Investigating Cellulose Nanocrystals’ Biocompatibility and Their Effects on Pseudomonas syringae pv. tomato Epiphytic Survival for Sustainable Crop Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Plant Growth Condition
2.2. CNC and Plant Compatibility
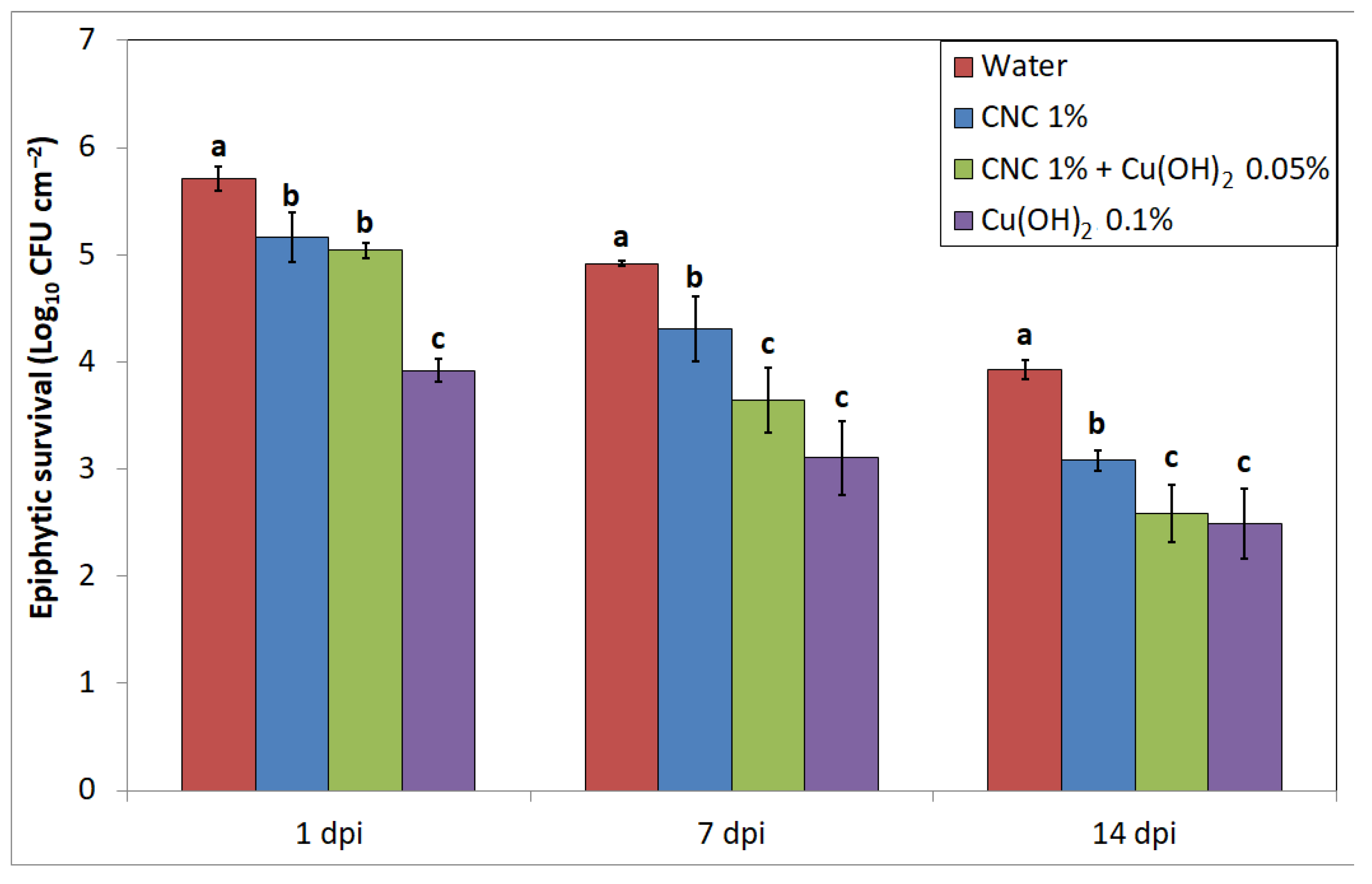
2.3. Effect of CNC on Pst Epiphytic Survival
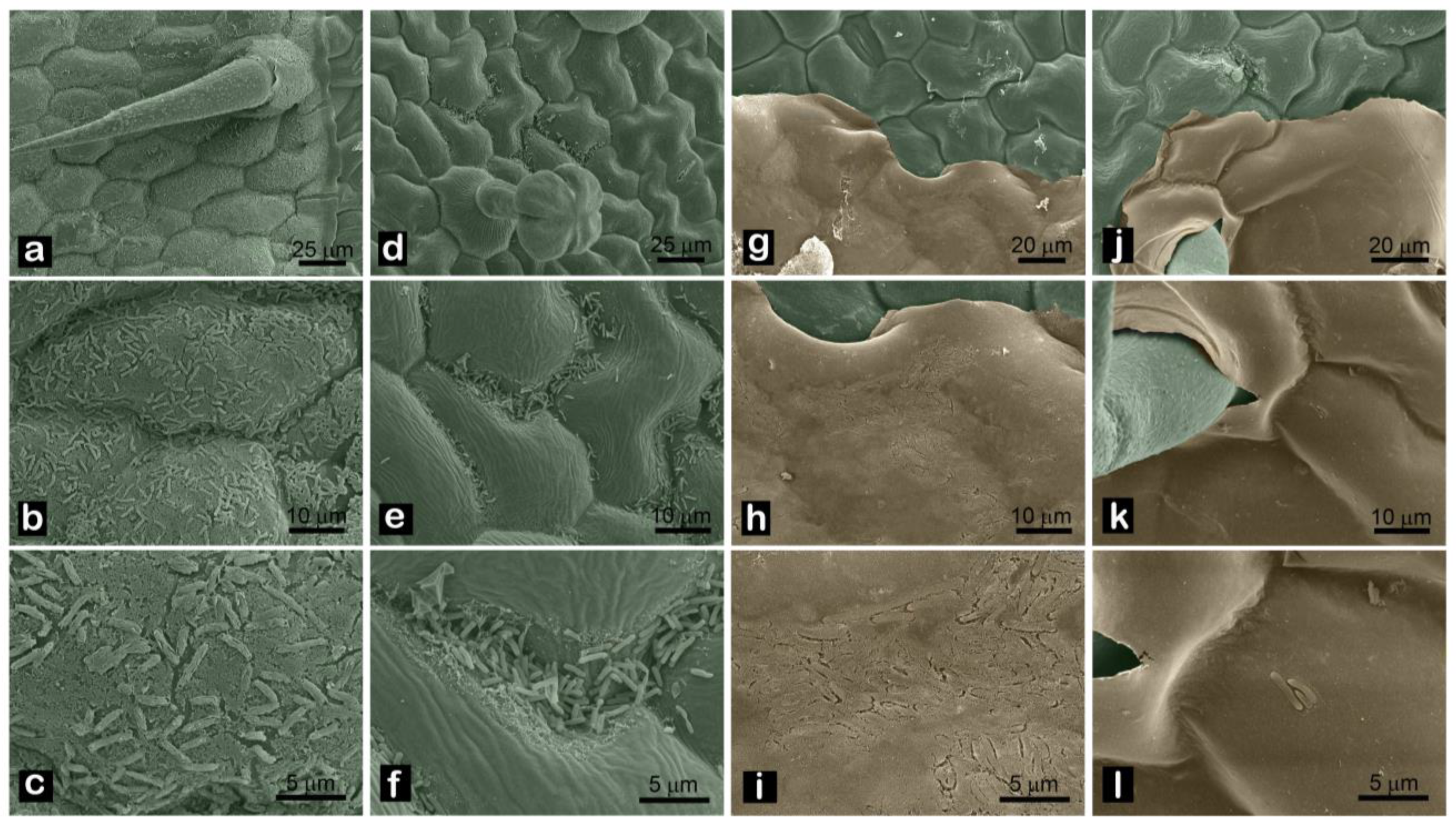
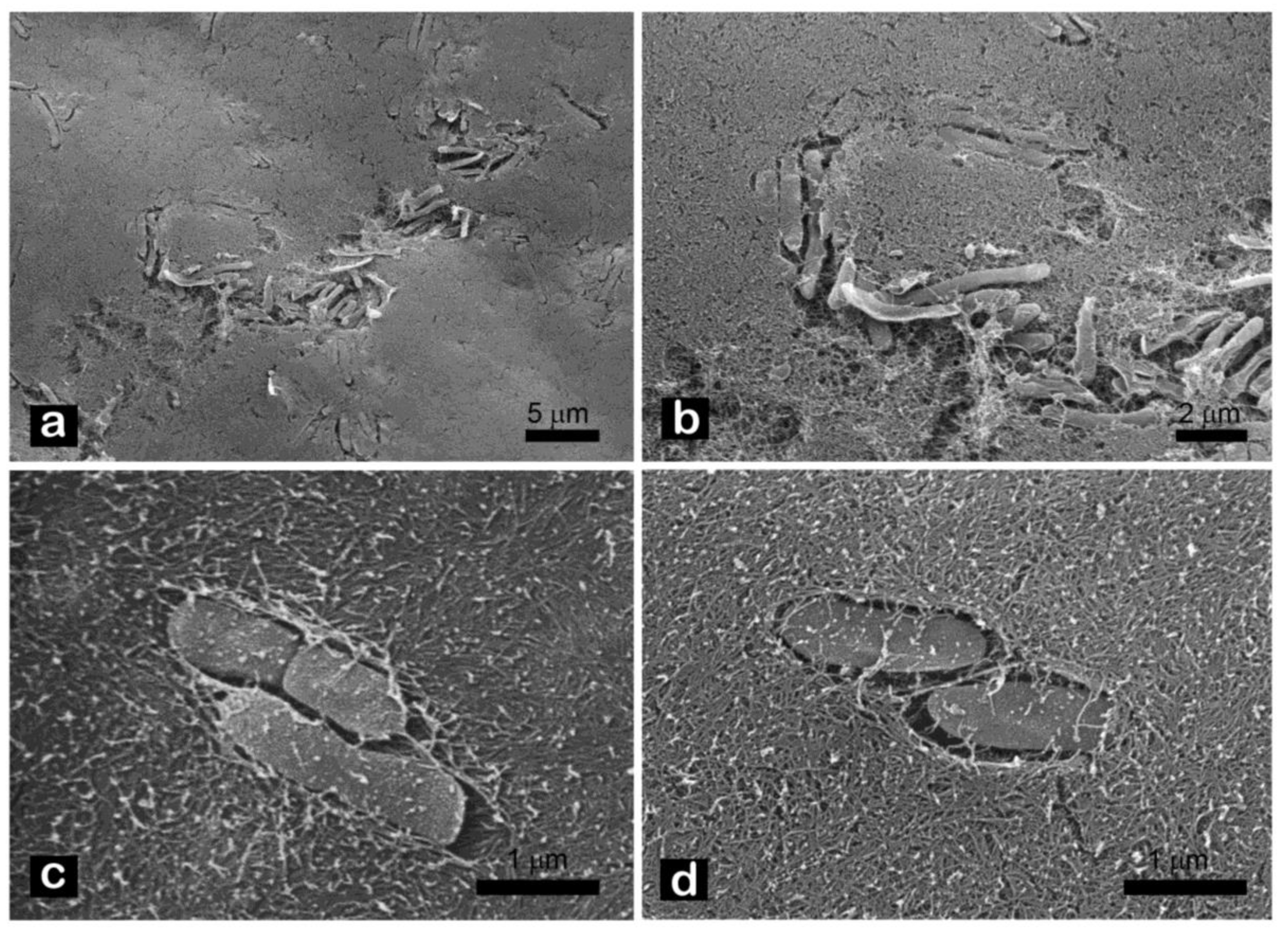
2.4. SEM Observation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- Abrahamian, P.; Jones, J.B.; Vallad, G.E. Efficacy of copper and copper alternatives for management of bacterial spot on tomato under transplant and field production. Crop. Prot. 2019, 126, 104919. [Google Scholar] [CrossRef]
- Keller, A.A.; Adeleye, A.S.; Conway, J.R.; Garner, K.L.; Zhao, L.; Cherr, G.N.; Hong, J.; Gardea-Torresdey, J.L.; Godwin, H.A.; Hanna, S. Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 2017, 7, 28–40. [Google Scholar] [CrossRef]
- Flemming, C.A.; Trevors, J.T. Copper toxicity and chemistry in the environment: A review. Water. Air. Soil Pollut. 1989, 44, 143–158. [Google Scholar] [CrossRef]
- Rippa, M.; Battaglia, V.; Cermola, M.; Sicignano, M.; Lahoz, E.; Mormile, P. Monitoring of the copper persistence on plant leaves using pulsed thermography. Environ. Monit. Assess. 2022, 194, 160. [Google Scholar] [CrossRef] [PubMed]
- Timilsina, S.; Liao, Y.Y.; Young, M.; Rosskopf, E.N.; Vallad, G.E.; Goss, E.M.; Santra, S.; Jones, J.B.; Hong, J.C.; Paret, M.L. Simulated Leaching of Foliar Applied Copper Bactericides on the Soil Microbiome Utilizing Various Beta Diversity Resemblance Measurements. Microbiol. Spectr. 2022, 10, e0148121. [Google Scholar] [CrossRef]
- Cooksey, D.A.; Azad, H.R.; Cha, J.S.; Lim, C.K. Copper resistance gene homologs in pathogenic and saprophytic bacterial species from tomato. Appl. Environ. Microbiol. 1990, 56, 431–435. [Google Scholar] [CrossRef]
- Griffin, K.; Gambley, C.; Brown, P.; Li, Y. Copper-tolerance in Pseudomonas syringae pv. tomato and Xanthomonas spp. and the control of diseases associated with these pathogens in tomato and pepper. A systematic literature review. Crop. Prot. 2017, 96, 144–150. [Google Scholar] [CrossRef]
- Bender, C.L.; Cooksey, D.A. Indigenous plasmids in Pseudomonas syringae pv. tomato: Conjugative transfer and role in copper resistance. J. Bacteriol. 1986, 165, 534–541. [Google Scholar] [CrossRef]
- Tamm, L.; Thuerig, B.; Apostolov, S.; Blogg, H.; Borgo, E.; Corneo, P.E.; Fittje, S.; De Palma, M.; Donko, A.; Experton, C.; et al. Use of Copper-Based Fungicides in Organic Agriculture in Twelve European Countries. Agronomy 2022, 12, 673. [Google Scholar] [CrossRef]
- Hofmann, T.; Lowry, G.V.; Ghoshal, S.; Tufenkji, N.; Brambilla, D.; Dutcher, J.R.; Gilbertson, L.M.; Giraldo, J.P.; Kinsella, J.M.; Landry, M.P.; et al. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food 2020, 1, 416–425. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Balestra, G.M.; Fortunati, E. Nanotechnology-Based Sustainable Alternatives for the Managements of Plant Diseases; Balestra, G.M., Fortunati, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-823394-8. [Google Scholar]
- Fiol, D.F.; Terrile, M.C.; Frik, J.; Mesas, F.A.; Álvarez, V.A.; Casalongué, C.A. Nanotechnology in plants: Recent advances and challenges. J. Chem. Technol. Biotechnol. 2021, 96, 2095–2108. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Poulose, A.; Parameswaranpillai, J.; George, J.J.; Gopi, J.A.; Krishnasamy, S.; Dominic, C.D.M.; Hameed, N.; Salim, N.V.; Radoor, S.; Sienkiewicz, N. Nanocellulose: A Fundamental Material for Science and Technology Applications. Molecules 2022, 27, 8032. [Google Scholar] [CrossRef]
- Barhoum, A.; Rastogi, V.K.; Mahur, B.K.; Rastogi, A.; Abdel-Haleem, F.M.; Samyn, P. Nanocelluloses as new generation materials: Natural resources, structure-related properties, engineering nanostructures, and technical challenges. Mater. Today Chem. 2022, 26, 101247. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Puglia, D.; Dominici, F.; Santulli, C.; Kenny, J.M.; Torre, L. Investigation of thermo-mechanical, chemical and degradative properties of PLA-limonene films reinforced with cellulose nanocrystals extracted from Phormium tenax leaves. Eur. Polym. J. 2014, 56, 77–91. [Google Scholar] [CrossRef]
- Schiavi, D.; Di Lorenzo, V.; Francesconi, S.; Giovagnoli, S.; Camaioni, E.; Balestra, G.M. Waste valorization by nanotechnology approaches for sustainable crop protection: A mini review. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1265, 012009. [Google Scholar] [CrossRef]
- De Lima, P.H.C.; Antunes, D.R.; de Lima Forini, M.M.; da Silva Pontes, M.; Mattos, B.D.; Grillo, R. Recent Advances on Lignocellulosic-Based Nanopesticides for Agricultural Applications. Front. Nanotechnol. 2021, 3, 809329. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Jiménez, A.; Gopakumar, D.A.; Puglia, D.; Thomas, S.; Kenny, J.M.; Chiralt, A.; Torre, L. Revalorization of sunflower stalks as novel sources of cellulose nanofibrils and nanocrystals and their effect on wheat gluten bionanocomposite properties. Carbohydr. Polym. 2016, 149, 357–368. [Google Scholar] [CrossRef]
- Hemmati, F.; Jafari, S.M.; Kashaninejad, M.; Barani Motlagh, M. Synthesis and characterization of cellulose nanocrystals derived from walnut shell agricultural residues. Int. J. Biol. Macromol. 2018, 120, 1216–1224. [Google Scholar] [CrossRef]
- García, A.; Gandini, A.; Labidi, J.; Belgacem, N.; Bras, J. Industrial and crop wastes: A new source for nanocellulose biorefinery. Ind. Crops Prod. 2016, 93, 26–38. [Google Scholar] [CrossRef]
- Mateo, S.; Peinado, S.; Morillas-Gutiérrez, F.; La Rubia, M.D.; Moya, A.J. Nanocellulose from agricultural wastes: Products and applications—A review. Processes 2021, 9, 1594. [Google Scholar] [CrossRef]
- Schiavi, D.; Ronchetti, R.; Di Lorenzo, V.; Vivani, R.; Giovagnoli, S.; Camaioni, E.; Balestra, G.M. Sustainable Protocols for Cellulose Nanocrystals Synthesis from Tomato Waste and Their Antimicrobial Properties against Pseudomonas syringae pv. tomato. Plants 2023, 12, 939. [Google Scholar] [CrossRef] [PubMed]
- Bergougnoux, V. The history of tomato: From domestication to biopharming. Biotechnol. Adv. 2014, 32, 170–189. [Google Scholar] [CrossRef]
- Xin, X.F.; He, S.Y. Pseudomonas syringae pv. tomato DC3000: A model pathogen for probing disease susceptibility and hormone signaling in plants. Annu. Rev. Phytopathol. 2013, 51, 473–498. [Google Scholar] [CrossRef]
- Preston, G.M. Pseudomonas syringae pv. tomato: The right pathogen, of the right plant, at the right time. Mol. Plant Pathol. 2000, 1, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Cazorla, F.M.; Arrebola, E.; Sesma, A.; Pérez-García, A.; Codina, J.C.; Murillo, J.; De Vicente, A. Copper resistance in Pseudomonas syringae strains isolated from mango is encoded mainly by plasmids. Phytopathology 2002, 92, 909–916. [Google Scholar] [CrossRef]
- Shenge, K.C.; Wydra, K.; Mabagala, R.B.; Mortensen, C.N. Assessment of strains of Pseudomonas syringae pv. tomato from Tanzania for resistance to copper and streptomycin. Arch. Phytopathol. Plant Prot. 2008, 41, 572–585. [Google Scholar] [CrossRef]
- McLeod, A.; Masimba, T.; Jensen, T.; Serfontein, K.; Coertze, S. Evaluating spray programs for managing copper resistant Pseudomonas syringae pv. tomato populations on tomato in the Limpopo region of South Africa. Crop Prot. 2017, 102, 32–42. [Google Scholar] [CrossRef]
- Moretti, C.; Bocchini, M.; Quaglia, M.; Businelli, D.; Orfei, B.; Buonaurio, R. Sodium Selenate: An Environmental-Friendly Means to Control Tomato Bacterial Speck Disease. Agronomy 2022, 12, 1351. [Google Scholar] [CrossRef]
- Mansilla, A.Y.; Albertengo, L.; Rodríguez, M.S.; Debbaudt, A.; Zúñiga, A.; Casalongué, C.A. Evidence on antimicrobial properties and mode of action of a chitosan obtained from crustacean exoskeletons on Pseudomonas syringae pv. tomato DC3000. Appl. Microbiol. Biotechnol. 2013, 97, 6957–6966. [Google Scholar] [CrossRef]
- Quattrucci, A.; Ovidi, E.; Tiezzi, A.; Vinciguerra, V.; Balestra, G.M. Biological control of tomato bacterial speck using Punica granatum fruit peel extract. Crop Prot. 2013, 46, 18–22. [Google Scholar] [CrossRef]
- Fanelli, V.; Cariddi, C.; Finetti-Sialer, M. Selective detection of Pseudomonas syringae pv. tomato using dot blot hybridization and real-time PCR. Plant Pathol. 2007, 56, 683–691. [Google Scholar] [CrossRef]
- Cuppels, D.A.; Louws, F.J.; Ainsworth, T. Development and evaluation of PCR-based diagnostic assays for the bacterial speck and bacterial spot pathogens of tomato. Plant Dis. 2006, 90, 451–458. [Google Scholar] [CrossRef]
- Schiavi, D.; Balbi, R.; Giovagnoli, S.; Camaioni, E.; Botticella, E.; Sestili, F.; Balestra, G.M. A green nanostructured pesticide to control tomato bacterial speck disease. Nanomaterials 2021, 11, 1852. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Cui, J. Graphene oxide loaded with copper oxide nanoparticles as an antibacterial agent against: Pseudomonas syringae pv. tomato. RSC Adv. 2017, 7, 38853–38860. [Google Scholar] [CrossRef]
- Elsharkawy, M.; Derbalah, A.; Hamza, A.; El-Shaer, A. Zinc oxide nanostructures as a control strategy of bacterial speck of tomato caused by Pseudomonas syringae in Egypt. Environ. Sci. Pollut. Res. 2020, 27, 19049–19057. [Google Scholar] [CrossRef] [PubMed]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [CrossRef]
- Orzali, L.; Valente, M.T.; Scala, V.; Loreti, S. Antibacterial Activity of Essential Oils and Trametes versicolor Extract against Clavibacter michiganensis subsp. michiganensis and Ralstonia solanacearum for Seed Treatment and Development of a Rapid In Vivo Assay. Antibiotics 2020, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated Digital Image Analysis for Rapid and Accurate Measurement of Leaf Area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Ben Abdallah, F.; Philippe, W.; Goffart, J.P. Comparison of optical indicators for potato crop nitrogen status assessment including novel approaches based on leaf fluorescence and flavonoid content. J. Plant Nutr. 2018, 41, 2705–2728. [Google Scholar] [CrossRef]
- Canzoniere, P.; Francesconi, S.; Giovando, S.; Balestra, G.M. Antibacterial activity of tannins towards Pseudomonas syringae pv. tomato, and their potential as biostimulants on tomato plants. Phytopathol. Mediterr. 2021, 60, 23–36. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Lessons learned: Are engineered nanomaterials toxic to terrestrial plants? Sci. Total Environ. 2016, 568, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Siddiqui, M.H., Mohammad, F., Al-Whaibi, M.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 97833-19145020. [Google Scholar]
- Ruttkay-Nedecky, B.; Krystofova, O.; Nejdl, L.; Adam, V. Nanoparticles based on essential metals and their phytotoxicity. J. Nanobiotechnol. 2017, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Guo, H. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Physiol. Biochem. 2017, 110, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Roman, M. Toxicity of cellulose nanocrystals: A review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Coelho, C.C.S.; Michelin, M.; Cerqueira, M.A.; Gonçalves, C.; Tonon, R.V.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.; Cabral, L.M.C.; Teixeira, J.A. Cellulose nanocrystals from grape pomace: Production, properties and cytotoxicity assessment. Carbohydr. Polym. 2018, 192, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, D.; Francesconi, S.; Taddei, A.R.; Fortunati, E.; Balestra, G.M. Exploring cellulose nanocrystals obtained from olive tree wastes as sustainable crop protection tool against bacterial diseases. Sci. Rep. 2022, 12, 6149. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, D.; Ronchetti, R.; Di Lorenzo, V.; Salustri, M.; Petrucci, C.; Vivani, R.; Giovagnoli, S.; Camaioni, E.; Balestra, G.M. Circular Hazelnut Protection by Lignocellulosic Waste Valorization for Nanopesticides Development. Appl. Sci. 2022, 12, 2604. [Google Scholar] [CrossRef]
- Fortunati, E.; Rescignano, N.; Botticella, E.; La Fiandra, D.; Renzi, M.; Mazzaglia, A.; Torre, L.; Kenny, J.M.; Balestra, G.M. Effect of poly(DL-lactide-co-glycolide) nanoparticles or cellulose nanocrystals-based formulations on pseudomonas syringae pv. tomato (Pst) and tomato plant development. J. Plant Dis. Prot. 2016, 123, 301–310. [Google Scholar] [CrossRef]
- Bracke, J.; Elsen, A.; Adriaenssens, S.; Schoeters, L.; Vandendriessche, H.; Van Labeke, M.C. Application of proximal optical sensors to fine-tune nitrogen fertilization: Opportunities for woody ornamentals. Agronomy 2019, 9, 408. [Google Scholar] [CrossRef]
- Guttenplan, S.B.; Kearns, D.B. Regulation of flagellar motility during biofilm formation. FEMS Microbiol. Rev. 2013, 37, 849–871. [Google Scholar] [CrossRef]
- Babelegoto, N.; Varvaro, L.; Cirulli, M. Epiphytic, and endophytic multiplication of Pseudomonas syringae pv. tomato (Okabel) Young et al. in susceptible and resistant tomato leaves. Phytopathol. Mediterr. 1988, 27, 138–144. [Google Scholar]
- Balestra, G.M.; Antonelli, M.; Fabi, A.; Varvaro, L. Effectiveness of natural products for in vitro and in vivo control of epiphytic populations of Pseudomonas syringae pv. tomato on tomato plants. J. Plant Pathol. 1999, 80, 251. [Google Scholar]
- D’Orazio, G.; Munizza, L.; Zampolli, J.; Forcella, M.; Zoia, L.; Fusi, P.; Di Gennaro, P.; La Ferla, B. Cellulose nanocrystals are effective in inhibiting host cell bacterial adhesion. J. Mater. Chem. B 2017, 5, 7018–7020. [Google Scholar] [CrossRef]
- Noronha, V.T.; Camargos, C.H.M.; Jackson, J.C.; Souza Filho, A.G.; Paula, A.J.; Rezende, C.A.; Faria, A.F. Physical membrane-stress-mediated antimicrobial properties of cellulose nanocrystals. ACS Sustain. Chem. Eng. 2021, 9, 3203–3212. [Google Scholar] [CrossRef]
- Sakata, N.; Shiraishi, N.; Saito, H.; Komoto, H.; Ishiga, T.; Usuki, G.; Yamashita, Y.; Ishiga, Y. Covering cabbage leaves with cellulose nanofiber confers resistance against Pseudomonas cannabina pv. alisalensis. J. Gen. Plant Pathol. 2023, 89, 53–60. [Google Scholar] [CrossRef]
- Silva, F.; Gracia, N.; McDonagh, B.H.; Domingues, F.C.; Nerín, C.; Chinga-Carrasco, G. Antimicrobial activity of biocomposite films containing cellulose nanofibrils and ethyl lauroyl arginate. J. Mater. Sci. 2019, 54, 12159–12170. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Application of Copper-Chitosan Nanoparticles Stimulate Growth and Induce Resistance in Finger Millet (Eleusine coracana Gaertn.) Plants against Blast Disease. J. Agric. Food Chem. 2018, 66, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Chauhan, D.; Sankararamakrishnan, N. Copper chitosan nanocomposite: Synthesis, characterization, and application in removal of organophosphorous pesticide from agricultural runoff. Environ. Sci. Pollut. Res. 2012, 19, 2055–2062. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Strayer-Scherer, A.L.; White, J.; Mukherjee, A.; De La Torre-Roche, R.; Ritchie, L.; Colee, J.; Vallad, G.E.; Freeman, J.H.; Jones, J.B.; et al. Nano-magnesium oxide: A novel bactericide against copper-tolerant xanthomonas perforans causing tomato bacterial spot. Phytopathology 2019, 109, 52–62. [Google Scholar] [CrossRef]
- Carvalho, R.; Duman, K.; Jones, J.B.; Paret, M.L. Bactericidal Activity of Copper-Zinc Hybrid Nanoparticles on Copper-Tolerant Xanthomonas perforans. Sci. Rep. 2019, 9, 20124. [Google Scholar] [CrossRef]
- Doan, H.K.; Ngassam, V.N.; Gilmore, S.F.; Tecon, R.; Parikh, A.N.; Leveau, J.H.J. Topography-Driven Shape, Spread, and Retention of Leaf Surface Water Impacts Microbial Dispersion and Activity in the Phyllosphere. Phytobiomes 2020, 4, 268–280. [Google Scholar] [CrossRef]
- Lindow, S.E.; Brandl, M.T. Microbiology of the Phyllosphere. Appl. Environ. Microbiol. 2003, 69, 1875–1883. [Google Scholar] [CrossRef]
- Xin, X.F.; Kvitko, B.; He, S.Y. Pseudomonas syringae: What it takes to be a pathogen. Nat. Rev. Microbiol. 2018, 16, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Rouse, D.I.; Nordheim, E.V.; Hirano, S.S.; Upper, C.D. A Model Relating the Probability of Foliar Disease Incidence to the Population Frequencies of Bacterial Plant Pathogens. Phytopathology 1985, 75, 505. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Varvaro, L. Epiphytic Pseudomonas savastanoi pv. savastanoi can infect and cause olive knot disease on Olea europaea subsp. cuspidata. Australas. Plant Pathol. 2013, 42, 219–225. [Google Scholar] [CrossRef]
- De Azevedo Souza, C.; Li, S.; Lin, A.Z.; Boutrot, F.; Grossmann, G.; Zipfel, C.; Somerville, S.C. Cellulose-derived oligomers act as damage-associated molecular patterns and trigger defense-like responses. Plant Physiol. 2017, 173, 2383–2398. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavi, D.; Taddei, A.R.; Balestra, G.M. Investigating Cellulose Nanocrystals’ Biocompatibility and Their Effects on Pseudomonas syringae pv. tomato Epiphytic Survival for Sustainable Crop Protection. Horticulturae 2023, 9, 525. https://doi.org/10.3390/horticulturae9050525
Schiavi D, Taddei AR, Balestra GM. Investigating Cellulose Nanocrystals’ Biocompatibility and Their Effects on Pseudomonas syringae pv. tomato Epiphytic Survival for Sustainable Crop Protection. Horticulturae. 2023; 9(5):525. https://doi.org/10.3390/horticulturae9050525
Chicago/Turabian StyleSchiavi, Daniele, Anna Rita Taddei, and Giorgio Mariano Balestra. 2023. "Investigating Cellulose Nanocrystals’ Biocompatibility and Their Effects on Pseudomonas syringae pv. tomato Epiphytic Survival for Sustainable Crop Protection" Horticulturae 9, no. 5: 525. https://doi.org/10.3390/horticulturae9050525
APA StyleSchiavi, D., Taddei, A. R., & Balestra, G. M. (2023). Investigating Cellulose Nanocrystals’ Biocompatibility and Their Effects on Pseudomonas syringae pv. tomato Epiphytic Survival for Sustainable Crop Protection. Horticulturae, 9(5), 525. https://doi.org/10.3390/horticulturae9050525







