Abstract
The sweetpotato whitefly, Bemisia tabaci MEAM1 Gennadius (Hemiptera: Aleyrodidae), and the complex of viruses it transmits are major limiting factors to squash production in the southeastern United States. At this time, insecticides are extensively relied upon for the management of whiteflies and, indirectly, whitefly-transmitted viruses. The development of a multi-faceted, integrated pest management (IPM) program is needed to increase the sustainability and profitability of squash production. Experiments in 2018 and 2019 evaluated the effects of insect exclusion netting (IEN) in combination with selected pesticides on whitefly population dynamics and virus incidence in greenhouse-grown squash seedlings. Field experiments from 2018 to 2021 evaluated the effects of mulch type (UV-reflective mulch, live mulch, and white plastic mulch), row covers, and insecticides on whitefly population dynamics, silver leaf disorder (SSL) intensity, virus symptom severity, and marketable yield. IEN significantly reduced whiteflies and virus incidence on squash seedlings in the greenhouse study. In the field mulch study, lower whitefly abundance and SSL intensity, as well as reduced virus symptom severity, were observed in plots with reflective mulch compared with white plastic or live mulch. In the insecticide/row cover study, whitefly abundance, SSL intensity, and virus symptom severity were lowest in the row cover and cyantraniliprole- and flupyradifurone-treated plots. Field plots with row covers and those with UV-reflective mulch consistently produced the greatest marketable yields. These findings demonstrate that growers can reduce whitefly and virus pressure and preserve yields in squash production in the southeastern United States by combining cultural and chemical tactics, including row covers, UV-reflective mulch, and select insecticides.
1. Introduction
Southeastern states, including Georgia and Florida, are among the top producers of fresh market yellow and zucchini squash (Cucurbita pepo L.) in the United States, with a market valued at USD 37.6 million in 2019 [1]. The majority of Georgia’s yellow and zucchini squash (hereafter referred to as squash) are grown in the fall season, during which pressure from pests and pathogens is typically higher than in the spring [2,3,4]. The sweetpotato whitefly, Bemisia tabaci Gennadius MEAM1 (Hemiptera: Aleyrodidae), is arguably one of the most important pests of squash. Feeding by whitefly nymphs results in squash silverleaf disorder (SSL), a reversible physiological disorder that reduces photosynthesis, stunts plants, and diminishes fruit quality [5,6,7,8]. Feeding by whiteflies also results in the transmission of plant viruses, which can be severely yield-limiting. Yield losses from B. tabaci and whitefly-transmitted viruses, combined with management actions, cost growers tens of millions of dollars each year [9,10,11].
A complex of at least two whitefly-transmitted viruses impacts squash in Georgia, viz., cucurbit leaf crumple virus (CuLCrV) and cucurbit yellow stunting disorder virus (CYSDV) [4]. Both viruses are relatively new to Georgia. CuLCrV was first documented in the U.S. in 1998–1999 in Arizona, Texas, and California, and it was found in Florida and Georgia in 2006 and 2009, respectively [12,13,14,15]. CYSDV was first observed in California and Arizona in 2006 and in Georgia in 2016 [16,17]. Cucurbit leaf crumple virus is in the genus Begomovirus and family Geminiviridae, and Cucurbit yellow stunting disorder virus is in the genus Crinivirus and family Closteroviridae [18,19]. CuLCrV is transmitted in a persistent and circulative manner by whiteflies [20,21,22,23]. The acquisition access period (AAP), inoculation access period (IAP), and latent period each take hours to days [24]. CYSDV is transmitted by whiteflies in a semi-persistent and non-circulative manner [25,26]. Criniviruses, such as CYSDV, have shorter AAPs and IAPs (minutes to hours) than begomoviruses, with no latent period before they can be inoculated to a susceptible host [25,27,28]. Whiteflies can acquire and transmit multiple viruses at once, and thus, both viruses are often observed as mixed infection in squash, resulting in more severe symptoms [4,24].
Squash seedling/transplant production in open greenhouses is a common practice that often leaves plants vulnerable to virus infection at their most susceptible stage. This can result in the establishment of vector reservoirs and virus inoculum sources once infected seedlings are transplanted into the field [29,30,31,32,33]. Resistant crop varieties offer the best protection against whiteflies and viruses, but there are no commercially available squash varieties with resistance to whiteflies and/or CuLCrV and CYSDV [3,34,35]. Insecticide use, often multiple applications per week, is the norm for whitefly and virus management, indirectly [35,36]. However, most insecticides, despite suppressing whiteflies, do not reduce virus transmission, as one or a few viruliferous whiteflies past the latent period can readily inoculate a susceptible squash plant within minutes of feeding [37,38].
The current management strategy of prophylactic and frequent insecticide applications can also increase risks of insecticide resistance development. Bemisia tabaci MEAM1 populations around the world have developed resistance to nearly all insecticide classes used [37,39,40,41,42]. Such indiscriminate insecticide applications pose risks to applicators, non-target organisms, and the environment, and can negatively impact the biological control of pests by natural enemies. Reflective mulch can disorient whiteflies and prevent landing on transplanted seedlings by reflecting visible and UV light [43,44,45,46]. Living mulches, such as buckwheat, clover, perennial peanut, yellow mustard, cowpea, and sorghum have also been shown to effectively reduce whitefly abundance, SSL intensity, and virus incidence in crops [32,47,48,49]. In addition, row covers and other methods of physical exclusion have been shown to be extremely effective in protecting greenhouse seedlings and direct-seeded plants in the first few weeks of the growing season [33,46,50,51,52,53]. Despite the effectiveness of reflective mulch and row covers, they are seldom used in commercial squash production in the southeastern U.S.
At this time, there is no single management tactic that is effective enough to suppress whiteflies and reduce the transmission of viruses in Georgia and other southeastern states. Therefore, an integrated pest management (IPM) program comprised of existing cultural and chemical tactics aimed at pre- and post-transplant protection is essential to limit yield losses and maintain sustainability. The first objective of this study was to evaluate the effect of insect exclusion netting (IEN), either alone or in combination with insecticides, in greenhouse production of squash seedlings. The second objective was to evaluate UV-reflective and live mulches, as well as insecticides in combination with row covers, under open field conditions. One greenhouse and two field trials were conducted over four field seasons (2018–2021) with the goal of developing a combination of reduced-risk tactics to mitigate yield loss and enhance sustainability in squash production.
2. Materials and Methods
2.1. Evaluation of Pre-Plant Seedling Protection Tactics (Greenhouse)
Greenhouse experiments were performed in 2018 and 2019 to evaluate the effects of insecticide/acibenzolar-S-methyl applications and physical exclusion of whiteflies on whitefly population dynamics and virus incidence in squash seedlings. Experiments were performed at the University of Georgia (UGA) Coastal Plain Experiment Station in Tifton, GA, USA, in a non-insect-proof, temperature-controlled polytunnel enclosed with double-layer plastic sheeting. Roll-up sides opened automatically for ventilation. Squash seeds (cultivar ‘Gold Star’; Johnny’s Selected Seeds, Fairfield, ME, USA) were planted in seed trays (~64 plants) with Sta-Green potting mix (Lowe’s®, Moorseville, NC, USA) and were arranged on top of greenhouse benches in a split-plot design with five replicates. The main plot factor was the presence or absence of IEN, which is an equivalent to row cover when used in the field. The subplot factor was insecticide/acibenzolar-S-methyl treatment (Table 1).

Table 1.
Pesticide treatments, years, and application rates from greenhouse and field experiments performed at the University of Georgia Coastal Plain Experiment Station in Tifton, GA, USA.
Benches were spaced 0.6 m apart, with a 1.8 m aisle down the center of the greenhouse. IEN (0.35 mm × 0.35 mm; Dubois Agrinovation, Saint-Rémi, QC, Canada) was installed over PVC hoops and completely enclosed the seedling trays in each main plot. Insecticides/acibenzolar-S-methyl were applied as soil drenches approximately one week after planting. Plants were irrigated at the soil line as needed. Greenhouse conditions were 24 °C, 60% relative humidity, and 13:11 (L:D) h photoperiod.
In 2018 and 2019, plants were sampled once for whitefly eggs, nymphs, and adults at approximately 15 days after planting. Adult whiteflies were counted in situ on the abaxial sides of two randomly selected leaves from each replicate. Two additional leaves were removed from each replicate and taken to the Virus-Vector Interactions Laboratory in Tifton, GA, where eggs and immature whiteflies were counted at 20X magnification under a dissection microscope (Leica Microsystems, Wetzlar, Germany). Leaf samples were stored in a refrigerator at 3–5 °C until processing. All plants were visually screened for virus symptoms two to three weeks after planting. All plants exhibiting virus symptoms were tested to confirm CuLCrV infection. DNA was extracted and polymerase chain reaction (PCR) was conducted using virus-specific primer sets and established protocols [24]. The percent virus incidence in each replicate was calculated.
2.2. Evaluation of Post-plant Protection Tactics (Field)
2.2.1. General
All field experiments were conducted at the UGA Coastal Plain Experiment Station in Tifton, GA, USA. Experimental plots were constructed in accordance with standard commercial practices. Rows were tilled with a KMC 6800 ripper bedder and fertilized with 10-10-10 fertilizer (500 lb./ac.) before beds were shaped. A tractor mounted Kennco micro-combo plastic layer (Kennco Manufacturing, Inc., Ruskin, FL, USA) was used to shape raised beds, lay irrigation tape, and apply plastic mulch (DNM Ag Supply, Inc., Calabasas, CA, USA) to each bed. Raised beds in all trials in all years were 0.81 m wide with 1.8 m wide row middles. Beds were fumigated with Pic-Clor 60 (Trical, Inc., Hollister, CA, USA) approximately three weeks prior to planting. Planting holes at 30.5 cm spacing were cut along the center of each bed using a hand-powered, spiked wheel planter. Plots were irrigated as needed.
2.2.2. Evaluation of Mulch Types
Field experiments were performed in 2018, 2019, 2020, and 2021 to evaluate the effects of UV-reflective plastic mulch, white plastic mulch, and live mulch on whitefly abundance, SSL intensity, virus symptom severity, and marketable yield. Three-row plots were used for this trial in all years. Plots (replicates) were approximately 6 m in length with 3 m buffers between adjacent plots in a row. Treatments in all years were arranged in a randomized complete block design with four replicates. Treatments included: (1) UV-reflective mulch; (2) live mulch (buckwheat, Fagopyrum esculentum) plus white plastic mulch; and (3) white plastic mulch. The white mulch treatment was considered the control/grower standard. Buckwheat seeds were seeded directly between and outside each row in the three-row plots, two to three weeks before squash was planted. In 2021, buckwheat was re-seeded one week after initial seeding due to low germination. Buckwheat plants were also irrigated through drip lines. Yellow squash seeds (cultivar ‘Gold Star’) were seeded directly into pre-made planting holes in late August to early September of each year.
Whitefly abundance was measured weekly for three to six weeks beginning in September of each year. In 2018 and 2019, adult samples were taken by gently turning ten squash leaves per plot and counting adult whiteflies in situ on the abaxial side of each leaf. In 2020 and 2021, adult samples were taken by turning five leaves per plot and taking an image of the abaxial side of each leaf using an iPhone 7/iPhone SE/iPhone XR camera (Apple®, Inc., Cupertino, CA, USA). Adult whiteflies were counted by examining sample photos on a desktop computer. To measure whitefly egg and nymph abundances, five leaves were randomly collected per plot, stored in labeled zipper bags (Great Value, Walmart, Bentonville, AR, USA), and transported in a cooler to the Virus-Vector Interactions Laboratory in Tifton, GA (2019) or Griffin, GA (2020 and 2021). Leaf samples were stored in a refrigerator at 3–5 °C until processing. The number of eggs and nymphs in a 2.54 cm2 area on the abaxial side of each leaf were counted at 20X magnification under a dissection microscope (Leica Microsystems, Wetzlar, Germany). Eggs and nymphs were only counted to an upper limit of 200 individuals per leaf. Abundances of whitefly eggs and nymphs were not measured in 2018.
SSL intensity and virus symptom severity ratings were performed once at the end of each season. The SSL intensity in each plot was rated on a 1–5 scale in all years, as follows: 1 = 0–20% of plants exhibiting SSL symptoms; 2 = 21–40% of plants exhibiting SSL symptoms; 3 = 41–60% of plants exhibiting SSL symptoms; 4 = 61–80% of plants exhibiting SSL symptoms; and 5 = 81–100% of plants exhibiting SSL symptoms. The severity of virus symptoms was rated on a 1–5 scale in all years, as follows: 1 = no visible symptoms; 2 = ≤ 50% of plants showing leaves with minimal curling symptoms and early chlorosis; 3 = > 50% of plants showing leaves with minimal curling and early chlorosis; 4 = ≤ 50% of plants showing leaves with severe curling, yellowing, or stunting; 5 = > 50% of plants showing severe curling, yellowing, or stunting [34]. Symptomatic plant samples were randomly selected from plots to confirm CuLCrV and/or CYSDV infection. DNA and RNA were extracted, and PCR and RT-PCR were conducted using virus-specific primer sets and established protocols [24].
In all years, yield was measured by harvesting squash fruits of marketable size (≥15 cm in length) every 2–4 days and classifying as “marketable” or “non-marketable” (non-marketable data not included in analysis). Fruits were considered non-marketable when they exhibited virus symptoms, such as green streaking and mosaic discoloration, wrinkling due to poor pollination, or distorted shape. The number of fruits in each category was recorded.
2.2.3. Evaluation of Row Covers and Insecticides
Field experiments were performed in 2018, 2019, 2020, and 2021 to evaluate the effects of insecticides and physical exclusion of whiteflies (row covers) on whitefly abundance, SSL intensity, virus symptom severity, and marketable yield. Single row plots were used in all years. Plots (replicates) were 6.0–7.6 m in length with 1.5–3.0 m buffers between adjacent plots within a row. Yellow squash seeds (cultivar ‘Gold Star’) were seeded directly into pre-made planting holes in late August to early September of each year. Treatments in all years were arranged in a randomized complete block design with four replicates. Insecticide treatments evaluated in all years are listed in Table 1. Row covers (0.35 mm × 0.35 mm; Dubois Agrinovation, Saint-Rémi, QC, Canada) were installed over PVC hoops at the time squash was planted and were removed three weeks after planting or at the emergence of female flowers, whichever occurred earlier. No insecticide sprays were undertaken for the row cover treatment until the covers were removed. Cyantraniliprole (Exirel; FMC Ag US, Philadelphia, PA, USA) was applied weekly following removal of row covers. All insecticides were applied as foliar sprays using a CO2-powered backpack sprayer at the rate of 300–468 L per hectare using cone tip nozzles (TeeJet Technologies, Wheaton, IL, USA). In each year, insecticide applications were made weekly for four to six weeks.
Whitefly abundance was measured weekly for three to seven weeks beginning in September of each year. Samples of adult and immature whiteflies were collected and processed as described under the mulch trial methods. The population dynamics of whitefly eggs and nymphs were not measured in 2018.
The SSL intensity and virus symptom severity were rated using the scales previously described. SSL intensity was not measured in 2019. Virus symptom severity was rated once at the end of each season. Symptomatic plant samples were randomly selected from plots to confirm CuLCrV and/or CYSDV infection. DNA and RNA were extracted, and PCR and RT-PCR were conducted using virus-specific primer sets and established protocols [24]. To measure yield in all years, squash fruits were harvested, classified as marketable/non-marketable, and counted as previously described.
2.3. Data Analysis
End-of-season ratings of virus symptom severity, SSL intensity, whitefly counts, and marketable yield were analyzed with a generalized linear mixed model using PROC GLIMMIX in SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). The number of eggs, nymphs, and adult whiteflies, as well as the number of marketable fruits, were fitted to a Poisson distribution. Virus symptom severity and SSL intensity data were fitted to a Gaussian distribution. For the greenhouse study, the use of IEN, insecticide/acibenzolar-S-methyl, and their interaction were considered fixed effects. For the mulch evaluation study, mulch type was considered as a fixed effect. For the insecticide study, treatment (insecticides, row cover) was the fixed effect. Treatment effects and their interaction (interaction term in greenhouse study only) were considered significant at p ≤ 0.05. Multiple mean comparisons were performed using the Tukey method. Means were considered to be significantly different at p ≤ 0.05.
3. Results
3.1. Evaluation of Pre-Plant Seedling Protection Tactics (Greenhouse)
The use of IEN on squash seedlings significantly impacted the number of whitefly eggs, nymphs, and adults observed (Table 2). Squash seedlings grown under IEN had fewer whiteflies compared with non-covered seedlings, with the mean number of whitefly adults being nearly zero in both years (Table 3).

Table 2.
Effect of treatments and their interaction on the number of whiteflies on squash seedlings grown in the greenhouse at the University of Georgia Coastal Plain Experiment Station in Tifton, GA, USA, in 2018 and 2019.

Table 3.
Effect of insect exclusion netting (IEN), insecticide/acibenzolar-S-methyl, and their interactions on the number of whitefly eggs, nymphs, and adults on squash seedlings grown in the greenhouse at the University of Georgia Coastal Plain Experiment Station in Tifton, GA, USA, in 2018 and 2019.
The insecticide/acibenzolar-S-methyl treatments and the interaction between IEN and insecticide/acibenzolar-S-methyl both had a significant effect on the number of adult whiteflies observed but not on the number of eggs and nymphs (Table 2). Among non-covered seedlings, the number of adult whiteflies was consistently lower on seedlings treated with Chenopodium ambrosioides near ambrosioides extract (Requiem Prime) and cyantraniliprole (Verimark), the two insecticides included in the study (Table 3). It is not surprising that acibenzolar-S-methyl (Actigard) had no effect on whitefly abundance, as this product induces plant resistance to pathogens and does not have direct pesticidal activity. The number of whiteflies did not vary significantly among insecticide/acibenzolar-S-methyl treatments in seedlings grown under IEN, as the IEN nearly eliminated whitefly abundance on plants.
In 2018, the use of IEN (F1,32 = 10.95, p > F = 0.0023) and insecticides/acibenzolar-S-methyl (F3,32 = 6.92, p > F = 0.0010) each had a significant effect on the percent incidence of CuLCrV. The interaction between IEN and insecticide/acibenzolar-S-methyl did not have an effect on CuLCrV (F3,32 = 1.17, p > F = 0.3367). The use of IEN reduced virus incidence in seedlings by half, compared with those grown without IEN (Table 4). However, CuLCrV incidence was higher in seedlings treated with acibenzolar-S-methyl and Chenopodium ambrosioides near ambrosioides extract compared with the non-treated control and cyantraniliprole. No CuLCrV was detected in the 2019 greenhouse experiment.

Table 4.
Effect of IEN, insecticide/acibenzolar-S-methyl, and their interaction on percent incidence of cucurbit leaf crumple virus (CuLCrV) in squash seedlings grown in the greenhouse at the University of Georgia Coastal Plain Experiment Station in Tifton, GA, USA, in 2018 a.
3.2. Evaluation of Post-Plant Protection Tactics (Field)
3.2.1. Evaluation of Mulch Types
Mulch type had a significant effect on the number of whitefly eggs, nymphs, and adults over the multi-year study (Table 5). There were significantly fewer whitefly nymphs and adults in squash grown on UV-reflective mulch compared with white plastic and live mulch (Figure 1). Fewer whitefly eggs were observed in plants grown on white plastic in 2019 and with live mulch and UV-reflective mulch in 2020, but the effects were inconsistent across years. Mulch type also had a significant effect on the intensity of SSL in two out of four years. In 2018 and 2021, SSL was less intense in plots with UV-reflective mulch (Figure 1). The intensity of SSL was not different in the other two years. The symptom severity of virus infection was lower among infected plants on plots with UV-reflective mulch in 2018 and in live mulch and UV-reflective mulch treatments in 2019 (Figure 1).

Table 5.
Effect of mulch type on whiteflies, squash silverleaf disorder (SSL) intensity, virus symptom severity, and marketable yield in yellow squash grown in 2018, 2019, 2020, and 2021 at the University of Georgia Coastal Plain Experiment Station in Tifton, GA, USA.
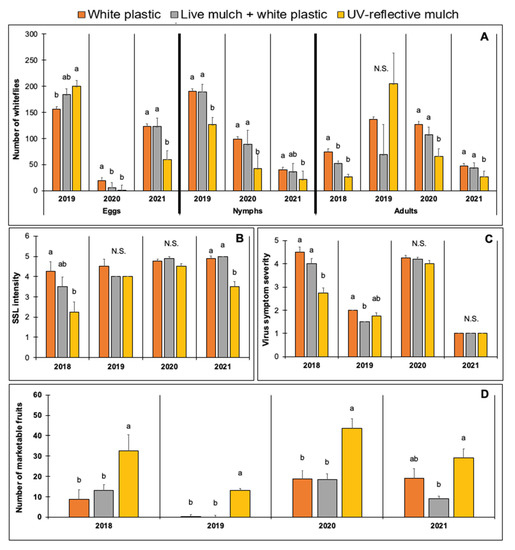
Figure 1.
Effect of mulch type on the number of immature whiteflies (per 2.54 cm2) and adult whiteflies (per leaf) (A). Squash silverleaf disorder (SSL) intensity (B). Severity of whitefly-transmitted virus symptoms (C). The number of marketable fruits per plot of yellow squash grown in field trials conducted in 2018, 2019, 2020, and 2021 at the University of Georgia Coastal Plain Experiment Station in Tifton, GA, USA (D). Data were subjected to analysis of variance using generalized linear mixed models, 𝛂 = 0.05. Treatment means followed by the same letter are not significantly different (Tukey, p < 0.05). N.S. = means not significantly different.
Mulch had a consistent effect on the number of marketable fruits harvested throughout all years. Plants grown in plots with UV-reflective mulch produced the greatest number of marketable squash fruits (Figure 1). In three out of four years of the study, the number of marketable fruits in plots with UV-reflective mulch was more than twice the amount harvested in the white plastic and live mulch plots.
3.2.2. Evaluation of Row Covers and Insecticides
The treatments had significant effects on abundances of whitefly eggs, nymphs, and adults (Table 6). The number of whitefly eggs was consistently greatest in non-treated plots and plots treated with Chromobacterium subtsugae (Grandevo WDG) (Figure 2). In 2019 and 2020, the fewest whitefly eggs were observed in plots with row covers. Compared with the non-treated control, significantly fewer whitefly eggs were observed in plots treated with imidacloprid (Admire Pro), cyantraniliprole (Exirel), flupyradifurone (Sivanto Prime), afidopyropen (Sefina), and spirotetramat plus pyriproxyfen (Senstar) in two out of the three years that whitefly eggs were recorded. Similarly, there were fewer nymphs in plots with row covers and those treated with cyantraniliprole, flupyradifurone, paraffinic oil (JMS Stylet-Oil), afidopyropen, and spirotetramat plus pyriproxyfen compared with the non-treated control (Figure 2). The abundances of nymphs in plots treated with Chromobacterium subtsugae and Chenopodium ambrosioides near ambrosioides extract were not significantly different than the non-treated control. The number of adult whiteflies also varied among treatments. In three out of the four years, significantly fewer adult whiteflies were recorded in plots with row covers and those treated with cyantraniliprole, flupyradifurone, Chenopodium ambrosioides near ambrosioides extract, and Paraffinic oil. The non-treated plots, as well as plots treated with imidacloprid and Chromobacterium subtsugae, had the greatest number of adult whiteflies (Figure 2).

Table 6.
Effect of treatment (insecticide or row cover) on whiteflies, squash silverleaf disorder (SSL) intensity, virus symptom severity, and marketable yield in yellow squash grown in 2018, 2019, 2020, and 2021 at the University of Georgia Coastal Plain Experiment Station in Tifton, GA, USA.
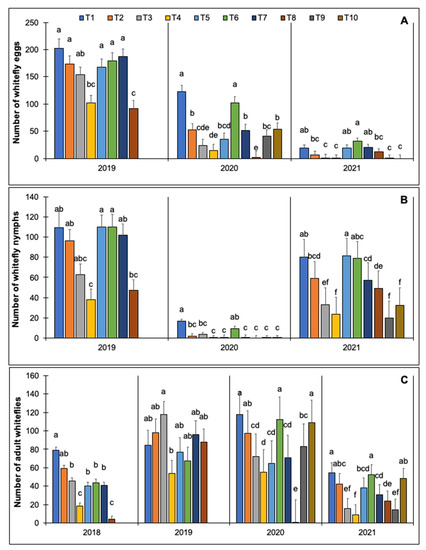
Figure 2.
Effect of insecticides and row covers followed by insecticide application on the number of whitefly eggs (per 2.54 cm2) (A). Nymphs (per 2.54 cm2) (B) and adults (per leaf) (C) observed in yellow squash grown in field trials conducted in 2018, 2019, 2020, and 2021 at the University of Georgia Coastal Plain Experiment Station in Tifton, GA, USA. Whitefly egg and nymph data were not collected in 2018. Only treatments T1-T8 were included in 2018 and 2019. All treatments (T1–T10) were included in 2020 and 2021. The treatments are as follows: T1 = non-treated control, T2 = imidacloprid (0.73 L/ha), T3 = cyantraniliprole (1.50 L/ha), T4 = flupyradifurone (1.02 L/ha), T5 = terpene constituents of Chenopodium ambrosioides near ambrosioides extract (7.01 L/ha), T6 = Chromobacterium subtsugae (3.36 kg/ha), T7 = paraffinic oil (14.03 L/ha), T8 = row cover followed by cyantraniliprole (1.50 L/ha), T9 = afidopyropen (1.02 L/ha), and T10 = spirotetramat plus pyriproxyfen (0.73 L/ha). Data were subjected to analysis of variance using generalized linear mixed models, 𝛂 = 0.05. Treatment means followed by the same letter are not significantly different (Tukey, p < 0.05).
The intensity of SSL varied among treatments in two out of the three years that data were collected (Table 6). In 2020 and 2021, SSL was less intense in plots with row covers and those treated with cyantraniliprole and flupyradifurone (Figure 3). In these three treatments, the mean SSL intensity was rated <1 at the end of the growing season. SSL was severe in plots treated with Chenopodium ambrosioides near ambrosioides extract, Chromobacterium subtsugae, paraffinic oil, and imidacloprid.
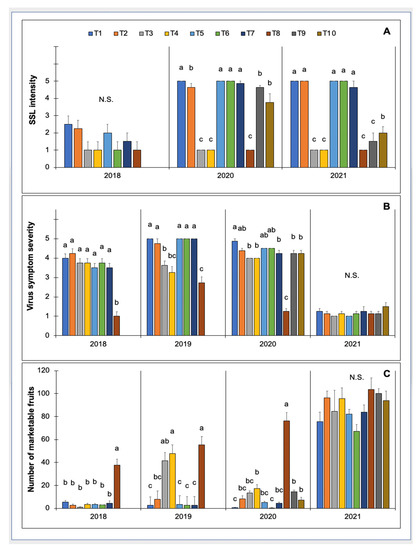
Figure 3.
Effect of insecticides and row cover followed by insecticide application on squash silverleaf disorder (SSL) intensity (data not collected in 2019) (A). Severity of whitefly-transmitted virus symptoms (B). The number of marketable fruits in yellow squash grown in field trials conducted in 2018, 2019, 2020, and 2021 at the University of Georgia Coastal Plain Experiment Station in Tifton, GA (C). Only treatments T1-T8 were included in 2018 and 2019. All treatments (T1–T10) were included in 2020 and 2021. The treatments are as follows: T1 = non-treated control, T2 = imidacloprid (0.73 L/ha), T3 = cyantraniliprole (1.50 L/ha), T4 = flupyradifurone (1.02 L/ha), T5 = terpene constituents of Chenopodium ambrosioides near ambrosioides extract (7.01 L/ha), T6 = Chromobacterium subtsugae (3.36 kg/ha), T7 = paraffinic oil (14.03 L/ha), T8 = row cover followed by cyantraniliprole (1.50 L/ha), T9 = afidopyropen (1.02 L/ha), and T10 = spirotetramat plus pyriproxyfen (0.73 L/ha). Data were subjected to analysis of variance using generalized linear mixed models, 𝛂 = 0.05. Treatment means followed by the same letter are not significantly different (Tukey, p < 0.05). N.S. = means not significantly different.
Treatments influenced virus symptom severity (Table 6). In three out of four years, virus symptoms were least severe among plots with row covers (Figure 3). In 2019 and 2020, plots treated with cyantraniliprole and flupyradifurone also had lower virus symptom severity compared with the non-treated control. The severity of virus symptoms did not vary significantly among treatments in 2021, due to low virus pressure.
Plots with row covers consistently produced the greatest number of marketable fruits in three out of four years (Figure 3). In contrast, the lowest yields were observed in plots treated with imidacloprid, Chenopodium ambrosioides near ambrosioides extract, Chromobacterium subtsugae, and paraffinic oil, and the non-treated control. The number of marketable fruits was not different among treatments in 2021.
4. Discussion
Bemisia tabaci MEAM1 and whitefly-transmitted viruses are the biggest limiting factors to profitable and sustainable yellow and zucchini squash production in Georgia, United States. In this study, several management tactics aimed at reducing the impacts of B. tabaci MEAM1 and whitefly-transmitted viruses were evaluated. This study demonstrated that adequate management measures in seedling greenhouses are key to minimizing virus inoculum sources in the field. Additionally, this study evaluated UV-reflective and live mulches, row covers, and insecticides in the field over four consecutive field seasons. The outcomes revealed that protection methods and insecticides helped manage whitefly populations and, to some extent, virus symptom severity. The benefits of these management tactics led to an increase in yield that was most prominent during the seasons when virus pressure was highest.
Whitefly-transmitted viruses are emerging worldwide [55], and the southeastern United States is no exception to this global trend. Mixed infections of CuLCrV, CYSDV, and cucurbit chlorotic yellows virus (CCYV) were found to be widespread in cucurbit fields in Georgia in 2021 [4,56]. Squash silverleaf disorder (SSL), a physiological disorder resulting from whitefly feeding, also affects squash production [5,43]. Yellow and zucchini squash varieties with resistance or tolerance to whiteflies and/or whitefly-transmitted viruses are not commercially available [34,35]. Current management programs for whiteflies and whitefly-transmitted viruses rely heavily on insecticides [37,42,57]. There is not a single management tactic available that can effectively reduce whitefly-mediated transmission of viruses to desirable levels. This study intended to offset this critically important need by evaluating a number of management tactics with the goal of developing IPM recommendations for yellow and zucchini squash production in the southeastern United States.
The first step to reducing virus pressure and the resulting reduction in yield is to avoid introducing virus-infected seedlings into the field. Squash seedlings are typically grown in open greenhouses without any insect exclusion materials. Consequently, virus-infected seedlings may be transplanted into fields, facilitating rapid secondary spread of viruses after planting. The IEN used in this study was effective in reducing whitefly abundance and virus incidence in the greenhouse study. The insecticides cyantraniliprole and terpene constituents of Chenopodium ambrosioides near ambrosioides extract also reduced the number of whitefly adults on seedlings, although cyantraniliprole alone reduced virus incidence. Acibenzolar-S-methyl, a salicylic acid analog, does not have insecticidal properties but is known to induce plant defenses against viruses by activating the salicylic acid pathway [58,59,60]. While fewer adult whiteflies were observed in the acibenzolar-S-methyl treatment compared with the non-treated control in our study, the former did not reduce virus incidence in squash seedlings. It is possible that the timing of our acibenzolar-S-methyl application could be optimized to improve virus protection [59], or that multiple applications may be necessary for suppressing CuLCrV-induced symptoms on squash seedlings [60]. The greenhouse experiment in this study demonstrated that seedling infection in open greenhouses could be minimized with the use of IEN in conjunction with insecticides.
In addition to transplant seedling protection, an effective IPM program should include tactics that reduce whitefly abundance and virus inoculum in the transplanted field. Previous studies have shown that different mulch types can have varying effects on whiteflies and virus transmission [38,43,45,49,61,62]. Summers and Stapleton [43] observed fewer whitefly adults and nymphs in squash plots with UV-reflective mulch than in bare-ground plots and found that reflective mulch was as effective for whitefly management as imidacloprid. In our study, the use of UV-reflective plastic mulch reduced the number of immature and adult whiteflies and significantly reduced SSL intensity (Figure 4). Marketable yield was doubled in plots with UV-reflective mulch compared with live mulch and white plastic. Reflective mulch may have encouraged yield increases by several complementary mechanisms: (1) by repelling whiteflies and reducing direct feeding injury and SSL intensity [43]; (2) by reducing virus transmission due to reduced feeding [38,45,61]; and (3) by radiating additional light energy onto plants, which augments photosynthesis and growth [45,63,64,65]. Although UV-reflective mulch has been shown to reduce virus pressure in squash [66,67], reduced virus severity was only detected in one year of our study. This was likely due to explosive whitefly populations in the experimental area and the fact that a single viruliferous whitefly can transmit the virus to a susceptible host plant [68,69,70].

Figure 4.
Reduced squash silverleaf disorder (SSL) intensity observed on yellow squash plants grown on UV-reflective mulch (left) compared with white plastic mulch (right) in a trial at the University of Georgia Coastal Plain Experiment Station in Tifton, GA, USA.
Live mulch intercropped with white plastic mulch only reduced adult whitefly abundance as effectively as the UV-reflective mulch in the 2018 season. This effect did not extend to abundances of whitefly eggs and nymphs, virus symptom severity, SSL intensity, or yield, and no effects of live mulch were observed in 2019, 2020, or 2021. Similarly, Frank and Liburd [48] did not observe reduced whitefly abundance or virus incidence in plots with live mulch. However, other studies have found that plantings with live mulch had comparable whitefly abundances, virus incidences, and yields to reflective mulch and even insecticide treatments [49,61]. Flowering live mulches can increase the abundances of natural enemies that attack whiteflies, such as hover flies (Diptera: Syrphidae), predatory wasps (Hymenoptera: Scoliidae, Sphecidae, Eumenidae, Vespidae), and lady beetles (Coleoptera: Coccinellidae [47,48,71,72,73]. Poor germination and growth of buckwheat in at least two years of our study due to heavy rains may have negatively impacted natural enemy populations and, subsequently, whitefly populations.
Insect-proof row covers installed immediately after planting were equally or more effective than insecticides in reducing whitefly abundance, SSL intensity, and virus symptom severity in squash plants. Additionally, protecting young plants with row covers dramatically increased marketable yield, while the insecticide treatments alone had little to no effect. Webb and Linda [33] and Costa et al. [51] saw similar reductions in whitefly abundance and SSL intensity, as well as increases in yield when squash was protected using row covers. Other studies have also seen virus incidence reduction with the use of row covers [50,51]. Row covers also exclude other pests that can cause additional injury and yield reductions [33,50]. The use of row covers in squash production is relatively new in the southeastern United States but is already practiced for frost protection in crops such as strawberries [74,75].
Insecticides have effectively reduced whitefly abundance and SSL intensity in squash in other studies [37,61,76]. Of the insecticides tested in our field trials, cyantraniliprole and flupyradifurone offered the best protection against adult whiteflies. Afidopyropen and spirotetramat plus pyriproxyfen also provided protection against whitefly nymphs. All four products have different modes of action (Table 1) and represent alternatives to commonly used neonicotinoids, such as imidacloprid. Some insecticides can also help prevent virus infection under certain conditions, including cyantraniliprole and flupyradifurone [36,39,53,77,78,79,80,81,82]. In this study, reduced virus symptom severity, but not virus incidence, was observed in plots treated with cyantraniliprole and flupyradifurone compared with the non-treated control. Virus infection was ubiquitous in the trials, often reaching 100%. Unlike many conventional insecticides that rely only on toxic activity against the vector, cyantraniliprole and flupyradifurone rapidly inhibit vector feeding, potentially limiting virus transmission [37,78,81,83,84,85,86]. Thus, insecticide applications remain an important part of IPM programs for whiteflies and viruses. However, reliance on insecticides is not recommended, as most insecticides do not impact virus incidence in the long-term, and insecticide resistance in whitefly populations is a major concern [37,38]. There is already evidence that B. tabaci MEAM1 populations in the southeastern United States have developed high levels of resistance to the commonly used neonicotinoids imidacloprid and thiamethoxam, with varying tolerances to pyrethroids, flupyradifurone, buprofezin, and dinotefuran [41,87,88].
With no “silver bullet” for managing whitefly-transmitted viruses in squash, a combination of multiple tactics with additive effects is the most effective management approach at this time. Combining the use of row covers and UV-reflective plastic mulch with effective insecticides may help achieve optimum control and ensure profitable yields until virus-resistant squash varieties become available [48,49,61,79,82]. Besides improving the management of whiteflies and viruses, integrating these cultural management practices can reduce reliance on insecticides and lessen resistance development risks [62]. These practices can be implemented by both small- and large-scale growers and may also be effective in other crops, such as watermelon, where squash vein yellowing virus, another whitefly-transmitted virus causing vine decline in watermelon, is common [79,80,89,90].
5. Conclusions
The protection of squash seedlings in greenhouses and in the field is essential for reducing whitefly and virus pressure and ensuring profitable yields. IEN/row covers were extremely effective for reducing whitefly feeding and virus symptom severity in both greenhouse and field settings. UV-reflective mulch also helped reduce whitefly pressure and SSL symptoms and led to increases in yield. Most of the insecticides tested were not as effective for managing whiteflies and viruses as the use of IEN/row covers. Until whitefly- or virus-resistant squash varieties become available, a combination of cultural and chemical tactics is required to mitigate virus-induced risks and yield losses in squash production in the southeastern United States.
Author Contributions
Conceptualization, R.S., B.D. and T.C.; methodology, R.S., B.D., C.B.C., A.L.B.R.d.S. and A.G.L.; formal analysis, C.B.C., R.S. and B.D.; investigation, A.G.L., C.B.C., S.L. and R.S.; resources, R.S., B.D. and A.L.B.R.d.S.; data curation, A.G.L., C.B.C., B.D. and R.S.; writing—original draft preparation, A.G.L. and C.B.C.; writing—review and editing, A.G.L., C.B.C., S.L., B.D., R.C.K.J., S.A., W.T. and R.S.; visualization, C.B.C. and A.G.L.; supervision, R.S., B.D., R.C.K.J., S.A., W.T. and A.L.B.R.d.S.; project administration, R.S., B.D. and A.L.B.R.d.S.; funding acquisition, R.S., B.D. and T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Georgia Commodity of Commission for Vegetables, Georgia Department of Agriculture-Specialty Crop Block Grant, USDA-SARE, USDA-Specialty Crops Research Initiative (USDA NIFA SCRI grant 2018-003391), and the UGA-USDA ARS cooperative agreement Number: 6080-22000-027-18-S.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Eric Evans, Mike Foster, Simmy McKeown, Yi-Ju Chen, Saptarshi Ghosh, Saurabh Gautam, Habibu Mugerwa, Ben Aigner, Mark Abney, and Sarah Bragg for their assistance with site preparation, experiment maintenance, technical support, sampling, and sample processing.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Stubbs, K. Georgia Farm Gate Value Report 2019; University of Georgia Center for Agribusiness and Economic Development: Athens, GA, USA, 2020; pp. 1–170. [Google Scholar]
- Srinivasan, R.; Riley, D.; Diffie, S.; Sparks, A.; Adkins, S. Whitefly population dynamics and evaluation of whitefly-transmitted tomato yellow leaf curl virus (TYLCV)-resistant tomato genotypes as whitefly and TYLCV reservoirs. J. Econ. Entomol. 2012, 105, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Coolong, T. Yellow squash and zucchini cultivar evaluation in Georgia. Horttechnology 2017, 27, 296–302. [Google Scholar] [CrossRef]
- Kavalappara, S.R.; Milner, H.; Konakalla, N.C.; Morgan, K.; Sparks, A.N.; McGregor, C.; Culbreath, A.K.; Wintermantel, W.M.; Bag, S. High throughput sequencing-aided survey reveals widespread mixed infections of whitefly-transmitted viruses in cucurbits in Georgia, USA. Viruses 2021, 13, 988. [Google Scholar] [CrossRef]
- Yokomi, R.K.; Hoelmer, K.A.; Osborne, L.S. Relationships between the sweetpotato whitefly and the squash silverleaf disorder. Phytopathology 1990, 80, 895–900. [Google Scholar] [CrossRef]
- Schuster, D.J.; Kring, J.B.; Price, J.F. Association of the sweetpotato whitefly with a silverleaf disorder of squash. HortScience 1991, 26, 155–156. [Google Scholar] [CrossRef]
- McAuslane, H.J.; Chen, J.; Bruce Carle, R.; Schmalstig, J. Influence of Bemisia argentifolii (Homoptera: Aleyrodidae) infestation and squash silverleaf disorder on zucchini seedling growth. J. Econ. Entomol. 2004, 97, 1096–1105. [Google Scholar] [CrossRef]
- Liburd, O.E.; Nyoike, T.W.; Razze, J.M. Biology and management of whiteflies in sustainable field production of cucurbits. EDIS 2008, 2008, 1–3. [Google Scholar] [CrossRef]
- Little, E.L. 2017 Georgia Plant Disease Loss Estimates; University of Georgia: Athens, GA, USA, 2019; pp. 1–21. [Google Scholar]
- Little, E.L. 2018 Georgia Plant Disease Loss Estimates; University of Georgia: Athens, GA, USA, 2020; pp. 1–20. [Google Scholar]
- Little, E.L. 2019 Georgia Plant Disease Loss Estimates; University of Georgia: Athens, GA, USA, 2021; pp. 1–29. [Google Scholar]
- Brown, J.K.; Idris, A.M.; Olsen, M.W.; Miller, M.E.; Isakeit, T.; Anciso, J. Cucurbit leaf curl virus, a new whitefly transmitted geminivirus in Arizona, Texas, and Mexico. Mexico. Plant Dis. 2000, 84, 809. [Google Scholar] [CrossRef]
- Guzman, P.; Sudarshana, M.R.; Seo, Y.-S.; Rojas, M.R.; Natwick, E.; Turini, T.; Mayberry, K.; Gilbertson, R.L. A new bipartite geminivirus (Begomovirus) causing leaf curl and crumpling in cucurbits in the Imperial Valley of California. Plant Dis. 2000, 84, 488. [Google Scholar] [CrossRef]
- Akad, F.; Webb, S.; Nyoike, T.W.; Liburd, O.E.; Turechek, W.; Adkins, S.; Polston, J.E. Detection of Cucurbit leaf crumple virus in Florida cucurbits. Plant Dis. 2008, 92, 648. [Google Scholar] [CrossRef]
- Larsen, R.; Kmiecik, K. First report of cucurbit leaf crumple virus in snap bean in Georgia. In Proceedings of the 2010 American Phytopathological Society Annual Meeting, Charlotte, NC, USA, 7–11 August 2010. [Google Scholar]
- Kuo, Y.-W.; Rojas, M.R.; Gilbertson, R.L.; Wintermantel, W.M. First report of cucurbit yellow stunting disorder virus in California and Arizona, in association with cucurbit leaf crumple virus and squash leaf curl virus. Plant Dis. 2007, 91, 330. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, K.R.; Dutta, B.; Coolong, T.; Sparks, A.N.; Adkins, S.; Srinivasan, R. First report of cucurbit yellow stunting disorder virus in cucurbits in Georgia, United States. Plant Health Prog. 2018, 19, 9–10. [Google Scholar] [CrossRef]
- Fiallo-Olivé, E.; Lett, J.M.; Martin, D.P.; Roumagnac, P.; Varsani, A.; Zerbini, F.M.; Navas-Castillo, J. ICTV virus taxonomy profile: Geminiviridae 2021. J. Gen. Virol. 2021, 102, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Bar-Joseph, M.; Candresse, T.; Maree, H.J.; Martelli, G.P.; Melzer, M.J.; Menzel, W.; Minafra, A.; Sabanadzovic, S. ICTV virus taxonomy profile: Closteroviridae. J. Gen. Virol. 2020, 101, 364–365. [Google Scholar] [CrossRef]
- Rosen, R.; Kanakala, S.; Kliot, A.; Pakkianathan, B.C.; Farich, B.A.; Santana-Magal, N.; Elimelech, M.; Kontsedalov, S.; Lebedev, G.; Cilia, M.; et al. Persistent, circulative transmission of begomoviruses by whitefly vectors. Curr. Opin. Virol. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Ghanim, M.; Morin, S.; Czosnek, H. Rate of Tomato yellow leaf curl virus translocation in the circulative transmission pathway of its vector, the whitefly Bemisia tabaci. Phytopathology 2001, 91, 188–196. [Google Scholar] [CrossRef]
- Cicero, J.M.; Brown, J.K. Anatomy of accessory salivary glands of the whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) and correlations to begomovirus transmission. Ann. Entomol. Soc. Am. 2011, 104, 280–286. [Google Scholar] [CrossRef]
- Czosnek, H.; Hariton-Shalev, A.; Sobol, I.; Gorovits, R.; Ghanim, M. The incredible journey of Begomoviruses in their whitefly vector. Viruses 2017, 9, 273. [Google Scholar] [CrossRef]
- Gautam, S.; Gadhave, K.R.; Buck, J.W.; Dutta, B.; Coolong, T.; Adkins, S.; Srinivasan, R. Virus-virus interactions in a plant host and in a hemipteran vector: Implications for vector fitness and virus epidemics. Virus Res. 2020, 286, 198069. [Google Scholar] [CrossRef]
- Célix, A.; Lopez-Sesé, A.; Almarza, N.; Gomez-Guillamon, M.L.; Rodriguez-cerezo, E. Characterization of cucurbit yellow stunting disorder virus, a Bemisia tabaci-transmitted closterovirus. Phytopathology 1996, 86, 1370–1376. [Google Scholar]
- Wisler, G.C.; Duffus, J.E.; Liu, H.; Li, R.H. Ecology and epidemiology of whitefly-transmitted closteroviruses. Plant Dis. 1998, 82, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Hogenhout, S.A.; Ammar, E.D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, X.; Wang, X.; Shi, Y.; Gu, Q.; Kuo, Y.W.; Falk, B.W.; Yan, F. Direct evidence for the semipersistent transmission of cucurbit chlorotic yellows virus by a whitefly vector. Sci. Rep. 2016, 6, 36604. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.J.; Stansly, P.A.; Polston, J.E. Expressions of plant damage of Bemisia. In Bemisia 1995: Taxonomy, Biology, Damage, Control and Management; Gerling, D., Mayer, R.T., Eds.; Intercept: Andover, GB, UK, 1996; pp. 153–165. [Google Scholar]
- Roy, A.; Acharyya, S.; Das, S.; Ghosh, R.; Paul, S.; Srivastava, R.K.; Ghosh, S.K. Distribution, epidemiology and molecular variability of the begomovirus complexes associated with yellow vein mosaic disease of mesta in India. Virus Res. 2009, 141, 237–246. [Google Scholar] [CrossRef]
- Levy, D.; Lapidot, M. Effect of plant age at inoculation on expression of genetic resistance to tomato yellow leaf curl virus. Arch. Virol. 2008, 153, 171–179. [Google Scholar] [CrossRef]
- Hooks, C.R.R.; Valenzuela, H.R.; Defrank, J. Incidence of pests and arthropod natural enemies in zucchini grown with living mulches. Agric. Ecosyst. Environ. 1998, 69, 217–231. [Google Scholar] [CrossRef]
- Webb, S.E.; Linda, S.B. Evaluation of spunbonded polyethylene row covers as a method of excluding insects and viruses affecting fall-grown squash in Florida. J. Econ. Entomol. 1992, 85, 2344–2352. [Google Scholar] [CrossRef]
- Candian, J.S.; Coolong, T.; Dutta, B.; Srinivasan, R.; Sparks, A.; Barman, A.; da Silva, A.L.B.R. Yellow squash and zucchini cultivar selection for resistance to cucurbit leaf crumple virus in the southeastern United States. Horttechnology 2021, 31, 504–513. [Google Scholar] [CrossRef]
- Luckew, A.; Meru, G.; Wang, Y.; Mwatuwa, R.; Kalischuk, M.; Luiz, A.; Ribeiro, B.; McGregor, C. Field evaluation of Cucurbita germplasm for resistance to whiteflies and whitefly-transmitted viruses. HortScience 2022, 57, 337–344. [Google Scholar] [CrossRef]
- Dempsey, M.; Riley, D.G.; Srinivasan, R. Insecticidal effects on the spatial progression of tomato yellow leaf curl virus and movement of its whitefly vector in tomato. J. Econ. Entomol. 2017, 110, 875–883. [Google Scholar] [CrossRef]
- Castle, S.; Palumbo, J.; Prabhaker, N. Newer insecticides for plant virus disease management. Virus Res. 2009, 141, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.G.; Srinivasan, R. Integrated management of tomato yellow leaf curl virus and its whitefly vector in tomato. J. Econ. Entomol. 2019, 112, 1526–1540. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.E.; Kanan, H.O.; Sugimoto, Y.; Ma, Y.Q.; Inanaga, S. Effect of imidacloprid on incidence of tomato yellow leaf curl virus. Plant Dis. 2001, 85, 84–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prabhaker, N.; Castle, S.; Perring, T.M. Baseline susceptibility of Bemisia tabaci B biotype (Hemiptera: Aleyrodidae) populations from California and Arizona to spirotetramat. J. Econ. Entomol. 2014, 107, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.A.; Nagle, C.A.; MacVean, C.A.; McKenzie, C.L. Susceptibility of Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae) to imidacloprid, thiamethoxam, dinotefuran and flupyradifurone in South Florida. Insects 2016, 7, 57. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Summers, C.G.; Stapleton, J.J. Use of UV reflective mulch to delay the colonization and reduce the severity of Bemisia argentifolii (Homoptera: Aleyrodidae) infestations in cucurbits. Crop Prot. 2002, 21, 921–928. [Google Scholar] [CrossRef]
- Polston, J.E.; Lapidot, M. Management of tomato yellow leaf curl virus: US and Israel perspectives. In Tomato Yellow Leaf Curl Virus Disease; Czosnek, H., Ed.; Springer: New York, NY, USA, 2007; pp. 251–262. [Google Scholar]
- Csizinszky, A.A.; Schuster, D.J.; Polston, J.E. Effect of ultraviolet-reflective mulches on tomato yields and on the silverleaf whitefly. HortScience 1999, 34, 911–914. [Google Scholar] [CrossRef]
- Lapidot, M.; Legg, J.P.; Wintermantel, W.M.; Polston, J.E. Management of whitefly-transmitted viruses in open-field production systems. In Advances in Virus Research; Loebenstein, G., Katis, N., Eds.; Academic Press: Burlington, NJ, USA, 2014; Volume 90, pp. 147–206. ISBN 9780128012468. [Google Scholar]
- Bugg, R.L.; Dutcher, J.D. Warm-season cover crops for pecan orchards: Horticultural and entomological implications. Biol. Agric. Hortic. 1989, 6, 123–148. [Google Scholar] [CrossRef]
- Frank, D.L.; Liburd, O.E. Effects of living and synthetic mulch on the population dynamics of whiteflies and aphids, their associated natural enemies, and insect-transmitted plant diseases in zucchini. Environ. Entomol. 2005, 34, 857–865. [Google Scholar] [CrossRef]
- Hilje, L.; Stansly, P.A. Living ground covers for management of Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) and tomato yellow mottle virus (ToYMoV) in Costa Rica. Crop Prot. 2008, 27, 10–16. [Google Scholar] [CrossRef]
- Perring, T.M.; Royalty, R.N.; Farrar, C.A. Floating row covers for the exclusion of virus vectors and the effect on disease incidence and yield of cantaloupe. J. Econ. Entomol. 1989, 82, 1709–1715. [Google Scholar] [CrossRef]
- Costa, H.S.; Johnson, M.W.; Ullman, D.E. Row covers effect on sweetpotato whitefly (Homoptera: Aleyrodidae) densities, incidence of silverleaf, and crop yield in zucchini. J. Econ. Entomol. 1994, 87, 1616–1621. [Google Scholar] [CrossRef]
- Hilje, L.; Costa, H.S.; Stansly, P.A. Cultural practices for managing Bemisia tabaci and associated viral diseases. Crop Prot. 2001, 20, 801–812. [Google Scholar] [CrossRef]
- Roberts, P.; Stansly, P.; Adkins, S.; Kousik, C.S.; Bruton, B. Management of whitefly populations for the control of watermelon vine decline in Florida. Phytopathology 2007, 97, S182. [Google Scholar]
- The IRAC Mode of Action Classification Online. Available online: https://irac-online.org/modes-of-action/ (accessed on 2 February 2022).
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Ann. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef]
- Kavalappara, S.R.; Milner, H.; Sparks, A.N.; McGregor, C.; Wintermantel, W.M.; Bag, S. First report of cucurbit chlorotic yellows virus in association with other whitefly-transmitted viruses in squash (Cucurbita pepo) in Georgia. Plant Dis. 2021, 105, 1862. [Google Scholar] [CrossRef]
- Gilbertson, R.L.; Rojas, M.; Natwick, E. Development of integrated pest management (IPM) strategies for whitefly (Bemisia tabaci)-transmissible geminiviruses. In The Whitefly, Bemisia Tabaci (Homoptera: Aleyrodidae) Interaction with Geminivirus-Infected Host Plants; Thompson, W.M.O., Ed.; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2011; pp. 323–356. ISBN 9789400715240. [Google Scholar]
- Görlach, J.; Volrath, S.; Knauf-Beiter, G.; Hengy, G.; Beckhove, U.; Kogel, K.H.; Oostendorp, M.; Staub, T.; Ward, E.; Kessmann, H.; et al. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell 1996, 8, 629–643. [Google Scholar] [CrossRef]
- Csinos, A.S.; Pappu, H.R.; McPherson, R.M.; Stephenson, M.G. Management of tomato spotted wilt virus in flue-cured tobacco with acibenzolar-S-methyl and imidacloprid. Plant Dis. 2001, 85, 292–296. [Google Scholar] [CrossRef]
- Kenney, J.R.; Grandmont, M.-E.; Mauck, K.E. Priming melon defenses with acibenzolar-s-methyl attenuates infections by phylogenetically distinct infected hosts. Viruses 2020, 12, 257. [Google Scholar] [CrossRef]
- Nyoike, T.W.; Liburd, O.E.; Webb, S.E. Suppression of whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae) and incidence of cucurbit leaf crumple virus, a whitefly-transmitted virus of zucchini squash new to Florida, with mulches and imidacloprid. Fla. Entomol. 2008, 91, 460–465. [Google Scholar] [CrossRef]
- Nasruddin, A.; Agus, N.; Saubil, A.; Jumardi, J.; Rasyid, B.; Siriniang, A.; Nasruddin, A.D.; Firdaus, F.; Said, A.E. Effects of mulch type, plant cultivar, and insecticide use on sweet potato whitefly population in chili pepper. Scientifica 2020, 2020, 6428426 . [Google Scholar] [CrossRef] [PubMed]
- Csizinszky, A.A.; Schuster, D.J.; Kring, J.B. Evaluation of color mulches and oil sprays for yield and for the control of silverleaf whitefly, Bemisia argentifolii (Bellows and Perring) on tomatoes. Crop Prot. 1997, 16, 475–481. [Google Scholar] [CrossRef]
- Mahmoudpour, M.A.; Stapleton, J.J. Influence of sprayable mulch colour on yield of eggplant (Solanum melongena L. cv. Millionaire). Sci. Hortic. 1997, 70, 331–338. [Google Scholar] [CrossRef]
- Vos, J.G.M.; Sumarni, N. Integrated crop management of hot pepper (Capsicum spp.) under tropical lowland conditions: Effects of mulch on crop performance and production. J. Hortic. Sci. 1997, 72, 415–424. [Google Scholar] [CrossRef]
- Summers, C.G.; Stapleton, J.J.; Newton, A.S.; Duncan, R.A.; Hart, D. Comparison of sprayable and film mulches in delaying the onset of aphid-transmitted virus diseases in zucchini squash. Plant Dis. 1995, 79, 1126–1131. [Google Scholar] [CrossRef]
- Murphy, J.F.; Eubanks, M.D.; Masiri, J. Reflective plastic mulch but not a resistance-inducing treatment reduced watermelon mosaic virus incidence and yield losses in squash. Int. J. Veg. Sci. 2009, 15, 3–12. [Google Scholar] [CrossRef]
- Mansour, A.; Al-Musa, A. Tomato yellow leaf curl virus: Host range and virus-vector relationships. Plant Pathol. 1992, 41, 122–125. [Google Scholar] [CrossRef]
- Mehta, P.; Wyman, J.A.; Nakhla, M.K.; Maxwell, D.P. Transmission of tomato yellow leaf curl geminivirus by Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 1994, 87, 1291–1297. [Google Scholar] [CrossRef]
- Li, M.; Hu, J.; Xu, F.C.; Liu, S.S. Transmission of tomato yellow leaf curl virus by two invasive biotypes and a Chinese indigenous biotype of the whitefly Bemisia tabaci. Int. J. Pest Manag. 2010, 56, 275–280. [Google Scholar] [CrossRef]
- Bugg, R.L.; Phatak, S.C.; Dutcher, J.D. Insects associated with cool-season cover crops in southern Georgia: Implications for pest control in truck-farm and pecan agroecosystems. Biol. Agric. Hortic. 1990, 7, 17–45. [Google Scholar] [CrossRef]
- McNeill, C.A.; Liburd, O.E.; Chase, C.A. Effect of cover crops on aphids, whiteflies, and their associated natural enemies in organic squash. Sustain. Agric. 2012, 36, 382–403. [Google Scholar] [CrossRef]
- Razze, J.M.; Liburd, O.E.; McSorley, R. Preference of Bemisia tabaci biotype B on zucchini squash and buckwheat and the effect of Delphastus catalinae on whitefly populations. Pest Manag. Sci. 2016, 72, 1335–1339. [Google Scholar] [CrossRef]
- Hochmuth, G.J.; Hochmuth, R.C.; Kostewicz, S.; Stall, W. Row Covers for Commercial Vegetable Culture in Florida; University of Florida: Gainesville, FL, USA, 1987; pp. 1–11. [Google Scholar] [CrossRef]
- Poling, E.B.; Fuller, H.P.; Perry, K.B. Frost/freeze protection of strawberries grown on black plastic mulch. HortScience 1991, 26, 15–17. [Google Scholar] [CrossRef]
- Nyoike, T.W.; Liburd, O.E. Effect of living (buckwheat) and UV reflective mulches with and without imidacloprid on whiteflies, aphids and marketable yields of zucchini squash. Int. J. Pest Manag. 2010, 56, 31–39. [Google Scholar] [CrossRef]
- Adkins, S.; Webster, C.G.; Kousik, C.S.; Webb, S.E.; Roberts, P.D.; Stansly, P.A.; Turechek, W.W. Ecology and management of whitefly-transmitted viruses of vegetable crops in Florida. Virus Res. 2011, 159, 110–114. [Google Scholar] [CrossRef]
- Caballero, R.; Schuster, D.J.; Peres, N.A.; Mangandi, J.; Hasing, T.; Trexler, F.; Kalb, S.; Portillo, H.E.; Marçon, P.C.; Annan, I.B. Effectiveness of cyantraniliprole for managing Bemisia tabaci (Hemiptera: Aleyrodidae) and interfering with transmission of tomato yellow leaf curl virus on tomato. J. Econ. Entomol. 2015, 108, 894–903. [Google Scholar] [CrossRef]
- Kousik, C.S.; Adkins, S.T.; Turechek, W.; Roberts, P.D. Use of reflective plastic mulch and insecticide sprays to manage viral watermelon vine decline in Florida, 2007. Plant Dis. Manag. Rep. 2008, 2, V169. [Google Scholar]
- Kousik, C.S.; Adkins, S.; Webster, C.G.; Turechek, W.W.; Stansly, P.; Roberts, P.D. Influence of insecticides and reflective mulch on watermelon vine deqcline caused by squash vein yellowing virus (SqVYV). Plant Health Prog. 2015, 16, 43–49. [Google Scholar] [CrossRef]
- Castle, S.; Palumbo, J.; Merten, P.; Cowden, C.; Prabhaker, N. Effects of foliar and systemic insecticides on whitefly transmission and incidence of cucurbit yellow stunting disorder virus. Pest Manag. Sci. 2017, 73, 1462–1472. [Google Scholar] [CrossRef]
- Smith, H.A.; Nagle, C.A.; MacVean, C.M.; Vallad, G.E.; Van Santen, E.; Hutton, S.F. Comparing host plant resistance, repellent mulches, and at-plant insecticides for management of Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae) and tomato yellow leaf curl virus. J. Econ. Entomol. 2019, 112, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.L.; Kennedy, G.G. The effect of three rates of cyantraniliprole on the transmission of tomato spotted wilt virus by Frankliniella occidentalis and Frankliniella fusca (Thysanoptera: Thripidae) to Capsicum annuum. Crop Prot. 2011, 30, 512–515. [Google Scholar] [CrossRef]
- Jacobson, A.L.; Kennedy, G.G. Effect of cyantraniliprole on feeding behavior and virus transmission of Frankliniella fusca and Frankliniella occidentalis (Thysanoptera: Thripidae) on Capsicum annuum. Crop Prot. 2013, 54, 251–258. [Google Scholar] [CrossRef]
- Cameron, R.; Lang, E.B.; Alvarez, J.M. Use of honeydew production to determine reduction in feeding by Bemisia tabaci (Hemiptera: Aleyrodidae) adults when exposed to cyantraniliprole and imidacloprid treatments. J. Econ. Entomol. 2014, 107, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Jeschke, P.; Velten, R.; Beck, M.E.; Ebbinghaus-Kintscher, U.; Thielert, W.; Wölfel, K.; Haas, M.; Kunz, K.; Raupach, G. Flupyradifurone: A brief profile of a new butenolide insecticide. Pest Manag. Sci. 2015, 71, 850–862. [Google Scholar] [CrossRef]
- Schuster, D.J.; Mann, R.S.; Toapanta, M.; Cordero, R.; Thompson, S.; Cyman, S.; Shurtleff, A.; Morris, R.F. Monitoring neonicotinoid resistance in biotype B of Bemisia tabaci in Florida. Pest Manag. Sci. 2010, 66, 186–195. [Google Scholar] [CrossRef]
- Caballero, R.; Cyman, S.; Schuster, D.J. Monitoring insecticide resistance in biotype b of Bemisia tabaci (Hemiptera: Aleyrodidae) in Florida. Fla. Entomol. 2013, 96, 1243–1256. [Google Scholar] [CrossRef]
- Webb, S.E.; Adkins, S.; Reitz, S.R. Semipersistent whitefly transmission of squash vein yellowing virus, causal agent of viral watermelon vine decline. Plant Dis. 2012, 96, 839–844. [Google Scholar] [CrossRef][Green Version]
- Dutta, B.; Myers, B.; Coolong, T.; Srinivasan, R.; Sparks, A. Whitefly-transmitted plant viruses in South Georgia. Univ. Georg. Coop. Ext. Bull. 2018, 1507, 1–7. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).