Abstract
This study explores the bioactive potential of young shoots from blackcurrant, European blueberry, and mountain cranberry, widely employed in gemmotherapy and phytotherapy, as rich sources of antioxidants, antimicrobial agents, and anti-inflammatory components. The primary aims of this study were to enhance the extraction conditions for bioactive compounds from blackcurrant young shoots using Modde software for experimental design, to conduct a comprehensive phytochemical analysis of blackcurrant, European blueberry, and mountain cranberry young shoot extracts through LC–MS analysis, and to evaluate the in vitro biological activities of these optimized extracts. The experimental design comprised multiple variables: extraction techniques, solvent type, extraction time, apparent pH, and the solvent-to-vegetal product ratio. The responses included total phenolic content, total flavonoid content, condensed tannin content, and total antioxidant activity determined through the DPPH assay. Furthermore, the antioxidant potential of the extracts was validated through in vitro cell culture experiments, in addition to the cytotoxicity assessments conducted on both normal and cancer cell lines. Extracts obtained through Ultra-Turrax extraction using 70% acetone displayed high levels of polyphenolic compounds and enhanced antioxidant potential, regardless of young shoots origin. LC–MS analysis revealed the predominant occurrence of chlorogenic acid, hyperoside, and isoquercitrin in all examined samples. The optimized extracts also displayed significant biological potential when evaluated in vitro on cell lines. These results provide valuable insights into the potent bioactive components present in these young shoot extracts, paving the way for further exploration in therapeutic applications.
1. Introduction
The historical utilization of plants in traditional medicine and the knowledge acquired from folk medicine have formed the foundation for extracting active compounds and investigating their potential applications across diverse industries [1,2]. Given the rapid development of research and technology, advances in medicine and in toxicological evaluations, plant extracts and natural bioactive compounds are gaining more and more ground and are increasingly preferred to synthetic antioxidants in the pharmaceutical, cosmetic, and food industries.
Berries are well known for their nutritional benefits and for their rich content of bioactive compounds imparting health benefits and have been the subject of many scientific studies in recent years [3,4,5]. Fruits of the Vaccinium and Ribes genera have been extensively studied for their antioxidant and antiproliferative effects [6,7,8,9,10,11,12,13,14,15,16,17]. Extracts obtained from different fruits of the Vaccinium genus (e.g., bilberry, blueberry, lingonberry, and cranberry) have shown remarkable antioxidant activity, being rich in flavonoids, catechins, polyphenolic acids, anthocyanidins and procyanidins, organic acids, and vitamins [6,7]. In in vitro tests, either the berry extracts, as such, or isolated fractions of these extracts with high content of bioactive compounds showed significant efficacy in inhibiting cell proliferation on various cancer cell lines such as colon cancer cells (Caco-2, HT-29, and HCT-116), oral squamous cell carcinoma (OSCC) cells, cervix epithelioid carcinoma, and breast adenocarcinoma [8,9,10,11]. Fruits of the genus Ribes (e.g., blackcurrant) are also rich in polyphenols, especially anthocyanins and phenolic acids, with antioxidant and antiproliferative action demonstrated on cancer cell lines such as SGC-7901 gastric cancer cells, Caco-2 colon carcinoma cells, HepG2 human liver cancer cells, murine B16F10 melanoma cells, A2780 ovarian cancer cells, or HeLa cervical cancer cells [12,13,14,15,16,17].
In addition to the fruits themselves, young shoots are used for medicinal purposes, with important therapeutic potential, although these plant parts are often neglected or considered as byproducts [18,19]. Although most research focuses on the fruit [20], recent studies have shown that blackcurrant leaves and buds have anti-inflammatory effects [19,21,22] and have an even greater antioxidant capacity compared to fruits [23].
The damaging effects of free radicals and oxidative stress on cellular systems are linked to the development of numerous diseases, including neurodegenerative diseases, cancer, cardiovascular disease, diabetes, and premature aging. Thus, bioactive compounds in plant extracts that possess antioxidant properties can combat free radical activity, counteract cellular oxidative stress, and attenuate or prevent associated inflammatory processes [4].
In phytotherapy, the extraction of bioactive compounds from plants is based on traditional methods, such as maceration, infusion, and decoction, with ancient historical origins. Tinctures, evolved from these practices, use alcohol as a solvent, with hydroalcoholic solutions with alcohol concentrations of 30–50% offering superior extraction capacity [24]. Gemmotherapy is distinguished from phytotherapy using extracts obtained from embryonic plant tissues in the growth phase, called meristematic (buds, shoots, roots, etc.). Gemmotherapeutic extracts are classically prepared according to the European Pharmacopoeia in glycerol mixtures of fresh plant material by maceration [24].
As alternatives to classical extraction methods, green extraction techniques have recently been developed, which are more efficient due to higher extraction yields, a considerable reduction in extraction time, used energy, and extraction costs, a reduction in the volume of organic solvents required, extractions at low temperatures, and extracts richer in bioactive compounds, including thermolabile compounds [25].
Due to the increased interest in the benefits of berries, there are recent studies using green extraction methods to obtain polyphenol-rich extracts from these natural sources, including leaves and buds or shoots, such as microwave-assisted extraction (MAE) [26], ultrasound-assisted extraction (UAE) [26,27], enzyme-assisted extraction (EAE) [27], and deep eutectic solvent (DES) extraction [26]. This class of compounds is of particular interest as they are considered to have the strongest antioxidant and antiradical properties [28].
Turbo-extraction (TE) by Ultra-Turrax is also considered a green extraction method because it requires a short extraction time (1–3 min), optimizes the interaction between plant material and solvents, increasing extraction efficiency, uses low amount of energy, and is considered a sustainable extraction method [29,30]. Recent studies have successfully applied this method for the isolation of polyphenolic compounds from walnut septum [29] and hazelnut involucre [31], neglected byproducts, which can be exploited in the food industry, cosmetics, and phytotherapy. To the best of our knowledge, this is the first study that has explored the young shoots of R. nigrum using UTE.
In the context of the current orientation towards “green chemistry”, plant parts considered as byproducts, such as young shoots, have the potential to provide a valuable source of bioactive compounds [32]. Furthermore, given the seasonal variations in phenolic compound content and antioxidant activity of different plant parts, there is an opportunity to study and determine the optimal harvest time and extraction conditions to maximize benefits [18].
On the other hand, with the development and widespread use of gemmotherapy, there has been an increased interest in investigating gemmotherapeutic extracts [33]. While blackcurrant, European blueberry (or bilberry), and mountain cranberry (or lingonberry) fruits and leaves have been phytochemically and biologically studied more often, there is little information in the literature on the phytochemical composition and application potential of gemmotherapeutic extracts obtained from these three species.
In vitro studies on cell cultures with extracts from young shoots (YSs) of Vaccinium spp. or Ribes spp. are scarce, and the literature is deficient in such data. Thus, the present study focused on young shoots from three species: Ribes nigrum L. (blackcurrant), Vaccinium myrtillus L. (European blueberry or bilberry), and Vaccinium vitis-idaea L. (mountain cranberry or lingonberry). Based on these premises, the aims of this work were (1) to optimize the extraction conditions of bioactive compounds from R. nigrum young shoots (RNYSs) using a rational experimental design and two green extraction methods, UAE and UTE; (2) to characterize the phytochemical profile of the extract obtained from RNYSs under the optimal extraction conditions identified, using validated high-performance liquid chromatography–tandem mass spectrometry (LC–MS) analytical methods; (3) to preliminary investigate the phytochemical profiles and health beneficial potential of V. myrtillus young shoots (VMYSs) and V. vitis-idaea young shoots (VVIYSs) in order to develop their optimal experimental conditions in further studies; (4) to evaluate the antioxidant and cytotoxic in vitro potential on normal and cancerous cell lines and to correlate the biological activity with the content of bioactive compounds.
2. Materials and Methods
2.1. Chemical and Reagents
Vanillin (99%), sodium carbonate, sodium acetate, potassium chloride, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) (97%), 2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazine (DPPH), 2,7 dichloro-fluorescein diacetate (DCFH-DA), and dimethyl sulfoxide (DMSO) were all acquired from Sigma Aldrich (Schnelldorf, Germany), and aluminum chloride (≥98%) from Carl Roth (Karlsruhe, Germany). Folin–Ciocâlteu reagent, hydrochloric acid (37%), acetic acid, acetone, ethanol, and methanol were obtained from Merck (Darmstadt, Germany). All reagents were of analytical purity, and HPLC-grade solvents were used.
All standards used for spectrophotometric assays and LC–MS analysis were obtained from Sigma Aldrich (Schnelldorf, Germany), except sinapic acid purchased from Carl Roth (Karlsruhe, Germany), and ferulic acid and gallic acid acquired from Merck (Darmstadt, Germany). Double-distilled, deionized water pro injections was purchased from Infusion Solution Laboratory of the Iuliu Haţieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania (IHUMPh).
2.2. Plant Material
In June 2022, young shoots from blackcurrant, bilberry, and lingonberry were harvested in the Obcinile Bucovinei region, located at an altitude of approximately 1000 m, within Suceava County, Romania (Table 1). Each of the three plant specimens was transported to the Department of Pharmaceutical Botany at the Faculty of Pharmacy, IHUMPh, for proper identification and subsequent inclusion in the Herbarium, where they received voucher numbers (R. nigrum L.—51.1.2.1.1; V. myrtillus L.—106.7.2.10; V. vitis-idaea L.—106.7.1.1).

Table 1.
Details regarding the harvesting location of selected species.
After harvesting, the plant material was purified by rinsing with tap water. The fresh YSs, with a length of 5–7.5 cm, from these three plant species were processed by chopping using a Thermomix (Vorwerk, Wuppertal, Germany). They were then vacuum-sealed and stored in a refrigerator at 4 °C until the time of analysis. Next, the RNYSs underwent lyophilization, beginning at −55 °C for 24 h, followed by 48 h at −25 °C, under a pressure of 200 mTorr, employing the Advantage 2.0 equipment from SP Scientific (Warminster, PA, USA). This lyophilized portion was utilized in the experimental design to evaluate the total phenolic content (TPC), total flavonoid content (TFC), condensed tannin content (CTC), total antioxidant activity (TAA) using the DPPH assay, and LC–MS analysis.
2.3. Preparation of Plant Extracts and Investigation of Optimal Experimental Conditions to Obtain Extracts Rich in Phytochemicals
An experimental design (Table 2) was developed using Modde Software, version 13.0.2 (Sartorius Stedim Data Analytics AB, Umeå, Sweden) to establish the optimal extraction conditions for polyphenolic compounds from RNYS samples. This design involved five independent variables (Table 3): extraction method (X1), which included UAE and UTE; stirring time (X2); extraction solvent (X3); apparent pH (X4); and solvent-to-plant product ratio (X5), as detailed in Table 2. Additionally, four dependent variables were considered: TPC, quantified in gallic acid equivalents (GAE) per gram of plant material dry weight (dw) (mg GAE/g dw); TFC, measured in quercetin equivalents (QE) per gram of dry weight (mg QE/g dw); CTC, expressed in catechin equivalents (CE) per gram of dry weight (mg CE/g dw); and TAA assessed using the DPPH test, reported in Trolox equivalents (TE) per gram of dry weight (mg TE/g dw).

Table 2.
Independent variables of the experimental design for assessing optimal conditions for the extraction of bioactive compounds from blackcurrant young shoots.

Table 3.
Independent and dependent variables of the experimental design used in the screening step.
Lyophilized RNYSs were ground and weighed according to the experimental plan and mixed with the extraction solvent in 25 mL Falcon tubes. The apparent pH of the considered extraction solvents was determined using a pH meter type 110 from Hana Instruments (Smithfield, RI, USA). The extracts prepared by UAE were obtained in two steps: (a) shaking for 2 min using a Vortex RX-3 (Velp Scientifica, Usmate, Italy); (b) actual extraction using an ultrasonic bath (Elmasonic S 180 (H), manufacturer Elma Schmidbauer GmbH, Singen, Germany), according to the experimental plan. Following extraction, each mixture was centrifuged for 15 min at 3000 rpm at room temperature using a Hettich Micro 22R centrifuge (manufactured by Andreas Hettich GmbH & Co., Tuttlingen, Germany). The extracts prepared through UTE also followed a two-stage process: (a) shaking for 2 min using a Vortex RX-3 and (b) actual extraction using an Ultra-Turrax homogenizer at 4000 rpm (T18; IKA Labortechnik, Staufen, Germany) for 1–3 min based on the experimental plan. After homogenization, each mixture was similarly centrifuged under the same conditions. The resulting supernatants, representing the extracted compounds, were collected and subsequently subjected to analysis for the determination of dependent variables as per the experimental plan, as well as phytochemical analysis through LC–MS.
Once the experimental plan was validated, the identified optimal extraction conditions were applied to obtain the optimal extract from young shoots of R. nigrum (blackcurrant). Under identical conditions, extracts were prepared from the young shoots of V. myrtillus (bilberry) and V. vitis-idaea (lingonberry) at a concentration of 10% (mass/volume). After centrifugation (at 3000 rpm for 15 min.), each resulting supernatant was divided into two equal fractions. The first fraction was used to determine TPC, TFC, CTC, TAA, and phytochemical analysis using LC–MS.
The second fraction was lyophilized after the solvent evaporation using a rotary evaporator (HEI-VAP Advantage Rotary evaporator HL/HB/G1, Heidolph Instruments GmbH & Co. KG, Schwabach, Germany). The lyophilization was performed at a pressure of 200 mTorr and a temperature of −25 °C for 48 h using the SP Scientific Virtis AdVantage 2.0 BenchTop lyophilizer (Model Advantage Plus EL-85, American Laboratory Trading Inc., East Lyme, CT, USA). These samples were later used for in vitro testing on cell lines.
2.4. Quantitative Determinations of Total Bioactive Compounds
2.4.1. Total Phenolic Content
The determination of TPC in the extracts followed a spectrophotometric method using the Folin–Ciocâlteu (FC) reagent, as outlined in the protocols described by Rusu et al. and Vlase et al. [30,31]. In brief, a 20 μL aliquot from each sample was placed in a 96-well plate and mixed with 80 μL of FC reagent (diluted at a 1:10 ratio). After a 3 min incubation period, 80 μL of a sodium carbonate solution (7.5% w/v) was added. The absorbance readings were taken at 760 nm against a reagent blank after a 30 min incubation in darkness at room temperature using a Synergy HT microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). The calibration curve was prepared with gallic acid as reference standard (0.01–0.16 mg/mL, R2 = 0.9998). The results were expressed as mg GAE/g dw.
2.4.2. Total Flavonoid Content
The spectrophotometric determination of the TFC in the extracts was carried out in 96-well plates, as previously detailed [30,31]. Here, 100 µL of extract was mixed with an equal volume of a 2% AlCl3 aqueous solution. The plate was then placed in darkness and incubated at room temperature for 15 min. Subsequently, the absorbance of each sample was measured at 420 nm against a blank, using the microplate reader. Quercetin was employed as the reference standard (0.313–60 µg/mL, R2 = 0.9967). The quantification of TFC was expressed as mg QE/g dw.
2.4.3. Condensed Tannin Content
The determination of CTC was conducted through a spectrophotometric approach within 96-well plates [31]. In this method, 50 µL of the extract was combined with 250 µL of a solution containing 0.5% vanillin prepared in 4% concentrated HCl in methanol. Subsequently, the plate was placed in darkness and incubated at 30 °C for 20 min, after which the absorbance was measured at 500 nm against a blank, with the same microplate reader. The calibration curve was established using catechin as reference standard (0.01–0.12 mg/mL, R2 = 0.9935), and the CTC was expressed as mg CE/g dw.
2.5. Determination of the Antioxidant Activity
The DPPH free radical scavenging capacity was conducted in a 96-well plate [30,31] by combining 10 µL of the extract with 9 µL of a 0.004% methanolic solution of DPPH and incubating the mixture in darkness for 30 min. The modification in absorbance was assessed at 517 nm, and the DPPH reduction capabilities were determined in comparison to the Trolox stock solution using the formula A = Astock solution − Asample. Here, Astock solution represents the absorbance of DPPH radical mixed with methanol, and Asample corresponds to the absorbance of DPPH radical mixed with the plant extract. The absorbance values of the samples were compared against a solvent blank utilizing the microplate reader. Trolox reagent served as the reference standard (0.025–0.3 mg/mL, R2 = 0.9942), and the outcomes were quantified as mg TE/g extract dw.
2.6. Phytochemical Analysis by LC–MS
2.6.1. Identification and Quantification of Polyphenolic Compounds
The phytochemical composition of the YS extracts was analyzed by LC–MS employing two validated analytical methods [29,34,35]. The equipment used included an Agilent Technologies 1100 HPLC Series system equipped with an autosampler, column thermostat, binary gradient pump, degasser, and UV detector, which was connected to an Agilent mass spectrometer, specifically, the Ion Trap 1100 SL model (LC/MSD Ion Trap VL, Agilent, Santa Clara, CA, USA) [36,37].
The first analytical method, with minor modifications (adding five new compound standards), was employed to identify 23 polyphenols in the YS extracts, namely, apigenin, caffeic acid, 4-O-caffeoylquinic acid, caftaric acid, chlorogenic acid, p-coumaric acid, ferulic acid, fisetin, gentisic acid, hyperoside, isoquercitrin, kaempferitrin, kaempferol, kaempferol-3-rhamnoside, luteolin, myricetin, patuletin, quercetin, quercitrin, rutoside, sinapic acid, vitexin, and vitexin 2-O-rhamnoside. In brief, chromatographic separation occurred on a reverse-phase analytical column (Zorbax SB-C18, 100 mm × 3.0 mm id, 3.5 μm, Agilent Technologies, Santa Clara, CA, USA) using a mobile phase consisting of a mixture of methanol and 0.1% acetic acid (v/v) in a binary gradient [38]. The elution process began with a linear gradient, commencing at 5% methanol and concluding at 42% methanol at 35 min. An isocratic elution of 42% methanol was maintained for the subsequent 3 min, followed by rebalancing the column with 5% methanol for the next 7 min [39]. The flow rate was set at 1 mL/min, the column temperature was held at 48 °C, and each sample was injected at a volume of 5 µL. Detection of the bioactive compounds occurred in both UV and MS modes. Initially, phenolic acids were detected at 330 nm for the first 17 min, and subsequently, flavonoids and their aglycones were identified at 370 nm up to 38 min. The MS system operated in negative mode using an electrospray ionization source (ESI) with specific settings [31,40].
The second validated LC–MS analytical method was employed to identify six additional polyphenols in the extracts: (+)-catechin, (−)-epicatechin, gallic acid, protocatechuic acid, syringic acid, and vanillic acid, as previously described [41,42]. The chromatographic separation, equipment, and mobile phase were consistent with the first method, with a specific binary gradient. Detection of these compounds was performed solely in MS mode, utilizing the same ESI source and settings as described in the first method [41].
For the identification of each bioactive compound in the plant extracts, the MS spectra/traces were compared with library spectra. After MS detection, the UV trace was utilized for quantification of the compounds. Quantification was based on the calibration curve of their respective standards.
Data acquisition and interpretation were carried out using DataAnalysis (v5.3) and ChemStation (vA09.03) software from Agilent (Santa Clara, CA, USA). The results were expressed in mg of bioactive compound per g of vegetal product.
2.6.2. Identification and Quantification of Procyanidins
A novel LC–MS analytical method was developed for the quantification of procyanidins in the extracts of RNYSs, VMYSs, and VVIYSs. The same equipment used for the determination of polyphenolic compounds was employed. The chromatographic separation was carried out using a Zorbax SB-C18 column (100 mm × 3.0 mm i.d., 3.5 µm) from Agilent Technologies. A gradient mixture of methanol and 0.1% acetic acid in water was utilized for separation (initiated with 8% methanol, increased to 20% methanol at 8 min, followed by a 3 min re-equilibration with 8% methanol). The chromatography was conducted at 45 °C with a flow rate of 1 mL/min, and a 5 µL injection volume was used.
Analyte detection was performed in MS/MS mode with negative ionization using an ion trap mass spectrometer equipped with an ESI source. The specific settings included a capillary voltage of 3000 V, a nebulizer pressure of 60 psi (nitrogen), a dry nitrogen gas flow rate of 12 L/min, and a dry gas temperature of 350 °C.
Under these chromatographic conditions, the retention times for the various procyanidins were as follows: 2.6 min for B3, 2.9 min for B1, 3.8 min for B4, 5.1 min for B2, 7.1 min for C1, and 7.6 min for A1.
For quantification purposes, the following mass spectrometry transitions were employed: m/z 575 > (m/z 407; 423; 447; 449; 450; 453; 539; 557) for A1 procyanidin, m/z 577 > m/z (407.2; 425.1; 451.1) for all four B procyanidins (as they share identical MS/MS spectra due to their isomeric nature), and m/z 865 > (407.2; 425.2; 451.2; 543.3; 577.3; 695.4; 713.3; 739.3) for C1 procyanidin. The calibration curves were found to be linear for all analytes within the concentration range of 0.1–100 µg/mL.
2.7. In Vitro Biological Activity on Cell Lines
2.7.1. Cell Culture
The intestinal cell line Caco-2 (CLS Cell Lines Service GmbH, Eppelheim, Germany) and the A549 and BJ cells (ATCC, Manassas, VA, USA) were used. Caco-2 cells were maintained in Eagle’s minimum essential medium (EMEM), while A549 and BJ cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM). All media were supplemented with 10% fetal bovine serum (FBS). The cell culture flasks were routinely incubated at 37 °C in a humidified incubator with 5% CO2 supplementation. The media were changed every 2 days, and the cells were subcultured or further used in experiments once they reached 80–90% confluence.
2.7.2. Preparation of Extract Solutions
Stock solutions of 100 mg/mL in dimethyl sulfoxide (DMSO) were prepared from each lyophilized plant extract. The stock solutions were further diluted in DMSO to obtain working solutions of 2.5, 6.25, 12.5, 18.75, 25, 37.5, 50, and 75 mg/mL. Consecutively, the working solutions were used to prepare the solutions with concentrations ranging from 20 to 800 µg/mL in cell culture media.
2.7.3. Viability Assays
The cytotoxicity of the extracts was evaluated by Alamar Blue (AB) assay as previously reported [43]. The cellular dependent conversion of resazurin to the fluorescent resorufin compound was measured at λexcitation = 530/25; λemission = 590/35, using a Synergy 2 Multi-Mode Microplate Reader. In brief, 5000 Caco-2 cells or BJ cells and 15000 A549 cells were seeded in 96-well plates to reach a confluency of 80% at the end of the experiment and left to attach to the wells for 24 h. The cells were subsequently exposed, for 24 h, to extracts dissolved in media at increasing concentrations ranging from 20 to 800 µg/mL. Following the exposure, the medium was removed, and the mentioned viability assay was performed, with the cells being incubated with a 200 µM solution of resazurin for 3 h. The experiments included three biological replicates. The results were expressed as relative values compared to the negative control (100%) (cells exposed to media containing 0.2% DMSO).
2.7.4. Dichloro-Fluorescein Diacetate Assay
The ability of the extracts to mitigate oxidative stress in Caco-2 cells was monitored using the reactive oxygen species (ROS)-sensitive DCFH-DA dye in non-stimulated and H2O2 stimulated conditions [34,44]. Caco-2 cells were selected as they were reported to be a more suitable model for the evaluation of antioxidant properties of dietary phenolics due to a retained active transport through the membrane [45].
Briefly, after a 24 h treatment with nontoxic concentrations of the extracts, the cells were washed with phosphate-buffered saline (PBS) and further incubated for 2 h with 50 µM DCFH-DA in Hanks’ Balanced Salt Solution (HBSS). Subsequently, the excess of DCFH-DA was washed, and the cells were either exposed to 250 µM H2O2 in HBSS (stimulated conditions) or to HBSS alone (non-stimulated conditions) for 2 h. The conversion of DCFH-DA to the fluorescent compound dichlorofluorescein (DCF) was measured using the microplate reader at λexcitation = 485/20; λemission = 528/20. The potency of the extracts to protect against oxidative stress was compared to the well-known antioxidant N-Acetyl Cysteine (NAC, 20 mM solution for cells treatment).
2.8. Statistical Analysis
All samples were analyzed in triplicate (n = 3) and the results were presented as the mean ± standard deviation (SD). For the statistical analysis, the Student’s t-test and one-way analysis of variance (ANOVA) were utilized, followed by post hoc testing with either the Holm– Šídák or Tukey's multiple comparisons methods. SigmaPlot 11.0 software was employed for statistical analysis and significance was considered at a p-value of ≤ 0.05.
3. Results
3.1. Outcomes of the Experimental Runs and Fitting the Data with the Models
The experimental design matrix for the extraction of bioactive compounds from RNYSs, consisting of 19 vegetal extracts, was generated using Modde software v13.0.2. Table 4 presents the factors presumed to impact the extraction yield, including extraction method and duration, solvent type, apparent pH, and the solvent-to-vegetal product ratio. Furthermore, it includes the responses examined, such as TPC, TFC, CTC, and TAA. This table offers a concise overview of the outcomes resulting from the experimental runs. The summarized results illustrate that these factors exerted varying degrees of influence, either positively or negatively, on the observed outcomes.

Table 4.
The experimental design matrix used during the screening step and the outcomes of TPC, TFC, CTC, and TAA of the Ribes nigrum L. young shoot extracts.
In the screening phase, the experimental design data were fitted using the partial least squares regression (PLS) method. Figure 1 displays the fitting parameters for the output variables, which include TPC, TFC, CTC, and TAA.
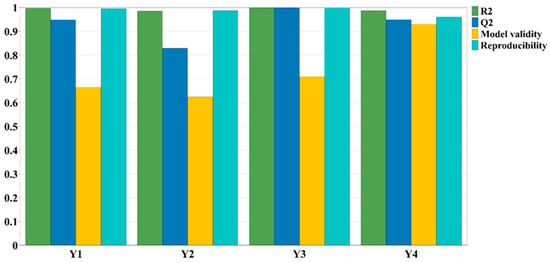
Figure 1.
The fitting parameters for the evaluated output variables.
3.1.1. The Influence of Experimental Conditions on Dependent Variables
As indicated in Table 4, it is evident that the working parameters have a noticeable impact on the extraction yield of bioactive compounds. The influence of these working conditions on TPC, TFC, CTC, and TAA is visually represented in Figure 2 through diagrams featuring scaled and centered coefficients.
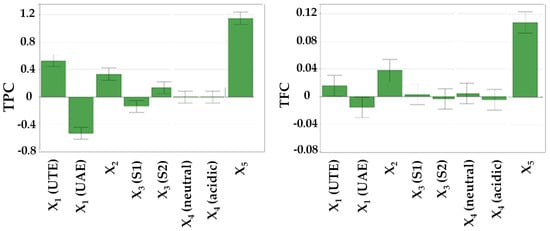
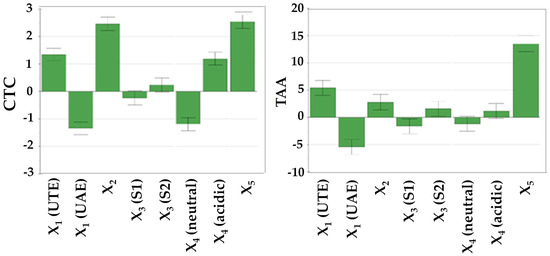
Figure 2.
The influence of working conditions on TPC, TFC, CTC, and TAA by DPPH assay upon R. nigrum L. extract during the screening step, depicted as scaled and centered coefficient plots. X1—extraction method; X2—stirring time; X3—extraction solvent; X4—solvent apparent pH; X5—solvent (mL):vegetal product (g) ratio.
Furthermore, Figure 3 illustrates the response surfaces used to predict the extraction yield of bioactive compounds from RNYS extracts concerning the assessed extraction parameters.
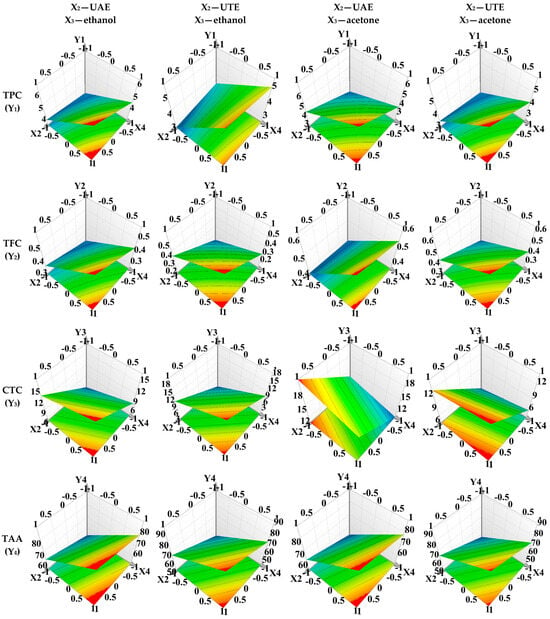
Figure 3.
Response surface for predicting the bioactive compounds recovery from RNYS extract with respect to X1—extraction method; X2—stirring time; X3—extraction solvent; X4—solvent apparent pH; X5—solvent (mL):vegetal product (g) ratio. The red areas on the graphics depict the extraction parameter ranges that ensure the highest extraction yield for the bioactive compounds under evaluation and maximum outcome for the evaluated dependent variables.
3.1.2. Investigation of Optimal Experimental Conditions to Obtain Extracts Rich in Phytochemicals for RNYSs
In the optimization step, the primary objective was to maximize the extraction of polyphenols, flavonoids, and condensed tannins, and to achieve significant antioxidant activity. Consequently, the Modde software provided the optimal extraction conditions as follows: UTE for 3 min, using a solvent composed of 70% acetone in water, maintaining a neutral apparent pH (6.4), and applying a solvent-to-vegetal product ratio of 20 (mL) to 2 (grams of YS). Under these conditions, a new extract was successfully obtained from the RNYSs. In the same conditions, with a plant product concentration of 10% (mass/volume), extracts from VMYSs and VVIYSs were also prepared.
For all these three extracts, a comprehensive series of determinations was conducted, including TPC, TFC, CTC, and TAA. Additionally, a detailed phytochemical profile was established, consisting in the identification and quantification of polyphenols (utilizing previously validated LC–MS analytical methods) and procyanidins, with the application of a newly developed analytical method. Moreover, the extracts’ antioxidant and cytotoxic potential were investigated in vitro through studies performed on cell lines.
3.2. Quantitative Determinations of Total Bioactive Compounds and Antioxidant Activity
Table 5 summarizes the results obtained for the spectrophotometric assays performed on the YS extracts. Statistical analysis was conducted to compare the results of TPC, TFC, CTC, and TAA among the optimized extracts of RNYSs, VMYSs, and VVIYSs (data are provided in Supplementary Material, Figures S1–S4).

Table 5.
Determination of TPC, TFC, CTC, and total antioxidant activity through DPPH assay for the RNYSs, VMYSs, and VVIYSs (mean value ± SD, n = 3).
3.3. Phytochemical Analysis by LC–MS
Table 6 displays the quantification results for individual phenolic acids and flavonoids identified within the extracts from the studied plant species.

Table 6.
Identification and quantification of bioactive compounds from young shoot extracts of selected species by LC/MS.
3.4. Biological Activities of the Young Shoot Extracts
3.4.1. Cytotoxic Potential
The results obtained in the cytocompatibility assays of the YS extracts on the three tested line cells are illustrated in Figure 4.
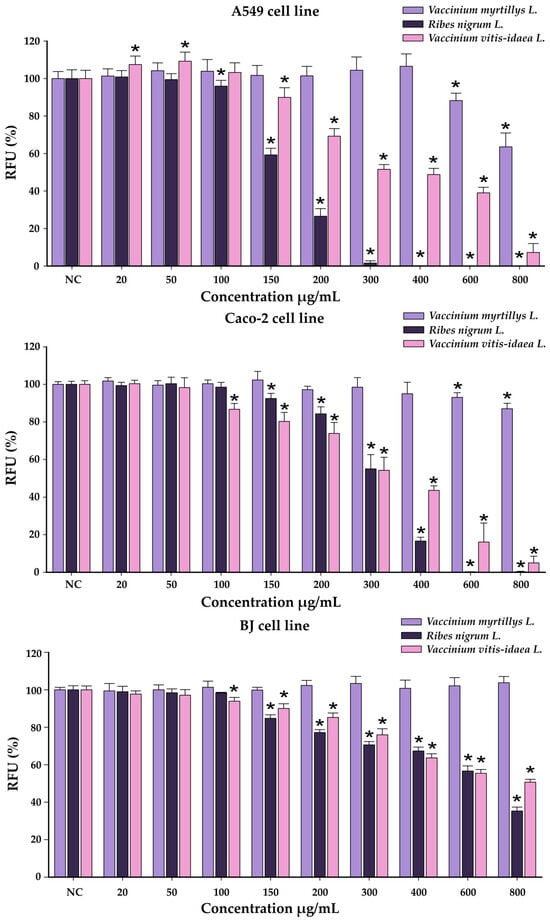
Figure 4.
Cytotoxicity of VMYSs, RNYSs, and VVIYSs on A549 cell line, Caco-2 cell line, and BJ cell line evaluated using Alamar Blue assay after a 24 h exposure. The results were expressed as the mean ± SD (n = 3) and as relative values compared to the negative control (NC) (100%). * p < 0.05 vs. NC (ANOVA + post hoc Holm–Šídák test).
For an easier comparison of biological potencies, IC50 values were calculated for all extracts on the evaluated cell lines (Table 7).

Table 7.
IC50 values (µg/mL) after exposure of A549, Caco-2 and BJ cells for VMYSs, RNYSs, and VVIYSs, evaluated by Alamar Blue assay.
3.4.2. Antioxidant Activity
The antioxidant effects of the YS extracts on Caco-2 cells are illustrated in Figure 5.
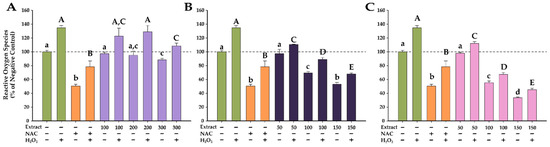
Figure 5.
Antioxidant effects of VMYSs (A), RNYSs (B), and VVIYSs (C), evaluated using DCFH-DA assay on Caco-2 cell line. Cells were incubated with the extracts at three noncytotoxic concentrations or NAC (20 mM) for 24 h and further loaded with 50 µM DCFH-DA. The antioxidant potential was measured in stimulated/non-stimulated conditions after a 3 h exposure in the presence and absence of 250 µM H2O2. Data are expressed as means ± SD (n = 3). All values are expressed as relative values compared to the negative control (100%). Different letters (a–d in non-stimulated conditions, and A–E in stimulated conditions) show statistically significant differences (ANOVA + Holm–Šídák post hoc test at p < 0.05).
4. Discussion
4.1. Fitting the Experimental Data with the Models
For all assessed variables, a very good fit was achieved, with R2 values ranging from 0.986 to 1.000. Moreover, an excellent model validity and reproducibility was observed. R2, a statistical parameter, characterizes the proportion of variation in the response explained by the chosen model. In a robust model, R2 should approach a value of 1. In contrast, Q2 signifies the percentage of response variation predicted by the model through cross-validation (values ranging from 0.829 to 0.999). High values for both parameters, with a difference of <0.3 between them, indicate strong predictive capability for a suitable model. In the selected model, a minimal difference between these two statistical parameters was found, further affirming the model’s reproducibility and validity [29,30,31].
Additionally, it was noticed that polyphenols, flavonoids, and catechins displayed good extraction yields with different solvent types. However, this factor had a minimal impact on the overall extraction yield, as depicted in Figure 2. Consequently, in the optimization phase, it was aimed to maximize TPC, TFC, CTC, and TAA. The optimal extraction conditions were, hence, identified as follows: UTE for 3 min, using a solvent composed of 70% acetone in water, maintaining a neutral apparent pH of 6.4, and applying a solvent-to-vegetal product ratio of 20 mL to 2 g of RNYSs.
4.2. The Influence of Experimental Conditions on Dependent Variables
In this experiment, the focus was on a less-explored plant matrix, namely, YSs of R. nigrum. The analysis results, as depicted in Table 4, clearly indicate that the measured responses (TPC, TFC, CTC, and TAA) were significantly influenced by the factors considered in the experimental design. For a more detailed understanding, contour plots that allow for curvature modelling were employed, thus enabling the visualization of nonlinear response surfaces, and thereby facilitating the prediction of extraction efficiency for each considered response, as illustrated in Figure 3.
The experimental parameters comprised a stirring time ranging from 1 to 3 min for UTE and 10 to 20 min for UAE. The apparent pH was maintained in the neutral to acidic range by including 1% acetic acid in the solvent mixture. The solvents employed were 50% ethanol in water (EtOH50) and 70% acetone in water (Ace70). The solvent-to-plant product ratio was also considered, ranging from 20:1 to 20:2.
Notably, the selection of an appropriate solvent is a critical aspect of plant product extraction techniques. In this study, it was opted for two polar solvents known to yield favorable results in phenolic extraction. As anticipated, based on prior research [8], the mixture of acetone and water, comprising a polar aprotic and relatively acidic solvent, and a polar protic solvent, respectively, outperformed a mixture of two polar protic solvents, namely, water and ethanol. Specifically, the results indicate that the best outcomes in terms of TPC, TFC, CTC, and TAA were achieved using a solvent composed of 70% acetone in water, underscoring the positive correlation between bioactive compound content and antioxidant activity. These findings align with previous research [31,46], where superior extraction yields for phenolic compounds translated into enhanced antioxidant activity and associated health benefits, particularly when a binary solvent mixture of water and acetone was employed.
In relation to the extraction technique, for all assessed output variables (TPC, TFC, CTC, and TAA), optimal extraction yields were observed when employing UTE as the extraction method (exerting a positive influence), in combination with a hydroalcoholic mixture containing 70% acetone (also exerting a positive influence). Conversely, the use of UAE as the extraction method (having a negative influence), particularly when combined with a hydroalcoholic mixture containing 50% ethanol (also having a negative influence), resulted in decreased recovery yields. Consequently, the extraction conditions that positively influenced the recovery of the output variables yielded the highest TPC at 80.25 ± 3.84 mg GAE/g dw (N13, run order 4, Table 4), the highest TFC at 7.22 ± 0.23 mg QE/g dw (N15, run order 17, Table 4), the highest CTC at 197.24 ± 23.18 mg CE/g dw (N13, run order 4, Table 4), and the greatest radical scavenging activity at 1488.62 ± 4.33 mg TE/g dw (N13, run order 4, Table 4).
From Table 4 and Figure 2, it becomes evident that the solvent-to-plant product ratio had the most substantial positive impact on the recovery of bioactive compounds. A higher proportion of plant products was associated with an augmented extraction yield of bioactive compounds and superior antioxidant capacity.
Furthermore, the stirring time exhibited statistically significant influence over TPC, TFC, CTC, and TAA (Table 4, Figure 2). Longer stirring times positively affected the extraction yield, particularly in the case of UTE. In contrast, when UAE was employed, longer stirring times led to reduced recovery yields.
To a lesser degree, solvent apparent pH influenced the output variables. Notably, higher TPC and TFC were obtained at a neutral apparent pH, while elevated CTC and TAA were observed when 1% acetic acid was added to the solvent mixture, ensuring an acidic apparent pH.
In the pursuit of optimized extracts with an appropriate phytocomplex and robust antioxidant activity, it was set out to maximize TPC, TFC, CTC, and TAA using the Modde software. The optimized extraction conditions derived from this approach included UTE for 3 min, employing a hydroalcoholic solvent mixture containing 70% acetone, maintaining a neutral apparent pH, and using a solvent-to-plant product ratio of 20:2.
4.3. Quantitative Determinations of Total Bioactive Compounds and Antioxidant Activity
This research conducted on extracts of RNYSs, VMYSs, and VVIYSs provides additional evidence that modern extraction techniques exert a substantial influence on bioactive compounds content and antioxidant potential [28,30,39,42].
Following the experimental determinations, the extracts yielded the results summarized in Table 5. The investigation of the RNYSs, VMYSs, and VVIYSs revealed variations in their phenolic content and antioxidant activity. In terms of TPC, RNYSs exhibited the highest concentration among the three species, registering 263.86 ± 18.74 mg GAE/g dw. Indeed, it was obtained under the optimal extraction condition using UTE, a modern and ecofriendly extraction method seldom applied on plant matrices. Vagiri et al. found a quantity of 45−56 mg GAE/g dw in blackcurrant buds using UAE, significantly less compared to this study [47]. Nonetheless, comparison with other literature data proved challenging due to variations in expressing the measurements. For instance, in the study by Tabart et al., polyphenols were extracted from blackcurrant buds by simple extraction for 1 h at 4 °C, yielding 45.1 ± 7.5 mg of chlorogenic acid equivalents (CAE) per gram of frozen weight (FW) [48]. Turrini et al. expressed their results in mg GAE/100 mL extract. They found TPC values of 415.56 ± 5.52 mg GAE/100 mL in an extract obtained by pulsed ultrasound-assisted extraction (PUAE) compared to 276.44 ± 3.85 mg GAE/100 mL in a glycerin macerate, both for a ratio of solid plant material (dw):solvent of 1:20 [49]. However, the UTE method is considered as a promising approach for obtaining polyphenolic-rich extracts from plant matrices, including young shoots. The RNYS extract prepared under the optimal identified conditions proved to be rich in polyphenolics.
VVIYSs levels of TPC followed those of blackcurrant with amounts of 114.43 ± 5.72 mg GAE/g dw, while VMYSs displayed a slightly lower TPC of 105.25 ± 7.36 mg GAE/g dw. For these two matrices, the optimal extraction conditions will be studied in further analyses. The investigations of their phytochemical profiles and health beneficial potential were the reasons these plant matrices were included along with RNYSs in this study.
The current findings underscore the rich phenolic composition of all three selected species. Tabart et al. comparatively analyzed extracts of leaves, buds, and berries from R. nigrum. The TPC values were 46.0, 45.1 mg, and 21.2 mg CAE/g frozen weight (FW), respectively [48]. In similar studies, phytochemical analyses indicated that R. nigrum leaves are valuable sources of polyphenols, showing contents up to fivefold higher than in fruits, while the antioxidant potential was correlated with the content of polyphenols [23]. These results were confirmed by another study that found the highest amount of TPC and TFC in extracts from leaves, followed by stems and berries [50]. In a study conducted by Bujor et al. the bilberry leaf extracts exhibited a TPC of 142.9 ± 19.2 mg GAE/g dry extract [51]. Additionally, in another study by the same author, the TPC of lingonberry leaf extracts was reported as 158.9 ± 6.0 mg GAE/g dry extract [52].
Concerning TFC, VMYS extracts demonstrated the highest TFC at 43.17 ± 3.02 mg QE/g dw, closely followed by RNYSs with 41.49 ± 1.65 mg QE/g dw, and VVIYSs with 38.06 ± 4.18 mg QE/g dw. This indicates a substantial presence of flavonoids in all three species. The outcomes shown for VMYSs were comparable with other studies performed on leaf extracts of Vaccinium species. In a study conducted by Ștefănescu et al., V. vitis-idaea leaf extract exhibited a TFC of 49.94 ± 8.03 mg QE/g plant material [53]. Dragana et al. found variable amounts of total flavonoids in V. myrtillus leaves, ranging from 43.08 ± 0.68 mg rutin equivalent (RUE)/g of extract (for aqueous extract) to 94.49 ± 3.61 mg RUE/g of extract (for ethyl acetate extract) [54]. Tabart et al. found in extracts obtained from R. nigrum leaves, buds, and berries, TFC amounts of 2.05, 2.15, and 0.50 mg QE/g FW, respectively [48]. Charpentier et al. obtained flavonoid content of concentrated bud macerates from R. nigrum of 150 mg/100 g fresh weight (fw) [55]. Other analyses on the same matrix found values of 126 mg/100 g [56] and 97 mg/100 g [57]. In a study conducted by Mikulic-Petkovsek et al., V. myrtillus harvested from shaded forests, characterized by low photosynthetic active radiation (PAR), exhibited lower levels of flavanols [58]. Conversely, blueberries from sun-exposed locations with high PAR displayed higher amounts of flavanols. This observation aligns with the findings of Jaakola et al., who reported significantly elevated levels of flavan-3-ols in bilberry leaves exposed to direct sunlight [59]. Considering that the collection site in Obcinile Bucovinei, Suceava county has substantial solar exposure, the high amount of TFC for the investigated species have a plausible explanation and align with prior findings.
Additionally, the evaluation of CTC in RNYSs highlighted blackcurrant as a significant source of condensed tannins, with 131.81 ± 7.90 mg CE/g dw. In contrast, VVIYSs and VMYSs displayed lower CTC values of 25.99 ± 1.03 mg CE/g dw and 18.8 ± 1.88 mg CE/g dw, respectively.
Furthermore, the assessment of TAA by DPPH assay underscored the robust antioxidant potential of RNYSs, with a TAA of 3.66 ± 0.40 mg TE/g dw. Similarly, VMYSs exhibited substantial antioxidant activity (3.45 ± 0.24 mg TE/g dw), while VVIYSs showed a TAA of 3.41 ± 0.17 mg TE/g dw. These results indicate significant antioxidant capabilities for all three YS extracts of the selected species and correlations with the TPC and TFC values, as these bioactive compounds are responsible for the radical scavenging potential [60]. In the study performed by Turrini et al., the radical scavenging activity (RSA) of the extracts from R. nigrum buds was also correlated with TPC values. For PUAE compared to glycerin macerate, TPC values were 415.56 ± 5.52 vs. 276.44 ± 3.85 mg/100 mL, while RSA values were 1158.58 ± 73.24 vs. 1137.04 ± 38.49 mg/100 mL, respectively [49].
Previous research has indicated that increased solar exposure at higher altitudes was associated with higher levels of ortho-dihydroxylated flavonoids and improved radical scavenging capacity [61,62]. Compounds like flavanols, flavonols, and caffeic acid derivatives contributed to strong antioxidant properties, primarily due to the presence of highly antioxidant 1,2-dihydroxyphenyl moieties. Soobrattee et al. ranked antioxidant activity in the following order: procyanidin dimer > flavan-3-ols > flavonols > hydroxycinnamic acids > simple phenolic acids [63]. The variations in reactivity noted among the extracts of YSs in the DPPH test may be ascribed to variances in their polyphenol compositions, including compounds containing dihydroxyphenyl moieties, differences in molecular sizes (e.g., flavanols), or the potential presence of unidentified substances exhibiting antioxidant activity.
4.4. Phytochemical Analysis by LC–MS
The phytochemical analysis permitted the identification and quantification of 15 compounds in YSs distributed in two major classes, phenolic acids and flavonoids (Table 6). The approach revealed that the most abundant secondary metabolites of RNYSs were hydroxycinnamic acids, among them, chlorogenic acid found in relatively high amounts, while gallic and p-coumaric acids were found in lesser concentrations. Chlorogenic acid was determined in all three matrices; the highest amount (4455.11 ± 356.40 μg/g) was quantified in VMYS extract, representing more than 10 times the amounts determined in extracts of RNYSs and VVIYSs. The same trend was noticed for p-coumaric and gallic acids, much higher values were found in VMYS extract. However, the extracts of RNYSs and VVIYSs were much richer in 4-O-caffeoilquinic acid. Moreover, the extracts of VVIYSs showed higher amounts of catechin and epicatechin, yet epigallocatechin was quantified only in RNYSs and VMYSs. Although these two plant matrices presented higher contents of TPC, the extracts of VVIYSs seemed to be richer in individual flavonoids.
A quantitative comparison between the outcomes of this study and the results obtained in other experiments was not possible due to the lack of data for YS matrix. Therefore, we examined several other matrices of R. nigrum, V. myrtillus, and V. vitis-idaea. Compared to our research, where the chlorogenic acid amount was 321.09 ± 28.89 µg/g plant product, Vagiri et al. found, in ethanolic extracts of R. nigrum leaves, amounts of chlorogenic acid ranging from 81 to 121 µg/g dw relative to leaf positions and harvest date [47], while Raudsepp et al. determined a considerably higher amount for this compound (14.93 mg/g dry leaves) [64]. This research group also detected catechin, epicatechin, and epigallocatechin in the leaves of Estonian R. nigrum.
In this study, three glycosides of quercetin (hyperoside, isoquercitrin, quercitrin), that contain antioxidant chemical structures that can scavenge free radicals responsible for oxidative chain reactions [65], were found in all three types of YSs, while rutoside, another quercetin glycoside, was quantified only in R. nigrum and V. vitis-idaea. In the leaves of R. nigrum, Vagiri et al. also identified quercetin-3-O-galactoside (hyperoside), quercetin-3-O-glucoside (isoquercitrin), and quercetin-3-O-rutinoside (rutoside) [47]. Hyperoside content from YSs in this study was comparable to the amount in leaves, 58.97 ± 3.53 vs. 57–81 μg/g, while the other two were higher in YSs, 280.91 ± 14.04 vs. 38–85 μg/g and 433.13 ± 21.66 vs. 99–229 μg/g, respectively. Nevertheless, another analysis of R. nigrum leaf extract showed high concentrations of quercitrin and rutoside, the two major glycosides of their ethanolic extract, of 19.47 and 3.99 mg/g dry leaves, respectively [64]. Moreover, besides hydroethanolic and methanolic extracts, infusion of R. nigrum leaves reported contents of phenolic acids and quercetin glycosides, especially isoquercitrin (9.3 ± 1.01 mg/100g dw) and rutoside (7.5 ± 1.4 mg/100g dw) [66].
Liu et al. characterized the chemical profile by HPLC-DAD and HPLC-ESI-MS in the aqueous acetone extracts from the leaves of V. myrtillus and V. vitis-idaea [67]. The total phenolics in V. myrtillus green leaves ranged from 9 to 77 mg/g dw and from 73 to 74 mg/g dw in red leaves, with chlorogenic acid accounting for the majority of the total content of phenolic compounds, from 2 to 66 mg/g dw depending on the growth period. Quercetin glycosides including hyperoside, isoquercitrin, quercitrin, and rutin were among the major phenolics in the extracts from both species [67].
Furthermore, quantification of compounds in Caucasian V. myrtillus leaf extracts demonstrated high contents of hyperoside (4.69 mg/g), isoquercitrin (12.02 mg/g), and quercitrin (2.77 mg/g) [68]. Both fruits and leaves of V. myrtillus were disclosed to contain catechin and epicatechin, hydroxycinnamic acids dominated by p-coumaric, ferulic, and caffeic acids, followed by gallic, ellagic, and syringic acids [7].
In the study of Hokkanen et al., phenolics from leaves and stems of V. vitis-idaea and V. myrtillus were studied using LC–MS [69]. The concentrations of most quercetin glycosides, including hyperoside, isoquercitrin, quercitrin, and rutoside, as well as total flavonols were higher in the extracts of V. vitis-idaea, while catechins were more abundant in V. myrtillus extracts [69].
Ieri et al. defined the phytochemical fingerprint of V. myrtillus and V. vitis-idaea buds and foliar tissues [70]. They concluded that TPC was higher in the young sprouts than in adult leaves regardless of the type of extraction. Hydroxycinnamic acids were the main class of compounds, independently of the age of the leaf tissue, corresponding to 93% of the total phenols in the glycerol–alcoholic bud extract. Interestingly, hydroxycynnamic acids were more concentrated in young tissues compared with adult leaf tissues (53.6 and 25.2 mg/g, respectively) [70], which, again, underlines the importance of our research.
It is worth mentioning that many factors, such as geographical location, cultivar type, growing conditions, or growth period, could impact the chemical composition of plant matrices [71]. Indeed, the mountainous region from which the analyzed plant samples were collected (Obcinile Bucovinei, at 1000 m altitude) and the mountain climate were correlated with their high polyphenolic content and phytochemical profile.
4.5. Biological Activities of the Young Shoot Extracts
4.5.1. Cytotoxic Potential
Among the extracts obtained from the YSs, the VMYS extract exhibited the highest cytocompatibility, statistically reducing cellular viability at the two highest tested concentrations in the case of the cancerous cells, while in the case of BJ cells, no statistical difference was observed. At the 800 µg/mL dosage, this bilberry extract reduced cellular viability of Caco-2 cells by approximately 15%, while in the case of A549 cells, the viability was decreased by 35%. Conversely, the VVIYS and RNYS extracts showed increased toxicity on all cell lines, leading to a dose-dependent decrease in cellular viability starting from a dose of 100–150 µg/mL, depending on the extract and the cellular line exposed. In comparison with normal cellular phenotype, cancerous cells displayed an increased susceptibility, at the highest tested dose both extracts decreasing the cellular viability by more than 90%. For BJ cells, the measured viability for the VVIYS and RNYS extracts at the highest dose was 50 and 35% of the negative control.
To evaluate if there is a statistical difference between the susceptibility of the cell lines and the cytotoxic potencies of the extracts, an ANOVA 3-way analysis was performed using as variables the cell type, type of the extract, and the concentration tested, and as the response the measured viability. The analysis indicated that all variables tested had a significant impact on the measured viability. As observed from the graphical representations, the analysis indicated that the VMYS extract displayed less toxicity than the other two extracts, with the cancerous cells being more sensitive to the cytotoxic activity of the extracts.
Comparable results were reported by Ginovyan et al. for an ethanolic extract of R. nigrum L. leaves in HT-29 and MCF-7 cells, with the cellular viability decreasing by more than 50% at exposure doses between 300 and 400 µg/mL. In addition, the authors reported that the observed cytotoxicity was dependent on the exposure duration with longer exposures being associated with increased cytotoxicity [72]. The anticancer effects were also noted for aqueous extract from blueberry leaves on the AGS gastric cancer cell line, as the cellular viability decreased by more than 50% at doses higher than 400 µg/mL. The leaf extract also presented an antimigrating potential, most probably due to the high content of anthocyanins. In the same study, the authors evaluated the cytotoxic effect of a similarly obtained extract from the fruits, which was shown to be less active on the cancerous cell line [73].
A selective cytotoxic effect towards the cancerous cell line was reported for a methanolic extract of R. idaeus shoots, with the extract displaying IC50 values of 110 μg/mL and 300 μg/mL towards HL-60 and HeLa cells, respectively. The authors supposed that this effect was related to the high sanguiin H-6 content, a primary ellagitannin present in the Rosaceae family [74].
Conversely, Ziemlewska et al. reported that water–glycerin extracts from the leaves of R. nigrum L. and V. myrtillus can have a beneficial effect on the viability of keratinocytes (HaCaT) and fibroblasts (BJ), depending on the exposure dose. At small/intermediate doses, the authors reported that the extracts increased the measured viability and the cellular metabolism. Interestingly, similar to the current study a higher cytotoxicity was observed for the RNYS extract compared to VMYS extract [19]. Similarly, Debnath-Canning et al. reported that fruit and leaf extract from V. angustifolium can reduce cellular damage and inflammation in microglial cells exposed to glutamate or α-synuclein and act as protective agents against neurodegenerative disorders [75].
4.5.2. Antioxidant Activity
The antioxidant activity of the extracts prepared from YSs was assessed on the Caco-2 cell line because these cells were reported to be a more suitable model for the evaluation of antioxidant properties of the dietary phenolics. Kellett et al. evaluated the antioxidant effects of quercetin and catechin in HepG2 and Caco-2 cells. While quercetin presented an antioxidant effect on both cell lines (increased effect on Caco-2 cells), the antioxidant effects of catechin were only noticeable on the Caco-2 cells. This could be explained by the fact that Caco-2 cells display tight junctions and permeability similar to small intestinal epithelial cells and a retained active transport through the membrane [76].
When cells were exposed to H2O2, there was a statistically significant increase in ROS, reaching approximately 135% of the negative control value. Conversely, exposure to NAC reduced ROS levels by 50% in both stimulated and non-stimulated conditions. In comparison with previously reported data from our group on the antioxidant effects of various plant extracts, exposure of Caco-2 cells to H2O2 induced a significantly lower increase in ROS. Exposure of A549, T47D-kBluc, MCF-7, and human gingival fibroblasts to the same concentration of H2O2 resulted in an approximately 300–500% increase in ROS, while in the present case, a modest increase of 30% was observed [34]. These results could be related to the increased barrier membrane function observed in Caco-2 cells [76].
In accordance with the observed effects for NAC, all extracts obtained from YSs exhibited antioxidant properties, with the blackcurrant and lingonberry extracts demonstrating exceptional antioxidant capabilities. The extracts effectively decreased ROS levels in both conditions in a dose-dependent manner, with lingonberry extracts exhibiting a stronger antioxidant activity than NAC at the highest tested dose. Regarding the VVIYS extract, it alone reduced ROS levels by approximately 65%, and in the presence of H2O2, the ROS content was reduced by 70%. The antioxidant properties observed in cell culture are congruent with the results obtained in the abiotic DPPH assay (Table 5). Bioactive phytocompounds can directly mitigate oxidative insults, as measured by the abiotic antioxidant assays (DPPH), or indirectly augment the cellular antioxidant capacity by increasing the synthesis of cellular antioxidants and the activity of the detoxifying enzymes.
Similar to the current results, Kwon et al. reported that the pre-exposure to a methanolic extract from the leaves of V. bracteatum Thunb. reduced ROS in BV-2 microglial cells exposed to lipopolysaccharides (LPS) in a dose-dependent manner [77].
Gao et al. reported that ethanolic extracts of fruits, leaves, and flower buds from V. dunalianum Wight reduced ROS in H2O2-stimulated PC12 cells by increasing the activity of the superoxide dismutase and catalase antioxidant enzymes. In addition, they reported that the extracts decreased the malondialdehyde (MDA) content and increased the level of reduced glutathione [78]. Compared with the fruit extracts, the extract from leaves had a similar antioxidant effect, indicating that other plant parts, such as the YSs tested in the current study, could prove to be a valuable resource of bioactive compounds. V. dunalianum Wight was also studied by Cheng et al., who investigated various fractions of a shoot extract obtained by using 80% methanol in water and then divided into fractions using various solvents (petroleum ether, chloroform, ethyl acetate, and n-butanol). These fractions, along with the remaining aqueous extract, were evaluated for their antioxidant potential through various assays, both in vitro and on HepG2 cells. The ethyl acetate fraction displayed notable antioxidant properties, including the ability to inhibit ROS formation and induce apoptosis in HepG2 cells exposed to H2O2. Furthermore, it dose-dependently enhanced the activity of antioxidant enzymes like superoxide-dismutase and catalase and increased glutathione (GSH) levels [79].
Chlorogenic acid, one of the more abundant phytochemicals in the YS extracts, has been shown to possess antioxidant and anti-inflammatory potential through the upregulation of an antioxidant cellular defense mechanisms, acting as an antioxidant in in vitro and in vivo models [80,81,82]. In addition, caffeoylquinic acids were previously reported to exhibit antioxidant properties effects on hepatic cells exposed to the pro-oxidant tert-butyl hydroperoxide (t-BHP). Congruent with the results reported by Gao et al. [78], caffeoylquinic acids can increase the cellular GSH level and alleviate BHP-induced DNA damage. All these bioactive compounds present a common indirect antioxidant mechanism by inducing the nuclear translocation of the Nrf2 and the activation of the Nrf2/ARE cell signaling pathway, leading to increased expression of antioxidant enzymes and cellular defense [83].
Tabart et al. explored the antioxidant capacity of R. nigrum extracts (shoots, leaves, and fruits) obtained through solvent extraction. They assessed antioxidant activity using the cellular antioxidant activity (CAA) assay on human EAHy926 cells and conducted erythrocyte resistance tests. The leaf extract displayed the highest antioxidant activity on EAHy926 cells, followed by the shoot and fruit extracts. In the erythrocyte resistance test, the shoot extract showed similar antioxidant activity to the leaf extract, both outperforming the fruit extract. Additionally, the researchers investigated how blackcurrant extracts (from leaves, berries, and shoots) influenced ROS production in stimulated equine neutrophils. The leaf extract exhibited the strongest antioxidant activity, followed by the shoot and berry extracts. Nevertheless, none of these extracts significantly reduced the total myeloperoxidase release from activated neutrophils compared to the control group [84].
5. Conclusions
The primary objective of this research was to investigate less-explored natural resources with diverse potential applications. Up to date, this is the first study that has explored the young shoots of R. nigrum. For this purpose, a green, modern, and efficient method, namely, turboextraction by Ultra-Turrax, was applied. To optimize the extraction of polyphenols, flavonoids, and condensed tannins, and to maximize antioxidant activity in this plant matrix, a rational experimental design was employed, which identified the most effective extraction parameters: Ultra-Turrax extraction for 3 min, using a solvent composed of 70% acetone in water, maintaining a neutral pH, and employing a solvent-to-vegetal product ratio of 20:2. The scope and the novelty of the study were extended by investigating extracts of young shoots from two other prominent species, V. myrtillus and V. vitis-idaea, prepared under the same experimental conditions. This expanded perspective underscores the diversity of phytochemical profiles within these extracts. The current findings underscore the remarkable antioxidant potential and cytotoxic activity of these extracts when investigated in vitro on cancer cell lines. This study highlights the biological potential of the investigated species, emphasizing the need for further studies to determine the optimal experimental conditions for V. myrtillus and V. vitis-idaea young shoots.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9111163/s1, Figure S1: Statistical comparison for results of total polyphenolic content (TPC) for optimized young shoots extracts of Ribes nigrum (RNYS), Vaccinium myrtillus (VMYS), and Vaccinium vitis-idaea (VVIYS). All values are expressed as mean ± SD (n = 3). Statistical analysis was performed using a one-way ANOVA test, with Tukey’s multiple comparisons post-test (**** p < 0.0001); Figure S2: Statistical comparison for results of total flavonoid content (TFC) for optimized young shoots extracts of Ribes nigrum (RNYS), Vaccinium myrtillus (VMYS), and Vaccinium vitis-idaea (VVIYS). All values are expressed as mean ± SD (n = 3). Statistical analysis was performed using a one-way ANOVA test, with Tukey’s multiple comparisons post-test. Statistical significance was considered for p < 0.05; Figure S3: Statistical comparison for results of condensed tannin content (CTC) for optimized young shoots extracts of Ribes nigrum (RNYS), Vaccinium myrtillus (VMYS), and Vaccinium vitis-idaea (VVIYS). All values are expressed as mean ± SD (n = 3). Statistical analysis was performed using a one-way ANOVA test, with Tukey’s multiple comparisons post-test (**** p < 0.0001); Figure S4: Statistical comparison for results of total antioxidant activity evaluated by DPPH assay (TAA) for optimized young shoots extracts of Ribes nigrum (RNYS), Vaccinium myrtillus (VMYS), and Vaccinium vitis-idaea (VVIYS). All values are expressed as mean ± SD (n = 3). Statistical analysis was performed using a one-way ANOVA test, with Tukey’s multiple comparisons post-test. Statistical significance was considered for p < 0.05.
Author Contributions
Conceptualization, M.-B.S. and D.-S.P.; methodology, I.T., I.F. and L.V.; software, I.T., I.F. and L.V.; validation, I.T., L.V. and I.F.; formal analysis and investigation, M.-B.S., L.M., I.F., A.-E.P., I.-V.C., M.E.R., L.V. and A.-M.V.; writing—original draft preparation, A.-M.V., M.-B.S., I.F., M.E.R. and D.-S.P.; writing—review and editing, A.-M.V., I.F., M.E.R. and D.-S.P.; visualization, A.-M.V., I.F., M.E.R. and D.-S.P.; supervision, I.T., L.V., I.F. and D.-S.P.; project administration, M.-B.S. and D.-S.P.; funding acquisition, M.-B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Iuliu Hațieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania, PhD grant PCD 882/57/13.01.2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant Flavonoids in Cancer Chemoprevention: Role in Genome Stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Ancillotti, C.; Ciofi, L.; Pucci, D.; Sagona, E.; Giordani, E.; Biricolti, S.; Gori, M.; Petrucci, W.A.; Giardi, F.; Bartoletti, R.; et al. Polyphenolic Profiles and Antioxidant and Antiradical Activity of Italian Berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. subsp. gaultherioides (Bigelow) S.B. Young. Food Chem. 2016, 204, 176–184. [Google Scholar] [CrossRef]
- Staszowska-Karkut, M.; Materska, M. Phenolic Composition, Mineral Content, and Beneficial Bioactivities of Leaf Extracts from Black Currant (Ribes nigrum L.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef]
- Martini, D.; Marino, M.; Venturi, S.; Tucci, M.; Klimis-Zacas, D.; Riso, P.; Porrini, M.; Del Bo’, C. Blueberries and Their Bioactives in the Modulation of Oxidative Stress, Inflammation and Cardio/Vascular Function Markers: A Systematic Review of Human Intervention Studies. J. Nutr. Biochem. 2023, 111, 109154. [Google Scholar] [CrossRef]
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Yang, J.; Li, Z.; Ni, X.; Tian, Y.; Li, H.; et al. Blueberry Fruit Valorization and Valuable Constituents: A Review. Int. J. Food Microbiol. 2022, 381, 109890. [Google Scholar] [CrossRef]
- Kowalska, K. Lingonberry (Vaccinium vitis-idaea L.) Fruit as a Source of Bioactive Compounds with Health-Promoting Effects—A Review. Int. J. Mol. Sci. 2021, 22, 5126. [Google Scholar] [CrossRef]
- Martău, G.A.; Bernadette-Emőke, T.; Odocheanu, R.; Soporan, D.A.; Bochiș, M.; Simon, E.; Vodnar, D.C. Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants. Molecules 2023, 28, 1533. [Google Scholar] [CrossRef]
- Aaby, K.; Grimmer, S.; Holtung, L. Extraction of Phenolic Compounds from Bilberry (Vaccinium myrtillus L.) Press Residue: Effects on Phenolic Composition and Cell Proliferation. LWT-Food Sci. Technol. 2013, 54, 257–264. [Google Scholar] [CrossRef]
- Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G.; Djilas, S.; Četojević-Simin, D. Dried Bilberry (Vaccinium myrtillus L.) Extract Fractions as Antioxidants and Cancer Cell Growth Inhibitors. LWT-Food Sci. Technol. 2015, 61, 615–621. [Google Scholar] [CrossRef]
- Schantz, M.; Mohn, C.; Baum, M.; Richling, E. Antioxidative Efficiency of an Anthocyanin Rich Bilberry Extract in the Human Colon Tumor Cell Lines Caco-2 and HT-29. J. Berry Res. 2010, 1, 25–33. [Google Scholar] [CrossRef]
- Mauramo, M.; Onali, T.; Wahbi, W.; Vasara, J.; Lampinen, A.; Mauramo, E.; Kivimäki, A.; Martens, S.; Häggman, H.; Sutinen, M.; et al. Bilberry (Vaccinium myrtillus L.) Powder Has Anticarcinogenic Effects on Oral Carcinoma In Vitro and In Vivo. Antioxidants 2021, 10, 1319. [Google Scholar] [CrossRef]
- Georgescu, C.; Frum, A.; Virchea, L.-I.; Sumacheva, A.; Shamtsyan, M.; Gligor, F.-G.; Olah, N.K.; Mathe, E.; Mironescu, M. Geographic Variability of Berry Phytochemicals with Antioxidant and Antimicrobial Properties. Molecules 2022, 27, 4986. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneha, K.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Jia, N.; Xiong, Y.L.; Kong, B.; Liu, Q.; Xia, X. Radical Scavenging Activity of Black Currant (Ribes nigrum L.) Extract and Its Inhibitory Effect on Gastric Cancer Cell Proliferation via Induction of Apoptosis. J. Funct. Foods 2012, 4, 382–390. [Google Scholar] [CrossRef]
- Miladinovic, B.; Faria, M.Â.; Ribeiro, M.; Sobral, M.M.C.; Ferreira, I.M.P.L.V.O. Delphinidin-3-Rutinoside from Blackcurrant Berries (Ribes nigrum): In Vitro Antiproliferative Activity and Interactions with Other Phenolic Compounds. Molecules 2023, 28, 1286. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and Antioxidant Properties of Anthocyanin Rich Extracts from Blueberry and Blackcurrant Juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef]
- Bishayee, A.; Háznagy-Radnai, E.; Mbimba, T.; Sipos, P.; Morazzoni, P.; Darvesh, A.; Bhatia, D.; Hohmann, J. Anthocyanin-Rich Black Currant Extract Suppresses the Growth of Human Hepatocellular Carcinoma Cells. Nat. Prod. Commun. 2010, 5, 1613–1618. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.-O.; Dommes, J. Antioxidant Capacity of Black Currant Varies with Organ, Season, and Cultivar. J. Agric. Food Chem. 2006, 54, 6271–6276. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of Cytotoxicity and Antioxidant Properties of Berry Leaves as By-Products with Potential Application in Cosmetic and Pharmaceutical Products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Ștefănescu, R.; Laczkó-Zöld, E.; Ősz, B.-E.; Vari, C.-E. An Updated Systematic Review of Vaccinium myrtillus Leaves: Phytochemistry and Pharmacology. Pharmaceutics 2022, 15, 16. [Google Scholar] [CrossRef]
- Téglás, T.; Mihok, E.; Cziáky, Z.; Oláh, N.-K.; Nyakas, C.; Máthé, E. The Flavonoid Rich Black Currant (Ribes nigrum) Ethanolic Gemmotherapy Extract Elicits Neuroprotective Effect by Preventing Microglial Body Swelling in Hippocampus and Reduces Serum TNF-α Level: Pilot Study. Molecules 2023, 28, 3571. [Google Scholar] [CrossRef] [PubMed]
- Magnavacca, A.; Piazza, S.; Cammisa, A.; Fumagalli, M.; Martinelli, G.; Giavarini, F.; Sangiovanni, E.; Dell’Agli, M. Ribes nigrum Leaf Extract Preferentially Inhibits IFN-γ-Mediated Inflammation in HaCaT Keratinocytes. Molecules 2021, 26, 3044. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of Phenolic Compounds and Antioxidant Potential between Selected Edible Fruits and Their Leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Health Care. European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2005; Volume 2, ISBN 978-92-871-5281-7. [Google Scholar]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Martinović, M.; Krgović, N.; Nešić, I.; Žugić, A.; Tadić, V.M. Conventional vs. Green Extraction Using Natural Deep Eutectic Solvents—Differences in the Composition of Soluble Unbound Phenolic Compounds and Antioxidant Activity. Antioxidants 2022, 11, 2295. [Google Scholar] [CrossRef] [PubMed]
- José Aliaño González, M.; Carrera, C.; Barbero, G.F.; Palma, M. A Comparison Study between Ultrasound–Assisted and Enzyme–Assisted Extraction of Anthocyanins from Blackcurrant (Ribes nigrum L.). Food Chem. X 2022, 13, 100192. [Google Scholar] [CrossRef] [PubMed]
- Marzullo, L.; Ochkur, O.; Orlandini, S.; Renai, L.; Gotti, R.; Koshovyi, O.; Furlanetto, S.; Del Bubba, M. Quality by Design in Optimizing the Extraction of (Poly)Phenolic Compounds from Vaccinium myrtillus Berries. J. Chromatogr. A 2022, 1677, 463329. [Google Scholar] [CrossRef]
- Rusu, M.; Gheldiu, A.-M.; Mocan, A.; Moldovan, C.; Popa, D.-S.; Tomuta, I.; Vlase, L. Process Optimization for Improved Phenolic Compounds Recovery from Walnut (Juglans regia L.) Septum: Phytochemical Profile and Biological Activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef] [PubMed]
- Vlase, A.-M.; Toiu, A.; Tomuță, I.; Vlase, L.; Muntean, D.; Casian, T.; Fizeșan, I.; Nadăș, G.C.; Novac, C.Ș.; Tămaș, M.; et al. Epilobium Species: From Optimization of the Extraction Process to Evaluation of Biological Properties. Antioxidants 2022, 12, 91. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizeșan, I.; Pop, A.; Gheldiu, A.-M.; Mocan, A.; Crișan, G.; Vlase, L.; Loghin, F.; Popa, D.S.; Tomuta, I. Enhanced Recovery of Antioxidant Compounds from Hazelnut (Corylus avellana L.) Involucre Based on Extraction Optimization: Phytochemical Profile and Biological Activities. Antioxidants 2019, 8, 460. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; José Núñez, M.; Parajó, J.C. Natural Antioxidants from Residual Sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Raiciu, A.D.; Mihele, D.E.; Ionita, C.; Nistorica, V.; Manea, S. Antimicrobial Activity of Ribes Nigrum, Rosmarinus Officinalis, Betula Pubescens, Salix Alba, Vaccinium myrtillus Gemoderivatives. Farmacia 2010, 58, 735–748. [Google Scholar]
- Rusu, M.E.; Fizesan, I.; Pop, A.; Mocan, A.; Gheldiu, A.-M.; Babota, M.; Vodnar, D.C.; Jurj, A.; Berindan-Neagoe, I.; Vlase, L.; et al. Walnut (Juglans regia L.) Septum: Assessment of Bioactive Molecules and In Vitro Biological Effects. Molecules 2020, 25, 2187. [Google Scholar] [CrossRef] [PubMed]
- Epure, A.; Pârvu, A.E.; Vlase, L.; Benedec, D.; Hanganu, D.; Gheldiu, A.-M.; Toma, V.A.; Oniga, I. Phytochemical Profile, Antioxidant, Cardioprotective and Nephroprotective Activity of Romanian Chicory Extract. Plants 2020, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Toiu, A.; Vlase, L.; Gheldiu, A.-M.; Vodnar, D.; Oniga, I. Evaluation of the Antioxidant and Antibacterial Potential of Bioactive Compounds from Ajuga Reptans Extracts. Farmacia 2017, 65, 351–355. [Google Scholar]
- Rauca, V.-F.; Vlase, L.; Casian, T.; Sesarman, A.; Gheldiu, A.-M.; Mocan, A.; Banciu, M.; Toiu, A. Biologically Active Ajuga Species Extracts Modulate Supportive Processes for Cancer Cell Development. Front. Pharmacol. 2019, 10, 334. [Google Scholar] [CrossRef]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.-M.; Nadăș, G.C.; Matei, I.A.; Filip, G.A.; Vlase, L.; Crișan, G. The Effect of Extraction Methods on Phytochemicals and Biological Activities of Green Coffee Beans Extracts. Plants 2023, 12, 712. [Google Scholar] [CrossRef]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.-M.; Nadăș, G.C.; Filip, G.A.; Vlase, L.; Crișan, G. Influences of Different Extraction Techniques and Their Respective Parameters on the Phytochemical Profile and Biological Activities of Xanthium spinosum L. Extracts. Plants 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Toiu, A.; Mocan, A.; Vlase, L.; Pârvu, A.E.; Vodnar, D.C.; Gheldiu, A.-M.; Moldovan, C.; Oniga, I. Comparative Phytochemical Profile, Antioxidant, Antimicrobial and In Vivo Anti-Inflammatory Activity of Different Extracts of Traditionally Used Romanian Ajuga genevensis L. and A. reptans L. (Lamiaceae). Molecules 2019, 24, 1597. [Google Scholar] [CrossRef]
- Toiu, A.; Mocan, A.; Vlase, L.; Pârvu, A.E.; Vodnar, D.C.; Gheldiu, A.-M.; Moldovan, C.; Oniga, I. Phytochemical Composition, Antioxidant, Antimicrobial and in Vivo Anti-Inflammatory Activity of Traditionally Used Romanian Ajuga laxmannii (Murray) Benth. (“Nobleman’s Beard”—Barba Împăratului). Front. Pharmacol. 2018, 9, 7. [Google Scholar] [CrossRef]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.-M.; Nadăș, G.C.; Novac, C.Ș.; Filip, G.A.; Vlase, L.; Crișan, G. Red Clover and the Importance of Extraction Processes—Ways in Which Extraction Techniques and Parameters Affect Trifolium pratense L. Extracts’ Phytochemical Profile and Biological Activities. Processes 2022, 10, 2581. [Google Scholar] [CrossRef]
- Pop, A.; Bogdan, C.; Fizesan, I.; Iurian, S.; Carpa, R.; Bacali, C.; Vlase, L.; Benedec, D.; Moldovan, M.L. In Vitro Evaluation of Biological Activities of Canes and Pomace Extracts from Several Varieties of Vitis vinifera L. for Inclusion in Freeze-Drying Mouthwashes. Antioxidants 2022, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.L.; Carpa, R.; Fizeșan, I.; Vlase, L.; Bogdan, C.; Iurian, S.M.; Benedec, D.; Pop, A. Phytochemical Profile and Biological Activities of Tendrils and Leaves Extracts from a Variety of Vitis vinifera L. Antioxidants 2020, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.-M.; Gouda, M.; Ye, X.-Q.; Shao, Z.-P.; Chen, J.-C. Evaluation of Proanthocyanidins from Kiwi Leaves (Actinidia chinensis) against Caco-2 Cells Oxidative Stress through Nrf2-ARE Signaling Pathway. Antioxidants 2022, 11, 1367. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.-M.; Tomuta, I.; Popa, D.-S. Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef]
- Vagiri, M.; Conner, S.; Stewart, D.; Andersson, S.C.; Verrall, S.; Johansson, E.; Rumpunen, K. Phenolic Compounds in Blackcurrant (Ribes nigrum L.) Leaves Relative to Leaf Position and Harvest Date. Food Chem. 2015, 172, 135–142. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Evers, D.; Dommes, J. Ascorbic Acid, Phenolic Acid, Flavonoid, and Carotenoid Profiles of Selected Extracts from Ribes nigrum. J. Agric. Food Chem. 2011, 59, 4763–4770. [Google Scholar] [CrossRef]
- Turrini, F.; Donno, D.; Beccaro, G.L.; Zunin, P.; Pittaluga, A.; Boggia, R. Pulsed Ultrasound-Assisted Extraction as an Alternative Method to Conventional Maceration for the Extraction of the Polyphenolic Fraction of Ribes Nigrum Buds: A New Category of Food Supplements Proposed by The Finnover Project. Foods 2019, 8, 466. [Google Scholar] [CrossRef]
- Cvetanović, A.; Zengin, G.; Zeković, Z.; Švarc-Gajić, J.; Ražić, S.; Damjanović, A.; Mašković, P.; Mitić, M. Comparative in Vitro Studies of the Biological Potential and Chemical Composition of Stems, Leaves and Berries Aronia Melanocarpa’s Extracts Obtained by Subcritical Water Extraction. Food Chem. Toxicol. 2018, 121, 458–466. [Google Scholar] [CrossRef]
- Bujor, O.-C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal Variations of the Phenolic Constituents in Bilberry (Vaccinium myrtillus L.) Leaves, Stems and Fruits, and Their Antioxidant Activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef]
- Bujor, O.-C.; Ginies, C.; Popa, V.I.; Dufour, C. Phenolic Compounds and Antioxidant Activity of Lingonberry (Vaccinium vitis-Idaea L.) Leaf, Stem and Fruit at Different Harvest Periods. Food Chem. 2018, 252, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical Composition and Biological Activities of the Nord-West Romanian Wild Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.) Leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Dragana, M.V.; Miroslav, R.P.; Branka, B.R.G.; Olgica, D.S.; Sava, M.V.; Ljiljana, R.Č. Antibacterial and Antioxidant Activities of Bilberry (Vaccinium myrtillus L.) in Vitro. Afr. J. Microbiol. Res. 2013, 7, 5130–5136. [Google Scholar] [CrossRef]
- Charpentier, T.; Boisard, S.; Le Ray, A.-M.; Bréard, D.; Chabrier, A.; Esselin, H.; Guilet, D.; Ripoll, C.; Richomme, P. A Descriptive Chemical Composition of Concentrated Bud Macerates through an Optimized SPE-HPLC-UV-MS2 Method—Application to Alnus glutinosa, Ribes nigrum, Rosa canina, Rosmarinus officinalis and Tilia tomentosa. Plants 2022, 11, 144. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerruti, A.K.; Bounous, G. Medicinal Plants, Chemical Composition and Quality: May Blackcurrant Buds and Blackberry Sprouts Be a New Polyphenol Source for Herbal Preparations? J. Appl. Bot. Food Qual. 2013, 86, 79–89. [Google Scholar] [CrossRef]
- Turrini, F.; Donno, D.; Boggia, R.; Beccaro, G.L.; Zunin, P.; Leardi, R.; Pittaluga, A.M. An Innovative Green Extraction and Re-Use Strategy to Valorize Food Supplement by-Products: Castanea Sativa Bud Preparations as Case Study. Food Res. Int. 2019, 115, 276–282. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A Comparison of Fruit Quality Parameters of Wild Bilberry (Vaccinium Myrtillus L.) Growing at Different Locations: Fruit Quality Parameters of Wild Bilberry. J. Sci. Food Agric. 2015, 95, 776–785. [Google Scholar] [CrossRef]
- Hohtola, A.; Jaakola, L.; Maatta-Riihinen, K.; Karenlampi, S. Activation of Flavonoid Biosynthesis by Solar Radiation in Bilberry (Vaccinium Myrtillus L.) Leaves. Planta 2004, 218, 721–728. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Koivuniemi, H.; Huttunen, S. Comparison of the Triterpenoid Content of Berries and Leaves of Lingonberry Vaccinium vitis-idaea from Finland and Poland. J. Agric. Food Chem. 2012, 60, 4994–5002. [Google Scholar] [CrossRef]
- Spitaler, R.; Winkler, A.; Lins, I.; Yanar, S.; Stuppner, H.; Zidorn, C. Altitudinal Variation of Phenolic Contents in Flowering Heads of Arnica Montana Cv. ARBO: A 3-Year Comparison. J. Chem. Ecol. 2008, 34, 369–375. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as Potential Antioxidant Therapeutic Agents: Mechanism and Actions. Mutat. Res. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Raudsepp, P.; Kaldmäe, H.; Kikas, A.; Libek, A.-V.; Püssa, T. Nutritional Quality of Berries and Bioactive Compounds in the Leaves of Black Currant (Ribes nigrum L.) Cultivars Evaluated in Estonia. J. Berry Res. 2010, 1, 53–59. [Google Scholar] [CrossRef]
- Mateș, L.; Rusu, M.E.; Popa, D.-S. Phytochemicals and Biological Activities of Walnut Septum: A Systematic Review. Antioxidants 2023, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Montoro, P.; Piacente, S. Detection and Comparison of Phenolic Compounds in Different Extracts of Black Currant Leaves by Liquid Chromatography Coupled with High-Resolution ESI-LTQ-Orbitrap MS and High-Sensitivity ESI-Qtrap MS. J. Pharm. Biomed. Anal. 2020, 179, 112926. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lindstedt, A.; Markkinen, N.; Sinkkonen, J.; Suomela, J.-P.; Yang, B. Characterization of Metabolite Profiles of Leaves of Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.). J. Agric. Food Chem. 2014, 62, 12015–12026. [Google Scholar] [CrossRef] [PubMed]
- Shamilov, A.A.; Olennikov, D.N.; Pozdnyakov, D.I.; Bubenchikova, V.N.; Garsiya, E.R.; Larskii, M.V. Caucasian Blueberry: Comparative Study of Phenolic Compounds and Neuroprotective and Antioxidant Potential of Vaccinium myrtillus and Vaccinium arctostaphylos Leaves. Life 2022, 12, 2079. [Google Scholar] [CrossRef]
- Hokkanen, J.; Mattila, S.; Jaakola, L.; Pirttilä, A.M.; Tolonen, A. Identification of Phenolic Compounds from Lingonberry (Vaccinium vitis-idaea L.), Bilberry (Vaccinium myrtillus L.) and Hybrid Bilberry (Vaccinium × Intermedium Ruthe L.) Leaves. J. Agric. Food Chem. 2009, 57, 9437–9447. [Google Scholar] [CrossRef]
- Ieri, F.; Martini, S.; Innocenti, M.; Mulinacci, N. Phenolic Distribution in Liquid Preparations of Vaccinium myrtillus L. and Vaccinium vitis idaea L. Phytochem. Anal. 2013, 24, 467–475. [Google Scholar] [CrossRef]
- Mourabit, Y.; El Hajjaji, S.; Taha, D.; Badaoui, B.; El Yadini, M.; Rusu, M.E.; Lee, L.-H.; Bouyahya, A.; Bourais, I. HPLC-DAD-ESI/MS Phytochemical Investigation, Antioxidant, and Antidiabetic Activities of Moroccan Rosa canina L. Extracts. Biocatal. Agric. Biotechnol. 2023, 52, 102817. [Google Scholar] [CrossRef]
- Ginovyan, M.; Bartoszek, A.; Koss-Mikołajczyk, I.; Kusznierewicz, B.; Andreoletti, P.; Cherkaoui-Malki, M.; Sahakyan, N. Growth Inhibition of Cultured Cancer Cells by Ribes nigrum Leaf Extract. AIMS Biophys. 2022, 9, 282–293. [Google Scholar] [CrossRef]
- Ribera-Fonseca, A.; Jiménez, D.; Leal, P.; Riquelme, I.; Roa, J.C.; Alberdi, M.; Peek, R.M.; Reyes-Díaz, M. The Anti-Proliferative and Anti-Invasive Effect of Leaf Extracts of Blueberry Plants Treated with Methyl Jasmonate on Human Gastric Cancer In Vitro Is Related to Their Antioxidant Properties. Antioxidants 2020, 9, 45. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical Composition and Biological Activity of Rubus Idaeus Shoots—A Traditional Herbal Remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Debnath-Canning, M.; Unruh, S.; Vyas, P.; Daneshtalab, N.; Igamberdiev, A.U.; Weber, J.T. Fruits and Leaves from Wild Blueberry Plants Contain Diverse Polyphenols and Decrease Neuroinflammatory Responses in Microglia. J. Funct. Foods 2020, 68, 103906. [Google Scholar] [CrossRef]
- Kellett, M.E.; Greenspan, P.; Pegg, R.B. Modification of the Cellular Antioxidant Activity (CAA) Assay to Study Phenolic Antioxidants in a Caco-2 Cell Line. Food Chem. 2018, 244, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Ma, S.-X.; Ko, Y.-H.; Seo, J.-Y.; Lee, B.-R.; Lee, T.H.; Kim, S.Y.; Lee, S.-Y.; Jang, C.-G. Vaccinium bracteatum Thunb. Exerts Anti-Inflammatory Activity by Inhibiting NF-κB Activation in BV-2 Microglial Cells. Biomol. Ther. 2016, 24, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-H.; Zhao, T.-R.; Liu, Y.-P.; Wang, Y.-F.; Cheng, G.-G.; Cao, J.-X. Phenolic Constituents, Antioxidant Activity and Neuroprotective Effects of Ethanol Extracts of Fruits, Leaves and Flower Buds from Vaccinium dunalianum Wight. Food Chem. 2022, 374, 131752. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-S.; Gu, Q.-H.; Zhang, J.-K.; Tao, J.-H.; Zhao, T.-R.; Cao, J.-X.; Cheng, G.-G.; Lai, G.-F.; Liu, Y.-P. Phenolic Constituents, Antioxidant and Cytoprotective Activities, Enzyme Inhibition Abilities of Five Fractions from Vaccinium dunalianum Wight. Molecules 2022, 27, 3432. [Google Scholar] [CrossRef]
- Yao, J.; Peng, S.; Xu, J.; Fang, J. Reversing ROS-mediated Neurotoxicity by Chlorogenic Acid Involves Its Direct Antioxidant Activity and Activation of Nrf2-ARE Signaling Pathway. BioFactors 2019, 45, 616–626. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. Chlorogenic Acid, a Polyphenol from Prunus domestica (Mirabelle), with Coupled Anxiolytic and Antioxidant Effects. J. Neurol. Sci. 2007, 262, 77–84. [Google Scholar] [CrossRef]
- Shah, M.-A.; Kang, J.-B.; Park, D.-J.; Kim, M.-O.; Koh, P.-O. Chlorogenic Acid Alleviates Neurobehavioral Disorders and Brain Damage in Focal Ischemia Animal Models. Neurosci. Lett. 2021, 760, 136085. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Simedrea, R.; Gheldiu, A.-M.; Mocan, A.; Vlase, L.; Popa, D.-S.; Ferreira, I.C.F.R. Benefits of Tree Nut Consumption on Aging and Age-Related Diseases: Mechanisms of Actions. Trends Food Sci. Technol. 2019, 88, 104–120. [Google Scholar] [CrossRef]
- Tabart, J.; Franck, T.; Kevers, C.; Pincemail, J.; Serteyn, D.; Defraigne, J.-O.; Dommes, J. Antioxidant and Anti-Inflammatory Activities of Ribes nigrum Extracts. Food Chem. 2012, 131, 1116–1122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).