Abstract
(1) Background: The purpose of this study was to compare the free volatile compounds of 18 Veronica species (Plantaginaceae), as previously analyzed by gas chromatography coupled with mass spectrometry, with their DNA sequences for internal transcribed spacers ITS2 and ITS1-5.8S-ITS2 of the nuclear ribosomal DNA. (2) Methods: Two sets of DNA sequence data were generated and used for phylogenetic analysis: ITS2 sequences (~360 bp) obtained by next-generation sequencing and ITS1-5.8S-ITS2 sequences (~580 bp) sequenced by the Sanger sequencing method. Clustering from previously analyzed free volatile compounds was performed by Ward’s method. (3) Results: Both sets of DNA sequence data showed that the 18 analyzed Veronica species were grouped into eight main groups corresponding to the following subgenera: Pentasepalae, Pocilla, Chamaedrys, Veronica, Beccabunga, Cochlidiosperma, Stenocarpon and Pseudolysimachium. Results of the clustering analysis of free volatile compounds showed better clustering when using microwave-extracted volatiles. Three clusters were detected with the following main compounds: hexahydrofarnesyl acetone, hexadecanoic acid, phytol, caryophyllene oxide and (E)-caryophyllene. (4) Conclusion: The phylogenetic analysis of ITS2 data obtained by NGS technology and ITS1-5.8S-ITS2 data obtained by Sanger sequencing resulted in the grouping of 18 Veronica species into eight subgenera, which is in accordance with the existing classification. Statistical testing showed that there was no correlation between such clustering of Veronica species and clustering that was based on free volatile compounds. The achieved results can be viewed in the light of parallel evolution among some of the species of the Veronica genus as well as the fact that volatile compound composition can be influenced by environmental factors or epigenetic modifications.
Keywords:
Veronica; volatile compounds; chemophenetic markers; molecular phylogeny; ITS1; ITS2; NGS; Sanger 1. Introduction
The genus Veronica of the family Plantaginaceae consists of 450 species, growing mainly in the temperate regions of the Northern Hemisphere, while a smaller number of species grow in the mountains of the tropical and temperate regions of the Southern Hemisphere regions [1,2]. Many species testify to the great ecological adaptability of the genus Veronica, in which species grow in wet and dry habitats, by the sea and in the mountains [3].
One of the first phytochemical studies of the genus Veronica was related to iridoid glycosides, which proved to be taxonomically important [4,5]. This genus was once placed in the family Scrophulariaceae based mainly on morphological characters, but based on the results of DNA sequence studies, it was transferred and is currently in the family Plantaginaceae [6]. Albach et al., Jensen et al. and Taskova et al. studied the iridoid glycosides of the genus Veronica and concluded that the distribution of these compounds in the different species of the genus is consistent with the molecular phylogeny of the genus, thus showing that the genus chemistry can serve as a good indicator of links between species and within the genus [7,8,9,10]. Advances in analytical methods, especially chromatography, followed by electronic detection methods, accelerated chemical studies to the point of metabolic profiling of plant species. In general, research on chemical compounds produced by plants is extremely important because these compounds ultimately affect not only the plant in which they are found but indirectly other plants in the environment as well as the environment as a whole [11]. Iridoid glycosides have been used as chemophenetic markers at different taxonomic levels [12,13,14,15,16]. As a part of research on chemophenetic markers for the genus Veronica, Taskova et al. isolated 16 iridoid glycosides and established a link between chemical composition and basic chromosome number [16]. Mehrvarz et al. studied the chemical composition of selected species of the genus Veronica, iridoids and flavonoids and investigated their significance for the systematic and phylogenetic assignment of these species. The analysis of four species of the genus Veronica (V. persica, V. polita, V. francispetae, V. siaretensis) showed a qualitatively constant composition of iridoids of all species, regardless of environmental conditions [17]. Crişan et al. studied 12 species of the genus Veronica with LC-MS, analyzing the content of aucubin and catalpol belonging to iridoid glycosides, and also confirmed their importance in chemophenetics [15]. Albach et al. studied the iridoid glycosides aucubin and catalpol in the genus Veronica species and the species Paederota lutea and, based on the composition of these compounds, concluded that these two genera Veronica and Paederota are related [18]. Molecular analyses showed that Paederota is a sister group next to Veronica, and the composition of iridoid glycosides supported this as the same compounds were detected in Paederota species [18]. This proves that iridoids are a very good marker for the chemophenetics of plant species, as confirmed by Saracoglu et al. in their studies [19].
The free volatile compounds, as specialized metabolites of the genus Veronica, have been much less studied than other metabolites such as glycosides, phenols and flavonoids. Only a few research studies could be found before our research [20,21,22,23]. Our recent studies on the composition of volatile compounds in 21 Croatian Veronica species indicate the diversity and richness of isolated metabolites, and for most of the Veronica species studied, these data were presented for the first time [24].
The molecular phylogeny and taxonomy of the genus Veronica are well studied [2,25,26,27,28,29]. Due to the parallel morphological evolution observed in this genus (a taxonomically distinct species develops similar morphological characters) [2], it is not recommended to rely solely on plant morphology for the identification and classification of species in the genus Veronica. Therefore, DNA analyses are recommended for reliable and accurate species identification. DNA barcoding regions that have been shown to be useful for the phylogenetics of Veronica are ITS1-5.8SrDNA-ITS2 (nuclear genes which are inherited from both parents) and trnL-trnF regions (genes from the chloroplast which are usually inherited from the female parent) [25].
Although the ITS1-5.8SrDNA-ITS2 region is probably the most popular molecular marker in plants for the DNA identification of plant species (DNA barcoding) this sequence can be hypervariable and therefore difficult to analyze when sequenced in a conventional way, using the Sanger method. This is particularly important for plants of hybrid and/or polyploid origin. Since the occurrence of polyploidy has been observed in several species of the genus Veronica [25], we hoped that with the new method of next-generation sequencing (NGS, next-generation sequencing), much more accurate identification of species would be possible. With this method, it is possible to sequence many more gene variants (markers) and obtain data of much better quality and higher resolution, revealing genetic diversity such as single nucleotide polymorphisms (SNPs), insertions/deletions (indels), homopolymeric regions and microsatellites, which in aggregate will allow accurate species identification. The NGS method is already used in many cases: the detection of ITS2 allelic variation in mosquitoes [30], diversity of rDNA unit in Nicotiana [31], authenticity of plant foods [32] and identification of medicinal plants [33].
This study provides an overview of the phytochemical composition of free volatile compounds of selected species of the genus Veronica from Croatia, which have been published previously; however, in this work, cluster classification based on free volatile compounds (FVCs) is performed. Moreover, this clustering is compared with molecular classification based on the ITS (internal transcribed spacer) regions of ribosomal genes. One of the main objectives of this study is to investigate whether free volatiles can also be a good chemophenetic marker for classification between species and between genera. Several recent studies indicated the diversity and richness of isolated metabolites from the genus Veronica, and some of them proved to be reliable taxonomic markers. The combination of such studies with molecular phylogenetic analyses provides a good basis for exploring the potential relationship between the distribution of free volatile compounds and phylogeny.
2. Materials and Methods
2.1. ITS2 and ITS1-5.8S-ITS2 Sequencing
2.1.1. DNA Extractions
Genomic DNAs of 18 Veronica species listed in Table 1 (locations shown in Figure 1) were extracted from young silica-dried leaves using NucleoSpin Plant II, Mini kit for DNA from plants (Macherey-Nagel™; Cat. No. 740770.5, 52355 Düren, Germany) according to the manufacturer’s instructions. For each species studied, DNA was separately isolated from three individuals. Voucher specimens were deposited at the Laboratory of Botany herbarium (HPMF-HR), Faculty of Science, University of Split, Croatia.

Table 1.
List of 18 Veronica species with location collection information used for this research (the same plant material was used for the previously published research [24]).

Figure 1.
Map of locations of material collection.
2.1.2. PCR Amplification and Sequencing by Sanger
The ITS1-5.8S-ITS2 region of 35S rDNA was amplified by PCR using the primers ITS-1 (5′GTTTCCGTAGGTGAACCTGC3′) described by Bezić et al. [34] and ITS-4 (5′TCCTCCGCTTATTGATATGC3′) described by White et al. [35]. The amplified products were visualized and confirmed by 1% agarose gel electrophoresis, extracted from the gel and isolated using a Plasmid Mini Kit (Qiagen, Hilden, Germany). Purified DNA was sent to Macrogen (Amsterdam, The Netherlands) for sequencing. The ITS1-5.8S-ITS2 sequences were deposited in GenBank under the following accession numbers: OQ564378-OQ564387 (Table S1).
2.1.3. Sequence Analysis
The DNA sequences were assembled and aligned in ClustalW implemented in MEGA11 [36], and the alignment was manually refined. Alignments in fasta format are available as Datas S1 and S2. To infer phylogenetic relationships from the newly obtained Veronica ITS sequences and other closely related Veronica species, the sequences were subjected to similarity search against the non-redundant nucleotide sequence database using the NCBI (National Centre for Biotechnology Information) BLASTN network service. Four species from the Plantaginaceae family were taken into analysis as outgroup species, and details of their GenBank accession numbers and publications are shown in Table S2 [8,10,25,28,37,38,39,40]. The final dataset of ITS2 sequences contained 57 sequences, and there were a total of 327 positions. The evolutionary history was inferred by using the Maximum Parsimony method implemented in MEGA 11 under default parameters. Tree #1 out of 5 most parsimonious trees (length = 323) is shown in Figure 2. All positions with less than 95% site coverage were eliminated, i.e., fewer than 5% alignment gaps, missing data and ambiguous bases were allowed at any position (partial deletion option).

Figure 2.
Evolutionary relationships of the studied Veronica species inferred from ITS2 sequence analysis by the Maximum Parsimony method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches. Subgeneric affiliation is shown.
The final dataset of ITS1-5.8S-ITS2 sequences contained 28 sequences, and there were a total of 528 positions in the final dataset. The evolutionary history was inferred by using the maximum likelihood method and general time-reversible model. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.4567)). All positions with less than 95% site coverage were eliminated, i.e., fewer than 5% alignment gaps, missing data and ambiguous bases were allowed at any position (partial deletion option). The bootstrap consensus tree was inferred from 500 replicates. Evolutionary analyses were conducted in MEGA11 [36].
2.1.4. NGS (Illumina Sequencing) of ITS2 Region and Data Analyses
Preparation of Illumina libraries, PCR and sequencing was outsourced to Novogene (Cambridge, UK). Target ITS2 region was amplified from genomic DNA extractions using ITS3 (5′ GCATCGATGAAGAACGCAGC 3′) and ITS4 (5′ TCCTCCGCTTATTGATATGC 3′) universal primers described by White et al. [35]. The same amount of PCR products from each sample was pooled, end-repaired, A-tailed and ligated with Illumina adapters. NovaSeq PE250 Sequencing System was used to sequence the libraries. Paired-end reads were assigned to samples based on their unique barcode, after which barcode and primer sequences were removed. Quality filtering of the raw reads was performed with Novogene proprietary pipeline and included discarding reads shorter than 60 bp, reads with >10% of uncertain nucleotides (N) and reads with >50% of low-quality nucleotides (Qscore ≤ 5). The quality of paired-end reads at every step was inspected with FastQC v0.11.8 [41]. Clean reads were merged using FLASH v1.2.7 [42], and chimeras were removed by comparing them to the Gold database using UCHIME algorithm v.7.0.1001 [43]. The resulting effective clean tags were further screened for contamination by comparison with the UNITE database [44] of the eukaryotic nuclear ribosomal ITS regions using SortMeRNA v2.1 [45]. Tags that did not match any of the Veronica plant species in the database were discarded from further analyses. Contigs (ribotypes) of the ITS2 region for each sample were assembled de novo as fragments using MIRA v5rc1 [46] in draft mode. Assembly in fragment mode is recommended for single gene projects or small plasmids. Since the tag number was high (mean 90,774, min. 32,731–max. 116,647), lossless digital normalization was enabled, and other assembly parameters were kept as default. Contigs that contained more than 20% of reads and displayed highest coverage were selected as representative ribotypes, and the longest of them were selected for phylogenetic analyses (Table S4). Purification of effective tags, quality control and assembly of contigs were performed using the resources of the Isabella computing cluster hosted by the University Computing Centre, University of Zagreb (SRCE), Croatia.
2.2. Review Protocol
In order to obtain data for the cluster analyses of volatile compounds, the data for the free volatile compounds (FVCs) for 18 selected Veronica species used for this paper were obtained from one previously published paper by our team [24]. The volatile compounds were isolated by two methods: hydrodistillation in a Clevenger-type apparatus (Šurlan, Medulin, Croatia) and microwave-assisted extraction (Milestone ‘ETHOS X’ microwave laboratory oven, 1900 W maximum, Sorisole, Italy) for 2.5 h using 30–50 g of dried plant material. The distillate consists of two layers: a lipophilic layer collected in a side tube using a pentane/diethyl ether trap and a water layer (hydrosol). For this study, we only used volatile compounds from lipophilic layer, because it is standard procedure in most papers when obtaining data for cluster analyses of volatile compounds [47,48,49,50,51]. For this paper, we presented percentages of FVCs from both extractions in Table 2. Percentages for FVCs were obtained with gas chromatography–mass spectrometry. Method for FVC analyses is presented in detail in paper by Dunkić et al. [24].

Table 2.
Free volatile compounds most abundant in all investigated Veronica species—Clevenger hydrodistillation (HD) and microwave extraction (MW) (relative percentage, %).
2.3. Statistical Analyses
Cluster Analyses Based on Free Volatile Compounds
Cluster analysis (CA) was based on chemical constituents obtained by classical extraction—hydrodistillation with Clevenger apparatus and microwave-assisted water extractions in an amount of at least 2%. Dendrograms of Euclidean distances were prepared according to Ward’s method to verify the affinities determined in the molecular analysis. CA was performed using the Statistica 7 software (StatSoft Inc., Tulsa, OK, USA).
The relationship between matrices of Euclidean distances describing chemical components of species and genetic distances obtained by Sanger and NGS methods was assessed using Mantel test as implemented in vegan package for R v4.2.2 [52]. Spearman rank correlation was chosen as a measure of association, and significance was calculated through 9999 permutations. Genetic distances for the Mantel test were calculated in MEGA11 [34] using Kimura 2-parameter model with 1000 bootstrap iterations [53].
3. Results
3.1. ITS2 and ITS1_5.8S-ITS2 Data Analyses
With the aim of evaluating the usefulness of next-generation sequencing (NGS) for the authentication of species from the genus Veronica, we performed NGS sequencing of the ITS2 region of 18 Veronica species (data available as BioProject PRJNA944611). Each species was represented by three plants. On average, 99086 paired-end reads were produced per sample (Table S3). After quality control, filtering and chimera removal, an average of 99.4% of reads remained and were used to generate effective clean tags. The proportion of contaminant non-Veronica species tags was generally low (4.8% on average), except for sample E (V. chamaedrys) where 30.9% of tags were removed (Table S3).
Out of 18 Veronica species, the identity of 11 species was successfully confirmed by blasting randomly chosen 5000 sequences for the ITS2 region, as these data were consistent with the results of anatomical-morphological identification. For the remaining seven species, identification based on the ITS2 region was doubtful and did not agree with their morphological identification. For these samples, an additional analysis of the complete ITS1-5.8S-ITS2 region was performed by the PCR amplification of this region and its classical Sanger sequencing (Table S1).
The number of reads/tags after the clean and merge procedures is shown in Table S2. Clean effective tags were assembled into anywhere between 21 to 237 contigs per sample, with one largest contig/ribotype recreated with the highest coverage and a length of approximately 360 nucleotides for most samples. Information on the assembled contigs supported by at least 20% of the reads for each sample is listed in Table S3, and their nucleotide sequences are given in fasta format in Data S1. These contigs/ribotypes that had the highest coverage were selected as representative ITS2 ribotypes, deposited in GenBank under unique GenBank accession numbers (Table S1), and the longest of those were chosen for phylogenetic analyses (Figure 2).
Both sets of DNA sequence data (ITS2 and ITS1-5.8S-ITS2) showed that the 18 analyzed Veronica species were divided into eight major groups corresponding to the following subgenera: Pentasepalae, Pocilla, Chamaedrys, Veronica, Beccabunga, Cochlidiosperma, Stenocarpon and Pseudolysimachium (Figure 2 and Figure 3). The most numerous subgenus was Beccabunga, which contained six species: V. beccabunga, V. catenata, V. acinifolia, V. serpyllifolia, V. anagaloides and V. anagalis-aquatica. This subgenus is much better supported in the phylogenetic tree with ITS1-5.8S-ITS2 sequences (bootstrap support = 99) (Figure 3) than in the tree containing only ITS 2 sequences (bootstrap support = 92) (Figure 2). Monophyletic group Cochlidiosperma, with two species V. cymbalaria and V. hederifolia, was the least supported group in both the ITS2 and the ITS1-5.8S-ITS2 phylogenetic trees (Figure 2 and Figure 3). The subgenera Stenocarpon and Pseudolysimachium are represented by only a single species V. saturejoides and V. longifolia, respectively.

Figure 3.
Evolutionary relationships of the studied Veronica species inferred from ITS1-5.8S-ITS2 sequence analysis by the maximum likelihood method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches. Subgeneric affiliation is shown.
A comparison of the two phylogenetic analyses and their results—the two phylogenetic trees—suggests that the analysis of the longer ITS1-5.8S-ITS2 sequences yielded better subgenus classification than the analysis of the ITS2 sequences. This is likely due to the fact that longer Sanger-generated sequences (~580 bp) produce more variable and informative sites (positions) than shorter NGS sequences (~360 bp). Thus, our results indicate that ITS2 data are of rather limited value for Veronica species identification and phylogenetic analysis.
3.2. Statistical Analyses
3.2.1. Cluster Analyses Based on Free Volatile Compounds
The complete table of volatile compounds of these Veronica species was previously published in a paper by Dunkić et al. [24]. Free volatile compounds were extracted with two methods of hydrodistillation, with the Clevenger apparatus and microwave hydrodistillation. Table 2 lists the compounds with the highest relative percentages for all species selected for genetic research. Cluster analyses were performed for this study, and the results are shown in Figure 4 and Figure 5. A total of 40 volatiles were used for this analysis. Figure 4 shows the clustering for the volatiles extracted with the Clevenger apparatus. Two main groups can be observed, which we described with five major compounds: phytol, hexahydrofarnesyl acetone, caryophyllene oxide, (E)-caryophyllene and hexadecanoic acid. The first group includes V. persica, V. acinifolia, V. chamaedrys, V. arvensis, V. cymbalaria, V. anagalloides, V. longifolia, V. austriaca ssp. jacquinii, V. polita, V. montana and V. anagallis-aquatica. This group cannot be described with only one chemotype, but we can observe three subgroups. The subgroup V. persica/V. acinifolia/V. chamaedrys can be described with compounds in the following order: phytol > hexahydrofarnesyl acetone > caryophyllene oxide > (E)-caryophyllene > hexadecanoic acid. The subgroup V. arvesis/V. cymbalaria/V. anagalloides/V. longifolia/V. austriaca ssp. jacquinii is defined with compounds in the following order: hexahydrofarnesyl acetone ≈ hexadecanoic acid ≈ caryophyllene oxide > (E)-caryophyllene ≈ phytol. The third subgroup V. polita/V. montana/V. anagallis-aquatica can be described with compounds in the following order: phytol ≈ hexahydrofarnesyl acetone > hexadecanoic acid ≈ caryophyllene oxide > (E)-caryophyllene. The second cluster consists of V. serpyllifolia, V. hederifolia, V. dalmatica, V. saturejoides, V. catenata, V. officinalis and V. beccabunga. This cluster is specified by the main compounds in the following order: phytol ≈ caryophyllene oxide ≈ hexahydrofarnesyl acetone > hexadecanoic acid > (E)-caryophyllene.

Figure 4.
Dendrograms of Euclidean distances using Ward’s method based on chemical constituents obtained by classical extraction—hydrodistillation with Clevenger apparatus in an amount equal to or greater than 2%. 1-phytol; 2-hexahydrofarnesyl acetone; 3- phytol/hexahydrofarnesyl acetone; 4-phytol/caryophyllene oxide/hexahydrofarnesyl acetone.
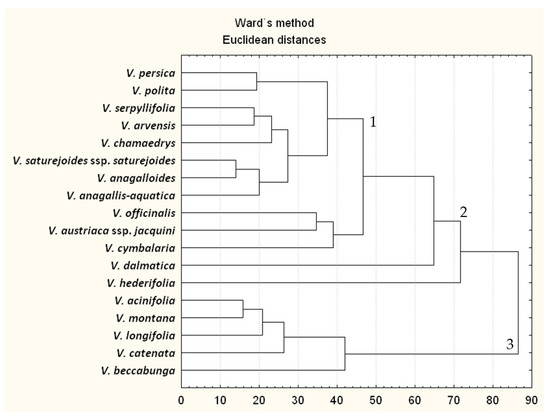
Figure 5.
Dendrograms of Euclidean distances using Ward’s method based on chemical constituents obtained by microwave-assisted water extractions in an amount equal to or greater than 2%. 1-hexahydrofarnesyl acetone; 2-phytol/caryophyllene oxide/hexahydrofarnesyl aceton; 3-phytol.
In Figure 5, which shows the clusters of volatiles extracted with a microwave apparatus, three clusters can be observed. The first cluster includes V. persica, V. polita, V. serpyllifolia, V. arvensis, V. chamaedrys, V. saturejoides, V. anagalloides and V. anagallis-aquatica. This cluster is specified by the major compounds in the following order: hexahydrofarnesyl acetone > phytol > hexadecanoic acid ≈ caryophyllene oxide > (E)-caryophyllene. The second cluster consists of V. officinalis, V. austriaca ssp. jacquinii, V. cymbalaria, V. dalmatica and V. hederifolia. This cluster is specified by major compounds in the following order: phytol ≈ caryophyllene oxide ≈ hexahydrofarnesyl acetone > hexadecanoic acid > (E)-caryophyllene. It can be seen that this type of percentage ratio of volatile compounds is observed in the second cluster for Clevenger isolation (species compared in both clusters are V. officinalis, V. dalmatica and V. hederifolia). The third cluster includes V. acinifolia, V. montana, V. longifolia, V. catenata and V. beccabunga. For all these species, the prevailing compound is phytol, and other compounds are evenly distributed in relative percentage.
3.2.2. Mantel Test
In order to compare the grouping of the 18 studied Veronica species based on the ITS sequences with the grouping based on volatile components, a Mantel test was performed to compare the Euclidean distances of volatile components and Kimura genetic distances from DNA sequence data (Data S3). However, the result showed that there was no correlation between these two sets of data, and it was not statistically significant.
4. Discussion
Although it was possible to identify most of the analyzed Veronica species studied from their ITS2 sequence data generated by NGS sequencing, more accurate identification was achieved by using the longer ITS1-5.8S-ITS2 sequences obtained by classical Sanger sequencing. In addition, better results of the phylogenetic analysis and clustering of Veronica species to eight subgenera were obtained based on ITS1-5.8S-ITS2 sequences than with data based on ITS2 sequences. This is likely due to the fact that longer sequences generated by the Sanger sequencing method (~580 bp) produced more variable and informative positions than shorter NGS sequences (~360 bp). However, the abundance of ITS2 sequences obtained with NGS technology, along with ITS1 sequences also sequenced with NGS for 18 Veronica species, which are not shown in this paper because they are less informative than the ITS2 sequences, will be used for the detailed analysis of the diversity of contigs/ribotypes within each species and their molecular evolution and genome structure, and such analyses are underway.
The results of the two phylogenetic analyses (based on ITS2 and ITS1-5.8S-ITS2) are largely consistent with previous publications. According to our and previously published data on molecular-phylogenetic analyses of the Veronica species [1,2,6,8,10,28,54,55], the species from Croatia selected for this research belong to eight subgenera: Pseudolysimachium (V. longifolia), Beccabunga (V. acinifolia, V. anagallis-aquatica, V. beccabunga, V. catenata, V. serpyllifolia, V. anagalloides), Veronica (V. montana, V. officinalis), Chamaedrys (V. arvensis, V. chamaedrys), Pentasepalae (V. austriaca, V. dalmatica), Stenocarpon (V. saturejoides), Pocilla (V. persica, V. polita) and Cochlidiosperma (V. cymbalaria, V. hederifolia).
Comparing the two clusters created based on the free volatile compounds (Figure 4 and Figure 5) and based on the molecular data for ITS regions (Figure 2), it can be said that clustering based on the volatile compounds from microwave extraction resulted in clusters that better fit the molecular clusters. The first cluster (Figure 5) consists of species belonging to Chamaedrys, Pocilla, Stenocarpon and Beccabunga subgenera. Chamaedrys and Pocilla are closely related in the molecular ITS clusters. The second cluster includes species from Cochlidiosperma, Veronica and Pentasepalae subgenera. The third cluster consists of species from Veronica and Beccabunga subgenera. In Figure 2, these two subgenera, Veronica and Beccabunga, are closely related. Compounds in these species that were present in all species regardless of the habitat and conditions under which they grew include hexahydrofarnesyl acetone, hexadecanoic acid, caryophyllene oxide and (E)-caryophyllene. Other compounds also present in almost all species samples are phytol, germacrene D and pentacosane. The different relative percentages and their mutual ratio are probably the result of the ecological conditions in which they live. Since no correlation was found between the ITS cluster and the volatile cluster, it can be concluded that these compounds are not good interspecies (belonging to the same genus) chemophenetic markers, but some of them can be defined as chemophenetic markers for the whole genus Veronica. To back up this result, we reviewed volatile compounds studied in other genera belonging to the family Plantaginaceae and found that compositions of volatiles differed in major compounds but had some of the same compounds as Veronica species. Hammami et al. studied essential oil of Plantago afra and found that the major constituents were thymol (14.3%), 3-[4-(t-Butyl) phenyl] furan-2,5-dione (12.7%), hexadecanoic acid (8.9%) and eudesmane [56]. Al-Mazroa et al. studied essential oils of Plantago amplexicaulis and Plantago boissieri, and the results showed the main composition for P. amplexicaulis was hexadecanoic acid and 3 -methyl undecane. P. boissieri major compounds were found to be bicyclo-2,2, 1-heptane,2-(2-propenyl and l-dodecane-3-ol [57]. Essential oil major constituents of Conobea scoparioides were found to be thymol methyl ether (62%), thymol (16%) and α-phellandrene (14%) in a study by de Lima et al. [58]. In another study by Brandao et al., Dizygostemon riparius essential oil major constituents were found to be endo-fenchyl acetate and endo-fenchol, followed by (E)-caryophyllene and caryophyllene oxide in smaller relative percentages [59]. Bajer et al. studied the essential oil composition of Plantago lanceolata leaves and found the following major compounds: hexadecanoic acid, linalool and pentyl vinyl ketone. They also identified hexahydrofarnesyl acetone but in small relative percentages (1.81–2.99%) [60]. In another study on the essential oils of Plantago lanceolata and Plantago major, the major compounds identified were different from those in Bajer et al.’s research: metaraminol, bifemelane, metossamina and pterin-6-carboxylic acid in P. lanceolata and 2-dodecen-1-yl (-) succinic anhydride, benzenemethanol, α-(1-aminoethyl)-2,5- dimethoxy, dl-phenylephrine and nortriptyline in P. major [61]. Fons et al. also studied Plantago lanceolata essential oil and found the major compounds of leaf essential oil to be oct-1-en-3-ol and (E)-4(3-oxo-2.6.6-trimethylcyclohex-2-en-1-yl)-3-buten-2-ol [62]. They found hexahydrofarnesyl acetone as a major compound but only in the fruits of this plant [62]. One more study on the Plantaginaceae family reported hexahydrofarnesyl acetone as a detected compound in the FVC composition. Roudbaraki et al. studied Digitalis nervosa leaf volatile compounds and found that the major compounds were trans-pinocamphone and hexadecanoic acid, followed by caryophyllene oxide and phytol [63]. Comparing all these results to the identified volatiles of Veronica species and their relative percentage, it can be concluded that the identified volatile compounds of the genus Veronica were not found outside the genus in this combination, at least not until this moment. Some major compounds mentioned earlier in these identified percentage ratios can be defined as chemophenetic markers for the genus Veronica. Hexahydrofarnesyl acetone is particularly noteworthy, which appears in high percentages in all Veronica species studied and appears in other Plantaginaceae genera in much smaller relative percentages than in Veronica species. Other reported identifications of this compound are studied in seeds or fruits, so they are not comparable to our study [62,64]. A similar situation is found with caryophyllene oxide, (E)-caryophyllene, phytol, germacrene D and pentacosane. After reviewing all these Plantaginaceae volatiles, it can be concluded that hexadecanoic acid is not a good chemophenetic marker as it appears in many other species outside the Veronica genus. Our study is not the first that found compounds that are chemophenetic markers for the genus but not for the subgenus level. Mehrvarz et al. found similar results in their study, in which they detected constant and characteristic iridoid and flavonoid profiles in selected Veronica species, which is useful in analyzing taxonomic problems at a specific level (intergenus level) [17] but not for distinguishing species belonging to the same genus. Reviewing the glycosides that were detected in the species of genus Veronica, Taskova et al. found that aucubin and catalpol were found in all the species they investigated (many of them were part of this study by our team: V. polita, V. persica, V. chamaedrys, V. cymbalaria, V. montana, V. officinalis, V. longifolia, V. anagallis-aquatica, V. peregrina, V. beccabunga, V. serpyllifolia, V. acinifolia) [10]. In their research, they also found no correlation between clade membership and phytochemical components due to intraclade variability. This is also the case with free volatile compounds from our research. Taskova et al. also concluded in their research of the New Zealand snow hebe that chemical profiles can provide valuable data for taxonomic problems at the subsection rank [65]. Further research on the free volatile compounds as chemophenetic markers could include some sections of the genus Veronica that grow in the Southern Hemisphere, such as Hebe, Parahebe, Heliohebe, Detzneria and Derwentia, as they were once considered to be different genera.
The Mantel test for the comparison between the Euclidean distances of volatile components and Kimura genetic distances from DNA sequence data showed that there was no correlation between the chemical and genetic data groups. Genetic distances are typically based on molecular markers that evolve slowly over time and are subject to random mutations. Moreover, the observed cases of parallel evolution and in the genus Veronica [2,37] can additionally complicate the correlation of genetic data with the chemical composition of volatile components. Volatile components as well as other plant metabolites may be influenced by more rapid changes such as changes in gene expression, environmental factors or epigenetic modifications. Therefore, it is important to consider multiple lines of evidence when studying the evolutionary relationships between organisms, including genetic, morphological and biochemical data. Further studies will shed more light on this interesting question.
5. Conclusions
This is the first report of the comparison between the free volatile compounds and DNA sequence data in Croatian Veronica species and a useful contribution to the better understanding of interspecies relationships in this genus. We did not find any correlations between the Veronica subgenera membership and the composition of free volatile compounds. This could be explained by the fact that volatile components as well as other plant metabolites may be influenced by more rapid changes such as changes in gene expression, environmental factors or epigenetic modifications. Major components identified by the classical (hydrodistillation) and green (microwave) methods of extraction regardless of the habitat isolated in all 18 species selected for this study were hexahydrofarnesyl acetone, hexadecanoic acid, phytol, (E)-caryophyllene and caryophyllene oxide. Since these compounds were not identified as major compounds in these relative percentage ratios in any other genera from the Plantaginaceae family, they could be considered as chemophenetic markers for the genus.
Supplementary Materials
The following supporting information can be downloaded at www.mdpi.com/xxx/s1eo. Table S1: List of taxa, GenBank accession numbers for the ITS2 and ITS1-5.8S-ITS2 sequences of Veronica species obtained in this study, Table S2: List of taxa, GenBank accession numbers for the ITS1-5.8S-ITS2 sequences and references for the previously published sequences used in this study, Table S3: Number of raw reads, effective tags and contigs/ribotypes obtained after cleaning, merging, chimera removal and assembly of ITS2 region amplicons of Veronica plants with NovaSeq Illumina platform, Table S4: Statistics of assembled contigs/ribotypes of ITS2 region of Veronica plants that incorporated > 20% of clean tags, Data S1: Sequences of assembled contigs/ribotypes of ITS2 region of Veronica plants that incorporated > 20% of clean tags, Data S2: Sequences of ITS1-5.8S-ITS2 region of Veronica species obtained in this study and combined with earlier published sequences from GenBank, Data S3: Results of Mantel test.
Author Contributions
Conceptualization, J.P. and M.N.; methodology, V.D., M.N. and J.P.; software, J.P., Ž.T. and D.K.; validation, J.P. and V.D.; formal analysis, J.P., M.N., V.D. and Ž.T.; investigation, V.D., M.N., Ž.F., Ž.T., D.K. and J.P.; data curation, J.P., M.N. and V.D.; writing—original draft preparation, M.N. and J.P.; writing—review and editing, M.N., V.D., D.K., Ž.T. and J.P.; visualization, M.N., D.K. and J.P.; supervision, V.D.; funding acquisition, V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the project “Croatian Veronica Species: Phytotaxonomy and Biological Activity” (CROVeS-PhyBA) (project number: IP-2020-02-8425) funded by the Croatian Science Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw sequence reads obtained with the NovaSeq Illumina platform for the ITS2 region were submitted to the NCBI Sequence Read Archive (SRA) and are available under BioProject PRJNA944611, biosample accessions SAMN33750235—SAMN33750287. The most representative ITS2 sequences from NGS datasets (dominant ribotype) were deposited in GenBank under accession numbers OQ594828- OQ594880, and the ITS1-5.8S-ITS2 sequences that were sequenced by the Sanger sequencing method were deposited in GenBank under accession numbers OQ564378-OQ564387.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Albach, D.C.; Martínez-Ortega, M.M.; Fischer, M.A.; Chase, M.W. A New Classification of the Tribe Veroniceae-Problems and a Possible Solution. Taxon 2004, 53, 429–452. [Google Scholar] [CrossRef]
- Albach, D.C.; Martínez-Ortega, M.M.; Chase, M.W. Veronica: Parallel Morphological Evolution and Phylogeography in the Mediterranean. Plant Syst. Evol. 2004, 246, 177–194. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Tayeboon, G.S.; Sharifi-Rad, J.; Iriti, M.; Varoni, E.M.; Razazi, S. Inhibitory Activity on Type 2 Diabetes and Hypertension Key-Enzymes, and Antioxidant Capacity of Veronica Persica Phenolic-Rich Extracts. Cell Mol. Biol. 2016, 62, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Grayer-Barkmeijer, R.J. A Chemosystematic Study of Veronica: Iridoid Glucosides. Biochem. Syst. Ecol. 1973, 1, 101–110. [Google Scholar] [CrossRef]
- Grayer, R.J.; Chase, M.W.; Simmonds, M.S.J. A Comparison between Chemical and Molecular Characters for the Determination of Phylogenetic Relationships among Plant Families: An Appreciation of Hegnauer’s “Chemotaxonomie Der Pflanzen”. Biochem. Syst. Ecol. 1999, 27, 369–393. [Google Scholar] [CrossRef]
- Albach, D.C.; Meudt, H.M.; Oxelman, B. Piecing Together the “New” Plantaginaceae. Am. J. Bot. 2005, 92, 297–315. [Google Scholar] [CrossRef]
- Albach, D.C.; Grayer, R.J.; Jensen, S.R.; Özgökce, F.; Veitch, N.C. Acylated Flavone Glycosides from Veronica. Phytochemistry 2003, 64, 1295–1301. [Google Scholar] [CrossRef]
- Albach, D.C.; Jensen, S.R.; Özgökce, F.; Grayer, R.J. Veronica: Chemical Characters for the Support of Phylogenetic Relationships Based on Nuclear Ribosomal and Plastid DNA Sequence Data. Biochem. Syst. Ecol. 2005, 33, 1087–1106. [Google Scholar] [CrossRef]
- Jensen, S.R.; Albach, D.C.; Ohno, T.; Grayer, R.J. Veronica: Iridoids and Cornoside as Chemosystematic Markers. Biochem. Syst. Ecol. 2005, 33, 1031–1047. [Google Scholar] [CrossRef]
- Taskova, R.M.; Albach, D.C.; Grayer, R.J. Phylogeny of Veronica—A Combination of Molecular and Chemical Evidence. Plant Biol. 2004, 6, 673–682. [Google Scholar] [CrossRef]
- Reynolds, T. The Evolution of Chemosystematics. Phytochemistry 2007, 68, 2887–2895. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.; Menichini, F.; Statti, G.; Menichini, F. Biological and Pharmacological Activities of Iridoids: Recent Developments. Mini-Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Harput, U.S.; Saracoglu, I.; Inoue, M.; Ogihara, Y. Phenylethanoid and Iridoid Glycosides from Veronica persica. Chem. Pharm. Bull. 2002, 50, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Harput, U.S.; Saracoglu, I.; Nagatsu, A.; Ogihara, Y. Iridoid Glucosides from Veronica hederifolia. Chem. Pharm. Bull. 2002, 50, 1106–1108. [Google Scholar] [CrossRef]
- Crişan, G.; Vlase, L.; Balica, G.; Muntean, D.; Ştefǎnescu, C.; Pǎltinean, R.; Tǎmaş, M.; Leucuţa, S. LC /MS Analysis of Aucubin and Catalpol of Some Veronica Species. Farmacia 2010, 58, 237–242. [Google Scholar]
- Taskova, R.; Peev, D.; Handjieva, N. Iridoid Glucosides of the Genus Veronica s.l. and Their Systematic Significance. Plant Syst. Evol. 2002, 231, 1–17. [Google Scholar] [CrossRef]
- Saeidi Mehrvarz, S.; Mahmoodi, N.O.; Asadian, R.; Bakhshi Khaniki, G. Iridoid and Flavonoid Patterns of the Genus Veronica Sect. Alsinebe Subsect. Agrestis (Benth.) Stroh (Lamiales) and Their Systematic Significance. Aust. J. Crop. Sci. 2008, 1, 1–5. [Google Scholar] [CrossRef]
- Albach, D.C.; Held Gotfredsen, C.; Jensen, S.R. Iridoid Glucosides of Paederota Lutea and the Relationships between Paederota and Veronica. Phytochemistry 2004, 65, 2129–2134. [Google Scholar] [CrossRef]
- Saracoǧlu, I.; Harput, Ü.Ş.; Ogihara, Y. Acylated Flavone Glycosides from Veronica pectinata var. glandulosa and V. persica. Turk. J. Chem. 2004, 28, 751–759. [Google Scholar]
- Li, F. Analysis of Chemical Constituents of Essential Oil in Veronica linariifolia by Gas Chromatography-Mass Spectrometry. Chin. J. Anal. Chem. 2002, 822–825. [Google Scholar]
- Kwak, J.H.; Kim, H.J.; Lee, K.H.; Kang, S.C.; Zee, O.P. Antioxidative Iridoid Glycosides and Phenolic Compounds from Veronica peregrina. Arch. Pharm. Res. 2009, 32, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Çelik, E.; Yuvali Çelik, G.; Meysun, A. Essential Oil Composition and Antibacterial Activity of Some Plant Species. J. Appl. Biol. Sci. 2010, 1, 45–48. [Google Scholar] [CrossRef]
- Ertas, A.; Boga, M.; Kizil, M.; Ceken, B.; Goren, A.C.; Hasimi, N.; Demirci, S.; Topcu, G.; Kolak, U. Chemical Profile and Biological Activities of Veronica thymoides Subsp. pseudocinerea. Pharm. Biol. 2015, 53, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Dunkić, V.; Nazlić, M.; Ruščić, M.; Vuko, E.; Akrap, K.; Topić, S.; Milović, M.; Vuletić, N.; Puizina, J.; Jurišić Grubešić, R.; et al. Hydrodistillation and Microwave Extraction of Volatile Compounds: Comparing Data for Twenty-One Veronica Species from Different Habitats. Plants 2022, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Andrés, B.M.; Low, E.; Albach, D.C.; Prebble, J.M.; Meudt, H.M.; Garnock-Jones, P.J. Is Genome Downsizing Associated with Diversification in Polyploid Lineages of Veronica? Bot. J. Linn. Soc. 2015, 178, 243–266. [Google Scholar] [CrossRef]
- Albach, D.C.; Meudt, H.M. Phylogeny of Veronica in the Southern and Northern Hemispheres Based on Plastid, Nuclear Ribosomal and Nuclear Low-Copy DNA. Mol. Phylogenet. Evol. 2010, 54, 457–471. [Google Scholar] [CrossRef]
- Bardy, K.E.; Albach, D.C.; Schneeweiss, G.M.; Fischer, M.A.; Schönswetter, P. Disentangling Phylogeography, Polyploid Evolution and Taxonomy of a Woodland Herb (Veronica chamaedrys Group, Plantaginaceae s.l.) in Southeastern Europe. Mol. Phylogenet. Evol. 2010, 57, 771–786. [Google Scholar] [CrossRef]
- Albach, D.C.; Von Sternburg, M.; Scalone, R.; Bardy, K.E. Phylogenetic Analysis and Differentiation of Veronica Subgenus Stenocarpon in the Balkan Peninsula. Bot. J. Linn. Soc. 2009, 159, 616–636. [Google Scholar] [CrossRef]
- Bardy, K.E.; Schönswetter, P.; Schneeweiss, G.M.; Fischer, M.A.; Albach, D.C. Extensive Gene Flow Blurs Species Boundaries among Veronica barrelieri, V. orchidea and V. spicata (Plantaginaceae) in Southeastern Europe. Taxon 2011, 60, 108–121. [Google Scholar] [CrossRef]
- Batovska, J.; Cogan, N.O.I.; Lynch, S.E.; Blacket, M.J. Using Next-Generation Sequencing for DNA Barcoding: Capturing Allelic Variation in ITS2. G3 Genes Genomes Genet. 2017, 7, 19–29. [Google Scholar] [CrossRef]
- Matyášek, R.; Renny-Byfield, S.; Fulneček, J.; Macas, J.; Grandbastien, M.A.; Nichols, R.; Leitch, A.; Kovařík, A. Next Generation Sequencing Analysis Reveals a Relationship between RDNA Unit Diversity and Locus Number in Nicotiana Diploids. BMC Genom. 2012, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Tejedor, A.; Leekitcharoenphon, P.; Aarestrup, F.M.; Otani, S. Evaluating the Usefulness of Next-Generation Sequencing for Herb Authentication. Food Chem. Mol. Sci. 2021, 3, 100044. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yao, H.; Han, J.; Liu, C.; Song, J.; Shi, L.; Zhu, Y.; Ma, X.; Gao, T.; Pang, X.; et al. Validation of the ITS2 Region as a Novel DNA Barcode for Identifying Medicinal Plant Species. PLoS ONE 2010, 5, e8432. [Google Scholar] [CrossRef] [PubMed]
- Bezić, N.; Šamanić, I.; Dunkić, V.; Besendorfer, V.; Puizina, J. Essential Oil Composition and Internal Transcribed Spacer (ITS) Sequence Variability of Four South-Croatian Satureja Species (Lamiaceae). Molecules 2009, 14, 925–938. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Albach, D.C.; Chase, M.W. Paraphyly of Veronica (Veroniceae; Scrophulariaceae): Evidence from the Internal Transcribed Spacer (ITS) Sequences of Nuclear Ribosomal DNA. J. Plant Res. 2001, 114, 9–18. [Google Scholar] [CrossRef]
- Muñoz-Centeno, L.M.; Albach, D.C.; Sánchez-Agudo, J.A.; Martínez-Ortega, M.M. Systematic Significance of Seed Morphology in Veronica (Plantaginaceae): A Phylogenetic Perspective. Ann. Bot. 2006, 98, 335–350. [Google Scholar] [CrossRef]
- Wagstaff, S.J.; Garnock-Jones, P.J. Evolution and Biogeography of the Hebe Complex (Scrophulariaceae) Inferred from ITS Sequences. N. Z. J. Bot. 1998, 36, 425–437. [Google Scholar] [CrossRef]
- Surina, B.; Pfanzelt, S.; Einzmann, H.J.R.; Albach, D.C. Bridging the Alps and the Middle East: Evolution, Phylogeny and Systematics of the Genus Wulfenia (Plantaginaceae). Taxon 2014, 63, 843–858. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Institute: Cambridge, UK, 2018. [Google Scholar]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, R.H.; Kõljalg, U. UNITE General FASTA Release for Eukaryotes 2; Version 16.10.2022; UNITE Community; Available online: https://doi.org/10.15156/BIO/2483914 (accessed on 14 March 2023).
- Kopylova, E.; Noé, L.; Touzet, H. SortMeRNA: Fast and Accurate Filtering of Ribosomal RNAs in Metatranscriptomic Data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Chevreux, B.; Pfisterer, T.; Drescher, B.; Driesel, A.J.; Müller, W.E.G.; Wetter, T.; Suhai, S. Using the MiraEST Assembler for Reliable and Automated MRNA Transcript Assembly and SNP Detection in Sequenced ESTs. Genome Res. 2004, 14, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Craft, J.D.; Satyal, P.; Setzer, W.N. The Chemotaxonomy of Common Sage (Salvia officinalis) Based on the Volatile Constituents. Medicines 2017, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Lim Ah Tock, M.J.; Kamatou, G.P.P.; Combrinck, S.; Sandasi, M.; Viljoen, A.M. A Chemometric Assessment of Essential Oil Variation of Three Salvia Species Indigenous to South Africa. Phytochemistry 2020, 172, 112249. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, J.S.; Zlatković, B.K.; Jovanović, S.Č.; Stojanović, G.S.; Marin, P.D.; Mitić, Z.S. Needle Volatiles as Chemophenetic Markers in Differentiation of Natural Populations of Abies alba, A. x Borisii-Regis, and A. cephalonica. Phytochemistry 2021, 183, 112612. [Google Scholar] [CrossRef]
- Kremer, D.; Dunkić, V.; Radosavljević, I.; Bogunić, F.; Ivanova, D.; Ballian, D.; Stešević, D.; Matevski, V.; Ranđelović, V.; Eleftheriadou, E.; et al. Phytochemicals and Their Correlation with Molecular Data in Micromeria and Clinopodium (Lamiaceae) Taxa. Plants 2022, 11, 3407. [Google Scholar] [CrossRef]
- Niederbacher, B.; Winkler, J.B.; Schnitzler, J.P. Volatile Organic Compounds as Non-Invasive Markers for Plant Phenotyping. J. Exp. Bot. 2015, 66, 5403–5416. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 14 March 2023).
- Kimura, M. A Simple Method for Estimating Evolutionary Rates of Base Substitutions through Comparative Studies of Nucleotide Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Doostmohammadi, M.; Bordbar, F.; Albach, D.C.; Mirtadzadini, M. Phylogeny and Historical Biogeography of Veronica Subgenus Pentasepalae (Plantaginaceae): Evidence for Its Origin and Subsequent Dispersal. Biol. 2022, 11, 639. [Google Scholar] [CrossRef]
- Albach, D.C.; Martınez-Ortega, M.M.; Fischer, M.A.; Chase, M.W. Evolution of Veroniceae: A Phylogenetic Perspective. Ann. Mo. Bot. Gard. 2004, 91, 275–302. [Google Scholar]
- Hammami, S.; Debbabi, H.; Jlassi, I.; Joshi, R.K.; Mokni, R. El Chemical Composition and Antimicrobial Activity of Essential Oil from the Aerial Parts of Plantago afra L. (Plantaginaceae) Growing Wild in Tunisia. South Afr. J. Bot. 2020, 132, 410–414. [Google Scholar] [CrossRef]
- Al-Mazroa, S.A.; Al-Wahaibi, L.H.; Mousa, A.A.; Al-Khathlan, H.Z. Essential Oil of Some Seasonal Flowering Plants Grown in Saudi Arabia. Arab. J. Chem. 2015, 8, 212–217. [Google Scholar] [CrossRef]
- De Lima, E.J.; Fontes, S.S.; Nogueira, M.L.; Silva, V.R.; Santos, L.D.S.; D’Elia, G.M.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.R.; Bezerra, D.P.; et al. Essential Oil from Leaves of Conobea scoparioides (Cham. & Schltdl.) Benth. (Plantaginaceae) Causes Cell Death in HepG2 Cells and Inhibits Tumor Development in a Xenograft Model. Biomed. Pharmacother. 2020, 129, 110402. [Google Scholar] [CrossRef]
- Brandão, C.M.; Cavalcante, K.S.B.; de M. Teles, R.; Georgiana, G.E.; Monteiro, O.S.; Andrade, E.H.A.; Maia, J.G.S. Composition and Larvicidal Activity of the Oil of Dizygostemon riparius (Plantaginaceae), a New Aromatic Species Occurring in Maranhão, Brazil. Chem. Biodivers. 2020, 17, e2000462. [Google Scholar] [CrossRef]
- Bajer, T.; Janda, V.; Bajerová, P.; Kremr, D.; Eisner, A.; Ventura, K. Chemical Composition of Essential Oils from Plantago lanceolata L. Leaves Extracted by Hydrodistillation. J. Food Sci. Technol. 2016, 53, 1576–1584. [Google Scholar] [CrossRef]
- Haghighi, S.R.; Yazdinezhad, A.; Bagheri, K.; Sharafi, A.; Haghighi, S.R.; Sharafi, A. Original Article Volatile Constituents and Toxicity of Essential Oils Extracted from Aerial Parts of Plantago lanceolata and Plantago major Growing in Iran. Pharm. Biomed. Res. 2022, 8, 205–224. [Google Scholar]
- Fons, F.; Rapior, S.; Gargadennec, A.; Andary, C.; Bessière, J.M. Volatile Components of Plantago lanceolata (Plantaginaceae). Acta Bot. Gall. 1998, 145, 265–269. [Google Scholar] [CrossRef]
- Roudbaraki, S.J.; Nori-Shargh, D. The Volatile Constituent Analysis of Digitalis nervosa Steud. & Hochst. Ex Benth. from Iran. Russ. Chem. Bull. 2016, 65, 1148–1150. [Google Scholar] [CrossRef]
- Seifi, H.; Masoum, S.; Seifi, S.; Ebrahimabadi, E.H. Chemometric Resolution Approaches in Characterisation of Volatile Constituents in Plantago ovata Seeds Using Gas Chromatography-Mass Spectrometry: Methodology and Performance Assessment. Phytochem. Anal. 2014, 25, 273–281. [Google Scholar] [CrossRef]
- Taskova, R.M.; Kokubun, T.; Ryan, K.G.; Garnock-Jones, P.J.; Jensen, S.R. Phenylethanoid and Iridoid Glycosides in the New Zealand Snow Hebes (Veronica, Plantaginaceae). Chem. Pharm. Bull. 2010, 58, 703–711. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).