Abstract
Garden cress is a vegetable crop in the Brassicaceae family that is appreciated for its nutraceutical and taste-giving components in minimally processed food chains. Due to its very short cycle, which depends on the range of production from microgreens to baby-leaf vegetables, this crop is threatened by soil-borne pathologies developing within the initial stages of germination and emergence. This study aims to evaluate the suppressive bio-compost as an innovative means to counteract the main telluric diseases of garden cress and reduce the risks of yield loss by adopting sustainable remedies and decreasing the dependence on synthetic fungicides. Therefore, eleven green composts obtained using both previously distilled and raw aromatic plant residues were analyzed for suppressive properties against Rhizoctonia solani and Sclerotinia sclerotiorum on sown garden cress. The biological active component of the composts, detected by CO2-release, FDA-hydrolysis and microbial counts, proved to be indispensable for pathogen control in vitro and in vivo, as demonstrated by the loss of suppressiveness after sterilization. Cross-polarization magic angle spinning 13C-nuclear magnetic resonance (CP-MAS-13C-NMR) was used to analyze the molecular distribution of organic C in composts. The results indicated the suitability of the feedstock used to make quality compost. The suppression levels shown by composts P1 (40% wood chips, 30% escarole and 30% a mixture of sage, basil, mint and parsley) and P2 (40% wood chips, 30% escarole and 30% a mixture of essential oil-free sage, basil and rosemary) are promising for the sustainable, non-chemical production of garden cress vegetables.
1. Introduction
Garden cress (Lepidium sativum L.) is an annual cruciferous herb cultivated in many temperate areas of the world and prized as a leafy vegetable and a microgreen for the ready-to-eat functional food sector [1,2]. The interest in this herb is due to its distinctive taste and richness in health-beneficial phytonutrients, including antioxidants, phenolic compounds and glucosinolates [3]. Its cultivation is characterized by very short cycles that end in the order of 10–20 days after sowing with the cutting of the fresh product, which can be followed by minimal processing, bagging and distribution [4,5]. In the microgreens chain, the time is shortened even further [6]. With a view to immediate consumption, it is therefore necessary to adopt cultivation systems and management protocols, particularly for phytosanitary aspects, that minimize or even exclude the use of synthetic chemicals. On the other hand, the cultivation of garden cress at the ground level is very vulnerable to polyphagous telluric pathogens that can proliferate in sick soils and/or intensive systems [7].
The use of suppressive compost can be a very advantageous strategy in the cultivation of this vegetable, both in terms of sustainability and quality. A quality compost with its biological (microbial community and excreta) and/or physicochemical (supramolecular structures and compounds) components can hinder disease development by interfering in pathogenesis through some key mechanisms schematically referred to as biological control, the induction of resistance and direct antifungal activity [8,9]. Therefore, the use of a highly suppressive compost in the production of baby-leaf vegetables and/or microgreens can drastically reduce the dependence on synthetic fungicides and promote healthy plant development [10]. Suppressiveness adds to the advantages of using compost in cress cultivation, e.g., nutrient supply and the stimulation of the synthesis of functional metabolites that improve organoleptic quality [11].
Over the years, many studies have been conducted to investigate the main mechanisms underlying compost suppressiveness, establishing the primacy of the microbiological component that is selected during the long composting process through the continuous feedback between the transformation of organic matrices by the microorganisms and the conditioning of the microbiota structure induced by the physicochemical nature of the organic matter [12]. Many of these studies have used garden cress as a model plant to characterize compost suppressiveness [12,13] (see Table 1), and, even earlier, it proved to be particularly suitable for testing phytotoxicity [14], demonstrating the potential for use in productive systems as well.

Table 1.
Percentage of control efficacy showed by many composts tested against soil-borne diseases of cress in previous studies. The asterisk indicates statistical significance (p < 0.05); ns indicates not significant, as compared to the infected reference.
Composting is proposed as a circular economy process aimed at valorizing agro-industrial organic waste through recycling and reuse in new crop cycles [20]. The systematic production of multifunctional composts with suppressive and biostimulating properties is a new perspective for the development of the sector, which can be found in plant materials with a well-defined phytochemical potential, a valuable resource. This may be the case in the aromatic plant sector, which can produce a large amount of noble waste for this purpose, both from the cultivation and dressing stages. This residual aromatic biomass can continue its productive life as a co-product of the distillation of essential oils, the production of aromatic waters and, then, through just composting [21].
The present study aims to evaluate the suppression in a collection of 11 composts produced from aromatic plant waste against two telluric diseases of cress: Rhizoctonia and Sclerotinia damping-off. By characterizing the microbiological and chemical properties and the molecular distribution of organic C in the composts, inferences are made about the mechanisms underlying disease suppression.
2. Materials and Methods
2.1. Bio-composts
In this study, eleven composts (P1–11) obtained by on-farm composting (singly or in complex) different feedstocks available in the aromatic plant chain—in a static pile periodically turned by hand, as previously illustrated by [22]—were used. The starting materials are listed in Table 2; they include both raw and previously hydrodistilled feedstock [21]. These composts were available as representative of a possible combination of biomasses from the herb sector included in valuable circular economy processes.

Table 2.
Main compost feedstock used to produce P1–P11 composts.
2.2. In Planta Compost Suppressiveness Assay
The compost suppression bioassay was conducted against the Rhizoctonia and Sclerotinia diseases of L. sativum, as was described previously by Pane et al. [17]. In particular, the experimental setup is summarized here:
- The eleven bio-composts, both sterile (twice autoclaved) and not sterile, were supplied at a rate of 30% (vol.) to a standard peat-based growing medium. Non-amended peat was used as a control.
- Isolates of Rhizoctonia solani (AG-4) and Sclerotinia sclerotiorum from the CREA microbial collection (at the Research Center for Vegetables and Ornamental Crops in Pontecagnano Faiano, Italy), maintained on a potato dextrose agar medium (PDA, Oxoid Ltd., Basingstoke, UK), were grown for 21 days on common millet seeds saturated with potato dextrose broth (PDB, Oxoid Ltd., Basingstoke, UK) (1/10 w/w) to prepare the pathogen inoculum to be incorporated into the substrate at a final concentration of 1% (w/w, dry weight).
- The experimental unit consisted of a mini plastic pot (7 cm diam., ~0.1 L vol.) filled with the different substrates, sown with 20 seeds of garden cress cv. Comune (Blumen, Milan, Italy) each and replicated five times for each treatment. Ultimately, there were 11 composts ×2 conditions (autoclaved and non-autoclaved) +2 controls (healthy and infected peat) for a total of 24 treatments, resulting in 120 pots and 2400 seeds. The experiment was repeated.
- The sown pots were placed in a climate chamber (25 °C) for 7 days to allow for the emergence of cress seedlings and the development of Sclerotinia and Rhizoctonia damping off (Figure 1).
 Figure 1. Photograph of healthy (A) and Rhizoctonia- (B) and Sclerotinia-diseased (C) cress seedlings sown in pots on autoclaved peat. Details of the damping-off by Rhizoctonia solani (D) and the wilting of cress seedlings covered by Sclerotinia sclerotiorum mold (E).
Figure 1. Photograph of healthy (A) and Rhizoctonia- (B) and Sclerotinia-diseased (C) cress seedlings sown in pots on autoclaved peat. Details of the damping-off by Rhizoctonia solani (D) and the wilting of cress seedlings covered by Sclerotinia sclerotiorum mold (E).
The incidence of Sclerotinia and Rhizoctonia diseases on the cress was recorded as the percentage of damping-off (DO%), according to Equation (1):
where HSo and HSi are the number of healthy seedlings in the non-amended control and in the ith compost mix, respectively.
2.3. Analysis of the Main Physico-Chemical and Biological Components of Compost
The electrical Conductivity and pH of the compost were determined according to the official methods of the Italian National Society of Soil Science [23]. The nitrate content in the compost was assessed by a colorimetric technique using Reflectoquant® strips read by a RQflex® 10 reflectometer (Merck, Darmstadt, Germany).
The phytotoxicity assay was carried out by assessing the germination rate of the cress after the exposure of 20 seeds to 4 mL of aqueous compost extracts at three different concentrations (50, 16.6 and 5 g L−1 of compost, dry weight). They were placed onto blotting paper in Petri plates and incubated at 25 °C for 5 days. After incubation, the number and root elongation of the seedlings were calculated in the germination index (GI%) according to Formula (1).
where the number (N°) and the mean root length (RL) of the germinated seedlings in both the water control (So) and the ith compost eluate (Si) are taken in account, respectively.
The population levels of the filamentous fungi and the total, spore-forming and pseudomonads-like bacteria in the composts were assessed by the plate counting of tenfold (10−1 to 10−7) serial dilutions of water suspensions. Fungal colonies were grown on PDA pH 6 and supplemented with 150 mg L−1 of nalidixic acid and 150 mg L−1 of streptomycin. The total bacteria were counted on a selective medium (glucose 1 g L−1, proteose peptone 3 g L−1, yeast extract 1 g L−1, K2PO4 1 g L−1, agar 15 g L−1) supplemented with actidione 100 mg L−1. Peudomonads were counted on a selective agar medium without iron that was supplemented with actidione [24]. Finally, spore-forming bacteria were counted on Nutrient Agar [25] previously heated at 90 °C for 10 min.
Basal respiration was expressed as the CO2 release rate of 10 g (dry weight) of compost at an 80% water holding capacity in a sealed 50 mL sterile plastic tube (Falcon, Oxnard, CA, USA), as measured with the CO2 Analyser IRGA SBA-4 OEM (PP Systems, Haverhill, MA, USA).
The rate of hydrolysis of the Fluorescein diacetate (FDA) from the compost (2.5 g) mixed with 0.2 M potassium phosphate buffered at pH 7.6 (15 mL) and then supplemented with 0.5 mL FDA solution (2 mg mL−1) was assessed after 2 h of dynamic incubation and after stopping the reaction by the addition of 15 mL CHCl3/CH3OH (2:1 vol.) with a UV-Vis spectrophotometer (model UV-1800, Shimadzu, Canby, OR, USA) at 490 nm.
2.4. Analysis of the Molecular Carbon Components of Compost
The finely powdered compost samples were analyzed by solid-state NMR spectroscopy (13C CPMAS NMR) on a Bruker AV300 Spectrometer equipped with a 4 mm wide-bore MAS probe by packing the homogenized organic substrates in 4 mm zirconium rotors with Kel-F caps. The technical parameters for the NMR acquisition were set as follows: a rotor spin rate of 13,000 Hz, 2 s of recycle time, 1 ms of contact time, 30 ms of acquisition time and 4000 scans. A conventional composite shaped “ramp” pulse on the 1H channel was used for the cross-polarization pulse sequence to account for the inhomogeneity of the Hartmann–Hann condition at a high rotor spin frequency. The Fourier transform was performed with a 4k data point and an exponential apodization of 200 Hz of line broadening.
Although the solid-state 13C CPMAS NMR is a powerful technique for the direct investigation of natural organic matter, the analysis of complex solid matrices involves the occurrence of unavoidable technical drawbacks, such as chemical shield anisotropy and dipolar coupling effects, with a loss in signal resolution. Solid-state NMR spectra are therefore characterized by a large signal broadening and an overlapping of carbon functionalities, partly addressed by the application of magic angle spinning and high-power 1H decoupling. Therefore, for the interpretation of solid state 13C NMR spectra, the different signals are conventionally grouped into six extended chemical shift regions that are representative of the main types of carbon functional groups: Alkyl-C: 0–45 ppm; Methoxyl-C: 45–60 ppm; O-Alkyl-C: 60–110 ppm; Aryl-C: 110–145 ppm; Phenol-C: 145–160 ppm; and Carboxyl-C: 190–160 ppm.
The relative contribution of each spectral region was determined by integration (MestreNova 6.2.0 software, [26]) and expressed as a percentage of the total area. The molecular features of organic substrates can be summarized by calculating the dimensionless structural index [17,27]. The Alkyl ratio (A/OA) compares the relative intensity between Alkyl-C and O-Alkyl-C (3):
The Hydrophobic Index (HB) corresponds to the comparison of hydrophobic apolar C functionalities and potentially more hydrophilic polar functional groups (4):
The Lignin ratio (LigR) is based on the relation between the C distribution in Methoxyl-C and the C-N region, as related to the O-Aryl-C components (5):
The A/OA and HB indices are mainly used as references to determine the biochemical stability of organic matrices and correlate the structural composition with the stabilization processes of organic materials [28,29]. The Lig R ratio is a useful indicator to discriminate between NMR signals due to lignin and other phenolic compounds (lower LigR) versus the prevalent contribution of peptidic moieties (larger LigR) in the 45–60 ppm range of the NMR spectra [17,30].
2.5. Statistical Analysis
All of the measured parameters were subjected to descriptive statistics. A two-way ANOVA was applied to the results of the in planta suppression assays for each pathogen to test the effects of the compost sample and sterilization treatment on the damping-off percentage and to the phytotoxicity assay to test the effects of the compost sample and eluate concentration on the cress germination index. The percentage data were arcsine transformed to satisfy the normality assumption of distribution. A one-way ANOVA was used to test for differences in the physico-chemical, chemical and microbiological characteristics among the samples.
3. Results
3.1. Compost Suppressiveness
The composts showed varying levels of suppressiveness against the two target soil-borne diseases (Figure 2).
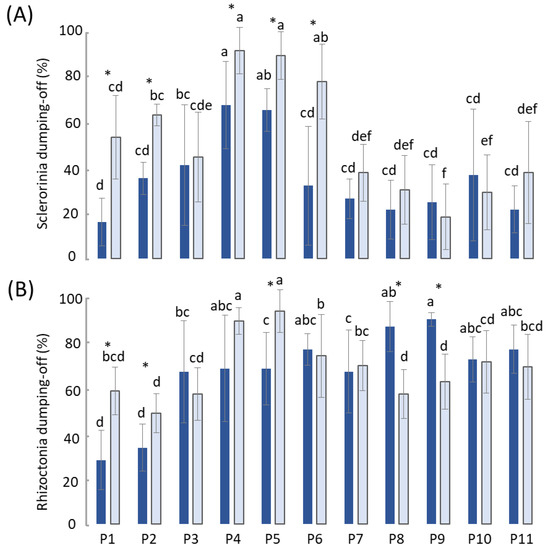
Figure 2.
Percentage of Sclerotinia (A) and Rhizoctonia (B) damping-off on cress seedlings in raw (dark blue bars) and autoclaved (light blue bars) composts diluted into peat. Lowercase lettering indicates significant differences (p ≤ 0.05), according to the Least Significant Difference post hoc test, among both the raw and autoclaved samples. Asterisk indicates significant differences (p ≤ 0.05), according to the Least Significant Difference post hoc test, between the raw and autoclaved samples of the same compost.
All of the raw composts were able to significantly (p < 0.001) reduce the damping-off caused by S. sclerotiorum on the cress in pot trials compared to the non-amended pots. Furthermore, with the exception of P4 and P5, the composts kept the disease severity below 50%; P1 was the most suppressive, sharing a statistically comparable level of suppression with a large group including P2 and P6 to P11. On the other hand, although Rhizoctonia damping-off was significantly (p < 0.001) reduced in 7 out of 11 cases compared to the control peat, the suppression was very low: only about 25% of the disease control capacity, except for P1 and P2, which proved to be the best performing raw composts, achieving over 60% disease control. The sterilization of the P1 and P2 composts drastically cancelled out suppression. Similarly, the sterilized composts P4, P5, P6, P8 and P9 significantly (p < 0.01) lost the suppressiveness that was expressed when they were raw. Indeed, the two-way ANOVA showed significant (p < 0.01) compost × sterilization interaction.
3.2. Phyitotoxicity and Biological Properties of Composts
The cress germination test on the compost eluates showed the profiles of their phytotoxicity potential with, on average, a concentration-dependent behavior (Figure 3). Statistics showed that the effect of the single factor, compost sample or concentration was significant (p < 0.001), whereas no significant interaction was found between them (compost × concentration). Composts P4 and P5 showed the lowest toxicity, as expressed by the germination index percentage, at the highest concentration analyzed. In parallel, the eluates of P3, P6 and P7 showed intermediate values of the parameter between 50 and 100%. Passing to the remaining composts, on average, lower germination index percentages were observed.
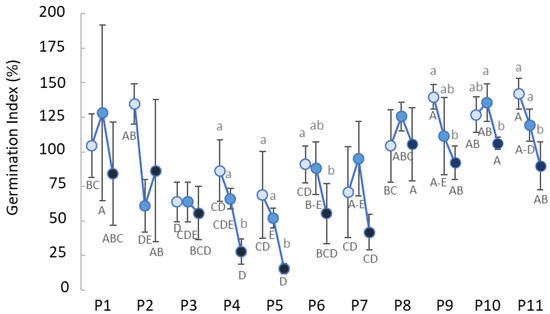
Figure 3.
Germination index of cress seeds exposed to compost water extracts (P1 to P11) at 50 (dark blue), 16.6 (medium blue) and 5 g L−1 (light blue). Lower and uppercase lettering indicate significant differences (p ≤ 0.05), according to the Least Significant Difference post hoc test, among the concentration within the same sample and the sample within the same concentration, respectively.
Table 3 shows the values of the main biological parameters measured on the composts. The largest levels of FDA hydrolysis were recorded in P4 and P6, followed by P1, P2, P5 and P7, which, together, form the top cluster regarding this enzymatic activity. These behaviors were not confirmed by the assessment of basal respiration, as the rate of CO2 release showed no significant differences among the samples. The population levels of total fungi were statistically highest in P8 and P11, followed by P9, while the remaining composts showed lower enumerations of culturable colonies. P9 showed the highest values of the total bacterial population, followed by P5, P10 and P11. Heat-resistant bacteria were significantly more numerous in composts P1, P2 and P4, while Pseudomonas-like bacteria were more numerous in P8, P9 and P10.

Table 3.
Some biological, microbiological, physico-chemical and chemical properties of the composts (P1–P11). Asterisks indicate that the differences indicated by lowercase lettering are statistically significant (p < 0.001). Non-significance is reported as ns.
3.3. Chemical and Molecular Properties of the Composts
The composts showed sub-alkaline pH values (>8.0) and variable levels of electrical conductivity. Similarly, nitrate availability showed values ranging from 2.13 ppm in P9 to 13.7 ppm in P6 (Table 3).
The 13C NMR spectra of the compost samples were characterized by an overall composition dominated by the aliphatic molecules of either alkyl-C or O-alkyl-C components (Figure 4).
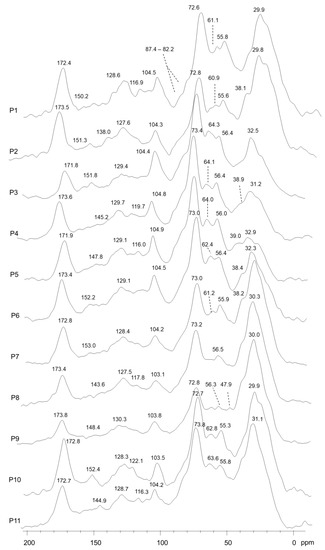
Figure 4.
13C-CPMAS-NMR of compost samples (P1–P11).
The carbon distribution ranged from 21.6 to 43.6% and from 26.8 to 42.4% of the total spectral area, respectively (Table 4). The various bands grouped beneath the 110–145 ppm spectral interval are related to the unsubstituted and C-substituted nuclei of phenyl carbons pertaining to the aromatic units of terpenoid, polyphenols, flavonoids and the lignin components of plant tissues [31,32,33]. Moreover, the signals shown in the alkyl-C interval (0–45 ppm) also include the various lipid compounds derived from plant tissues and the microbial biomass [17]. The intense and broad bands centered around 30–33 ppm (Figure 4) are related to the overlapping of different bulk methylene (CH2) segments of predominantly straight-chain molecules of wax and cutin components [27,34], as well as those of cyclic aliphatic components such as terpene derivatives and sterols derived from the aromatic plant used in compost-starting biomasses [32,35].

Table 4.
Relative C distribution (%) over the chemical shift regions (ppm) and structural index in the NMR spectra of compost samples (P1–P11).
The shoulders evident at 39–40 ppm (Figure 4) can be also associated with CH2, tertiary (CH) and quaternary (C-R) carbons in the assembled rings of terpenoid compounds [33,36].
The subsequent chemical shift region between 46 and 60 ppm, marked by the signal at 56 ppm (Figure 4), includes typical resonances found in plant-derived organic materials consisting of the contribution of various groups represented by methoxyl substituents on the aromatic rings of guaiacyl and syringyl units in lignin, or by C-N bonds in peptidic moieties [17,27].
The various peaks in the O-alkyl-C region (60–110 ppm) are currently assigned to monomeric units in the oligo and polysaccharide chains of plant tissues [28,30]. The less intense shoulders at 62/64 ppm represent the out-of-plane carbon 6 of cyclic carbohydrate conformation, followed by the sharp signal around 72 ppm due to the coalescence of the chemical shift of hydroxylated carbons in position 2, 3 and 5 in the pyranoside structure, whereas the band shift at 104 ppm is the di-O-alkyl frequency of anomeric carbons (Figure 4). In addition to the monomers of the saccharide chains, the O-alkyl regions of the NMR spectra may also include a contribution from the carbohydrate moieties of the glycosidic components in the structures of the flavonoids of aromatic plants [31,37]. The reduced evidence of carbon 4 involved in the β 1→4 glycosidic bond at 82/88 ppm suggested the decomposition of the ether bond of the polysaccharides chain in composting processes, as well as the incorporation of mannan, xylan and arabinan derivatives into the hemicellulose structures of the initial fresh plant biomass [29,30].
The phenolic aromatic region (140–160 ppm) indicates the presence of O-substituted ring carbon derived from various aromatic structures [17,28]. Finally, the wide peak at 174 ppm (Figure 4) indicated the presence of carboxyl groups in aliphatic and aromatic acids, amide groups in amino acid moieties and acetyl substituents in the carbohydrate components of hemicelluloses and flavonoids.
4. Discussion
The potential application of composts and organic materials in garden cress cultivation has long been investigated with the aims to reduce peat in growing media [38], improve soil properties [39] and upgrade the nutritional quality of yield [40]. On the other hand, cress is also plentifully used in studies carried out to test the disease suppressiveness, maturity and agronomic suitability of compost. The current paper presents a comprehensive examination of bio-composts from aromatic plant residues applied to the soilless cultivations of cress as a protective means against the most common soil-borne pathogens. The study revealed that the feedstocks may affect compost features and functionality and provide benefits for crop protection.
In view of composting, aromatic plant waste differs from other crop residues, mainly because of its significant content of essential oil. This plant constituent is made up of a rich mixture of compounds that generally exhibit marked antimicrobial and phytotoxic activity, amplified by their volatility, lipophilicity and very low lethal concentration [41,42]. Essential oils contained in aromatic plants may interfere with the dynamics of microorganisms devoted to the composting, causing potential start-up difficulties, process slowdowns, negative impacts on microbial development and, secondarily, prolonged phytotoxic effects when the compost is applied to the plants [43]. However, the aware composting of these vegetable matrices well assembled with other complementary ingredients, as a sound remedy to modulate the cited microbial conditioning aspects, can lead to a high agronomic and functional value-end product. From a circular economy perspective, however, the removal/recovery of the essential oils from the raw material by means of hydrodistillation is a further way of valorizing aromatic plant leftovers, drawing attention to the concept of by-products [21]. This study, by examining the composts obtained from the raw and oil-free aromatic matrices of different botanical origins, sheds light on the consequences for the potential composts’ suppressive functions.
The bioassays unequivocally demonstrated a high ability to suppress Rhizoctonia and Sclerotinia diseases on the cress of the raw composts P1 and P2, respectively obtained from the following two groups of ingredients: wood chips, escarole and a mixture of sage, basil, mint and parsley and wood chips, 30% escarole and a mixture of essential oil-free sage, basil and rosemary. Furthermore, after generating the biological vacuum in them by autoclaving, the two high-performing composts lost their ability to stop the pathogenesis dynamics, indicating the crucial role played by resident microorganisms in conferring biological control properties. This is corroborated by the high levels of the general enzymatic activity recorded by FDA hydrolysis, the respiration rate (although without statistical significance) and microbial counting profiles. Interestingly, these two composts showed the highest levels of thermal-resistant bacteria populations, including Bacillus-like cells, which are widely reported among the main functional microbial groups conferring suppressiveness to composts [44]. As matter of the fact, the composts assessed for suppressiveness in this study were previously used as a useful source for the stepwise selection of new strains of Bacillus spp. antagonistic to soil-borne pathogens [22]. In addition, the phytotoxicity of P1 and P2 was low, and the cress germination assay showed irregular behavior with the dose, suggesting an antagonistic toxic/nutritional effect of eluates. The pot assays indicated that R. solani is more difficult to control with suppressive composts than S. sclerotiorum, likely due to the insidious nature of the pathogen and the residual phytotoxicity of the non-suppressive composts that weaken the plant. This could correspond to a specific mechanism—the suppressive activity slightly related to the population levels of the Bacillus-like bacteria. On the contrary, against Sclerotinia damping-off, the success in biocontrol by composts has been broader, although keeping the same pattern of the Rhizoctonia bioassay regarding the most and the least suppressive ones.
There is no evidence about the modulating effects of the essential oil extraction from feedstock on the levels of disease suppressiveness; rather, the ability to counteract pathogens seems to be more influenced by the botanical diversity of the ingredients mixed into the pile. For example, both the mono-matrix composts of basil leftovers showed the lowest suppressiveness. In contrast, both the raw and essential oil-extracted rosemary-based composts showed a particularity to significantly increase suppressiveness after sterilization, possibly indicating the release or formation of highly antifungal compounds after heating. While the use of vapor heating may enhance the release of antimicrobial components in aromatic plants [27], the metabolic products of rosemary, such as flavonoids and phenolic derivates, show consistent thermal stability and bioactive performance upon thermal treatment [45,46]. It is therefore conceivable that the preliminary autoclave sterilization process of fresh biomass may have promoted the release of aromatic oligomers during the composting of rosemary-based matrices, thus enhancing the suppressive activity of the final processed composts.
Interestingly, on average, the greatest reduction in suppressiveness passing from the raw to the corresponding autoclaved compost is observed in the multi-matrix composts, suggesting a role of the carbon source complexity in the structure and functionality of the developing microbial communities. From this perspective, although the EC, pH and nitrate concentrations of the composts were in the typical range for green composts, the molecular distribution of organic carbon along the samples reflect these considerations.
Despite the broadening of the signal produced by the CPMAS technique, the NMR spectra of the compost samples allowed for an adequate evaluation of specific functional groups related to the characteristic plant components in the different chemical shift regions. The relative distribution of C in the chemical shift regions and the structural indices in the NMR spectra of the compost samples suggest a differentiation of maturity into two main groups based on the initial composition of the starting substrates. The inclusion of a higher number of poplar residues (P1) and the exclusive use of the rosemary (P8, P9) and sage (P10, P11) biomass promoted a significant incorporation of lipid compounds. On the contrary, the halving of the structural fraction and the increase in fresh biomass, consisting mainly in basil and parsley (P3, P4, P6), enabled the final prevalence of the carbohydrates and polysaccharides. This is highlighted by the distinct values found for the comparison of the aliphatic compositions carried out with the Alkyl ratio, while no significant differences were found in the overall relative distribution of the aromatic compounds. The larger residual presence of carbohydrates in the basil and/or parsley-based composts, likely due to the proliferative effects on harmful microorganisms [47], may explain the complete absence and very low suppressiveness, respectively, in the P4 and P5 composts and in the P6 and P3 composts, which also exhibited a lower germination index than the other compost samples, as an indication of their low degradation.
The bioactive properties of composts in supporting crop development through biostimulation or suppressive effects are usually associated with the combination of microbial composition and molecular characteristics [12,17,27]. Regarding chemical components, although an unambiguous relationship between structure and activity has not yet been clarified, the bioactive functionalities of compost and its derivates are mainly related to the stage of humification and the content of humified fraction. These structural characteristics are evidenced by increased stabilization properties summarized in the NMR analysis by the Hydrophobic Index and Alkyl ratio [34]. The relative preservation of apolar compounds during the dynamics of natural organic materials such as litter, soil organic matter, compost, digestates, etc. are in fact closely correlated with the advancement of carbon stabilization and the so-called humification process [29,34]. Bioactive properties are thus triggered by depolymerized organic molecules released by the intense humification process, mainly represented by aromatic and phenolic compounds and decomposed peptide moieties [48]. In contrast, the retention of more intact lignocellulose residues indicated the lower intensity of humification and the reduced availability of less decomposed active fragments [30,49]. A remarkable shared effect on the composting process was also shown by the biomasses subjected to the preliminary removal of essential oils. Almost all the extracted final composts showed a final composition characterized by an increase in the HB and A/OA indexes and/or a decrease in the Lignin ratio compared to the corresponding non-extracted samples. The trend of a decreasing Lignin ratio is related to the improved organic stabilization of the composted biomass. In fact, despite the extraction of essential oils and the consequent release of aromatic and phenolic compounds, which constitute the denominator of LigR, almost all of the de-oiled mature composts showed a steady decrease in this structural parameter. This change suggests the better fragmentation of the larger proteinaceous material underlying the peak area at 45–60 ppm combined with the constant maintenance or relative increase of the O-aryl-C signals of lignin fragments in the 145–160 ppm region, thus leading to final LigR values in the extracted biomasses comparable to those usually found in the humified fraction of stable mature composts [17,30]
Essential oils from aromatic plants are recognized as effective antioxidant and antimicrobial agents with suppressive properties against a wide range of microorganisms [21,22,27]. It is therefore conceivable that the preliminary extraction of the oil fraction may have favored the development of microbial activity in processed biomass, thus promoting an organic matter dynamic associated with compost humification.
5. Conclusions
The composting of aromatic plant residues is a suitable method to improve the circularity of this production chain and agricultural systems in general. The extraction of the essential oil does not affect the suppressive functions, so feedstock without essential oil, as well as raw materials, can potentially promote the formation of quality composts, especially in a complex combination with ingredients of different botanical origins.
Cress cultivation can benefit from the application of suppressive composts to reduce the dependence on fungicides for disease management and improve rapid soilless producing systems such as ready-to-eat salads and microgreens.
Author Contributions
Conceptualization, C.P. and M.Z.; methodology, C.P., R.S. and M.Z.; formal analysis, C.P. and R.S.; investigation, C.P., R.S. and M.C.; data curation, C.P. and R.S.; writing—original draft preparation, C.P. and R.S.; writing—review and editing, C.P., R.S., M.C., E.D.F. and M.Z.; project administration and funding acquisition, E.D.F. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Campania Region via the EU FEASR funding program PSR 2007–2014 measure 124 through the project “Gestione innovative degli scarti di coltivazione e lavorazione nella filiera delle erbe aromatiche” (acronym: “Polieco 2”).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nicola, S.; Hoeberechts, J.; Fontana, E. Ebb-and-flow and floating systems to grow leafy vegetables: A review for rocket, corn salad, garden cress and purslane. Acta Hortic. 2007, 747, 585–593. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; De Pascale, S. Sprouts, microgreens and edible flowers as novel functional foods. Agronomy 2021, 11, 2568. [Google Scholar] [CrossRef]
- Santos, J.; Oliveira, M.B.P.P.; Ibáñez, E.; Herrero, M. Phenolic profile evolution of different ready-to-eat baby-leaf vegetables during storage. J. Chromatog. A 2014, 1327, 118–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, T.; Grover, K.; Grewal, I.S. Development and sensory evaluation of ready to eat supplementary food using garden cress (Lepidium sativum) seeds. J. Appl. Nat. Sci. 2016, 8, 1501–1506. [Google Scholar] [CrossRef]
- Ciesielska, K.; Ciesielski, W.; Kulawik, D.; Oszczęda, Z.; Tomasik, P. Cultivation of cress involving water treated under different atmospheres with low-temperature, low-pressure glow plasma of low frequency. Water 2020, 12, 2152. [Google Scholar] [CrossRef]
- Keutgen, N.; Hausknecht, M.; Tomaszewska-Sowa, M.; Keutgen, A.J. Nutritional and sensory quality of two types of cress microgreens depending on the mineral nutrition. Agronomy 2021, 11, 1110. [Google Scholar] [CrossRef]
- Ogórek, R. Enzymatic activity of potential fungal plant pathogens and the effect of their culture filtrates on seed germination and seedling growth of garden cress (Lepidium sativum L.). Eur. J. Plant Pathol. 2016, 145, 469–481. [Google Scholar] [CrossRef]
- Mehta, C.M.; Palni, U.; Franke-Whittle, I.H.; Sharma, A.K. Compost: Its role, mechanism and impact on reducing soil-borne plant diseases. Waste Manag. 2014, 34, 607–622. [Google Scholar] [CrossRef]
- Pane, C.; Zaccardelli, M. Principles of compost-based plant diseases control and innovative new developments. In Composting for Sustainable Agriculture; Maheshwari, D., Ed.; Sustainable Development and Biodiversity; Springer: Cham, Switzerland, 2014; Volume 3. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Egea-Gilabert, C.; Santísima-Trinidad, A.B.; Ros, M.; Pascual, J.A. Agro-industry composts as growing medium for growing baby-leaf lettuces in a floating system—Added-value to suppress Pythium irregulare. Acta Hortic. 2019, 1242, 791–798. [Google Scholar] [CrossRef]
- Morau, A.; Piepho, H.-P.; Fritz, J. Growth responses of garden cress (Lepidium sativum L.) to biodynamic cow manure preparation in a bioassay. Biol. Agric. Hortic. 2020, 36, 16–34. [Google Scholar] [CrossRef] [Green Version]
- Pane, C.; Piccolo, A.; Spaccini, R.; Celano, G.; Villecco, D.; Zaccardelli, M. Agricultural waste-based composts exhibiting suppressivity to diseases caused by the phytopathogenic soil-borne fungi Rhizoctonia solani and Sclerotinia minor. Appl. Soil Ecol. 2013, 65, 43–51. [Google Scholar] [CrossRef]
- Pane, C.; Spaccini, R.; Piccolo, A.; Scala, F.; Bonanomi, G. Compost amendments enhance peat suppressiveness to Pythium ultimum, Rhizoctonia solani and Sclerotinia minor. Biol. Control 2011, 56, 115–124. [Google Scholar] [CrossRef]
- Aslam, D.N.; Horwath, W.; Vander Gheynst, J.S. Comparison of several maturity indicators for estimating phytotoxicity in compost-amended soil. Waste Manag. 2008, 28, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Ventorino, V.; Parillo, R.; Testa, A.; Viscardi, S.; Espresso, F.; Pepe, O. Chestnut green waste composting for sustainable forest management: Microbiota dynamics and impact on plant disease control. J. Environ. Manag. 2016, 166, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Ronga, D.; Francia, E.; Allesina, G.; Pedrazzi, S.; Zaccardelli, M.; Pane, C.; Tava, A.; Bignami, C. Valorization of vineyard by-products to obtain composted digestate and biochar suitable for nursery grapevine (Vitis vinifera L.) production. Agronomy 2019, 9, 420. [Google Scholar] [CrossRef] [Green Version]
- Pane, C.; Spaccini, R.; Piccolo, A.; Celano, G.; Zaccardelli, M. Disease suppressiveness of agricultural greenwaste composts as related to chemical and bio-based properties shaped by different on-farm composting methods. Biol. Control 2019, 137, 104026. [Google Scholar] [CrossRef]
- Pane, C.; Sorrentino, R.; Scotti, R.; Molisso, M.; Di Matteo, A.; Celano, G.; Zaccardelli, M. Alpha and beta-diversity of microbial communities associated to plant disease suppressive functions of on-farm green composts. Agriculture 2020, 10, 113. [Google Scholar] [CrossRef] [Green Version]
- Bignami, C.; Melegari, F.; Zaccardelli, M.; Pane, C.; Ronga, D. Composted solid digestate and vineyard winter prunings partially replace peat in growing substrates for micropropagated highbush blueberry in the nursery. Agronomy 2022, 12, 337. [Google Scholar] [CrossRef]
- De Corato, U. Agricultural waste recycling in horticultural intensive farming systems by on-farm composting and compost-based tea application improves soil quality and plant health: A review under the perspective of a circular economy. Sci. Tot. Environ. 2020, 738, 139840. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Roscigno, G.; Pane, C.; Celano, G.; Di Matteo, M.; Mainente, M.; Vuotto, A.; Mencherini, T.; Esposito, T.; Vitti, A.; et al. Essential oils and quality composts sourced by recycling vegetable residues from the aromatic plant supply chain. Ind. Crop. Prod. 2021, 162, 113255. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Pane, C.; Caputo, M.; Durazzo, A.; Lucarini, M.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A.; De Feo, V. Sage species case study on a spontaneous Mediterranean plant to control phytopathogenic fungi and bacteria. Forests 2020, 11, 704. [Google Scholar] [CrossRef]
- Violante, P. Metodi di Analisi Chimica del Suolo; Angeli, F., Ed.; Italian Ministry of Agriculture: Milan, Italy, 2000; p. 536. [Google Scholar]
- Scher, F.M.; Baker, R. Effect of Pseudomonas putida and a synthetic iron chelator on induction of soil suppressiveness to Fusarium Wilt pathogens. Phytopathology 1982, 72, 1567–1573. [Google Scholar] [CrossRef]
- Sadfi, N.; Cherif, M.; Fliss, I.; Boudabbous, A.; Antoun, H. Evaluation of bacterial isolates from salty soils and Bacillus thuringiensis strains for the biocontrol of Fusarium dry rot of potato tubers. J. Plant Pathol. 2001, 83, 101–118. [Google Scholar]
- Mestrelab Research, MestReNova Manual 2010. Manual of MestreNova 6.2. Available online: https://mestrelab.com/ (accessed on 19 June 2022).
- Verrillo, M.; Cozzolino, V.; Spaccini, R.; Piccolo, A. Humic substances from green compost increase bioactivity and antibacterial properties of essential oils in Basil leaves. Chem. Biol. Technol. Agric. 2021, 8, 28. [Google Scholar] [CrossRef]
- Bento, L.R.; Spaccini, R.; Cangemi, S.; Mazzei, P.; de Freitas, B.B.; de Souza, A.E.O.; Moreira, A.B.; Ferreira, O.P.; Piccolo, A.; Bisinoti, M.C. Hydrochar obtained with by-products from the sugarcane industry: Molecular features and effects of extracts on maize seed germination. J. Environ. Manag. 2021, 281, 111878. [Google Scholar] [CrossRef] [PubMed]
- Fregolente, L.G.; Dos Santos, J.V.; Vinci, G.; Piccolo, A.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C.; Spaccini, R. Insights on molecular characteristics of hydrochars by 13C-NMR and off-line TMAH-GC/MS and assessment of their potential use as plant growth promoters. Molecules 2021, 26, 1026. [Google Scholar] [CrossRef]
- de Aquino, A.M.; Canellas, L.P.; da Silva, A.P.S.; Canellas, N.O.; da S Lima, L.; Olivares, F.L.; Piccolo, A.; Spaccini, R. Evaluation of molecular properties of humic acids from vermicompost by 13C-CPMAS-NMR spectroscopy and thermochemolysis–GC–MS. J. Anal. Appl. Pyrol. 2019, 141, 104634. [Google Scholar] [CrossRef]
- Yang, F.; Qi, Y.; Liu, W.; Li, J.; Wang, D.; Fang, L.; Zhang, Y. Separation of five flavonoids from aerial parts of Salvia Miltiorrhiza bunge using HSCCC and their antioxidant activities. Molecules 2019, 24, 3448. [Google Scholar] [CrossRef] [Green Version]
- Kadir, A.; Zheng, G.; Zheng, X.; Jin, P.; Maiwulanjiang, M.; Gao, B.; Aisa, H.A.; Yao, G. Structurally diverse diterpenoids from the roots of Salvia deserta based on nine different skeletal types. J. Nat. Prod. 2021, 84, 1442–1452. [Google Scholar] [CrossRef]
- Aydin, S.K.; Ertaş, A.; Boğa, M.; Erol, E.; Toraman, G.O.A.; Saygı, T.K.; Halfon, B.; Topçu, G. Di-, and triterpenoids isolation and lc-ms analysis of salvia marashica extracts with bioactivity studies. Rec. Nat. Prod. 2021, 15, 463–475. [Google Scholar] [CrossRef]
- Martinez-Balmori, D.; Spaccini, R.; Aguiar, N.O.; Novotny, E.H.; Olivares, F.L.; Canellas, L.P. Molecular characteristics of humic acids isolated from vermicomposts and their relationship to bioactivity. J. Agr. Food Chem. 2014, 62, 11412–11419. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Brito, C.; Joseph-Nathan, P.; Burgueño-Tapia, E.; Martínez-Otero, D.; Nieto-Camacho, A.; Calzada, F.; Yépez-Mulia, L.; Esquivel, B.; Quijano, L. Structure and absolute configuration of abietane diterpenoids from Salvia clinopodioides: Antioxidant, antiprotozoal, and antipropulsive activities. J. Nat. Prod. 2019, 82, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Yaris, E.; Balur Adsız, L.; Yener, I.; Tuncay, E.; Yilmaz, M.A.; Akdeniz, M.; Kaplaner, E.; First, M.; Ertas, A.; Kolak, U. Isolation of secondary metabolites of two endemic species: Salvia rosifolia Sm. and Salvia cerino-pruinosa Rech. f. var. elazigensis (Lamiaceae). J. Food Meas. Charact. 2021, 15, 4929–4938. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Brede, C. Flavonoids and other phenolics in herbs commonly used in Norwegian commercial kitchens. Food Chem. 2020, 309, 125678. [Google Scholar] [CrossRef]
- Gajdoš, R. Effects of two composts and seven commercial cultivation media on germination and yield. Compost Sci. Util. 1997, 5, 16–37. [Google Scholar] [CrossRef]
- Shao-qi, Z.; Wei-dong, L.; Xiao, Z. Effects of heavy metals on planting watercress in kailyard soil amended by adding compost of sewage sludge. Process Saf. Environ. 2010, 88, 263–268. [Google Scholar]
- Tuncay, Ö.; Esiyok, D.; Yamur, B.; Okur, B. Yield and quality of garden cress affected by different nitrogen sources and growing period. Afr. J. Agric. Res. 2011, 6, 608–617. [Google Scholar]
- Pauli, A. Antimicrobial properties of essential oil constituents. Int. J. Arom. 2001, 11, 126–133. [Google Scholar] [CrossRef]
- De Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic activities of mediterranean essential oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef] [Green Version]
- Greff, B.; Lakatos, E.; Szigeti, J.; Varga, L. Co-composting with herbal wastes: Potential effects of essential oil residues on microbial pathogens during composting. Crit. Rev. Environ. Sci. Technol. 2021, 51, 457–511. [Google Scholar] [CrossRef]
- Pane, C.; Villecco, D.; Campanile, F.; Zaccardelli, M. Novel strains of Bacillus, isolated from compost and compost-amended soils, as biological control agents against soil-borne phytopathogenic fungi. Biocontrol Sci. Technol. 2012, 22, 1373–1388. [Google Scholar] [CrossRef]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014, 142, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Doudin, K.; Al-Malaika, S.; Sheena, H.H.; Tverezovskiy, V.; Fowler, P. New genre of antioxidants from renewable natural resources: Synthesis and characterisation of rosemary plant-derived antioxidants and their performance in polyolefins. Polym. Degrad. Stab. 2016, 130, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Palese, A.M.; Pane, C.; Villecco, D.; Zaccardelli, M.; Altieri, G.; Celano, G. Effects of organic additives on chemical, microbiological and plant pathogen suppressive properties of aerated municipal waste compost teas. Appl. Sci. 2021, 11, 7402. [Google Scholar] [CrossRef]
- Scaglia, B.; Nunes, R.R.; Rezende, M.O.O.; Tambone, F.; Adani, F. Investigating organic molecules responsible of auxin-like activity of humic acid fraction extracted from vermicompost. Sci. Tot. Environ. 2016, 562, 289–295. [Google Scholar] [CrossRef]
- Castaño, R.; Borrero, C.; Avilés, M. Organic matter fractions by SP-MAS 13C NMR and microbial communities involved in the suppression of Fusarium wilt in organic growth media. Biol. Cont. 2011, 58, 286–293. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).