholar] [CrossRef]
- Campbell, I.D.; Marzook, M.; Marinescu, M.; Offer, G.J. How Observable Is Lithium Plating? Differential Voltage Analysis to Identify and Quantify Lithium Plating Following Fast Charging of Cold Lithium-Ion Batteries. J. Electrochem. Soc. 2019, 166, A725–A739. [Google Scholar] [CrossRef]
- Ando, K.; Matsuda, T.; Miwa, T.; Kawai, M.; Imamura, D. Degradation mechanism of all-solid-state lithium-ion batteries with argyrodite Li7−xPS6−xClx sulfide through high-temperature cycling test. Batter. Energy 2023, 2, 20220052. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.I.; Wohlfahrt-Mehrens, M. Li plating as unwanted side reaction in commercial Li-ion cells—A review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Juarez-Robles, D.; Vyas, A.A.; Fear, C.; Jeevarajan, J.A.; Mukherjee, P.P. Overcharge and Aging Analytics of Li-ion Cells. J. Electrochem. Soc. 2020, 167, 090547. [Google Scholar] [CrossRef]
- Che, Y.; Zheng, Y.; Forest, F.E.; Sui, X.; Hu, X.; Teodorescu, R. Predictive health assessment for lithium-ion batteries with probabilistic degradation prediction and accelerating aging detection. Reliab. Eng. Syst. Saf. 2024, 241, 109603. [Google Scholar] [CrossRef]
- Janakiraman, U.; Garrick, T.R.; Fortier, M.E. Review—Lithium Plating Detection Methods in Li-Ion Batteries. J. Electrochem. Soc. 2020, 167, 160552. [Google Scholar] [CrossRef]
- Ren, Y.; Widanage, D.; Marco, J. A Plating-Free Charging Scheme for Battery Module Based on Anode Potential Estimation to Prevent Lithium Plating. Batteries 2023, 9, 294. [Google Scholar] [CrossRef]
- Koleti, U.R.; Rajan, A.; Tan, C.; Moharana, S.; Dinh, T.Q.; Marco, J. A study on the influence of lithium plating on battery degradation. Energies 2020, 13, 3458. [Google Scholar] [CrossRef]
- Diallo, M.S.; Shi, T.; Zhang, Y.; Peng, X.; Shozib, I.; Wang, Y.; Miara, L.J.; Scott, M.C.; Tu, Q.H.; Ceder, G. Effect of solid-electrolyte pellet density on failure of solid-state batteries. Nat. Commun. 2024, 15, 858. [Google Scholar] [CrossRef]
- Gu, J.; Chen, X.; He, Z.; Liang, Z.; Lin, Y.; Shi, J.; Yang, Y. Decoding Internal Stress-Induced Micro-Short Circuit Events in Sulfide-Based All-Solid-State Li-Metal Batteries via Operando Pressure Measurements. Adv. Energy Mater. 2023, 13, 2302643. [Google Scholar] [CrossRef]
- Agubra, V.; Fergus, J. Lithium ion battery anode aging mechanisms. Materials 2013, 6, 1310–1325. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Whitacre, J.F. Inhomogeneous aging of cathode materials in commercial 18650 lithium ion battery cells. J. Energy Storage 2021, 35, 102244. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, Y.; Hu, Y.; Choe, S.Y. Electrochemical-thermal modeling of lithium plating/stripping of Li(Ni0.6Mn0.2Co0.2)O2/Carbon lithium-ion batteries at subzero ambient temperatures. J. Power Sources 2019, 418, 61–73. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, X.; Han, G.; Dai, H.; Zhu, J.; Wang, X.; Tang, X.; Ye, J. Lithium plating on the anode for lithium-ion batteries during long-term low temperature cycling. J. Power Sources 2021, 484, 229312. [Google Scholar] [CrossRef]
- Saxena, S.; Roman, D.; Robu, V.; Flynn, D.; Pecht, M. Battery stress factor ranking for accelerated degradation test planning using machine learning. Energies 2021, 14, 723. [Google Scholar] [CrossRef]
- Liu, G.; Wan, W.; Nie, Q.; Zhang, C.; Chen, X.; Lin, W.; Wei, X.; Huang, Y.; Li, J.; Wang, C. Controllable Long-term Lithium Replenishment for Enhancing Energy Density and Cycle Life of Lithium-ion Batteries. Energy Environ. Sci. 2024, 17, 1163–1174. [Google Scholar] [CrossRef]
- Wang, L.; Yin, S.; Zhang, C.; Huan, Y.; Xu, J. Mechanical characterization and modeling for anodes and cathodes in lithium-ion batteries. J. Power Sources 2018, 392, 265–273. [Google Scholar] [CrossRef]
- Fathiannasab, H.; Zhu, L.; Chen, Z. Chemo-mechanical modeling of stress evolution in all-solid-state lithium-ion batteries using synchrotron transmission X-ray microscopy tomography. J. Power Sources 2021, 483, 229028. [Google Scholar] [CrossRef]
- Kemeny, M.; Ondrejka, P.; Mikolasek, M. Comprehensive Degradation Analysis of NCA Li-Ion Batteries via Methods of Electrochemical Characterisation for Various Stress-Inducing Scenarios. Batteries 2023, 9, 33. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A review on the key issues of the lithium ion battery degradation among the whole life cycle. eTransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Xu, J. Critical Review on cathode–electrolyte Interphase Toward High-Voltage Cathodes for Li-Ion Batteries. Nano-Micro Lett. 2022, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ikuhara, Y.H.; Fisher, C.A.J.; Huang, R.; Kuwabara, A.; Moriwake, H.; Kohama, K.; Ikuhara, Y. Oxygen loss and surface degradation during electrochemical cycling of lithium-ion battery cathode material LiMn2O4. J. Mater. Chem. A 2019, 7, 8845–8854. [Google Scholar] [CrossRef]
- Arabolla Rodríguez, R.; González Montiel, M.; Della Santina Mohallem, N.; Mosqueda Laffita, Y.; Andrey Montoro, L.; Avila Santos, M.; León Ramírez, H.; Pérez-Cappe, E.L. The role of defects on the Jahn-teller effect and electrochemical charge storage in nanometric LiMn2O4 material. Solid State Ion. 2021, 369, 115707. [Google Scholar] [CrossRef]
- Kim, S.; Huang, H.Y.S. Mechanical stresses at the cathode-electrolyte interface in lithium-ion batteries. J. Mater. Res. 2016, 31, 3506–3512. [Google Scholar] [CrossRef]
- Gao, X.; He, P.; Ren, J.; Xu, J. Modeling of contact stress among compound particles in high energy lithium-ion battery. Energy Storage Mater. 2019, 18, 23–33. [Google Scholar] [CrossRef]
- Son, S.B.; Zhang, Z.; Gim, J.; Johnson, C.S.; Tsai, Y.; Kalensky, M.; Lopykinski, S.; Kahvecioglu, O.; Yang, Z.; Montoya, A.T.; et al. Transition Metal Dissolution in Lithium-Ion Cells: A Piece of the Puzzle. J. Phys. Chem. C 2023, 127, 1767–1775. [Google Scholar] [CrossRef]
- Frankenberger, M.; Trunk, M.; Seidlmayer, S.; Dinter, A.; Dittloff, J.; Werner, L.; Gernhäuser, R.; Revay, Z.; Märkisch, B.; Gilles, R.; et al. SEI growth impacts of lamination, formation and cycling in lithium ion batteries. Batteries 2020, 6, 21. [Google Scholar] [CrossRef]
- Shinagawa, C.; Ushiyama, H.; Yamashita, K. Multiscale Simulations for Lithium-Ion Batteries: SEI Film Growth and Capacity Fading. J. Electrochem. Soc. 2017, 164, A3018–A3024. [Google Scholar] [CrossRef]
- Roy, P.K.; Shahjalal, M.; Shams, T.; Fly, A.; Stoyanov, S.; Ahsan, M.; Haider, J. A Critical Review on Battery Aging and State Estimation Technologies of Lithium-Ion Batteries: Prospects and Issues. Electronics 2023, 12, 4105. [Google Scholar] [CrossRef]
- Wang, L.F.; Geng, M.M.; Ding, X.N.; Fang, C.; Zhang, Y.; Shi, S.S.; Zheng, Y.; Yang, K.; Zhan, C.; Wang, X.D. Research progress of the electrochemical impedance technique applied to the high-capacity lithium-ion battery. Int. J. Miner. Metall. Mater. 2021, 28, 538–552. [Google Scholar] [CrossRef]
- Hunter, J.C. Preparation of a new crystal form of manganese dioxide: λ-MnO2. J. Solid State Chem. 1994, 48, 173–174. [Google Scholar] [CrossRef]
- Li, W. Review—An Unpredictable Hazard in Lithium-ion Batteries from Transition Metal Ions: Dissolution from Cathodes, Deposition on Anodes and Elimination Strategies. J. Electrochem. Soc. 2020, 167, 090514. [Google Scholar] [CrossRef]
- Vermeer, W.; Chandra Mouli, G.R.; Bauer, P. A Comprehensive Review on the Characteristics and Modeling of Lithium-Ion Battery Aging. IEEE Trans. Transp. Electrif. 2022, 8, 2205–2232. [Google Scholar] [CrossRef]
- Mikheenkova, A.; Gustafsson, O.; Misiewicz, C.; Brant, W.R.; Hahlin, M.; Lacey, M.J. Resolving high potential structural deterioration in Ni-rich layered cathode materials for lithium-ion batteries operando. J. Energy Storage 2023, 57, 106211. [Google Scholar] [CrossRef]
- Love, C.T.; Korovina, A.; Patridge, C.J.; Swider-Lyons, K.E.; Twigg, M.E.; Ramaker, D.E. Review of LiFePO 4 Phase Transition Mechanisms and New Observations from X-ray Absorption Spectroscopy. J. Electrochem. Soc. 2013, 160, A3153–A3161. [Google Scholar] [CrossRef]
- Zhan, C.; Wu, T.; Lu, J.; Amine, K. Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes-A critical review. Energy Environ. Sci. 2018, 11, 243–257. [Google Scholar] [CrossRef]
- Vu, T.T.; Cheon, H.J.; Shin, S.Y.; Jeong, G.; Wi, E.; Chang, M. Hybrid electrolytes for solid-state lithium batteries: Challenges, progress, and prospects. Energy Storage Mater. 2023, 61, 102876. [Google Scholar] [CrossRef]
- Liao, X.; Huang, Q.; Mai, S.; Wang, X.; Xu, M.; Xing, L.; Liao, Y.; Li, W. Understanding self-discharge mechanism of layered nickel cobalt manganese oxide at high potential. J. Power Sources 2015, 286, 551–556. [Google Scholar] [CrossRef]
- Wandt, J.; Freiberg, A.; Thomas, R.; Gorlin, Y.; Siebel, A.; Jung, R.; Gasteiger, H.A.; Tromp, M. Transition metal dissolution and deposition in Li-ion batteries investigated by operando X-ray absorption spectroscopy. J. Mater. Chem. A 2016, 4, 18300–18305. [Google Scholar] [CrossRef]
- Rynearson, L.; Antolini, C.; Jayawardana, C.; Yeddala, M.; Hayes, D.; Lucht, B.L. Speciation of Transition Metal Dissolution in Electrolyte from Common Cathode Materials. Angew. Chem. 2024, 136, e202317109. [Google Scholar] [CrossRef]
- Huang, D.; Engtrakul, C.; Nanayakkara, S.; Mulder, D.W.; Han, S.D.; Zhou, M.; Luo, H.; Tenent, R.C. Understanding Degradation at the Lithium-Ion Battery Cathode/Electrolyte Interface: Connecting Transition-Metal Dissolution Mechanisms to Electrolyte Composition. ACS Appl. Mater. Interfaces 2021, 13, 11930–11939. [Google Scholar] [CrossRef]
- Krupp, A.; Beckmann, R.; Diekmann, T.; Ferg, E.; Schuldt, F.; Agert, C. Calendar aging model for lithium-ion batteries considering the influence of cell characterization. J. Energy Storage 2022, 45, 103506. [Google Scholar] [CrossRef]
- Dai, Q.; Kelly, J.C.; Gaines, L.; Wang, M. Life Cycle Analysis of Lithium-Ion Batteries for Automotive Applications. Batteries 2019, 5, 48. [Google Scholar] [CrossRef]
- Hou, J.; Qu, S.; Yang, M.; Zhang, J. Materials and electrode engineering of high capacity anodes in lithium ion batteries. J. Power Sources 2020, 450, 227697. [Google Scholar] [CrossRef]
- Lucu, M.; Martinez-Laserna, E.; Gandiaga, I.; Liu, K.; Camblong, H.; Widanage, W.D.; Marco, J. Data-driven nonparametric Li-ion battery ageing model aiming at learning from real operation data—Part B: Cycling operation. J. Energy Storage 2020, 30, 101410. [Google Scholar] [CrossRef]
- Serhan, M.; Sprowls, M.; Jackemeyer, D.; Long, M.; Perez, I.D.; Maret, W.; Tao, N.; Forzani, E. Total iron measurement in human serum with a smartphone. In Proceedings of the AIChE Annual Meeting Conference Proceedings, San Francisco, CA, USA, 15–20 November 2019; pp. 1–3. [Google Scholar]
- Lee, Y.K. Effect of transition metal ions on solid electrolyte interphase layer on the graphite electrode in lithium ion battery. J. Power Sources 2021, 484, 229270. [Google Scholar] [CrossRef]
- Xu, B.; Oudalov, A.; Ulbig, A.; Andersson, G.; Kirschen, D.S. Modeling of lithiumion battery degradation for cell life assessment. IEEE Trans. Smart Grid 2018, 9, 1131–1140. [Google Scholar] [CrossRef]
- Lee, C.H.; Wu, Z.Y.; Hsu, S.H.; Jiang, J.A. Cycle Life Study of Li-Ion Batteries with an Aging-Level-Based Charging Method. IEEE Trans. Energy Convers. 2020, 35, 1475–1484. [Google Scholar] [CrossRef]
- Campion, C.L.; Li, W.; Lucht, B.L. Thermal Decomposition of LiPF6-Based Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A2327. [Google Scholar] [CrossRef]
- Xu, J.; Deshpande, R.D.; Pan, J.; Cheng, Y.-T.; Battaglia, V.S. Electrode Side Reactions, Capacity Loss and Mechanical Degradation in Lithium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A2026–A2035. [Google Scholar] [CrossRef]
- Pinson, M.B.; Bazant, M.Z. Theory of SEI Formation in Rechargeable Batteries: Capacity Fade, Accelerated Aging and Lifetime Prediction. ECS Meet. Abstr. 2013, MA2013-01, 405. [Google Scholar] [CrossRef]
- Alsagri, A.S. An innovative design of solar-assisted carnot battery for multigeneration of power, cooling, and process heating: Techno-economic analysis and optimization. Renew. Energy 2023, 210, 375–385. [Google Scholar] [CrossRef]
- Alsagri, A.S.; Rahbari, H.R.; Wang, L.; Arabkoohsar, A. Thermo-economic optimization of an innovative integration of thermal energy storage and supercritical CO2 cycle using artificial intelligence techniques. Process Saf. Environ. Prot. 2024, 186, 1373–1386. [Google Scholar] [CrossRef]
- Alsagri, A.S.; Arabkoohsar, A.; Khosravi, M.; Alrobaian, A.A. Efficient and cost-effective district heating system with decentralized heat storage units, and triple-pipes. Energy 2019, 188, 116035. [Google Scholar] [CrossRef]
- Wood, S.M.; Fang, C.; Dufek, E.J.; Nagpure, S.C.; Sazhin, S.V.; Liaw, B.; Meng, Y.S. Predicting Calendar Aging in Lithium Metal Secondary Batteries: The Impacts of Solid Electrolyte Interphase Composition and Stability. Adv. Energy Mater. 2018, 8. [Google Scholar] [CrossRef]
- Dose, W.M.; Temprano, I.; Allen, J.P.; Björklund, E.; O’Keefe, C.A.; Li, W.; Mehdi, B.L.; Weatherup, R.S.; De Volder, M.F.L.; Grey, C.P. Electrolyte Reactivity at the Charged Ni-Rich Cathode Interface and Degradation in Li-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 13206–13222. [Google Scholar] [CrossRef]
- Fermín-Cueto, P.; McTurk, E.; Allerhand, M.; Medina-Lopez, E.; Anjos, M.F.; Sylvester, J.; dos Reis, G. Identification and machine learning prediction of knee-point and knee-onset in capacity degradation curves of lithium-ion cells. Energy AI 2020, 1. [Google Scholar] [CrossRef]
- Aris, A.M.; Shabani, B. An Experimental Study of a Lithium Ion Cell Operation at Low Temperature Conditions. Energy Procedia 2017, 110, 128–135. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Li, H.; Zhang, M. Review on state of charge estimation methods for Li-ion batteries. Trans. Electr. Electron. Mater. 2017, 18, 136–140. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, S.; Luo, L.; Ding, M.S.; Zheng, J.; Cartmell, S.S.; Wang, C.M.; Xu, K.; Zhang, J.G.; Xu, W. Wide-Temperature Electrolytes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 18826–18835. [Google Scholar] [CrossRef]
- Gao, F.; Tang, Z. Kinetic behavior of LiFePO4/C cathode material for lithium-ion batteries. Electrochim. Acta 2008, 53, 5071–5075. [Google Scholar] [CrossRef]
- Almeida, C.S. Analysis of the co-dispersion structure of health-related indicators, the center of the subject’s sense of health, and the elderly people living at home. Rev. Bras. Linguística Apl. 2016, 5, 1689–1699. [Google Scholar]
- Kalaga, K.; Rodrigues, M.T.F.; Trask, S.E.; Shkrob, I.A.; Abraham, D.P. Calendar-life versus cycle-life aging of lithium-ion cells with silicon-graphite composite electrodes. Electrochim. Acta 2018, 280, 221–228. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, H.; Yu, L.; Fan, W.Z.; Huang, D. Lithium difluorophosphate as an additive to improve the low temperature performance of LiNi0.5Co0.2Mn0.3O2/graphite cells. Electrochim. Acta 2016, 221, 107–114. [Google Scholar] [CrossRef]
- Ren, D.; Smith, K.; Guo, D.; Han, X.; Feng, X.; Lu, L.; Ouyang, M.; Li, J. Investigation of Lithium Plating-Stripping Process in Li-Ion Batteries at Low Temperature Using an Electrochemical Model. J. Electrochem. Soc. 2018, 165, A2167–A2178. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Y.; Zou, C.; Yao, K.; Xia, Y.; Gao, F. A novel framework for Lithium-ion battery modeling considering uncertainties of temperature and aging. Energy Convers. Manag. 2019, 180, 162–170. [Google Scholar] [CrossRef]
- Hahn, S.L.; Storch, M.; Swaminathan, R.; Obry, B.; Bandlow, J.; Birke, K.P. Quantitative validation of calendar aging models for lithium-ion batteries. J. Power Sources 2018, 400, 402–414. [Google Scholar] [CrossRef]
- Röder, P.; Stiaszny, B.; Ziegler, J.C.; Baba, N.; Lagaly, P.; Wiemhöfer, H.D. The impact of calendar aging on the thermal stability of a LiMn2O4-Li(Ni1/3Mn1/3Co1/3)O2/graphite lithium-ion cell. J. Power Sources 2014, 268, 315–325. [Google Scholar] [CrossRef]
- Wright, R.B.; Motloch, C.G.; Belt, J.R.; Christophersen, J.P.; Ho, C.D.; Richardson, R.A.; Bloom, I.; Jones, S.A.; Battaglia, V.S.; Henriksen, G.L.; et al. Calendar- and cycle-life studies of advanced technology development program generation 1 lithium-ion batteries. J. Power Sources 2002, 110, 445–470. [Google Scholar] [CrossRef]
- Thomas, E.V.; Bloom, I.; Christophersen, J.P.; Battaglia, V.S. Statistical methodology for predicting the life of lithium-ion cells via accelerated degradation testing. J. Power Sources 2008, 184, 312–317. [Google Scholar] [CrossRef]
- Liu, K.; Ashwin, T.R.; Hu, X.; Lucu, M.; Widanage, W.D. An evaluation study of different modelling techniques for calendar ageing prediction of lithium-ion batteries. Renew. Sustain. Energy Rev. 2020, 131, 110017. [Google Scholar] [CrossRef]
- Gismero, A.; Stroe, D.I.; Schaltz, E. Calendar Aging Lifetime Model for NMC-based Lithium-ion Batteries Based on EIS Measurements. In Proceedings of the 2019 Fourteenth International Conference on Ecological Vehicles and Renewable Energies, Monte-Carlo, Monaco, 8–10 May 2019. [Google Scholar] [CrossRef]
- Ning, G.; Haran, B.; Popov, B.N. Capacity fade study of lithium-ion batteries cycled at high discharge rates. J. Power Sources 2003, 117, 160–169. [Google Scholar] [CrossRef]
- Cordoba-Arenas, A.; Onori, S.; Guezennec, Y.; Rizzoni, G. Capacity and power fade cycle-life model for plug-in hybrid electric vehicle lithium-ion battery cells containing blended spinel and layered-oxide positive electrodes. J. Power Sources 2015, 278, 473–483. [Google Scholar] [CrossRef]
- Lam, L.; Bauer, P. Practical capacity fading model for Li-ion battery cells in electric vehicles. IEEE Trans. Power Electron. 2013, 28, 5910–5918. [Google Scholar] [CrossRef]
- Sun, T.; Xu, B.; Cui, Y.; Feng, X.; Han, X.; Zheng, Y. A sequential capacity estimation for the lithium-ion batteries combining incremental capacity curve and discrete Arrhenius fading model. J. Power Sources 2021, 484, 229248. [Google Scholar] [CrossRef]
- Kim, J.H.; Woo, S.C.; Park, M.S.; Kim, K.J.; Yim, T.; Kim, J.S.; Kim, Y.J. Capacity fading mechanism of LiFePO4-based lithium secondary batteries for stationary energy storage. J. Power Sources 2013, 229, 190–197. [Google Scholar] [CrossRef]
- Li, J.; Gee, A.M.; Zhang, M.; Yuan, W. Analysis of battery lifetime extension in a SMES-battery hybrid energy storage system using a novel battery lifetime model. Energy 2015, 86, 175–185. [Google Scholar] [CrossRef]
- Suri, G.; Onori, S. A control-oriented cycle-life model for hybrid electric vehicle lithium-ion batteries. Energy 2016, 96, 644–653. [Google Scholar] [CrossRef]
- Bui, T.M.N.; Sheikh, M.; Dinh, T.Q.; Gupta, A.; Widanalage, D.W.; Marco, J. A Study of Reduced Battery Degradation through State-of-Charge Pre-Conditioning for Vehicle-to-Grid Operations. IEEE Access 2021, 9, 155871–155896. [Google Scholar] [CrossRef]
- Guena, T.; Leblanc, P. How depth of discharge affects the cycle life of lithium-metal-polymer batteries. In Proceedings of the INTELEC 06-Twenty-Eighth International Telecommunications Energy Conference, Providence, RI, USA, 10–14 September 2006. [Google Scholar] [CrossRef]
- Redondo-Iglesias, E.; Venet, P.; Pelissier, S. Efficiency Degradation Model of Lithium-Ion Batteries for Electric Vehicles. IEEE Trans. Ind. Appl. 2019, 55, 1932–1940. [Google Scholar] [CrossRef]
- Madani, S.S.; Schaltz, E.; Kær, S.K. Effect of current rate and prior cycling on the coulombic efficiency of a lithium-ion battery. Batteries 2019, 5, 57. [Google Scholar] [CrossRef]
- Chiang, Y.H.; Sean, W.Y.; Ke, J.C. Online estimation of internal resistance and open-circuit voltage of lithium-ion batteries in electric vehicles. J. Power Sources 2011, 196, 3921–3932. [Google Scholar] [CrossRef]
- Lin, N.; Jia, Z.; Wang, Z.; Zhao, H.; Ai, G.; Song, X.; Bai, Y.; Battaglia, V.; Sun, C.; Qiao, J.; et al. Understanding the crack formation of graphite particles in cycled commercial lithium-ion batteries by focused ion beam—Scanning electron microscopy. J. Power Sources 2017, 365, 235–239. [Google Scholar] [CrossRef]
- Chen, X.; Shen, W.; Vo, T.T.; Cao, Z.; Kapoor, A. An overview of lithium-ion batteries for electric vehicles. In Proceedings of the 2012 10th International Power & Energy Conference (IPEC), Ho Chi Minh City, Vietnam, 12–14 December 2012; pp. 230–235. [Google Scholar] [CrossRef]
- Fanoro, M.; Božanić, M.; Sinha, S. A Review of the Impact of Battery Degradation on Energy Management Systems with a Special Emphasis on Electric Vehicles. Energies 2022, 15, 5889. [Google Scholar] [CrossRef]
- Yang, F.; Xie, Y.; Deng, Y.; Yuan, C. Impacts of battery degradation on state-level energy consumption and GHG emissions from electric vehicle operation in the United States. Procedia CIRP 2019, 80, 530–535. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Hall, J.C.; Lin, T.; Brown, G.; Biensan, P.; Bonhomme, F. Decay processes and life predictions for lithium ion satellite cells. In Proceedings of the Collection Technology Paper, 4th International Energy Conversion Engineering Conference and Exhibit (IECEC), San Diego, CA, USA, 26–29 June 2006; Volume 1, pp. 632–642. [Google Scholar] [CrossRef]
- Marongiu, A.; Roscher, M.; Sauer, D.U. Influence of the vehicle-to-grid strategy on the aging behavior of lithium battery electric vehicles. Appl. Energy 2015, 137, 899–912. [Google Scholar] [CrossRef]
- Ziv, B.; Borgel, V.; Aurbach, D.; Kim, J.-H.; Xiao, X.; Powell, B.R. Investigation of the Reasons for Capacity Fading in Li-Ion Battery Cells. J. Electrochem. Soc. 2014, 161, A1672–A1680. [Google Scholar] [CrossRef]
- Lawder, M.T.; Northrop, P.W.C.; Subramanian, V.R. Model-Based SEI Layer Growth and Capacity Fade Analysis for EV and PHEV Batteries and Drive Cycles. J. Electrochem. Soc. 2014, 161, A2099–A2108. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, S.; Li, X.; Cui, Y.; Lai, X.; Wang, X.; Ma, Y.; Zheng, Y. A Novel Capacity Estimation Approach for Lithium-Ion Batteries Combining Three-Parameter Capacity Fade Model with Constant Current Charging Curves. IEEE Trans. Energy Convers. 2021, 36, 2574–2584. [Google Scholar] [CrossRef]
- Myung, S.T.; Hitoshi, Y.; Sun, Y.K. Electrochemical behavior and passivation of current collectors in lithium-ion batteries. J. Mater. Chem. 2011, 21, 9891–9911. [Google Scholar] [CrossRef]
- Fares, R.L.; Webber, M.E. What are the tradeoffs between battery energy storage cycle life and calendar life in the energy arbitrage application? J. Energy Storage 2018, 16, 37–45. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Noelle, D.J.; Wang, M.; Le, A.V.; Shi, Y.; Qiao, Y. Internal resistance and polarization dynamics of lithium-ion batteries upon internal shorting. Appl. Energy 2018, 212, 796–808. [Google Scholar] [CrossRef]
- Canals Casals, L.; Schiffer Gonzalez, A.M.; Garcia, B.; Llorca, J. PHEV Battery Aging Study Using Voltage Recovery and Internal Resistance from Onboard Data. IEEE Trans. Veh. Technol. 2016, 65, 4209–4216. [Google Scholar] [CrossRef]
- Tröltzsch, U.; Kanoun, O.; Tränkler, H.R. Characterizing aging effects of lithium ion batteries by impedance spectroscopy. Electrochim. Acta 2006, 51, 1664–1672. [Google Scholar] [CrossRef]
- Yang, F.; Wang, D.; Zhao, Y.; Tsui, K.L.; Bae, S.J. A study of the relationship between coulombic efficiency and capacity degradation of commercial lithium-ion batteries. Energy 2018, 145, 486–495. [Google Scholar] [CrossRef]
- Patil, M.A.; Tagade, P.; Hariharan, K.S.; Kolake, S.M.; Song, T.; Yeo, T.; Doo, S. A novel multistage Support Vector Machine based approach for Li ion battery remaining useful life estimation. Appl. Energy 2015, 159, 285–297. [Google Scholar] [CrossRef]
- Kassem, M.; Delacourt, C. Postmortem analysis of calendar-aged graphite/LiFePO4 cells. J. Power Sources 2013, 235, 159–171. [Google Scholar] [CrossRef]
- Darma, M.S.D.; Lang, M.; Kleiner, K.; Mereacre, L.; Liebau, V.; Fauth, F.; Bergfeldt, T.; Ehrenberg, H. The influence of cycling temperature and cycling rate on the phase specific degradation of a positive electrode in lithium ion batteries: A post mortem analysis. J. Power Sources 2016, 327, 714–725. [Google Scholar] [CrossRef]
- Palacín, M.R. Understanding ageing in Li-ion batteries: A chemical issue. Chem. Soc. Rev. 2018, 47, 4924–4933. [Google Scholar] [CrossRef] [PubMed]
- Uddin, K.; Perera, S.; Widanage, W.D.; Somerville, L.; Marco, J. Characterising lithium-ion battery degradation through the identification and tracking of electrochemical battery model parameters. Batteries 2016, 2, 13. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]
- Zhang, X.; Winget, B.; Doeff, M.; Evans, J.W.; Devine, T.M. Corrosion of Aluminum Current Collectors in Lithium-Ion Batteries with Electrolytes Containing LiPF6. J. Electrochem. Soc. 2005, 152, B448. [Google Scholar] [CrossRef]
- Lewerenz, M.; Fuchs, G.; Becker, L.; Sauer, D.U. Irreversible calendar aging and quantification of the reversible capacity loss caused by anode overhang. J. Energy Storage 2018, 18, 149–159. [Google Scholar] [CrossRef]
- Guo, W.; He, M. An optimal relevance vector machine with a modified degradation model for remaining useful lifetime prediction of lithium-ion batteries. Appl. Soft Comput. 2022, 124, 108967. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; Zhang, B.; Liu, M.; Wang, J.; Liu, C.; Zhang, Y. Method of Predicting SOH and RUL of Lithium-Ion Battery Based on the Combination of LSTM and GPR. Sustainability 2022, 14, 11865. [Google Scholar] [CrossRef]
- Tagade, P.; Hariharan, K.S.; Ramachandran, S.; Khandelwal, A.; Naha, A.; Kolake, S.M.; Han, S.H. Deep Gaussian process regression for lithium-ion battery health prognosis and degradation mode diagnosis. J. Power Sources 2020, 445, 227281. [Google Scholar] [CrossRef]
- Rauf, H.; Khalid, M.; Arshad, N. Machine learning in state of health and remaining useful life estimation: Theoretical and technological development in battery degradation modelling. Renew. Sustain. Energy Rev. 2022, 156, 111903. [Google Scholar] [CrossRef]
- May, G.; El-Shahat, A. Battery-degradation model based on the ANN regression function for EV applications. In Proceedings of the 2017 IEEE Global Humanitarian Technology Conference (GHTC), San Jose, CA, USA, 19–22 October 2017; pp. 1–3. [Google Scholar] [CrossRef]
- Pang, B.; Chen, L.; Dong, Z. Data-Driven Degradation Modeling and SOH Prediction of Li-Ion Batteries. Batteries 2022, 15, 5580. [Google Scholar] [CrossRef]
- MAlmuhaylan, M.R.; Ghumman, A.R.; Al-Salamah, I.S.; Ahmad, A.; Ghazaw, Y.M.; Haider, H.; Shafiquzzaman, M. Evaluating the impacts of pumping on aquifer depletion in arid regions using MODFLOW, ANFIS and ANN. Water 2020, 12, 2297. [Google Scholar] [CrossRef]
- Kang, L.W.; Zhao, X.; Ma, J. A new neural network model for the state-of-charge estimation in the battery degradation process. Appl. Energy 2014, 121, 20–27. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X. Microgrid Optimal Energy Scheduling Considering Neural Network Based Battery Degradation. IEEE Trans. Power Syst. 2024, 39, 1594–1606. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Garg, A.; Guo, L. Enhancing real-time degradation prediction of lithium-ion battery: A digital twin framework with CNN-LSTM-attention model. Energy 2024, 124, 129681. [Google Scholar] [CrossRef]
- Jafari, S.; Byun, Y.C. A CNN-GRU Approach to the Accurate Prediction of Batteries’ Remaining Useful Life from Charging Profiles. Computers 2023, 12, 219. [Google Scholar] [CrossRef]
- Cui, S.; Joe, I. A Dynamic Spatial-Temporal Attention-Based GRU Model with Healthy Features for State-of-Health Estimation of Lithium-Ion Batteries. IEEE Access 2021, 9, 27374–27388. [Google Scholar] [CrossRef]
- Nan, J.; Deng, B.; Cao, W.; Tan, Z. Prediction for the Remaining Useful Life of Lithium–Ion Battery Based on RVM-GM with Dynamic Size of Moving Window. World Electr. Veh. J. 2022, 13, 25. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, N.; Ji, Y.; Niu, M.; Wang, Y. Lithium-ion batteries remaining useful life prediction based on BLS-RVM. Energy 2021, 234, 121269. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Wang, Z.; Dong, P. Remaining useful life prediction for lithium-ion batteries based on a hybrid model combining the long short-term memory and Elman neural networks. J. Energy Storage 2019, 21, 510–518. [Google Scholar] [CrossRef]
- Cong, X.; Zhang, C.; Jiang, J.; Zhang, W.; Jiang, Y. A Hybrid Method for the Prediction of the Remaining Useful Life of Lithium-Ion Batteries with Accelerated Capacity Degradation. IEEE Trans. Veh. Technol. 2020, 69, 12775–12785. [Google Scholar] [CrossRef]
- Nash, W.; Drummond, T.; Birbilis, N. A review of deep learning in the study of materials degradation. npj Mater. Degrad. 2018, 2, 37. [Google Scholar] [CrossRef]
- Park, K.; Choi, Y.; Choi, W.J.; Ryu, H.Y.; Kim, H. LSTM-Based Battery Remaining Useful Life Prediction with Multi-Channel Charging Profiles. IEEE Access 2020, 8, 20786–20798. [Google Scholar] [CrossRef]
- Chinomona, B.; Chung, C.; Chang, L.K.; Su, W.C.; Tsai, M.C. Long short-term memory approach to estimate battery remaining useful life using partial data. IEEE Access 2020, 8, 165419–165431. [Google Scholar] [CrossRef]
- Li, W.; Sengupta, N.; Dechent, P.; Howey, D.; Annaswamy, A.; Sauer, D.U. One-shot battery degradation trajectory prediction with deep learning. J. Power Sources 2021, 506, 230024. [Google Scholar] [CrossRef]
- Nutakki, T.U.; Alghassab, M.A.; Dutta, A.K.; Abdullaeva, B.S.; Alkhalaf, S.; Alharbi, F.S.; Ghandour, R.; Al Barakeh, Z.; Knani, S. Thermo-environmental multi- investigation and ANN-based optimization of a novel heat integration criteria system integrated with a marine engine generating liquefied hydrogen. Case Stud. Therm. Eng. 2024, 56, 104240. [Google Scholar] [CrossRef]
- Lu, J.; Xiong, R.; Tian, J.; Wang, C.; Hsu, C.W.; Tsou, N.T.; Sun, F.; Li, J. Battery degradation prediction against uncertain future conditions with recurrent neural network enabled deep learning. Energy Storage Mater. 2022, 50, 139–151. [Google Scholar] [CrossRef]
- Ren, L.; Dong, J.; Wang, X.; Meng, Z.; Zhao, L.; Deen, M.J. A Data-Driven Auto-CNN-LSTM Prediction Model for Lithium-Ion Battery Remaining Useful Life. IEEE Trans. Ind. Inform. 2021, 17, 3478–3487. [Google Scholar] [CrossRef]
- Li, Z.; Liu, F.; Yang, W.; Peng, S.; Zhou, J. A Survey of Convolutional Neural Networks: Analysis, Applications, and Prospects. IEEE Trans. Neural Netw. Learn. Syst. 2022, 33, 6999–7019. [Google Scholar] [CrossRef]
- Costa, N.; Sánchez, L.; Anseán, D.; Dubarry, M. Li-ion battery degradation modes diagnosis via Convolutional Neural Networks. J. Energy Storage 2022, 55, 105558. [Google Scholar] [CrossRef]
- Haris, M.; Hasan, M.N.; Qin, S. Degradation Curve Prediction of Lithium-Ion Batteries Based on Knee Point Detection Algorithm and Convolutional Neural Network. IEEE Trans. Instrum. Meas. 2022, 71, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).