Optimization of Infrared Postharvest Treatment of Barhi Dates Using Response Surface Methodology (RSM)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Infrared Treatment (IR)
2.3. Experimental Design
2.4. Determination of Total Soluble Solids (TSS)
2.5. Color
2.6. Hardness
2.7. Bioactive Properties (TPC and DPPH)
2.8. Glucose
2.9. Microbial Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Model Fitting
3.2. Effect of IR Treatment and Storage Condition on the TSS, Hardness, and ΔE Values of Barhi Dates
3.3. Effect of IR Treatment and Storage Condition on the Total Viable Count of Barhi Dates
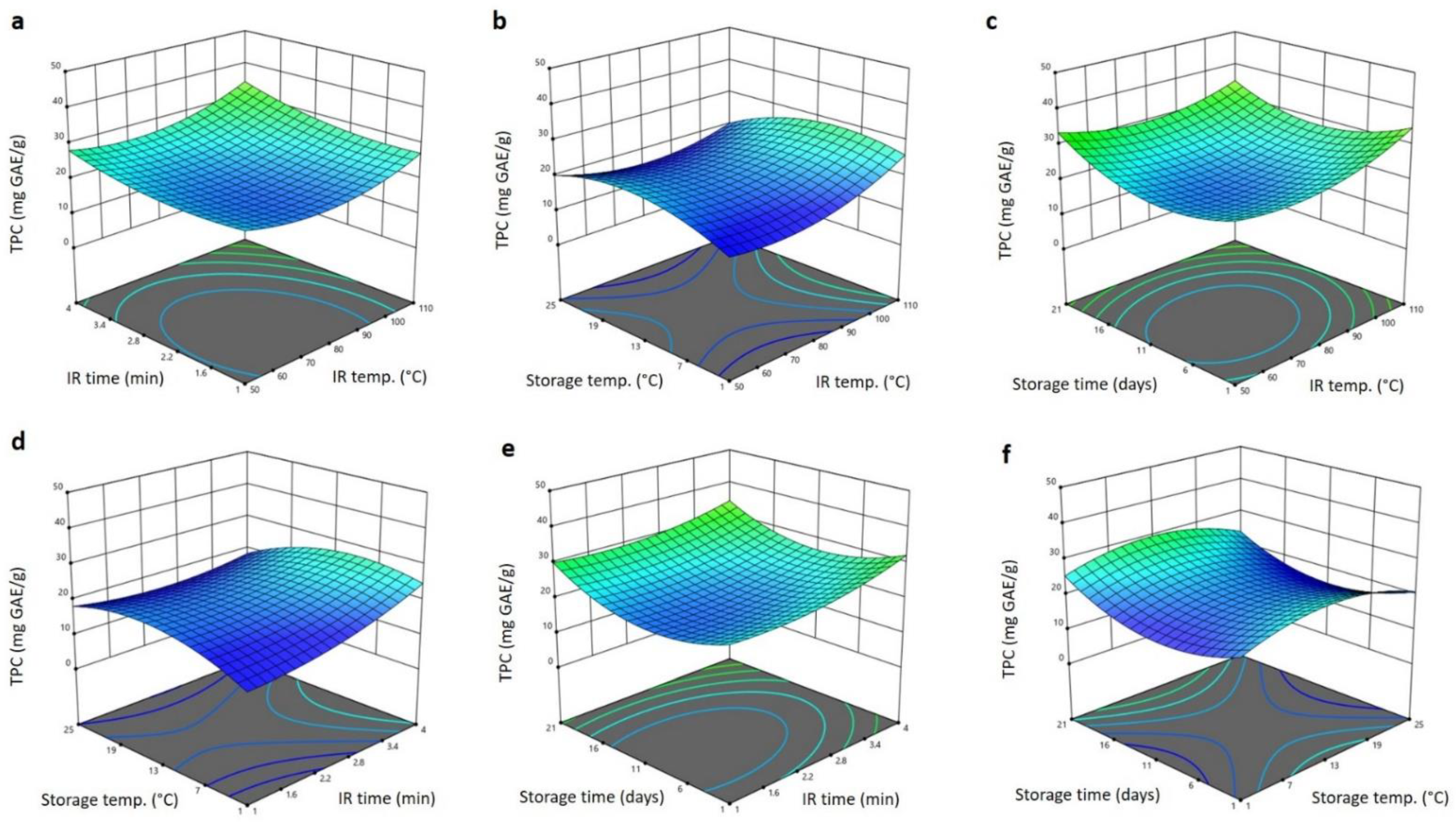
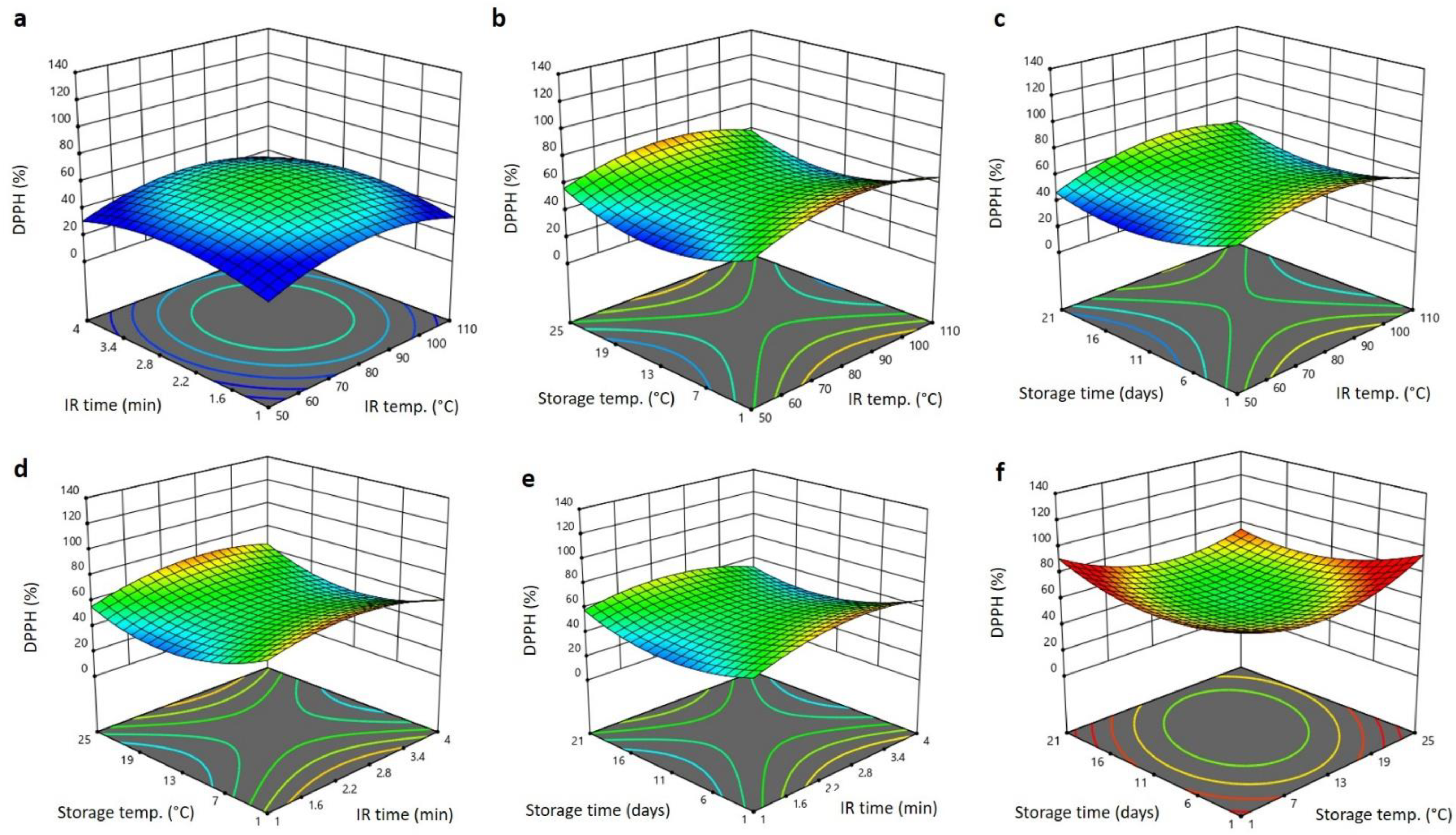
3.4. Effect of IR Treatment and Storage Condition on the Bioactive Properties of Barhi Dates
3.5. Effect of IR Treatment and Storage Condition on Glucose Content of Barhi Dates
3.6. Optimization of IR Treatment and Storage Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, Z.F.R.; Al Shaibani, F.Y.Y.; Kaur, N.; Maqsood, S.; Schmeda-Hirschmann, G. Improving fruit quality, bioactive compounds, and storage life of date palm (Phoenix dactylifera L., cv. Barhi) using natural elicitors. Horticulturae 2021, 7, 293. [Google Scholar] [CrossRef]
- Atia, A.; Abdelkarim, D.; Younis, M.; Alhamdan, A. Effects of pre-storage dipping in calcium chloride and salicylic acid on the quality attributes of stored Khalal Barhi dates. Int. J. Agric. Biol. Eng. 2020, 13, 206–212. [Google Scholar] [CrossRef]
- Alhamdan, A.M.; Fickak, A.; Atia, A.R. Evaluation of sensory and texture profile analysis properties of stored Khalal Barhi dates nondestructively using Vis/NIR spectroscopy. J. Food Process Eng. 2019, 42, e13215. [Google Scholar] [CrossRef]
- Fekry, W.M.E.; Rashad, Y.M.; Alaraidh, I.A.; Mehany, T. Exogenous Application of Melatonin and Methyl Jasmonate as a Pre-Harvest Treatment Enhances Growth of Barhi Date Palm Trees, Prolongs Storability, and Maintains Quality of Their Fruits under Storage Conditions. Plants 2022, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Alhamdan, A.; Hassan, B.; Alkahtani, H.; Abdelkarim, D.; Younis, M. Freezing of fresh Barhi dates for quality preservation during frozen storage. Saudi J. Biol. Sci. 2018, 25, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Atia, A.; Abdelkarim, D.; Younis, M.; Alhamdan, A. Effects of calcium chloride and salicylic acid postharvest treatments on the quality of Khalal Barhi dates at different ripening levels during cold storage. J. Food Meas. Character. 2018, 12, 1156–1166. [Google Scholar] [CrossRef]
- El-Gioushy, S.F.; El-Masry, A.M.; Fikry, M.; El-Kholy, M.F.; Shaban, A.E.; Sami, R.; Algarni, E.; Alshehry, G.; Aljumayi, H.; Benajiba, N.; et al. Utilization of Active Edible Films (Chitosan, Chitosan Nanoparticle, and CaCl2) for Enhancing the Quality Properties and the Shelf Life of Date Palm Fruits (Barhi Cultivar) during Cold Storage. Coatings 2022, 12, 255. [Google Scholar] [CrossRef]
- Al-Redhaiman, K.N. Chemical changes during storage of Barhi dates under controlled atmospheric conditions. Hortic. Sci. 2005, 40, 1413–1415. [Google Scholar]
- Alsawmahi, O.N.; Al-Juhaimi, F.Y.; Alhamdan, A.M.; Ghafoor, K.; Mohamed Ahmed, I.A.; Hassan, B.H.; Ehmed, K.A.; Abdelkarim, D.; Younis, M.; Alashmawe, N.; et al. Enzyme activity, sugar composition, microbial growth and texture of fresh Barhi dates as affected by modified atmosphere packaging. J. Food Sci. Technol. 2018, 55, 4492–4504. [Google Scholar] [CrossRef]
- Alsawmahi, O.N.; Al-Juhaimi, F.Y.; Alhamdan, A.M.; Ghafoor, K.; Adiamo, O.Q.; Mohamed Ahmed, I.A.; Hassan, B.H.; Ehmed, K.A.; Babiker, E.E.; Abdelkarim, D.; et al. Phenolic, tannin, antioxidant, color, and sensory attributes of Barhi date (Phoenix dactylifera) fruit stored in modified atmosphere packages. J. Food Biochem. 2018, 42, e12576. [Google Scholar] [CrossRef]
- Alhamdan, A.; Hassan, B.; Alkahtani, H.; Abdelkarim, D.; Younis, M. Cryogenic freezing of fresh date fruits for quality preservation during frozen storage. J. Saudi Soc. Agric. Sci. 2018, 17, 9–16. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Khurana, H.K.; Jun, S.; Irudayaraj, J.; Demirci, A. Infrared Heating in Food Processing: An Overview. Comp. Rev. Food Sci. Food Saf. 2008, 7, 2–12. [Google Scholar] [CrossRef]
- Aboud, S.A.; Altemimi, A.B.; Al-HiIphy, A.R.S.; Yi-Chen, L.; Cacciola, F. A Comprehensive Review on Infrared Heating Applications in Food Processing. Molecules 2019, 24, 4125. [Google Scholar] [CrossRef] [PubMed]
- Ciric, A.; Krajn, B.; Heath, D.; Ogrin, N. Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem. Toxicol. 2020, 135, 110976. [Google Scholar] [CrossRef]
- Almusallam, I.A.; Mohamed Ahmed, I.A.; Babiker, E.E.; Al Juhaimi, F.Y.; Fadimu, G.J.; Osman, M.A.; Al Maiman, S.A.; Ghafoor, K.; Alqah, H.A.S. Optimization of ultrasound-assisted extraction of bioactive properties from date palm (Phoenix dactylifera L.) spikelets using response surface methodology. LWT-Food Sci. Technol. 2021, 140, 110816. [Google Scholar] [CrossRef]
- Maskan, M. Kinetics of color change of kiwifruits during hot air and microwave drying. J. Food Engineer. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Li, B.; Du, W.; Jin, J.; Du, Q. Preservation of (-)-epigallocatechin-3 gallateantioxidant properties loaded in heat treated β-lactoglobulin nanoparticles. J. Agric. Food Chem. 2012, 60, 3477–3484. [Google Scholar] [CrossRef]
- Bouhlali, E.T.; Derouich, M.; Meziani, R.; Bourkhis, B.; Filali-Zegzouti, Y.; Alem, C. Nutritional, mineral and organic acid composition of syrups produced from six Moroccan date fruit (Phoenix dactylifera L.) varieties. J. Food Comp. Anal. 2020, 93, 103591. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Fadimu, G.J.; Ghafoor, K.; Babiker, E.E.; Al-Juhaimi, F.; Abdulraheem, R.A.; Adenekan, M.K. Ultrasound-assisted process for optimal recovery of phenolic compounds from watermelon (Citrullus lanatus) seed and peel. J. Food Meas. Charact. 2020, 14, 1784–1793. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, S.; Liao, M. Optimization of ultrasonic extraction of phenolic compounds from Euryale ferox seed shells using response surface methodology. Ind. Crops Prod. 2013, 49, 837–843. [Google Scholar] [CrossRef]
- Yao, L.; Fan, L.; Duan, Z. Effect of different pretreatments followed by hot-air and far-infrared drying on the bioactive compounds, physicochemical property and microstructure of mango slices. Food Chem. 2020, 305, 125477. [Google Scholar] [CrossRef] [PubMed]
- Bassey, E.J.; Cheng, J.-H.; Sun, D.-W. Improving drying kinetics, physicochemical properties and bioactive compounds of red dragon fruit (Hylocereus species) by novel infrared drying. Food Chem. 2022, 375, 131886. [Google Scholar] [CrossRef] [PubMed]
- Rahmawati, L.; Saputra, D.; Sahim, K.; Priyanto, G. Effect of infrared radiation on chemical and physical properties of duku’s peel. Potravin. Slovak J. Food Sci. 2018, 12, 744–755. [Google Scholar] [CrossRef][Green Version]
- Nathakaranakule, A.; Jaiboon, P.; Soponronnarit, S. Far-infrared radiation assisted drying of longan fruit. J. Food Engineer 2010, 100, 662–668. [Google Scholar] [CrossRef]
- Ee, C.T. Drying Kinetics and Quality of Dried Kedondong Fruits Undergoing Hybrid Drying Processes. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2021. [Google Scholar]
- Doymaz, İ.; Karasu, S.; Baslar, M. Effects of infrared heating on drying kinetics, antioxidant activity, phenolic content, and color of jujube fruit. Food Meas. 2016, 10, 283–291. [Google Scholar] [CrossRef]
- Byrne, B.; Dunne, G.; Bolton, D.J. Thermal inactivation of Bacillus cereus and Clostridium perfringens vegetative cells and spores in pork luncheon roll. Food Microbiol. 2006, 23, 803–808. [Google Scholar] [CrossRef]
- Oduola, A.A.; Bowie, R.; Wilson, S.A.; Shad, Z.M.; Atungulu, G.G. Impacts of broadband and selected infrared wavelength treatments on inactivation of microbes on rough rice. J. Food Saf. 2019, 40, e12764. [Google Scholar] [CrossRef]
- Maqsood, S.; Adiamo, O.; Ahmad, M.; Mudgil, P. Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chem. 2020, 308, 125522. [Google Scholar] [CrossRef]
- Tomsik, A.; Pavlic’, B.; Vladic’, J.; Ramic’, M.; Brindza, J.; Vidovic, S. Optimization of ultrasound-assisted extraction of bioactive compounds from wild garlic (Allium ursinum L.). Ultrason. Sonochem. 2016, 29, 502–511. [Google Scholar] [CrossRef]
- Vega Gálvez, A.; Uribe, E.; Poblete, J.; García, V.; Pastén, A.; Aguilera, L.E.; Stucken, K. Comparative study of dehydrated papaya (Vasconcellea pubescens) by different drying methods: Quality attributes and effects on cells viability. J. Food Meas. Charact. 2021, 15, 2524–2530. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Han, X.; Ni, Y.; Zhao, D.; Hao, J. Quality evaluation and drying kinetics of shitake mushrooms dried by hot air, infrared and intermittent microwave–assisted drying methods. LWT-Food Sci. Technol. 2019, 107, 236–242. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, Z.; Swinny, E.E. Sugars and organic acids in Japanese plums (Prunus salicina Lindell) as influenced by maturation, harvest date, storage temperature and period. Int. J. Food Sci. Technol. 2009, 44, 1973–1982. [Google Scholar] [CrossRef]




| Independent Variables | Level | ||||
|---|---|---|---|---|---|
| IR temperature, °C (X1) | 50 (−1) | 70 (−0.333) | 90 (0.333) | 110 (1) | |
| IR time, min (X2) | 1 (−1) | 2 (−0.333) | 3 (0.333) | 4 (1) | |
| Storage temperature, °C (X3) | 1 (−1) | 5 (−0.667) | 15 (0.167) | 25 (1) | |
| Storage time, days (X4) | 1 (−1) | 6 (−0.5) | 11 (0) | 16 (0.5) | 21 (1) |
| Factors | TSS | Hardness | ΔE | TVC | TPC | DPPH | Glucose |
|---|---|---|---|---|---|---|---|
| Intercept | |||||||
| β0 | 63.440 | 121.345 | 18,723.132 | 10.034 | 34.148 | 56.140 | 16.480 |
| Linear | |||||||
| X1 (β1) | −0.676 | 1.845 ** | 17,695.333 *** | 0.371 ** | −0.541 ** | 2.482 *** | −1.712 *** |
| X2 (β2) | −2.077 * | −11.794 | −9940.188 *** | −0.199 ** | −4.363 *** | 26.765 *** | −3.133 *** |
| X3 (β3) | 0.343 | 0.158 | −15,610.622 ** | −0.103 *** | 1.859 | −2.644 *** | 0.441 ** |
| X4 (β4) | 0.315 | −3.605 *** | −16,368.966 ** | −0.494 ** | −0.939 *** | −2.644 *** | 1.931 ** |
| Interaction | |||||||
| X1X2 (β12) | −0.0108 | 0.144 | 71,706.607 *** | −0.004 | 0.024 * | −0.012 *** | −0.849 ** |
| X1X3 (β13) | −0.002 | 0.009 | −7040.482 | −0.009 | −0.006 *** | −0.045 *** | −0.052 |
| X1X4 (β14) | −0.031 | 0.036 | −27,111.007 *** | 0.001 | −0.006 *** | 0.011 *** | −2.343 *** |
| X2X3 (β23) | −0.047 * | −0.074 | −273,690.111 *** | −0.010 | −0.165 *** | 0.191 *** | 2.511 *** |
| X2X4 (β24) | 0.015 | −0.352 | −7111.538 *** | 0.014 * | −0.052 ** | −0.280 *** | 1.868 *** |
| X3X4 (β34) | 0.013 ** | −0.040 | 6640.601 *** | 0.005 ** | −0.002 * | −0.055 *** | 1.262 *** |
| Quadratic | |||||||
| X12 (β11) | 0.048 *** | −0.0189 | 46,099.111 *** | −0.021 * | 0.005 ** | −0.015 *** | 6.081 *** |
| X22 (β22) | 0.592 | 1.624 | −15,913.125 | 0.188 | 1.387 ** | −4.875 *** | 1.001 |
| X32 (β33) | −0.018 * | 0.032 | −35,305.703 *** | 0.009 | −0.038 *** | 0.116 *** | −8.370 *** |
| X42 (β44) | −0.013 | −0.161 | −3967.777 *** | 0.013 * | 0.079 *** | 0.131 *** | 11.839 *** |
| Model F-value | 3.040 | 15.630 | 5.630 | 37.280 | 195.87 | 116.20 | 320.11 |
| p-value | 0.025 | 0.0012 | 0.0001 | 0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Mean | 37.05 | 114.64 | 34.64 | 4.150 | 28.51 | 60.34 | 27.45 |
| C.V. % | 3.850 | 2.890 | 2.660 | 4.260 | 1.520 | 1.752 | 1.470 |
| Adeq. precision | 6.842 | 11.485 | 11.485 | 19.026 | 9.936 | 9.336 | 70.987 |
| R2 | 0.924 | 0.963 | 0.914 | 0.967 | 0.999 | 0.919 | 0.996 |
| Adjusted R2 | 0.850 | 0.921 | 0.891 | 0.960 | 0.994 | 0.891 | 0.913 |
| Std. Dev. | 1.430 | 14.35 | 14.35 | 0.177 | 0.433 | 0.133 | 0.404 |
| F-value (Lack of Fit) | 2.250 | 0.005 | 0.005 | 4.080 | 1.760 | 0.254 | 1.240 |
| p-value (Lack of Fit) | 0.194 | 0.948 | 0.948 | 0.099 | 0.242 | 0.094 | 0.316 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelkarim, D.O.; Ahmed, K.A.; Younis, M.; Yehia, H.M.; El-Abedein, A.I.Z.; Alhamdan, A.; Ahmed, I.A.M. Optimization of Infrared Postharvest Treatment of Barhi Dates Using Response Surface Methodology (RSM). Horticulturae 2022, 8, 342. https://doi.org/10.3390/horticulturae8040342
Abdelkarim DO, Ahmed KA, Younis M, Yehia HM, El-Abedein AIZ, Alhamdan A, Ahmed IAM. Optimization of Infrared Postharvest Treatment of Barhi Dates Using Response Surface Methodology (RSM). Horticulturae. 2022; 8(4):342. https://doi.org/10.3390/horticulturae8040342
Chicago/Turabian StyleAbdelkarim, Diaeldin O., Khaled A. Ahmed, Mahmoud Younis, Hany M. Yehia, Assem I. Zein El-Abedein, Abdulla Alhamdan, and Isam A. Mohamed Ahmed. 2022. "Optimization of Infrared Postharvest Treatment of Barhi Dates Using Response Surface Methodology (RSM)" Horticulturae 8, no. 4: 342. https://doi.org/10.3390/horticulturae8040342
APA StyleAbdelkarim, D. O., Ahmed, K. A., Younis, M., Yehia, H. M., El-Abedein, A. I. Z., Alhamdan, A., & Ahmed, I. A. M. (2022). Optimization of Infrared Postharvest Treatment of Barhi Dates Using Response Surface Methodology (RSM). Horticulturae, 8(4), 342. https://doi.org/10.3390/horticulturae8040342









