Abstract
Potassium (K) is the most important element for fruit quality improvement. This study aimed at determining the best K fertilizer type that can promote grape growth and nutrient uptake. Specifically, four types of K fertilizers (complex fertilizer, potassium nitrate, potassium sulfate, and potassium dihydrogen phosphate) were applied to grapevines grown in plastic pots, and then their effects on grape growth and nutrient uptake were explored. Results showed that the complex fertilizer and potassium nitrate treatments increased the biomass of the grapevine plants, whereas the other fertilizers had no significant effects on the biomass. Only the potassium nitrate treatment increased the contents of photosynthetic pigments in grapevine leaves. The complex fertilizer and potassium nitrate treatments increased the total N content in the grapevine plants to some extent, whereas the other fertilizer treatments decreased the total N content to some extent. It was also evident that all four K fertilizers increased the total P and K contents in the grapevine plants. Compared to the control, the complex fertilizer, potassium nitrate, potassium sulfate, and potassium dihydrogen phosphate treatments increased the scion total P content by 20.18%, 9.77%, 12.52%, and 30.81%, respectively, and increased the scion total K content by 15.37%, 8.41%, 20.15%, and 26.48%, respectively. In addition, correlation and grey relational analyses showed that the rootstock stem total N content, rootstock root biomass, and soil alkali-hydrolyzable N concentration were the top three indicators most closely associated with the scion total N content, whereas the rootstock root total P content, soil available P concentration, and rootstock stem total P content were the top three indicators most closely associated with the scion total P content. Additionally, the rootstock root total K content, soil available K concentration, and rootstock root total P content were the top three indicators most closely associated with the scion total K content. Overall, the different K fertilizers can all promote the uptake of P and K by grapevine plants, and the potassium dihydrogen phosphate fertilizer is the best choice.
1. Introduction
Fertilizer application is an important horticultural cultivation practice because it improves soil fertility, promotes the growth of horticulture crops, and improves fruit quality [1]. Studies have shown that reasonable fertilization measures during the process of fruit production are directly associated with the yield and quality of fruits [2,3]. Among the nitrogen (N), phosphorus (P), and potassium (K) macro elements, K was shown to be the most important element for fruit quality improvement [4] and was previously found to be the “quality” element [5,6]. Thus, K fertilizer treatment has become a critical cultivation practice for fruit production.
Plants take up K from the soil mainly through their roots [7], and also through mycorrhizal symbiosis of the roots [8]. The K content in plants varies from species to species, the same plant at different stages of growth, and in different organ tissues [9]. Accumulating evidence has revealed that K is involved in various physiological metabolic roles through out the plant life cycle [1,6,10], including promoting the activities of numerous enzymes [11], participating in osmoregulation [12,13], maintaining the balance of anions and cations [14,15], enhancing plant photosynthesis and assimilating product transport [16,17], metabolizing reactive oxygen species [18], improving the plant’s disease resistance and stress tolerance [19], and improving resistance to external abiotic stresses [20]. Consequently, all of these factors improve the fruit yield and the intrinsic and extrinsic fruit quality [6,21,22]. For the photosynthetic physiology of crops, for example, K can increase the carbon dioxide uptake by crop leaves by promoting the opening of the stomata, and increase the amount of chlorophyll, thus increasing the activity of photosynthesis-related enzymes, which, in turn, improves the photosynthesis of crops [23]. Application of K fertilizer can increase the chlorophyll content in rice, maize, wheat, and grape [24,25,26,27]. Previous studies have demonstrated that the interaction of different nutrients with K during the fertilization and nutrient uptake by fruit plants is vital for nutrient balance [28,29]. Notably, the uptake of K depends on the nutritional status of N, especially the form of N supply. For example, NH4+ distributes K to leaves more easily than NO3−, but K translocation across the membrane is also competitively inhibited by NH4+ [30]. In addition, excess N has an antagonistic effect on the accumulation of P and K [31], and P translocation is limited by the supply of K [32]. Studies have also reported that a suitable K fertilizer can improve the utilization of P fertilizer, whereas higher levels of K application increase the N uptake and inhibit the P uptake [33,34]. Moreover, the excessive supply of P leads to the competitive inhibition of Ca2+, which, in turn, inhibits the K uptake, ultimately leading to a decrease in the quality of fruits [34]. Therefore, choosing suitable fertilizer types and the optimal timing of K fertilizer application are important to the quality of fruits.
Grape (Vitis vinifera L.), a perennial vine fruit plant, produces berry fruits that have rich nutritional value [35]. Grapes were one of the four earliest cultivated and most widely distributed fruits [36]. The berry fruits of grape are critical K sinks, and the amount of K fertilizer required increases with the grape’s growth and development [37]. Therefore, the application of appropriate amounts of K fertilizer during the growth and development of grape berry fruits can increase the yield and quality of the fruits [38]. However, given the wide variety of K fertilizers available in agricultural production, the most suitable K fertilizer for grape production has not yet been reported [39,40].
In this experiment, four types of K fertilizers were applied to grapevine plants, and their effects on the growth of and nutrient uptake by the grapevines were explored. The aim of this study was to determine the best K fertilizer type that would promote grape growth and nutrient uptake.
2. Materials and Methods
2.1. Materials
This study used the ‘Shine Muscat’ variety of grape (a hybrid from Japan, with female parent ‘Akitsu-21’ and male parent ‘Hakunan’) for the grafted grapevines. The rootstock used was that of the ‘Beta’ grape (a hybrid from the United States of America, with female parent ‘Carver’ and male parent ‘Concord’), with the rootstock stem measuring about 10 cm in height. The annual grafted grapevines were collected, at a height of 30 cm, from the Chongzhou Modern Agriculture Research and Development Base of Sichuan Agricultural University, Chongzhou, Chengdu, Sichuan, China in February 2021.
2.2. Experimental Design
The experiment was conducted in a greenhouse at the Chengdu Campus of Sichuan Agricultural University, within a subtropical humid climate. Soil samples (of fluvo-aquic soil) were collected from farmlands around the Chengdu Campus of Sichuan Agricultural University, Chengdu, Sichuan, China. The soil had the following basic physicochemical properties: pH value (6.39), alkaline hydrolysis N concentration (95.58 mg/kg), available P concentration (90.23 mg/kg), and available K concentration (120.22 mg/kg). First, soil samples were crushed, and 10 kg of the soil was put into each plastic pot (height × diameter = 25 cm × 30 cm). Thereafter, grapevines were transplanted into the pots in March 2021, with each pot containing one plant. The pots were watered daily to ensure that the soil moisture content was maintained at 80% of the field capacity. When the plants grew to 100 cm in height (April 2021), the different potassium fertilizers were applied to the soil. The complex fertilizer was produced by Lomon Land Agriculture Co., Ltd. (Deyang, China), and contained a total nutrient content of ≥45%, with N:P2O5:K2O = 15:15:15. The potassium nitrate was produced by Huichuang Agricultural Development (Yunnan) Co., Ltd. (Kunming, China), and contained a total nutrient content of ≥59.5%, with N:K2O = 13.5:46.0. The potassium sulfate was produced by Sinochem Fertilizer Co., Ltd. (Chengdu, China), and contained K2O ≥ 52%. The potassium dihydrogen phosphate was produced by Sichuan Jiuhe Chemistry Co., Ltd. (Yibin, China), contained K2O ≥ 34% and P2O5 ≥ 52%. There were five treatments used in this experiment: no fertilizer application (control), application of complex fertilizer, application of potassium nitrate, application of potassium sulfate, and application of potassium dihydrogen phosphate. The different potassium fertilizers were applied at a level of 0.6 g K2O per kilogram of soil (6 g K2O per pot) [41,42], and the converted fertilizer applications in total were as follows: complex fertilizer, 40.00 g; potassium nitrate, 13.04 g; potassium sulfate, 11.54 g; and potassium dihydrogen phosphate, 17.65 g. Each treatment was replicated three times (nine pots per treatment). Each fertilizer was divided into three equal portions, which were applied at an interval of seven days. During each application, the fertilizer was dissolved in 250 mL tap water and then used to irrigate the pot. The whole plants were harvested, and the soil in the pots was collected two months after the initial fertilizer irrigation in order to determine the indicators.
2.3. Determination of Indicators
In June 2021, the mature leaves (one month old) at the middle of the shoots were collected in order to determine the contents of photosynthetic pigments (chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid). The photosynthetic pigment content was extracted using the acetone-ethanol extraction method, and a spectrophotometer (Summit, Shanghai, China) was used to determine absorbency at 663, 645, and 470 nm wavelengths; the contents of chlorophyll a, chlorophyll b, and carotenoid were then calculated according to Li et al. (2022) [43] and Hao et al. (2004) [44]. The total chlorophyll content was calculated as chlorophyll a content + chlorophyll b content [43]. Subsequently, all of the plants were divided into rootstock roots, rootstock stems, and scions, and then subjected to heating at 110 °C for 15 min, followed by drying at 80 °C to constant weight. The biomass (dry weight) of each of the plant sample parts was weighed using an electronic balance, and then the samples were finely ground. In order to determine the total N, P, and K contents, the dried ground samples were digested in H2SO4/H2O2 (5:1, v/v) solution. The total N content was determined by the Kjeldahl method, the total P content was determined by molybdenum-antimony anti-colorimetry, and the total K content was determined by flame photometry, according to Bao (2000) [45]. On the other hand, soil samples were collected, air-dried, and passed through a 1.0 mm sieve for chemical analysis. The soil alkali-hydrolyzable N concentration was determined by the alkali diffusion method, the soil available P concentration was determined by molybdenum-antimony anti-colorimetry after the extraction of sodium bicarbonate, and the soil available K concentration was determined by flame photometry after the extraction of ammonium acetate, according to Bao (2000) [45]. Finally, the soil pH value was determined using a pH meter with a soil/water ratio of 1:2.5 [45].
2.4. Statistical Analyses
The SPSS 20.0.0 software (IBM, Chicago, IL, USA) was used to analyze the data. Data for three replications were normalized and subjected to a homogeneity test, followed by one-way analysis of variance (ANOVA) and Duncan’s Multiple Range Test (p < 0.05). Pearson’s correlation was used to compare the relationships among the different indicators. Grey relational analysis was applied in order to analyze the relationships of the scion N, P, and K content with other indicators, according to the method described by Lin et al. (2023) [46] and Zhang et al. (2022) [47].
3. Results
3.1. Biomass (Dry Weight) of Grapevine
The potassium nitrate treatment increased the rootstock root, rootstock stem, and scion biomasses of the grapevine plants by 32.64%, 19.69%, and 31.97%, respectively, compared to the control (Figure 1A–C). The complex fertilizer treatment increased the rootstock root and scion biomasses of the grapevine plants by 19.55% and 18.86%, respectively, compared to the control, but had no significant effect on the rootstock stem biomass. The potassium sulfate and potassium dihydrogen phosphate treatments had no significant effects on the biomasses of the above samples.
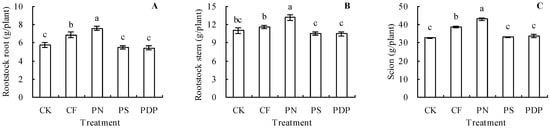
Figure 1.
Biomass (dry weight) of grapevine. (A) Rootstock root biomass; (B) rootstock stem biomass; (C) scion biomass. Different lowercase letters indicate significant differences among the treatments (Duncan’s Multiple Range Test, p < 0.05). CK—control; CF—complex fertilizer; PN—potassium nitrate; PS—potassium sulfate; PDP—potassium dihydrogen phosphate.
3.2. Photosynthetic Pigment Content in Grapevine Leaves
Results showed that the potassium nitrate treatment increased the chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid contents in grapevine leaves by 7.82%, 7.89%, 7.84%, and 8.24%, respectively, compared to the control (Table 1). All other treatments had no significant effects on the contents of these photosynthetic pigments in grapevine leaves.

Table 1.
Photosynthetic pigment content in grapevine leaves.
3.3. Total N Content in Grapevine
Compared to the control, the complex fertilizer treatment increased the rootstock root, rootstock stem, and scion total N content in the grapevine plants by 7.41%, 36.42%, and 18.41%, respectively (Figure 2A–C). The potassium nitrate treatment had no significant effects on the total N content in the rootstock roots or rootstock stems of the grapevine plants, but increased the scion total N content compared to the control. The potassium sulfate and potassium dihydrogen phosphate treatments decreased the total N content in various grapevine organs, to some extent, compared to the control.
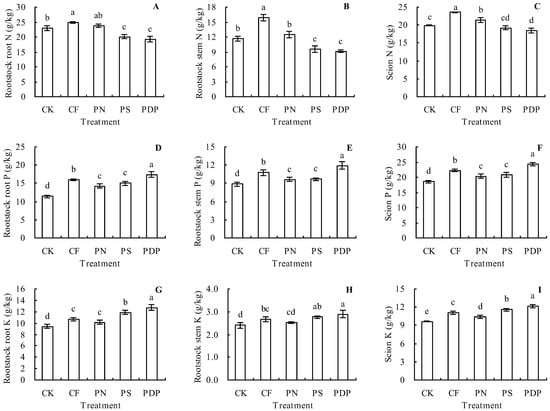
Figure 2.
Total nutrient (N, P, and K) contents in grapevine plants. (A) Rootstock root total N content; (B) rootstock stem total N content; (C) scion total N content; (D) rootstock root total P content; (E) rootstock stem total P content; (F) scion total P content; (G) rootstock root total K content; (H) rootstock stem total K content; (I) scion total K content. Different lowercase letters indicate significant differences among the treatments (Duncan’s Multiple Range Test, p < 0.05). CK—control; CF—complex fertilizer; PN—potassium nitrate; PS—potassium sulfate; PDP—potassium dihydrogen phosphate.
3.4. Total P Content in Grapevine
Results revealed that the four fertilizer treatments increased the total P content in various grapevine organs (Figure 2D–F). Specifically, the compound fertilizer, potassium nitrate, potassium sulfate, and potassium dihydrogen phosphate treatments increased the rootstock root total P content by 39.65%, 24.10%, 30.83%, and 52.31%, respectively, increased the rootstock stem total P content by 21.56%, 8.92%, 9.37%, and 34.65%, respectively, and increased the scion total P content by 20.18%, 9.77%, 12.52%, and 30.81%, respectively, compared to the control.
3.5. Total K Content in Grapevine
The four fertilizer treatments increased the total K content in the rootstock roots and scions of the grapevine plants (Figure 2G,I). Compared to the control, the complex fertilizer, potassium nitrate, potassium sulfate, and potassium dihydrogen phosphate treatments increased the rootstock root total K content by 12.82%, 7.52%, 25.53%, and 35.06%, respectively, and increased the scion total K content by 15.37%, 8.41%, 20.15%, and 26.48%, respectively. In addition, results showed that the complex fertilizer, potassium sulfate, and potassium dihydrogen phosphate treatments all increased the rootstock stem total K content compared to the control, whereas the potassium nitrate treatment had no significant effect (Figure 2H).
3.6. Soil pH Value and Available Nutrient Concentration
The complex fertilizer treatment decreased the soil pH value, whereas the potassium dihydrogen phosphate treatment increased the soil pH value, compared to the control. However, the potassium nitrate and potassium sulfate treatments had no significant effects on the soil pH value (Table 2). With regard to the soil alkali-hydrolyzable N concentration, the complex fertilizer and potassium nitrate treatments increased the soil alkali-hydrolyzable N concentration compared to the control, whereas the potassium sulfate and potassium dihydrogen phosphate treatments had no significant effects. Notably, the four fertilizer treatments increased the soil available P and available K concentrations.

Table 2.
Soil pH value and available nutrient concentration.
3.7. Correlation and Grey Relational Analyses
According to the correlation analysis, the rootstock root total N content exhibited a highly significant (p < 0.01) or significant (0.01 ≤ p < 0.05) positive correlation with the biomasses of the various organs, chlorophyll b content, total chlorophyll content, carotenoid content, and soil alkali-hydrolyzable N concentration, and exhibited a highly significant (p < 0.01) or significant (0.01 ≤ p < 0.05) negative correlation with the total K content in various organs, soil pH value, soil available P concentration, and soil available K concentration (Table 3). The rootstock stem total N content exhibited a highly significant (p < 0.01) or significant (0.01 ≤ p < 0.05) positive correlation with the biomasses of the various organs, total chlorophyll content, and soil alkali-hydrolyzable N concentration, and had a highly significant (p < 0.01) or significant (0.01 ≤ p < 0.05) negative correlation with the rootstock root total K content and soil pH value. The scion total N content exhibited a highly significant (p < 0.01) or significant (0.01 ≤ p < 0.05) positive correlation with the biomasses of the various organs, total chlorophyll content, carotenoid content, and soil alkali-hydrolyzable N concentration, and had a highly significant (p < 0.01) negative correlation with the soil pH value.

Table 3.
Correlations among the different indicators.
In addition, the total P content in various organs had a highly significant (p < 0.01) positive correlation with the total K content in various organs. The total P and K contents had a highly significant (p < 0.01) positive correlation with the soil available P concentration and soil available K concentration. The total N, P, and K contents in various organs had a highly significant (p < 0.01) positive correlation with the N, P, and K contents in other organs.
Moreover, grey relational analysis was used to further analyze the grey relationships of the scion total N, P, and K contents with all other indicators (Figure 3A–C). Results indicated that the scion total N content, scion total P content, or scion total K content had a grey correlation with all other indicators. The rootstock stem total N content, rootstock root biomass, and soil alkali-hydrolyzable N concentration were the top three indicators most closely associated with the scion total N content, since they had grey correlation coefficient values higher than 0.50 (Figure 3A). The rootstock root total P content, soil available P concentration, and rootstock stem total P content, with grey correlation coefficient values higher than 0.50, were the top three indicators most closely associated with the scion total P content (Figure 3B). Furthermore, the rootstock root total K content, soil available K concentration, and rootstock root total P content, with grey correlation coefficient values higher than 0.50, were the top three indicators most closely associated with the scion total K content (Figure 3C).
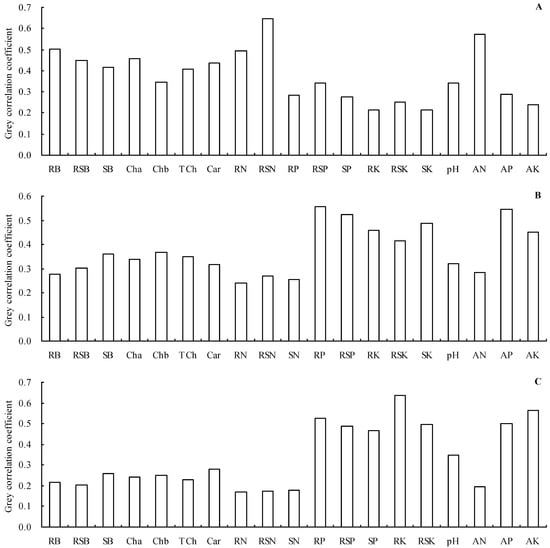
Figure 3.
Grey correlation coefficients. (A) Grey correlation coefficient of the biomass, photosynthetic pigment content, rootstock root total N content, rootstock stem total N content, total P content, and total K content with the scion total N content; (B) grey correlation coefficient of the biomass, photosynthetic pigment content, total N content, rootstock root total P content, rootstock total P content, and total K content with the scion total P content; (C) grey correlation coefficient of the biomass, photosynthetic pigment content, total N content, total P content, rootstock root total K content, and rootstock stem total K content with the scion total K content. RB—rootstock root biomass; RSB—rootstock stem biomass; SB—scion biomass; Cha—chlorophyll a content; Chb—chlorophyll b content; TCh—total chlorophyll content; Car—carotenoid content; RN—rootstock root total N content; RSN—rootstock stem total N content; SN—scion total N content; RP—rootstock root total P content; RSP—rootstock stem total P content; SP—scion total P content; RK—rootstock root total K content; RSK—rootstock stem total K content; SK—scion total K content; pH—soil pH value; AN—alkali-hydrolyzable N concentration; AP—available P concentration; AK—available K concentration.
4. Discussion
The application of different chemical fertilizers to soil can promote the growth, increase the yield, and improve the quality of crops [1,2,3]. The application of different K fertilizers has varying effects on plant growth [48], and these effects are associated with the concentration of K and the interaction between elements [29]. Applying potassium nitrate, potassium sulfate, or potassium chloride fertilizers promote potato growth and increase its biomass and tuber yield, with the highest tuber yield observed after the potassium sulfate application [49]. With regard to grapes, one study revealed that the reasonable application of potassium fertilizer improved their fruit yield and quality [50]. However, to date, the effects of applying different types of K fertilizers on grapes have not yet been reported. In the present study, the complex fertilizer and potassium nitrate treatments increased the biomasses of the rootstock roots, rootstock stems, and scions of grapevine plants, whereas the potassium sulfate and potassium dihydrogen phosphate treatments had no significant effects. These results suggest that different types of K fertilizers confer different effects on grapevine growth. Previous studies have reported that the nutritional growth of grapevines is dominant, and N has an important role in its growth [51]. The form of N in potassium nitrate is nitrate N, an effective form of N that can be directly absorbed and used by plants; thus, the potassium nitrate application promotes the growth of plants [39]. Complex fertilizer, which also contains N, has also been shown to have a positive effect on plant growth [52,53]. On the other hand, potassium sulfate and potassium dihydrogen phosphate contain no N. This explains why the complex fertilizer and potassium nitrate treatments promoted the growth of the grapevine plants, whereas the potassium sulfate and potassium dihydrogen phosphate treatments did not show significant effects. In addition, results demonstrated that the potassium nitrate treatment increased the contents of photosynthetic pigments in grapevine leaves, whereas the complex fertilizer, potassium sulfate, and potassium dihydrogen phosphate treatments had no significant effects. This may be attributed to the fact that N is an important component of the chlorophyll molecule [54], and thus it can regulate photosynthesis by affecting chloroplast development, chlorophyll biosynthesis, and photosynthetic enzyme activity [55]. The nitrate N contained in the potassium nitrate used in this experiment is one of the main forms of N, and has a fast onset of action after absorption by plants, whereas the N in the complex fertilizer is amide N, which has a slow onset of action [56,57]. Therefore, the potassium nitrate treatment increased the photosynthetic pigment contents in grapevine leaves, whereas the complex fertilizer treatment had no significant effect.
The nutrients N, P, and K for crops are mainly absorbed from the soil [4]. The application of different chemical fertilizers to soil can increase the soil available nutrient concentration, thereby promoting the nutrient uptake by crops [4,5,6]. Applying different types of fertilizers increases the effective state of nutrients in the soil, and also affects the soil pH value to some extent under the action of plant roots [31]. There are also antagonistic, synergistic, and interactive effects among different nutrient elements in the soil, which directly affect the uptake of nutrients and nutrient utilization by plants. Notably, these effects are also associated with the plant type and growth stage [29,32]. Previous studies have shown that the soil pH value and the concentrations of soil available N, P, and K are affected, to varying degrees, by the application of different types of K fertilizers [41,42,49]. In this study, the soil pH value was decreased by the complex fertilizer treatment and increased by the potassium dihydrogen phosphate treatment, whereas there were no significant effects conferred by the potassium nitrate and potassium sulfate treatments. The complex fertilizer and potassium nitrate treatments increased the soil alkali-hydrolyzable N concentration, whereas the potassium sulfate and potassium dihydrogen phosphate treatments had no significant effects. The four fertilizer treatments all increased the soil available P and K concentrations. These results are consistent with the findings of previous studies [29,32,34], which suggest that applying different K fertilizers alters the soil pH value and soil available nutrient concentration, and benefits the uptake of P and K by grapevines, whereas the complex fertilizer and potassium nitrate applications benefit N uptake by grapevines. It has also been reported that the interaction of elements in the soil promotes the uptake of different nutrients by plants to some extent [29]. The application of both potassium sulfate and potassium chloride increase the N, P, and K contents in apple leaves to some extent, but decrease the calcium and magnesium contents [58]. Moreover, other studies have demonstrated that different types of K fertilizers can increase the nutrient uptake of N, P, and K by sweet potato and potato [41,42]. In this study, results showed that the complex fertilizer and potassium nitrate treatments increased the N content in various grapevine organs to some extent, whereas the potassium sulfate and potassium dihydrogen phosphate treatments inhibited the N content in the organs. This may be attributed to the fact that the complex fertilizer and potassium nitrate contained N, which increased the soil available N (alkali-hydrolyzable N) concentration, thereby promoting N uptake by the grapevines. In contrast, the potassium sulfate and potassium dihydrogen phosphate had no N, which resulted in lower soil and plant N content compared to the control, due to a nutrient imbalance caused by the limitation of N input [59]. These results differed from the findings of previous studies [41,42,58], which may be attributed to the varying nutrient requirements of different plants at different growth stages [41,42]. In addition, applying the four fertilizers (complex fertilizer, potassium nitrate, potassium sulfate, and potassium dihydrogen phosphate) increased the P and K contents in the grapevine plants, which is consistent with previous studies [41,42,58]. This result suggests that the application of K fertilizer promotes the P uptake by grapes, which is associated with the alteration of soil P and K solubilizing microorganisms by potassium fertilizer [28], and is beneficial to improving the quality of grape berries [37,38]. Moreover, correlation analysis showed that the N content in different grapevine organs was negatively correlated with the soil pH value, and positively correlated with the soil alkali-hydrolyzable N concentration, biomass, and total chlorophyll content, further suggesting that nitrogenous fertilizers promote grapevine growth by increasing the soil available N concentration. The P and K contents in different grapevine organs were positively correlated with each other and with soil available P and K concentrations, which further suggests that P and K had either interactive or synergistic effects. Furthermore, the grey relational analysis indicated that the rootstock stem total N content, rootstock root biomass, and soil alkali-hydrolyzable N concentration were closely associated with the scion total N content, whereas the rootstock root total P content, soil available P concentration, and rootstock stem total P content were closely associated with the scion total P content. In addition, the rootstock root total K content, soil available K concentration, and rootstock root total P content were closely associated with the scion total K content. Therefore, it was evident that the soil available nutrient concentrations were closely associated with the scion nutrient uptake by grapevine plants.
5. Conclusions
The complex fertilizer and potassium nitrate treatments promoted grapevine growth by increasing the biomass and photosynthetic pigment content, whereas the potassium sulfate and potassium dihydrogen phosphate treatments had no significant effect on grapevine growth. The complex fertilizer and potassium nitrate treatments promoted the N uptake by grapevine to some extent, whereas the other fertilizer treatments inhibited the N uptake to some extent. Moreover, the four types of K fertilizers promoted the uptake of P and K by grapevine plants. Future studies should explore the effects of different types of K fertilizers on the quality of grape berries.
Author Contributions
Conceptualization, X.L.; investigation, W.H., J.W., Q.D., D.L., H.X. and L.L.; writing—original draft preparation, W.H.; writing—review and editing, X.L.; supervision, X.L.; project administration, X.L.; funding acquisition, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Sichuan Provincial Science and Technology Program (2022ZHCG0105; 2020JDPT0004), and the Sichuan Fruit Innovation Team (sccxtd-04).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Upon request, the data will be provided.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rawat, J.; Sanwal, P.; Saxena, J. Potassium Solubilizing Microorganisms for Sustainable Agriculture; Spinger: Varanasi, India, 2016. [Google Scholar]
- Bindraban, P.S.; Dimkpa, C.; Nagarajan, L.; Roy, A.; Rabbinge, R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biol. Fertil. Soils 2015, 51, 897–911. [Google Scholar] [CrossRef]
- Blidariu, C.; Sala, F. Influence of organic and mineral fertilization on sugar content in Italian Riesling grape variety. J. Hortic. For. Biotechnol. 2012, 16, 251–254. [Google Scholar]
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the grape (Vitis vinifera L.) berry: Transport and function. Front. Plant Sci. 2017, 8, 1629. [Google Scholar] [CrossRef]
- He, P.C. Vinology; China Agricultural Press: Beijing, China, 2001. [Google Scholar]
- Villette, J.; Cuéllar, T.; Verdeil, J.; Delrot, S.; Gaillard, I. Grapevine potassium nutrition and fruit quality in the context of climate change. Front. Plant Sci. 2020, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Alemán, F.; Martínez, V.; Rubio, F. K+ uptake in plant roots: The systems involved, their regulation and parallels in other organisms. J. Plant Physiol. 2014, 171, 688–695. [Google Scholar] [CrossRef]
- Garcia, K.; Zimmermann, S.D. The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 2014, 5, 337. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture-status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Hafsi, C.; Debez, A.; Abdelly, C. Potassium deficiency in plants: Effects and signaling cascades. Acta Physiol. Plant. 2014, 36, 1055–1070. [Google Scholar] [CrossRef]
- Oosterhuis, D.M.; Loka, D.A.; Kawakami, E.M.; Pettigrew, W.T. Chapter three—The physiology of potassium in crop production. Adv. Agron. 2014, 126, 203–233. [Google Scholar]
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 1–25. [Google Scholar] [CrossRef]
- Chérel, I.; Gaillard, I. The complex fine-tuning of K+ fluxes in plants in relation to osmotic and ionic abiotic stresses. Int. J. Mol. Sci. 2019, 20, 715. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Andrianteranagna, M.; Cuéllar, T.; Chérel, I.; Gibrat, R.; Boeglin, M.; Moreau, B.; Paris, N.; Verdeil, J.L.; Zimmermann, S.D.; et al. Characterization of the grapevine Shaker K+ channel VvK3.1 supports its function in massive potassium fluxes necessary for berry potassium loading and pulvinus-actuated leaf movements. New Phytol. 2019, 222, 286–300. [Google Scholar] [CrossRef]
- White, P.J.; Karley, A.J. Cell Biology of Metals and Nutrients; Springer: Heidelberg, Germany, 2010. [Google Scholar]
- Zahoor, R.; Dong, H.; Abid, M.; Zhao, W.; Wang, Y.; Zhou, Z. Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ. Exp. Bot. 2017, 137, 73–83. [Google Scholar] [CrossRef]
- Zahoor, R.; Zhao, W.; Dong, H.; Snider, J.L.; Abid, M.; Iqbal, B. Potassium improves photosynthetic tolerance to and recovery from episodic drought stress in functional leaves of cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 119, 21–32. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, N.S.; Zhao, J.J.; Guo, Y.P.; Zhao, Z.Y.; Mei, L.X. Potassium fertilization improves apple fruit (Malus domestica Borkh. Cv. Fuji) development by regulating trehalose metabolism. J. Hortic. Sci. Biotechnol. 2017, 92, 539–549. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Nahar, K.; Hossain, M.S.; Mahud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Wakeel, A.; Gul, M.; Zörb, C. Soil Science: Agricultural and Environmental Prospectives I; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Zhou, M.; Mao, X.; Chen, H.; Bai, M.; Liu, K.Y.; Yang, G.S. Research advances on potassium nutrition and berry accumulation in grapevines. J. Fruit Sci. 2017, 34, 752–761. [Google Scholar]
- Sun, S.; Yang, J.; Zhang, S.Y.; Zhang, F.Q.; Ding, S.L. Research progress on relationship between potassium nutrition and photosynthesis physiology & fruit quality of fruit trees. Guangdong Agric. Sci. 2006, 33, 126–129. [Google Scholar]
- Abbasi, G.H.; Akhtar, J.; Ahmad, R.; Jamil, M.; Anwar-ul-Haq, M.; Ali, S.; Ijaz, M. Potassium application mitigates salt stress differentially at different growth stages in tolerant and sensitive maize hybrids. Plant Growth Regul. 2015, 76, 111–125. [Google Scholar] [CrossRef]
- Gautam, P.; Lal, B.; Tripathi, R.; Shahid, M.; Baig, M.J.; Maharana, S.; Puree, C.; Nayak, A.K. Beneficial effects of potassium application in improving submergence tolerance of rice (Oryza sativa L.). Environ. Exp. Bot. 2016, 128, 18–30. [Google Scholar] [CrossRef]
- Zafar, S.; Ashraf, M.Y.; Saleem, M. Shift in physiological and biochemical processes in wheat supplied with zinc and potassium under saline condition. J. Plant Nutr. 2018, 41, 19–28. [Google Scholar] [CrossRef]
- Zhou, M.; Zeng, B.; Zhao, Y.; Bai, M.; Yang, G. Effects of potassium on the photosynthesis of Vitis davidii Foex. J. Hunan Agric. Univ. 2017, 43, 156–160. [Google Scholar]
- Han, H.S.; Lee, K.D. Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ. 2006, 52, 130–136. [Google Scholar] [CrossRef]
- Bernardi, A.C.D.C.; Carmello, Q.A.D.C.; Carvalho, S.A.D.; Machado, E.C.; Medina, C.L.; Gomes, M.D.M.D.A.; Lima, D.M. Nitrogen, phosphorus and potassium fertilization interactions on the photosynthesis of containerized citrus nursery trees. J. Plant Nutr. 2015, 38, 1902–1912. [Google Scholar] [CrossRef]
- Zhang, F.; Niu, J.; Zhang, W.; Chen, X.; Li, C.; Yuan, L.; Xie, J. Potassium nutrition of crops under varied regimes of nitrogen supply. Plant Soil 2010, 335, 21–34. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Yang, X.; Ge, M.; Ma, Q.; Wei, H.; Dai, Q.; Huo, Z.; Xu, K.; Luo, D. Accumulation and utilization of nitrogen, phosphorus and potassium of irrigated rice cultivars with high productivities and high N use efficiencies. Field Crops Res. 2014, 161, 55–63. [Google Scholar] [CrossRef]
- Malvi, U.R. Interaction of micronutrients with major nutrients with special reference to potassium. Karnataka J. Agric. Sci. 2011, 24, 106–109. [Google Scholar]
- Reid, J.B.; Trolove, S.N.; Tan, Y.; Johnstone, P.R. Nitrogen or potassium preconditioning affects uptake of both nitrate and potassium in young wheat (Triticum aestivum). Ann. Appl. Biol. 2016, 168, 66–80. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Xia, S.; Wang, L.; Liu, C.; Zhang, R.; Fan, Z.; Chen, F.; Liu, Y. Interactions between N, P and K fertilizers affect the environment and the yield and quality of satsumas. Glob. Ecol. Conserv. 2019, 19, e00663. [Google Scholar] [CrossRef]
- Kandylis, P. Grapes and their derivatives in functional foods. Foods 2021, 10, 672. [Google Scholar] [CrossRef]
- Cao, S.Y.; Xie, S.X.; Fang, J.G.; Lu, X.P. A Map History of Local Grape Varieties in China; China Forestry Press: Beijing, China, 2018. [Google Scholar]
- Ramos, M.C.; Romero, M.P. Potassium uptake and redistribution in Cabernet Sauvignon and Syrah grape tissues and its relationships with grape quality parameters. J. Sci. Food Agric. 2017, 97, 3269–3277. [Google Scholar] [CrossRef]
- Sustr, M.; Soukup, A.; Tylova, E. Potassium in root growth and development. Plants 2019, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Pushpavathi, Y.; Satisha, J.; Satisha, G.C.; Reddy, M.L. Influence of potassium fertilization on yield, petiole and soil nutrient status of table grape cv. Sharad seedless. J. Plant Nutr. 2021, 44, 2218–2227. [Google Scholar]
- Gu, N.; Zhao, L.P.; Zhao, X.M. A review and perspective on slow and controlled release fertilizer in China. Appl. Mech. Mater. 2014, 535, 222–225. [Google Scholar] [CrossRef]
- Yang, Q.F.; Xu, D.Y.; Chen, H.; Wu, J.J. Effects of different potassium treatments on nutrient absorption and utilization and yield of starch-type sweet potato. Hunan Agric. Sci. 2021, 50, 30–33, 37. [Google Scholar]
- Gao, X.; Li, C.L.; Zhang, M.; Wang, R. Effects of potassium fertilizer type and rate on yield and quality of potato. J. Soil Water Conserv. 2014, 28, 143–148. [Google Scholar]
- Li, Z.; Fan, R.; Peng, X.; Shu, J.; Liu, L.; Wang, J.; Lin, L. Salicylic acid alleviates selenium stress and promotes selenium uptake of grapevine. Physiol. Mol. Biol. Plants 2022, 28, 625–635. [Google Scholar] [CrossRef]
- Hao, Z.B.; Cang, J.; Xu, Z. Plant Physiology Experiment; Harbin Institute of Technology Press: Harbin, China, 2004. [Google Scholar]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Lin, L.; Li, Z.; Wang, J.; Liang, D.; Xia, H.; Lv, X.; Tang, Y.; Wang, X.; Deng, Q.; Liao, M. 24-epibrassinolide promotes selenium uptake in grapevine under selenium stress. Sci. Hortic. 2023, 308, 111564. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Q.; Xu, X.; Liao, M.; Lin, L.; Hu, R.; Luo, X.; Wang, Z.; Wang, J.; Deng, Q.; et al. An amino acid fertilizer improves the emergent accumulator plant Nasturtium officinale R. Br. phytoremediation capability for cadmium-contaminated paddy soils. Front. Plant Sci. 2022, 13, 1003743. [Google Scholar] [CrossRef]
- Ye, X.F.; Zhu, H.B.; Jin, D.M.; Liu, G.S.; Wang, Y.Y.; Cui, S.Y.; Yu, Q.W.; Zhang, S. Influence of different potassium fertilizer on some enzyme activities of growing flue-cured tobacco. Acta Agric. Boreali-Sin. 2007, 22, 67–70. [Google Scholar]
- Deng, L.S.; Lin, C.L.; Gong, L.; Li, Z.H.; Tu, P.F.; Zhang, C.L. Effect of different potassium fertilizers on growth and production of potato under fertigation. J. South China Agric. Univ. 2010, 31, 12–14, 27. [Google Scholar]
- Yan, S.Y.; Lu, J.; Song, K.; Li, B.; Yang, J.H.; Zhang, C. Effects of potassium fertilization on the quality of ‘Muscat Hamburg’ grapes. J. Tianjin Agric. Univ. 2022, 29, 14–18. [Google Scholar]
- Cocco, A.; Mercenaro, L.; Muscas, E.; Mura, A.; Nieddu, G.; Lentini, A. Multiple effects of nitrogen fertilization on grape vegetative growth, berry quality and pest development in mediterranean vineyards. Horticulturae 2021, 7, 530. [Google Scholar] [CrossRef]
- Duncan, E.G.; O’Sullivan, C.A.; Roper, M.M.; Biggs, J.S.; Peoples, M.B. Influence of co-application of nitrogen with phosphorus, potassium and sulphur on the apparent efficiency of nitrogen fertiliser use, grain yield and protein content of wheat. Field Crops Res. 2018, 226, 56–65. [Google Scholar] [CrossRef]
- Duncan, E.G.; O’Sullivan, C.A.; Roper, M.M.; Palta, J.; Whisson, K.; Peoples, M.B. Yield and nitrogen use efficiency of wheat increased with root length and biomass due to nitrogen, phosphorus, and potassium interactions. J. Plant Nutr. Soil Sci. 2018, 181, 364–373. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Wang, W.; Chu, C.C. Towards understanding of nitrogen use efficiency in plants. Chin. Bull. Life Sci. 2018, 30, 1060–1071. [Google Scholar]
- Hu, M.J.; Guo, Y.P.; Shen, Y.G.; Zhang, L.C. Environmental regulation of Citrus photosynthesis. Chin. J. Appl. Ecol. 2006, 17, 535–540. [Google Scholar]
- Wickert, E.; Marcondes, J.; Lemos, M.V.; Lemos, E.G.M. Nitrogen assimilation in Citrus based on CitEST data mining. Genet. Mol. Biol. 2007, 30, 810–818. [Google Scholar] [CrossRef]
- Mo, L.Y.; Wu, L.H.; Tao, Q.N. Research advances on GS/GOGAT cycle in higher plants. Plant Nutr. Fertil. Sci. 2001, 7, 223–231. [Google Scholar]
- Chen, C.; Tong, Y.A.; Lu, Y.L.; Gao, Y.M. Effects of different potassium fertilizers on production, quality and storability of Fuji apple. J. Plant Nutr. Fertil. 2016, 22, 216–224. [Google Scholar]
- Marques, D.J.; Broetto, F.; Lobato, A.K.D.S.; Silva, E.C.D.; Carvalho, J.G.D.; Vila, F.W.D.; Alves, G.A.R.; Andrade, I.P. Photosynthetic pigments, nitrogen status, and flower behavior in eggplant exposed to different sources and levels of potassium. Sci. Res. Essays 2013, 8, 67–74. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).