Abstract
Healing and acclimatization are critical in vegetable grafting under controlled environments. Here, we investigated the impacts of LED light qualities on the morphological traits and photosynthetic performance of grafted tomato seedlings. Seeds of the tomatoes “DRW 7806 F1” and “Maxifort” (Solanum lycopersicum × Solanum habrochaites) used as scion and rootstock were planted in 104-cell plug trays into a mixture of cocopeat and perlite (volume ratio: 3 to 1). Survival ratio, above- and underground growth, photosynthetic performance, soluble carbohydrate content, pigmentation, and antioxidant enzymes activity were evaluated following 20 days of exposure to different light qualities, including white (35% B, 49% intermediate spectra, 16% R) light as control, blue, red, and a combination of red (68%) and blue with the same light intensity of 75 ± 5 µmol m−2 s−1. The lowest scion diameter, leaf area, root and shoot dry weight, SPAD value, and the highest scion length and amount of soluble carbohydrate were detected in R-exposed seedlings. Moreover, R-exposed seedlings showed leaf epinasty and reduced photosynthetic performance. On the other hand, RB-exposed seedlings showed the highest leaf area, shoot and root dry weight, plant fresh and dry weight, scion stem diameter and photosystem II efficiency. In addition, superoxide dismutase activity was increased in R-exposed seedlings, while guaiacol peroxidase activity was enhanced in seedlings grown in RB. In conclusion, a combination of R and B is suggested as the suitable light spectrum to promote plant growth and photosynthetic performance in grafted tomato seedlings.
1. Introduction
Grafted plants for horticultural purposes have been commercially used since the 1920s [1]. They have been extensively utilized in vegetables to improve yield and fruit quality [2,3]. Specifically, grafting is widely practiced in Cucurbitaceae and Solanaceae [4]. Grafted seedlings and transplants are tolerant to undesirable environmental variations such as biotic and abiotic stresses [5] and have higher plant vigor and yield than non-grafted ones. The production of grafted vegetables consists of three main stages: (i) the production of rootstocks and scions, (ii) grafting, and finally (iii) healing and acclimatization [6]. Healing and acclimatization are crucial as the newly cut grafted seedlings lack vascular connections. Therefore, the air’s relative humidity (RH) should usually be more than 90%. Otherwise, the scion will be desiccated, and the graft will fail. Temperature is another crucial factor in this step, affecting transpiration, respiration, and even air RH. Although healing and acclimatization are practiced in conventional tunnel systems and greenhouse conditions, several reasons have motivated producers to use indoor multilayer vertical systems under artificial light-emitting diodes (LED). Among all the benefits, the vertical growth pattern realizes higher yields per unit area, higher control over sanitation, less dependency on the outside environment, and more energy efficiency [7]. Improving the environmental factors is vital for seedling production during the healing and acclimatization stages [8]. Therefore, to improve the growth and marketability of grafted transplants, it is crucial to accurately control the temperature, light, and relative humidity during the healing and acclimatization process [9].
Many researchers have reported the results of environmental improvement in the healing of grafted transplant production under controlled conditions [10,11]. Light, as a signal, regulates gene expression and photomorphogenesis in plants [12,13]. Moreover, different light parameters such as intensity, quality and duration of radiation can change plant morphological and physiological characteristics [14]. Artificial light technology’s rapid development has led to its expansion in closed horticultural systems [15]. LEDs are new light sources for plant research and production in controlled environments [16]. Among the advantages of using LED lights are their long life, small size, less energy dissipation, and capability to dim the intensity [17]. The possible manipulation of light quality is also ongoing with the development of LEDs [18,19]. Today, the industry benefits from LED fixtures that can be easily programmed for a desired spectrum and intensity. Moreover, the main advantage is that LEDs have the highest energy use efficiency compared with all other plant lighting lamps. The lifetime of LEDs has been reported to be about 100,000 h, and this number is still rising [20].
LEDs are also considered optimum tools for plant science lighting research due to their technical advantages over traditional lighting sources. This is because they can produce the most compatible light wavelength with plant photoreceptors, so these light sources can more effectively influence plants’ physiology, morphology, and development [21]. Different light qualities impose specific reactions on the plants. For example, the maximum absorption spectra by chlorophyll pigments in the photosynthetic apparatus is in the range of red and blue light waveband ranges [22]. R and B light strongly affect the chemical contents, structure, and photosynthetic performance of the plants [23]. R light changes the plant’s anatomy and induces shoot and branch elongation, while stomatal opening, chlorophyll synthesis, and chloroplast maturation occur mainly under B light [24]. These findings illustrate the importance of RB light on the performance of PSII.
Moreover, photoreceptors and their signaling pathways influence the content and function of secondary metabolites [25]. Plants have several photoreceptors, each absorbing a specific spectrum of light. Phytochromes are generally more sensitive receptors of R light, while phototropin and cryptochromes are B light photoreceptors [26]. Photosynthetic performance is affected by all lighting environments and the light quality during cultivation [19]. Chlorophyll fluorescence is a practical approach to assess the photosynthetic efficiency of plants, as it allows for evaluating the disposition of energy resulting from chlorophyll excitation.
Furthermore, chlorophyll fluorescence information can provide insights into the functional and structural aspects of the plant’s photosynthetic apparatus [27,28]. Finally, the method of chlorophyll fluorescence induction is employed to determine the ultimate fate of energy absorbed by chlorophyll molecules. A non-destructive method to evaluate the efficiency of biophysical phases in the different steps of the electron transport system is the fast chlorophyll fluorescence induction curve (OJIP) [29,30]. This protocol is obtained based on the energy flow in the thylakoid membrane and can be used to evaluate the performance of the photosynthetic apparatus in response to stresses [30,31].
Given the species- and cultivar-specific nature of plant responses to light, it is essential to investigate the impact of lighting conditions on grafted seedlings with varying rootstock and scion combinations beyond those that have already been researched. For instance, B light has been shown to induce stomatal opening and subsequently increase the transpiration rates [32]. As a result, rootstocks with enhanced water-pumping capabilities are more effective in such conditions [33]. In other words, the rootstock cultivar can influence how the scion responds to the light source. Hence, conducting additional studies on grafted seedlings exposed to various lighting environments is essential. In addition to the standard morphological and biochemical parameters, it is beneficial to evaluate the functionality of the photosynthetic apparatus concerning various lighting conditions using contemporary techniques such as OJIP analysis and chlorophyll fluorescence imaging. This strategy provides a better understanding of the underlying mechanisms contributing to the superior performance of specific lighting conditions compared to others [19,30].
The present study was conducted to understand the impact of light quality on the survival, morphology, carbohydrates, photosynthetic pigment content, and photosynthetic performance of grafted tomato seedlings via OJIP analysis and chlorophyll fluorescence imaging during healing and acclimatization. These parameters not only help in determining the quality of the produced grafted seedlings, but they also help in understanding the underlying physiological aspects of plant–light reactions specific to grafted seedlings. In addition, studying light quality for specific combinations of scion and rootstocks could lead to the growth of LED-equipped vertical systems that rely solely on artificial lighting during healing and acclimatization.
2. Materials and Methods
2.1. Plant Materials and Environmental Conditions
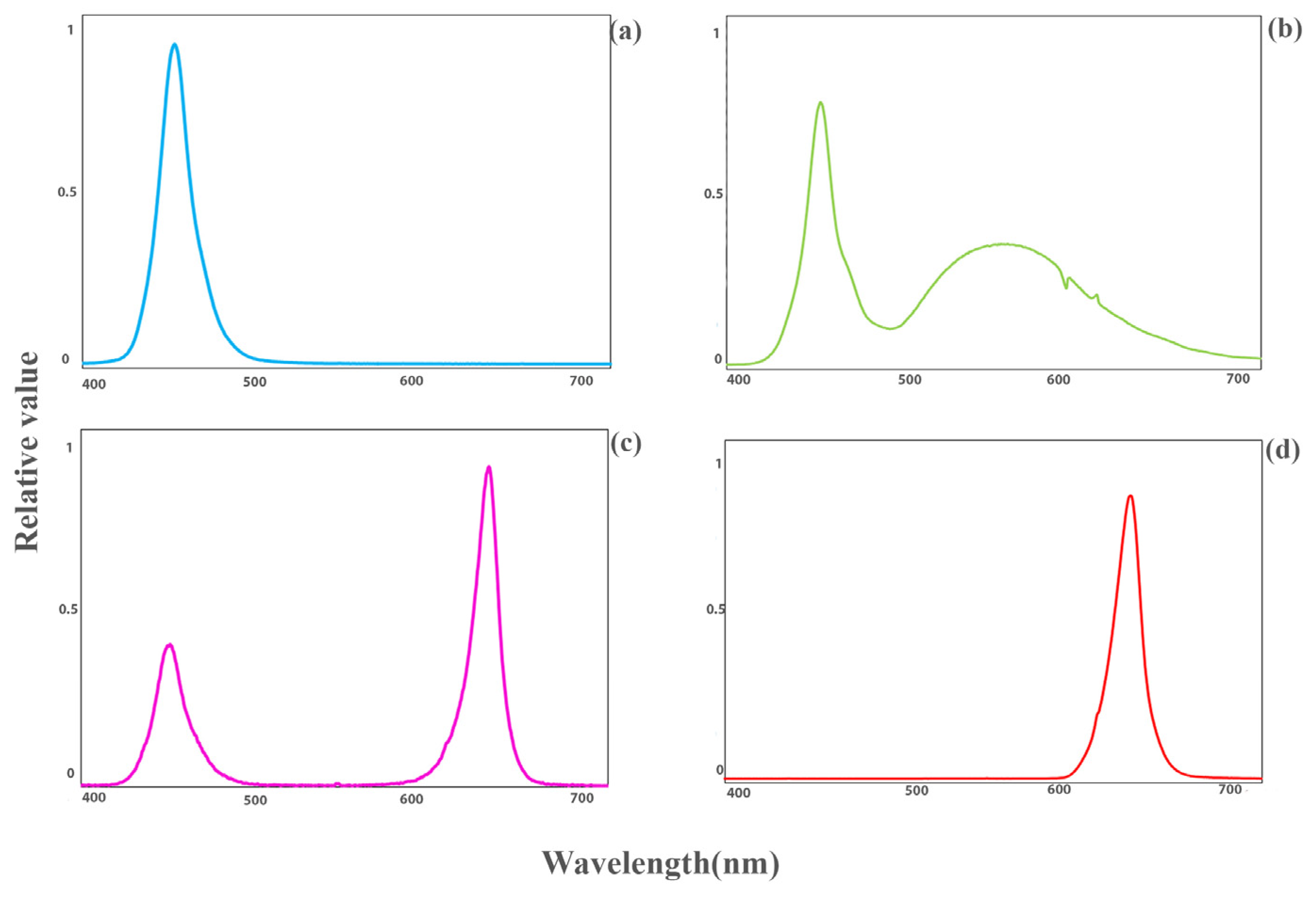
Seeds of the tomatoes “DRW 7806 F1” and “Maxifort” (Solanum lycopersicum × Solanum habrochaites) used as scion and rootstock, respectively, were planted in 104-cell plug trays into a mixture of cocopeat and perlite (volume ratio: 3 to 1). The trays were placed in a greenhouse in the agricultural research faculty of Tehran University, in Karaj campus, Iran. The substrate’s moisture was kept near the maximum water-holding capacity, so there was around 30% leachate after every fertigation. Environmental factors such as day/night temperature, relative humidity and photoperiod were adjusted to 25/20 °C, 70% ± 5% and 12 h, respectively. After 20 days, when the diameter of the rootstock and scion reached about 2–3 mm, the grafting process was performed manually by the splice grafting method. The scion was placed on the rootstock hypocotyl by using a plastic clip. The grafted plants were transferred to specific healing cabinets with three floors equipped with LED lights. The transplants were kept in darkness (D) after grafting for three days to prevent leaf dehydration. After that, the LED lights were turned on. All plants were subjected to controlled environmental conditions, including a 16 h photoperiod, a day/night temperature of 25/20 °C, and a photosynthetic photon flux density (PPFD) of 75 ± 5 µmol m−2 s−1, which was provided by LED modules (Iraneon Co in Birjand, South Khorasan, Iran). The spectra of the light were monitored using a Sekonic light meter (Sekonic C-7000, Tokyo, Japan). Besides white (W; 35% blue (400–500 nm), 49% green (500–600 nm), and 16% red (600–700 nm)) light as control, other light qualities, including red (R; 600–700 nm), blue (B; 400–500 nm), and a combination of red and blue (RB; 32% blue (400–500 nm) and 68% red (600–700 nm)); as represented in Figure 1 were used. Relative air humidity was moderately decreased, being 98–100% (days 0–3), 90–95% (days 4–5), 85–90% (days 6–7), 80% (days 8–9) and 70% (days 10–14). Twenty days after grafting, data collection and measurements were carried out. Samples were selected from young leaves that were expanded and grown under direct light.
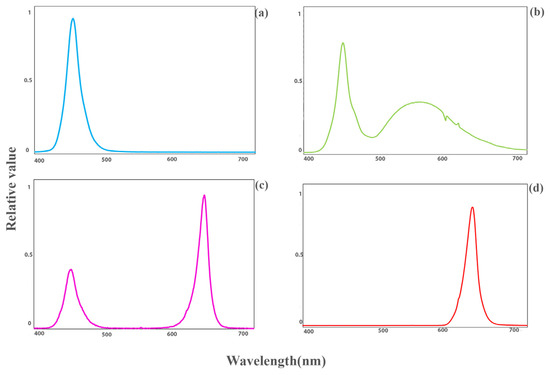
Figure 1.
Light spectra of blue ((a); 400–500 nm), white ((b); 35% blue (400–500 nm), 48% intermediate (500–600 nm), and 16% red (600–700 nm)) as control, red and blue ((c); 32% blue (400–500 nm), and 68% red (600–700 nm)), and red ((d); 600–700 nm) light quality regimes under study.
2.2. Morphological Parameters
The assessment of morphological parameters included scion length (measured from the graft union to the apical meristem), scion diameter (measured at a point 1 cm above the graft union), number of nodes, leaf area, and shoot and root dry and fresh weights. Dry weight was determined by placing the samples in an air-drying oven for 72 h at 80 °C [34,35]. For the assessment of the leaf area, the leaves were scanned using an HP Scanjet G4010 scanner (Palo Alto, CA, USA) and then analyzed using the Digimizer software (version 4.1.1.0, MedCalc Software, Ostend, Belgium), as described in reference [19,35].
2.3. Determination of Soluble Carbohydrates
Liquid nitrogen was used to freeze leaf samples for the determination of soluble carbohydrates. First, 0.2 g of plant tissue was weighed and combined with 7 mL of 70% ethanol (w/v) on ice for 5 min and then centrifuged at four °C for 10 min (6700× g). In the next step, 200 mL of supernatant was added to 1 mL of anthrone compound. Finally, the absorbance value was determined according to van Doorn’s method [36] using a spectrophotometer (PowerWave XS Microplate Reader, BioTek Instruments, Inc., Winooski, VT, USA) at 625 nm.
2.4. Antioxidant Enzymes Activity
The activity of the antioxidant enzymes including superoxide dismutase (SOD) and guaiacol peroxidase (GPX) was evaluated in fully expanded leaves of grafted tomatoes 20 days after grafting. These enzymes play a crucial role in protecting plants against oxidative stress. The method described by Dhindsa et al. [37] was employed, which is based on the ability of SOD to inhibit the photochemical reduction of nitro blue tetrazolium (NBT). The reaction was initiated by exposing the samples to a fluorescent lamp (50 W, 60 cm) for 10 min, pausing the reaction by turning off the light and covering the tubes with a black cloth. The absorbance of the reaction mixture was then determined at 560 nm.
To measure GPX activity in grafted tomatoes, the method described by Flocco and Giulietti [38] was employed. Guaiacol oxidation in the presence of hydrogen peroxide (H2O2) was used to determine the GPX activity. Initially, 100 mg of each root and shoot sample was weighed and homogenized in 100 mM phosphate buffer (pH 6) using a mortar. The homogenate was centrifuged for 5 min at 11,000 rpm and 5 °C. Next, 10 μL of the supernatant was added to 3 mL of the reaction mixture containing 3.6 mM hydrogen peroxide, 200 mM potassium phosphate buffer (pH 7.4), and 31 mM guaiacol and immediately stirred. The increase in absorbance was then measured, and the kinetic assessment of absorbance was determined over 2 min at 15 s intervals using a spectrophotometer at 470 nm. The peroxidase activity unit (U) was defined as the amount of enzyme that oxidized 1 μmol of hydrogen peroxide per minute.
2.5. Determination of Hydrogen Peroxide Content
The hydrogen peroxide (H2O2) content was measured by spectrophotometry after the reaction of H2O2 with potassium iodide (KI). The reaction mixture consisted of leaf extract supernatant, 2 mL of reagent (1 M KI w/v in fresh double-distilled H2O), 0.5 mL of 0.1% TCA, and 0.5 mL of 100 mM K-phosphate buffer. The blank was prepared with 0.1% TCA without leaf extract. The reaction was carried out for 1 h under dark conditions, and the absorbance was measured at 390 nm. The amount of H2O2 was determined using a standard curve generated with known concentrations of H2O2, according to Patterson et al. [39].
2.6. SPAD Determination
The non-destructive method of chlorophyll measurement via SPAD as a handheld device that easily and quickly measures the photosynthetic pigments was employed using a SPAD-502 (Konica Minolta Corp., Solna, Sweden). This device estimates leaf chlorophyll content by transmitting the red and infrared light spectrum (i.e., 650 and 940 nm, respectively) through the leaf. In each replicate, three points were measured for leaf chlorophyll content [40].
2.7. Chlorophyll Fluorescence Imaging
The maximum quantum yield of PSII (Fv/Fm) was measured using a chlorophyll fluorescence imaging technique. Leaves from sample plants of each light treatment were used for this purpose. Before the assessment, the leaves were dark-adapted for 20 min by switching off LEDs. Images were then recorded during short measuring flashes in darkness. At the end of these flashes, the samples were exposed to a saturating light pulse (3900 μmol m−2 s−1), which led to the transitory saturation of photochemistry and reduction of QA− of PSII. The measurements were taken using a FluorCam FC 1000-H (Photon Systems Instruments, Drásov, Czech Republic) [19,41,42,43].
2.8. Analysis of the OJIP Test
The polyphasic Chl a fluorescence (OJIP) transients were measured using a handheld Fluorpen FP 100-MAX (Photon Systems Instruments, Drasov, Czech Republic) to assess the overall health and functionality of photosynthetic apparatus of tomato plants after a relatively long 20-day healing and acclimatization period. The measurements were taken on young, fully expanded leaves of tomato seedlings after 20 min of dark adaptation.
The fluorescence levels were measured at four different timescales, specifically at the O-step (F0), J-step (Fj), I-step (Fi), and P-step (Fm). The parameters relevant to the OJIP test were computed based on the methods outlined by Strasser et al. [29]. The O, J, I, and P steps refer to specific points on the fluorescence transient curve that reflect different stages of energy transfer within photosystem II (PSII). For example, the F0 measurement is taken at the O-step and reflects the initial fluorescence intensity, while Fm is taken at the P-step and represents the maximum fluorescence intensity. The Fj and Fi measurements correspond to intermediate points on the curve and reflect the formation of the closed PSII reaction center and the accumulation of QA− on the acceptor side of PSII, respectively. Based on the protocol, two successive series of fluorescence data were recorded: one during the short measuring flashes in the darkness (F0) and the other at the time of exposure to saturating flash (Fm). The Fv/Fm was calculated using the following equation:
Fv/Fm = (Fm − F0)/Fm
Higher F0 and lower Fv/F0, Fv/Fm, and Fm/F0 show exposure to higher stress severity [44,45]. The following calculation parameters were used: relative variable fluorescence in step J (VJ), the maximum quantum yield of primary photochemistry (φP0), the quantum yield of energy dissipation (φD0) and performance index on the absorption basis (PIABS). Relative variable fluorescence in step J (VJ) was calculated using the following equation:
VJ = (FJ − F0)/(Fm − F0)
Performance index on the absorption basis (PIABS), the maximum quantum yield of primary photochemistry (φP0) and the quantum yield of energy dissipation (φD0) were also calculated using the following equations:
PIABS = (RC/ABS) × (φP0/1 − φP0) × (Ψ0/1 − Ψ0)
φP0 = Fv/Fm = 1 − (F0/Fm)
φD0 = 1 − φP0 = (F0/Fm)
The probability that a trapped exciton proceeds an electron through the electron transport chain beyond QA− (Ψ0) was calculated using the following equation:
Ψ0 = 1 − VJ
These parameters were calculated according to previous studies [29,46].
2.9. Statistical Analysis
A completely randomized design ANOVA was used to analyze the treatment effects of four different light treatments. Three chambers were used for each light treatment, resulting in 12 chambers. Each chamber contained one 104-cell seedling trays. Mean and standard error data were compared using Duncan’s multiple range test at a probability level of p ≤ 0.05. The data were analyzed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Morphology of Grafted Seedlings Was Improved by RB Light Regime
The results showed that the morphology of grafted tomato transplants was influenced by light quality. However, all seedlings survived under light treatments regardless of spectral quality (Table 1). R light-grown plants had the maximum stem length. Exposing the seedlings to different light spectra did not remarkably influence the node number, while R-exposed seedlings had the thinnest scion stem diameter. Due to the small size of the leaves in the plants grown under R light, these seedlings had the lowest leaf area. The lowest root, shoot and plant dry weights were detected in R-grown seedlings, while RB-exposed seedlings showed the maximum fresh and dry weight. The maximum scion stem diameter was obtained in RB-exposed seedlings. However, there was no significant difference in scion stem diameter among RB-, B- and W-exposed seedlings.

Table 1.
The morphological and growth parameters of grafted tomato seedlings exposed to different light regimes for 20 days under a light intensity of 75 ± 5 µmol m−2 s−1 PPFD. The light quality treatments included white light (W) as control, blue (B), red (R), and a combination of red (68%) and blue (RB) lights. The spectral composition of the RB light treatment can be found in Figure 1. Values with the same letters are not significantly different at p ≤ 0.05, according to Duncan’s multiple range tests.
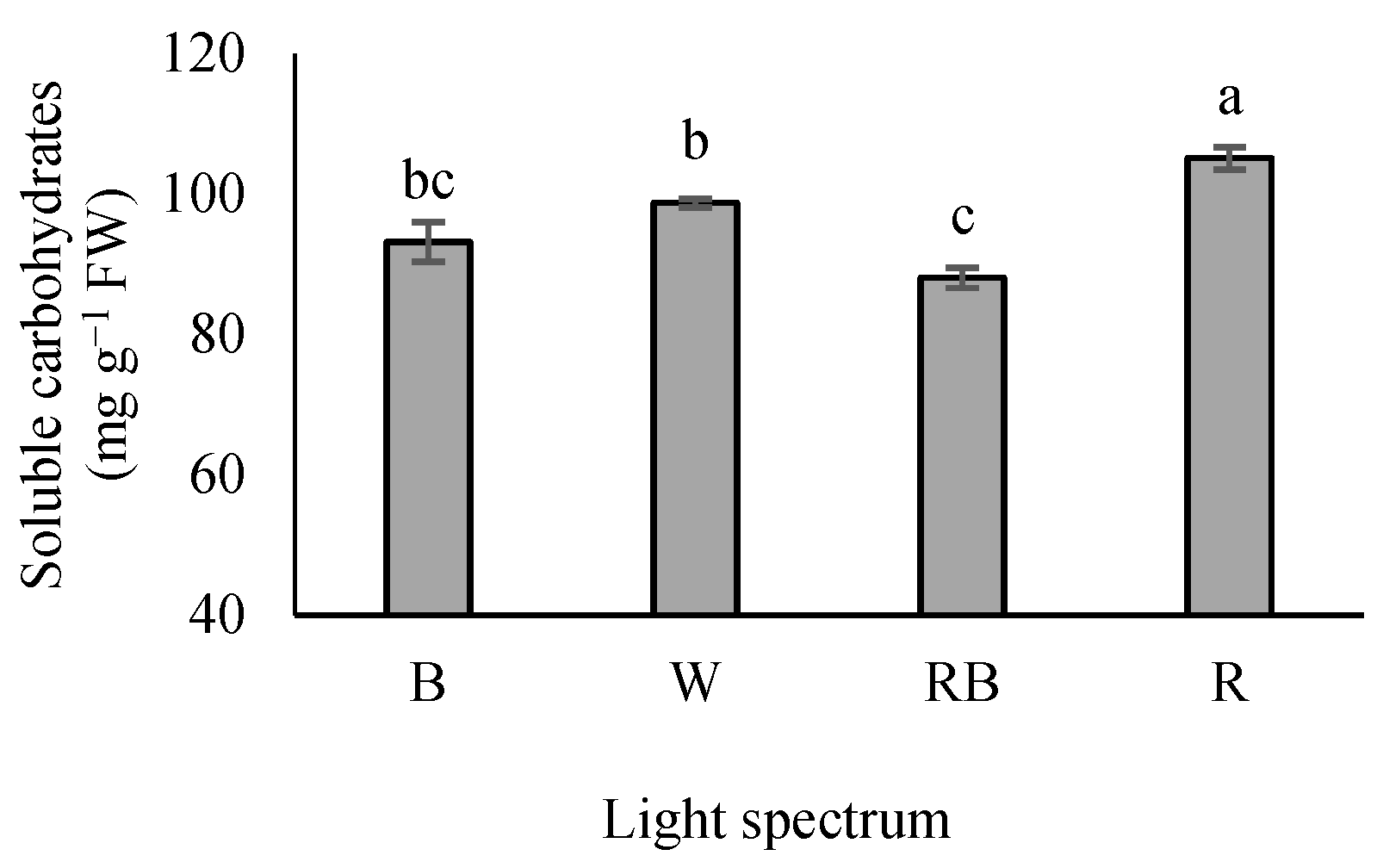
3.2. Leaf-Soluble Carbohydrates
Seedlings grown under R light showed a maximum soluble carbohydrate level, whereas their minimum contents were recorded in RB-exposed seedlings. Seedlings grown under B and W lights had an intermediate state. Additionally, seedlings exposed to B light showed no significant difference with either W or RB lights (Figure 2).
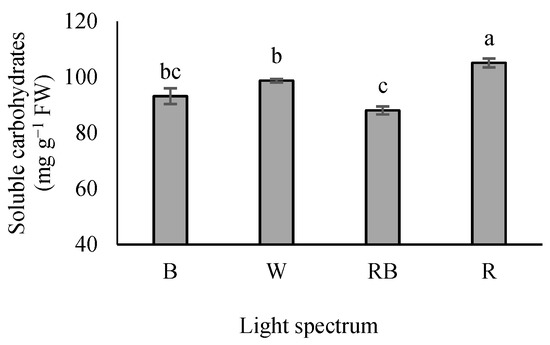
Figure 2.
Soluble leaf carbohydrate levels in the leaves of grafted tomato seedlings exposed to different light qualities (including white (W; 35% B, 49% intermediate spectra, 16% R) light as control, blue (B), red (R), and a combination of red (68%) and blue (RB) (see the correspondence spectrum in Figure 1)), with 75 ± 5 µmol m−2 s−1 PPFD for 20 days. Columns with the same letters are not significantly different at p ≤ 0.05, according to Duncan’s multiple range tests. Bars represent the mean value of three replications ± SEM.
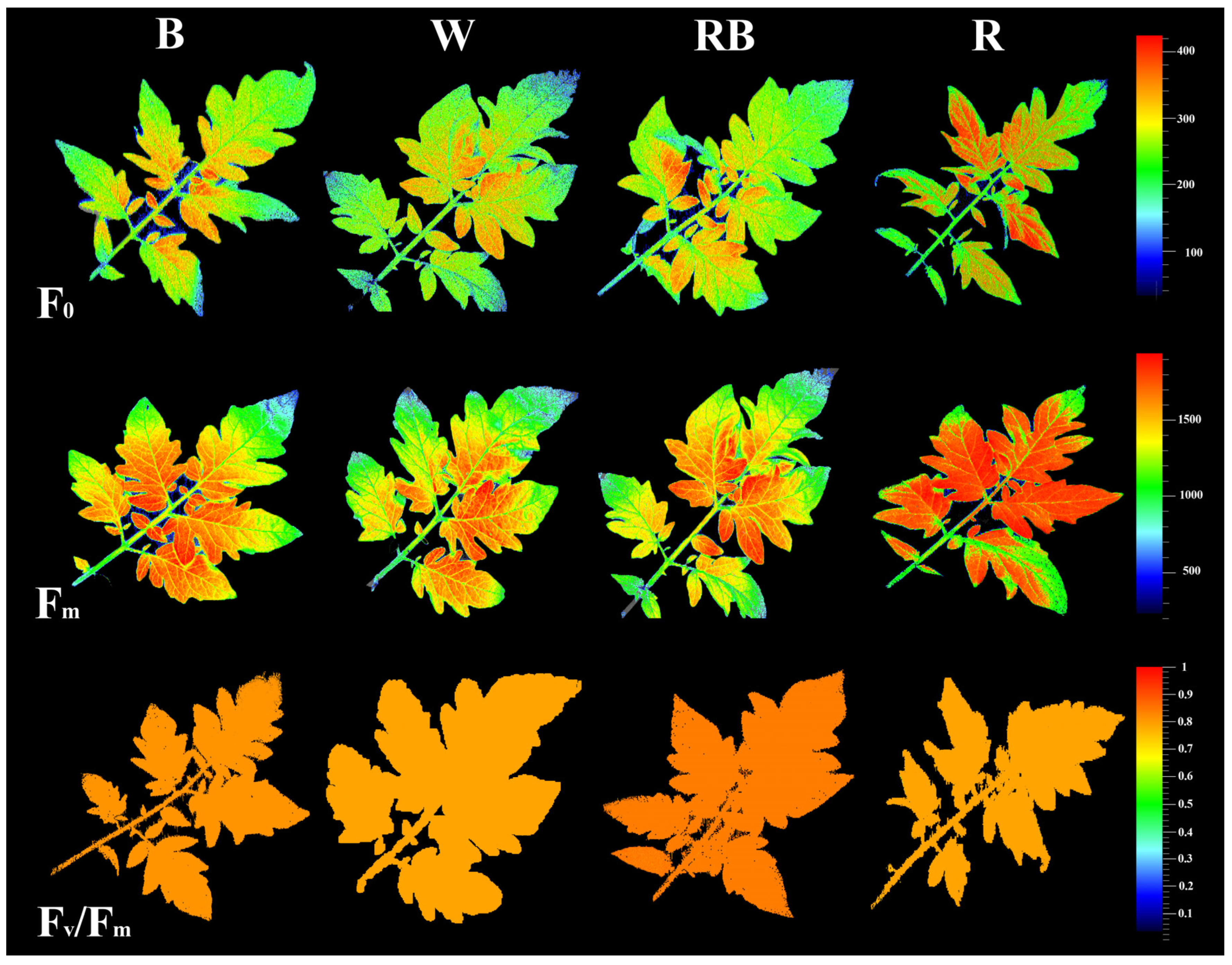
3.3. Chlorophyll Fluorescence Imaging
The impacts of light on photosynthetic functionality in the healing and acclimatization stages were evaluated by assessment of the spatial pattern of fluorescence emission through pseudo-color images of F0 (minimum fluorescence), Fm (maximum fluorescence), and Fv/Fm (maximal quantum yield of PSΠ photochemistry) (Figure 3). The highest F0, Fm and lowest Fv/Fm were recorded in R-exposed seedlings, whereas RB-exposed seedlings exhibited the highest Fv/Fm.
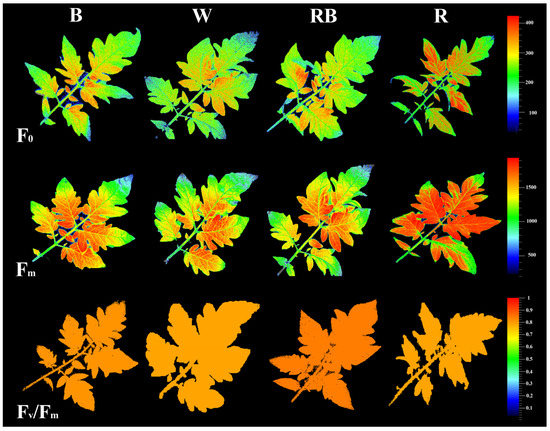
Figure 3.
Representative images of fluorescence parameters including F0, Fm, and Fv/Fm exhibited by leaves sampled from grafted tomato seedlings exposed to different light qualities (including white (W; 35% B, 49% intermediate spectra, 16% R) light as control, blue (B), red (R), and a combination of red (68%) and blue (RB), (see the correspondence spectrum in Figure 1)), with 75 ± 5 µmol m−2 s−1 PPFD for 20 days.
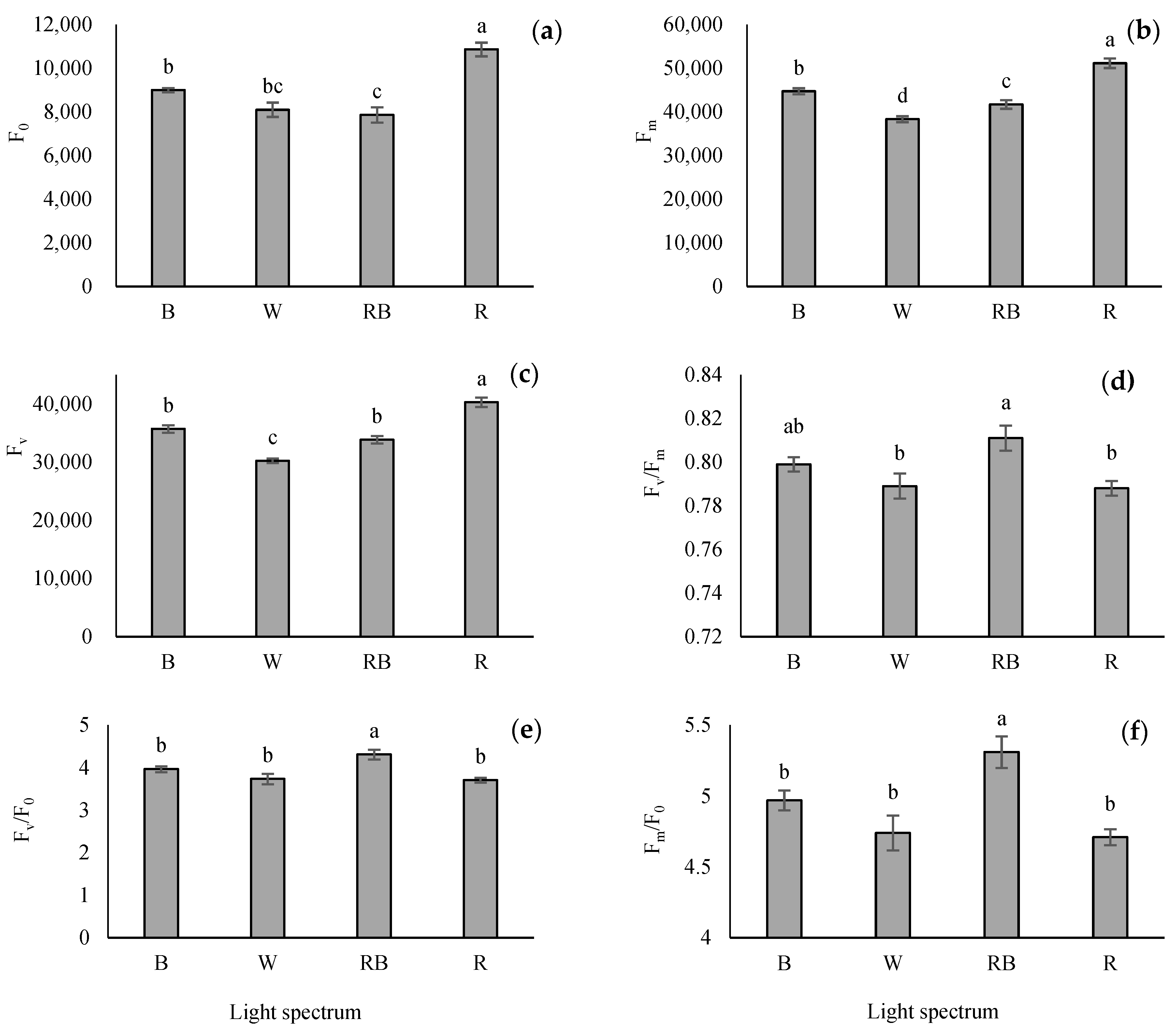
3.4. Evaluation of Parameters Obtained from the OJIP Protocol
Seedlings grown under R light had the highest F0, FJ, FI, and Fm compared with other light treatments. Among the light treatments, the maximum Fv/F0 and Fm/F0 were obtained under RB-exposed seedlings, while the minimum of these parameters was detected in W, B and R treatments. Overall, different light treatments significantly affected the maximum quantum yield of PSII (Fv/Fm). Based on the results, the maximum Fv/Fm was obtained from RB-light treatment (Figure 4).
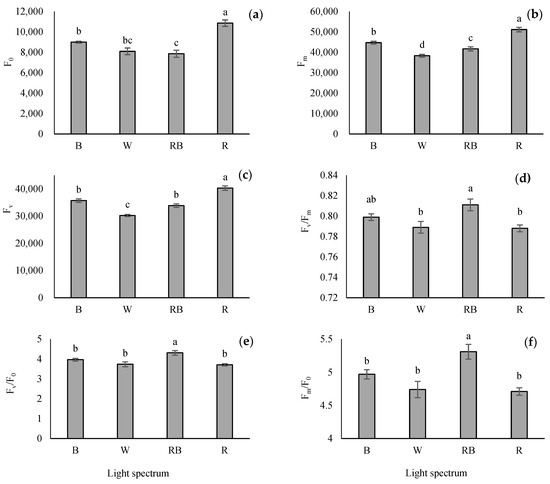
Figure 4.
The intensity of chlorophyll fluorescence in the OJIP-test including minimum fluorescence when all PSΠ reaction centers are open (F0; (a)), maximum fluorescence when all PSΠ reaction centers are closed (Fm; (b)), variable fluorescence of the dark-adapted leaf (Fv; (c)), the maximal quantum yield of PSΠ photochemistry (Fv/Fm; (d)), Fv/F0 (e) and Fm/F0 (f) from the fluorescence transient exhibited by leaves sampled from grafted tomato seedlings exposed to different light qualities (including white (W; 35% B, 49% intermediate spectra, 16% R) light as control, blue (B), red (R), and a combination of red (68%) and blue (RB), (see the correspondence spectrum in Figure 1)), with 75 ± 5 µmol m−2 s−1 PPFD for 20 days. Columns with the same letters are not significantly different at p ≤ 0.05, according to Duncan’s multiple range tests. Bars represent the mean value of three replications ± SEM.
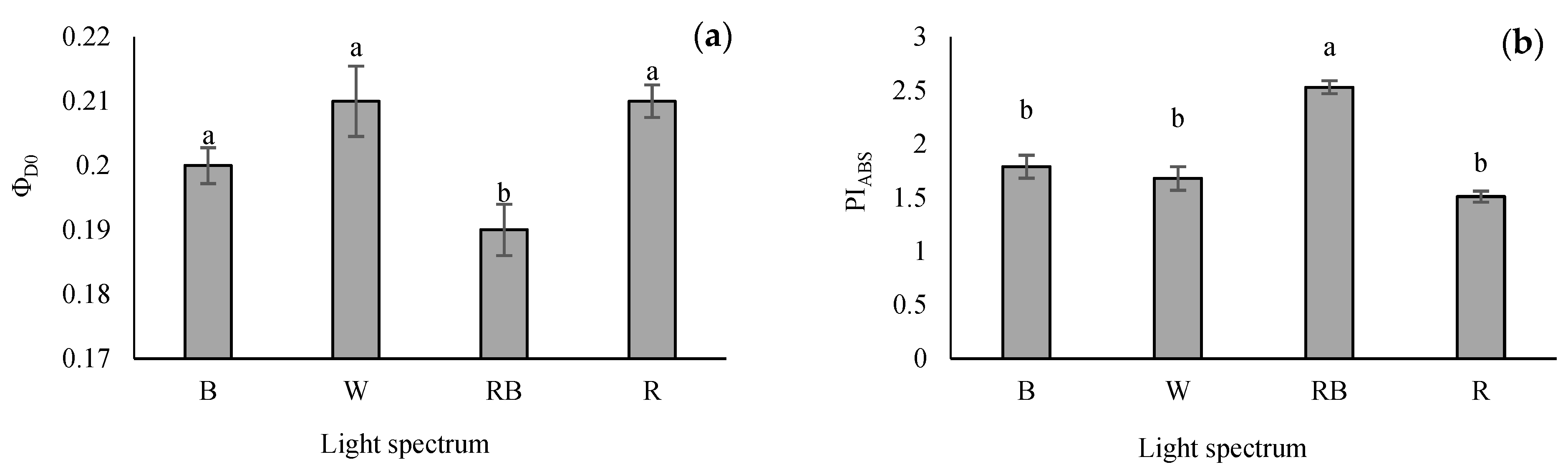
The lowest quantum yield of energy dissipation (φD0) was recorded in RB-grown seedlings, whereas the highest φD0 value was obtained from R and W light treatments. However, there was no remarkable difference in the φD0 value of seedlings grown under W, B and R treatments (Figure 5a). The performance index on the absorption basis (PIABS) is the validity index of PSII. This parameter was enhanced significantly in RB treatment. However, no differences were recorded in W-, B- and R-exposed seedlings (Figure 5b).
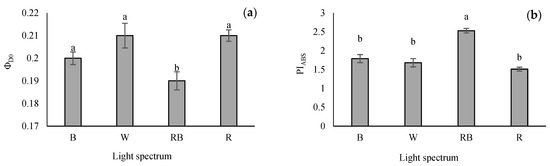
Figure 5.
The quantum yield of energy dissipation (φD0; (a)), and performance index in light absorption basis (PIABS; (b)) from the fluorescence transient exhibited by leaves sampled from grafted tomato seedlings exposed to different light qualities (including white (W; 35% B, 49% intermediate spectra, 16% R) light as control, blue (B), red (R), and a combination of red (68%) and blue (RB), (see the correspondence spectrum in Figure 1)), with 75 ± 5 µmol m−2 s−1 PPFD for 20 days. Columns with the same letters are not significantly different at p ≤ 0.05, according to Duncan’s multiple range tests. Bars represent the mean value of three replications ± SEM.
3.5. Chlorophyll Index
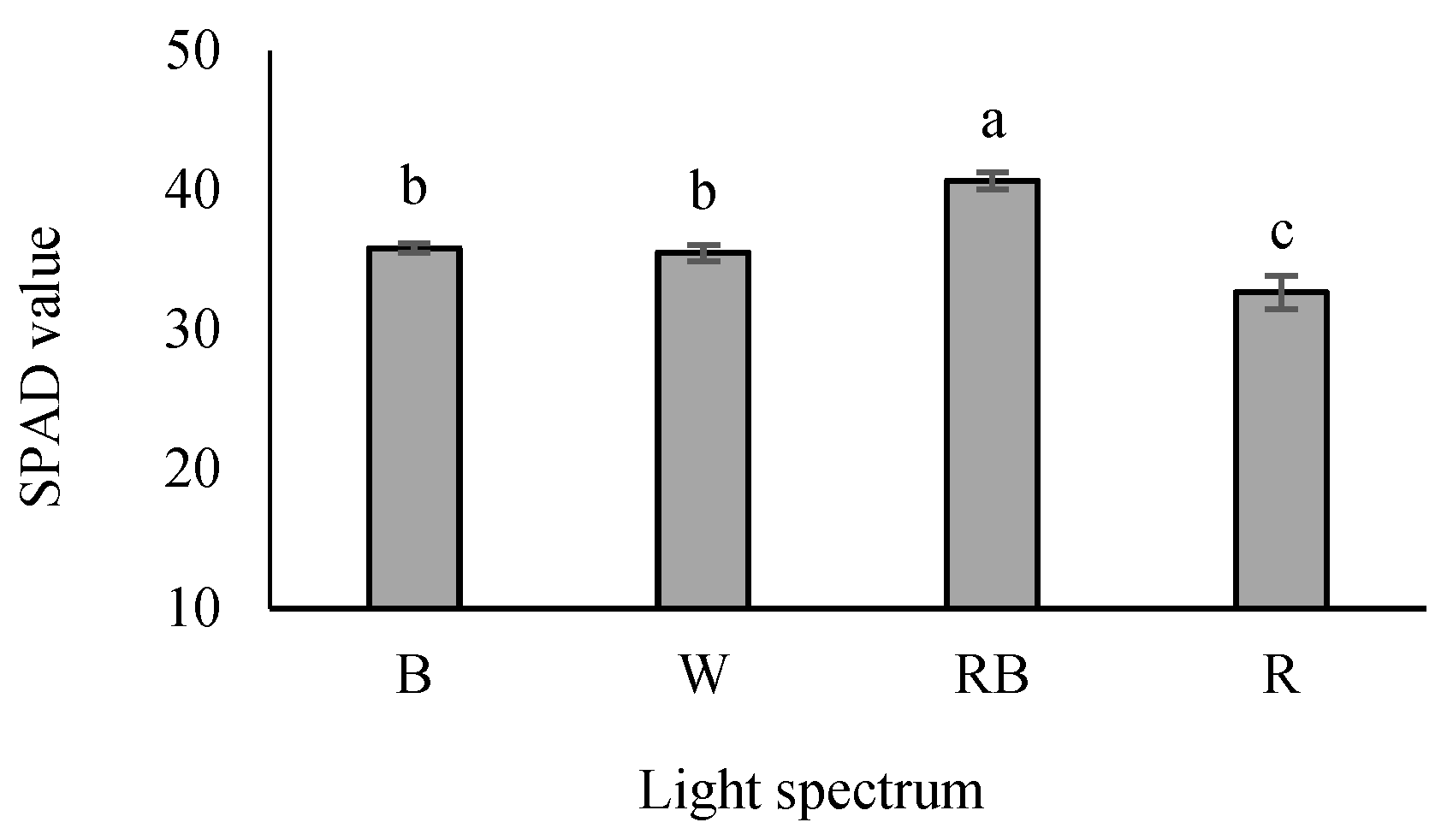
The SPAD value was the highest in RB-exposed seedlings, while its lowest value was detected under R-exposed seedlings. An intermediate SPAD value was also recognized in W- and B-exposed seedlings (Figure 6).
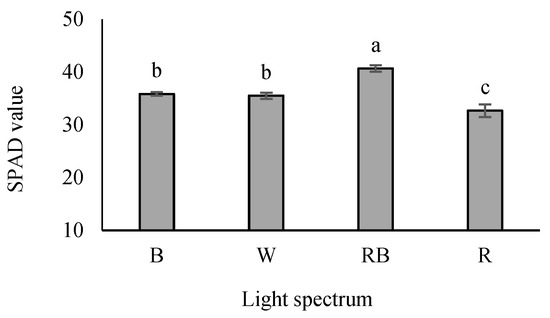
Figure 6.
SPAD values in grafted tomato seedlings exposed to different light qualities (including white (W; 35% B, 49% intermediate spectra, 16% R) light as control, blue (B), red (R), and a combination of red (68%) and blue (RB), (see the correspondence spectrum in Figure 1)), with 75 ± 5 µmol m−2 s−1 PPFD for 20 days. Columns with the same letters are not significantly different at p ≤ 0.05, according to Duncan’s multiple range tests. Bars represent the mean value of three replications ± SEM.
3.6. Antioxidant Enzymes Activity
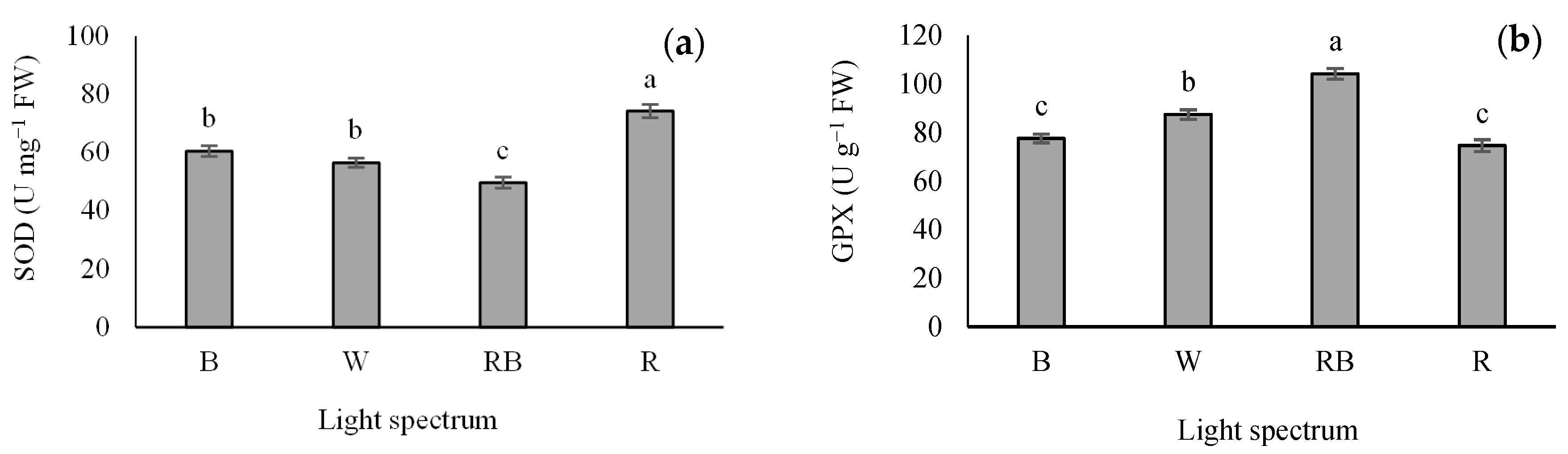
Different light treatments significantly influenced the activity of SOD and GPX. R light increased SOD activity in the seedlings. The lowest activity of SOD was detected in the RB treatment (Figure 7a), while the maximum GPX activity was detected in RB-exposed seedlings. Additionally, the lowest GPX activity was recorded in B- and R-exposed seedlings (Figure 7b).
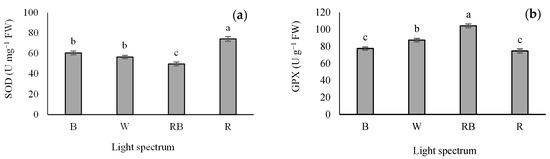
Figure 7.
Superoxide dismutase (SOD; (a)) and guaiacol peroxidase (GPX; (b)) activity in grafted tomato seedlings exposed to different light qualities (including white (W; 35% B, 49% intermediate spectra, 16% R) light as control, blue (B), red (R), and a combination of red (68%) and blue (RB), (see the correspondence spectrum in Figure 1)), with 75 ± 5 µmol m−2 s−1 PPFD for 20 days. Columns with the same letters are not significantly different at p ≤ 0.05, according to Duncan’s multiple range tests. Bars represent the mean value of three replications ± SEM.
3.7. Determination of Hydrogen Peroxide
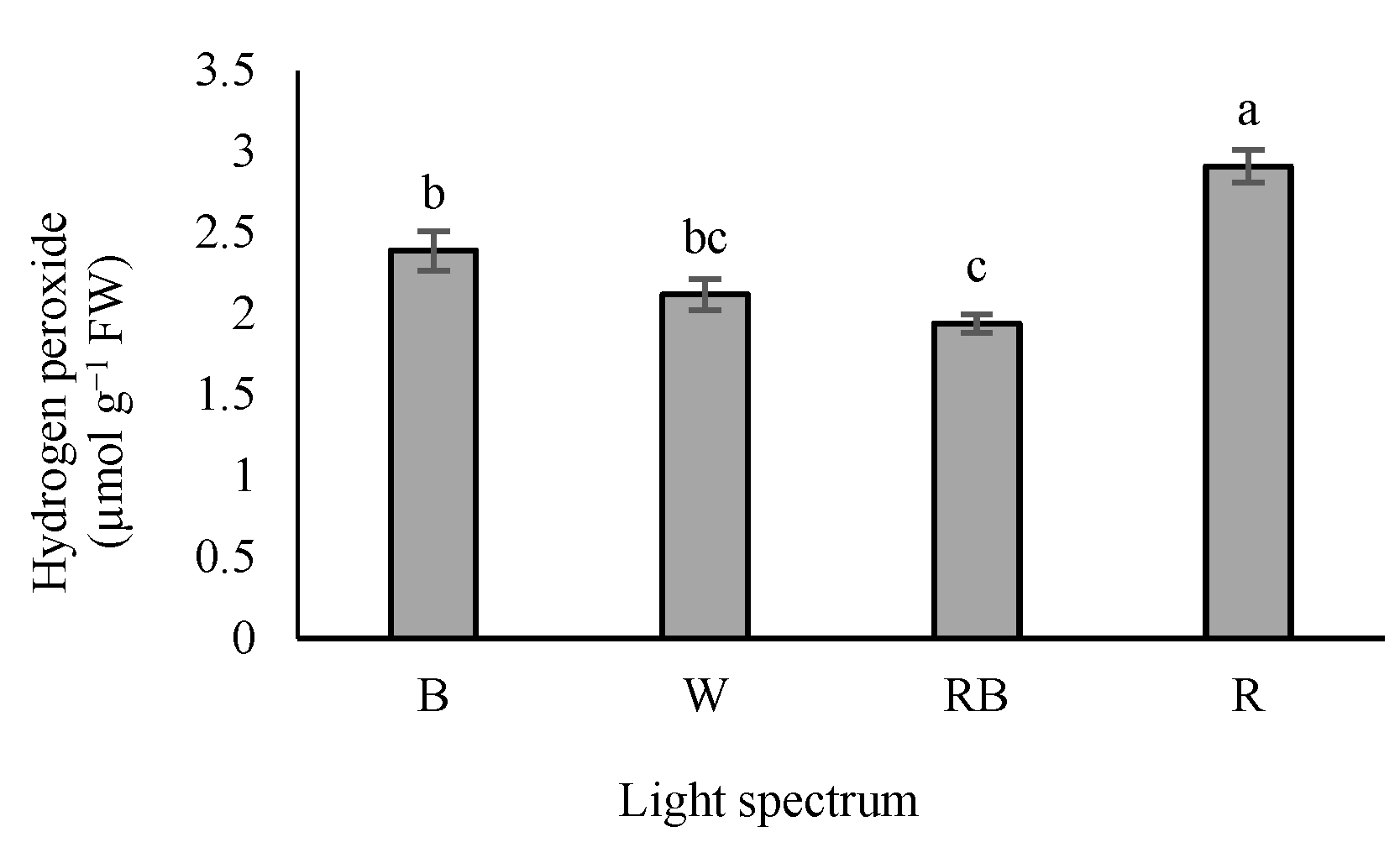
According to our results, H2O2 accumulation was the highest in seedlings exposed to R light, while exposure to W and RB lights reduced H2O2 levels in the leaves (Figure 8).
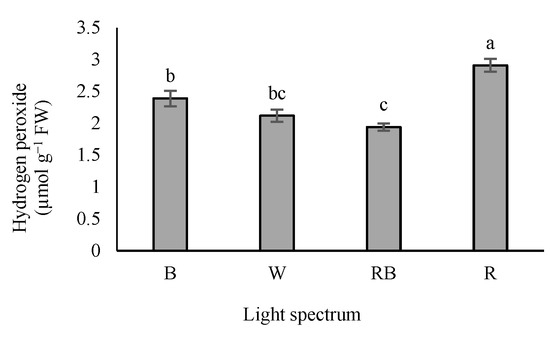
Figure 8.
Hydrogen peroxide (H2O2) level in grafted tomato seedlings exposed to different light qualities (including white (W; 35% B, 49% intermediate spectra, 16% R) light as control, blue (B), red (R), and a combination of red (68%) and blue (RB), (see the correspondence spectrum in Figure 1)), with 75 ± 5 µmol m−2 s−1 PPFD for 20 days. Columns with the same letters are not significantly different at p ≤ 0.05, according to Duncan’s multiple range tests. Bars represent the mean value of three replications ± SEM.
4. Discussion
4.1. Plant Fresh and Dry Weight and Stem Diameter Reduced in R Light
Seedlings’ photomorphogenesis is a strategic behavior to facilitate adaptation to environmental conditions. This process is directly affected by light, regulated by special receptors such as phytochromes for R light and cryptochromes and phototropin for B light. The signals released by the receptors induce physiological and biochemical variations in different growth processes [47]. In the present study, different lighting conditions resulted in a clear morphological response in different parts of the plants (Table 1). For example, R-exposed seedlings were characterized with the longest scion compared with the other light treatments, while the node number remained unaffected. These results are supported by previous studies showing that R light usually promotes internode elongation compared with monochromatic B light [8,22,48,49]. Many researchers have reported decreased stem length under B light exposure. B-light photoreceptors, i.e., cryptochromes, prevent stem elongation. Phytochromes, which are more sensitive acceptors to R light, stimulate cell division and development. Induction of the appearance of the short stem by B light and the long stem by R light has been previously reported in different plant species such as Arabidopsis [47,50], Norway spruce [51], Chrysanthemum [52] and soybean [53].
As a primary light source, the R light influences stem elongation via the role of phytohormones such as Gibberellins (GAs) or through blocking negative-GA signaling components in the tissue, which leads to the elongation of stem internodes. Furthermore, B light causes compactness by upregulating the negative-GA signaling components [51,54,55,56,57]. It has been shown that cryptochromes (i.e., sensitive photoreceptors to B) play critical roles in regulating hypocotyl cell elongation. Gibberellin synthesis decreases under B light, and thus the stem length decreases [56,57].
Our findings are also supported by previous studies in Arabidopsis thaliana and tomato [56,57]. In this case, Ouzounis et al. [58] reported that hypocotyl length and plant height were reduced by more prolonged exposure to B light. Liu et al. [59] showed that cherry tomato seedlings grown under R light treatment had more extraordinary stem lengths than those exposed to B light alone and B and R combinations. Chen et al. [60] reported that the plant height of rice seedlings grown under R light was 50% taller than B-exposed samples.
Stem diameter in R-exposed seedlings was lower than those under other light spectra. The higher stem diameter was in RB-exposed seedlings. However, no significant difference was detected among seedlings exposed to W, B and RB lights (Table 1). The higher stem diameter in seedlings grown under B light is probably because of the phytohormones status [61]. The influences of light regimes on grafted seedling quality have been previously documented [19]. As an essential indicator for seedling quality, the leaf area in RB-exposed seedlings was the widest compared with other treatments. The difference in RB- and R-exposed plants’ leaf area was linked to the leaf area rather than leaf number [8,53]. Moosavi-Nezhad et al. [19] reported that W- and RB-exposed watermelon seedlings had more expansive leaf areas than seedlings exposed to R light. Yousef et al. [48] also reported that the red and blue light combination (R7:B3) significantly increased leaf area. This study recorded the lowest root, shoot and plant dry weight in R-light-exposed seedlings, while RB-exposed seedlings represented the maximum shoot and plant dry weight (Table 1). Fang et al. [53] showed that soybean seedlings had higher root and total dry weight under mixed R and B lights than those exposed to R light alone. In addition, a greenhouse study showed that the shoot dry weight in tomato seedlings grown under RB light (R92%) was more than those cultivated under monochromatic R light [62]. It is notable that RB light treatment enhanced the tomato seedling biomass, compared with monochromic R and B light (Table 1). This could be because the peak absorption of chlorophyll occurs in the R and B wavelength range, and thus plants grown under RB light have the highest photosynthetic efficiency [24,63].
Many studies have shown that light and its quality are vital for seedling survival during the healing and acclimatization stages (~0–10 days) [48,57,64]. Light during the healing stage is necessary for grafted seedlings to stimulate callus induction and vascular junction formation [10,64]. The absence of light causes callus formation disturbances and leaves abscission of the grafted seedlings’ survival [65]. Due to the lack of carbon absorption in dark conditions, plants use carbohydrates in the shoot to survive [66]. In the absence of light for an extended period, plants must consume the reserves of starch, lipids and amino acids to maintain their metabolism [67,68,69]. After 12 days of dark treatment, the surviving seedlings showed inferior shoot development and a considerably weaker root system than those exposed to light [19]. Light application enhanced the survival ratio in this study without differences among the spectra (Table 1). Based on the obtained results, R light caused an unfavorable increase in the internode elongation and a decrease in stem diameter, leaf area and biomass in grafted tomato seedlings, reducing their quality. In contrast, RB light improved seedlings’ growth, quality and marketability.
4.2. Red Light-Induced Carbohydrate Accumulation
Among light treatments, R-exposed seedlings showed the maximum soluble carbohydrate concentration, while their lowest concentration was detected in RB-exposed seedlings (Figure 2). It has been reported that R-exposed watermelon seedlings had the highest soluble carbohydrate concentration [19]. Carbohydrate content affects auxin biosynthesis and transport, possibly representing the leaf epinasty under R light [70]. Damage to the photosynthetic apparatus reduces carbohydrate accumulation. However, it seems that enhanced carbohydrate levels in R-grown seedlings are probably due to the reduced translocation of carbohydrates outside the leaves [71]. This change is generally related to the down-regulation of photosynthesis [72] and consequently blocks sucrose synthesis and sugar phosphates’ cytosol accumulation [73].
4.3. Red Light-Induced SOD Enzymes Activity and H2O2 Accumulation
It is well-known that antioxidant enzymes play critical roles in scavenging the oxidative stress resulting from reactive oxygen species (ROS) accumulation in plants [74,75,76]. Antioxidant enzymes (e.g., SOD, APX, GPX and CAT) are known as essential factors in protecting plants from the damages caused by oxidative stress. SOD acts as the first line of defense that converts free radical oxygen into H2O2; then, the generated H2O2 is converted into H2O by CAT, GPX and APX. Accordingly, CAT, GPX and APX have the same activity [77]. The results of the present study indicated that seedlings exposed to R light increased SOD activity (Figure 7a). The maximum activity of GPX was observed in seedlings exposed to RB, whereas the lowest GPX activity was recorded in seedlings exposed to B and R lights (Figure 7b). H2O2 accumulation was the highest in R light treatment, while exposure to W and RB light treatments led to decreased H2O2 accumulation (Figure 8). Bayat et al. [78] reported a parallel correlation between SOD activity and H2O2 production, while CAT and APX negatively regulate H2O2 production. Therefore, it can be stated that the accumulation of H2O2 due to the stress could be related to the higher activity of SOD and lower activity of CAT and APX. In this experiment, higher activity of SOD and accumulation of H2O2 and lower activity of GPX were detected in R-exposed seedlings. In comparison, lower activity of SOD and accumulation of H2O2 and higher activity of GPX were observed in RB-exposed plants. On the other hand, the highest and the lowest soluble carbohydrates concentration were detected in R- and RB-exposed seedlings, respectively. Our results showed a positive correlation between soluble carbohydrate content and SOD activity. Therefore, reducing H2O2 accumulation and the activity of the GPX enzyme in RB light protected the photosynthesis apparatus and improved plant growth and development.
4.4. Photosynthetic Functionality of Tomato Grafted Seedlings Enhanced by RB Light
The OJIP analysis facilitates understanding the relationship between the structure and performance of photosynthetic apparatus and supports quick assessments of plant vitality [46]. The maximum quantum yield of PSII (Fv/Fm) is considered an appropriate indicator for the evaluation of the effects of environmental conditions on photosynthesis functionality [79]. As a result, R, B and W lights increased the quantum yield of energy dissipation (φD0) in PSII compared to RB light (Figure 5a). A higher value of this parameter represents the higher conversion of energy to heat. Plants also use this strategy to protect cells against damage caused by light severity [80].
In the present study, the values of the biophysical parameters associated with the PSII efficiency, such as Fv/F0, Fm/F0 and Fv/Fm, were the highest under RB light. In contrast, the lowest value of these parameters was detected in seedlings exposed to monochromic R light. However, no significant change was detected under W, B and R treatments. Chen et al. [81] reported that lower Fv/Fm levels caused by R light are due to the decreased photochemical activity resulting from the PSII reaction centers inactivation and damage to the D1 protein.
The negative impacts of monochromic R light on the functioning of the electron transport chain of the photosynthetic apparatus have been reported for other plant species such as cucumber [82,83], tomato [62,84], watermelon [19], saffron [85] and chrysanthemum [22]. These adverse effects are evaluated mainly by evaluating the performance index according to the light energy absorption (PIABS). This parameter unifies the energy fluxes from the first stage of the light absorption to the plastoquinone reduction, giving sufficient and quantitative data on plants’ state and vitality [29]. In many studies, this parameter is the most accurate parameter for photosynthetic performance assessment in plants [30,78,86]. A lower level of PIABS possibly means a low potential for developing the trans-thylakoid proton gradient [46]. Additionally, the reduction in PIABS can be caused by the suppression of electron transfer resulting from decreased PSII functionality [87]. The present study recorded the lowest quantum yield of energy dissipation (φD0) and highest performance index on the absorption basis (PIABS) in RB-grown seedlings. Therefore, RB light treatment caused an increase in PSII photochemical performance in grafted tomato seedlings.
Moreover, the reduction in PSII photochemical performance in R-exposed seedlings could be due to the lower maximum quantum yield of PSII (Fv/Fm) [88,89]. The negative impacts of R light (known as red light syndrome) have been widely reported [82,90,91,92]. On the other hand, RB light can improve photosynthesis functioning and performance by reducing energy dissipation and elevating electron transport in the electron transport chain [57,63]. These findings illustrated the importance of RB light on the performance of PSII of the tomato grafted seedlings. Standard photosynthetic functionality needs a specific B-to-R golden ratio in the overall spectra.
Our results illustrated that the SPAD value was affected by light regimes, so RB-exposed seedlings had the highest value, while it was the lowest in R-exposed seedlings. Zheng and Van Labeke [93] reported that the total chlorophyll content is higher under a light intensity of 100 μmol m−2 s−1, and the total chlorophyll is higher under B and RB treatments. Additionally, it has been reported that R monochromatic light has reduced the chlorophyll content in the scion leaf [19]. In contrast, the influence of monochromatic R light on chlorophyll status is linked to enhanced carbohydrate accumulation. Generally, chlorophyll depletion and chloroplast deformation increase carbohydrate accumulation [94]. Hence, the marketability of seedlings grown under monochromatic R light is expected to be lower due to reduced greening. In contrast, grafted seedlings under RB light have higher quality.
5. Conclusions
The current study concentrated on light quality’s effects on the growth, primary metabolites and photosynthetic performance of tomato-grafted seedlings. Seedlings grown under R light showed the minimum leaf area, root, shoot fresh and dry weights and occurrence of leaf epinasty. This syndrome reduces the interception of light and thus reduces the quality of seedlings. In addition, a decrease in photosynthetic functionality was detected under the R light treatment. Seedlings grown under R light contained the maximum F0, Fm and Fv, whereas the maximum Fv/F0 and Fm/F0 were obtained under RB-exposed seedlings. Different light treatments significantly affected the maximum quantum yield of PSII. Therefore, the maximum Fv/Fm was obtained from RB light treatment. PIABS and φD0 showed a negative correlation. The lowest quantum yield of energy dissipation (φD0) and highest performance index on the absorption basis (PIABS) was recorded in RB-grown seedlings.
In contrast, the highest φD0 value was obtained from R and W light treatments. A positive correlation was observed between soluble carbohydrates and H2O2 concentration. R-grown seedlings had the highest soluble carbohydrates and H2O2 concentration, whereas the maximum GPX activity was noted in RB-exposed seedlings. The reduction of H2O2 accumulation and the activity of the GPX enzyme in RB light protected the photosynthesis apparatus and improved plant growth and development. SOD activity increased, while GPX activity decreased in R-exposed seedlings. The highest leaf area and plant, root, and shoot dry weights were recorded in RB-exposed seedlings. The results showed that leaf area is highly correlated with shoot dry weight. RB-exposed seedlings had the highest leaf area and shoot dry weight, while the lowest values of these two traits were found in R-exposed seedlings. RB light promoted tomato-grafted seedlings’ photosynthetic performance by affecting electron flow between PSII and PSI. B- and RB-exposed seedlings were similar in some of the evaluated morphological traits (such as in scion stem diameter, root fresh and dry weights). Therefore, RB light is recommended as the lighting environment for producing tomato-grafted seedlings during the healing and acclimatization stage.
Author Contributions
Conceptualization, S.S., H.A., R.S., S.H.N. and M.M.-N.; methodology, S.S., H.A., R.S. and M.M.-N.; software, S.S.; validation, R.S., S.A. and N.S.G.; formal analysis, S.S.; investigation, S.S.; resources, H.A., R.S. and S.A., data curation, H.A., R.S. and M.M.-N.; writing—original draft preparation, S.S.; writing—review and editing, M.M.-N., N.S.G. and S.A.; visualization, S.S. and M.M.-N.; supervision, H.A.; project administration, H.A.; funding acquisition, S.A. and N.S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
We thank the laboratory staff for their contributions, continued diligence, and dedication to their craft. The authors express deep gratitude to Saeid Eilkhani for his help during the construction of the growth chambers. The valuable comments of the editor and three anonymous reviewers are also greatly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| B | blue |
| R | red |
| RB | red and blue |
| W | white |
| D | darkness |
| OJIP | polyphasic chlorophyll fluorescence induction curve |
| FW | Fresh weight |
| PPFD | photosynthetic photon flux density |
| PSII | photosystem II |
| SOD | superoxide dismutase |
| GPX | guaiacol peroxidase |
| H2O2 | hydrogen peroxide |
| RC | reaction center |
| NBT | nitro blue tetrazolium |
| KI | potassium iodide |
| TCA | Trichloroacetic acid |
References
- King, S.R.; Davis, A.R.; Zhang, X.; Crosby, K. Genetics, Breeding and Selection of Rootstocks for Solanaceae and Cucurbitaceae. Sci. Hortic. 2010, 127, 106–111. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Colla, G. Vegetable Grafting: A Toolbox for Securing Yield Stability under Multiple Stress Conditions. Front. Plant Sci. 2018, 8, 2255. [Google Scholar] [CrossRef]
- Gruda, N.; Savvas, D.; Colla, G.; Rouphael, Y. Impacts of Genetic Material and Current Technologies on Product Quality of Selected Greenhouse Vegetables—A Review. Eur. J. Hortic. Sci. 2018, 83, 319–328. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A.; Siomos, A.; Menexes, G.; Dangitsis, C.; Kintzonidis, D. Assessing Quantitative Criteria for Characterization of Quality Categories for Grafted Watermelon Seedlings. Horticulturae 2019, 5, 16. [Google Scholar] [CrossRef]
- Abdelmageed, A.H.A.; Gruda, N. Influence of Grafting on Growth, Development and Some Physiological Parameters of Tomatoes under Controlled Heat Stress Conditions. Eur. J. Hortic. Sci. 2009, 74, 16–20. [Google Scholar]
- Colla, G.; Pérez-Alfocea, F.; Schwarz, D. Vegetable Grafting: Principles and Practices; CAB International: Oxfordshire, UK, 2017; ISBN 9781780648972. [Google Scholar]
- Moosavi-Nezhad, M.; Salehi, R.; Aliniaeifard, S.; Winans, K.S.; Nabavi-Pelesaraei, A. An Analysis of Energy Use and Economic and Environmental Impacts in Conventional Tunnel and LED-Equipped Vertical Systems in Healing and Acclimatization of Grafted Watermelon Seedlings. J. Clean. Prod. 2022, 361, 132069. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A.; Siomos, A.S.; Fotelli, M.N.; Kintzonidis, D. Bichromatic Red and Blue LEDs during Healing Enhance the Vegetative Growth and Quality of Grafted Watermelon Seedlings. Sci. Hortic. 2020, 261, 109000. [Google Scholar] [CrossRef]
- Dong, W.; Zhou, Z.C.; Bu, Y.L.; Zhuo, J.Q.; Chen, L.Z.; Li, Y.Z. Research and Application of Grafted Seedlings Healing Room. Acta Hortic. 2015, 1086, 51–58. [Google Scholar] [CrossRef]
- Lee, K.M.; Lim, C.S.; Muneer, S.; Jeong, B.R. Functional Vascular Connections and Light Quality Effects on Tomato Grafted Unions. Sci. Hortic. 2016, 201, 306–317. [Google Scholar] [CrossRef]
- Lang, K.M.; Nair, A.; Litvin, A.G. An Alternative Healing Method for Grafted Tomato Transplants: The Effect of Light Exclusion and Substrate Temperature on Plant Survival and Growth. Horttechnology 2020, 30, 677–684. [Google Scholar] [CrossRef]
- Klem, K.; Gargallo-Garriga, A.; Rattanapichai, W.; Oravec, M.; Holub, P.; Veselá, B.; Sardans, J.; Peñuelas, J.; Urban, O. Distinct Morphological, Physiological, and Biochemical Responses to Light Quality in Barley Leaves and Roots. Front. Plant Sci. 2019, 10, 1026. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Nieuwendijk, N.M.; Pantazopoulou, C.K.; Pierik, R. Light Signalling Shapes Plant–Plant Interactions in Dense Canopies. Plant Cell Environ. 2021, 44, 1014–1029. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of Different Light Intensities on Chlorophyll Fluorescence Characteristics and Yield in Lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Academic Press: San Diego, CA, USA, 2019; ISBN 9780128166918. [Google Scholar]
- Li, H.; Tang, C.; Xu, Z. The Effects of Different Light Qualities on Rapeseed (Brassica napus L.) Plantlet Growth and Morphogenesis in Vitro. Sci. Hortic. 2013, 150, 117–124. [Google Scholar] [CrossRef]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of Phenolic Acid and Flavonoid Synthesis to Blue and Blue-Violet Light Depends on Plant Species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Hajilou, J.; Tabatabaei, S.J. Photosynthetic and Growth Responses of Olive to Proline and Salicylic Acid under Salinity Condition. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 579–585. [Google Scholar] [CrossRef]
- Moosavi-Nezhad, M.; Salehi, R.; Aliniaeifard, S.; Tsaniklidis, G.; Woltering, E.J.; Fanourakis, D.; Żuk-Gołaszewska, K.; Kalaji, H.M. Blue Light Improves Photosynthetic Performance during Healing and Acclimatization of Grafted Watermelon Seedlings. Int. J. Mol. Sci. 2021, 22, 8043. [Google Scholar] [CrossRef]
- Viršile, A.; Olle, M.; Duchovskis, P. LED Lighting in Horticulture. In Light Emitting Diodes for Agriculture: Smart Lighting; Springer Singapore: Singapore, 2017; pp. 113–147. ISBN 9789811058073. [Google Scholar]
- Meiramkulova, K.; Tanybayeva, Z.; Kydyrbekova, A.; Turbekova, A.; Aytkhozhin, S.; Zhantasov, S.; Taukenov, A. The Efficiency of Led Irradiation for Cultivating High-Quality Tomato Seedlings. Sustainability 2021, 13, 9426. [Google Scholar] [CrossRef]
- Seif, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Shomali, A.; Fanourakis, D.; Li, T.; Woltering, E. Monochromatic Red Light during Plant Growth Decreases the Size and Improves the Functionality of Stomata in Chrysanthemum. Funct. Plant Biol. 2021, 48, 515–528. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, B.; Li, J.; Tang, H.; Tang, J.; Yang, Z. Formation and Change of Chloroplast-Located Plant Metabolites in Response to Light Conditions. Int. J. Mol. Sci. 2018, 19, 654. [Google Scholar] [CrossRef]
- Shin, K.S.; Murthy, H.N.; Heo, J.W.; Hahn, E.J.; Paek, K.Y. The Effect of Light Quality on the Growth and Development of in Vitro Cultured Doritaenopsis Plants. Acta Physiol. Plant. 2008, 30, 339–343. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Carbohydrate Accumulation and Sucrose Metabolism Responses in Tomato Seedling Leaves When Subjected to Different Light Qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]
- Whitelam, G.C.; Halliday, K.J. Light and Plant Development; Garry, C., Whitelam, K.J.H., Eds.; Wiley: Hoboken, NJ, USA, 2007; ISBN 9780470988893. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Papageorgiou, G., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Kalaji, H.M.; Govindjee; Bosa, K.; Kościelniak, J.; Zuk-Gołaszewska, K. Effects of Salt Stress on Photosystem II Efficiency and CO2 Assimilation of Two Syrian Barley Landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanism, Regulation & Adaptation; Taylor and Francis: London, UK, 2000; pp. 443–480. ISBN 0748408215. [Google Scholar]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Rapacz, M.; Sasal, M.; Kalaji, H.M.; Kościelniak, J. Is the OJIP Test a Reliable Indicator of Winter Hardiness and Freezing Tolerance of Common Wheat and Triticale under Variable Winter Environments? PLoS ONE 2015, 10, e0134820. [Google Scholar] [CrossRef]
- Kinoshita, T.; Doi, M.; Suetsugu, N.; Kagawa, T.; Wada, M.; Shimazaki, K.I. Phot1 and Phot2 Mediate Blue Light Regulation of Stomatal Opening. Nature 2001, 414, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.C.; Alcaraz-López, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological Aspects of Rootstock-Scion Interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Estaji, A.; Kalaji, H.M.; Karimi, H.R.; Roosta, H.R.; Moosavi-Nezhad, S.M. How Glycine Betaine Induces Tolerance of Cucumber Plants to Salinity Stress? Photosynthetica 2019, 57, 753–761. [Google Scholar] [CrossRef]
- Moosavi-Nezhad, M.; Alibeigi, B.; Estaji, A.; Gruda, N.S.; Aliniaeifard, S. Growth, Biomass Partitioning, and Photosynthetic Performance of Chrysanthemum Cuttings in Response to Different Light Spectra. Plants 2022, 11, 3337. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, W.G. Water Relations of Cut Flowers: An Update. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; Volume 40, pp. 55–106. [Google Scholar]
- Dhindsa, R.S.; Plumb-dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Flocco, C.G.; Giulietti, A.M. In Vitro Hairy Root Cultures as a Tool for Phytoremediation Research. In Phytoremediation: Methods and Reviews; Springer: Berlin/Heidelberg, Germany, 2007; pp. 161–173. [Google Scholar]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of Hydrogen Peroxide in Plant Extracts Using Titanium(IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Hoel, B.O.; Solhaug, K.A. Effect of Irradiance on Chlorophyll Estimation with the Minolta SPAD-502 Leaf Chlorophyll Meter. Ann. Bot. 1998, 82, 389–392. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Van Meeteren, U. Natural Variation in Stomatal Response to Closing Stimuli among Arabidopsis Thaliana Accessions after Exposure to Low VPD as a Tool to Recognize the Mechanism of Disturbed Stomatal Functioning. J. Exp. Bot. 2014, 65, 6529–6542. [Google Scholar] [CrossRef]
- Ashrostaghi, T.; Aliniaeifard, S.; Shomali, A.; Azizinia, S.; Koohpalekani, J.A.; Moosavi-Nezhad, M.; Gruda, N.S. Light Intensity: The Role Player in Cucumber Response to Cold Stress. Agronomy 2022, 12, 201. [Google Scholar] [CrossRef]
- Moradi, S.; Kafi, M.; Aliniaeifard, S.; Moosavi-Nezhad, M.; Pedersen, C.; Gruda, N.S.; Salami, S.A. Monochromatic Blue Light Enhances Crocin and Picrocrocin Content by Upregulating the Expression of Underlying Biosynthetic Pathway Genes in Saffron (Crocus sativus L.). Front. Hortic. 2022, 1, 960423. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, A. Applications of Chlorophyll Fluorescence Imaging Technique in Horticultural Research: A Review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J. Govindjee Chlorophyll a Fluorescence Induction: Can Just a One-Second Measurement Be Used to Quantify Abiotic Stress Responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Goltsev, V.N.; Żuk-Golaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781498764506. [Google Scholar]
- Yu, X.; Liu, H.; Klejnot, J.; Lin, C. The Cryptochrome Blue Light Receptors. Arab. Book 2010, 8, e0135. [Google Scholar] [CrossRef]
- Yousef, A.F.; Ali, M.M.; Rizwan, H.M.; Ahmed, M.A.A.; Ali, W.M.; Kalaji, H.M.; Elsheery, N.; Wróbel, J.; Xu, Y.; Chen, F. Effects of Light Spectrum on Morphophysiological Traits of Grafted Tomato Seedlings. PLoS ONE 2021, 16, e0250210. [Google Scholar] [CrossRef]
- Nguyen, D.T.P.; Kitayama, M.; Lu, N.; Takagaki, M. Improving Secondary Metabolite Accumulation, Mineral Content, and Growth of Coriander (Coriandrum sativum L.) by Regulating Light Quality in a Plant Factory. J. Hortic. Sci. Biotechnol. 2020, 95, 356–363. [Google Scholar] [CrossRef]
- Neff, M.M.; Fankhauser, C.; Chory, J. Eight: An Indicator of Time and Place. Genes Dev. 2000, 14, 257–271. [Google Scholar] [CrossRef]
- Yang, F.O.; Mao, J.F.; Wang, J.; Zhang, S.; Li, Y. Transcriptome Analysis Reveals That Red and Blue Light Regulate Growth and Phytohormone Metabolism in Norway Spruce [Picea abies (L.) Karst.] . PLoS ONE 2015, 10, e0127896. [Google Scholar] [CrossRef]
- Dierck, R.; Dhooghe, E.; Van Huylenbroeck, J.; Van Der Straeten, D.; De Keyser, E. Light Quality Regulates Plant Architecture in Different Genotypes of Chrysanthemum Morifolium Ramat. Sci. Hortic. 2017, 218, 177–186. [Google Scholar] [CrossRef]
- Fang, L.; Ma, Z.; Wang, Q.; Nian, H.; Ma, Q.; Huang, Q.; Mu, Y. Plant Growth and Photosynthetic Characteristics of Soybean Seedlings Under Different LED Lighting Quality Conditions. J. Plant Growth Regul. 2021, 40, 668–678. [Google Scholar] [CrossRef]
- Lau, O.S.; Deng, X.W. Plant Hormone Signaling Lightens up: Integrators of Light and Hormones. Curr. Opin. Plant Biol. 2010, 13, 571–577. [Google Scholar] [CrossRef]
- El Kayal, W.; Allen, C.C.G.; Ju, C.J.T.; Adams, E.; King-Jones, S.; Zaharia, L.I.; Abrams, S.R.; Cooke, J.E.K. Molecular Events of Apical Bud Formation in White Spruce, Picea Glauca. Plant Cell Environ. 2011, 34, 480–500. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Walton, L.J.; Hayward, A.; Emery, R.J.N.; Pharis, R.P.; Reid, D.M. Interactions between Plant Hormones and Light Quality Signaling in Regulating the Shoot Growth of Arabidopsis Thaliana Seedlings. Botany 2012, 90, 237–246. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Shi, Q.; Yang, F.; Wei, M. Mixed Red and Blue Light Promotes Tomato Seedlings Growth by Influencing Leaf Anatomy, Photosynthesis, CO2 Assimilation and Endogenous Hormones. Sci. Hortic. 2021, 290, 110500. [Google Scholar] [CrossRef]
- Ouzounis, T.; Fretté, X.; Rosenqvist, E.; Ottosen, C.O. Spectral Effects of Supplementary Lighting on the Secondary Metabolites in Roses, Chrysanthemums, and Campanulas. J. Plant Physiol. 2014, 171, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Xiao-ying, L.; Zhi-gang, X.; Tao-tao, C.; Shi-rong, G. Growth and Photosynthesis of Cherry Tomato Seedling Exposed to Different Low Light of LED Light Quality. Acta Bot. Boreali-Occident. Sincia 2010, 30, 725–732. [Google Scholar]
- Chen, C.C.; Huang, M.Y.; Lin, K.H.; Wong, S.L.; Huang, W.D.; Yang, C.M. Effects of Light Quality on the Growth, Development and Metabolism of Rice Seedlings (Oryza sativa L.). Res. J. Biotechnol. 2014, 9, 15–24. [Google Scholar]
- Wang, L.; Chen, X.; Wang, Q.; Hao, J.; Lan, J. Effect of Different Light of LED Light Quality on Growth and Antioxidant Enzyme Activities of Ganoderma Lucidum. Zhongguo Zhongyao Zazhi 2011, 36, 2471–2474. [Google Scholar] [CrossRef]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F.M. Adding Blue to Red Supplemental Light Increases Biomass and Yield of Greenhouse-Grown Tomatoes, but Only to an Optimum. Front. Plant Sci. 2019, 9, 2002. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, X.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. Photosynthetic Characteristics and Chloroplast Ultrastructure of Welsh Onion (Allium fistulosum L.) Grown under Different LED Wavelengths. BMC Plant Biol. 2020, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hong, Y.; Zhang, X. Effect of Light Quality on Calluses Induction and Differentiation of Capsicum Annuum. J. Hunan Agric. Univ. 2009, 35, 615–617. [Google Scholar]
- Vu, N.T.; Kim, Y.S.; Kang, H.M.; Kim, I.S. Influence of Short-Term Irradiation during Pre- and Post-Grafting Period on the Graft-Take Ratio and Quality of Tomato Seedlings. Hortic. Environ. Biotechnol. 2014, 55, 27–35. [Google Scholar] [CrossRef]
- Zavala, J.A.; Ravetta, D.A. Allocation of Photoassimilates to Biomass, Resin and Carbohydrates in Grindelia Chiloensis as Affected by Light Intensity. Field Crops Res. 2001, 69, 143–149. [Google Scholar] [CrossRef]
- Smith, A.M.; Stitt, M. Coordination of Carbon Supply and Plant Growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef]
- Fan, J.; Yu, L.; Xu, C. A Central Role for Triacylglycerol in Membrane Lipid Breakdown, Fatty Acid β-Oxidation, and Plant Survival under Extended Darkness. Plant Physiol. 2017, 174, 1517–1530. [Google Scholar] [CrossRef]
- Usadel, B.; Bläsing, O.E.; Gibon, Y.; Poree, F.; Höhne, M.; Günter, M.; Trethewey, R.; Kamlage, B.; Poorter, H.; Stitt, M. Multilevel Genomic Analysis of the Response of Transcripts, Enzyme Activities and Metabolites in Arabidopsis Rosettes to a Progressive Decrease of Temperature in the Non-Freezing Range. Plant Cell Environ. 2008, 31, 518–547. [Google Scholar] [CrossRef]
- Pham, M.D.; Hwang, H.; Park, S.W.; Cui, M.; Lee, H.; Chun, C. Leaf Chlorosis, Epinasty, Carbohydrate Contents and Growth of Tomato Show Different Responses to the Red/Blue Wavelength Ratio under Continuous Light. Plant Physiol. Biochem. 2019, 141, 477–486. [Google Scholar] [CrossRef]
- Sæbø, A.; Krekling, T.; Appelgren, M. Light Quality Affects Photosynthesis and Leaf Anatomy of Birch Plantlets in Vitro. Plant Cell Tissue Organ Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Jeannette, E.; Reyss, A.; Grégory, N.; Gantet, P.; Prioul, J.L. Carbohydrate Metabolism in a Heat-Girdled Maize Source Leaf. Plant Cell Environ. 2000, 23, 61–69. [Google Scholar] [CrossRef]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of Light Quality on CO2 Assimilation, Chlorophyll-Fluorescence Quenching, Expression of Calvin Cycle Genes and Carbohydrate Accumulation in Cucumis Sativus. J. Photochem. Photobiol. B Biol. 2009, 96, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Sharma, P.K. High-Light–Induced Changes on Photosynthesis, Pigments, Sugars, Lipids and Antioxidant Enzymes in Freshwater (Nostoc Spongiaeforme) and Marine (Phormidium Corium) Cyanobacteria. Photochem. Photobiol. 2006, 82, 702–710. [Google Scholar] [CrossRef]
- Distelbarth, H.; Nägele, T.; Heyer, A.G. Responses of Antioxidant Enzymes to Cold and High Light Are Not Correlated to Freezing Tolerance in Natural Accessions of Arabidopsis Thaliana. Plant Biol. 2013, 15, 982–990. [Google Scholar] [CrossRef]
- Xu, F.; Shi, L.; Chen, W.; Cao, S.; Su, X.; Yang, Z. Effect of Blue Light Treatment on Fruit Quality, Antioxidant Enzymes and Radical-Scavenging Activity in Strawberry Fruit. Sci. Hortic. 2014, 175, 181–186. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of Growth under Different Light Spectra on the Subsequent High Light Tolerance in Rose Plants. AoB Plants 2018, 10, ply052. [Google Scholar] [CrossRef]
- Lee, T.Y.; Woo, S.Y.; Kwak, M.J.; Inkyin, K.; Lee, K.E.; Jang, J.H.; Kim, I.R. Photosynthesis and Chlorophyll Fluorescence Responses of Populus Sibirica to Water Deficit in a Desertification Area in Mongolia. Photosynthetica 2016, 54, 317–320. [Google Scholar] [CrossRef]
- Falqueto, A.R.; da Silva Júnior, R.A.; Gomes, M.T.G.; Martins, J.P.R.; Silva, D.M.; Partelli, F.L. Effects of Drought Stress on Chlorophyll a Fluorescence in Two Rubber Tree Clones. Sci. Hortic. 2017, 224, 238–243. [Google Scholar] [CrossRef]
- Chen, Y.E.; Mao, H.T.; Wu, N.; Din, A.M.U.; Khan, A.; Zhang, H.Y.; Yuan, S. Salicylic Acid Protects Photosystem Ii by Alleviating Photoinhibition in Arabidopsis Thaliana under High Light. Int. J. Mol. Sci. 2020, 21, 1229. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue Light Dose-Responses of Leaf Photosynthesis, Morphology, and Chemical Composition of Cucumis Sativus Grown under Different Combinations of Red and Blue Light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue Light Alleviates ‘Red Light Syndrome’ by Regulating Chloroplast Ultrastructure, Photosynthetic Traits and Nutrient Accumulation in Cucumber Plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Wang, L.W.; Li, Y.; Xin, G.F.; Wei, M.; Mi, Q.H.; Yang, Q.C. Effects of Different Proportions of Red and Blue Light on the Growth and Photosynthesis of Tomato Seedlings. Chin. J. Appl. Ecol. 2017, 28, 1595–1602. [Google Scholar] [CrossRef]
- Moradi, S.; Kafi, M.; Aliniaeifard, S.; Salami, S.A.; Shokrpour, M.; Pedersen, C.; Moosavi-Nezhad, M.; Wróbel, J.; Kalaji, H.M. Blue Light Improves Photosynthetic Performance and Biomass Partitioning toward Harvestable Organs in Saffron (Crocus sativus L.). Cells 2021, 10, 1994. [Google Scholar] [CrossRef]
- Mathur, S.; Mehta, P.; Jajoo, A. Effects of Dual Stress (High Salt and High Temperature) on the Photochemical Efficiency of Wheat Leaves (Triticum Aestivum). Physiol. Mol. Biol. Plants 2013, 19, 179–188. [Google Scholar] [CrossRef]
- Bukhov, N.G.; Drozdova, I.S.; Bondar, V.V.; Mokronosov, A.T. Blue, Red and Blue plus Red Light Control of Chlorophyll Content and CO2 Gas Exchange in Barley Leaves: Quantitative Description of the Effects of Light Quality and Fluence Rate. Physiol. Plant. 1992, 85, 632–638. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Keresztes, Á.; Sárvári, É.; Jaglarz, A.; Fischer, W. Multiple Effects of Chromate on Spirodela Polyrhiza: Electron Microscopy and Biochemical Investigations. Plant Biol. 2003, 5, 315–323. [Google Scholar] [CrossRef]
- Oukarroum, A.; Madidi, S.E.; Schansker, G.; Strasser, R.J. Probing the Responses of Barley Cultivars (Hordeum vulgare L.) by Chlorophyll a Fluorescence OLKJIP under Drought Stress and Re-Watering. Environ. Exp. Bot. 2007, 60, 438–446. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Seif, M.; Arab, M.; Mehrjerdi, M.Z.; Li, T.; Lastochkina, O. Growth and Photosynthetic Performance of Calendula Officinalis under Monochromatic Red Light. Int. J. Hortic. Sci. Technol. 2018, 5, 123–132. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of Photosynthesis after the “red Light Syndrome” in Cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.O. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Zheng, L.; Van Labeke, M.C. Effects of Different Irradiation Levels of Light Quality on Chrysanthemum. Sci. Hortic. 2018, 233, 124–131. [Google Scholar] [CrossRef]
- Krapp, A.; Quick, W.P.; Stitt, M. Ribulose-1,5-Bisphosphate Carboxylase-Oxygenase, Other Calvin-Cycle Enzymes, and Chlorophyll Decrease When Glucose Is Supplied to Mature Spinach Leaves via the Transpiration Stream. Planta 1991, 186, 58–69. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).