Abstract
Far-red light (FR; wavelength: 700–800 nm) is known for its effects on plant morphology and photosynthesis. However, its effects on stomatal responses and transpiration are not well understood. This study investigated the effects of FR on stomatal development and evapotranspiration in sweet basil grown under red, blue, and green (RGB) light-emitting diodes (LED). FR was provided for 10 days at 0, 30, 100, and 130 μmol·m−2·s−1 with the same base light (RGB ratio of 6:2:2; a photosynthetic photon flux density of 200 μmol·m−2·s−1). Evapotranspiration was measured using a load cell, and stomatal development was monitored microscopically. FR increased the height of sweet basil mostly due to the shade avoidance syndrome. The photosynthetic rate was not improved with FR, probably due to insufficient base light intensity. Despite similar leaf area and root growth, daily evapotranspiration increased with FR, resulting in higher water use. Although the stomatal density and guard cell area were similar across treatments, the stomatal aperture area was larger in plants with FR, thus increasing evapotranspiration. In conclusion, FR with the base RGB light intensity of 200 μmol·m−2·s−1 enhanced the evapotranspiration of sweet basil by regulating stomatal opening, but it did not enhance photosynthesis.
1. Introduction
Climate change owing to global warming is altering precipitation regimes worldwide, leading to an increase in the frequency and intensity of extreme drought events [1]. Concomitantly, the world population is expected to increase significantly by the year 2050 [2]; hence, the global food demand will continue to increase, and food security will have to be ensured with various efficient technologies and policies. In response, plant factories with artificial lighting (PFAL) are expected to provide crop cultivation for stable crop production in the future [3], relying on a range of techniques with efficient control of environments. In particular, an efficient amount of artificial light with the optimal combination of light spectra has been extensively researched in PFAL. For stable plant production in PFAL, securing sufficient and effective light intensity, wavelength, and duration for plants growing under artificial light sources is essential. Mitchell et al. [4] reviewed several previous studies that focused on the effects of light intensity, light quality, and photoperiod on the growth and development of several plant species using light-emitting diodes (LEDs).
LEDs have been acknowledged as the most effective artificial light source to readily achieve separate control of light intensity and quality [3]; thus, many researchers have studied the morphological and physiological responses of plants under various light-quality combinations of LEDs. Among the various light spectra, red light is considered a primary component of photosynthetically active radiation (PAR) because of its high relative quantum efficiency in photosynthesis [5]. However, using red light alone leads to a reduction in photosynthetic rate and stomatal conductance, and the symptoms can be relieved with blue light [6,7]. Furthermore, a greater proportion of blue than red light can reduce water use efficiency and increase stomatal conductance [8]. Additionally, green light can affect plant morphology and evapotranspiration; plants tend to grow taller in shaded environments upon the addition of green light [9] and green light combined with blue light enhances stomatal development, thereby increasing the water use amount [10].
Recently, studies on far-red light (FR; 700–800 nm) with PAR have been repeatedly reporting their significant roles in plant photosynthesis and growth. The shade avoidance syndrome is a representative morphological effect of far-red light, typically inducing stem and leaf elongation under a low red-to-far-red light (R:FR) ratio. This ratio determines how the ratio of phytochromes interacts with Phytochrome Interacting Factors (PIFs), which can induce light-regulated gene expression [11,12]. Recently, many researchers have studied the relationship between phytochromes (PIFs) and phytohormones. Wang et al. [13] put several studies together that R:FR could induce the regulation of phytohormones such as auxin, gibberellin, or brassinosteroid, which are closely related to phenotypic changes in shade avoidance syndrome. Further, the light of wavelengths longer than 700 nm added to red light can increase photosynthetic rates [14]. It has been reported that far-red light added to red light increases the quantum yield of photosystem II (PSII) [15,16] by accelerating PSII reaction center reopening. Therefore, Zhen et al. [17] suggested that far-red light should be included in the PAR range as extended PAR.
Previous studies have primarily focused on the impact of far-red light on the light reaction of photosynthesis, while its effect on stomatal regulation, which is equally important for plant photosynthesis and transpiration, has received less attention. Therefore, the objective of this research is to investigate the stomatal response and evapotranspiration of sweet basil under far-red light conditions during 10 days of plant growth.
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
Sweet basil (Ocimum basilicum L.) seeds (Asia Seed Co., Seoul, Republic of Korea) were sown in 200-cell plug trays filled with a germinating substrate (Sunshine Mix #5; Sun Gro Horticulture, Agawam, MA, USA) and grown in a greenhouse at Korea University, in Seoul, Korea. After 3 weeks, seedlings were transplanted into 10 cm round plastic pots filled with a soil-less substrate (Sunshine Mix #4; Sun Gro Horticulture) mixed with a controlled-release fertilizer (Multicote 6; NPK 14-14-14, Haifa Chemicals, Israel) at a rate of 4 g·L−1. Transplanted seedlings were grown for 2 weeks in a greenhouse, and 96 plants with similar growth were transferred into a customized plant growth chamber equipped with an LED dimming system and acclimated for 2 weeks before the treatment. During acclimation, average temperature, relative humidity, and CO2 concentration in the chamber were 25.0 ± 0.3 °C, 74.7 ± 6.6%, and 599 ± 199 μmol·mol−1 (mean ± SD), respectively. LED lights were provided at a photosynthetic photon flux density (PPFD) of 200 μmol·m−2·s−1 at the canopy level, with a photoperiod of 16/8 h. Plants were irrigated daily with an unlimited water supply during the acclimation and treatment periods. After treatment, average temperature, relative humidity, and CO2 concentration were 25.0 ± 0.3 °C, 74.4 ± 8.8%, and 603 ± 136 μmol·mol−1, respectively. Plants were automatically irrigated every day at 6 AM for 240 s (133 mL) to each pot.
2.2. Light Treatments
To provide specific light-quality conditions, we used a customized LED dimming system equipped with six LED bars, each consisting of blue (B; 400–485 nm; peak = 451 nm), green (G; 485–600 nm; peak = 524 nm), red (R; 600–700 nm; peak = 664 nm), and far-red (FR; 700–800 nm; peak = 728 nm) LED chips (ESLEDs, Seoul, Korea). To achieve the desired ratios and intensities of the R, G, B, and FR diodes at the plant canopy level, a spectroradiometer (SS-110; Apogee Instruments, Logan, UT, USA) was utilized to monitor light spectra. The spectroradiometer was positioned 15 cm away from the LED bars and provided the necessary information to adjust the diodes. Base lighting was provided with a quantum intensity R:G:B ratio of 6:2:2, adjusting PPFD to 200 μmol·m−2·s−1. For FR treatments, four different levels of FR were provided, i.e., 0, 30, 100, and 130 μmol·m−2·s−1 (FR0, FR30, FR100, and FR130, respectively) with the following R:FR ratios: infinite, 4, 1.2, and 0.9, respectively (Figure 1). As plants grew, the plant canopy level was readjusted every other day using scissor lifts to correct incident light intensity and quality.
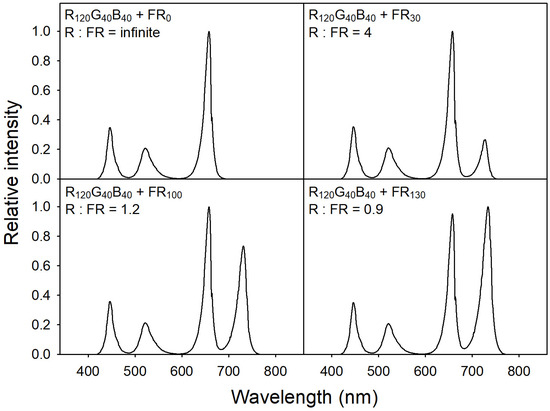
Figure 1.
The relative intensities of light treatments with blue (B; 400–485 nm; peak = 451 nm), green (G; 485–600 nm; peak = 524 nm), red (R; 600–700 nm; peak = 664 nm), and far-red (FR; 700–800 nm; peak = 728 nm). The proportion of red, green, and blue was 6:2:2. Base PPFD was 200 μmol·m−2·s−1. FR treatments were 0, 30, 100, and 130 μmol·m−2·s−1, and the R:FR ratios were infinite, 4, 1.2, and 0.9, respectively.
2.3. Growth Measurement and Photosynthetic Parameters
To measure plant morphological and physiological changes under the different FR treatments, general growth and photosynthetic parameters were measured at 0, 1, 4, 7, and 10 days after treatment (DAT). Measured plant growth parameters included plant height, shoot and root dry weights, and leaf area, which was measured using a leaf area meter (LI-3100; LI-COR, Lincoln, NE, USA). Relative chlorophyll content was determined with three spots on the uppermost fully expanded leaf using a SPAD meter (SPAD-502; Minolta Corporation Ltd., Osaka, Japan). The photosynthetic rate was measured in the uppermost fully expanded leaf using a portable photosynthesis system (CIRAS-3, PP Systems, Amesbury, MA, USA). When measuring photosynthetic parameters, cuvette temperature, relative humidity, and CO2 concentration were set at 25 °C, 75%, and 580 μmol·mol−1, respectively, and ambient light was used to investigate the FR effects according to the treatments. The maximum quantum yield (Fv/Fm) of PSII and the quantum yield of PSII under actinic light conditions (ΦPSII) were measured using a chlorophyll fluorometer (Mini-PAM-II; Heinz Walz GmbH, Effeltrich, Germany). Fv/Fm was measured after the leaves were dark-adapted for 20 min.
2.4. Measurement of Evapotranspiration
To investigate the evapotranspiration of sweet basils, 16 individually calibrated load cells (BCL-1L; CAS Scale Korea, Seoul, Korea) were connected to a data logger (CR1000; Campbell Scientific, Logan, UT, USA) via a multiplexer (AM 16/32B; Campbell Scientific). One pot per experimental unit was mounted on the load cell, and the weight of each pot was measured every 10 s. The average value and changes in pot weight (Δweight) were recorded every 30 min, and daily evapotranspiration was determined based on Δweight data [10].
2.5. Analysis of Stomatal Density and Size
Each one of the uppermost fully expanded leaves from the plants in each experimental unit was used for microscopic observation. Leaves were soaked in 94.5% ethyl alcohol for 24 h and then fixed. After fixation, transparent nail polish was applied to the abaxial leaf surface for transfer to microscope slides using clear tape. Two imprints were made per leaf. Images of the stomata were captured using a light microscope (CX31; Olympus Inc., Tokyo, Japan) equipped with a digital camera (YJO-301; Daemyung Optical, Seoul, Korea) and analyzed using the S-EYE software (version 1.6.0.11; Daemyung Optical, Seoul, Republic of Korea). Three photographs spanning an area of 0.8 mm2 were taken at distinct locations from each imprint to determine stomatal density. All stomata in the images were counted, and the average stomatal density was expressed as the number of stomata over 0.8 mm2. Stomatal aperture sizes were calculated with the long and short radii considered an ellipse, and guard cell sizes were calculated with the long and short radii of whole stomata, subtracting stomatal aperture size. Three stomata were selected in the range of 0.8 mm2 for calculating the corresponding size and the average stomatal size.
2.6. Experimental Design and Statistical Analysis
The experiment was laid in a randomized complete block design with four treatments (TRT) and four blocks, with six plants in each experimental unit. Differences in parameter means among treatments were analyzed with a two-way (TRT and DAT as fixed effects; block as a random effect) analysis of variance (ANOVA) followed by pair-wise comparison with least-square means at α = 0.05 using PROC MIXED of statistical analysis software (SAS 9.4; SAS Institute, Cary, NC, USA). The average daily evapotranspiration values for each treatment were compared using Tukey’s honestly significant difference at α = 0.05.
3. Results
3.1. Shoot and Root Growth
Growth of sweet basil increased with increasing DAT (PDAT < 0.001), but FR treatments only affected plant height and shoot dry weight, and the FR effect on plant height was dependent on DAT (PDAT*TRT < 0.001; Figure 2A). FR-treated plants were taller than those without FR from DAT 7, and plants under FR100 and FR130 were taller than those under FR30 (Figure 2A). Despite changes in plant height, leaf area did not differ across treatments throughout the experimental period (Figure 2B). FR-treated plants had greater shoot dry weight than FR0 from DAT 7, but plants under FR30 had similar shoot dry weights of FR0. Plants under FR100 and FR130 had larger shoot dry weights than those under FR0 or FR30 at both DAT 7 and DAT 10 (Figure 2C). Root dry weight increased over time throughout the experimental period, without significant differences among treatments (Figure 2D).
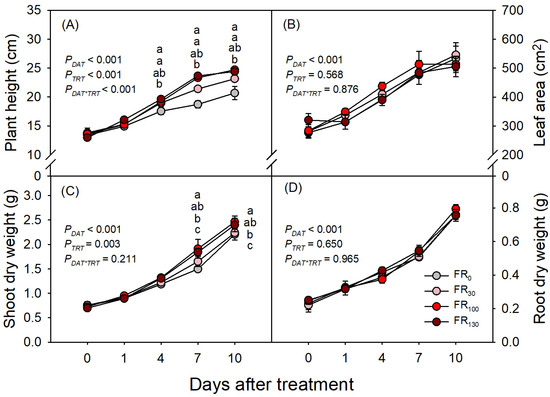
Figure 2.
(A) Plant height, (B) leaf area, (C) shoot dry weight, and (D) root dry weight of sweet basil at 0, 1, 4, 7, and 10 DAT under FR treatments of 0, 30, 100, and 130 μmol·m−2·s−1 with base RGB LED light (R:G:B = 6:2:2) at 200 μmol·m−2·s−1. PDAT, PTRT, and PDAT*TRT are the p values following two-way ANOVA with DATs and treatments (TRTs). Means followed by the same letter within the same DAT are not significantly different following pair-wise comparison at α = 0.05. Error bars indicate standard error (n = 4).
3.2. Photosynthetic Rate and Quantum Yield of PSII
Although several previous studies have reported the enhancement of plant photosynthesis with FR, our study on sweet basil showed no significant increase in photosynthesis with FR. Until DAT 7, the photosynthetic rates of sweet basil showed no significant differences across treatments, but FR-treated plants rather showed lower photosynthetic rates compared to those under FR0 at DAT 10 (Figure 3A). Meanwhile, Fv/Fm and ΦPSII showed no significant differences across treatments over the experimental period (Figure 3C,D). However, the relative chlorophyll content (SPAD) of sweet basil under all FR treatments was lower than that of plants under FR0 at DAT 10, consistently with the abovementioned results on a photosynthetic rate decrease under FR treatment (Figure 3B).
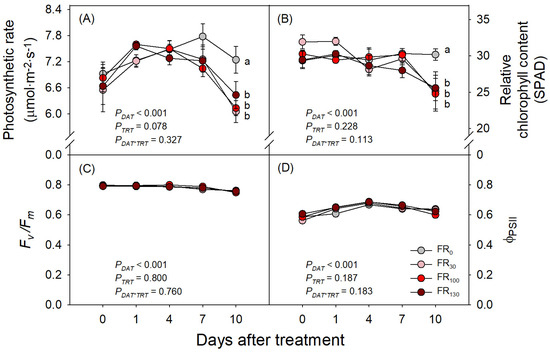
Figure 3.
(A) Photosynthetic rate, (B) relative chlorophyll content, (C) Fv/Fm, and (D) ΦPSII of sweet basil at 0, 1, 4, 7, and 10 DAT under FR treatments of 0, 30, 100, and 130 μmol·m−2·s−1 with base RGB LED light (R:G:B = 6:2:2) at 200 μmol·m−2·s−1. PDAT, PTRT, and PDAT*TRT are the p values following two-way ANOVA with DATs and treatments (TRTs). Means followed by the same letter within the same DAT are not significantly different following pair-wise comparison at α = 0.05. Error bars indicate standard error (n = 4).
3.3. Daily Evapotranspiration and Stomatal Development
FR treatments also increased evapotranspiration of sweet basil regardless of DAT (Figure 4A; PTRT = 0.004; PDAT*TRT = 1.000). When FR was applied, daily evapotranspiration was greater than that of FR0 (Figure 4B). Even among FR levels, FR100-treated plants showed greater daily evapotranspiration than FR30-treated plants, suggesting the enhancing effect of FR levels on evapotranspiration. In the results of the microscopic analysis of stomata, the stomatal densities of all treatments initially increased from DAT 1 to DAT 4. However, stomatal densities after DAT 4, guard cell areas, and stomatal aperture areas of all treatments decreased as DAT increased (Figure 5). FR treatment did not affect stomatal density or guard cell area, but stomatal aperture areas under the FR treatments were larger than those under FR0 treatment. The FR100 treatment resulted in a larger stomatal aperture area than FR0 at DAT 4, and the FR130 treatment resulted in a larger stomatal aperture area than FR0 at DAT 10 (Figure 5C).
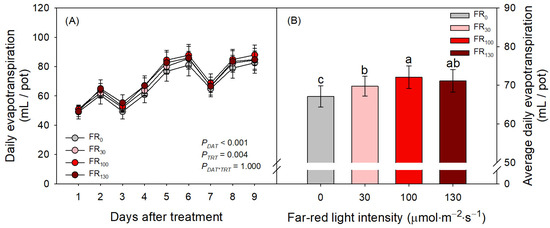
Figure 4.
(A) Daily evapotranspiration at each day after treatment (DAT) and (B) average daily evapotranspiration of sweet basil through all experimental periods under FR treatments at 0, 30, 100, and 130 μmol·m−2·s−1 with base RGB LED light (R:G:B = 6:2:2) at 200 μmol·m−2·s−1. PDAT, PTRT, and PDAT*TRT are the p values following two-way ANOVA with DATs and treatments (TRTs). Means of average daily evapotranspiration followed by the same letter are not significantly different following Tukey’s honestly significant difference at α = 0.05. Error bars indicate standard error (n = 4).
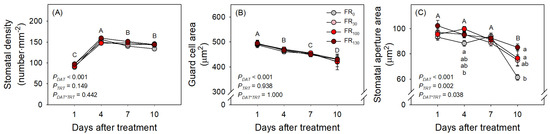
Figure 5.
(A) Stomatal density, (B) guard cell area, and (C) stomatal aperture area in leaves of sweet basil seedlings at 1, 4, 7, and 10 DAT under FR treatments at 0, 30, 100, and 130 μmol·m−2·s−1 with base RGB LED light (R:G:B = 6:2:2) at 200 μmol·m−2·s−1. PDAT, PTRT, and PDAT*TRT are the p values following two-way ANOVA with DATs and treatments (TRTs). Capital letters indicate mean separations using DAT. Means followed by the same letter are not significantly different following pair-wise comparison at α = 0.05. Error bars indicate standard error (n = 24).
4. Discussion
Increased plant height with increasing levels of FR was primarily attributed to the well-known shade avoidance syndrome [18,19,20]. The higher levels of FR, which entail a lower R:FR ratio, induce plants to be relatively taller compared with those that used FR0, as the amount of active phytochrome (photoreceptor protein, Pfr) decreases, thus allowing it to decrease to interact with a suppressor of stem cell elongation, which leads to upregulating auxin and gibberellic acid biosynthetic pathways, causing stem cell elongation [21]. In this study, increased plant height mostly affected a shoot dry weight increase, whereas FR treatment did not affect leaf area. In contrast, Park and Runkle [22] reported that FR induced leaf expansion of four bedding plants (geranium, petunia, snapdragon, and impatiens); however, those plants were grown from young seedlings for a month, and the FR effect on their growth may have enhanced overall growth over a longer period. In contrast, our study was conducted only for 10 days with fully grown sweet basil, which might not be sufficient time to provide the changes in leaf area attributed to FR treatment. In addition, FR did not affect root dry weight (Figure 2D), indicating that the overall growth change induced with FR in sweet basil was mainly shoot elongation, as a shade avoidance syndrome. Nevertheless, no differences in leaf area or root growth among treatments could override the effect of leaf area or roots on transpiration to distinguish the effect of FR per se on the evapotranspiration of the sweet basil in the current study.
Recently, the development of LED applications for PFAL environments has improved the interest of researchers and producers in using FR, such that a considerable number of related studies have confirmed that FR does enhance photosynthesis in many species [15,16,23]. Furthermore, Zhen et al. [17] suggested revising the PAR range to extended PAR (e-PAR) to include FR as an essential component of the best radiation for plant photosynthesis. However, the positive effect of FR on photosynthesis might depend on the species and base light intensity, as each species has its light compensation and saturation points. The light response curves from previous research showed that FR did not increase photosynthesis in cucumber [24] or tomato [25] at approximately 200 μmol·m−2·s−1 PPFD, while photosynthesis enhancement with FR occurred under higher light intensity levels.
In our study, chlorophyll fluorescence parameters of sweet basil were similar among treatments, regardless of DAT, suggesting that FR was ineffective for photosynthetic enhancement of sweet basil under the base light intensity used (200 μmol·m−2·s−1). On top of that, we consistently maintained an optimal vapor pressure deficit (VPD) level (approximately 0.85–0.9 kPa) and relatively higher CO2 concentration (approximately 600 μmol·mol−1). While the stomatal aperture areas in the FR treatments were higher, potentially affecting the evapotranspiration of sweet basil, it is important to consider that both VPD and CO2 concentration around the leaves can also significantly impact evapotranspiration [26]. Thus, maintaining optimal VPD levels and higher CO2 concentration might play a crucial role in regulating gas exchange during photosynthesis. Furthermore, intercellular CO2 concentration (Ci) across the treatments exhibited no significant differences at each DAT. These observations suggest that all plants in the various treatments effectively absorbed an adequate amount of CO2 for photosynthesis.
Conversely, FR treatment reduced the photosynthetic rate at DAT 10 compared with FR0 (Figure 3), likely due to the reduced relative chlorophyll content with FR treatments, whereas plants at FR0 consistently maintained relative chlorophyll content. Similarly, Meng and Runkle [27] and Zhang et al. [28] found that FR reduced the relative chlorophyll content of various species, which might play a pivotal role in reducing the photosynthetic rate [29]. In addition, FR0 displayed a higher photosynthetic rate despite having lower stomatal aperture areas. This phenomenon can be elucidated by examining the SPAD values. Therefore, the observed reduction in photosynthesis of sweet basil upon treatment with FR was mostly owing to lower relative chlorophyll content, but further investigations are needed to understand how FR precisely influences the reduction in the SPAD value. Additionally, providing FR was ineffective in enhancing the photosynthesis of sweet basil in a low-light-intensity, high-humidity, and higher-CO2-concentration environment.
Although FR did not enhance photosynthesis in this study, it increased daily evapotranspiration. Previously, Lim and Kim [10] reported that both blue and green light altered stomatal density, thus affecting the water use amount of sweet basil in the PFAL system. Blue light can also affect guard cells by altering the starch concentration in the cell to regulate osmotic levels [30]. All treatments showed stomatal density increasing from DAT 1 to DAT 4 (Figure 5A). According to Lake et al. [31], mature leaves have the capacity to increase their stomatal density when exposed to elevated CO2 concentrations. Sweet basil plants were subjected to relatively higher CO2 concentrations throughout the acclimation and experimental period. This prolonged exposure to elevated CO2 levels could plausibly explain the observed increase in stomatal density from DAT 1 to DAT 4. However, all treatments showed stomatal aperture areas decreasing through the experimental period (Figure 5C). Sweet basil was initially cultivated in a greenhouse environment and subsequently acclimated to a controlled LED chamber. This transition involved a reduction in light intensity and an increase in CO2 concentration, as the greenhouse provided higher light intensity and lower CO2 concentration in comparison to the LED chamber. This shift in environmental conditions, as elucidated by Esmaeili et al. [32] and Ghorbanzadeh et al. [33], is known to induce changes in stomatal aperture sizes. Specifically, plants exposed to lower light intensity tend to exhibit smaller stomatal apertures, signifying a potential alteration in the overall stomatal aperture area upon transfer from the greenhouse to the LED chamber. Furthermore, it has been reported that an increase in CO2 concentration can cause stomatal closure through the modulation of ion channel activity in guard cells [34]. This physiological response can also contribute to a reduction in the overall stomatal aperture areas transferring from the greenhouse to the chamber.
Despite that the stomatal density and guard cell area were not significantly affected with FR treatment, plants with FR hindered the reduction in stomatal aperture areas compared to plants under FR0. This finding might indicate that FR provided a relatively larger stomatal opening, thereby increasing daily evapotranspiration more than that recorded for FR0. The photosynthetic enhancement with FR observed in previous studies might be partially attributed to the effect of FR on stomatal opening, whereby gas exchange was enhanced. However, the base light intensity used in the experiments reported herein might not be sufficient to enhance photosynthesis of sweet basil. Therefore, further studies with a higher base light intensity might be required to elucidate the effect of FR on the photosynthesis of sweet basil, along with their stomatal changes. Nevertheless, a greater stomatal opening caused with FR treatment might be the only factor to increase daily evapotranspiration in this study, as the treatment did not affect leaf area, root dry weight, or other stomata-related parameters strongly related to evapotranspiration. In addition, FR can be transmitted through leaves [35]; thus, whole-plant rather than single-leaf evapotranspiration might be significantly affected.
5. Conclusions
Despite being fully grown, sweet basil plants exhibited increased height after receiving FR treatment. However, contrary to previous reports, sweet basil did not exhibit enhanced photosynthesis with FR, possibly because the base light intensity of PAR was insufficient. Thus, when providing sweet basil with FR in PFAL, it is crucial to consider the appropriate base light intensity for photosynthetic enhancement. While FR did not impact stomatal density or guard cell size, it did increase stomatal opening, leading to higher evapotranspiration rates in sweet basil. In summary, the effects of FR on morphological change, stomatal opening, and evapotranspiration should be reconsidered for high-quality production of sweet basil with high energy and water use efficiency.
Author Contributions
Conceptualization, J.K.; Data curation, S.K.A.; Formal analysis, J.U.P.; Funding acquisition, J.K.; Investigation, J.U.P.; Methodology, S.K.A. and J.K.; Supervision, J.K.; Validation, S.K.A.; Writing—original draft, J.U.P. and S.K.A.; Writing—review and editing, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Korea Smart Farm R&D Foundation (KoSFarm), Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) (421027-04).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- NOAA. Annual 2021 Global Climate Report. Available online: https://www.ncdc.noaa.gov/sotc/global/202113 (accessed on 27 November 2022).
- FAO. How to Feed the World in 2050. Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 22 November 2022).
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Mitchell, C.A.; Dzakovich, M.P.; Gomez, C.; Lopez, R.; Burr, J.F.; Hernández, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S.; et al. Light-emitting diodes in horticulture. In Horticultural Reviews; Janick, J., Ed.; Wiley: Hoboken, NJ, USA, 2015; Volume 43, pp. 1–88. [Google Scholar]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Savvides, A.; Fanourakis, D.; van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 2012, 63, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of photosynthesis after the ‘red light syndrome’ in cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Clavijo-Herrera, J.; Van Santen, E.; Gómez, C. Growth, water-use efficiency, stomatal conductance, and nitrogen uptake of two lettuce cultivars grown under different percentages of blue and red light. Horticulturae 2018, 4, 16. [Google Scholar] [CrossRef]
- Zhang, T.; Maruhnich, S.A.; Folta, K.M. Green light induces shade avoidance symptoms. Plant Physiol. 2011, 157, 1528–1536. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J. Light quality affects water use of sweet basil by changing its stomatal development. Agronomy 2021, 11, 303. [Google Scholar] [CrossRef]
- Lorrain, S.; Allen, T.; Duek, P.D.; Whitelam, G.C.; Fankhauser, C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008, 53, 312–323. [Google Scholar] [CrossRef]
- Quail, P.H. Phytochrome-interacting factors. Semin. Cell Dev. Biol. 2000, 11, 457–466. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Liu, Y.; Fan, S.; Ma, Q. Progress of research on the regulatory pathway of the plant shade-avoidance syndrome. Front. Plant Sci. 2020, 11, 439. [Google Scholar] [CrossRef]
- Emerson, R.; Chalmers, R.; Cederstrand, C. Some factors influencing the long-wave limit of photosynthesis. Proc. Natl. Acad. Sci. USA 1957, 43, 133–143. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Haidekker, M.; van Iersel, M.W. Far-red light enhances photochemical efficiency in a wavelength-dependent manner. Physiol. Plant. 2019, 167, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; van Iersel, M.; Bugbee, B. Why far-red photons should be included in the definition of photosynthetic photons and the measurement of horticultural fixture efficacy. Front. Plant Sci. 2021, 12, 1158. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Whitelam, G. The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997, 20, 840–844. [Google Scholar] [CrossRef]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015. [Google Scholar]
- Tan, T.; Li, S.; Fan, Y.; Wang, Z.; Raza, M.A.; Shafiq, I.; Wang, B.; Wu, X.; Yong, T.; Wang, X. Far-red light: A regulator of plant morphology and photosynthetic capacity. Crop J. 2021, 10, 300–309. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Kono, M.; Kawaguchi, H.; Mizusawa, N.; Yamori, W.; Suzuki, Y.; Terashima, I. Far-red light accelerates photosynthesis in the low-light phases of fluctuating light. Plant Cell Physiol. 2020, 61, 192–202. [Google Scholar] [CrossRef]
- Shibuya, T.; Endo, R.; Kitamura, Y.; Kitaya, Y.; Hayashi, N. Potential photosynthetic advantages of cucumber (Cucumis sativus L.) seedlings grown under fluorescent lamps with high red: Far-red light. Hortic. Sci. 2010, 45, 553–558. [Google Scholar] [CrossRef]
- Ji, Y.; Ouzounis, T.; Courbier, S.; Kaiser, E.; Nguyen, P.T.; Schouten, H.J.; Visser, R.G.; Pierik, R.; Marcelis, L.F.; Heuvelink, E. Far-red radiation increases dry mass partitioning to fruits but reduces Botrytis cinerea resistance in tomato. Environ. Exp. Bot. 2019, 168, 103889. [Google Scholar] [CrossRef]
- Driesen, E.; Van den Ende, W.; De Proft, M.; Saeys, W. Influence of Environmental Factors Light, CO2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review. Agronomy 2020, 10, 1975. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Zhang, M.; Park, Y.; Runkle, E.S. Regulation of extension growth and flowering of seedlings by blue radiation and the red to far-red ratio of sole-source lighting. Sci. Hortic. 2020, 272, 109478. [Google Scholar] [CrossRef]
- Puangbut, D.; Jogloy, S.; Vorasoot, N. Association of photosynthetic traits with water use efficiency and SPAD chlorophyll meter reading of Jerusalem artichoke under drought conditions. Agric. Water Manag. 2017, 188, 29–35. [Google Scholar] [CrossRef]
- Lawson, T.; Matthews, J. Guard cell metabolism and stomatal function. Annu. Rev. Plant Biol. 2020, 71, 273–302. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.A.; Quick, W.P.; Beerling, D.J.; Woodward, F.I. Signals from mature to new leaves. Nature 2001, 411, 154. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.; Aliniaeifard, S.; Dianati Daylami, S.; Karimi, S.; Shomali, A.; Didaran, F.; Telesiński, A.; Sierka, E.; Kalaji, H.M. Elevated light intensity compensates for nitrogen deficiency during chrysanthemum growth by improving water and nitrogen use efficiency. Sci. Rep. 2022, 12, 10002. [Google Scholar] [CrossRef]
- Ghorbanzadeh, P.; Aliniaeifard, S.; Esmaeili, M.; Mashal, M.; Azadegan, B.; Seif, M. Dependency of Growth, Water Use Efficiency, Chlorophyll Fluorescence, and Stomatal Characteristics of Lettuce Plants to Light Intensity. J. Plant Growth Regul. 2021, 40, 2191–2207. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Jia, B.; Zhou, G. Elevated-CO2 Response of Stomata and Its Dependence on Environmental Factors. Front. Plant Sci. 2016, 7, 657. [Google Scholar] [CrossRef]
- Lambers, H.; Oliveira, R.S. Plant Physiological Ecology, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).