Physico-Chemical Characterization, Phenolic Compound Extraction and Biological Activity of Grapevine (Vitis vinifera L.) Canes
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Climate Conditions of the Vineyard
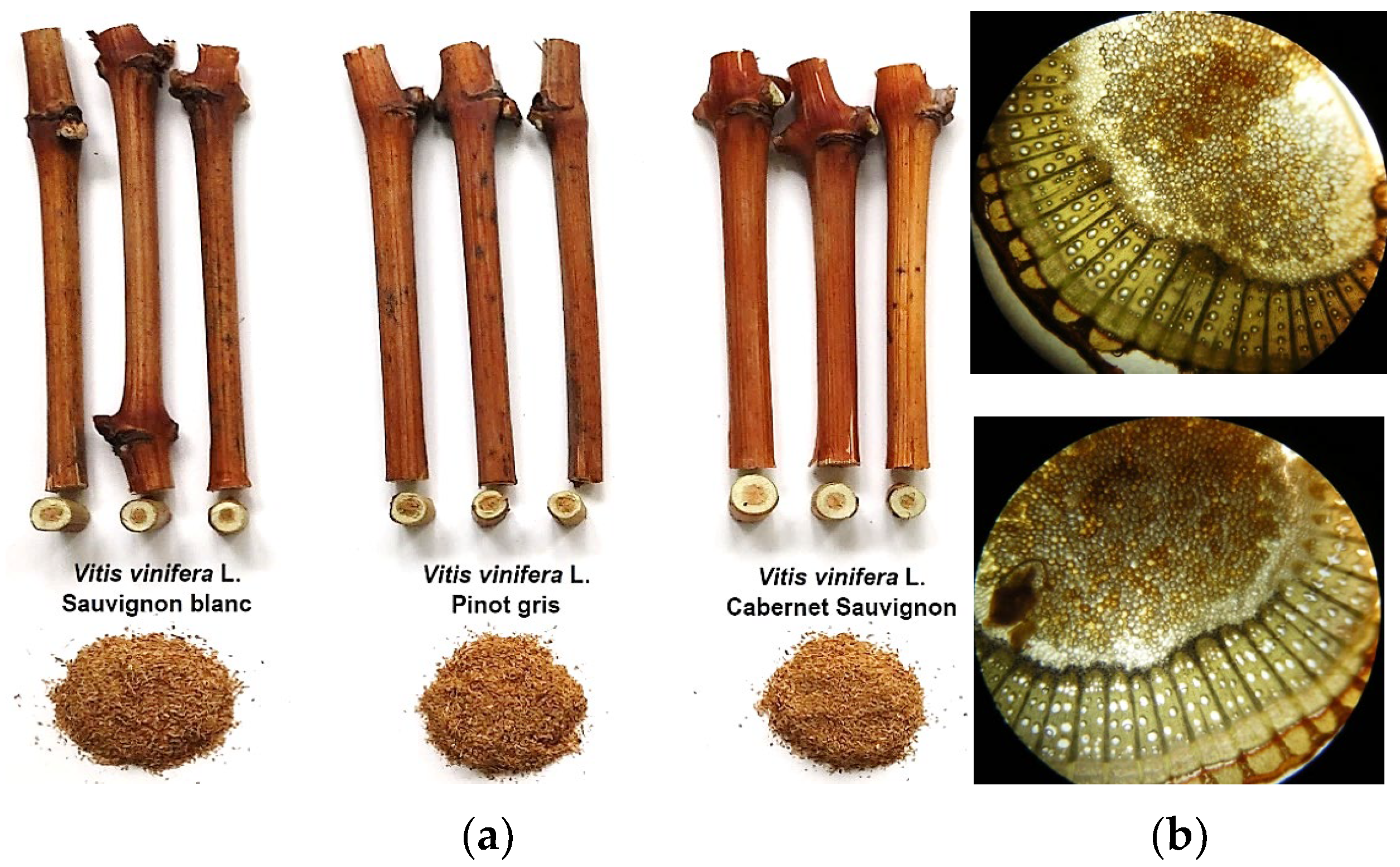
2.2. Biological Material
2.3. Physico-Chemical Determinations
2.4. Extraction Procedures
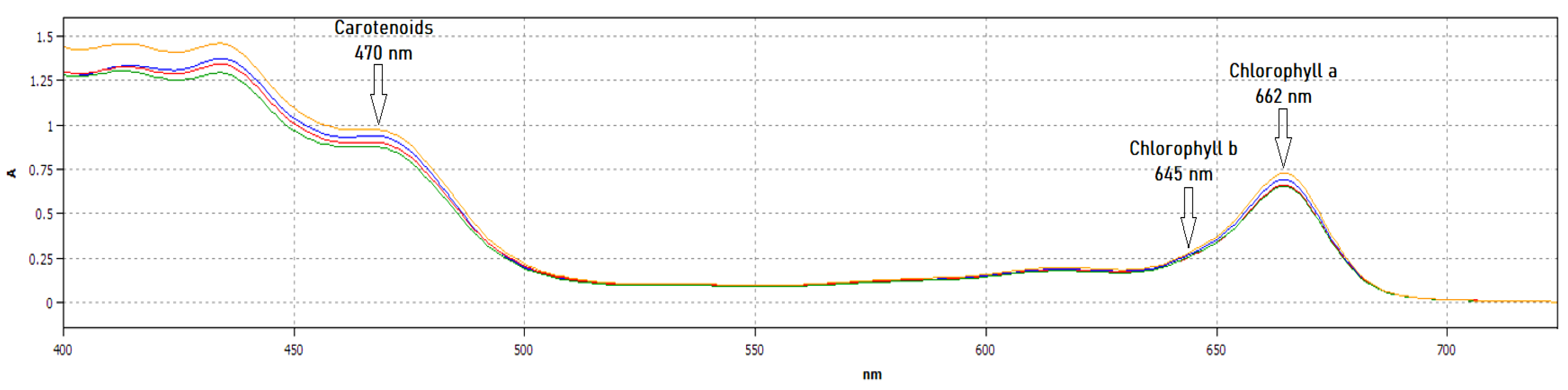
2.5. Chlorophyll and Carotenoid Content
2.6. Total Phenolic and Flavonoid Content
2.7. Polyphenolic Extract Purification
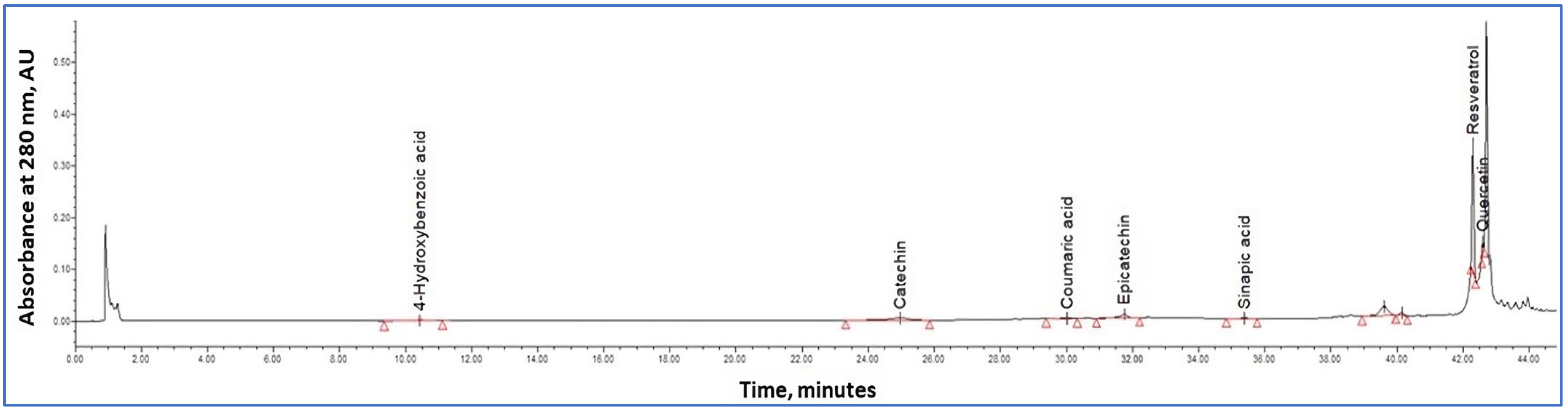
2.8. HPLC Procedure
2.9. DPPH Scavenging Activity
2.10. Phosphomolybdenum Assay
2.11. Ferric Reducing Power
2.12. Phenanthroline Assay
2.13. Antimicrobial Activity Assay
2.14. Statistical Procedures
3. Results and Discussion
3.1. Physico-Chemical Characterization of Grapevine Canes
3.2. Determination of the Extraction Parameters
3.2.1. Solvent Concentration
3.2.2. Extraction Time
3.2.3. Temperature
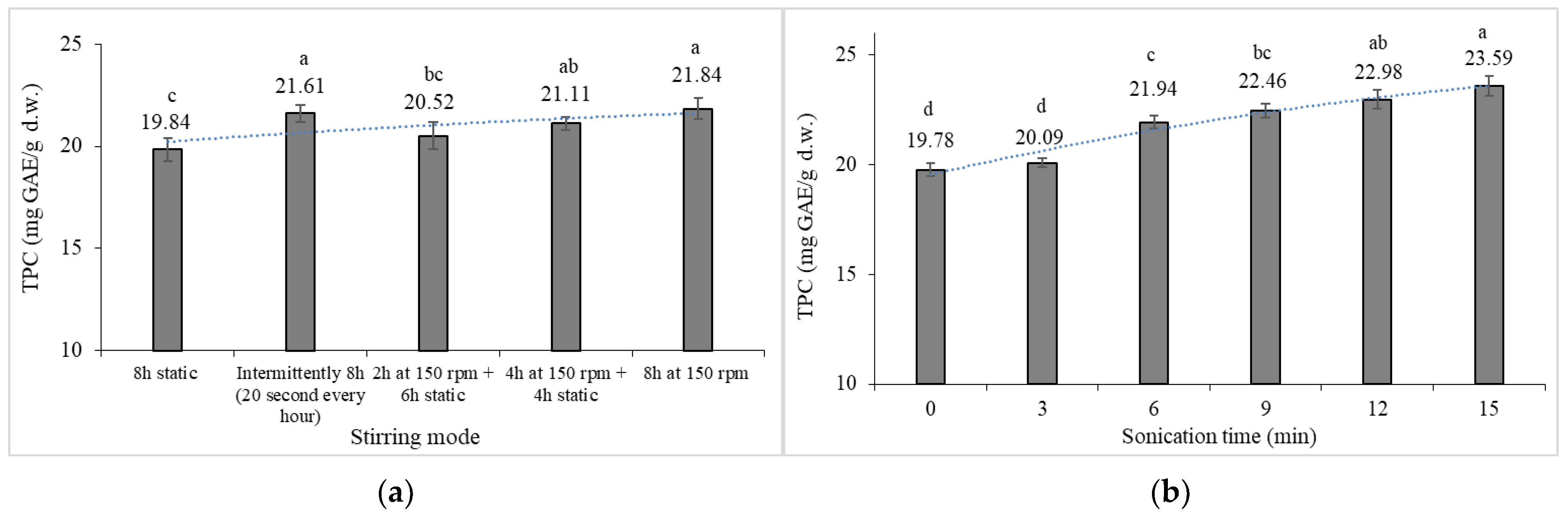
3.2.4. Stirring Conditions
3.2.5. Ultrasound Application
3.3. Obtaining the Crude and Purified Polyphenolic Extracts
3.4. Chlorophyll and Carotenoid Content
3.5. HPLC-PDA Polyphenolic Profile
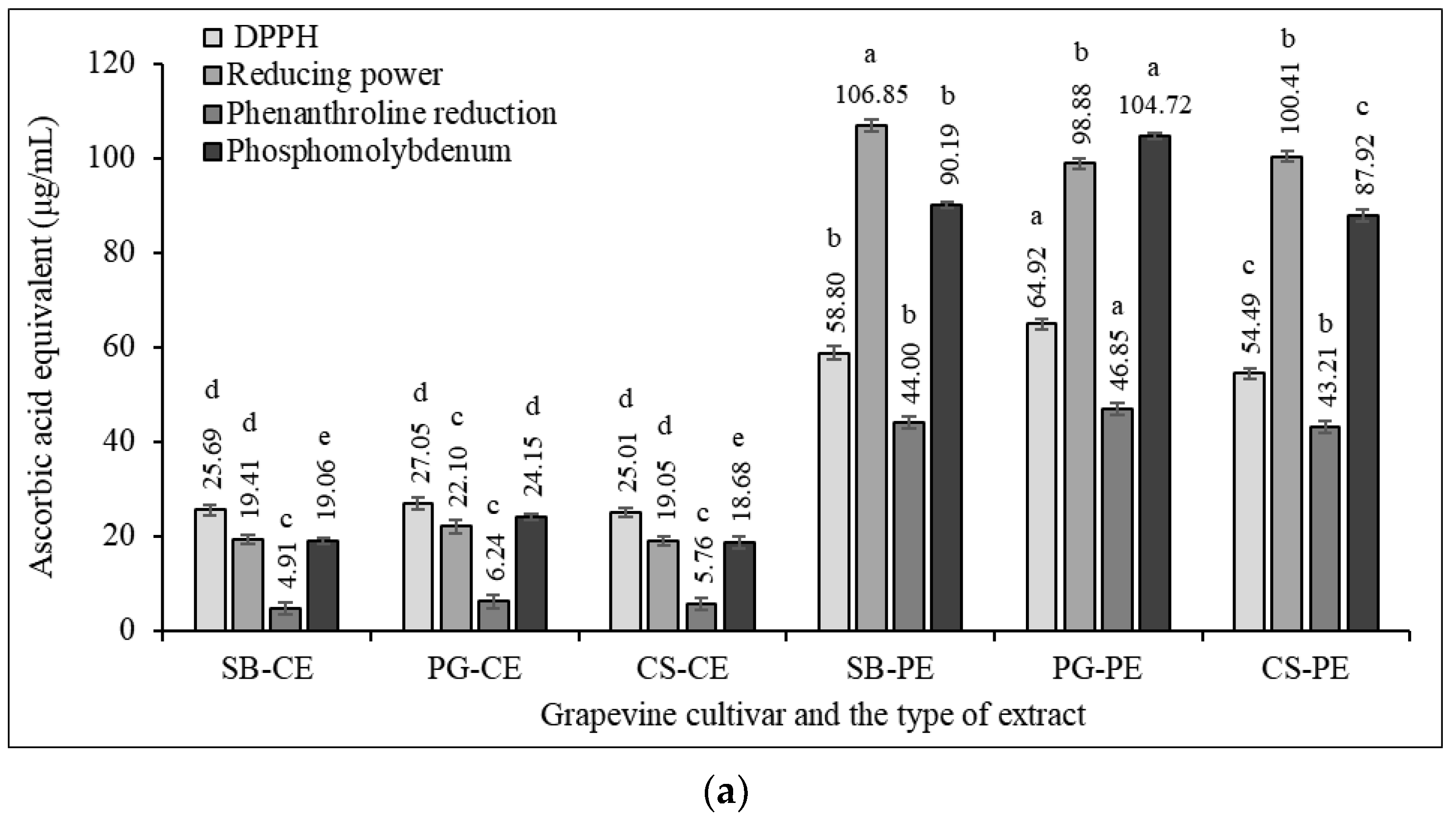
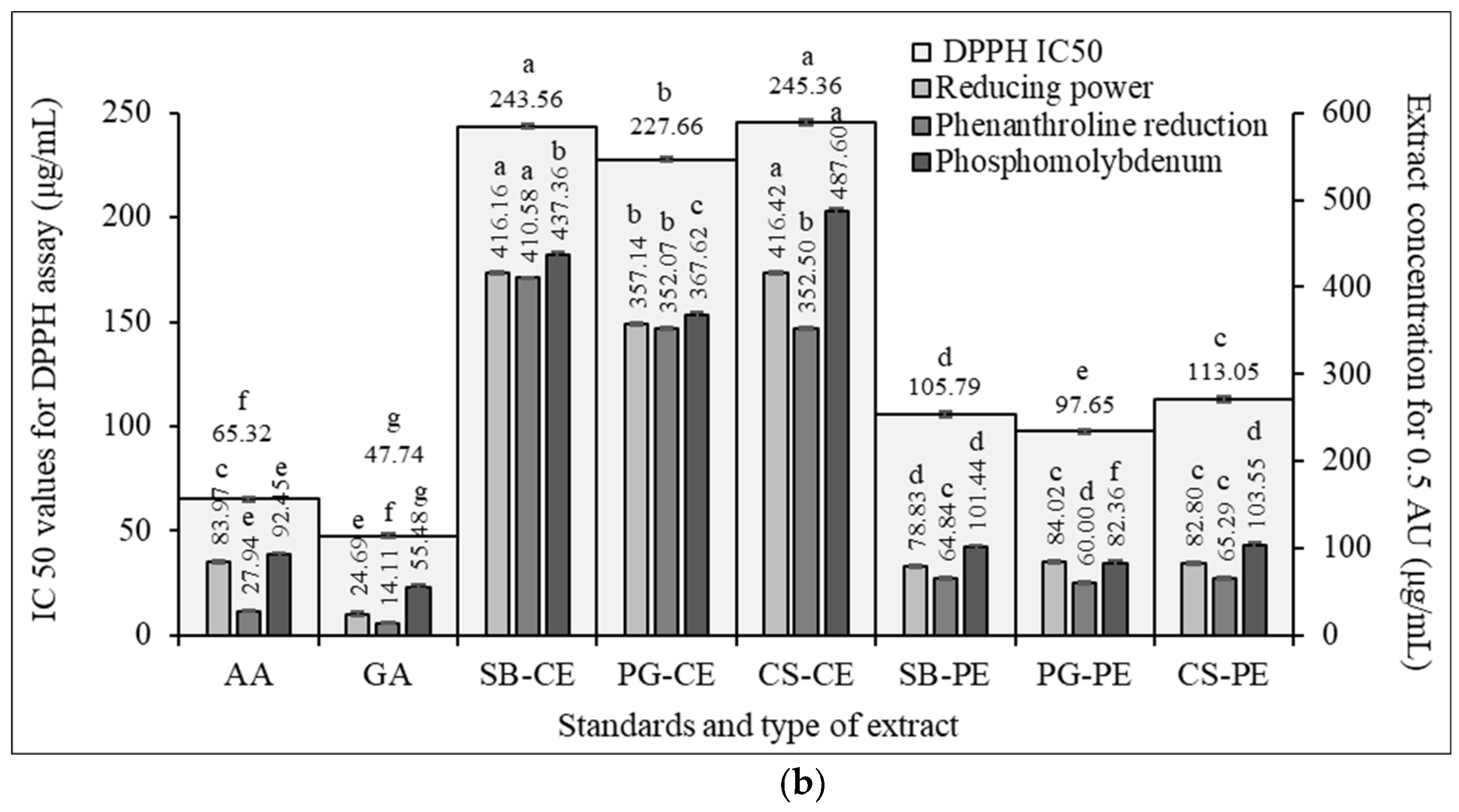
3.6. Antioxidant and Antiradical Potential
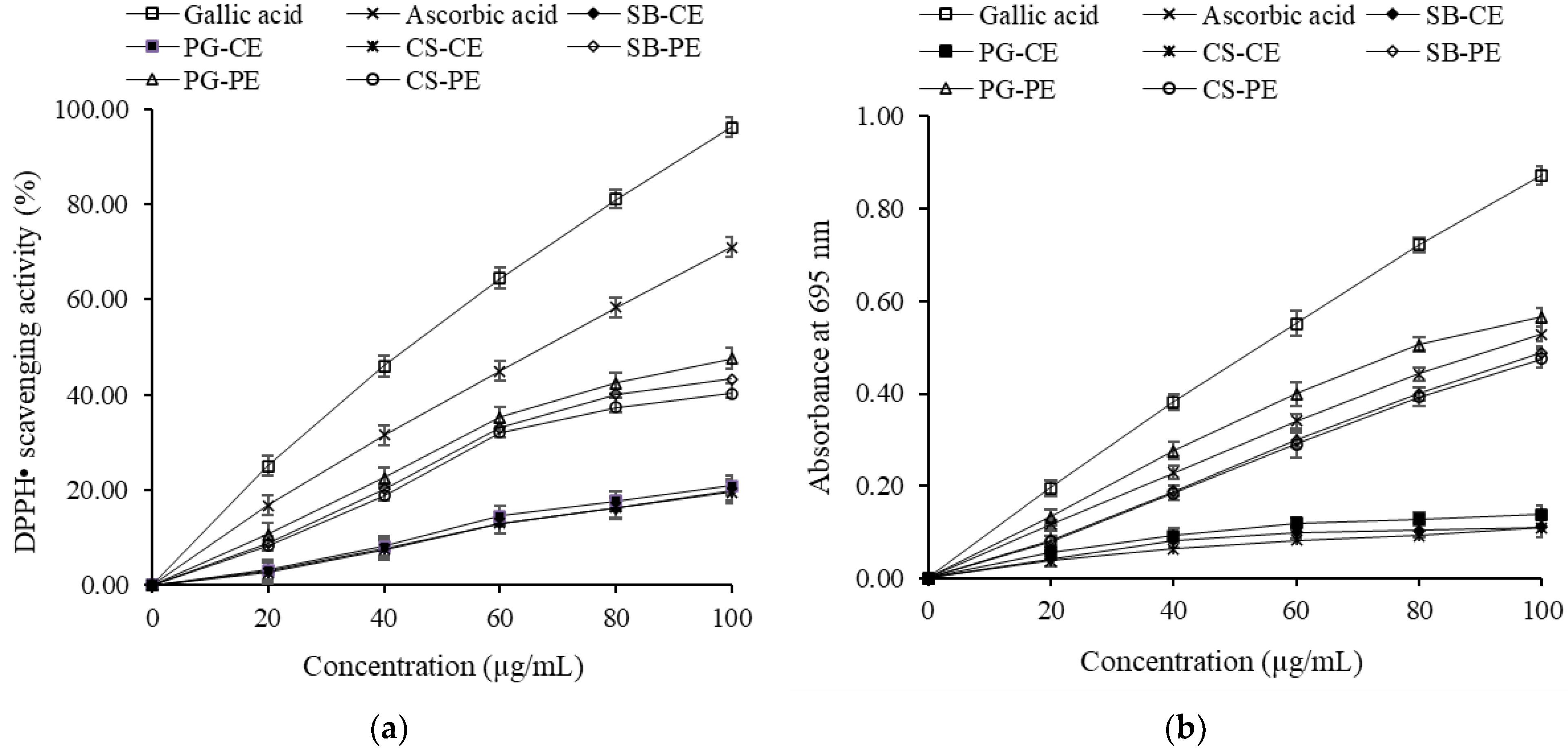
3.6.1. DPPH Scavenging Activity
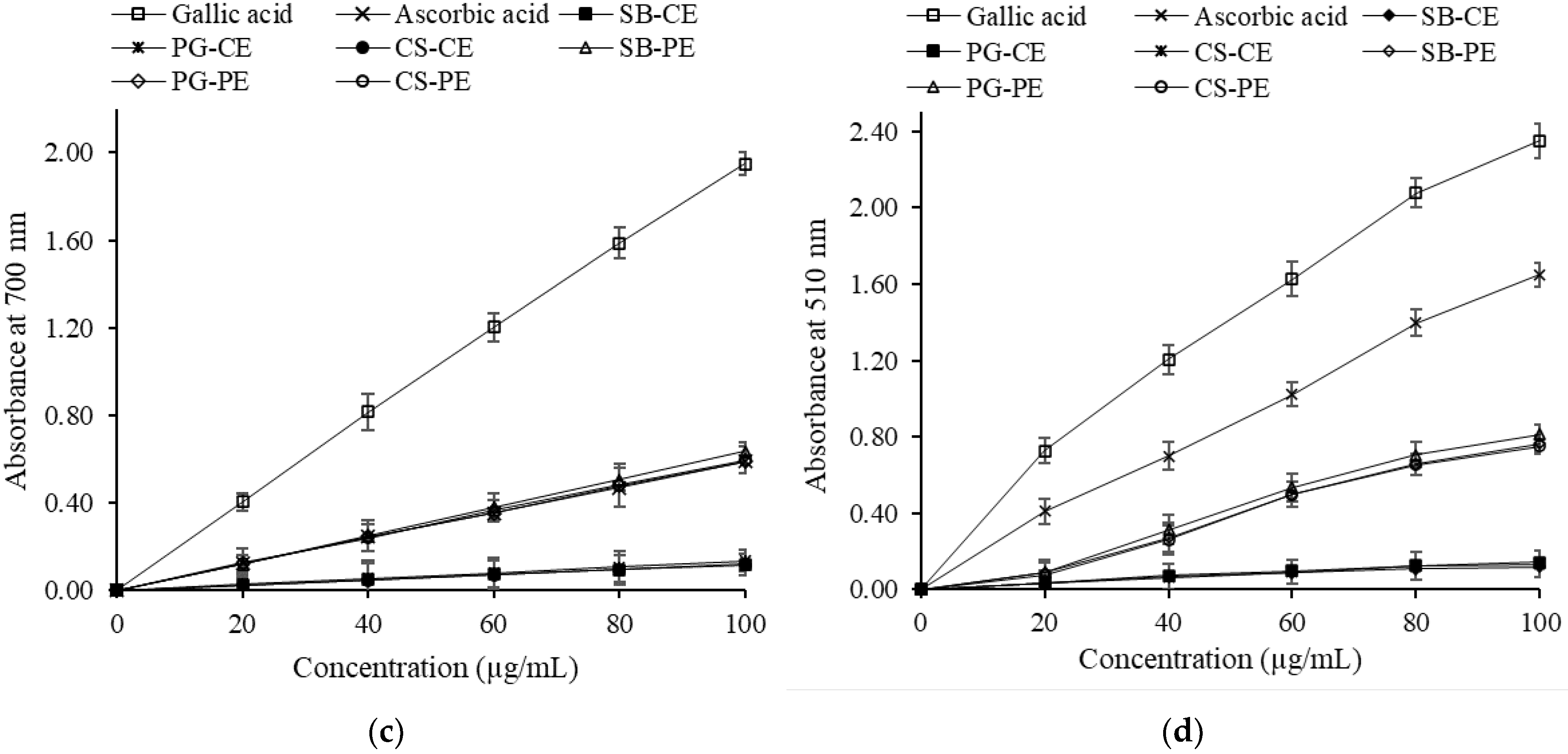
3.6.2. Phosphomolybdenum Assay
3.6.3. Ferric Reducing Power
3.6.4. Phenanthroline Assay
3.6.5. Antioxidant/Antiradical Activity and Phenolic Compounds’ Correlation
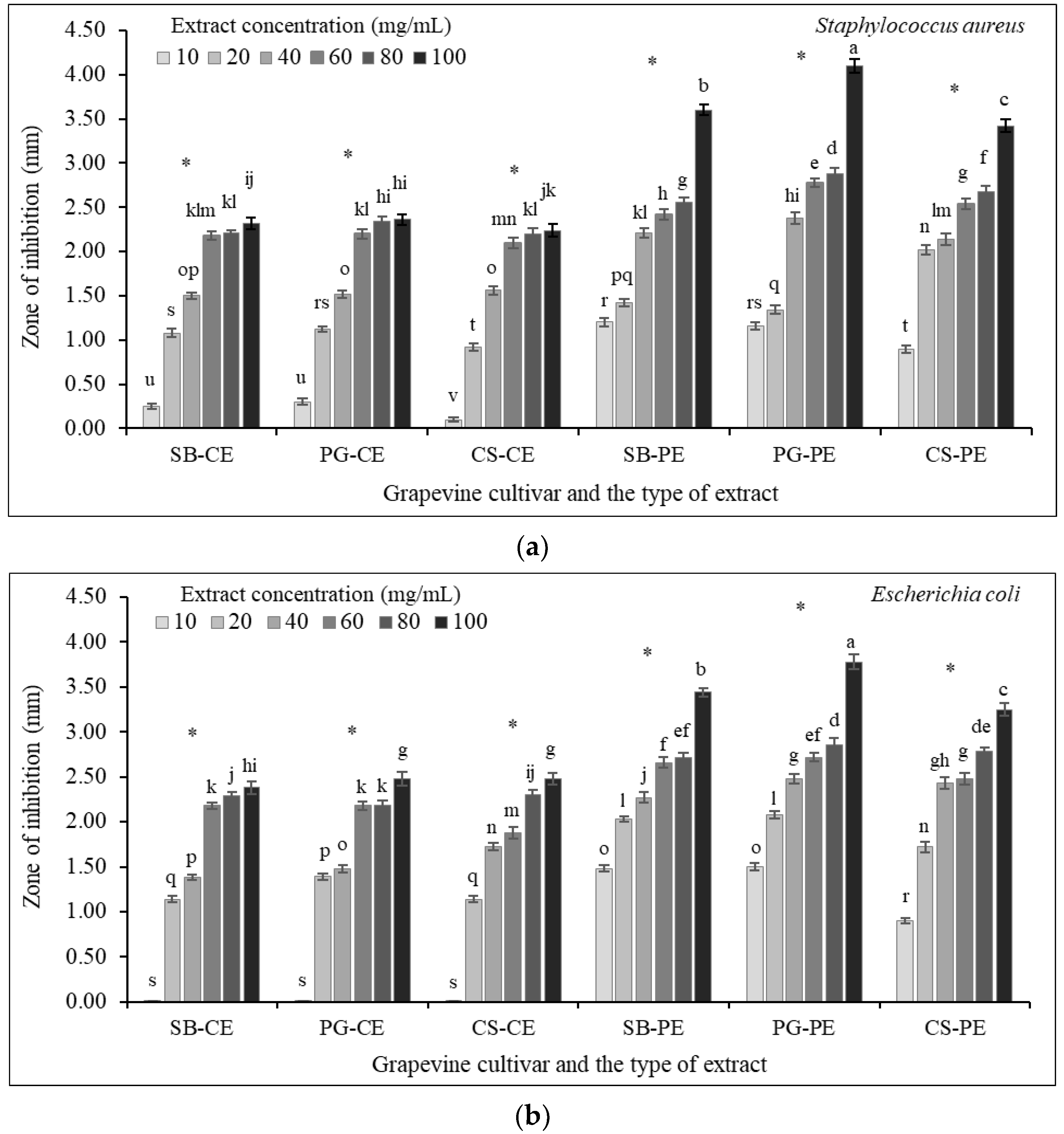
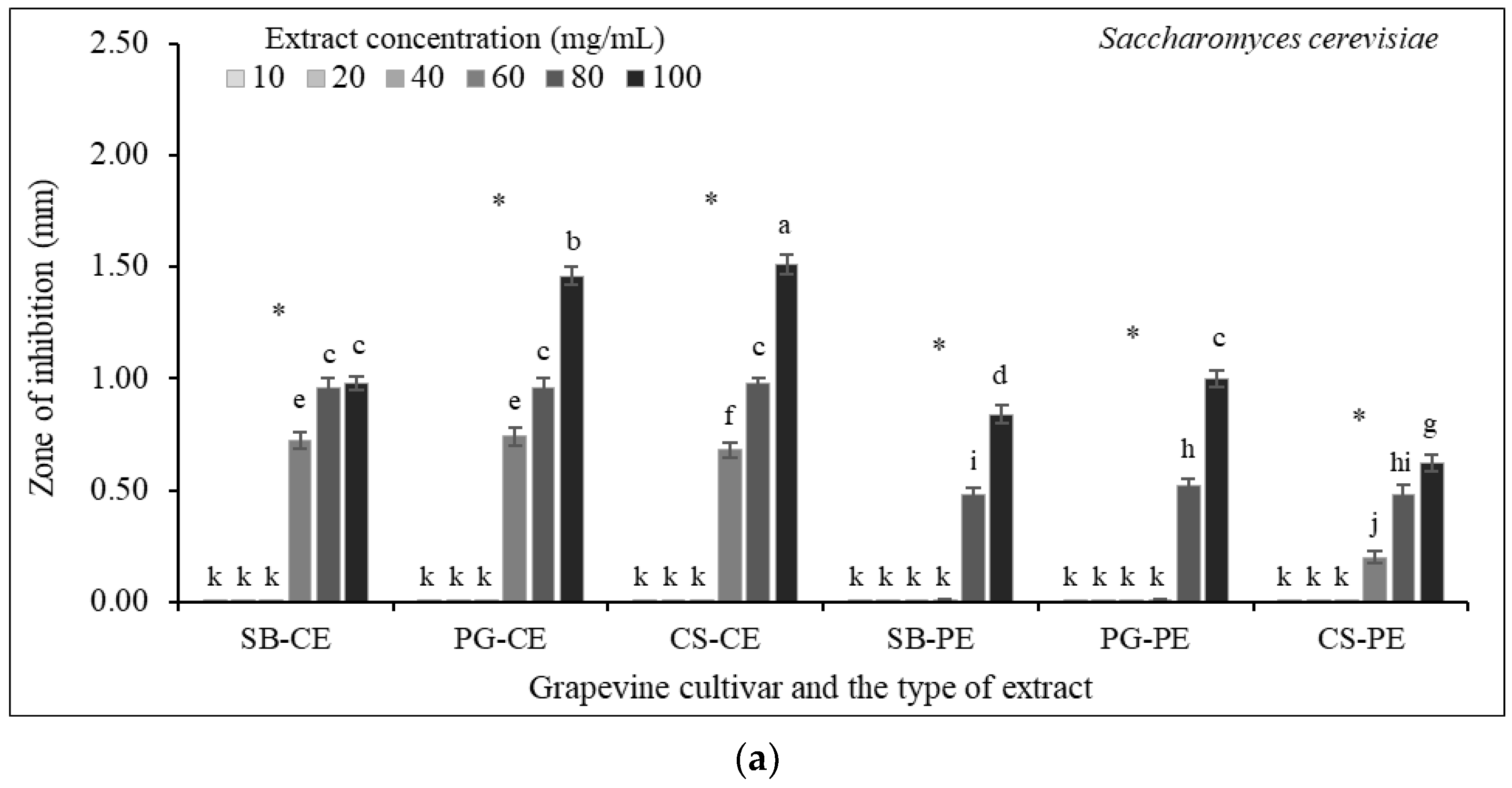
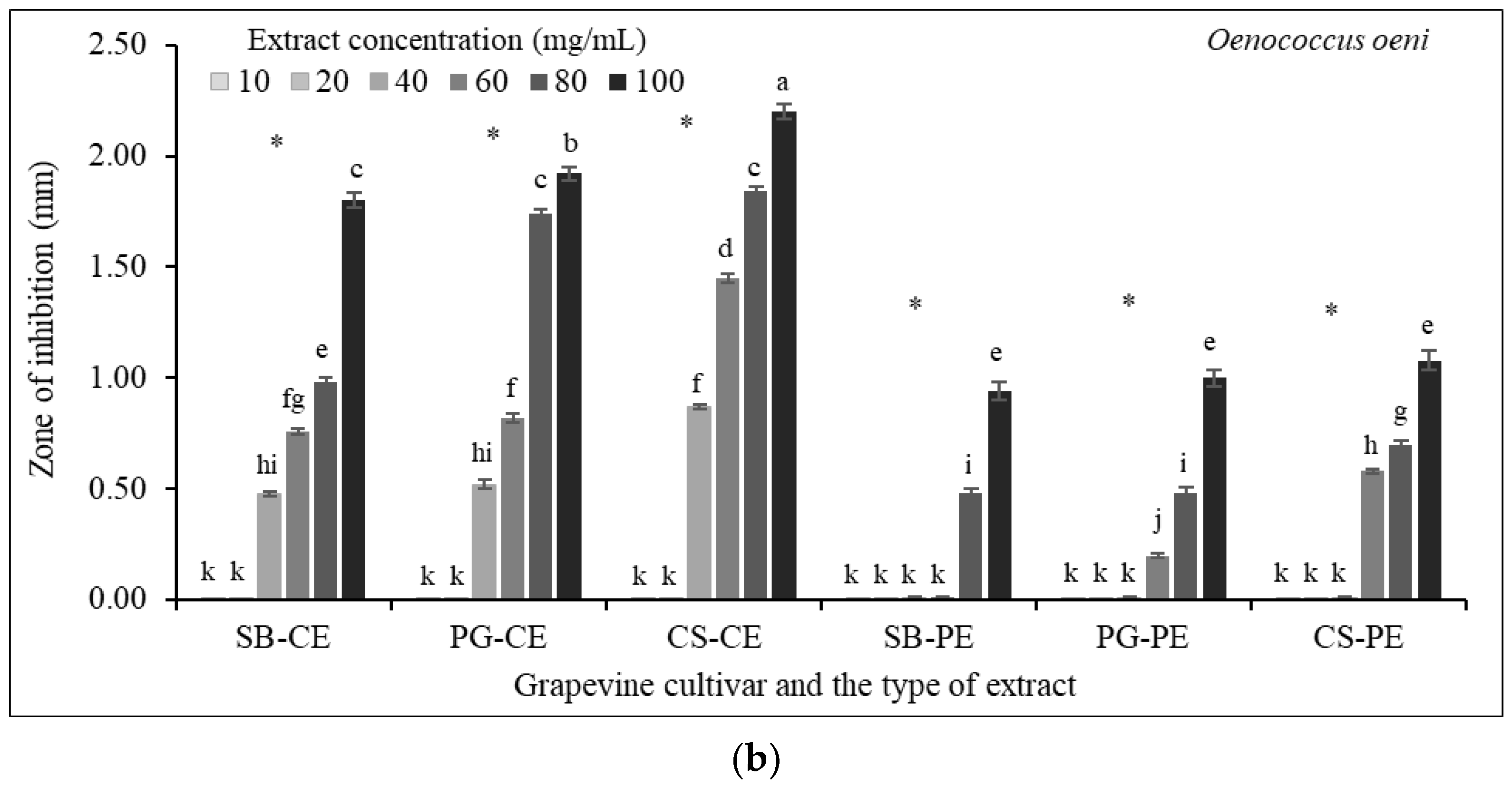
3.7. Antibacterial Susceptibility Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filimon, V.R.; Filimon, R.M.; Patraş, A.; Rotaru, L. Grape quality and ornamental potential of interspecific cultivars for temperate climate vineyards. J. Hortic. Sci. Biotechnol. 2020, 95, 65–75. [Google Scholar] [CrossRef]
- FAO-OIV (Food and Agriculture Organization of the United Nations and International Organisation of Vine and Wine. FAO–OIV Focus 2016. Table and Dried Grapes. 2016. Available online: https://www.oiv.int/public/medias/4911/fao-oiv-grapes-report-flyer.pdf (accessed on 4 January 2018).
- OIV (International Organisation of Vine and Wine). State of the World Vine and Wine Sector 2021. 2022. Available online: https://www.oiv.int/sites/default/files/documents/eng-state-of-the-world-vine-and-wine-sector-april-2022-v6_0.pdf (accessed on 11 February 2023).
- Aliaño-González, M.J.; Gabaston, J.; Ortiz-Somovilla, V.; Cantos-Villar, E. Wood waste from fruit trees: Biomolecules and their applications in agri-food industry. Biomolecules 2022, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Goldammer, T. Grape Grower’s Handbook: A Guide to Viticulture for Wine Production, 3rd ed.; Apex Publishers: Centreville, VA, USA, 2018; Available online: http://www.wine-grape-growing.com/ (accessed on 12 March 2020).
- Noviello, M.; Caputi, A.F.; Squeo, G.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Vine shoots as a source of trans-resveratrol and ε-viniferin: A study of 23 Italian varieties. Foods 2022, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, R.; Zalacain, A.; Pardo, F.; Alonso, G.L.; Salinas, M.R. Moscatel vine-shoot extracts as a grapevine biostimulant to enhance wine quality. Food Res. Int. 2017, 98, 40–49. [Google Scholar] [CrossRef]
- Pavela, R.; Waffo-Teguo, P.; Biais, B.; Richard, T.; Mérillon, J.-M. Vitis vinifera canes, a source of stilbenoids against Spodoptera littoralis larvae. J. Pest Sci. 2017, 90, 961–970. [Google Scholar] [CrossRef]
- Cruz, S.; Raposo, R.; Ruiz-Moreno, M.J.; Garde-Cerdán, T.; Puertas, B.; Gonzalo-Diago, A.; Moreno-Rojas, J.M.; Cantos-Villar, E. Grapevine-shoot stilbene extract as a preservative in white wine. Food Packag. Shelf Life 2018, 18, 164–172. [Google Scholar] [CrossRef]
- Cebrián-Tarancón, C.; Fernández-Roldán, F.; Alonso, G.L.; Salinas, R.M. Classification of vine-shoots for use as enological additives. J. Sci. Food Agric. 2022, 102, 724–731. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Sustainability of wine production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef]
- Zwingelstein, M.; Draye, M.; Besombes, J.L.; Piot, C.; Chatel, G. Viticultural wood waste as a source of polyphenols of interest: Opportunities and perspectives through conventional and emerging extraction methods. Waste Manag. 2020, 102, 782–794. [Google Scholar] [CrossRef]
- Bagchi, D.; Swaroop, A.; Preuss, H.G.; Bagchi, M. Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: An overview. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2014, 768, 69–73. [Google Scholar] [CrossRef]
- Kiskova, T.; Kubatka, P.; Büsselberg, D.; Kassayova, M. The plant-derived compound resveratrol in brain cancer: A review. Biomolecules 2020, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Kubyshkin, A.; Shevandova, A.; Petrenko, V.; Fomochkina, I.; Sorokina, L.; Kucherenko, A.; Gordienko, A.; Khimich, N.; Zyablitskaya, E.; Makalish, T.; et al. Anti-inflammatory and antidiabetic effects of grape-derived stilbene concentrate in the experimental metabolic syndrome. J. Diabetes Metab. Disord. 2020, 19, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, S.; Bottini, R.; Fontana, A. Background and perspectives on the utilization of canes’ and bunch stems’ residues from wine industry as sources of bioactive phenolic compounds. J. Agric. Food Chem. 2023, 71, 8699–8730. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Avello, D.; Lozano-Castellón, J.; Mardones, C.; Pérez, A.J.; Saéz, V.; Riquelme, S.; von Baer, D.; Vallverdú-Queralt, A. Phenolic profile of grape canes: Novel compounds identified by LC-ESI-LTQ-Orbitrap-MS. Molecules 2019, 24, 3763. [Google Scholar] [CrossRef]
- Badjah Hadj Ahmed, A.Y.; Wabaidur, S.M.; Siddiqui, M.R.; Alothman, Z.A.; Obeid, M.S.; Khan, M.R.; AL-Tamrah, S.A. Simultaneous determination of twenty-five polyphenols in multifloral and cactus honeys using solid-phase extraction and high-performance liquid chromatography with photodiode array detection. Eur. Food Res. Technol. 2016, 242, 943–952. [Google Scholar] [CrossRef]
- Tan, L.; Ji, T.; Jiang, G.; Hu, F. Simultaneous identification and quantification of five flavonoids in the seeds of Rheum palmatum L. by using accelerated solvent extraction and HPLC–PDA–ESI/MSn. Arab. J. Chem. 2019, 12, 1345–1352. [Google Scholar] [CrossRef]
- Soural, I.; Vrchotová, N.; Tříska, J.; Balík, J.; Horník, Š.; Cuřínová, P.; Sýkora, J. Various extraction methods for obtaining stilbenes from grape cane of Vitis vinifera L. Molecules 2015, 20, 6093–6112. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Biais, B.; Richard, T.; Puertas, B.; Waffo-Teguo, P.; Merillon, J.-M.; Cantos-Villar, E. Grapevine cane’s waste is a source of bioactive stilbenes. Ind. Crops Prod. 2016, 94, 884–892. [Google Scholar] [CrossRef]
- Rodríguez-Cabo, T.; Rodríguez, I.; Ramil, M.; Cela, R. Assessment of alcoholic distillates for the extraction of bioactive polyphenols from grapevine canes. Ind. Crops Prod. 2018, 111, 99–106. [Google Scholar] [CrossRef]
- Rajha, H.N.; Jaoude, N.A.; Louka, N.; Maroun, R.G.; Vorobiev, E. Industrial byproducts valorization through energy saving processes. Alkaline extraction of polyphenols from vine shoots. In Proceedings of the International Conference on Renewable Energies for Developing Countries, Beirut, Lebanon, 26–27 November 2014; pp. 89–94. [Google Scholar] [CrossRef]
- Delgado-Torre, M.P.; Ferreiro-Vera, C.; Priego-Capote, F.; Pérez-Juan, P.M.; Luque de Castro, M.D. Comparison of accelerated methods for the extraction of phenolic compounds from different vine-shoot cultivars. J. Agric. Food Chem. 2012, 60, 3051–3060. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Vine-shoot waste aqueous extracts for re-use in agriculture obtained by different extraction techniques: Phenolic, volatile, and mineral compounds. J. Agric. Food Chem. 2014, 62, 10861–10872. [Google Scholar] [CrossRef] [PubMed]
- Rayne, S.; Karacabey, E.; Mazza, G. Grape cane waste as a source of trans-resveratrol and transviniferin: High-value phytochemicals with medicinal and anti-phytopathogenic applications. Ind. Crops Prod. 2008, 27, 335–340. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Fang, Y.; Li, X.; Meng, J.; Wang, H.; Li, H.; Zhang, Z.; Guo, Z. Occurrence and estimation of trans-resveratrol in one-year-old canes from seven major Chinese grape producing regions. Molecules 2011, 16, 2846–2861. [Google Scholar] [CrossRef]
- Ju, Y.; Zhang, A.; Fang, Y.; Liu, M.; Zhao, X.; Wang, H.; Zhang, Z. Phenolic compounds and antioxidant activities of grape canes extracts from vineyards. Span. J. Agric. Res. 2016, 14, e0805. [Google Scholar] [CrossRef]
- Filimon, R.; Filimon, V.R.; Patraş, A.; Rotaru, L. Études sur L’extraction de Composes Phénoliques de Vigne Baguettes. Lucr. Şt. USAMV Iaşi—Ser. Hortic. 2018, 61, 421–426. [Google Scholar]
- Dorosh, O.; Moreira, M.M.; Pinto, D.; Peixoto, A.F.; Freire, C.; Costa, P.; Rodrigues, F.; Delerue-Matos, C. Evaluation of the extraction temperature influence on polyphenolic profiles of vine-canes (Vitis vinifera) subcritical water extracts. Foods 2020, 9, 872. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Marrufo-Curtido, A.; Vela, C.; Palma, M. Microwave-assisted extraction of stilbenes from woody vine material. Food Bioprod. Process. 2017, 103, 18–26. [Google Scholar] [CrossRef]
- Zachová, Z.; Tříska, J.; Vrchotová, N.; Balík, J.; Sajfrtová, M.; Sovová, H. Combining high-pressure methods for extraction of stilbenes from grape cane. J. Supercrit. Fluids 2018, 142, 38–44. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Jackman, R.L.; Smith, J.L. Anthocyanins and betalains. In Natural Food Colorants, 2nd ed.; Hendry, G.A.F., Houghton, J.D., Eds.; Chapman & Hall: London, UK, 1996; pp. 244–309. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, C.Y. Extraction and isolation of polyphenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. I1.2.1–I1.2.12. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. CRFS 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Fang, Y.; Wang, H.; Li, H.; Zhang, Z. Free-radical scavenging properties and reducing power of grape cane extracts from 11 selected grape cultivars widely grown in China. Molecules 2011, 16, 10104–10122. [Google Scholar] [CrossRef] [PubMed]
- Kodeš, Z.; Vrublevskaya, M.; Kulišová, M.; Jaroš, P.; Paldrychová, M.; Pádrová, K.; Lokočová, K.; Palyzová, A.; Maťátková, O.; Kolouchová, I. Composition and biological activity of Vitis vinifera winter cane extract on Candida biofilm. Microorganisms 2021, 9, 2391. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef]
- Filimon, R.M.; Rotaru, L.; Filimon, V.R. Effects of exogenous growth regulators on agrobiological, technological and physiological characteristics of an interspecific grapevine cultivar. Biol. Agric. Hortic. 2023, 39, 91–114. [Google Scholar] [CrossRef]
- Filimon, R.V.; Filimon, R.M.; Nechita, A.; Băetu, M.M.; Rotaru, L.; Arion, C.; Patraş, A. Assessment of quality characteristics of new Vitis vinifera L. cultivars for temperate climate vineyards. Acta Agric. Scand. B Soil Plant Sci. 2017, 67, 405–415. [Google Scholar] [CrossRef]
- VIVC (Vitis International Variety Catalogue). The Julius Kühn Institute, Federal, Research Centre for Cultivated Plants, Germany. 2018. Available online: http://www.vivc.de/ (accessed on 21 February 2019).
- ASRO. STAS 220/10-85; Material Săditor Viticol. Metode Pentru Verificarea Calităţii [Vineyard Planting Material. Methods for Quality Control]. Romanian Standards Association (ASRO): Bucharest, Romania, 1985. Available online: https://magazin.asro.ro/ro/standard/21261 (accessed on 4 March 2018).
- Rustioni, L.; Herrera, J.C.; Failla, O.; Peterlunger, E.; Sivilotti, P. Stem starch reserves studied by on-solid reactions coupled with reflectance detections in water stressed grapevines. Vitis 2019, 58, 47–51. [Google Scholar] [CrossRef]
- Moonmun, D.; Majumder, R.; Lopamudra, A. Quantitative phytochemical estimation and evaluation of antioxidant and antibacterial activity of methanol and ethanol extracts of Heliconia rostrata. Indian J. Pharm. Sci. 2017, 79, 79–90. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. AJEV 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Tibiri, A.; Sawadogo, W.R.; Ouedraogo, N. Evaluation of antioxidant activity, total phenolic and flavonoid contents of Entada africana Guill. et Perr. (Mimosaceae) organ extracts. Res. J. Med. Sci. 2010, 4, 81–87. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. The Chemistry of Wine. Stabilization and Treatments. In Handbook of Enology, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; Volume 2. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Packialakshmi, B.; Sudha, G.; Charumathy, M. Free radical scavenging and antioxidant efficacy of ethanolic fractions from an edible medicinal mushroom—Auricularia polytricha. Int. J. Pharm. 2017, 7, 108–114. [Google Scholar]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Japanese J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Szydlowska-Czerniaka, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szlyk, E. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta 2008, 76, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2002, 48, 5–16. [Google Scholar] [CrossRef]
- Mogana, R.; Adhikari, A.; Tzar, M.N.; Ramliza, R.; Wiart, C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement Med. Ther. 2020, 20, 55. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Microbiol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Balasubrahmanyam, V.R.; Eifert, J.; Diofasi, L. Nutrient reserves in grapevine canes as influenced by cropping levels. Vitis 1978, 17, 23–29. [Google Scholar] [CrossRef]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Göktürk Baydar, N. Chemical composition of grape canes. Ind. Crops Prod. 2011, 34, 994–998. [Google Scholar] [CrossRef]
- Burzo, I.; Dejeu, L.; Şerdinescu, A.; Bădulescu, L. Fiziologia plantelor de cultură. In Fiziologia Viţei de vie [The Physiology of Crop Plants. Grapevine Physiology]; Elisavaros: Bucharest, Romania, 2005; Volume 3. [Google Scholar]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid solid-liquid dynamic extraction (RSLDE): A powerful and greener alternative to the latest solid-liquid extraction techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Richard, T.; Cantos-Villar, E. Grapevine cane extracts: Raw plant material, extraction methods, quantification, and applications. Biomolecules 2020, 10, 1195. [Google Scholar] [CrossRef] [PubMed]
- Cacace, J.; Mazza, G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J. Food Sci. 2003, 68, 240–248. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Volpe, F.; Moler, J.A.; Esparza, I.; Ancín-Azpilicueta, C. Impact of extraction conditions on the phenolic composition and antioxidant capacity of grape stem extracts. Antioxidants 2019, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Houillé, B.; Besseau, S.; Delanoue, G.; Oudin, A.; Papon, N.; Clastre, M.; Simkin, A.J.; Guérin, L.; Courdavault, V.; Giglioli-Guivarch, N.; et al. Composition and tissue-specific distribution of stilbenoids in grape canes are affected by downy mildew pressure in the vineyard. J. Agric. Food Chem. 2015, 63, 8472–8477. [Google Scholar] [CrossRef] [PubMed]
- Ewald, P.; Delker, U.; Winterhalter, P. Quantification of stilbenoids in grapevine canes and grape cluster stems with a focus on long-term storage effects on stilbenoid concentration in grapevine canes. Food Res. Int. 2017, 100, 326–331. [Google Scholar] [CrossRef]
- Che Sulaiman, I.S.; Basri, M.; Fard Masoumi, H.R.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem. Cent. J. 2017, 11, 54. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction-An innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Ikawa, M.; Schaper, T.D.; Dollard, C.A.; Sasner, J.J. Utilization of Folin-Ciocalteu phenol reagent for the detection of certain nitrogen compounds. J. Agric. Food Chem. 2003, 51, 1811–1815. [Google Scholar] [CrossRef]
- Agbor, G.A.; Vinson, J.A.; Donnelly, P.E. Folin-Ciocalteau reagent for polyphenolic assay. Int. J. Food Sci. Nutr. Diet. 2014, 3, 147–156. [Google Scholar] [CrossRef]
- Bastola, K.; Guragain, Y.; Bhadriraju, V.; Vadlani, P. Evaluation of standards and interfering compounds in the determination of phenolics by Folin-Ciocalteu assay method for effective bioprocessing of biomass. Am. J. Anal. Chem. 2017, 8, 416–431. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- González-Silva, N.; Nolasco-González, Y.; Aguilar-Hernández, G.; Sáyago-Ayerdi, S.G.; Villagrán, Z.; Acosta, J.L.; Montalvo-González, E.; Anaya-Esparza, L.M. Ultrasound-assisted extraction of phenolic compounds from Psidium cattleianum leaves: Optimization using the response surface methodology. Molecules 2022, 27, 3557. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-H. Quantification and Analysis of Proteins—Chapter 8. In Diagnostic Molecular Biology; Shen, C.-H., Ed.; Academic Press: London, UK, 2019; pp. 187–214. [Google Scholar] [CrossRef]
- Pfanz, H.; Aschan, G.; Langenfeld-Heyser, R.; Wittmann, C.; Loose, M. Ecology and ecophysiology of tree stems: Corticular and wood photosynthesis. Naturwissenschaften 2002, 89, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Eugène, E.A.; Ganiyou, A.; Denis, Y.K.; Ané, A.; Sawaliho, B.E.H. Catechin and epicatechin. What’s the more reactive? Comput. Chem. 2022, 10, 53–70. [Google Scholar] [CrossRef]
- Lambert, C.; Richard, T.; Renouf, E.; Bisson, J.; Waffo-Téguo, P.; Bordenave, L.; Ollat, N.; Mérillon, J.-M.; Cluzet, S. Comparative analyses of stilbenoids in canes of major Vitis vinifera L. cultivars. J. Agric. Food Chem. 2013, 61, 11392–11399. [Google Scholar] [CrossRef]
- Nèmeth, G.; Molnár, Z.; Podmaniczky, P.; Nyitrai-Sárdy, D.; Kállay, M.; Dunai, A.; Kocsis, L. Trans-resveratrol content in grape cane and root of different scion-rootstock combinations. Mitt. Klosterneuburg Rebe Wein Obstb. Fruchteverwert. 2017, 67, 256–264. [Google Scholar]
- Loupit, G.; Prigent, S.; Franc, C.; De Revel, G.; Richard, T.; Cookson, S.J.; Valls Fonayet, J. Polyphenol profiles of just pruned grapevine canes from wild Vitis accessions and Vitis vinifera cultivars. J. Agric. Food Chem. 2020, 68, 13397–13407. [Google Scholar] [CrossRef]
- Tříska, J.; Vrchotová, N.; Balík, J.; Soural, I.; Sotolář, R. Variability in the content of trans-resveratrol, trans-ε-viniferin and r2-viniferin in grape cane of seven Vitis vinifera L. varieties during a three-year study. Molecules 2017, 22, 928. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Rodrigues, F.; Dorosh, O.; Pinto, D.; Costa, P.C.; Švarc-Gajić, J.; Delerue-Matos, C. Vine-canes as a source of value-added compounds for cosmetic formulations. Molecules 2020, 25, 2969. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Dorosh, O.; Silva, S.; Silva, A.M.; Grosso, C.; Vieira, E.F.; Rodrigues, F.; Fernandes, V.C.; Peixoto, A.F.; Freire, C.; et al. Subcritical water extraction of phenolic compounds from vineyard pruning residues: Evaluation of chemical composition and bioactive properties. Biol. Life Sci. Forum 2021, 6, 27. [Google Scholar] [CrossRef]
- Squillaci, G.; Vitiello, F.; Mosca, L.; La Cara, F.; Cacciapuoti, G.; Porcelli, M.; Morana, A. Polyphenol extract from “Greco” grape canes: Characterization, antioxidant capacity, and antitumor effects on Cal-33 and JHU-SCC-011 head and neck squamous cell carcinoma. Molecules 2022, 27, 2576. [Google Scholar] [CrossRef]
- Thoo, Y.Y.; Ho, S.K.; Liang, J.Y.; Ho, C.W.; Tan, C.P. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from Mengkudu (Morinda citrifolia). Food Chem. 2010, 120, 290–295. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar J. 2011, 10 (Suppl. S1), S4. [Google Scholar] [CrossRef]
- Burlakova, E.B.; Alesenko, A.V.; Molochkina, E.M.; Palmina, N.P.; Khrapova, N.G. Bioantioxidants in Radiation Damages and Malignant Growth; Izd. Nauka: Moscow, Russia, 1975. (In Russian) [Google Scholar]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Fatiha, M.; Abdelkader, T. Study of antioxidant activity of pyrimidinium betaines by DPPH radical scavenging method. J. Anal. Pharm. Res. 2019, 8, 33–36. [Google Scholar] [CrossRef]
- Olugbami, J.O.; Gbadegesin, M.A.; Odunola, O.A. In vitro evaluation of the antioxidant potential, phenolic and flavonoid contents of the stem bark ethanol extract of Anogeissus leiocarpus. Afr. J. Med. Med. Sci. 2014, 43 (Suppl. S1), 101–109. [Google Scholar]
- Wan, C.; Yu, Y.; Zhou, S.; Liu, W.; Tian, S.; Cao, S. Antioxidant activity and free radical-scavenging capacity of Gynura divaricata leaf extracts at different temperatures. Pharmacogn. Mag. 2011, 7, 40–45. [Google Scholar] [CrossRef]
- Kaushik, U.; Lachake, P.; Shreedhara, C.S.; Aswatha Ram, H.N. In-vitro antioxidant activity of extracts of Avipattikar churna. Pharmacologyonline 2009, 3, 581–589. [Google Scholar]
- Jayanthi, P.; Lalitha, P. Reducing power of the solvent extracts of Eichhornia crassipes (Mart.) Solms. Int. J. Pharm. Pharm. Sci. 2011, 3 (Suppl. S3), 126–128. [Google Scholar]
- Yefrida, Y.; Suyani, H.; Alif, A.; Efdi, M.; Aziz, H. Modification of phenanthroline method to determine antioxidant content in tropical fruits methanolic extract. Res. J. Chem. Environ. 2018, 22, 28–35. [Google Scholar]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Park, S.; Brown, S.; Pfaller, M.; Perlin, D.S. Evaluation of CLSI M44-A2 disk diffusion and associated breakpoint testing of caspofungin and micafungin using a well-characterized panel of wild-type and fks hot spot mutant Candida isolates. Antimicrob. Agents Chemother. 2011, 55, 1891–1895. [Google Scholar] [CrossRef]
- Horváth, G.; Bencsik, T.; Ács, K.; Kocsis, B. Sensitivity of ESBL-producing Gram-negative bacteria to essential oils, plant extracts, and their isolated compounds—Chapter 12. In Antibiotic Resistance; Kon, K., Rai, M., Eds.; Academic Press: London, UK, 2016; pp. 239–269. [Google Scholar] [CrossRef]
- Koh, G.Y.; Chou, G.; Liu, Z. Purification of a water extract of Chinese sweet tea plant (Rubus suavissimus S. Lee) by alcohol precipitation. J. Agric. Food Chem. 2009, 57, 5000–5006. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Taleb, H.; Maddocks, S.E.; Morris, R.K.; Kanekanian, A.D. The antibacterial activity of date syrup polyphenols against S. aureus and E. coli. Front Microbiol. 2016, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Rizwana, H.; Alwhibi, M.S.; Soliman, D.A. Research article antimicrobial activity and chemical composition of flowers of Matricaria aurea a native herb of Saudi Arabia. Int. J. Pharmacol. 2016, 12, 576–586. [Google Scholar] [CrossRef]
- Rathi, P.V.; Ambhore, D.; Jamode, P.; Katkar, P.; Kamble, P. Antimicrobial activity of Henna leaves against Staphylococcus aureus and Escherichia coli. World J. Pharm. Pharm. Sci. 2017, 6, 981–990. [Google Scholar] [CrossRef]
- Fu, L.; Lu, W.; Zhou, X. Phenolic compounds and in vitro antibacterial and antioxidant activities of three tropic fruits: Persimmon, guava, and sweetsop. Biomed Res Int. 2016, 2016, 4287461. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Eibes, G.; Moreira, M.T.; Dávila, I.; Labidi, J.; Gullón, P. Antioxidant and antimicrobial activities of extracts obtained from the refining of autohydrolysis liquors of vine shoots. Ind. Crops Prod. 2017, 107, 105–113. [Google Scholar] [CrossRef]
- Maroun, R.G.; Rajha, H.N.; Vorobiev, E.; Louka, N. 7—Emerging technologies for the recovery of valuable compounds from grape processing by-products. In Handbook of Grape Processing By-Products: Sustainable Solutions; Galanakis, C.M., Ed.; Elsevier Academic Press: London, UK, 2017; pp. 155–181. [Google Scholar] [CrossRef]
- Mencarelli, A.; Cavalli, R.; Greco, R. Analysis of the main physical and chemical characteristics of the vine shoots of three vine varieties from Veneto (Italy). J. Agric. Eng. 2022, 53, 1396. [Google Scholar] [CrossRef]



| Features | Cultivar | ||
|---|---|---|---|
| Sauvignon Blanc | Pinot Gris | Cabernet Sauvignon | |
| Diameter (cm) | 0.68 ± 0.11 a | 0.74 ± 0.08 a | 0.81 ± 0.10 a |
| Moisture (%) | 41.80 ± 0.88 b | 44.40 ± 0.91 a | 46.10 ± 0.74 a |
| Total dry matter (%) | 58.20 ± 0.88 b | 55.60 ± 0.91 a | 53.90 ± 0.74 a |
| Starch (%) | 5.60 ± 0.28 a | 6.32 ± 0.41 a | 5.54 ± 0.32 a |
| Sugars (%) | 6.43 ± 0.22 b | 7.66 ± 0.28 a | 7.08 ± 0.32 a |
| Total carbohydrates (%) | 12.03 ± 0.50 b | 13.98 ± 0.69 a | 12.62 ± 0.64 ab |
| Minerals (%) | 4.02 ± 0.16 a | 3.94 ± 0.11 a | 3.73 ± 0.20 a |
| Parameter | Grapevine cultivar | ||
|---|---|---|---|
| Sauvignon Blanc | Pinot Gris | Cabernet Sauvignon | |
| Chl a (μg/g d.w.) | 58.03 ± 0.57 a | 53.84 ± 0.42 c | 56.41 ± 0.38 b |
| Chl b (μg/g d.w.) | 16.12 ± 0.19 c | 16.79 ± 0.14 b | 17.15 ± 0.22 a |
| Car (x + c) (μg/g d.w.) | 27.71 ± 0.21 a | 22.97 ± 0.26 c | 24.63 ± 0.41 b |
| Chl a/b | 3.60 ± 0.04 a | 3.21 ± 0.02 c | 3.29 ± 0.02 b |
| Chl/Car | 2.68 ± 0.03 c | 3.08 ± 0.01 a | 2.99 ± 0.03 b |
| Phenolic compounds (μg/g d.w.) | Cultivar | ||
|---|---|---|---|
| Sauvignon Blanc | Pinot Gris | Cabernet Sauvignon | |
| 4-hydroxybenzoic acid | 1.50 ± 0.09 b | 5.68 ± 0.41 a | 6.34 ± 0.30 a |
| Catechin | 321.11 ± 2.07 b | 334.58 ± 2.72 a | 273.50 ± 2.17 c |
| Coumaric acid | 2.69 ± 0.32 b | 10.18 ± 1.54 a | 9.13 ± 0.64 a |
| Epicatechin | 271.41 ± 4.21 a | 177.99 ± 4.08 b | 169.91 ± 4.25 b |
| Sinapic acid | 11.68 ± 1.25 | n.d. | n.d. |
| Resveratrol | 282.19 ± 4.14 b | 419.01 ± 4.10 a | 425.60 ± 5.98 a |
| Quercetin | 77.99 ± 2.50 a | 54.49 ± 4.02 b | 72.75 ± 2.96 a |
| Sum of individual polyphenols | 968.57 ± 14.58 b | 1001.93 ± 19.93 a | 957.23 ± 16.87 b |
| Phenolic Group | DPPH | FRP | PR | PA | ||||
|---|---|---|---|---|---|---|---|---|
| CE | PE | CE | PE | CE | PE | CE | PE | |
| TPC | −0.9689 | −0.9831 | 0.4877 | −0.9630 | −0.9866 | −0.2506 | −0.9887 | −0.9883 |
| Fl. | −0.9670 | −0.9862 | 0.4946 | −0.9676 | −0.9878 | −0.2676 | −0.9899 | −0.9854 |
| Nfl. | −0.9724 | −0.9794 | 0.4754 | −0.9577 | −0.9842 | −0.2322 | −0.9865 | −0.9910 |
| ∑HPLC | - | −0.9703 | - | 0.4827 | - | −0.9856 | - | −0.9878 |
| Sample | Staphylococcus aureus | Escherichia coli | ||||
|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC Index | MIC (mg/mL) | MBC (mg/mL) | MIC Index | |
| SB-CE | 80 ± 0 | 110 ± 0 | 1.38 | 90 ± 0 | 120 ± 0 | 1.33 |
| PG-CE | 80 ± 0 | 100 ± 0 | 1.25 | 90 ± 0 | 110 ± 0 | 1.22 |
| CS-CE | 80 ± 0 | 110 ± 0 | 1.38 | 100 ± 0 | 120 ± 0 | 1.20 |
| SB-PE | 60 ± 0 | 100 ± 0 | 1.67 | 70 ± 0 | 110 ± 0 | 1.57 |
| PG-PE | 60 ± 0 | 90 ± 0 | 1.50 | 70 ± 0 | 100 ± 0 | 1.43 |
| CS-PE | 60 ± 0 | 100 ± 0 | 1.67 | 70 ± 0 | 120 ± 0 | 1.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filimon, R.V.; Bunea, C.I.; Bora, F.D.; Filimon, R.M.; Dunca, S.I.; Rózsa, S.; Ciurlă, L.; Patraș, A. Physico-Chemical Characterization, Phenolic Compound Extraction and Biological Activity of Grapevine (Vitis vinifera L.) Canes. Horticulturae 2023, 9, 1164. https://doi.org/10.3390/horticulturae9111164
Filimon RV, Bunea CI, Bora FD, Filimon RM, Dunca SI, Rózsa S, Ciurlă L, Patraș A. Physico-Chemical Characterization, Phenolic Compound Extraction and Biological Activity of Grapevine (Vitis vinifera L.) Canes. Horticulturae. 2023; 9(11):1164. https://doi.org/10.3390/horticulturae9111164
Chicago/Turabian StyleFilimon, Răzvan Vasile, Claudiu Ioan Bunea, Florin Dumitru Bora, Roxana Mihaela Filimon, Simona Isabela Dunca, Sándor Rózsa, Liliana Ciurlă, and Antoanela Patraș. 2023. "Physico-Chemical Characterization, Phenolic Compound Extraction and Biological Activity of Grapevine (Vitis vinifera L.) Canes" Horticulturae 9, no. 11: 1164. https://doi.org/10.3390/horticulturae9111164
APA StyleFilimon, R. V., Bunea, C. I., Bora, F. D., Filimon, R. M., Dunca, S. I., Rózsa, S., Ciurlă, L., & Patraș, A. (2023). Physico-Chemical Characterization, Phenolic Compound Extraction and Biological Activity of Grapevine (Vitis vinifera L.) Canes. Horticulturae, 9(11), 1164. https://doi.org/10.3390/horticulturae9111164








