Abstract
Maintaining a diverse diet is essential for the preservation of one’s health and may contribute to the fight against significant civilization diseases such as obesity or diabetes. Sweet potato can be fitted into a diverse diet and serve as a functional food with its antioxidant content. Therefore, it is important to know how the production technology alters the content and composition of these antioxidant compounds. The objective of this study was to collect information on how the increased potassium dominant fertilizer levels and also the additional high-dose fertigation can affect the phytonutrient contents and yields in an orange- and a purple-fleshed sweet potato cultivar. Field experiments were conducted in the Hungarian county of Heves in two consecutive growing seasons in 2021–2022. Different doses of potassium-predominant fertilizer were applied to an orange flesh (Beauregard) and a purple flesh (Stokes Purple) varieties of sweet potatoes. Different application techniques were used in the form of base and top dressing at different rates. The effect on yield and polyphenol content of the different fertilizer rates was investigated. Analytical studies were carried out by high-performance liquid chromatography (HPLC). In the case of the Beauregard variety, in addition to identifying the carotenoids, we also performed their quantitative determination. We found that 87% of the carotenoid content was ß-carotene. The total anthocyanin content was investigated for the purple variety—Stokes Purple, for which a new extraction method was developed. In our study, the split dosages, when the pre-planting fertilizer was supplemented with additional liquid fertilization, resulted in 36 and 30.5% higher yields in the Beauregard in Experiment I and Experiment II, respectively, compared to the untreated control plots over the two years. The additional liquid fertilizer increased the yield to a lesser extent when compared to treatments received only pre-planting fertilizer. As for Experiment III, the split dosages resulted in 34.4% higher yields in the Stokes Purple compared to the control plots. However, the additional liquid fertilizer was not effective at all when the plants received a double dose of pre-planting fertilizer in the case of Stokes Purple. Total carotenoid (Experiment I–II) was higher and anthocyanin yield per plant (Experiment III) was significantly higher in the split-dosage treatment than in the untreated control plots.
1. Introduction
Sweet potato is the seventh most important food crop worldwide, after wheat, rice, maize, potato, barley, and manioc. Sweet potato is particularly significant in the poor regions of the world; thus, it is the fourth most important food crop in developing countries. It is grown mostly in tropical and subtropical regions, where its storage root and leaves are consumed by people and also livestock. In developed countries, including Hungary, due to the changing consumption trends, functional food ingredients that have a positive physiological effect through their components are coming to the fore. Sweet potato is one of the most valuable vegetables in this respect.
Currently, China is the biggest, sweet potato grower with 51.8 million tons, with a bit more than 22 t ha−1 average yield [1]. At present, in Hungary, sweet potato is grown in almost 200 ha, mostly in scattered areas, with a 20–25 t ha−1 average yield [2].
Sweet potato, like other root vegetables such as sugar beet, potato, and cassava, is a plant with significant potassium need because the leaves, storage root, and stem absorb a considerable amount from the soil. Potassium is an essential nutrient for sweet potatoes because it improves root yield and storability. With potassium, we can increase the number, size, quality, and unit weight of storage roots. For optimal growth and adequate yield, it is recommended that the minimum K2O level should be twice the amount of nitrogen applied [3]. Depending on the soil, a threefold or even higher dose can be applied. The lack of K2O significantly reduces crop yield, while the lack of P2O5 does not affect it significantly. The crop adapts well to the low level of P2O5 because due to the mycorrhiza association on the root, P2O5 becomes accessible [3]. Based on research made in Japan, sweet potato with an average yield of 13 t ha−1 absorbs 70 kg ha−1 N, 20 kg ha−1 P2O5, and 110 kg ha−1 K2O from the soil, but it strongly depends on the length of the vegetation and the agroclimatic conditions [3].
Several factors can cause the lack of K2O, for instance, the nutrient availability of the different soil types or the diverse agricultural practices [4,5], and the increasing problem of using valuable stalk residues in bioethanol production, which would improve the nutrient supply capacity of soils [6]. Potassium deficiency is one of the most important abiotic stress factors that affect plant growth and development and limits plant productivity and quality, mainly when it occurs at the initial stage of phenology [7].
Beauregard sweet potato variety is characterized by light rose skin with moderately deep orange flesh. This variety was developed by the Louisiana Agricultural Experiment station. It is resistant to soil rot, Fusarium wilt, Rhizopus soft rot, and Fusarium root rot [8]. The Stokes Purple variety is characterized by brownish skin and deep-purple flesh with white and violet striations. Its high anthocyanin content contributes to its culinary and health-preserving significance. It was developed in Stokes Country, North Carolina.
Sweet potato is severely sensitive to unfavorable growing conditions, including potassium deficiency [9]. There are genotypic differences in resistance mechanisms, that result in different physiological responses to potassium deficiency [10,11]. According to Sidike et al. [12], each genotype utilizes potassium differently during vegetation. Wang et al. [13]. found significant differences in the intraspecific differences between genotypes of sweet potato for storage root-, dry matter- and biomass yield, as responses to potassium supply.
Among the sweet potato varieties, purple sweet potatoes contain large amounts of polyphenols (e.g., anthocyanins) [14]. The valuable compounds found in various purple sweet potato extracts have been shown to have remarkable and wide-ranging biological activities, such as hepatoprotective effects [15], high antioxidant activity [16], and memory-improving properties [17]. In addition, purple sweet potato dyes are sought after by the food industry as a low-cost product due to their non-toxicity, unique color, and positive nutritional benefits. Considering the trends in the food and beverage market, there is a public demand for pigmented vegetables that can be used as alternatives to synthetic compounds; however, agronomic practices should be researched to enhance the health benefits of these products through improved pigmentation of raw materials [18].
The yield of extracts obtained from purple sweet potato is low. Zhenzhou [19] started to develop a new approach to improve the extraction of coloring phytonutrient yield from purple sweet potato. Several methods (e.g., pure water extraction and traditional solvent extraction) have been described in the literature for the extraction of polyphenols from purple sweet potato [20], observing low extraction yields. A high temperature of 80 °C was used to extract the valuable compounds (anthocyanins), which was successful [21]. Although traditional solvent extraction techniques are the most common, there is a need to develop alternative procedures and methods to increase efficiency and selectivity while avoiding or minimizing the use of toxic solvents (e.g., methanol).
Recently, many new technological solutions have been tried to extract anthocyanins to increase their quantity. The potential of ultrasound- or microwave-assisted extraction (MAE) to overcome the problems encountered in conventional extraction solutions (CSE) has been investigated [22,23,24]. Among these technologies, ultrasound-assisted extraction (UAE) has proven to be an intriguing tool for the extraction of polyphenols from raw materials of plant-based food, as it is inexpensive compared to other alternative techniques. It requires low equipment and maintenance costs, and we can minimize the use of toxic solvents such as methanol use [19].
After the extraction procedure to identify the anthocyanin level, destructive methods can be used with high punctuality in the case of purple sweet potato. For identification, we use high-performance liquid chromatography (HPLC). Purple sweet potato contains anthocyanins in large quantities in its storage roots, of which cyanidin and peonidin are the main anthocyanins. Anthocyanins are excellent quality natural food antioxidants which is a preventive solution for lifestyle-related diseases because they have significant anticarcinogenic and antidiabetic effects [25].
Ginting [26] compared the yield, dry matter, and anthocyanin content of three different purple sweet potatoes, where the anthocyanin content of Antin-1 according to the fresh weight was 7.96 mg 100 g−1, and the yield was 33.2 t ha−1. Antin-2 has a higher yield potential of 37.1 t ha−1, the anthocyanin content is 130.19 mg 100 g−1, and the dry matter content of the storage roots is 32.6 percent by weight. The yield of Antin-3 is 30.6 t ha−1 and contains a very high proportion of anthocyanin: 150.67 mg 100 g−1, while its dry matter content is 29.7%. Nevertheless, the most important antioxidant component in the orange-fleshed cv. Beauregard are carotenoids, which have notable phenolic compounds as well, especially in the skin [27]. In addition, the main antioxidant compounds in the purple-fleshed cv. Stokes Purple are polyphenolic compounds, especially anthocyanins [28]. There were about 42 anthocyanin monomers identified in purple-fleshed sweet potatoes, according to a review [29]. Even the leaves of sweet potatoes contain anthocyanins [30].
Presumably, the raised potassium-predominant fertilizer levels are more effective when applied in split dosages in increasing yield and phytonutrient contents. The aim of this work was to investigate the effect of different potassium-predominant fertilizer rates—especially when the pre-planting fertilization was supplemented with a high amount of potassium-predominant fertigation as top dressing—on yield, carotenoids and phenolic content of two different sweet potato genotypes which, were the orange-fleshed ‘Beauregard’ and the purple-fleshed ‘Stokes Purple’ variety. In the case of the Beauregard variety, in addition to identifying the carotenoids, we also performed their quantitative determination. In the case of the purple sweet potato, the anthocyanin content was measured. A new method was developed for the extraction of anthocyanins.
2. Materials and Methods
2.1. Meteorology Data
Data in Figure 1 show that the weather conditions during the sweet potato planting season in 2021, in the second half of May, were marked by frequent movements of fronts and the weather was colder than average, which was not favorable for the planting. During the first days of June, daily mean temperatures remained below the long-term average. From the 13th to the 15th, the weather was cooler again, especially in the early hours of the morning, and then a positive anomaly prevailed throughout the rest of the month. The low temperatures caused great stress in the formation of batata storage roots. In the second half of the month, the daily mean temperature raised to such an extent that heat warnings were issued successively. In July, the daily mean temperature was above the long-term average, with the exception of the first and the beginning of the third decade. At the beginning of August, average daily temperatures remained below the long-term average, as a wavy frontal system shaped our weather. In the harvest season (September and October) average daily temperatures raised rapidly, so we had warmer days than usual. It helped the storage roots to grow and the harvest.
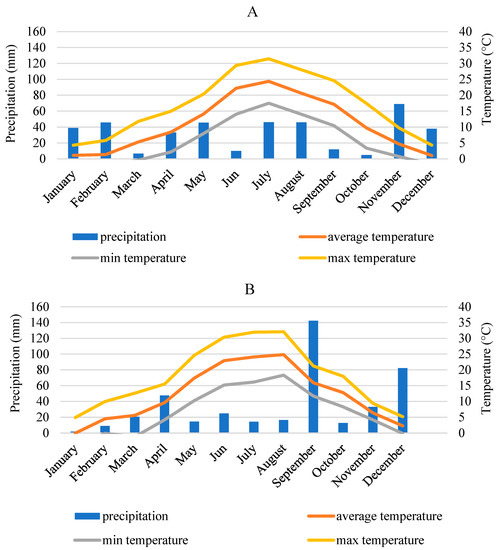
Figure 1.
Meteorology data show the minimum, maximum and average temperature, besides the summarized monthly precipitation amount (columns) in 2021 (A) and 2022 (B) (Hungarian Meteorological Service).
The 2022 growing season was the warmest year on record in Hungary. It was 2 °C warmer than the reference average between 1991 and 2020, and 0.5 °C warmer than the previous record year from 2003. In the growing season of 2022, there were several heat alerts during the summer. On 21 occasions, the national average daily mean temperature reached or went above 25 °C. Rainfall during the winter was 60–70% below average. The spring was not favorable either, with a little amount of rainfall. This contributed greatly to the hot, dry summer, which is favorable for tropical, subtropical plants such as sweet potatoes. The national average rainfall during the summer was 137 mm. In the study area, this value was 55.7 mm.
2.2. Field Experiment
The trial was set on a field in Heves, Heves county in Hungary, characterized by sandy soil, in 2021 and 2022. The physical texture of the soil is represented as having a content of clay 10%, silt 10%, and 80% and organic matter content below 1%.
The results of the pre-experiment soil analysis are presented in Table 1. (Mertcontrol HL-LAB, Debrecen, Hungary). The field trial consisted of 4 treatments in 4 repetitions on two different genotypes. Fifty sweet potato seedlings were planted within one plot, and the plots were repeated four times. A total of 1200 orange-fleshed ‘Beauregard’ and 1200 purple-fleshed sweet potatoes (‘Stokes Purple’) were examined in the experiment.

Table 1.
Results of the soil analysis (0–30 cm layer) conducted before the experiment.
The seedlings were planted on ridges covered with agro foil (plastic mulch) to eliminate weeds. The seedlings were planted at a distance of 30 cm, and the row spacing was 80 cm. Irrigation was carried out with drip tape, each plot received the same amount of irrigation water. The harvest took place on the 125th day after planting.
For basal (pre-planting fertilizer) complex N-P-K fertilizer YaraMila Cropcare 8-11-23 (Yara Hungária Ltd., Veszprém, Hungary) was applied. Potassium sulfate was applied as a basal (BP-I, BP-II, LP-I, LP-II) and top dressing/nutrient (BL-I, BL-II, LL-I, LL-II) solution. During basal dressing, potassium sulfate (23% K2O) was implemented on the ridges before planting. The potassium sulfate nutrient solution (51% K2O) was implemented as a top dressing (liquid fertilization) YaraTera Krista SOP (Yara Hungária Ltd., Veszprém, Hungary) in the case of individual rows. MgSO4 was only applied with a nutrient solution. Different K2O amounts of 0.625 and 1.375 kg per plot were applied as basal dressing in Experiment I-III. and Experiment II-IV., respectively. On the 45th, 64th, 72nd, 79th, and 88th days after planting, an additional 0.375 kg (51% K2O) of potassium sulfate was implemented with a nutrient solution in five equal doses, each time, per plot (50 plants on 12 m2). During the entire vegetation period, the plot treated with the nutrient solution (50 sweet potatoes/plot) received 1.875 kg (51% K2O) more potassium sulfate than the basal-dressed plots. Magnesium sulfate (MgSO4)—YaraTera Krista MgS—(Yara Hungária Ltd., Veszprém, Hungary) with 16% Mg content; sulfur (SO3) with 30% S content was applied 79th, 88th, and 93rd days after planting in three equal doses of 0.0875 kg per plot. A total of 0.2625 kg of MgSO4 (16% Mg) and SO3 (30% S) were implemented in the nutrient solution plot (50 sweet potatoes/plot). The total amount of active substances are presented in Table 2.

Table 2.
The total amount of active substances fertilizer applied in the treatments.
The total yield of the different plots was measured after the harvest following the heat treatment called curing, which is required for the recovery of the sweet potato skin. This postharvest technique is the most important step for the long-term storage of sweet potato storage roots [31]. Heat treatments have been used as a non-chemical means to modify the postharvest quality and reduce pathogen levels and disease development of sweet potato products.
2.3. Laboratory Experiment
2.3.1. Samples
Fresh sweet potato samples were obtained from the experimental field in Heves. The storage roots were analyzed in triplicate, the fresh material was homogenized in a warring blender, before the extraction.
2.3.2. Chemicals Used
HPLC grade organic solvents used in the HPLC analyses were from Merck (Budapest, Hungary), while analytical grade solvents and other chemicals used were from WVR (Debrecen, Hungary). Standard carotenoids such as lutein (95%), β-carotene (93%), lycopene (90%), and β-apo-8′-carotenal (96%) were purchased from Sigma-Aldrich via Merck (Budapest, Hungary). Stock solutions of 1 mg/mL for standard carotenoids such as lutein, β-carotene, lycopene, and 8-apo-β-carotenal were prepared in 2:1 methanol-acetone. The working solutions were made by diluting the stock solutions with HPLC-grade acetone.
2.3.3. Extraction of Carotenoids
Carotenoids were extracted according to a previously published protocol [32]. Five grams of sweet potato tubers were crushed in a crucible mortar in presence of quartz sand and 0.5 g of ascorbic acid. The solvent extraction started with the addition of 20 mL methanol to bind the water. The supernatant was decanted into a 100 mL Erlenmeyer flask and residues were extracted carefully by the gradual addition of 60 mL of 1:6 methanol-n-hexane with continuous mixing. The mixture was transferred carefully to the flask containing the first supernatant and shaken vigorously by hand. To achieve phase separation 1–2 mL of water was added and the mixture was shaken mechanically for 10–15 min. The phases were separated in a separating funnel and the n-hexane phase containing carotenoids was passed through an anhydrous Na2SO4 to a round-bottom flask. The solvent was then removed under vacuum at a maximum of 40 °C using a rotary evaporator. The residues were re-dissolved in 10 mL of HPLC grade acetone and further purified and cleaned through a 0.22 µm glass fiber syringe filter before injection into the HPLC column.
Separation of carotenoids was performed on a core type C-30, 2.6 µm, 150 × 4.6 mm column (Accucore from Thermo-Fischer Scientific, Walthan, MA, USA) with gradient elution consisting of (A): 2% water in methanol and (B): tert-butyl-methyl-ether. The elution started with 97% A and 3% B, changed to 35% B in A in 25 min, stayed isocratic for 5 min, and turned to 97% A and 3% B% in 5 min. The flow rate was 0.6 mL/min and carotenoids were detected between 190 and 700 nm using a diode-array detector.
The peaks were identified based on a comparison of their spectral characteristics and retention times with those of available standard materials such as lutein, β-carotene, and lycopene as well as with the literature data [33,34,35]. Quantitative determination was performed by integration of each peak area at the maximum absorption wavelength provided by DAD and relating it to that of the internal standard (8-apo-β-carotenal), which was spiked to the samples at known concentration before extraction. In addition, available standard lutein, β-carotene, and all trans-lycopene were used as external standards to emphasize their quantification.
2.3.4. HPLC Determination of Phenolics
Five grams of freshly harvested sweet potato storage roots were taken and crushed in a crucible mortar in presence of 1–2 g of quartz sand. The phenolic compounds were extracted by the addition of boiled ethanol containing 40% ortho-phosphoric acid solution. The macerate was then transferred to an Erlenmeyer flask and shaken for 15 min at 80 °C followed by ultrasonication for 5 min at 80 °C in a water-bath ultrasonic device (model RK-165-BH Bendelin Sonorex, Berlin, Germany). The extract was centrifuged for 5 min at 5000 rpm (M-Universal, MPW Med. Instrument, Warszawa, Poland). The supernatant was decantated and purified by passing through a 22 µm, 13 mm glass fiber syringe filter before injection into the HPLC apparatus.
2.3.5. HPLC Instrument and Conditions
A Chromaster Hitachi HPLC instrument containing a Model 5160 gradient pump, a Model 5260 autosampler, a Model 5310 column oven, and a Model 5430 diode-array detector was used with EZChrome Elite software for operation and data processing.
The separation of phenolic compounds was performed on Ascentis phosphor-conditioned C18 phase (C18-PCP, from Supelco, Bellefonte, PA, USA) with gradient elution of 1% ortho-phosphoric acid (A) and acetonitrile (B) according to a recently developed protocol (Under publication). The gradient elution started with 1% B in A, changed to 20% B in 20 min, stayed isocratic for 10 min, changed to 30% B in 5 min, stayed isocratic for 10 min, and, finally, turned to 1% B in 5 min. The DAD detection was between 190 nm and 700 nm. The quantification was based on recording the area at the maximum absorbance wavelength of each compound and relating it to that of the standard solution.
Stock solutions for different phenolics (Sigma-Aldrich via Merck, Budapest, Hungary) were prepared by dissolving 2–3 mg in 10 mL absolute ethanol or methanol and diluted 10 times with 40% ethanol in 1% ortho-phosphoric acid. The working solutions were used for calibration curves, identification, and quantification of phenolic compounds. In the case of no standard being available, the compounds were tentatively identified on the basis of a comparison of their spectral characteristics and chromatographic behavior with the literature data.
In the case of anthocyanins, only delphinidine chloride was available, therefore all anthocyanins detected on the HPLC profile were quantified as delphinidine chloride equivalent.
2.4. Statistical Analysis
Anova and Tukey posthoc tests were used to reveal differences among treatments, and in the case of the Beauregard variety, the relationship between total carotenoid and total yield was examined with linear regression, and the same was used in the case of Stokes Purple assessing the relationship between total anthocyanins and total yield.
3. Results and Discussion
3.1. The Effect of Different Fertilizer Dosages on the Yield of Beauregard
The high potassium-predominant fertilizer application had a positive effect on the yield (Figure 2). The split dosage BL-I treatments in Experiment I, where the plants received 0.625 kg of potassium-rich pre-planting fertilizer and then another 1.875 kg divided by four doses of top dressing (liquid fertilization) achieved higher yield (0.76 kg per plant in 2021 and 1.61 kg per plant in 2022 growing season) than BC (0.42 kg per plant in 2021 and 1.17 kg per plant in 2022) which was the untreated control plot. As a result of the BL-I treatment (Experiment I), the average yield increased by 36.1% compared to the BC. Under BL-II treatments (Experiment II), split dosages of raised pre-planting and liquid fertilizer were used and resulted in 30.5% higher yield (BL-II-0.62 kg per plant in 2021 and 1.64 kg per plant in 2022) than BC (0.42 kg per plant in 2021 and 1.17 kg per plant in 2022), this is a similar outcome that showed in Experiment I. The additional liquid fertilizer increased the yields by 23.3% over the two years. From the perspective of yield, the raised levels of pre-planting fertilizers were not effective. Important to note, that the 2021 growing season was cold, which is not favorable for sweet potato growth thus, the yield results were not reaching the standards of an ordinary year. The second year (2022) was more favorable for Beauregard sweet potato production than 2021, thus higher yields were reached under all treatments (Figure 1).
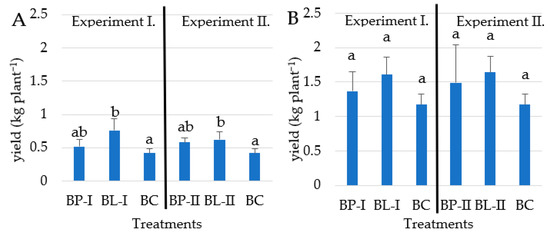
Figure 2.
The effect of different fertilizer dosages on the Beauregard yield in 2021 (A) and 2022 (B) growing seasons (n = 4). Error bars represent SD. Different letters indicate statistical differences (p < 0.05).
The maximum commercial yield of the Beauregard cultivar was achieved when 85 kg ha−1 K2O was applied in a study conducted in Brazil [36]. Abd El-Baky [37] found the highest yield of 1.19–1.49 kg plant−1, in the different growing seasons with a 150 kg fed−1 (357.2 kg ha−1) K2O fertilization level, in Egypt. In another experiment, conducted in Brazil, the highest marketable yields were achieved when potassium fertilizers were applied in one dose 30 or 60 days after planting using KCl or K2SO4, respectively [38]. According to Harvey et al. [39], profitable production of Beauregard can be achieved under 174 kg ha−1 K2O; however, the maximum K content of leaves and roots could be measured at 269 and 404 kg ha−1 K2O doses.
3.2. Influence of Different K2O Predominant Fertilizer Treatments on the Carotenoids
Table 3 and Table 4 show the difference between the carotenoid compounds identified in the orange-fleshed Beauregard sweet potato for the two different experiments in two consecutive years. The main identified carotenoids were the β-carotene, cis-β-carotene, β-cryptoxanthin, α-cryptoxanthin, luteo-chrome, cis-luteo-chrome, β-carotene-epoxide, and mutatochrome. β-carotene represented the highest amount (87% of the total carotenoid concentration in general). Others reported that about 99% of the carotenoid composition is β-carotene in orange-fleshed sweet potatoes [40]. The dominance and high level of ß-carotene were reported by Ishiguro [41] as well, in orange-fleshed sweet potato varieties. The second and third highest concentration levels were reached by the cis-ß-carotene and α-cryptoxanthin, respectively, in 2021 (Table 3). In 2021, the content of ß-carotene and the second-most important carotenoid α-cryptoxanthin were significantly higher in Experiment I under the BL-I treatments, than in the BP-I and BC. Although in the 2022 growing season, neither showed any statistically significant difference between treatments.

Table 3.
The difference between the carotenoid compounds identified in Beauregard sweet potato for different nutrient doses in the 2021 growing season (n = 4).

Table 4.
The difference between the carotenoid compounds identified in Beauregard sweet potato for different nutrient doses in the 2022 growing season (n = 4).
In Experiment II, the different treatments did not show significant differences for any of the carotenoid components in the 2021 growing season, but in the 2022 growing season the ß-carotene concentration was significantly higher in BL-II treatment, than in BP-II and BC.
If we compare the 2021 and 2022 growing seasons in Experiment I and Experiment II, the second most important carotenoid component after ß-carotene was α-cryptoxanthin in 2021, while it was luteo-chorme in 2022 (Table 3 and Table 4).
In Experiment I, in the 2021 growing season the maximum total carotenoid value was reached in the BL-I split-dosage treatment with an average 198.8 µg g−1 treatment, which is significantly (p > 0.05) higher than the value obtained by BP-I and BC, which reached 140.7 and 147.7 µg g−1 concentration, respectively. In 2022 the highest total carotene concentration was also achieved under BL-I treatment with an average of 159.1 µg g−1, but the difference between treatments was not significant. Experiment II showed similar results to Experiment I, where the split-dosage fertilizer treatment BL-II achieved the highest total carotene concentration in both growing seasons. BL-II treatment of total carotene concentration was 175.1 µg g−1 in 2021 which is higher than BP-I, but the difference was not significant. In the 2022 growing season, the highest concentration was found (205.4 µg g−1) under BL-II treatment and was significantly higher compared to BP-II (126 µg g−1) and BC (149.5 µg g−1) treatments (Figure 3).
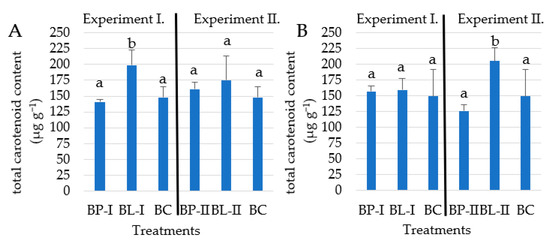
Figure 3.
The difference between the total carotenoid concentration in Beauregard sweet potato for different nutrient doses in 2021 (A) and 2022 (B) growing seasons. Values marked with different letters are significantly different at p ≤ 0.05 level.
Due to the more favorable weather conditions for sweet potato production, the nutrient utilization was better, so the higher dose of fertilizer (BL-II) contributed to the highest average total carotenoid (Figure 3). The use of a higher amount of potassium-predominant fertilizer resulted in an increase in the total and ß-carotene content compared to the untreated control (BC), which is the same as the finding of other studies [37,42].
The average ß-carotene concentration was 140.6 µg g−1 from the fresh samples which is 7 times higher than the amount measured in another study in a different orange-fleshed cultivar with a different method [43]. Teow et al. [44] reported 52% lower ß-carotene concentration from the same variety.
In Experiment I, as a result of the BL-I treatment, the average total carotenoid yield per plant was 151 mg g−1, which was significantly (p > 0.001) higher than the value of 62 mg measured in the control BC treatment, which was represented with the lowest value in the growing season of 2021. The highest carotenoid yield per plant was achieved due to the split K2O predominant dosage for BL-I (256.2 mg g−1) similarly in the growing season of 2022 (Figure 4).
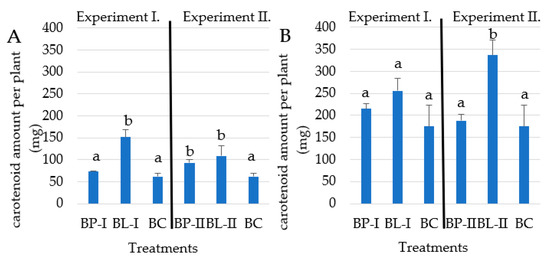
Figure 4.
The total carotenoid yield per plant in the 2021 (A) and 2022 (B) growing seasons. Values marked with different letters are significantly different at p ≤ 0.05 level.
In Experiment II, the BL-II split-dosage K2O predominant fertilizer treatment resulted in significantly higher total carotenoid yield per plant, compared to the BC in both growing seasons. In the 2022 growing season, the highest average total carotenoid yield per plant was 336.9 mg g−1, which was 51.9% higher than the BC control plot value of 174.9 mg g−1 under the BL-II treatment.
The split fertilizer dosage applied under BL-I and BL-II treatments resulted in the highest total carotenoid yield per plant, significantly higher than BC in three and higher than BP-I and BP-II in two cases (Figure 4). Generally lower carotenoid contents were measured in every treatment, in the first year, compared to the second experimental year.
The relationship of yield and carotenoid content considering the data of Experiment I and II in a linear regression model the results showed high correlation (R2 = 0.85) in the 2021 growing season; however, low correlation was detected (R2 = 0.22) in the 2022 growing season. When the results of both growing seasons were evaluated in a single linear regression model, no correlation was found since the two growing seasons were very different resulting in varying levels of yields and carotenoid contents.
3.3. Root Yield Results of Stokes Purple
It is clearly supported by the results that potassium played an important role in the yield of purple-fleshed sweet potato (Figure 5). The application of K2O predominant fertilizer improved the yield compared to the control plot in both years in the case of the purple-fleshed variety in Experiment III and IV. In the 2021 growing season, the highest value of 0.18 kg plant−1 was measured under the LL-I treatment which was 80% higher than the value of 0.1 kg plant−1 measured in the control plot (Figure 5). The growing season of 2022 was favorable for sweet potato production, so the yields were significantly higher than in the first year. The highest value was measured under the LL-I treatment as well in the 2022 growing season, reaching 0.62 kg plant−1. The LL-I treatment resulted in 100% higher yields compared to LC with 0.31 kg plant−1 yield.
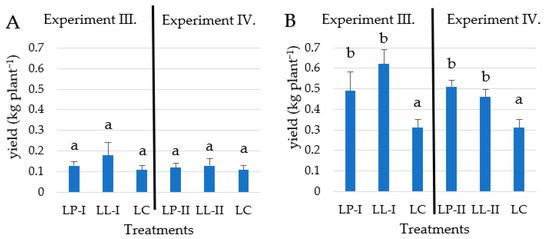
Figure 5.
The effect of different fertilizer doses on the yield of Stokes Purple in the 2021 (A) and 2022 (B) growing seasons (n = 4). Different letters indicate significant differences at p < 0.05 level.
In Experiment IV, the highest yields were measured under LP-II treatment in the 2022 growing season. The variation between yields was minimal in 2021. The double-dose pre-planting treatment exceeded the LC by almost 40% in 2022. High levels of K2O predominant split-dosage fertilizer applied in LL-II treatments reduced the Stokes Purple sweet potato yields in the 2022 growing season compared to LP-II treatment (Figure 5).
In Experiment IV., the split-dosage fertilizer treatment resulted in a lower yield, than in Experiment III, which suggested that an excessive amount of fertilizer caused an inhibitory effect on the yield production of Stokes Purple sweet potato.
Balanced nutrient supply, as the results of LL-I treatment (Experiment III) was the most effective from the perspective of the Stokes Purple sweet potato yield. According to results found in another study [45], the optimum storage root yield of sweet potato is achieved by applying 150–300 kg K2O kg ha−1. In our experiments the maximum yield was measured under higher, levels of K2O fertilizer thus it should be highly dependent on soil properties (Table 2).
3.4. Total Anthocyanin Content and Yield of the Stokes Purple
In Experiments III and IV, the anthocyanin content of storage roots in the control plot was significantly higher than in the treated plots (p < 0.05) in the 2021 growing season (Figure 6). Due to the stress caused by the lack of nutrient supply, we measured the highest anthocyanin value of 620.2 µg g−1 in the control treatment in this study. However, different results were measured in Experiment III in the 2022 growing season.
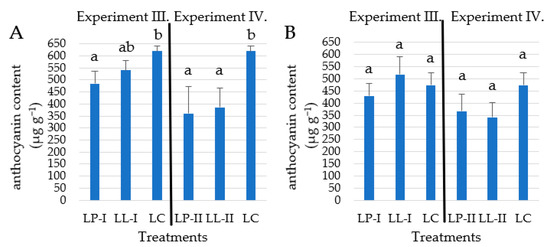
Figure 6.
Total anthocyanin content of storage roots of Stokes Purple in the 2021 (A) and 2022 (B) growing seasons (n = 4). Different letters indicate significant differences at p < 0.05 level.
Anthocyanin formation is significantly influenced by environmental factors such as abiotic stress. The growing season of 2021 was unfavorable for batata cultivation, also confirmed by the lower yields. In Experiment III, under the LL-I treatment, the anthocyanin content was 540.3 µg g−1, which was not clearly distinguished statistically from the concentration in the control 620.2 µg g−1, despite the 13% difference (Figure 6). Other studies reported around 13 100–13 420 µg g−1 (calculated for dry weight) total anthocyanin content from different purple-fleshed sweet potato cultivars [46,47]. Peng et al. [48] reported 793–3 650 µg g−1 (calculated for dry weight) total anthocyanin content from commercially available, purple-fleshed sweet potatoes. A very high level of total anthocyanin content (30 446 µg g−1 (calculated for dry weight)) was reported from the Korean cultivar ‘Sinjami’ [49].
According to Zheng et al. [50], the polyphenol substances were easy to accumulate by the abiotic stresses in the storage roots of sweet potatoes. The favorable growing season of 2022 resulted in fewer anthocyanin maximums than in the 2021 growing season (Figure 6), where the average temperature was lower. It can be said that fertilization clearly not increased the anthocyanin content of storage roots, in contrast to the abiotic stress, such as colder weather, which was observed in the 2021 growing season. Additionally, it must be noted, that the storage roots under the LL-I treatment in 2022 reached higher levels of anthocyanins than in other treatments.
Figure 7 shows that the yield of anthocyanin amount per plant was the highest under the LL-I treatment 97.3 mg plant−1 in 2021 and 322.9 mg plant−1 in 2022. Higher storage root yields influenced the anthocyanin amount per plant more than the concentrations. Root yields were not correlated with anthocyanin content at all, which accedes to the results of acquired in another study [51]. The yield and concentration data were evaluated with linear regression, which showed no correlation in Experiment III. In Experiment IV, the relationship of yield and anthocyanins content showed a negligible correlation R2 = 0.21. From the anthocyanin amount point of view, the LL-I split potassium-predominant fertilizer treatment was the optimal one in Experiment III, which resulted in the highest yield and the highest amount of anthocyanin that could be produced per plant in our experiments (Figure 7).
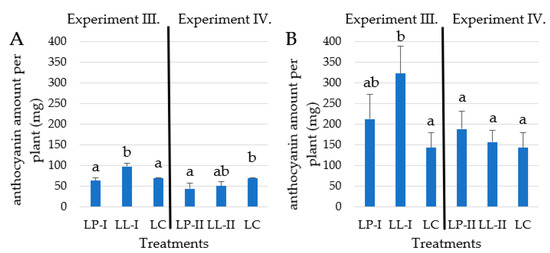
Figure 7.
The total anthocyanin yield per plant in 2021 (A) and 2022 (B) growing season (n = 4). Different letters indicate significant differences at p < 0.05 level.
4. Conclusions
In the case of the Beauregard variety, the divided application of K2O predominant fertilizer in Experiment I and Experiment II, as pre-planting and top dressing fertigation increased the yield compared to the untreated control in both growing seasons. The split-dosage treatment which received additional fertigation (BL-I and BL-II) averaged over two growing seasons increased the yield of the Beauregard variety compared to the untreated control (BC). The total carotenoid content was also positively affected by the additional fertigation over pre-planting fertilization. The carotenoid compounds in the orange-fleshed Beauregard sweet potato were successfully identified during the study. The main identified carotenoids were the β-carotene, cis-β-carotene, β-cryptoxanthin, α-cryptoxanthin, luteo-chrome, cis-luteo-chrome, β-carotene-epoxide, and mutatochrome. As the dominant component, β-carotene represented 87% of total carotenoid concentration. The effect of K2O predominant fertilization levels on the different carotenoid components was not clear. The total carotenoid concentration of sweet potatoes is not clearly related to yield. Weather and abiotic factors play a more important role in the development of total carotenoid yield. However, the different fertilization levels affected the total carotenoid concentration as the highest concentrations were measured in BL-I and BL-II (Experiment I and Experiment II) treatment in the two years, respectively.
For the purple variety—Stokes Purple, the LL-I (Experiment III) split-dosage treatment was the most effective, when single-dose pre-planting was combined with additional fertigation, regardless of the effect of different growing seasons. The LL-I treatment averaged over two growing seasons increased the yield by 104% of the Stokes Purple variety compared to the untreated control (LC). The double dose of potassium-predominant-fertilizer applied was not favorable for the Stokes Purple sweet potato variety in Experiment IV. It resulted in less yield production when compared to single-dose pre-planting and fertigation combination and it was less beneficial to anthocyanin content values as well. Under the LL-I split potassium-predominant fertilizer treatment the highest yield was achieved along with the highest amount of anthocyanin that can be produced per plant, even if the application of potassium-predominant fertilizers did not significantly increase the total anthocyanin content of sweet potato storage roots in these experiments. The conclusions are limited to these presented findings and direct suggestions cannot be formulated for growers until these will be supplemented with a cost–profit analysis. In the future, the identification and quantification of the anthocyanin compounds of ‘Stokes Purple’ should be examined.
Author Contributions
Conceptualization, L.H. and H.G.D.; methodology, Z.P.; software, M.É. and H.G.D.; investigation, A.N., Z.P. M.É. and V.B.; data curation, S.T.; writing—original draft preparation, V.B.; writing—review and editing, S.T.; visualization, V.B.; supervision, L.H. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the ÚNKP-22-3-I-MATE/9 and ÚNKP-22-4 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food Agriculture Organization of the United Nations, F.A.O.S.T.A.T. Available online: https://www.fao.org/statistics/data-collection/en/ (accessed on 2 March 2023).
- Hungarian Chamber of Agriculture. Available online: https://www.nak.hu/nyitolap (accessed on 2 March 2023).
- Degras, L. Sweet Potato. The Tropical Agriculturalist; Macmillan Publishers Ltd.: London, UK, 2003. [Google Scholar]
- Rengel, Z.; Damon, M. Crops and genotypes differ in efficiency of potassium uptake and use. Physiol. Plant. 2008, 13, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture– status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Römheld, V.; Kirkby, A. Research on potassium in agriculture: Needs and prospects. Plant Soil 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Tang, Z.-H.; Zhang, A.-J.; Wei, M.; Chen, X.-G.; Liu, Z.-H.; Li, H.-M.; Ding, Y.-F. Physiological response to potassium deficiency in three sweet potato (Ipomoea batatas [L.] Lam.) genotypes differing in potassium utilization efficiency. Acta Physiol. Plant. 2015, 37, 184. [Google Scholar] [CrossRef]
- Rolston, L.H.; Clark, C.A.; Cannon, J.M.; Randle, W.M.; Riley, E.G.; Wilson, P.W.; Robbins, M.L. Beauregard’ Sweet Potato. Hortscience 1987, 22, 1338–1339. [Google Scholar] [CrossRef]
- Tang, Z.H.; Zhang, Y.G.; Wei, M.; Chen, X.G.; Shi, X.M.; Zhang, A.J.; Li, H.M.; Ding, Y.F. Screening and evaluation indicators for low potassium-tolerant and potassium efficient sweetpotato (Ipomoea batatas L.) varieties (lines). Acta Agron. Sin. 2014, 40, 542–549. (In Chinese) [Google Scholar] [CrossRef]
- Reddy, K.; Zhao, D.L. Interactive effects of elevated CO2 and potassium deficiency on photosynthesis, growth, and biomass partitioning of cotton. Field Crops Res. 2005, 94, 201–213. [Google Scholar] [CrossRef]
- Wang, N.; Hua, H.; Eneji, A.E.; Li, Z.; Duan, L.; Tian, X. Genotypic variations in photosynthetic and physiological adjustment to potassium deficiency in cotton (Gossypium hirsutum). J. Photochem. Photobiol. B Biol. 2012, 110, 1–8. [Google Scholar] [CrossRef]
- George, M.S.; Lu, G.; Zhou, W. Genotypic variation for potassium uptake and utilization efficiency in sweet potato (Ipomoea batatas L.). Field Crop. Res. 2002, 77, 7–15. [Google Scholar] [CrossRef]
- Wang, J.D.; Wang, H.Y.; Zhang, Y.C.; Zhou, J.M.; Chen, X.Q. Intraspecific variation in potassium uptake and utilization among sweetpotato (Ipomoea batatas L.) genotypes. Field Crop Res. 2015, 170, 76–82. [Google Scholar] [CrossRef]
- Wang, X.-G.; Zhao, X.-H.; Jiang, C.-J.; Li, C.-H.; Cong, S.; Wu, D.; Chen, Y.-Q.; Yu, H.-Q.; Wang, C.-Y. Effects of potassium deficiency on photosynthesis and photoprotection mechanisms in soybean (Glycine max (L.) Merr.). J. Integr. Agric. 2015, 14, 856–863. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Choi, J.M.; Chung, Y.C.; Jeong, H.G. Protective mechanisms of anthocyanins from purple sweet potato against tert-butyl hydroperoxide-induced hepatotoxicity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 2081–2089. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, J.; Zheng, Y.; Hu, B.; Fan, S.; Wu, D.; Liu, C. Purple sweet potato color protects mouse liver against d-galactose-induced apoptosis via inhibiting caspase-3 activation and enhancing PI3K/Akt pathway. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48, 2500–2507. [Google Scholar] [CrossRef]
- Lu, D.; Wu, Y.; Zheng, B.; Hu, W.; Cheng, Z. Zhang Purple sweet potato color attenuates domoic acid-induced cognitive deficits by promoting estrogen receptor-α-mediated mitochondrial biogenesis signaling in mice. Free. Radic. Biol. Med. 2012, 52, 646–659. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Sampaio, S.L.; Di Gioia, F.; Tzortzakis, N.; Rouphael, Y.; Kyriacou, M.C.; Ferreira, I. Grown to be Blue—Antioxidant Properties and Health Effects of Colored Vegetables. Part I: Root Vegetables. Antioxidants 2019, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Guan, Q.; Koubaa, M.; Barba, F.J.; Roohinejad, S.; Cravotto, G.; Yang, X.; Li, S.; He, J. He-HPLC-DAD-ESI-MS2 analytical profile of extracts obtained from purple sweet potato after green ultrasound-assisted extraction. Food Chem. 2017, 215, 391–400. [Google Scholar] [CrossRef]
- de Aguiar Cipriano, P.; Ekici, R.C.; Barnes, C.; Gomes, S.T. Talcott Pre-heating and polyphenol oxidase inhibition impact on extraction of purple sweet potato anthocyanins. Food Chem. 2015, 180, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Bridgers, E.N.; Chinn, M.S.; Truong, V.-D. Extraction of anthocyanins from industrial purple-fleshed sweet potatoes and en-zymatic hydrolysis of residues for fermentable sugars. Ind. Crops Prod. 2010, 32, 613–620. [Google Scholar] [CrossRef]
- Kovačević, D.B.; Putnik, P.; Dragović-Uzelac, V.; Pedisić, S.; Jambrak, A.R.; Herceg, Z. Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 2016, 190, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Koubaa, M.; Roselló-Soto, E.; Žlabur, J.Š.; Jambrak, A.R.; Brnčić, M.; Grimi, N.; Barba, F.J. Current and new insights in the sustainable and green recovery of nutritionally valuable compounds from Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2015, 63, 6835–6846. [Google Scholar] [CrossRef]
- Jiao, Y.; Jiang, Y.; Zhai, W.; Yang, Z. Studies on antioxidant capacity of anthocyanin extract from purple sweet potato (Ipomoea batatas L.). Afr. J. Biotechnol. 2012, 11, 7046–7054. [Google Scholar]
- Ginting, E.; Yulifianti, R.; Jusuf, M.; Mejaya, M.J. Identifikasi Sifat Fisik, Kimia, dan Sensoris Klon-klon Harapan Ubijalar Kaya Antosianin. J. Penelit. Pertan. Tanam. Pangan 2015, 34, 69–78. [Google Scholar] [CrossRef]
- Padda, M.S.; Picha, D.H. Phenolic composition and antioxidant capacity of different heat-processed forms of sweetpotato cv. Beauregard. Int. J. Food Sci. Technol. 2008, 43, 1404–1409. [Google Scholar] [CrossRef]
- Truong, V.-D.; Deighton, N.; Thompson, R.T.; McFeeters, R.F.; Dean, L.O.; Pecota, K.V.; Yencho, G.C. Characterization of Anthocyanins and Anthocyanidins in Purple-Fleshed Sweetpotatoes by HPLC-DAD/ESI-MS/MS. J. Agric. Food Chem. 2009, 58, 404–410. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, S.; Zhao, G.; Ye, F. Destabilisation and stabilisation of anthocyanins in purple-fleshed sweet potatoes: A review. Trends Food Sci. Technol. 2021, 116, 1141–1154. [Google Scholar] [CrossRef]
- Su, X.; Griffin, J.; Xu, J.; Ouyang, P.; Zhao, Z.; Wang, W. Identification and quantification of anthocyanins in purple-fleshed sweet potato leaves. Heliyon 2019, 5, e01964. [Google Scholar] [CrossRef]
- Picha, D.H. Carbohydrate Changes in Sweet Potatoes During Curing and Storage. J. Am. Soc. Hortic. Sci. 1987, 112, 89–92. [Google Scholar] [CrossRef]
- Daood, H.G.; Bencze Gy Palotás, G.; Pék, Z.; Sidikov, A.; Helyes, L. HPLC analysis of carotenoids from tomatoes using cross-linked C18 column and MS detection. J. Chromatogr. Sci. 2014, 52, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chen, B.H. Determination of carotenoids in tomato juice by liquid chromatography. J. Chromatogr. A 2003, 1012, 103–109. [Google Scholar] [CrossRef] [PubMed]
- de Rosso, V.V.; Mercadante, A.Z. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef]
- de Faria, A.F.; de Rosso, V.V.; Mercadante, A.Z. Carotenoid composition of jackfruit (Artocarpus heterophyllus), determined by HPLC-PDA-MS/MS. Plant Foods Hum. Nutr. 2009, 64, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.B.C.; Mc Nascimento, S.; Silva, A.S.; Vargas, P.F. Agronomic performance of sweet potato with different potassium fertilization rates. Hortic. Bras. 2016, 34, 588–592. [Google Scholar] [CrossRef]
- Abd El-Baky, A.; Ahmed, A.A.; El-Nemr, M.A.; Zaki, M.F. Effect of potassium fertilizer and foliar zinc application on yield and quality of sweet potato. Res. J. Agric. Biol. Sci. 2010, 6, 386–394. [Google Scholar]
- da Silva, L.D.; Oliveira, A.P.D.; Cruz, J.M.D.L.; Sousa, V.F.D.O.; Silva, A.J.D.; Silva, M.C.D. Sweet potato yield in response to different potassium sources and splitting of fertilization. Rev. Bras. De Eng. Agrícola E Ambient. 2022, 26, 527–532. [Google Scholar] [CrossRef]
- Harvey, L.M.; Shankle, M.W.; Morris, C.J.; Hall, M.A.; Chatterjee, A.; Harvey, K.M. Sweet Potato (Ipomoea batatas L.) Response to Incremental Application Rates of Potassium Fertilizer in Mississippi. Horticulturae 2022, 8, 831. [Google Scholar] [CrossRef]
- Laveriano-Santos, E.P.; López-Yerena, A.; Jaime-Rodríguez, C.; González-Coria, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Romanyà, J.; Pérez, M. Sweet Potato Is Not Simply an Abundant Food Crop: A Comprehensive Review of Its Phytochemical Constituents, Biological Activities, and the Effects of Processing. Antioxidants 2022, 11, 1648. [Google Scholar] [CrossRef]
- Ishiguro, K.; Yoshinaga, M.; Kai, Y.; Maoka, T.; Yoshimoto, M. Composition, content and antioxidative activity of the carotenoids in yellow-fleshed sweetpotato (Ipomoea batatas L.). Breed. Sci. 2010, 60, 324–329. [Google Scholar] [CrossRef]
- Lauriea, S.M.; Faber, M.; van Jaarsveldb, P.J.; Lauriea, R.N.; du Plooya, C.P.; Modisanea, P.C. β-Carotene yield and productivity of orange-fleshed sweet potato (Ipomoea batatas L. Lam.) as influenced by irrigation and fertilizer application treatments. Sci. Hortic. 2012, 142, 180–184. [Google Scholar] [CrossRef]
- Drapal, M.; Fraser, P.D. Determination of carotenoids in sweet potato (Ipomoea batatas L., Lam) tubers: Implications for accurate provitamin A determination in staple sturdy tuber crops. Phytochemistry 2019, 167, 112102. [Google Scholar] [CrossRef] [PubMed]
- Teow, C.C.; Truong, V.D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Lu J-w Chen, F.; Xu Y-s Wan, Y.-f.; Liu, D.-b. Sweet potato response to potassium. Better Crops Int. 2001, 15, 10–12. [Google Scholar]
- Su, L.; Yin, J.J.; Charles, D.; Zhou, X.R. Antioxidant properties, phenolic profiles and anthocyanin contents of purple sweet potato (Ipomoea batatas L.) as affected by hot-air drying and two different storage modes. Food Chem. 2019, 294, 18–27. [Google Scholar]
- Kim, H.W.; Kim, J.B.; Cho, S.M.; Chung, M.N.; Lee, Y.M.; Chu, S.M.; Che, J.H.; Kim, S.N.; Kim, S.Y.; Cho, Y.S.; et al. Anthocyanin changes in the Korean purple-fleshed sweet potato, Shinzami, as affected by steaming and baking. Food Chem. 2012, 130, 966–972. [Google Scholar] [CrossRef]
- Peng, J.; Wang, K.; Ma, C.; Long, J.; Tu, K.; Pan, L. Determination of anthocyanin and moisture content of purple sweet potatoes during drying process by their optical properties in the 400–1050 nm range. Food Chem. 2021, 359, 129811. [Google Scholar] [CrossRef] [PubMed]
- Im, Y.R.; Kim, I.; Lee, J. Phenolic Composition and Antioxidant Activity of Purple Sweet Potato (Ipomoea batatas (L.) Lam.): Varietal Comparisons and Physical Distribution. Antioxidants 2021, 10, 462. [Google Scholar] [CrossRef]
- Zheng, S.H.; Yamazaki, N.; Nakamoto, H.; Yoshikado, T.; Arima, S. Relationship between polyphenol content of tubers and cultivating conditions in sweet potato (Iponomea batatas). Coast. Bioenviron.-Saga Univ. (Jpn.) 2008, 37–42, ISSN: 1348-7175. [Google Scholar]
- Tang, W.; Zhang, Y.G.; Liu, Y.J.; Wang, X.; Kou, M.; Yan, H.; Ma, D.F.; Li, Q. Quantifying cultivation technique and growth dynamics of purple-fleshed sweetpotato (Ipomoea batatas L.) in China. Field Crops Res. 2018, 227, 41–48. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).