Abstract
Mango is a climacteric fruit that requires efficient postharvest technologies to maintain quality during storage and transportation. This study aimed to investigate the effect of biodegradable packaging from chitosan (CS) incorporated with graphene oxide (GO) on the quality, bioactive compounds and antioxidant activity of cold-stored ‘Tommy Atkins’ mangoes. Mangoes harvested at physiological maturity were stored without packaging or in CS, CS-GO or non-biodegradable polyethylene (PE) packaging at 12.0 °C and 89% relative humidity for 42 days. The results show that GO improved the water barrier and mechanical properties of CS packaging. All packaging delayed fruit ripening by reducing the respiration rate, mass loss, softening and changes in color, soluble solids, titratable acidity and beta-carotene content, also preserving the mangoes’ visual appearance. In addition, all packaging maintained higher ascorbic acid, yellow flavonoid, phenolic compounds and antioxidant activity levels in the fruit, compared to non-packed ones. Chitosan packaging is a promising, eco-friendly alternative for the preservation of quality, bioactive compounds and antioxidant activity of cold-stored ‘Tommy Atkins’ mangoes, extending their postharvest life by at least 14 days.
1. Introduction
Mangoes (Mangifera indica L.) are a tropical fruit with an attractive color, delicious taste and exotic flavor, and are also a rich source of nutrients and phytochemicals, such as provitamin A carotenoids, ascorbic acid and phenolic compounds [1,2]. These bioactive compounds provide benefits for the human body by acting as natural antioxidants, inhibiting oxidation damage caused by reactive oxygen species [3].
Mango production takes place in more than 100 countries, with an increasing international trade and a high demand in the mainstream market outlets in most of the developed countries [4]. Brazil is among the largest of the mango-exporting countries, with harvests of high-quality fruit taking place all year long. Tommy Atkins is the leading exporting cultivar in Brazil, as a well-known mango genotype with great international appeal, especially due to its ability to withstand longer transportation and shelf life, compared to other genotypes [1,5,6]. These traits also make Tommy Atkins the most widely grown mango cultivar in the world.
Mango is a highly perishable climacteric fruit, characterized by rapid and intense ripening-related changes, including increased respiration and ethylene production, softening, conversion of starch to sugars, degradation of organic acids and chlorophylls and synthesis of carotenoids [4]. Several technologies have been investigated and applied to extend mangoes’ postharvest life [5,7,8,9,10,11]. Refrigeration at 10–13 °C is one of the most efficient techniques to maintain quality and extend the postharvest life of ‘Tommy Atkins’ mangoes without leading to chilling injury [12]. However, refrigeration alone may not be enough to export mangoes to distant markets, whose transport can take more than 30 days, limiting the worldwide mango distribution from the production regions to consumers.
In addition to refrigeration, modified atmosphere (MA) can also be used to maintain quality and extend mango postharvest life [5]. MA delays fruit ripening and senescence and preserves quality by reducing oxygen concentration and increasing carbon dioxide levels around the fruit as a result of its respiration and metabolic activity [13]. Traditionally used MA packaging films involve non-degradable materials, such as polyethylene (PE), which contributes to many environmental problems.
The application of biopolymers as edible coatings is a simple, non-toxic and environmentally friendly alternative for preserving mango quality, through formation of a transparent film on the fruit surface that acts as a barrier to water, oxygen and carbon dioxide, providing MA conditions to the fruit. The interest in biopolymers as eco-friendly and sustainable substitutes for petroleum-based plastics is strongly increasing because of their high biodegradability, low cost and ease to obtain from renewable resources [14,15].
Chitosan (CS) is a low-cost polymer derived from animal and fungal sources that has been tested for application in food, agriculture and pharmaceutical industries. The use of CS for food packaging has been successfully gaining attention due to its excellent film-forming ability associated with antioxidant and antimicrobial properties [16,17,18]. Besides many advantages over synthetic polymers, CS may have weak mechanical and moisture-barrier properties [19], both of which could be improved via the incorporation of nanoparticles to the film-forming solutions.
Graphene oxide (GO) is derived from graphene via the chemical modification of graphite, resulting in a molecule with various oxygen functional groups [20]. Amide linkages tend to be formed between the CS’s highly reactive amine groups and GO’s carboxylic acid groups, being expected that GO incorporates well into the biopolymeric CS matrix [14]. A homogeneous distribution and incorporation of GO into the CS matrix can enhance the mechanical, thermal, structural, morphological and barrier properties of the CS films, as previously reported in other studies [15,21].
Recently, CS-GO-based biodegradable packaging has been developed for fruit conservation using the casting method, resulting in an eco-friendly and high-performance packaging material that effectively maintain the quality and extend the postharvest life of melons [22]. Although MA conditions can have a strong effect on maintaining the quality and extending the postharvest life of mangoes, studies must be undertaken to determine the potential use of biodegradable packaging to make exporting mangoes to distant markets, that are still limited by the use of regular refrigerated containers, possible. Therefore, this study aimed to investigate the effect of biodegradable packaging from chitosan (CS) incorporated with graphene oxide (GO) on the quality, bioactive compounds and antioxidant activity of cold-stored ‘Tommy Atkins’ mangoes.
2. Materials and Methods
2.1. Fruit Material
‘Tommy Atkins’ mangoes were obtained from a commercial orchard in Petrolina, PE, Brazil (9°03′04.6″ S latitude, 40°17′46.5″ W longitude, and altitude of 376 m). Mangoes were harvested at the point of physiological maturity, represented by full shoulders at the fruit stem end and a predominant light-green skin color [23]. Mangoes without diseases or injuries were selected for uniformity of size, color and shape. Then, the fruit was washed, sanitized with a 200 ppm (v/v) sodium hypochlorite solution for 15 min and dried at room temperature before analysis.
2.2. Chemicals
An 85% deacetylated chitosan (CS) was purchased from Polymar (Fortaleza, Brazil). Graphene oxide (GO), gallic acid, DPPH, ABTS, Trolox, Folin–Ciocalteu’s reagent, acetic acid, methanol, acetone, hexane, potassium persulfate, hydrochloric acid and anhydrous sodium carbonate were provided by Sigma-Aldrich (San Luis, MO, USA). Polyethylene (PE) packaging was acquired from Nissan Steel Industry (Kyoto, Japan).
2.3. Preparation and Characterization of CS-GO Biodegradable Packaging
Biodegradable packaging was prepared according to Paiva et al. [22]. Briefly, the CS (2%, w/v) and the GO at 0% and 0.25% (w/w) were dissolved in 1% acetic acid (v/v), followed by moderate stirring for 12 h. The film-forming solutions were homogenized in an ultrasonic bath for 10 min. Then, 240 g of each solution were transferred to an acrylic plate of 32 × 32 cm and dried for 10 h at 50 °C. Each package consisted of two overlapped films, which were then sealed in the edges, with an opening to insert the fruit, using a sealing machine.
For each formulation, five films were used in the characterization of the water vapor transmission rate (WVTR) and mechanical properties. The WVTR was measured in quintuplicates, following the methodology of Sun et al. [24], in accordance with the American Society for Testing and Materials (ASTM) E96-00 method. Permeation measuring cells were filled with about 5 mL of distilled water, and square film samples (2 × 2 cm) were placed over the cells. The cell–film sets were weighed and placed in a desiccator containing silica gel, with internal temperature of 25 °C and 50% relative humidity. Cell-films were weighed every hour for eight hours, and the following equation was used to calculate the WVTR (Equation (1)):
where WVTR is the water vapor transmission rate (g/m2/s); M is the mass of water permeating through the film (g); A is the permeation area (m2); and t is the permeation time (s).
WVTR = M/(At)
The mechanical properties tensile strength (σ, in MPa), elongation at break (ε, in %) and Young’s modulus (Є, in MPa) were measured in film samples of 50 mm × 5 mm using a mechanical testing machine (model DL5000/10000, EMIC, São José dos Pinhais, Brazil), which operated according to ASTM D882-8312, at a test speed of 5 mm/min and an application force of 5 kN.
2.4. Postharvest Treatments
Mangoes were divided in four treatments, being CS-based biodegradable packaging (with and without GO), PE-based non-degradable packaging and the control (unpacked fruit). Two mangoes were inserted in each package, which was sealed using a sealing machine, creating conditions of modified atmosphere (MA). Then, the mangoes were stored at 12.0 ± 0.5 °C and 89 ± 3% relative humidity, in the central position at the end of a cold chamber (GEFRIO, Fortaleza, Brazil), with dimensions of 3 × 2 × 3 m (length × width × height), and air flow of 2 m s−1 [12]. A total of 96 fruit were stored for 42 days, simulating conditions of long-distance shipping. Every 14 days, six fruit per treatment were randomly sampled for analysis. About 50 g of each sample was stored at −85 °C for later bioactive compounds and antioxidant activity analyses.
2.5. Determination of Quality Parameters, Bioactive Compounds and Antioxidant Activity
2.5.1. Respiration Rate
Respiration rate of mangoes was measured with a gas analyzer model PA 7.0 (WITT, Witten, Germany). Each fruit was placed for 1 h into an airtight container, and the final CO2 concentrations were recorded. Respiration rate was expressed as mg/kg/h.
2.5.2. Mass Loss
Mass loss was calculated by multiplying the difference between the initial mass and the mass at the end of the storage by 100 and dividing by the initial mass. Mass loss was expressed as percentage.
2.5.3. Skin and Pulp Color
Color was determined in skin and pulp tissues with a colorimeter model CR-400 (Konica Minolta, Tokyo, Japan). The color was expressed as hue angle (°h), where 0/360° represents red, 90° represents yellowish green, 180° represents turquoise blue and 270° represents violet.
2.5.4. External Appearance
External appearance was evaluated with a 9-point visual scale according to Lima et al. [25], where higher values represent better appearance and lower incidence of injuries, spots or rot, and 5 is the limit of acceptability, expressing fruit with 10% spots.
2.5.5. Pulp Firmness
Pulp firmness was measured with a texture analyzer TA.XTPlus (Stable Micro Systems, Godalming, UK), equipped with a 6 mm stainless steel probe and set for a 10 mm penetration distance. Pulp firmness results were obtained from the average of two measurements performed in the equatorial region of each fruit without the skin, and were expressed in N.
2.5.6. Soluble Solid Content (SSC)
Soluble solid content (SSC) was determined in the juice using a digital refractometer model PAL-1 (Atago, Tokyo, Japan). The results were expressed in percentage.
2.5.7. Titratable Acidity (TA)
Titratable acidity (TA) was measured by titrating 5 mL of juice diluted in 45 mL of distilled water with 0.1 N sodium hydroxide solution to an end point of pH 8.1, using an automatic titrator model 848 (Metrohm, Herisau, Switzerland). The results were expressed in g of citric acid per 100 g.
2.5.8. Beta-Carotene Content
Beta-carotene content was determined according to the method of Nagata and Yamashita [26]. One gram of mango pulp was added to test tubes with 10 mL of acetone:hexane (4:6, v/v). The solution was stirred for 1 min using a homogenizer model T18 digital (IKA, Guangzhou, China). An aliquot of the supernatant was transferred to quartz cuvettes and was read on a spectrophotometer at 663, 645, 505 and 453 nm. Beta-carotene content was determined according to Equation (2):
where A663, A645, A505 and A453 refer to absorbances at 663, 645, 505 and 453 nm, respectively.
β-carotene (mg/100 g) = (0.216 A663 − 1.22 A645 − 0.304 A505 + 0.452 A453) × 10
2.5.9. Ascorbic Acid Content
Ascorbic acid (AsA) content was determined according to the AOAC method [27]. A total of 5 mL of mango juice was diluted in 100 mL of 0.5% (w/v) oxalic acid, which was then titrated with 0.02% (v/v) 2,6-dichlorophenol indophenol 0.02% (v/v) until reaching light pink color. The results were expressed as mg of AsA/100 g.
2.5.10. Total Phenolic Compounds (TPC) Content
For the preparation of the phenolic extract, 15 g of mango pulp was weighed in centrifuge tubes and left to extract in 20 mL of 50% methanol (v/v) for 1 h. The samples were later centrifuged at 10,000× g for 15 min at 4 °C. The supernatant was filtered and transferred to a 50 mL volumetric flask. The residue was extracted in 20 mL of 70% acetone (v/v) for 1 h. Centrifugation was repeated and the supernatant was filtered and added to the volumetric flask containing the supernatant of the first extraction. The final volume was then completed to 50 mL with distilled water.
TPC content was determined via the Folin–Ciocalteu method [27]. An aliquot of the phenolic extract was mixed with 1 mL of Folin–Ciocalteu reagent (1:3, v/v), 2 mL of 20% (w/v) sodium carbonate solution and 2 mL of distilled water in test tubes, which were shaken in a Vortex mixer and left to rest for 30 min, protected from light. Absorbance at 700 nm was measured using a spectrophotometer. Gallic acid was used to develop a standard calibration curve, and the results were expressed as g of gallic acid equivalents (GAE)/100 g.
2.5.11. Yellow Flavonoid (YF) Content
For determination of YF content, one gram of mango pulp was extracted in 10 mL of a 95% (v/v) ethanol/1.5 N HCl (85:15) solution [28]. Samples were homogenized for 1 min in an ULTRA-TURRAX homogenizer (model T18 digital, IKA, Guangzhou, China). The extract was transferred to a 50 mL balloon protected from light, conserved for 16 h under refrigeration and then filtered (Whatman No. 1 filter paper).
YF content was quantified by reading the extract absorbance at 374 nm on a spectrophotometer, using an absorption coefficient of 76.6. Results were expressed in mg/100 g.
2.5.12. Antioxidant Activity (AOX)
AOX was determined using the methods based on the capacity of the phenolic extract to scavenge the ABTS•+ and the DPPH• radicals [29].
In the ABTS•+ method [30], the radical was generated by reaction of the ABTS stock solution (7 mmol L−1) with potassium persulfate (140 mmol L−1), kept in the dark for 16 h at ambient temperature. Prior to the analysis, the mixture was diluted in ethanol until reaching an absorbance of 0.700 ± 0.005 at 734 nm. Three different dilutions for each fruit extract were prepared in triplicate. In the dark, 30 µL of each dilution were added to 3 mL of the ABTS radical, followed by homogenization in a Vortex mixer. The samples were read at 734 nm after 6 min of the addition of the radical. A standard Trolox curve was used, and the results were expressed as μM of Trolox per g of fresh mass.
In the DPPH• method [31], three dilutions of each extract were prepared in triplicate. In the dark, 100 μL of each extract dilution was added to 3.9 mL of the DPPH• radical (0.06 mM). The mixture was stirred in a Vortex mixer and then left to rest in the dark. As a control, 100 μL of the control solution (50% methanol, 70% acetone and water, in the 4:4:2 ratio) was used instead of phenolic extract. The readings were carried out at 515 nm, 45 min after the addition of the DPPH• radical, considering absorbance stabilization. The AOX was calculated as the extract concentration required to reduce the initial concentration of the DPPH• radical by 50% (EC50), with values expressed as mg fresh mass/mL of DPPH•.
2.6. Statistical Analysis
The experiment was performed using a completely randomized design, in a split-plot arrangement (4 × 4), with three replications of two mangoes. The plots were represented by packaging conditions (CS, CS-GO, PE and control), while the subplots consisted of storage times (0, 14, 28 and 42 days). The data were submitted to two-way ANOVA, and the least significant difference (LSD) test (p ≤ 0.05) was used to compare the means between packaging types.
A principal component analysis (PCA) based on the correlation matrix among the variables was applied to summarize the data into few principal components responsible for most of data variance.
Statistical analyses were performed in R 4.0.2 (R Development Core Team, Vienna, Austria) and are detailed in Supplementary Material S1.
3. Results
3.1. Water Vapor Transmission Rate and Mechanical Properties of the Packaging
The incorporation of GO reduced the water vapor transmission rate (WVTR) of CS-based films by 35% (p ≤ 0.05). The tensile strength and the Young’s modulus of the CS film were increased by 21% and 19% through the addition of GO (p ≤ 0.05), respectively. The elongation break was not influenced by the presence of GO (p > 0.05), which averaged 5.70% and 5.81% in the packaging with 0% and 0.25% GO, respectively (Table 1).

Table 1.
Water vapor transmission rate (WVTR), tensile strength (σ), elongation at break (ε) and Young’s modulus (Є) of chitosan (CS) and graphene oxide (GO)-based biodegradable packaging.
3.2. Fruit Quality Parameters
All quality parameters were significantly (p ≤ 0.05) influenced by packaging treatment and storage period, as well as by the interaction between the two factors.
Mangoes in all treatments exhibited increased respiration rates during storage. However, after the 14th day of storage, the unpacked fruit showed a higher respiration rate than packed ones (p ≤ 0.05). At 42 days of storage, fruit in all packaging had about 40% lower respiration rate than unpacked fruit (Figure 1a).
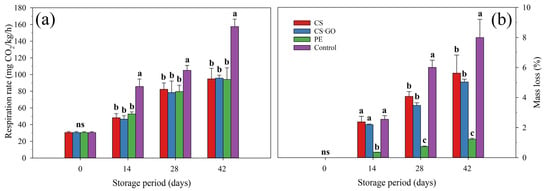
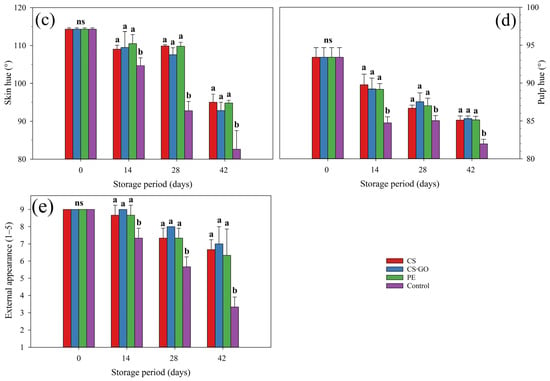
Figure 1.
Effect of packaging conditions on respiration rate (a), mass loss (b), skin hue (c), pulp hue (d) and external appearance (e) of ‘Tommy Atkins’ mangoes stored at 12 °C for 42 days. The values followed by different letters, in each storage day, are statistically different (p ≤ 0.05) according to the LSD test. ns indicates non-significant differences between packaging conditions (p > 0.05). Vertical bars represent the mean ± standard deviation of three replications per treatment (n = 3).
The packaging resulted in reduced mass loss (p ≤ 0.05) compared with the control treatment. Synthetic packaging provided a much lower mass loss, compared to the other treatments (p ≤ 0.05), with only 1.22% at 42 days. At the same day, mass losses of mangoes stored in CS and CS-GO-based packaging were 5.62% and 5.03%, respectively, values statistically lower (p ≤ 0.05) than those observed in the control fruit (7.99%) (Figure 1b).
The hue angle of both the skin and pulp of ‘Tommy Atkins’ mangoes decreased during storage, which was more pronounced in unpacked fruit. At harvest, mangoes had a green skin color (h° = 114.3), which gradually changed to yellow (h° = 82.6) in unpacked mangoes after 42 days of storage; during the same period, packed fruit showed a higher (p ≤ 0.05) average skin hue (h° = 94.2), indicating a yellowish-green color (Figure 1c). Pulp hue values of control mangoes decreased from 93.4° at harvest to 82.0° at the 42nd day of storage, which was statistically lower (p ≤ 0.05) than the average of 85.2° found in packed fruit at the same day (Figure 1d).
At the 14th day of storage, the unpacked fruit already presented an external appearance inferior to the packed ones (p ≤ 0.05), whose difference significantly remained until the end of storage. On the last day, the control fruit had an average score of 3.3 for external appearance, classifying them as unsuitable for commercialization due to the high severity of spots, injuries and wrinkles. On the same evaluation day, packed fruit had external appearance classified as regular/good, averaging 6.7 (Figure 1e).
Pulp firmness decreased over storage in all treatments, but this reduction was more intense in control fruit, which differed at 14 days of storage from packed ones (p ≤ 0.05). Mangoes have shown a pulp firmness of 73.0 N at harvest, decreasing to 2.8 N and 5.0 N after 42 days of storage in unpacked and packed fruit, respectively (Figure 2a).
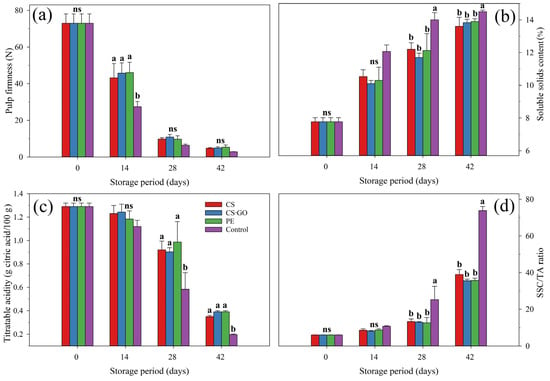
Figure 2.
Effect of packaging conditions on pulp firmness (a), soluble solid content (SSC) (b), titratable acidity (TA) (c) and SSC/TA ratio (d) of ‘Tommy Atkins’ mangoes stored at 12 °C for 42 days. The values followed by different letters, on each storage day, are statistically different (p ≤ 0.05) according to the LSD test. ns indicates non-significant differences between packaging conditions (p > 0.05). Vertical bars represent the mean ± standard deviation of three replications per treatment (n = 3).
Starting at 7.8% at harvest, the soluble solid content (SSC) increased until 14.5% and 13.8% in unpacked and packed mangoes, respectively (Figure 2b). Packed fruit have shown a lower SSC at 14 and 28 days of storage (p ≤ 0.05), compared to control fruit.
Titratable acidity (TA) decreased in all treatments during storage. However, this reduction was more intense in unpacked fruit, differing from other treatments at 28 and 42 days of storage (p ≤ 0.05) (Figure 2c). ‘Tommy Atkins’ mangoes were harvested with 1.29 g of citric acid/100 g, which decreased to 0.20 and 0.38 g of citric acid/100 g after 42 days of storage in unpacked and packed fruit, respectively.
The SSC/TA ratio increased in all treatments, but in unpacked fruit, the increase was 1126% over storage, while packed mangoes showed a statistically lower (p ≤ 0.05) increase of 546%, 489%, and 492%, for fruit in CS, CS-GO and PE packaging, respectively (Figure 2d).
3.3. Beta-Carotene, Ascorbic Acid, Total Phenolic Compounds and Yellow Flavonoids
The interaction between storage time and packaging significantly (p ≤ 0.05) influenced the β-carotene content in mangoes. Fruit showed an average β-carotene content of 0.23 mg/100 g at harvest, with an increase with storage time, regardless of packaging condition. However, unpacked fruit had statistically higher (p ≤ 0.05) β-carotene content than those found in packed fruit at 28 and 42 days of storage. The highest mean β-carotene content was found in the control treatment at the 42nd day (115.12 mg/100 g) (Figure 3a).
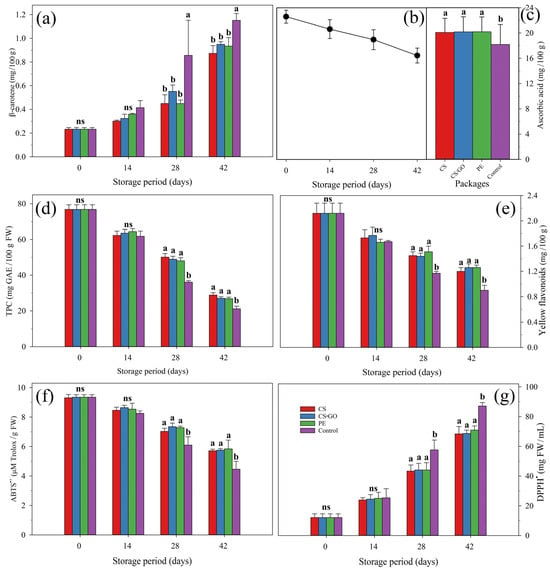
Figure 3.
Effect of packaging conditions on β-carotene (a), ascorbic acid (AsA) (b,c), total phenolic compounds (TPC) (d) and yellow flavonoids (YF) (e) content and total antioxidant activity (AOX) via ABTS•+ (f) and DPPH• (g) radical capture methods of ‘Tommy Atkins’ mangoes stored at 12 °C for 42 days. The values followed by different letters, in each storage day, are statistically different (p ≤ 0.05) according to the LSD test. ns indicates non-significant differences between packaging conditions (p > 0.05). Vertical bars represent the mean ± standard deviation of three replications per treatment (n = 3).
Packaging treatment and storage time significantly (p ≤ 0.05) affected the ascorbic acid (AsA) content in ‘Tommy Atkins’ mangoes, while the interaction between both was nonsignificant (p > 0.05). Mangoes showed, at the point of harvest, an average AsA content of 22.6 mg/100 g, which decreased over storage until averaging 16.5 mg/100 g at 42 days of storage (Figure 3b). The mean AsA content in packed fruit was 20.2 mg/100 g, which was 11% higher (p ≤ 0.05) than that found in unpacked fruit (18.2 mg/100 g) (Figure 3c).
The total phenolic compound (TPC) content in mangoes was significantly (p ≤ 0.05) influenced by storage time and packaging conditions and by the interaction between them. At harvest, mangoes had a mean TPC content of 76.88 mg GAE/100 g FW, which decreased in all the samples during storage as a natural effect of fruit ripening. However, from 28 days onwards, packed fruit had significantly higher (p ≤ 0.05) TPC content than unpacked ones (Figure 3d).
Yellow flavonoid (YF) content was influenced by storage time and packaging conditions, as well as by the interaction between both (p ≤ 0.05). YF content in mangoes was 2.12 mg/100 g at the time of harvest, decreasing over storage, more markedly in control. At the end of the storage period, packed fruit averaged 1.24 mg/100 g for YF content, while unpacked fruit showed a 27% lower (p ≤ 0.05) content (0.90 mg/100 g) (Figure 3e).
3.4. Antioxidant Activity
Antioxidant activity (AOX) of ‘Tommy Atkins’ mangoes showed a significant effect (p ≤ 0.05) due to storage time and packaging conditions, as well as the interaction between them, which was observed in both the ABTS•+ and DPPH• methods. A decrease was detected in fruit AOX in both ABTS•+ (Figure 3f) and DPPH• (Figure 3g) methods during storage. However, a higher (p ≤ 0.05) antioxidant activity was observed in packed fruit, compared to control fruit, which was reported from 28 days onwards in both methods (Figure 3f,g).
The correlation between ABTS•+ and DPPH• values was significantly strong and negative (−0.970) (Figure 4). The ABTS•+ radical-scavenging activity is equivalent to that of Trolox; therefore, higher μM values of Trolox/g correspond to higher antioxidant activities. On the other hand, DPPH• value is measured as the extract concentration required to reduce the initial concentration of the free radical by 50%, so lower values of mg/mL correspond to higher AOX.
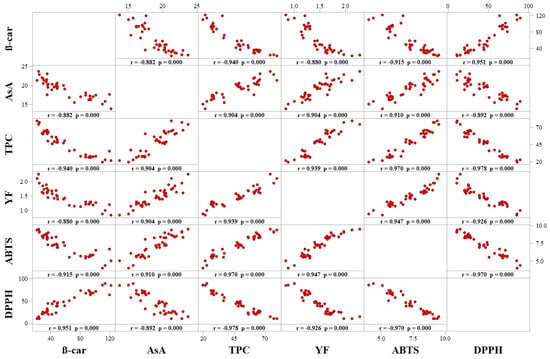
Figure 4.
Pearson’s correlation coefficient between bioactive compounds and antioxidant activities (ABTS•+ and DPPH•) of ‘Tommy Atkins’ mangoes stored at 12 °C for 42 days.
The contents of AsA (r = 0.910), TPC (r = 0.970) and YF (r = 0.947) had strong significant and positive (p ≤ 0.05) correlations with the AOX, according to the ABTS•+ assay. Similarly, these compounds also showed strong significant (p ≤ 0.05) correlations with the antioxidant activity measured with the DPPH• method. Coefficients of correlation between DPPH• and the contents of AsA, TPC and YF were −0.892, −0.978 and −0.926, respectively.
In contrast, β-carotene content showed significant opposite correlations with both methods of antioxidant activity analyses, being negative with ABTS•+ (−0.915) and positive with DPPH• (0.951) (Figure 4).
3.5. Principal Component Analysis
The data variability was explained in 92.02% by the first two principal components (PCs). PC1 was responsible for 88.00% of the total variance in the dataset and was positively correlated with the SSC, respiration rate, mass loss, SSC/TA ratio, β-carotene and DPPH•. In contrast, the external appearance, skin and pulp hue, TA, pulp firmness, AsA, YF, TPC and ABTS•+ were negatively correlated with PC1 (Figure 5).
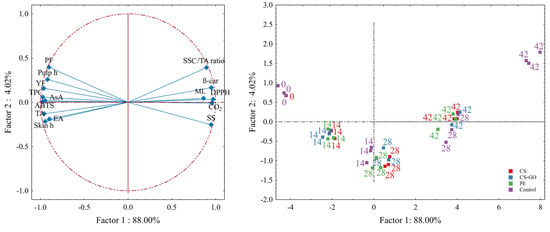
Figure 5.
Principal component analysis plot of data from different quality attributes, bioactive compounds and antioxidant properties of ‘Tommy Atkins’ mangoes stored at 12 °C for 42 days.
The negative axes of PC1 were highly correlated with mangoes at harvest (0 day) and unpacked mangoes at 14 days of storage, which presented quality parameters typical of unripe fruit. Conversely, the positive axis of PC1 had a strong correlation with control fruit at 28 days and fruit of all treatments at 42 days, which indicates fruit of a more advanced ripening stage (Figure 5).
4. Discussion
‘Tommy Atkins’ is one of the mango varieties most valued by mango-exporting countries, due to its longer shelf life and ability to withstand long transportation compared to other varieties [1,6]. Even so, ‘Tommy Atkins’ mangoes require adoption of postharvest technologies to enable the export of high-quality fruit to the final destination, whose transport can take more than 30 days. The ideal storage temperature for ‘Tommy Atkins’ mangoes ranges from 10 to 13 °C, in order to reduce fruit metabolism and conserve fruit quality without lead to chilling injury [12]. However, due to the high perishability of mangos, other technologies are required in addition to cold storage to increase fruit postharvest life.
In our study, the adoption of a passive modified atmosphere (MA) through the use of biodegradable packaging was tested as an eco-friendly technology for mango export. In passive MAs, the packaging acts as a barrier against gas exchanges and moisture loss from the fruit to the environment [11], where the desired in-pack gas levels (low O2 and high CO2) are established naturally from the process of fruit respiration. The effectiveness of MA in preservation of fruit quality depends on factors related to fruit (species, variety, maturity stage and mass), packaging material (thickness and permeability to moisture, O2 and CO2) and storage conditions (temperature and moisture). In addition, the exposure of fruit to low oxygen concentrations increases the risk of stress-related physiological disorders that reduce fruit quality and acceptance by consumers [32].
CS has been extensively used in the creation of MA conditions for fruit conservation because its film-forming properties, which reduces gas exchanges between the fruit and the atmosphere [17,18]. However, CS has a significant water affinity, which can result in the weakening of the mechanical properties of materials based on this polymer [16]. According to our results, the incorporation of GO reduced the water vapor transmission rate (WVTR) of CS-based films. The WVTR represents the ability of a film to block the passage of water across its surface. This property is influenced by environmental factors such as temperature and relative humidity, film structure, including its thickness and area, difference in pressure and concentration gradient across the film [19]. Low WVTR values are favorable for the development of eco-friendly coatings or films to enhance the shelf life of food products, including fresh fruit [24]. Molecules of GO tend to fill the structural gaps when incorporated in the CS polymeric chain [33]. According to Ahmed et al. [21], the decrease in the diffusion of water vapor through the film is related to the creation of a tortuous path, due to the excellent dispersion of GO in the polymeric matrix.
The mechanical properties (tensile strength and Young’s modulus) of the CS packaging were improved by the addition of GO, confirming the previous results of Paiva et al. [22]. The mechanical properties represent the resistance of the packaging to breakage and consequently reflect their potential to protect food from contact with the external environment [19]. For fresh fruit and vegetables, packaging is an important means of reducing gas exchanges with the external environment, thus reducing fruit respiration and metabolism and extending their shelf life. In this context, the CS-based biodegradable packaging is more suitable for use throughout refrigerated transportation, as a way to preserve the quality of the mangoes until they arrive to their final destination than for the transport of the fruit by the consumer. In this case, the biodegradable packaging does not require mechanical strength equivalent to commercial PE-based packaging for transport use [22].
Unpacked mangoes showed higher respiration rates than packed ones throughout the storage period. This result is in accordance with previous studies that also observed an effect of CS on reducing mango respiratory metabolism [34,35,36]. The decrease of fruit respiration rate is attributed to the gas barrier properties of the polymers, CS and PE, which reduce the availability of oxygen and concentrate the carbon dioxide surrounding the fruit, suppressing fruit metabolism.
Mass loss of mangoes was reduced via packaging treatments, especially PE-based packaging, which reduced the mass loss of fruit by 6.5-fold compared to the control at the end of storage. The positive effect of MA conditions on preventing mass loss has also been reported in mangoes coated with CS [8,37] or packed in plastic film [38]. The mass loss is related to fruit respiration and transpiration and results in quality losses that affect consumer acceptance. Packaging acts as a barrier with a protective function for the mango’s surface, blocking the movement of moisture and solutes and improving mass retention [35].
Color is an important quality parameter for mango consumers, directly influencing their acceptance. ‘Tommy Atkins’ mangoes are characterized by a dark-red, blush skin color that covers much of the fruit’s surface, with green and orange-yellow accents [23]. Mango ripening is accompanied by color changes from green to yellow-orange in the skin as a result of chlorophyll degradation concomitant with the accumulation of carotenoids in the mesocarp tissue [39]. However, the skin color may not have a good relationship with the internal quality of the fruit in some mango varieties [40]. In this context, pulp color changes are uniform during fruit ripening, so it is considered an adequate maturity index for mangoes [41]. Skin and pulp hue angles decreased as the duration of storage increased, which confirms color changes typical of mango ripening. In both fruit tissues, bagged fruit had higher hue values than control fruit, representing a delay in fruit ripening as a result of MA conditions. A previous study with lettuce revealed that MA regulated the expression of pheophorbide a oxygenase, a key enzyme in the chlorophyll degradation pathway [42].
The packaging reduced fruit softening, in agreement with Oliveira et al. [8], who reported the retention of firmness in chitosan-coated ‘Tommy Atkins’ mangoes compared to uncoated ones. Fruit softening is related to the solubilization of pectic substances, starch hydrolysis and transpiration [37]. Excessive softening is the major undesirable change that influences the marketability of mango [9]. Alterations in gaseous composition inside the packaging promoted by MA tends to reduce the activity of cell-wall-degrading enzymes [13], delaying the loss of firmness in packed mangoes.
Mango ripening is accompanied by the hydrolyzation of starch into simple sugars (glucose, fructose and sucrose), which increases fruit SSC and sweetness perception. Concomitantly, organic acids are used as respiratory substrates, decreasing fruit acidity [13]. The delay in these biochemical changes in packed fruit in relation to unpacked ones could be related to the reduced available oxygen for fruit upon MA conditions, which reduces fruit respiration [37].
β-carotene is a pro-vitamin A lipophilic molecule with important biological functions related to antioxidant activity, providing protection to human organism against cancer, cardiovascular diseases and diabetes by scavenging the oxygen free radicals [43]. As the major carotenoid in the pulp of ‘Tommy Atkins’ mangoes [44], β-carotene was quantified in our study to determine changes in fruit ripening. β-carotene content increased in all samples throughout the storage period, with a higher content in unpacked fruit. These results corroborate those presented in the literature, where mangoes stored under MA accumulated significantly less carotenoids over storage [45]. The changes in fruit metabolism promoted by packaging include the inhibition of ethylene synthesis, which in turn stimulates carotenoid biosynthesis by enhancing the transcription of phytoene synthase [46].
AsA (or vitamin C) is a natural water-soluble vitamin that acts as a potent antioxidant and free-radical scavenger [47]. AsA is the most abundant antioxidant compound in all plant tissues, and has a key role in crucial reactions throughout plant development, including its action as a promoter of enhanced pectin solubilization and depolymerization of polysaccharides during the ripening of climacteric fruit [48]. In our study, AsA content decreased in ‘Tommy Atkins’ mangoes during cold storage, as a result of the increased enzymatic degradation by ascorbic acid oxidase, which catalyzes the oxidation reaction of L-ascorbic acid to dehydroascorbic acid (DHA) [49].
Conditions of low oxygen availability promoted by packaging tend to delay the enzyme activity and oxidation reactions of AsA, conserving its content longer in packed fruit. Similar results were found in mangoes in MA conditions through the use of coatings based on guar-Spirulina platensis-Aloe vera extract [50], alginate [51] and CS-zein-cinnamaldehyde nano-cellulose [35]. The retention of AsA in mangoes after harvest is strictly related to high fruit quality, considering the high antioxidant and free-radical scavenging properties of AsA and its action as fruit preserver, through the inhibition of polyphenol oxidase activity, which oxidizes diphenols to quinones and leads to browning after wounding. AsA also acts as a promoter of enhanced pectin solubilization and depolymerization of polysaccharides during the ripening of climacteric fruit [52].
Phenolic compounds are a group of phytochemicals widely distributed in plants, well known for their beneficial biological effects, including the scavenging and neutralizing of reactive oxygen species, which are partially responsible for the incidence of chronic diseases [1,2]. Phenolic compounds also contribute to important organoleptic properties of fruits and vegetables, such as astringency, color, bitterness and flavor [50]. TPC content decreased throughout storage, as a natural effect of fruit ripening, so the preservation of these compounds is important in order to avoid a loss of fruit quality. By limiting the gas exchange between mangoes and the external environment, the packaging decreased polyphenol oxidase activity, which occurs in the presence of molecular oxygen and is responsible for undesirable enzymatic browning reactions and losses of nutraceutical value in fruit. The preservation of TPC in mangoes under MA was also observed in market conditions (>20 °C) up to four weeks in studies on fruit packed with polypropylene [50] and coated with guar-Spirulina platensis-Aloe vera extract [53].
Flavonoids are the major class of phenolic compounds, with the same biological functions, including antioxidant, antiviral, antifungal, anti-angiogenic, anti-tumorigenic, antidiabetic, and immunomodulatory bioproperties [54]. YF content decreased with the progression of the storage period, but with lower significant losses of these compounds in packaged mangoes. ‘Banganapalli’ and ‘Totapuri’ mangoes also exhibited higher flavonoid content when stored in a polypropylene-based packaging [55]. A similar effect was observed in ‘Mahali’ mangoes by Rastegar et al. [51], who applied an alginate-based coating and observed a 1.7-fold higher flavonoid content in coated fruit compared with the control fruit.
AOX represents the ability of phytochemicals to scavenge free radicals and inhibit oxidation, thus providing benefits to the immune system [29]. The AOX was measured by the capture of ABTS•+ and DPPH• radicals from the pulp of ‘Tommy Atkins’ mangoes. In both methods, AOX decreased in all samples during storage, more markedly in unpacked mangoes. The preservation of the AOX of packed mangoes is a consequence of the delay in fruit ripening and the lower degradation of bioactive compounds during storage. Previous studies have also reported beneficial effects of MA in maintaining the antioxidant activity of mangoes during storage [45,50,51,53].
A principal component analyses (PCA) was carid out in order to summarize the data obtained from all quality parameters, bioactive compounds and AOX, evaluated on ‘Tommy Atkins’ mangoes, with the aim of assessing the effects of different packaging conditions and 42 days of storage at 12 °C on the quality of the fruit. The results demonstrate the delay in fruit ripening and the preservation of bioactive compounds provided by the packaging of ‘Tommy Atkins’ mangoes.
The postharvest quality of packed fruit was extended by at least 14 days compared to unpacked fruit stored under the same conditions. Moreover, due to the health-protective effects of mangoes which are of high interest in mangoes, postharvest treatments such as packaging stand out as an easy and effective method of preserving the phenolic compounds and antioxidant activity of the fruit, with the advantage that the CS-based packaging is eco-friendly and biodegradable.
5. Conclusions
The incorporation of GO improved the water-barrier and mechanical properties of CS packaging. The incorporation of all the types of packaging delayed the ripening of ‘Tommy Atkins’ mangoes by reducing their respiration rate, mass loss and softening, slowing changes in color, SSC, TA and beta-carotene and preserving the appearance of the fruit. The packaging also demonstrated a positive effect on preserving the bioactive compounds in the mangoes. The postharvest quality of the packed fruit was extended by at least 14 days compared to unpacked ones stored in the same conditions, with the advantage that the CS packaging is eco-friendly and biodegradable.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae9101145/s1, Table S1: F-values of analysis of variance for quality attributes of ‘Tommy Atkins’ mangoes stored in different packaging conditions for 42 days at 12 °C. One, two and three asterisks represent F-values at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively, while ns represents non-significant F-value; Table S2: F-values of analysis of variance for bioactive compounds and antioxidant activity of ‘Tommy At-kins’ mangoes stored in different packaging conditions for 42 days at 12 °C. One, two and three asterisks represent F-values at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively, while ns represents non-significant F-value; Table S3: Eigenvalues and variances of the principal components that represent 15 variables evaluated in ‘Tommy Atkins’ mangoes stored in different packaging conditions for 42 days at 12 °C; Table S4: Loadings in principal components 1 and 2 for 15 variables evaluated in ‘Tommy Atkins’ mangoes stored in different packaging conditions for 42 days at 12 °C.
Author Contributions
Conceptualization, J.C.V., S.T.d.F. and E.M.M.A.; methodology, J.C.V., S.T.d.F., R.H.d.L.L., F.K.G.d.S. and E.M.M.A.; formal analysis, J.C.V.; investigation, J.C.V., M.A.R.F. and C.d.S.R.C.; data curation, J.C.V.; writing—original draft preparation, J.C.V., M.A.R.F. and C.d.S.R.C.; writing—review and editing, S.T.d.F., R.H.d.L.L., F.K.G.d.S. and E.M.M.A.; supervision, S.T.d.F. and E.M.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq, grant no. 131701/2019-3), the Coordination for the Improvement of Higher Education Personnel (CAPES, Financing Code 001), the Brazilian Agricultural Research Corporation (Embrapa, grant no. 22.13.06.025.00.04.007), the Federal Rural University of the Semi-Arid Region (UFERSA) and the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE, grant no. APQ-1046-5.01/22).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgments
The authors thank José Gustavo Lima de Almeida and Cristiane Alves de Paiva for technical support in the preparation and characterization of the properties of the packaging. We also thank the company, Agrodan, for providing the mangoes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sousa, A.S.B.; Silva, M.C.A.; Lima, R.P.; Meireles, B.R.L.A.; Cordeiro, A.T.M.; Santos, E.F.S.; Amaro, A.L.; Pintado, M.M.E.; Silva, S.M. Phenolic Compounds and Antioxidant Activity as Discriminating Markers and Adding Value of Mango Varieties. Sci. Hortic. 2021, 287, 110259. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Fernandez-Lafuente, R.; Berenguer-Murcia, Á.; Meza-Gordillo, R.; Gutiérrez, L.F.; Pacheco, N.; Cuevas-Bernardino, J.; Ayora-Talavera, T. Phenolic Compounds in Mango Fruit: A Review. J. Food Meas. Charact. 2022, 16, 619–636. [Google Scholar] [CrossRef]
- Win, S.T.; Setha, S. Enhancement of Anti-Inflammatory and Antioxidant Activities of Mango Fruit by Pre- and Postharvest Application of Salicylic Acid. Horticulturae 2022, 8, 555. [Google Scholar] [CrossRef]
- Singh, Z.; Singh, R.K.; Sane, V.A.; Nath, P. Mango–Postharvest Biology and Biotechnology. Crit. Rev. Plant Sci. 2013, 32, 217–236. [Google Scholar] [CrossRef]
- Santos, L.F.; Vilvert, J.C.; Souza, T.A.; Silva Alves, J.; Silva Ribeiro, T.; Neuwald, D.A.; Freitas, S.T. Minimum O2 Levels During Storage to Inhibit Aerobic Respiration and Prolong the Postharvest Life of ‘Tommy Atkins’ Mangoes Produced in Different Growing Seasons. Sci. Hortic. 2023, 318, 112094. [Google Scholar] [CrossRef]
- Costa, C.S.R.; Lima, M.A.C.; Lima Neto, F.P.; Silva Costa, A.E.; Vilvert, J.C.; Martins, L.S.S.; Santos Musser, R. Genetic Parameters and Selection of Mango Genotypes Using the FAI-BLUP Multitrait Index. Sci. Hortic. 2023, 317, 112049. [Google Scholar] [CrossRef]
- Freitas, S.T.; Guimarães, Í.T.; Vilvert, J.C.; Amaral, M.H.P.; Brecht, J.K.; Marques, A.T.B. Mango Dry Matter Content at Harvest to Achieve High Consumer Quality of Different Cultivars in Different Growing Seasons. Postharvest Biol. Technol. 2022, 189, 111917. [Google Scholar] [CrossRef]
- Ntsoane, M.L.; Zude-Sasse, M.; Mahajan, P.; Sivakumar, D. Quality Assesment and Postharvest Technology of Mango: A Review of its Current Status and Future Perspectives. Sci. Hortic. 2019, 249, 77–85. [Google Scholar] [CrossRef]
- Oliveira, K.Á.R.; Conceição, M.L.; Oliveira, S.P.A.; Lima, M.S.; Galvão, M.S.; Madruga, M.S.; Magnani, M.; Souza, E.L. Postharvest Quality Improvements in Mango Cultivar Tommy Atkins by Chitosan Coating with Mentha piperita L. Essential Oil. J. Hortic. Sci. Biotechnol. 2020, 95, 260–272. [Google Scholar] [CrossRef]
- Rastegar, S.; Hassanzadeh Khankahdani, H.; Rahimzadeh, M. Effects of Melatonin Treatment on the Biochemical Changes and Antioxidant Enzyme Activity of Mango Fruit during Storage. Sci. Hortic. 2020, 259, 108835. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmed, Z.F.R.; Hussain, S.B.; Omar, A.E.-D.K.; Amin, M.; Javed, S.; Ali, A.; Ullah, S.; Razzaq, K.; Rajwana, I.A.; et al. On-Tree Fruit Bagging and Cold Storage Maintain the Postharvest Quality of Mango Fruit. Horticulturae 2022, 8, 814. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Li, W.; Shao, Y. Comparative Analyses of Ripening, Texture Properties and Cell Wall Composition in Three Tropical Fruits Treated with 1-Methylcyclopropene during Cold Storage. Horticulturae 2023, 9, 126. [Google Scholar] [CrossRef]
- Vasconcelos, O.C.M.; Duarte, D.; Silva, J.C.; Mesa, N.F.O.; Mederos, B.J.T.; Freitas, S.T. Modeling ‘Tommy Atkins’ Mango Cooling Time Based on Fruit Physicochemical Quality. Sci. Hortic. 2018, 244, 413–420. [Google Scholar] [CrossRef]
- Seymour, G.; Poole, M.; Giovannoni, J.; Tucker, G. The Molecular Biology and Biochemistry of Fruit Ripening; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Das, L.; Das, P.; Bhowal, A.; Bhattachariee, C. Synthesis of Hybrid Hydrogel Nano-Polymer Composite using Graphene Oxide, Chitosan and PVA and its Application in Waste Water Treatment. Environ. Technol. Innov. 2020, 18, 100664. [Google Scholar] [CrossRef]
- Maraschin, T.G.; Correa, R.S.; Rodrigues, L.F.; Balzarettid, N.M.; Malmonge, J.A.; Galland, G.B.; Basso, N.R.S. Chitosan Nanocomposites with Graphene-Based Filler. Mater. Res. 2019, 22, e20180829. [Google Scholar] [CrossRef]
- Han Lyn, F.; Tan, C.P.; Zawawi, R.M.; Nur Hanani, Z.A. Physicochemical Properties of Chitosan/ Graphene Oxide Composite Films and Their Effects on Storage Stability of Palm-Oil Based Margarine. Food Hydrocoll. 2021, 117, 106707. [Google Scholar] [CrossRef]
- Parvin, N.; Rahman, A.; Roy, J.; Rashid, M.H.; Paul, N.C.; Mahamud, M.A.; Imran, S.; Sakil, M.A.; Uddin, F.M.J.; Molla, M.E.; et al. Chitosan Coating Improves Postharvest Shelf-Life of Mango (Mangifera indica L.). Horticulturae 2023, 9, 64. [Google Scholar] [CrossRef]
- Silva, K.G.d.; Cavalcanti, M.T.; Martinsa, L.P.; Alves, R.d.C.; Lucena, F.A.d.; Santos, M.S.A.; Silva, S.X.d.; Costa, F.B.d.; Moreira, I.d.S.; Pereira, E.M. Coatings Based on Gelatin and Chitosan in the Conservation of Papaya (Carica papaya L.) Minimally Processed. Horticulturae 2023, 9, 729. [Google Scholar] [CrossRef]
- Jakubowska, E.; Gierszewska, M.; Nowaczyk, J.; Olewnik-Kruszkowska, E. The Role of a Deep Eutectic Solvent in Changes of Physicochemical and Antioxidative Properties of Chitosan-Based Films. Carbohydr. Polym. 2021, 255, 117527. [Google Scholar] [CrossRef]
- Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites. Molecules 2020, 25, 1148. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Arfat, Y.A.; Thai, T.L.A. Mechanical, Thermal, Structural and Barrier Properties of Crab Shell Chitosan/Graphene Oxide Composite Films. Food Hydrocoll. 2017, 71, 141–148. [Google Scholar] [CrossRef]
- Paiva, C.A.; Vilvert, J.C.; Menezes, F.L.G.; Leite, R.H.L.; Santos, F.K.G.; Medeiros, J.F.; Aroucha, E.M.M. Extended Shelf-Life of Melons using Chitosan and Graphene Oxide Based Biodegradable Bags. J. Food Process. Preserv. 2020, 44, e14871. [Google Scholar] [CrossRef]
- National Mango Board. Available online: https://www.mango.org/ (accessed on 20 July 2023).
- Sun, Q.; Sun, C.; Xiong, L. Mechanical, Barrier and Morphological Properties of Pea Starch and Peanut Protein Isolate Blend Films. Carbohydr. Polym. 2013, 98, 630–637. [Google Scholar] [CrossRef]
- Lima, A.B.; Silva, S.M.; Rocha, A.; Nascimento, L.C.; Ramalho, F.S. Conservação Pós-Colheita de Manga “Tommy Atkins” Orgânica sob Recobrimentos Bio-Orgânicos. Rev. Bras. Frutic. 2012, 34, 704–710. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 21st ed.; AOAC: Rockville, MD, USA, 2019. [Google Scholar]
- Francis, F.J. Analysis of Anthocyanins. In Anthocyanins as Food Colors; Markakis, P., Ed.; Academic Press: New York, NY, USA, 1982; pp. 181–207. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Łysiak, G.P.; Rutkowski, K.; Walkowiak-Tomczak, D. Effect of Storage Conditions on Storability and Antioxidant Potential of Pears cv. ‘Conference’. Agriculture 2021, 11, 545. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango Leaf Extract Incorporated Chitosan Antioxidant Film for Active Food Packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Silva, G.M.C.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The Chitosan Affects Severely the Carbon Metabolism in Mango (Mangifera indica L. cv. Palmer) Fruit During Storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Gu, C.; Zhu, D.; Huang, Y.; Luo, Y.; Zhou, Q. Development and Characterization of an Edible Chitosan/Zein-Cinnamaldehyde Nano-Cellulose Composite Film and its Effects on Mango Quality During Storage. LWT 2021, 140, 110809. [Google Scholar] [CrossRef]
- Camatari, F.O.S.; Santana, L.C.L.A.; Carnelossi, M.A.G.; Alexandre, A.P.S.; Nunes, M.L.; Goulart, M.O.F.; Narain, N.; Silva, M.A.A.P. Impact of Edible Coatings Based on Cassava Starch and Chitosan on the Post-Harvest Shelf Life of Mango (Mangifera indica) ‘Tommy Atkins’ Fruits. Food Sci. Technol. 2018, 38, 86–95. [Google Scholar] [CrossRef]
- Eshetu, A.; Ibrahim, A.M.; Forsido, S.F.; Kuyu, C.G. Effect of Beeswax and Chitosan Treatments on Quality and shelf life of Selected Mango (Mangifera indica L.) Cultivars. Heliyon 2019, 5, e01116. [Google Scholar] [CrossRef]
- Costa, J.D.S.; Figueiredo Neto, A.; Almeida, F.A.C.; Costa, M.S. Conservation of ‘Tommy Atkins’ Mangoes Stored Under Passive Modified Atmosphere. Rev. Caatinga 2018, 31, 117–125. [Google Scholar] [CrossRef]
- Yungyuen, W.; Vo, T.T.; Uthairatanakij, A.; Ma, G.; Zhang, L.; Tatmala, N.; Kaewsuksaeng, S.; Jitareerat, P.; Kato, M. Carotenoid Accumulation and the Expression of Carotenoid Metabolic Genes in Mango during Fruit Development and Ripening. Appl. Sci. 2021, 11, 4249. [Google Scholar] [CrossRef]
- Vilvert, J.C.; Freitas, S.T.; Ferreira, M.A.R.; Costa, E.B.S.; Aroucha, E.M.M. Sample Size for Postharvest Quality Traits of ‘Palmer’ Mangoes. Rev. Bras. Frutic. 2021, 43, e014. [Google Scholar] [CrossRef]
- Vanoli, M.; Rizzolo, A.; Grassi, M.; Spinelli, L.; Torricelli, A. Modeling Mango Ripening during Shelf Life Based on Pulp Color Nondestructively Measured by Time-Resolved Reflectance Spectroscopy. Sci. Hortic. 2023, 310, 111714. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Fan, J.; Liu, H.; Zhou, J.; Chang, X. iTRAQ Proteomics Reveals Changes in the Lettuce (Lactuca sativa L. Grand Rapid) Proteome Related to Colour and Senescence under Modified Atmosphere Packaging. J. Sci. Food Agric. 2018, 99, 1908–1918. [Google Scholar] [CrossRef]
- Bhatt, T.; Patel, K. Carotenoids: Potent to Prevent Diseases Review. Nat. Prod. Bioprospecting 2020, 10, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ruales, J.; Baenas, N.; Moreno, D.A.; Stinco, C.M.; Meléndez-Martínez, A.J.; García-Ruíz, A. Biological Active Ecuadorian Mango ‘Tommy Atkins’ Ingredients–An Opportunity to Reduce Agrowaste. Nutrients 2018, 10, 1138. [Google Scholar] [CrossRef]
- Sousa, F.F.; Pinsetta Junior, J.S.; Oliveira, K.T.E.F.; Rodrigues, E.C.N.; Andrade, J.P.; Mattiuz, B.-H. Conservation of ‘Palmer’ Mango with an Edible Coating of Hydroxypropyl Methylcellulose and Beeswax. Food Chem. 2021, 346, 128925. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, Q.; Wang, Y.; Qin, L.; Shi, Y.; Wang, X.; Wang, R. NtDREB-1BL1 Enhances Carotenoid Biosynthesis by Regulating Phytoene Synthase in Nicotiana tabacum. Genes 2022, 13, 1134. [Google Scholar] [CrossRef] [PubMed]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic Acid: The Chemistry Underlying its Antioxidant Properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Mellidou, I.; Georgiadou, E.C.; Kaloudas, D.; Kalaitzis, P.; Fotopoulos, V.; Kanellis, A.K. Vitamins. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E., Ed.; Academic Press: New York, NY, USA, 2019; pp. 359–384. [Google Scholar]
- Mazurek, A.; Włodarczyk-Stasiak, M. A New Method for the Determination of Total Content of Vitamin C, Ascorbic and Dehydroascorbic Acid, in Food Products with the Voltammetric Technique with the Use of Tris(2-carboxyethyl)phosphine as a Reducing Reagent. Molecules 2023, 28, 812. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Rastegar, S. Preservation of Mango Fruit with Guar-Based Edible Coatings Enriched with Spirulina platensis and Aloe vera Extract During Storage at Ambient Temperature. Sci. Hortic. 2020, 265, 109258. [Google Scholar] [CrossRef]
- Rastegar, S.; Hassanzadeh Khankahdani, H.; Rahimzadeh, M. Effectiveness of Alginate Coating on Antioxidant Enzymes and Biochemical Changes during Storage of Mango Fruit. J. Food Biochem. 2019, 43, e12990. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Deryło, K.; Tchórzewska, D.; Zdunek, A. Changing of Biochemical Parameters and Cell Wall Polysaccharides Distribution during Physiological Development of Tomato Fruit. Plant Physiol. Biochem. 2017, 119, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Fareed, M.I.; Rashid, H.; Aziz, H.; Ehsan, N.; Khalid, S.; Ghaffar, I.; Ali, R.; Gul, A.; Hakeem, K.R. Flavonoids and Their Biological Secrets. In Plant and Human Health, Volume 2: Phytochemistry and Molecular Aspects; Ozturk, M., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2019; pp. 579–605. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Sellamuthu, P.S.; Emmanuel, R.S. Use of Modified Atmosphere Packaging Combined with Essential Oils for Prolonging Post-Harvest Shelf Life of Mango (cv. Banganapalli and cv. Totapuri). LWT 2021, 148, 111662. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).