Abstract
Pecan nuts (Carya illinoinensis) provide a wide range of bioactive compounds (particularly polyphenols) that improve the nutritional quality of diets. This study aimed to monitor the evolution of polyphenolic compounds (total phenols, total flavonoids, total flavanols, total condensed tannins, and total o-diphenols), the phenolic profile, the antioxidant activity, and the sugar concentration during pecan nut development in three Tunisian cultivars. Condensed tannins (41.98–221.13 mg catechin assay equivalents g−1 FW) were the dominant class of phenolics at all maturity stages, followed by total phenols (12.25–57.92 mg gallic acid equivalents g−1 FW). Ellagic acid and catechin were the most abundant phenolics at all maturity stages. The highest phenolic content and antioxidant activity were found at an early stage of ripening but as maturity progressed, a decreasing trend was observed. Sucrose (6.09–30.79 mg g−1 FW) was the predominant sugar followed by fructose and then glucose. A decreasing and later increasing trend of total carbohydrate concentration was detected during nut development. A Canonical Discriminant Analysis of the data succeeded in separating the three pecan cultivars due to their unique characteristics. Overall, the cultivar and the degree of maturity are the major factors controlling the chemical composition and antioxidant capacity of pecan nuts. This study provides more information on the optimal period when the maximum concentration of these health-enhancing compounds is found for use in food, nutraceutical, cosmetic, and pharmaceutical applications.
1. Introduction
Free radicals are potentially noxious agents causing oxidative stress. They are linked to severe damage to several macromolecules including nucleic acids, lipids, carbohydrates, and proteins [1,2]; provoke several diseases such as cancer, cardiovascular diseases, atherosclerosis, diabetes, neurodegenerative disorders, and autoimmune disorders; and are implicated in aging and other ailments [2,3]. Natural antioxidants are strong inhibitors of free radicals, and several studies have demonstrated the inverse relation between natural antioxidant intake and chronic disease appearance [1,2]. Antioxidants, such as vitamins, carotenoids, and polyphenols, are considered therapeutic substances. They are reducing agents that, in small quantities, scavenge free radicals and protect against oxidative, stress-related illnesses [1,2]. Antioxidant molecules may also be used in cosmetic, pharmaceutical, and industrial applications [3,4].
Polyphenols are the most widely dispersed and effective natural antioxidants. They exhibit antioxidant, anti-diabetic, anticancer, anti-inflammatory, cardioprotection, anti-atherosclerotic, antimicrobial, neuroprotective, and anti-aging effects [2,5].
Interestingly, pecan nuts (C. illinoinensis) are considered nutritionally valued wellness nuts. Their consumption provides a large spectrum of phenolic compounds, including flavonoids, phenols, condensed tannins, flavanols, and o-diphenols [6]. The high antioxidant potential of C. illinoinensis is mainly attributed to the abundance of condensed tannins, phenols, and flavonoids [6]. Pecan nut kernels contain high amounts of ellagic acid, catechin, and gallic acid [6] that can effectively quench free radicals [7,8,9]. The consumption of pecan nuts has risen with the growing interest of consumers in their great prophylactic and therapeutic effects [10], as they have been associated with a reduction in the risk of chronic diseases [10,11]. In addition to polyphenols, pecan nut kernels contain moderate amounts of carbohydrates, which serve as energy sources, with sucrose being the most abundant one [6]. Overall though, pecans are among the nuts with low levels of carbohydrates, suitable for a low-sugar-intake diet.
The aim of this work was, primarily, to monitor qualitatively and quantitatively the changes in phenolic profile, antioxidant capacity, and carbohydrate composition of three Tunisian cultivars, Mahan, Moore, and Burkett, during the ripening process and, secondly, to determine the optimal period when pecan nuts accumulate the maximum level of these health-promoting compounds. The knowledge achieved from evaluating the phenolic profile of pecan nuts at different stages of maturity can be used to encourage the consumption of pecan nuts as a source of health-enhancing compounds and to extend the industrial uses of these nuts as functional ingredients to formulate various healthy food products. We recently published a study about the effect of cultivar and harvest year on the composition of phytochemicals and carbohydrates of over-mature pecan nuts [6]. The current study, therefore, provides complementary information on the influence of the ripening stage on these compounds. To our knowledge, the literature on the evolution of phenolic compounds during pecan nut ripening is very scarce, while there are no reports available on their total flavanol or total o-diphenol content.
2. Materials and Methods
2.1. Reagent and Standard
HPLC-grade-quality acetone, acetic acid, acetonitrile, ethyl acetate, methanol, and 2.2-diphenly-1-picrylhydrazyl (DPPH) were purchased from Fischer Scientific (Waltham, MA, USA). Phenolic compounds (gallic acid, catechin, caffeic acid, vanillin, and ellagic acid), Trolox, Folin Ciacalteu reagent, as well as all other reagents of an analytical grade were purchased from Sigma-Aldrich (St. Louis, MI, USA).
2.2. Pecan Samples
Pecan nuts of three cultivars (Burkett, Mahan, and Moore) were harvested from a restricted zone of the INRGREF (National Institute for Research in Rural Engineering, Water and Forest) experimental farm, Mateur, northern Tunisia (Latitude: 37°15′ N, Longitude: 09°48′ E, Altitude: 5 m above sea level). Pecan nuts were harvested by hand at weekly intervals from mid-September (the beginning of pecan nut fruiting) until the last week of October (the maturity) in 2013 (Table 1). At each sampling event, the fruits were collected from the same 13-year-old trees (3 trees) from all sides around the canopy of the trees, and then they were stored at −20 °C in laminated bags until a further analysis.

Table 1.
Temporal distribution of sampling dates.
2.3. Phenolic Extraction
The extraction of phenolic compounds was performed according to the modified procedure of Kornsteiner et al. [12]. Pecan kernels (200 mg) were homogenized and extracted with 80% (v/v) acetone in deionized water (5 mL) in a water bath at 40 °C, with periodic agitation for 30 min. The homogenate was centrifuged at 4000 rpm for 6 min, the supernatant was removed, and the residue was collected and re-extracted in the same way. The two supernatants were combined and 5 mL was collected for evaluation of phenolic fractions and antioxidant activity.
2.4. Estimation of Total Phenols (TP)
Total phenol content (TPC) was assessed according to Tsantili et al. [13] with slight modifications. Gallic acid was used as a standard. An extract of each maturity stage (50 µL) was mixed with 3.95 mL of deionized water and 250 µL of Folin Ciacalteu reagent. After 1 min, 750 µL of a saturated Na2CO3 solution (20 g 100 mL−1) was added and the mixture was vortexed again and incubated for 2 h at room temperature. The absorbance was read at a wavelength of 760 nm using a spectrophotometer (Helios γ, Unicam) (Houston, TX, USA). The results were expressed as mg of gallic acid equivalents per gram of fresh weight (mg GAE g−1 FW) using a four-point calibration curve of the gallic acid standard.
2.5. Estimation of Total Flavonoids (TFoids)
The content of TFoids was determined according to the method of Bamdad et al. [14] with some modifications. Catechin was used as a standard. An extract of each maturity stage (500 µL) was mixed with 2 mL of deionized water and 150 µL of NaNO2. After 5 min, 150 µL of AlCl3 was added and the mixture was allowed to react for 6 min followed by the addition of 1 mL of NaOH (1 N) and 1.2 mL of H2O. The absorbance was determined at 510 nm. The results were expressed as mg of catechin equivalents per gram of fresh weight (mg CE g−1 FW), using a four-point calibration curve of the catechin standard.
2.6. Estimation of Total Condensed Tannins (TCT)
The TCT content was determined following a method described by de la Rosa et al. [15] using catechin as a standard. An extract of each maturity stage (0.5 mL) was added to 2.5 mL of 0.5% (w/v) vanillin in acidified methanol (4% HCl, v/v). The mixture was incubated at room temperature in the dark for 20 min. For each sample and standard, blanks were prepared for background subtraction by mixing 0.5 mL of the sample (or standard) with 2.5 mL of acidified methanol. The absorbance was read at 500 nm. The results were expressed as mg of catechin equivalents per gram of fresh weight (mg CE g−1 FW), using a four-point calibration curve of the catechin standard.
2.7. Determination of Total Flavanols (TFols)
The content of TFols was assessed according to the method of Arnous et al. [16]. Catechin was used as a standard. Approximately, 200 µL of the extract was mixed with 1 mL of chromogen reagent (100 mg of 4-dimethylaminocinnamaldehyde in 100 mL of 1 N HCl in methanol). The mixture was vortexed and then allowed to react for 10 min. The absorbance was read at 640 nm. The results were expressed as mg of catechin equivalents per gram of fresh weight (mg CE g−1 FW), using a four-point calibration curve of the catechin standard.
2.8. Estimation of Total o-Diphenols (ToDs)
The content of ToDs was measured following the procedure of Roussos and Pontikis [17]. Caffeic acid was used as a standard. An extract of each maturity stage (50 µL) was mixed with 450 µL of distilled water. After vortexing, 0.5 mL of a phosphate buffer (0.1 M, pH 5.8) was added. The mixture was vortexed again and 1 mL of Na2MoO4 5H2O 5% (w/v) was added and stirred. The mixture was allowed to react for 15 min. The absorbance was read at 370 nm. The results were expressed as mg of caffeic acid equivalents per gram of fresh weight (mg CAE g−1 FW), using a four-point calibration curve of the caffeic acid standard.
2.9. HPLC-DAD Analysis of Individual Phenolic Compounds
Extracts of phenolic compounds from each stage of maturity were evaporated to dryness under a stream of nitrogen, dissolved in 0.5 mL of methanol (HPLC grade), and filtered through a nylon syringe filter (0.45 µm) before a high-performance liquid chromatography (HPLC) analysis. Twenty microliter aliquots of the final samples were injected into an HP 1050 HPLC (Santa Clara, CA, USA) system equipped with a Supelco Discovery column (C18 5 μm, 150 × 4.6 mm) (St. Louis, MI, United States) and a diode array detector (DAD HP 1050) (Santa Clara, CA, USA).
Before the HPLC analysis, an additional hydrolysis treatment was required to verify and ensure the phenolic profile. The hydrolysis was performed according to the procedure described by Roussos and Pontikis [18]. Briefly, 0.2 mL from the final samples was hydrolyzed with 0.2 mL of 12 N HCl for 18 h at 110 °C in a reaction vial, the hydrolysate was extracted with ethyl acetate, which was then evaporated to dryness under a stream of nitrogen, and the residue was diluted in methanol, filtered, and analyzed with HPLC as described below.
A gradient of the mobile phase (solvent A: HPLC-grade water with 2% v/v acetic acid; solvent B: acetonitrile/methanol/acetic acid = 80:20:1) was used at a flow rate of 0.5 mL min−1. The gradient elution was applied as follows: 0–5 min A 95%, 25 min A 92%, 35–55 min A 80%, and at 60 min A 50%. The DAD was working at 260, 280, 325, and 350 nm. The spectra were recorded from 220 to 400 nm.
Identification of individual phenolic compounds was achieved by comparing their retention times and spectra with those of the pure standards. Quantification was achieved using the external standard method, using a four to five-point calibration curve. The results were expressed as micrograms of phenolic compound per gram of fresh weight (µg g−1 FW).
2.10. Estimation of Antioxidant Activity
2.10.1. DPPH (2,2-Diphenly-1-picrylhydrazyl) Radical Scavenging Activity
DPPH radical scavenging activity was assessed according to Ferreira et al. [19] with slight modifications. Trolox was used as a standard. A properly diluted extract (0.1 mL) of each maturity stage was mixed with 2 mL of the DPPH solution (0.1 mM) dissolved in methanol. The mixture was vortexed and allowed to stand in the dark for 60 min at room temperature. The absorbance was read at 517 nm and the results were expressed as micromoles of Trolox equivalent per gram of fresh weight (μmol TE g−1 FW), using a five-point calibration curve of Trolox standard.
2.10.2. Ferric Reducing Antioxidant Power (FRAP)
FRAP was measured as described by Benzie and Strain [20]. Trolox was used as a standard. A properly diluted extract (50 µL) of each maturity stage was mixed with 1.5 mL of a warm FRAP reagent. The mixture was kept to react for 5 min at 37 °C. The absorbance was read at 593 nm and the results were expressed in micromoles of Trolox equivalent per gram of fresh weight (μmol TE g−1 FW), using a five-point calibration curve of the Trolox standard.
2.11. Carbohydrate Analysis
The extraction and analysis of individual carbohydrates were carried out according to Roussos et al. [21]. The HPLC analysis was performed using a Waters 510 (Milford, MA 01757, USA) isocratic pump at a flow rate of 0.6 mL min−1 of water. For the separation of individual carbohydrates, a Hamilton HC-75 cation exchange column, calcium form (Ca2+) (4.1 × 250 mm, 9 µm) (Hamilton, Bonaduz, Switzerland), set at 80 °C was used and the carbohydrates were detected using a refractive index detector (HP 1047A) (Santa Clara, CA, USA). Under these conditions, the inositol + fructose peak was quantified as fructose following prior investigations on pecan nuts. Total carbohydrates were determined with the total sum of individual values of fructose, glucose, and sucrose.
2.12. Data Analysis
The experiment was designed as a completely randomized design with three replications of one tree each. The factors assayed were the cultivar (3 levels) and the sampling date (6 levels). The findings were reported as means ± standard deviation and analyzed using the analysis of variance (ANOVA). The statistical analysis was carried out using System XLSTAT (version 2020). Differences between ripening stages and cultivars were evaluated using a two-way ANOVA with Duncan’s test at the 5% significance level. Correlation coefficients were determined using Pearson’s correlation. A Canonical Discriminant Analysis (CDA) was carried out on analytical data using all available data (phenolic and carbohydrate concentration and antioxidant capacity) to examine possible discrimination of pecan nut cultivars based on their biochemical composition. Three biological replications were used in all assays and two technical ones per biological sample.
3. Results and Discussion
3.1. Variations in Different Classes of Phenolics during Pecan Nut Maturation
Pecan nuts were harvested as unripe (20 weeks after flowering date (WAFD), 21 WAFD, and 22 WAFD) when the exocarp (husk) was green and close; half-ripe (23 WAFD and 24 WAFD) when the husk was green and open, revealing the seed, which included the endocarp (shell) enclosing the kernel; and fully ripe (25 WAFD). Pecan nuts were considered fully ripe in autumn, in late October/early November, when the husk was dry, brown, and fully opened. The variations in total phenol, total flavonoid, total flavanol, total condensed tannin, and total o-diphenol concentration in Mahan, Moore, and Burkett cultivars are displayed in Table 2. All classes of polyphenols were found at all maturity stages. Changes in the level of total phenols (TP) during the ripening of C. illinoinensis were obvious, as seen in Table 2.

Table 2.
Changes of total phenol, total flavonoid, total flavanol, total condensed tannin, and total o-diphenol concentrations during the ripening of three cultivars.
The trends of TP accumulation were similar in Moore and Burkett cultivars. Total phenols were detected at the highest levels in immature pecan nuts (20 WAFD). Afterward, their amounts dropped significantly from 102.59 to 12.25 mg GAE g−1 FW and from 54.64 to 10.59 mg GAE g−1 FW, in Burkett and Moore cultivars, respectively. However, the Mahan cultivar revealed an accumulation pattern different from the other cultivars. Indeed, TP content increased significantly from 16.53 mg GAE g−1 FW to reach an utmost content of 22.61 mg GAE g−1 FW at 22 WAFD and then declined substantially to attain a lower concentration (13.92 mg GAE g−1 FW) at maturity. Jia et al. [22] evaluated the TP content throughout the ripening process in five pecan nut cultivars and they also reported a decreasing pattern. Quantitatively, the values presented by these authors were expressed on a defatted kernel weight basis (using ellagic acid as a standard) so it was not feasible to establish a direct comparison with the present results.
A similar decline in the level of total phenols during maturation has also been observed in walnut (Juglans regia L.) kernels [23,24,25,26], in cashews (Anacardium occidentale L.) [27], and in peaches (Prunus persica) [28] but not in hazelnuts [24], indicating the effect of species on the evolution of secondary metabolites in the nut.
In addition to ripeness, the variation in the level of TP was strongly influenced by the cultivar. Interestingly, the Mahan cultivar showed the lowest TP content at an early stage but the highest one at maturity, similar to the report of Ferrari et al. [29], where Mahan presented a high level of phenols, higher than that of many other cultivars. On the other hand, the unripe Burkett cultivar had the highest TP level among cultivars. This cultivar-dependent difference is in agreement with the findings of earlier studies [22,29,30] as well as studies on walnuts [25]. The findings are consistent with the earlier data found for the Mahan cultivar [6], suggesting that Mahan possesses the greatest total phenol content among over-mature nuts picked in the second week from husk split, after the seed had fallen to the ground (26 WAFD). Kornsteiner et al. [12] assessed the TP content in various nuts and reported that walnuts contain a high concentration of the total phenol level, followed by pecan nuts, while other nuts like pistachio nuts, hazelnuts, and almonds contain lower concentrations of these bioactive molecules.
Pecan nut kernels were found to be a significant source of total phenols, particularly at an early stage of growth. Indeed, stages 20, 22, and 24 WAFD presented high levels of total phenols and could have potential industrial and cosmetic applications.
Similarly to total phenols, the ripening process significantly affected the total flavonoids’ (TFoids) concentration too (Table 2). The trends of TFoids’ accumulation displayed some variations among the three cultivars. In the Mahan cultivar, the highest level (12.74 mg CE g−1 FW) was detected at an intermediate stage (22nd WAFD). In Moore and Burkett cultivars, the greatest amounts were detected at an early stage (20th WAFD). But, these contents decreased significantly from 66.81 to 8.17 mg CE g−1 FW in the Burkett cultivar, reflecting an 8.2-fold difference, and from 34.70 to 8.19 mg CE g−1 FW in the Moore cultivar, reflecting a 4.2-fold difference. Jia et al. [22] have also reported a decrease in flavonoid content during the development of various pecan nut cultivars. Quantitatively, our findings cannot be compared with the published data of Jia et al. [22], because of variations in extraction methods and analytical procedures. Similar behavior was also observed during the ripening of walnuts (Juglans regia L.) [25], pistachio nuts (Pistacia vera L.) [31], and peach (Prunus persica L.) mesocarp [32].
The variability in flavonoids’ level was also substantially affected by the cultivar, which is in agreement with a prior study [22]. The immature Burkett cultivar had TFoids’ content 1.9- to 6.9-fold higher than that of immature Moore and Mahan cultivars, respectively. At maturity, the highest flavonoid content was recorded in the Mahan cultivar though.
The first stage of maturity, irrespective of the cultivar, was the one characterized by the greatest level of flavonoids. This stage (20 WAFD) presented substantial levels of natural flavonoids and could be of interest for exploitation in pharmaceutical and cosmetic applications.
Until now, no reports are available on how the levels of flavanols change during pecan nut ripening. The patterns of total flavanols’ (TFols) accumulation showed variations among the three cultivars (Table 2). Moore and Burkett cultivars exhibited substantial initial levels of flavanols, which underwent a significant decline from 12.36 to 2.27 mg CE g−1 FW in the Moore cultivar and from 27.48 to 3.60 mg CE g−1 FW in the Burkett cultivar. Overall, TFols were 5.4- to 7.6-fold higher at an early stage compared to maturity, in Moore and Burkett cultivars, respectively. Nevertheless, the TFols showed slight fluctuations during the development of the Mahan cultivar. The highest level (5.91 mg CE g−1 FW) was detected at an intermediate stage (22 WAFD). Such a decreasing trend of total flavanols has also been reported in apples [33].
The early stages exhibited high levels of flavanols and therefore could have potential nutraceutical, cosmetic, and pharmaceutical uses. Moreover, the change in flavanol concentration during ripening was highly dependent on the cultivar. The immature Burkett cultivar exhibited a level of TFols 2.2- to 6.2-fold higher than that of immature Moore and Mahan cultivars, respectively, while the Mahan cultivar reached its highest value at the mature stage.
To the best of our knowledge, there have been no prior studies on developmental changes in o-diphenol content during pecan nut maturation. Important changes occurred in ToDs’ content during nut development (Table 2). The trends of ToDs’ evolution exhibited differences among the three cultivars. In Burkett and Moore cultivars, the highest levels were detected at the first stage (20th WAFD). However, the concentration decreased significantly from 21.15 to 1.77 mg CAE g−1 FW in the Burkett cultivar and from 9.98 to 2.08 mg CAE g−1 FW in the Moore cultivar. Overall, ToDs were 4.8- to 11.9-fold higher at the immature stage compared to the mature stage, in Moore and Burkett cultivars, respectively. However, the ToDs’ concentration exhibited slight fluctuations during the maturation of the Mahan cultivar. The regressive tendency of total o-diphenols during ripening has also been found in peaches (Prunus persica L.) [28]. Along with ripeness, the level of ToDs varied significantly among cultivars. The o-diphenol content was 2.1- to 10.5-fold higher in unripe Burkett compared to unripe Moore and Mahan cultivars, respectively, whereas the Mahan cultivar showed the highest value at maturity.
Based on the results of this study, it seems that total phenols, flavonoids, o-diphenols, and flavanols in pecan nut kernels are actively synthesized at an early stage, which according to Machado et al. [34] may be explained with the substantial activity of phenylalanine ammonia-lyase (PAL), the regulatory enzyme of the biosynthesis of phenylpropanoids. The great content of these polyphenols in C. illinoinensis at an early stage may be also attributed to their involvement in the self-defense mechanisms against pathogen invasion and predators [32]. Throughout the ripening process, the level of these compounds decreased significantly. In the current study, the concentrations of total phenols, total flavonoids, and total flavanols at complete maturity (25 WAFD) were very close to those reported in our previous work [6], performed on over-mature nuts. This substantial decline could be related to either a reduction in PAL activity [34,35] or to elevated catabolism. The correlation between polyphenol levels and PAL activity during maturation has already been observed [34], justifying the previous assumption.
Condensed tannins were the dominant class of polyphenols at each maturity stage. Major variations in the concentration of total condensed tannins (TCT) were observed during the development of C. illinoinensis (Table 2). The tendency of condensed tannin evolution was similar in Burkett and Moore cultivars. Their concentration decreased significantly from 413.37 to 36.12 mg CE g−1 FW, reflecting an 11.4-fold difference, and from 186.13 to 32.79 mg CE g−1 FW, reflecting a 5.7-fold difference, in Burkett and Moore cultivars, respectively. However, the Mahan cultivar displayed a different accumulation pattern, as the condensed tannins accumulated significantly from 63.89 mg CE g−1 FW to the maximum of 95.00 mg CE g−1 FW at 22 WAFD and then decreased significantly to a lower content (57.04 mg CE g−1 FW) at maturity. The significant decrease in condensed tannins can be explained with the fact that these substances play a crucial role in the defense of fruits against pathogens at an early stage of maturity [35,36]. Throughout ripening, the decline of these components could be attributed to the fact that they may form complexes with proteins, metal ions, and other compounds [37,38,39]. The reducing trends of TCT observed in this study are similar to those already reported by Jia et al. [22] in C. illinoinensis. In this latter study, the values were expressed on a defatted kernel weight basis so it is difficult to establish a quantitative comparison to our fresh weight basis. Following our present data, many studies have also shown reducing tendencies of TCT during the maturation of different fruits, such as pistachio nuts [40] and peach pulps [35].
The level of TCT differed significantly among cultivars, which is coherent with prior results on pecan nut cultivars [22,29,30,41,42]. The highest amount of condensed tannins was detected in the unripe Burkett cultivar, which was six times greater than that in the unripe Mahan cultivar, which presented the lowest concentration. The first stage of maturity (20 WAFD) may be considered a valuable source of total condensed tannins for nutraceutical, industrial, and pharmaceutical interests.
At maturity, TCT contents were surprisingly low when compared with our previous study [6]. This may be attributed to the maturity degree since our prior study was carried out on over-mature fruits and since the effects of geographical and climatic factors were eliminated.
3.2. Variations in Individual Phenolic Compounds during Pecan Nut Maturation
Identification of the individual phenolics present in Mahan, Moore, and Burkett cultivars was carried out using the HPLC-DAD technique and five phenolic compounds were identified in pecan nut kernels—gallic acid, catechin, epicatechin, an ellagic acid derivative, and ellagic acid (Figure 1)—similar to that reported by Flores-Cordova et al. [42]. The hydrolysis process proved to be efficient for the detection, confirmation, and quantification of the ellagic acid derivative, as described previously [6]. All compounds were found at all ripening stages. Individual phenolic compounds varied greatly during pecan nut development (Table 3).
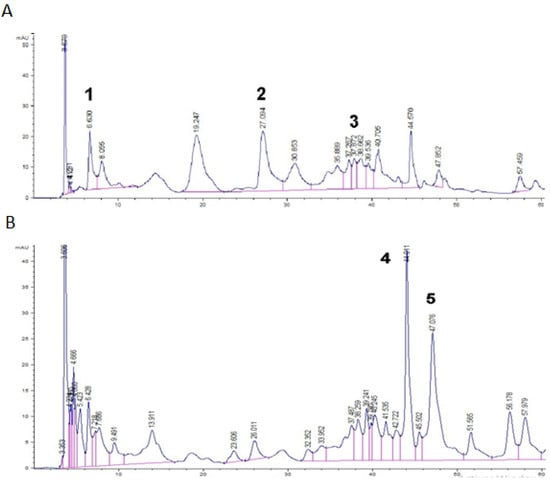
Figure 1.
HPLC chromatograms of Carya illinoinensis recorded at 280 nm (A) and 260 nm (B). Peak identification: (1) gallic acid; (2) catechin; (3) epicatechin; (4) ellagic acid derivative; and (5) ellagic acid.

Table 3.
Changes of individual phenolic concentrations during the ripening of three cultivars.
Overall, catechin and ellagic acid were the major phenolic compounds at all maturity stages. The highest amounts of individual phenolics were generally detected in immature pecan nuts. At an early stage, catechin was the predominant compound in Moore (1733.73 µg g−1 FW) and Burkett (3372.64 µg g−1 FW) cultivars, whereas ellagic acid was the major one with a concentration of 624.00 µg g−1 FW in the Mahan cultivar. During ripening, both compounds decreased significantly. At maturity, ellagic acid became the major one in the three cultivars. Regarding the ellagic acid derivative, gallic acid, and epicatechin, their concentration also exhibited a decreasing pattern in Moore and Burkett cultivars. However, they increased until intermediate-maturity stages and then declined until complete maturity in the Mahan cultivar. It is important to notice that the epicatechin level was very high at the early stage of maturity, especially in the Burkett cultivar (390.83 µg g−1 FW). This amount decreased almost 128.6-fold, reaching 3.04 µg g−1 FW at maturity. Epicatechin exhibits several biological properties, notably antioxidant, anti-inflammatory, anti-tumor, anti-diabetic, antiviral, and antibacterial activities [43,44]. It also prevents cardiovascular and cerebrovascular diseases, as well as metabolic disorders, and protects against neurodegenerative diseases [44,45], indicating that nuts at early stages of maturation could be a valuable source of this nutraceutical.
Our findings are in agreement, to a certain extent, with previous data obtained on five cultivars of pecan nuts [22], reporting the decreasing patterns of individual phenolic compounds as well as with that reported by Persic et al. [24] and Pycia et al. [25] in walnuts. The decline of individual phenolics during maturation could be due to limitations of the primary metabolism in mature fruits, inducing a deficiency of substrates that is fundamental for the biosynthesis of phenolic compounds [46]. On the other hand, this decrease could be related to conjugation, polymerization, and/or oxidation reactions occurring during ripening [32,47].
According to Bashir et al. [48], the decrease in phenolic compounds during ripening in the guava edible part was associated with increased polymerization and hydrolysis of tannins, which is associated with a decrease in astringency, while the utilization of phenolic compounds as substrates for the biosynthesis of other compounds cannot be excluded. On the other hand, the peel presented higher values of phenolic compounds during ripening, which the authors attributed to the need for efficient protection against pests and diseases. This could also be the case in pecan nuts, although we did not measure husk and shell phenolic compounds.
Furthermore, our findings showed that the change in phenolic profile during maturity was substantially affected by cultivars and that the predominance of specific phenolic compounds depended both on the maturity stage as well as on the cultivar.
In the present study, the values of individual phenolic compounds found at maturity were considerably lower than those reported previously [6]. This difference might be explained with the maturity stage.
3.3. Variations in Antioxidant Activity during Pecan Nut Maturation
Several methods are used to evaluate the antioxidant capacity of a plant extract. In the current study, DPPH and FRAP assays were employed to assess the antioxidant activity during the ripening of the three pecan nut cultivars, similar to Wei et al. [26] and Villarreal-Lozoya et al. [41].
The greatest antioxidant activity based on the DPPH assay was detected at early stages (Table 4). The radical scavenging activity varied from 382.41 to 130.20 μmol TE g−1 FW in the Moore cultivar, reflecting a 2.9-fold difference, and from 451.82 to 132.90 μmol TE g−1 FW in the Burkett cultivar, reflecting a 3.4-fold difference. However, the radical scavenging activity in the Mahan cultivar increased from 198.60 to 229.87 μmol TE g−1 FW at 22 WAFD and then decreased to 191.24 87 μmol TE g−1 FW at maturity. Likewise, the highest antioxidant activities based on the FRAP assay were 396.17 and 686.95 μmol TE g−1 FW at an early stage (20 WAFD) then dropped to lower values of 94.05 and 89.58 μmol TE g−1 FW for Moore and Burkett cultivars, respectively, at maturity.

Table 4.
Changes of antioxidant activity (expressed as μmol TE g−1 FW) during the ripening of three cultivars.
According to Pycia et al. [25] working with walnuts, the climatic and soil conditions, the cultivar, the conditions of harvesting and storage of nuts, as well as the extraction method of antioxidant molecules have a great role in the determination of these bioactive compounds and therefore in the estimation of the antioxidant capacity of the nut.
The decline in the antioxidant capacity of C. illinoinensis during maturation could be related to the decrease in polyphenolic levels. This trend has also been observed in Chinese pecan nuts [22] as well as in pistachio nuts [40] and walnuts [23,25,26].
The antioxidant activity, estimated with both DPPH and FRAP assays, greatly varied among cultivars too. The mature Mahan nuts showed the strongest antioxidant activity among cultivars, regarding the same maturity stage. Nevertheless, the immature Burkett cultivar recorded the highest antioxidant activity among the three cultivars at the immature stage. This cultivar-dependent difference is in accordance with previous studies on pecan nuts [22,30,41,42].
Positive and significant correlations have been previously observed between phenolic compounds and antioxidant activity [6,26,29,41,42,49]. Here also, the antioxidant activity assessed with DPPH and FRAP was closely correlated with the concentration of polyphenols (Figure 2).
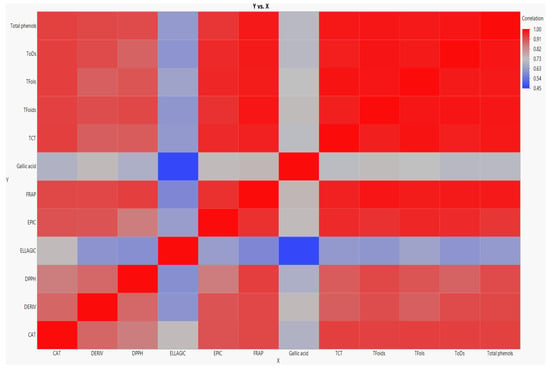
Figure 2.
Heat map of Pearson’s correlation coefficient among phenolics and DPPH and FRAP assays. Gradient color barcodes at the right indicate the minimum value in blue and the maximum in red.
The TP and TFoid contents displayed the greatest positive correlation with DPPH (r = 0.95) and FRAP (r = 0.99) methods (Figure 2), as also reported by Fu et al. [49] in walnuts. On the other hand, weaker relations were detected for TFols, ToDs, and TCT (Figure 2). Among the individual phenolics detected, the strongest linear correlation with antioxidant capacity was noticed for epicatechin (r = 0.82 and r = 0.95 for DPPH and FRAP assays, respectively) followed by the ellagic acid derivative. The lowest one was recorded for ellagic acid (r = 0.58 and r = 0.63 for DPPH and FRAP methods, respectively) (Figure 2). It is noteworthy that correlations of polyphenols and individual phenolics with FRAP were greater than with DPPH.
Overall, TP, TFoids, epicatechin, and the ellagic acid derivative were likely the main contributors to the potent antioxidant activity of C. illinoinensis, even though TCT, ellagic acid, and catechin were the main compounds. These findings are partially in concordance with the previous study of Jia et al. [22], who found that TP, catechin, and epicatechin exhibited the greatest relations with antioxidant activity. Linear correlations between antioxidant capacity and total phenols and flavonoids have also been found in various nuts in the literature [13,25,50].
It seems thus that pecan nut kernels could be a valuable source of natural antioxidants with great industrial and pharmaceutical importance, especially at an early stage of maturation.
3.4. Variations in Carbohydrates during Pecan Nut Development
Carbohydrate composition varied significantly during pecan nut development (Table 5). The highest amounts of carbohydrates were detected at an early stage (20 WAFD). The sucrose concentration decreased significantly from 32.56 mg g−1 FW in the Mahan cultivar and from 30.40 mg g−1 FW in the Burkett cultivar to reach a minimum of 6.05 mg g−1 FW and 5.47 mg g−1 FW in Mahan and Burkett cultivars, respectively, at 22 WAFD and then increased significantly to achieve higher contents at maturity. The same pattern was observed in the Moore cultivar but with lower content (5.99 mg g−1 FW) detected at 21 WAFD. A different pattern was found by Singanusong et al. [51] who reported that the sucrose content increased throughout the ripening process.

Table 5.
Changes of carbohydrate composition (expressed as mg g−1 FW) during the ripening of three cultivars.
The amounts of fructose and glucose showed the same regressive pattern in Moore and Burkett cultivars (Table 5). Their concentration decreased significantly from immature to mature fruits. However, the Mahan cultivar exhibited a different evolution pattern. Indeed, the contents increased significantly to reach a maximum at an intermediate ripening stage (23 WAFD) and then dropped significantly to attain lower contents at the last stage.
In all three cultivars, the evolution trend of total carbohydrates was similar, to a certain extent, to that of sucrose. Indeed, the total carbohydrate concentration decreased significantly to reach a minimum at the intermediate ripening stage and then increased significantly to attain higher contents at the last stage. This trend could be explained with the accumulation of total lipids during maturation [51,52], which requires a great level of energy supplied from the existing sugars [53]. Our findings are different from those of Wood and McMeans [52] who reported a decreasing tendency of total carbohydrates during pecan nut ripening. Such a decreasing-increasing trend of total carbohydrates during development has also been reported in hazelnuts by Cristofori et al. [54].
At the first stage of maturity (20 WAFD), sucrose was the major carbohydrate in Moore and Mahan cultivars except for the Burkett cultivar, where fructose was the predominant one. During maturation, both compounds varied significantly. At the last stage, sucrose became the major one in all three cultivars. The Burkett cultivar exhibited the highest total carbohydrate content at an early stage but the lowest one at maturity. These findings revealed that the predominance of specific forms of carbohydrates depended on the degree of maturity and cultivar of pecan nuts.
Compared to our prior study [6], the levels of carbohydrates found, here, at the maturity stage were different, probably because the previous study was carried out on over-mature fruits.
3.5. Canonical Discriminant Analysis (CDA)
A Canonical Discriminant Analysis (CDA) was employed to examine all the analytical data concurrently and to assess the effect of the cultivar. Moore and Mahan cultivars were closely located (at the positive side of Function 1), while the Burkett cultivar was well segregated at the negative side of Function 1 (Figure 3), which could be attributed to the high levels of some of the phytochemicals assayed during the first stage and their subsequent, significant decrease during maturation.
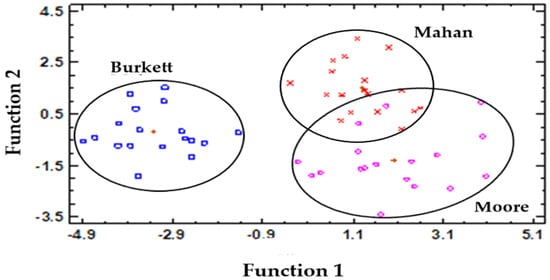
Figure 3.
Scatterplot of the canonical discriminant functions of the three pecan nut cultivars based on the analytical biochemical data.
Therefore, the biochemical parameters assayed in this study could be used to distinguish Burkett, Mahan, and Moore cultivars. Overall, the CDA effectively separated Burkett from the other cultivars, based on the measured variables of the present trial.
In the literature, a discriminant analysis has also been applied to differentiate the cultivars, years, and provenance of several nuts [55,56,57] with variable efficiency.
4. Conclusions
Polyphenols, antioxidant capacity, and sugars were qualitatively and quantitatively influenced by the cultivar and maturity stage. During the development of pecan nuts, three trends of polyphenols were found: (i) decrease during maturation, (ii) slight fluctuations during maturation (flavanols and o-diphenols), and (iii) increase reaching an utmost level at the intermediate ripening stage. Based on our results, Burkett and Moore cultivars displayed identical evolution trends during their development, while the Mahan cultivar behaved differently. Furthermore, a decreasing-increasing trend of total carbohydrates was also detected. The highest amounts of polyphenols, antioxidant activity, and sugars were found in the nuts of the Burkett cultivar at an early stage and of the Mahan cultivar at maturity. The CDA efficiently separated Burkett from the other two cultivars, based on the data of biochemical analyses (i.e., phenolics, antioxidant capacity, and carbohydrates during the different developmental stages). The present findings indicate that immature pecan nuts are a valuable natural source of antioxidants to be used for nutraceutical, cosmetic, and pharmaceutical purposes. Therefore, the harvest period must be planned rigorously to obtain the greatest amount of bioactive compounds. For the future, more research is deemed necessary to assess the effect of the genotype, the maturity stage, the agronomical practices employed, as well as the extraction techniques on all the parameters analyzed in this trial as well as on vitamins and pecan nut oil, which also have a great impact on human health.
Author Contributions
Conceptualization, I.B. and S.B.; methodology, I.B. and P.A.R.; software, I.B. and P.A.R.; validation, I.B. and P.A.R.; formal analysis, I.B., A.T. and E.N.; investigation, I.B. and P.A.R.; resources, A.A.; data curation, I.B.; writing—original draft, I.B.; writing—review and editing, P.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Tunisian Ministry of Higher Education and Scientific Research.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We gratefully thank the National Institute of Meteorology for providing information about the region of Mateur.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.N.V.R. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Paniwnyk, L.; Hassan, S. Polyphenols as natural antioxidants: Sources, extraction and applications in food, cosmetics and drugs. In Plant Based “Green Chemistry 2.0”; Li, Y., Chemat, F., Eds.; Green Chemistry and Sustainable Technology; Springer: Singapore, 2019; pp. 197–235. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Bouali, I.; Tsafouros, A.; Ntanos, E.; Albouchi, A.; Boukhchina, S.; Roussos, P.A. Inter cultivar and temporal variation of phenolic compounds, antioxidant activity and carbohydrate composition of pecan (Carya illlinoinensis) kernels grown in Tunisia. Hortic. Environ. Biotechnol. 2020, 61, 183–196. [Google Scholar] [CrossRef]
- Hayes, J.E.; Allen, P.; Brunton, N.; O’Grady, M.N.; Kerry, J.P. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: Olive leaf extract (Olea europaea L.), lutein, sesamol and ellagic acid. Food Chem. 2011, 126, 948–955. [Google Scholar] [CrossRef]
- Colon, M.; Nerin, C. Role of catechins in the antioxidant capacity of an active film containing green tea, green coffee, and grapefruit extracts. J. Agric. Food Chem. 2012, 60, 9842–9849. [Google Scholar] [CrossRef]
- Rajan, V.K.; Muraleedharan, K. A computational investigation on the structure, global parameters and antioxidant capacity of a polyphenol, Gallic acid. Food Chem. 2017, 220, 93–99. [Google Scholar] [CrossRef]
- Atanosov, A.G.; Sabharanjak, S.M.; Zengin, G.; Mollica, A.; Szostak, A.; Simirgiotis, M.; Huminiecki, Ł.; Horbanczuk, O.K.; Nabavi, S.M.; Mocan, A. Pecan nuts: A review of reported bioactivities and health effects. Trends Food Sci. Technol. 2017, 71, 246–257. [Google Scholar] [CrossRef]
- Rajaram, S.; Burke, K.; Connell, K.; Myint, T.J.; Sabate, J. A monounsaturated fatty acid-rich pecan enriched diet favorably alters the serum lipid profile of healthy men and women. J. Nutr. 2001, 131, 2275–2279. [Google Scholar] [CrossRef]
- Kornsteiner, M.; Wagner, K.H.; Elmadfa, I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006, 98, 381–387. [Google Scholar] [CrossRef]
- Tsantili, E.; Konstantinidis, K.; Christopoulos, M.V.; Roussos, P.A. Total phenolics and flavonoids and total antioxidant capacity in pistachio (Pistachia vera L.) nuts in relation to cultivars and storage conditions. Sci. Hortic. 2011, 129, 694–701. [Google Scholar] [CrossRef]
- Bamdad, F.; Kadivar, M.; Keramat, J. Evaluation of phenolic content and antioxidant activity of Iranian caraway in comparison with clove and BHT using model systems and vegetable oil. Int. J. Food. Sci. Technol. 2006, 41, 20–27. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Alvarez-Parrilla, E.; Shahidi, F. Phenolic compounds and antioxidant activity of kernels and shells of Mexican pecan (Carya illinoinensis). J. Agric. Food Chem. 2011, 59, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Roussos, P.A.; Pontikis, C.A. Phenolic compounds in olive explants and their contribution to browning during the establishment stage in vitro. Gartenbauwissenschaft 2001, 66, 298–303. [Google Scholar]
- Roussos, P.A.; Pontikis, C.A. Changes of free, soluble conjugated and bound polyamine titers of jojoba explants under sodium chloride salinity in vitro. J. Plant Physiol. 2007, 164, 895–903. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth. Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Roussos, P.A.; Vemmos, S.N.; Pontikis, C.A. The role of carbohydrates on the salt tolerance of Jojoba [Simmondsia chinensis (Link)] expiants in vitro. Eur. J. Hortic. Sci. 2005, 70, 278–282. [Google Scholar]
- Jia, X.; Luo, H.; Xu, M.; Zhai, M.; Guo, Z.; Qiao, Y.; Wang, L. Dynamic changes in phenolics and antioxidant capacity during pecan (Carya illinoinensis) kernel ripening and its phenolics profiles. Molecules 2018, 23, 435. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Masoodi, F.A.; Baba, W.N.; Khan, A.A.; Ganie, B.A. Effect of different ripening stages on walnut kernel quality: Antioxidant activities, lipid characterization and antibacterial properties. J. Food Sci. Technol. 2017, 54, 3791–3801. [Google Scholar] [CrossRef] [PubMed]
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Solar, A.; Veberic, R. Changes in phenolic profiles of red-colored pellicle walnut and hazelnut kernel during ripening. Food Chem. 2018, 252, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Pycia, K.; Kapusta, I.; Jaworska, G. Impact of the degree of maturity of walnuts (Juglans regia L.) and their variety on the antioxidant potential and the content of tocopherols and polyphenols. Molecules 2019, 24, 2936. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Li, Y.; Sun, D.; Chen, Q.; Fu, M.; Zhao, H.; Chen, X.; Huang, Y.; Xu, H. Odor, tastes, nutritional compounds and antioxidant activity of fresh-eating walnut during ripening. Sci. Hortic. 2022, 293, 110744. [Google Scholar] [CrossRef]
- Lopes, M.M.A.; Miranda, M.R.A.; Moura, C.F.H.; Filho, J.E. Bioactive compounds and total antioxidant capacity of cashew apples (Anacardium occidentale L.) during the ripening of early dwarf cashew clones. Ciênc. Agrotecnol. 2012, 36, 325–332. [Google Scholar] [CrossRef]
- Dabbou, S.; Lussiana, C.; Maatallah, S.; Gasco, L.; Hajlaoui, H.; Flamini, G. Changes in biochemical compounds in flesh and peel from Prunus persica fruits grown in Tunisia during two maturation stages. Plant Physiol. Biochem. 2016, 100, 1–11. [Google Scholar] [CrossRef]
- Ferrari, V.; Gil, G.; Heinzen, H.; Zoppolo, R.; Ibáñez, F. Influence of Cultivar on Nutritional Composition and Nutraceutical Potential of Pecan Growing in Uruguay. Front. Nutr. 2022, 9, 868054. [Google Scholar] [CrossRef]
- Prabhakar, H.; Sharma, S.; Kong, F. Effects of Postharvest Handling and Storage on Pecan Quality. Food Rev. Int. 2020, 38, 1485–1512. [Google Scholar] [CrossRef]
- Ballistreri, G.; Arena, E.; Fallico, B. Influence of ripeness and drying process on the polyphenols and tocopherols of Pistacia vera L. Molecules 2009, 14, 4358–4369. [Google Scholar] [CrossRef]
- Guizani, M.; Maatallah, S.; Dabbou, S.; Serrano, M.; Hajlaoui, H.; Helal, A.N.; Kilani-Jaziri, S. Physiological behaviors and fruit quality changes in five peach cultivars during three ripening stages in a semi-arid climate. Acta Physiol. Plant. 2019, 41, 154. [Google Scholar] [CrossRef]
- Bizjak, J.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R. Changes in primary metabolites and polyphenols in the peel of “Braeburn” apples (Malus domestica Borkh.) during advanced maturation. J. Agric. Food Chem. 2013, 61, 10283–10292. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Felizardo, C.; Fernandes-Silva, A.A.; Nunes, F.M.; Barros, A. Polyphenolic compounds, antioxidant activity and l-phenylalanine ammonia-lyase activity during ripening of olive cv. “Cobrançosa” under different irrigation regimes. Food Res. Int. 2013, 51, 412–421. [Google Scholar] [CrossRef]
- Belhadj, F.; Somrani, I.; Aissaoui, N.; Messaoud, C.; Boussaid, M.; Marzouki, M.N. Bioactive compounds contents, antioxidant and antimicrobial activities during ripening of Prunus persica L. varieties from the North West of Tunisia. Food Chem. 2016, 204, 29–36. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Ul-Haq, I.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Assis, J.S.; Maldonado, R.; Muñoz, T.; Escribano, M.I.; Merodio, C. Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Analysis of tannins in seeds and skins of Shiraz grapes throughout berry development. Aust. J. Grape Wine Res. 2003, 9, 15–27. [Google Scholar] [CrossRef]
- Zeng, X.; Du, Z.; Sheng, Z.; Jiang, W. Characterization of the interactions between banana condensed tannins and biologically important metal ions (Cu2+, Zn2+ and Fe2+). Food Res. Int. 2019, 123, 518–528. [Google Scholar] [CrossRef]
- Zarei, M.; Davarynejad, G.; Abedi, B.; Kafi, M.; Biabani, A. Changes in physical properties, chemical composition and antioxidant activity of four pistachio cultivars at ten maturity stages. Adv. Environ. Biol. 2014, 8, 106–115. [Google Scholar]
- Villarreal-Lozoya, J.E.; Lombardini, L.; Cisneros-Zevallos, L. Phytochemical constituents and antioxidant capacity of different pecan [Carya illinoinensis (Wangenh.) K. Koch] cultivars. Food Chem. 2007, 102, 1241–1249. [Google Scholar] [CrossRef]
- Flores-Córdova, M.A.; Sánchez, E.; Muñoz-Márquez, E.; Ojeda-Barrios, D.L.; Soto-Parra, J.M.; Preciado-Rangel, P. Phytochemical composition and antioxidant capacity in Mexican pecan nut. Emir. J. Food Agric. 2017, 29, 346–350. [Google Scholar] [CrossRef]
- Martín, M.Á.; Fernández-Millán, E.; Ramos, S.; Bravo, L.; Goya, L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol. Nutr. Food Res. 2013, 58, 447–456. [Google Scholar] [CrossRef]
- Qu, Z.; Liu, A.; Li, P.; Liu, C.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Advances in physiological functions and mechanisms of (−)-epicatechin. Crit. Rev. Food Sci. Nutr. 2020, 61, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Bernatova, I. Biological activities of (−)-epicatechin and (−)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Seraglio, S.K.T.; Schulz, M.; Nehring, P.; Betta, F.D.; Valese, A.C.; Daguer, H.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Nutritional and bioactive potential of Myrtaceae fruits during ripening. Food Chem. 2018, 239, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Gruz, J.; Ayaz, F.A.; Torun, H.; Strnad, M. Phenolic acid content and radical scavenging activity of extracts from medlar (Mespilus germanica L.) fruit at different stages of ripening. Food Chem. 2011, 124, 271–277. [Google Scholar] [CrossRef]
- Bashir, H.A.; Abu-Goukh, A.B.A. Compositional changes during guava fruit ripening. Food Chem. 2003, 80, 557–563. [Google Scholar] [CrossRef]
- Fu, M.; Qu, Q.; Yang, X.; Zhang, X. Effect of intermittent oven drying on lipid oxidation, fatty acids composition and antioxidant activities of walnut. LWT Food Sci. Technol. 2016, 65, 1126–1132. [Google Scholar] [CrossRef]
- Yang, J.; Liu, R.H.; Halim, L. Antioxidant and antiproliferative activities of common edible nut seeds. LWT Food Sci. Technol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Singanusong, R.; Mason, R.L.; D’Arcy, B.R.; Nottingham, S.M. Compositional changes of Australia-grown Western Schley pecans [Carya illinoinensis (Wangenh.) K. Koch] during Maturation. J. Agric. Food Chem. 2003, 51, 406–412. [Google Scholar] [CrossRef]
- Wood, B.W.; McMeans, J.L. Carbohydrates and fatty acids in developing pecan fruit. J. Am. Soc. Hortic. Sci. 1982, 107, 47–50. [Google Scholar] [CrossRef]
- Yang, B.M.; Yao, L.X.; Li, G.L.; He, Z.H.; Zhou, C.M. Dynamic changes of nutrition in litchi foliar and effects of potassium–nitrogen fertilization ratio. J. Soil Sci. Plant Nutr. 2015, 15, 98–110. [Google Scholar] [CrossRef]
- Cristofori, V.; Bertazza, G.; Bignami, C. Changes in kernel chemical composition during nut development of three Italian hazelnut cultivars. Fruits 2015, 70, 311–322. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Morais, C.L.M.; Lima, K.M.G.; Leite, G.W.P.; Oliveira, G.S.; Casagrande, I.P.; Neto, J.P.S.; Teixeira, G.H.A. Using Intact Nuts and Near Infrared Spectroscopy to Classify Macadamia Cultivars. Food Anal. Methods 2017, 11, 1857–1866. [Google Scholar] [CrossRef]
- Cristofori, V.; Ferramondo, S.; Bertazza, G.; Bignami, C. Nut and kernel traits and chemical composition of hazelnut (Corylus avellana L.) cultivars. J. Sci. Food Agric. 2008, 88, 1091–1098. [Google Scholar] [CrossRef]
- Amorello, D.; Orecchio, S.; Pace, A.; Barreca, S. Discrimination of almonds (Prunus dulcis) geographical origin by minerals and fatty acids profiling. Nat. Prod. Res. 2015, 30, 2107–2110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).