Abstract
Phalaenopsis is an orchid genus of great economic value in world floriculture. In vitro clonal propagation is the only large-scale feasible method for Phalaenopsis propagation, but it is difficult because of the low multiplication rate. The aim of this study was to evaluate the effect of types and concentrations of N6-benzyladenine (6-BA) and gibberellic acid (GA3) on the in vitro multiplication of shoots from inflorescence nodal segments (INS) of Phalaenopsis hybrids. INS with one axillary bud were inoculated in New Dogashima Medium with different combinations of BA and GA3. The results show that the treatment containing 1.0 mg L−1 BA and 1.5 mg L−1 GA3 showed the higher percentage of live inflorescence segments (71.48%) and a number of shoots (1.68 shoots/INS). The highest 6-BA concentration (4 mg L−1) tested in this study resulted in the best shoot multiplication rate (4.3). Contamination and browning of the INS tissues were the main difficulties identified for clonal propagation of Phalaenopsis. Successful in vitro rooting occurred on half-strength Murashige and Skoog medium (100%), and acclimatization (100%) was obtained independent of the substrates. However, the best gains in number of roots, leaves, chlorophyll content, and fresh weight of plantlets were achieved using vermiculite.
1. Introduction
The family Orchidaceae is one of the three largest angiosperm families, with almost 28,000 species [1], as well as thousands of hybrids from different regions of the world [2]. Among the more than 700 genera of this family [1], the genus Phalaenopsis has been appointed as the orchid with the greatest commercial importance in world floriculture, being the most commercialized as a cut or pot flower and with the greatest economic value to the world floriculture industry [3], especially due to the diversity of colors and floral architecture [4], as well as the high durability of their flowers [5], allowing transport to distant sites of production.
In vitro propagation of Phalaenopsis by seeds can generate a large number of seedlings from protocorms and has applicability in the development of new cultivars and species conservation [6], but the high genetic variability and the lack of uniformity in vegetative and reproductive development [7] make the commercial production of seedlings economically uninteresting. Thus, commercial production of Phalaenopsis orchids has been based on hybrids obtained from controlled manual crosses [8] and post-selected for improved characteristics of horticultural and market interest [9], followed by in vitro cloning of superior hybrid genotypes using shoot proliferation initiated by inflorescence stalks [10,11].
Thus, the production of clonal plantlets can be considered as one of the main technological steps for Phalaenopsis cultivation, with predominance of hybrid plants that serve as mother plants for in vitro cloning. Only this technique could be considered reliable for guaranteeing the uniformity of ornamental and horticultural characteristics of interest in flower production [12].
Protocorm-like bodies (PLBs) derived from different types of somatic tissues represent a clonal method used to produce large numbers of plantlets from few mother plants [11,13]. However, regeneration of PLBs from different tissues and in different genotypes has shown as the main limitation the occurrence of different frequencies (0–51.7%) of somaclonal variations (SVs), including SVs associated with flower development—e.g., absence of flower parts of Phalaenopsis ‘PH908’ [3].
In Phalaenopsis, direct shoot induction from flower stalk segments was used for this purpose, but a limited number of commercial genotypes were tested and reported [14]. Additionally, the use of this type of explant from selected hybrids of adult Phalaenopsis allowed the production of clonal shoots from dormant buds contained in inflorescences, with or without reduced somaclonal variations, and allowed the production of in vitro leaves aiming at induction, proliferation, and regeneration of protocorm-like bodies (PLBs) into plantlets [15,16].
Some plant genotypes presented high recalcitrance to regeneration when flowering occurred [17], with recent studies pointing to a possible interface between the florigen mRNA and the inhibition of tissue/organ regeneration [18]. However, in plants, flowering development is divided into two main stages: flowering induction and the effective development of inflorescences and flowers. The developing or developed floral organs and tissues have been used, aiming at in vitro regeneration, and have until now interesting applications in different important crops, such as the induction and regeneration of the somatic embryogenesis pathway [19,20]. In addition, floral organ in some species is the unique viable explant used as an alternative to apical shoot meristem, such as in Phalaenopsis, a monopodial orchid in which the assessment of shoot tips results in the death of the mother plant [21]. Thus, in Phalaenopsis, the previously developed buds present in nodes of flowering stalks make this tissue a realistic method for shoot induction by the activation of previously formed buds contained in each node being used as explant [11,13].
Despite these benefits, the greatest limitation of this technique has been associated with the low yield of in vitro shoot multiplication and has as a consequence increased the high cost of micropropagated plantlets [10]. In this context, endogenous factors (such as genotype) as well as exogenous factors (such as culture media, phytoregulators, and growing conditions) are preponderant for increasing the multiplication rate, aiming at increasing the efficiency of micropropagation and plantlet production in Phalaenopsis [11,16].
The main questions to be addressed by this research are: Do commercial hybrids of Phalaenopsis respond at the same level of model species, such as Phal. amabilis conventionally used for in vitro regeneration? Is there a specific importance of different phytoregulator classes, such as gibberellic acid (GA3) and N6-benzyladenine (6-BA) on bud sprouting and shoot proliferation of Phalaenopsis hybrids? Does the 6-BA used in the multiplication phase have residual influence on plantlet development during the rooting phase? What changes occur in acclimatization of Phalaenopsis clonal plantlets when acclimatized using the most common substrates in world floriculture? Considering these justifications, in this research, the effects of 6-BA and GA3 on the establishment and shoot proliferation of commercial Phalaenopsis orchid hybrids were evaluated using young inflorescence segments as initial explants. In addition, an experiment aiming at improvements in the multiplication phase using different concentrations of 6-BA was also designed. Finally, clonal plantlets were subjected to acclimatization in different substrates to evaluate plantlet development.
2. Material and Methods
2.1. Establishment and Multiplication of Inflorescence Stalks of Two Cultivars of Phalaenopsis
Inflorescences of two Phalaenopsis hybrid cultivars called ‘PH501’ and ‘PH908’ from the breeding program developed by the author J.C.C. at DBPVA/UFSCar were cultivated under greenhouse conditions, using 65% shading, irrigated three times a week (3.0 mm water/irrigation) and fertigated with Poly-Feed® (19% N, 19% P2O5, 19% K2O, 1000 ppm Fe, 500 ppm Mn) at a concentration of 1 g L−1 foliar fertilizer with weekly applications.
Young floral stalks, 10–15 cm long, were collected and divided into segments of approximately 3.0 cm containing one axillary bud and submitted to asepsis in 70% alcohol for two minutes, followed by 20 min in sodium hypochlorite (2.0–2.5% active chlorine), and again immersed in 70% alcohol for 1 min. Subsequently, three washes were performed with autoclaved deionized water.
After asepsis, the borders of the inflorescence segment and the bract covering the bud were removed. Explants were introduced into 250 mL flasks containing 40 mL of New Dogashima Medium (NDM) [13] supplemented with 20 g L−1 sucrose and 0.1 g L−1 inositol, with the addition of 6-benzyladenine (6-BA), gibberellic acid (GA3), and naphthaleneacetic acid (NAA), described as follows: control without phytoregulators (PGRs); 6-BA 1.0 mg L−1, GA3 1.5 mg L−1, and NAA 0.1 mg L−1 (complete PGRs); 6-BA 0.1 mg L−1 (reduced 10×), GA3 1.5 mg L−1, and NAA 0.1, and; 6-BA 1.0, GA3 0.15 (reduced 10×), and NAA 0.1 mg L−1.
The pH of each culture medium was adjusted to 5.7 ± 0.05, and the culture media were solidified with 7.0 g L−1 plant agar. The culture medium in 250 mL flasks, covered with polypropylene caps, was autoclaved (Prismatec, Brazil) at 120 °C and 1 kgf cm−2 for 20 min. The inflorescence segments were transplanted every 60 days, until completing three subcultures of in vitro multiplication in each treatment with culture media.
All flasks were kept in a growth room under cool white fluorescent light tubes with photon flux density of 54–57 μmol m−2 s−1, photoperiod of 14 h, and temperature of 25 ± 2 °C.
Evaluations were conducted in two different phases: 60 days after inoculation of inflorescence segments under in vitro conditions after asepsis. In this phase, evaluations were carried out for percentage of contamination and living inflorescence segments, type of bud development, whether in vegetative shoot (Ve) or new inflorescence (Ge), and also after the first to third subculture of INS, called the multiplication phase, in which the multiplication rate (number of shoots obtained/segment inoculated) was evaluated.
The experiment was conducted in a factorial arrangement of two (genotypes) and four (phytoregulators) with 7 replications at the in vitro establishment phase and with 11 replications for each subculture. Each replicate consisted of a flask with four inflorescence segments. The experiment in the multiplication phase was repeated for three times, consisting of three subsequent subcultures of explants.
2.2. Improvements in the Shoot Proliferation Phase of Phalaenopsis ‘PH501’
An additional experiment was also carried out with the cultivar Phalaenopsis ‘PH501’ in order to increase the yield of shoots obtained by subculture cycle. This cultivar was chosen because of better commercial characteristics than ‘PH908’, such as larger flowers and branched inflorescences. The culture medium used in this step was Murashige and Skoog [22] with half the concentration of macronutrients (MS½), maintaining the original concentrations of microelements and vitamins and aminoacidic, and supplemented with 20 g L−1 sucrose, 0.1 g L−1 inositol, and concentrations of cytokinin 6-BA as treatments: 1, 2, 3, and 4 mg L−1, with addition of 2.4 g L−1 Gelrite (Duchefa, Netherlands). In total, six replications were used per treatment, each repetition consisting of a flask containing three apical shoots.
All flasks containing the shoots were kept in a growth room for 90 days under LED lighting with wavelengths in the blue (450 nm) and red (660 nm) (1:1.5) (Audax electronics, São Bernardo do Campo, Brazil), 40–42 µmol m−2 s−1 using a 16-h photoperiod and a temperature of 26 ± 1 °C. The experiment was repeated twice.
After 90 days of cultivation, the multiplication rate, the number of new leaves emerged, and the fresh mass of shoots were evaluated in the multiplication phase, as well as the number of leaves and roots obtained from the rooting phase of each treatment, to evaluate the 6-BA residual effects in the later stages of micropropagation, such as rooting and acclimatization.
2.3. Rooting of Shoots and Acclimatization of Plantlets Derived from Inflorescence Segments
Shoots obtained were rooted in Murashige and Skoog [22] medium containing half of the macronutrients (MS½) and supplemented with 20 g L−1 sucrose, 0.1 g L−1 inositol, 25 mL L−1 fresh coconut water, and 1 g L−1 activated charcoal and solidified with 2.4 g L−1 Gelrite and pH adjusted to 5.7 ± 0.05. Plants were cultivated in a growth room under cool white fluorescent light tubes with photon flux density of 55–57 μmol m−2 s−1, photoperiod of 14 h, and temperature of 25 ± 2 °C for 60 days.
Acclimatization was performed in 50-cell (40 mm × 40 mm × 90 mm depth) plastic trays and transferred to a greenhouse under 65% shading (Freshnet®, Agronet, Brazil) and irrigated using micro sprinklers three times a week (3.0 mm water/each irrigation), plus one fertilization a week by fertigation (0.5 L/m2) using the follow concentration of salts (mg L−1): 293 Ca(NO3)2*4H2O, 55 KNO3, 9 NH4NO3, 109 KH2PO4, 71 K2SO4, 96 MgSO4*7H2O, and a micronutrient source of 25 mg L−1 of chelated minerals (Apex Mix®, Apex Agro, Valinhos, Brazil).
Substrates tested were the coconut powder, sphagnum, and vermiculite for acclimatization of Phalaenopsis ‘Ph501′ clonal plantlets. Fresh mass, number of leaves and roots, and chlorophyll a, b, and a + b fluorescence in leaves (Clorofilog, Falker®, Porto Alegre, Brazil) were measured in plants at the beginning of acclimatization (Cf initial—day zero) and in plastic trays containing each substrate after 60 days of cultivation (Cf final). The chlorophyll content index was measured using the chlorophyll meter Clorofilog® (Falker, Porto Alegre, Brazil).
At the end of the 60 days of cultivation under greenhouse conditions, plants were removed from the substrates, and the same parameters were evaluated, aiming to detect gains/differences obtained in each substrate used.
The experiment was conducted in a completely randomized design with three treatments (substrates) and 10 replications (plants). The results were tested by analysis of variance (ANOVA), and then the treatments were compared by Tukey averages comparison test at 5% probability. These analyses were run in Agroestat software [23].
3. Results
3.1. Establishment Using Inflorescence Nodal Segment Explants
Young inflorescence segments with 10–15 cm in length can be successfully used for in vitro shoot induction of the two hybrids of Phalaenopsis ‘Ph908’ and ‘Ph501’. Buds present in inflorescence nodal segments (INS) showed two types of development: the shoots (SH) with production of new sprouts or; the generative development, which results in the formation of new inflorescences (NI) (Figure 1E). The percentage of INS of ‘PH501’ developed into NI was close to 75%, while in ‘PH908’, the majority developed into SH, 85% INS (Table 1). The different combinations of phytoregulators had no influence on the type of development, SH, or NI (Table 1).

Figure 1.
Micropropagation of Phalaenopsis hybrids using inflorescence nodal segments (INS): size of inflorescences used (A), segmentation (B), elimination of dead tissue margins after asepsis (C), and inoculation of INS in culture medium (D); (E) 90 days after in vitro cultivation of INS with vegetative (Ve) shoots or generative (Ge), obtained in the different inflorescence-type of Phalaenopsis ‘PH501’ and ‘PH908’; (F) late and persistent contamination by unidentified microorganism (in red circle) causing the death of INS and shoots in culture medium; acclimatized plantlets of ‘PH501’ after 60 days in different substrates: vermiculite (vr), sphagnum (sp), and coconut powder (cp).

Table 1.
Percentage of live inflorescence nodal segments (INS), of shoots in generative and vegetative shoots, and yield of shoots per segments of inflorescence in the in vitro establishment of flower stalks of two cultivars of Phalaenopsis.
The treatments containing 1.0 mg L−1 BA caused an increase in the percentage of live explants (67.8–78.6%) and shoot proliferation (1.92–2.12 shoots/explant), compared with the control (Table 1). The cultivar ‘PH501’ showed a higher percentage of live segments (76.8%) and number of shoots (1.81), compared with the cultivar ‘PH908’ (44.6 and 1.21, respectively), with no significant interaction between genotype and phytoregulators (Table 1).
3.2. Multiplication or Shoot Proliferation Phase
The culture medium containing the phytoregulators 6-BA, GA3, and NAA at 1.0, 1.5, and 0.1 mg L−1, respectively, resulted in higher explant survival (71.5%) and higher number of shoots (1.68). The combination of 6-BA with GA3 had an additive effect to increase shoot proliferation of Phalaenopsis hybrids (Table 2). The continuous use of this culture medium also homogenized the in vitro development and multiplication of shoots along the subcultures (Table 2).

Table 2.
Percentage of live inflorescence nodal segments (INS) and shoot multiplication rate (SM) in three different subcultures in multiplication phase and the mean of the three multiplications.
Using shoot proliferation, all the leaves were excised, keeping only the apical region containing the leaf primordia and the proximal region of developed leaves, instead of intact plants. This procedure was performed and promoted a greater number of shoots from the axillary buds than intact individual shoots (data not shown).
The 6-BA concentration was directly correlated with increased multiplication rate or shoot proliferation in Phalaenopsis; and the best multiplication rate was achieved with the highest concentration of 6-BA tested: 4.0 mg L−1 (Table 2; Figure 2). Cytokinin 6-BA also increased fresh mass gain up to a concentration of 3.0 mg L−1 (2.79 g/flask and 0.93 g/plantlet) and the number of leaves up to a concentration of 4.0 mg L−1 (9.4 leaves/flask and 3.1 leaves/plantlet) (Figure 2).
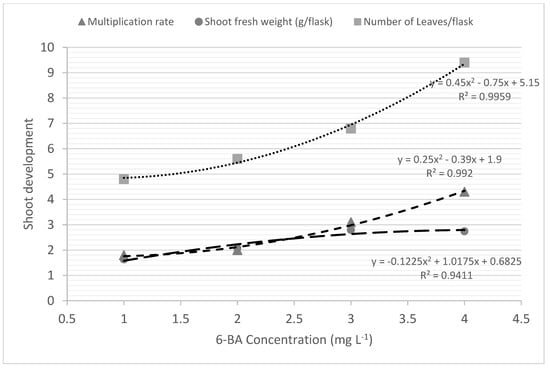
Figure 2.
In vitro shoot proliferation of Phalaenopsis cv. ‘PH501’ in concentrations of 6-BA added to culture medium.
The best development of plantlets, considering the number of leaves and roots produced at the rooting phase, was also obtained from 6-BA-derived shoots (Figure 3).
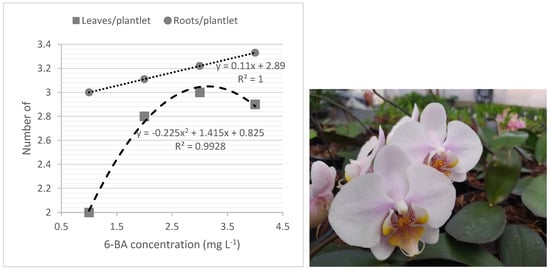
Figure 3.
Residual effects of 6-BA treatments in number of leaves and roots of plantlets of Phalaenopsis ‘PH501’ after 90 d of subculture in rooting medium under free-BA medium (left) and 18-month-old flowering plants of micropropagated Phalaenopsis ‘PH501’ (right).
3.3. Rooting of Shoots and Acclimatization of Plantlets Derived from Inflorescence Segments
The MS ½ culture medium was also effective for in vitro multiplication (Figure 2) and the in vitro rooting (Figure 3), with 100% rooted and survived plantlets for both Phalaenopsis hybrids. All plantlets (100%) survived the acclimatization phase, regardless of the substrate used. Vermiculite resulted in the highest increase in root number (1.9/plant) compared with sphagnum and coconut chips substrates, 0.9 and 0.7 roots/plant, respectively. Similarly, the highest increase in leaf number and fresh mass was also found using vermiculite, followed by sphagnum moss substrate (Table 3). In general, chlorophyll content index (a, b, a + b) from all treatments were reduced after transfer from in vitro to ex vitro acclimatization conditions, verified by the negative values (Table 3). In vermiculite, the lowest reductions in all chlorophyll content index (−9.0/−9.4 and −18.4) were found, while in coconut powder a major reduction was observed (−15.0/−15.3 and −30.3).

Table 3.
Gains in number of leaves and roots, fresh weight, and differences in chlorophyll content index (Falker index) of 60-day acclimatized plantlets (final) of Phalaenopsis ‘Ph501′ under greenhouse conditions compared with initial plantlets acclimatized (day zero).
4. Discussion
We report the successful micropropagation of Phalaenopsis hybrids using inflorescence nodal segments from young inflorescences, an interesting alternative explant to this monopodial orchid genus of high commercial importance in the world of floriculture.
4.1. Inflorescence Nodal Segments Could Be Used for Initiate Micropropagation
The differential characteristics of types of inflorescence, branched (PH501) or simple (PH908) in Phalaenopsis cultivars, seem to be a factor controlling post-development of INS buds after inoculation under in vitro conditions, similar to those observed for the architecture of inflorescences of petunias [24] and legumes [25]. INS buds of cultivar ‘PH501’ with branched inflorescences (Figure 1—PH501) developed new in vitro inflorescences (Figure 1E—Ge), while in ‘PH908’ with single inflorescence (Figure 1—PH908), INS buds directly developed into shoots under in vitro conditions (Figure 1E—Ve). The characteristic of new inflorescence development increased the multiplication rate of cultivar ‘PH501’ (1.81), compared with ‘PH908’ (1.21) (Table 1), mainly due to the formation of new nodal segments, which were sectioned and subcultured on a new culture medium for multiplication.
However, after the first subculture at the multiplication phase, only vegetative shoots were developed from all explants, and in both ‘PH501’ and ‘PH908’ cultivars (Table 2). The 6-BA added as a phytoregulator after the first subculture of INS, at the multiplication phase, resulted in a unique vegetative development of these explants since this phytoregulator was conventionally used to increase shoot proliferation and development in different orchid species [26], including Phalaenopsis [27], also with production of protocorm-like bodies (PLBs) and shoot regeneration from inflorescence shoot tips using 6-BA as the main phytoregulator [13]. Although PLBs resulted in higher proliferation rates than direct shoot proliferation, different studies associated PLBs with increases in somaclonal variations in Phalaenopsis [3,28].
The PGR-free culture medium resulted in low survival of explants and low multiplication rates, showing that dormant buds in inflorescence segments do not contain the required types and quantities of hormones—e.g., cytokinins, for spontaneous shoot induction and proliferation, indicating the importance of adding phytoregulators such as 6-BA in culture medium, aiming at rapid shoot induction. N6-Benzyladenine (6-BA), from 1 to 10 mg L−1, is an effective cytokinin for Phalaenopsis micropropagation and can also be used to obtain shoot proliferation and PLBs induction and proliferation from different explants, including inflorescence tissues [13,29,30].
The main limitation of this phase was the high contamination rate—on average 32% INS, caused by the development of a persistent colony with milky color (Figure 1A), which was not characterized. Tanaka et al. [31] also reported the contamination of flower-stalk cuttings under in vitro conditions. Contamination causes loss of time, financial, and genetic resources, as well as the risk of contamination of other flasks present in the same growth room [32,33] and also increases the costs of producing Phalaenopsis in vitro plantlets [10].
4.2. Effects of Phytoregulators on In Vitro Development of Phalaenopsis
Cytokinins, such as BA, are characterized by inducing shoots from inactive axillary buds [34] and are directly involved in cell division [35]. BA is the cytokinin most commonly used in plant tissue culture [36], and it is also efficient in promoting shoot development from inflorescence segments of Phalaenopsis, producing multiple new shoots at the multiplication phase. Our results also show that GA3 acts by improving and homogenizing shoot development and multiplication and is frequently correlated with the elongation of cells and stimulation of the growth of different types of organs [37]. In addition, it is also able to increase the number of shoots from axillary buds [38], similar to that observed in the present experiment with Phalaenopsis. Similarly, the combination of 6-BA with GA3 was able to induce faster in vitro shoot production and increased the mean number of in vitro shoots in Guizotia abyssinica [39].
The better development and proliferation of shoots in this culture medium containing higher concentrations of GA3 and 6-BA may be related to the effect of GA3 as an anti-browning agent, as has been reported using in vitro leaf segments of Phalaenopsis as explants [40]. Browning is one of the major problems affecting in vitro cultivation of Phalaenopsis and is frequently associated with high content of phenolics and increases in polyphenol oxidase activity (PPO) [41]. In the present study, we also observed browning of explants and also the culture medium, which intensified after 10–14 days of culture of the INS and along the multiplication phase (Figure 1C). The high rate of tissue browning, and its association with phenolic oxidation, has been previously reported in the genus Phalaenopsis and was associated with PPO activity [42] and is caused by physical damage to tissue, with phenolic oxidation being toxic to plant tissues and in some cases leading to plant death [43]. However, GA3 was not enough to improve shoot multiplication since the treatment containing the higher concentration of GA3, combined with the lowest 6-BA concentration (0.1 mg L−1) resulted in reduced explant survival and in vitro shoot multiplication of Phalaenopsis cultivars (Figure 1F).
4.3. INS-Derived Shoots Increased Shoot Proliferation Rate
Due to the low multiplication rate observed in previous experiments using inflorescence nodal segments as explants, an additional experiment was carried out with shoots derived from these explants, aiming to evaluate the effect of 6-BA concentrations on in vitro multiplication rate. This experiment with shoots derived from INS of ‘PH501’ and using different concentrations of 6-BA, with maintenance of the phytoregulators GA3 and NAA at 1.5 and 0.1 mg L−1, respectively, showed the positive correlation between the concentration of 6-BA and shoot proliferation rate and resulted in up to 4.3 shoots/explant using 4.0 mg L−1 6-BA (Figure 2). This is more than the double the shoot multiplication rate obtained using the concentration of 1.0 mg L−1 6-BA (Figure 2; Table 2).
There are few studies demonstrating the micropropagation of commercial cultivars of Phalaenopsis from inflorescence nodal segments and in vitro shoots used as explants. However, the results obtained in actual experiment differ from those reported by Chen and Piluek [44], in which there was a lower number of new shoots of Phalaenopsis hybrid at BAP concentrations from 10 µM (2.22 mg L−1) to 20 µM (4.44 mg L−1) (1.5–1.8 shoots/shoot), obtaining a multiplication rate similar to that obtained in the present experiment (4.3 shoots/shoot) only at the concentration of 40 µM (8.9 mg L−1), equivalent to more than double the concentration tested here (4.0 mg L−1) (Figure 2).
Chen and Piluek [44] also used the phenylurea thidiazuron (TDZ), a cytokinin-like compound, and demonstrated that this reagent was more effective than BAP for sprout proliferation in Phalaenopsis. Farrokhzad et al. [45] also observed high rates of shoot proliferation (5.86–5.93) in shoots derived from stalk internodes of Phalaenopsis amabilis cultivated in culture medium containing TDZ at 2.0 mg L−1, under low light intensity (40–80 µmol m−2 s−1). Nevertheless, highlights about recent associations between the use of TDZ and the occurrence of somaclonal variations and other physiology changes in plantlets derived from TDZ treatments were also observed in orchid micropropagation [46,47,48].
4.4. Rooting, Elongation, and Acclimatization of Phalaenopsis
Although 6-BA addition inhibited rooting and reduced the size of shoots at the multiplication phase, the shoots derived from 6-BA treatments resulted in better development of plantlets in the phytoregulator-free rooting culture medium, with improved shoot-quality. This is the opposite of that reported by different authors with micropropagated species, in which this cytokinin would be associated with a subsequent inhibition of in vitro rooting due to the formation of stable conjugates with this cytokinin in the plant [49]. However, the recent literature has shown that 6-BA-derived shoots resulted in better rooting and shoot quality in rooting and elongation phases, compared with shoots derived from 6-BA-free culture medium [50].
Our results show a strong influence of substrate and the development of plantlets after acclimatization. Values in Table 3 indicate the gains or differences in values between plants cultivated for 60 days under greenhouse conditions (after acclimatization) and at the moment of acclimatization. Due to large differences between in vitro and ex vitro environmental conditions, acclimatization leads plantlets to different types of stress, such as water content-, osmotic-, nutritional-, phytosanitary-, and photosynthetic-related stress [51].
Venturieri and Arbieto [52] concluded that only sphagnum promoted 100% survival for acclimatization with larger leaves (32% major) and roots (>24.7% major) of in vitro-derived seedlings of Phalaenopsis amabilis, followed by vermiculite, which represented a good substrate with 92.59% survival. The highest water-holding capacity was pointed out as the main difference between sphagnum, vermiculite, and other substrates [52].
However, based on the new data obtained in the present experiment, vermiculite proved to be an interesting substrate for acclimatization of micropropagated Phalaenopsis plantlets, promoting greater increases in all growth parameters evaluated, followed by sphagnum, and coconut powder (Table 3). In addition, plantlets grown in vermiculite presented the lowest value of index of chlorophyll content loss during acclimatization (Chl a −9.0, Chl b −9.4, and Chl a+b −18,4) and among all substrates used.
Chlorophyll fluorescence determination using chlorophyll meters is an easy, non-destructive technique, but this measurement is relative and requires being carried out under normalized conditions [53]. The chlorophyll content index could also be used to measure the stress suffered by plantlets, and it is associated with important enzymes stress activity [54]. Using these two concepts, there was an interesting and positive association between plantlet development and the chlorophyll content index in acclimatized plantlets of Phalaenopsis, in which the best development of Phalaenopsis plantlets was found under acclimatization with the lowest reduction in the index of chlorophyll content index (Table 3). This represents an interesting, non-destructive tool for monitoring Phalaenopsis plantlet health under acclimatization conditions.
Approximately 30 acclimatized plants from the 4.0 mg L−1 6-BA (best treatment for shoot proliferation) were cultivated in 15 cm diameter plastic pots until the flowering stage, using pine bark and coconut chips as substrate 1:1 (v/v), in order to observe the vegetative and reproductive development of plants. No relevant morphological differences were observed between the mother plants of cultivars used and those obtained from micropropagation (Figure 3), demonstrating that the technique allows for the efficient production of true-to-type plants.
The technique developed here with inflorescence nodal segments used as explants, followed by proliferation of INS-derived shoots, can also be applied to produce protocorm-like bodies (PLBs), replacing the use of leaves obtained from in vitro seedlings, which has the disadvantages of having non-clonal origin, aiming induction, and regenerating clonal plantlets [11,55].
5. Conclusions
The in vitro establishment and multiplication of Phalaenopsis hybrid cultivars is of interest for large-scale commercial production of plantlets, and the in vitro cultivation of inflorescence nodal segments as explants is an interesting alternative to the production of clonal plantlets of this important commercial orchids since other sources of explants, such as axial or apical buds, lead to the death of mother-donor plants. The addition of 6-BA and GA3 to the culture medium was positive. The use of shoots derived from INS in increased concentration of 6-BA up to 4.0 mg L−1 added to culture media resulted in the best shoot proliferation and the quality of plantlets at rooting and acclimatization stages. The chlorophyll content index proved to be a powerful tool for monitoring the development of micropropagated Phalaenopsis plantlets under acclimatization conditions. None of the plantlets acclimatized after 180 days of greenhouse growth and until flowering, presented any type of morphological variation, proving the clonal origin of plantlets obtained using the protocol developed.
Author Contributions
Author Contributions Conceptualization, J.C.C. and C.A.Z.; methodology, J.C.C., C.A.Z. and D.M.G.; software, C.A.Z. and D.M.G.; validation, C.A.Z., J.C.C. and W.N.D.; formal analysis, C.A.Z. and D.M.G.; investigation, J.C.C. and C.A.Z.; resources, J.C.C.; data curation, C.A.Z., D.M.G. and J.C.C.; writing—original draft preparation, C.A.Z. and D.M.G.; writing—review and editing, J.C.C. and W.N.D.; supervision, J.C.C.; project administration, J.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data associated with this study may also be found at https://repositorio.ufscar.br/handle/ufscar/10325?show=full#:~:text=Para%20isso%20a%20micropropaga%C3%A7%C3%A3o%2C%20por,produ%C3%A7%C3%A3o%20de%20mudas%20de%20Phalaenopsis (accessed on 13 April 2022).
Acknowledgments
J.C.C. thanks CNPQ for the process number 311083/2018-8 and the São Paulo Research Foundation (FAPESP) for the process number 2018/20673-3. C.A.Z. and W.N.D. thank Coordenação de Pessoal de Nível Superior for their MasterScience scholarships.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its anual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- RHS (Royal Horticultural Society). Search the International Orchid Register. 2016. Available online: http://apps.rhs.org.uk/horticulturaldatabase/orchidregister/orchidregister.asp (accessed on 10 February 2022).
- Cardoso, J.C.; Zanello, C.A.; Chen, J.-T. An Overview of Orchid Protocorm-Like Bodies: Mass Propagation, Biotechnology, Molecular Aspects, and Breeding. Int. J. Mol. Sci. 2020, 21, 985. [Google Scholar] [CrossRef]
- Khoddamzadeh, A.A.; Sinniah, U.R.; Kadir, M.A.; Kadzimin, S.B.; Mahmood, M.; Sreeeramanan, S. In vitro induction and proliferation of protocorm-like bodies (PLBs) from leaf segments of Phalaenopsis bellina (Rchb.f.) Christenson. Plant Growth Regul. 2011, 65, 381–387. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Martinelli, A.; Teixeira da Silva, J. A novel approach for the selection of Cattleya hybrids for precocious and season-independent flowering. Euphytica 2016, 210, 143–150. [Google Scholar] [CrossRef]
- Paek, K.Y.; Hahn, E.J.; Park, S.Y. Micropropagation of Phalaenopsis Orchids via Protocorms and Protocorm-Like Bodies. In Plant Embryo Culture. Methods in Molecular Biology (Methods and Protocols); Thorpe, T., Yeung, E., Eds.; Humana Press: Passaic, NJ, USA, 2011; Volume 710. [Google Scholar]
- Ishii, Y.; Takamura, T.; Goi, M.; Tanaka, M. Callus induction and somatic embryogenesis of Phalaenopsis. Plant Cell Rep. 1998, 17, 446–450. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Liang, F.; Jiang, S.H.; Wan, M.F.; Ma, J.; Zhang, X.Y.; Cui, B. Differential protein expression in Phalaenopsis under low temperature. Appl. Biochem. Biotechnol. 2015, 175, 909–924. [Google Scholar] [CrossRef]
- Tang, C.-Y.; Chen, W.-H. Breeding and Development of New Varieties in Phalaenopsis; Chen, W.-H., Chen, H.-H., Eds.; Orchid Biotech: Bhubaneswar, India; World Scientific: Singapore, 2007; pp. 1–22. [Google Scholar]
- Chen, C. Cost analysis of plant micropropagation of Phalaenopsis. Plant Cell Tissue Organ Cult. 2016, 126, 167–175. [Google Scholar] [CrossRef]
- Zanello, C.A.; Cardoso, J.C. PLBs induction and clonal plantlet regeneration from leaf segment of commercial hybrids of Phalaenopsis. J. Hort. Sci. Biotechnol. 2019, 94, 627–631. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, Y.E.; Yoon, Y.J.; Jeong, C.-S.; Lian, M.L.; Paek, K.-Y.; Park, S.-Y. High endoreduplicated floral organs of somaclonal variants in clonally propagated Phalaenopsis ‘Spring Dancer’. Plant Cell Tissue Organ Cult. 2016, 126, 67–77. [Google Scholar] [CrossRef]
- Tokuhara, K.; Mii, M. Micropropagation of Phalaenopsis and Doritaenopsis by culturing shoot tips of flower stalk buds. Plant Cell Rep. 1993, 13, 7–11. [Google Scholar] [CrossRef]
- Kosir, P.; Skof, S.; Luthar, Z. Direct shoot regeneration from nodes of Phalaenopsis orchids. Acta Agric. Slov. 2004, 83, 233–242. [Google Scholar]
- Balilashaki, K.; Naderi, R.; Kalantari, S.; Soorni, A. Micropropagation of Phalaenopsis amabilis cv. Cool ‘Breeze’ with using of flower stalk nodes and leaves os sterile obtained from node cultures. Int. J. Farm. Allied Sci. 2014, 3, 823–829. [Google Scholar]
- Khatun, K.; Nath, U.K.; Rahman, M.S. Tissue culture of Phalaenopsis: Present status and future prospects. J. Adv. Biotechnol. Exp. Therap. 2020, 3, 273–285. [Google Scholar] [CrossRef]
- Reuveni, M. Sex and Regeneration. Biology 2021, 10, 937. [Google Scholar] [CrossRef]
- Kutsher, Y.; Fisler, M.; Faigenboim, A.; Reuveni, M. Florigen governs shoot regeneration. Sci. Rep. 2021, 11, 13710. [Google Scholar] [CrossRef]
- de Almeida, N.V.; Rivas, E.B.; Cardoso, J.C. Somatic embryogenesis from flower tepals of Hippeastrum aiming regeneration of virus-free plants. Plant Sci. 2022, 317, 111191. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Curtolo, M.; Latado, R.R.; Martinelli, A.P. Somatic embryogenesis of a seedless sweet orange (Citrus sinensis (L.) Osbeck). Cell. Dev. Biol. Plant 2017, 53, 619–623. [Google Scholar] [CrossRef]
- Chugh, S.; Guha, S.; Rao, I.U. Micropropagation of orchids: A review on the potential of different explants. Sci. Hort. 2009, 122, 507–520. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Maldonado Junior, W. AgroEstat—Sistema Para Análises Estatísticas de Ensaios Agronômicos; FCAV/UNESP: Jaboticabal, Brazil, 2011. [Google Scholar]
- Souer, E.; Krol, A.; Kloos, D.; Spelt, C.; Bliek, M.; Mol, J.; Koes, R. Genetic control of branching pattern and floral identityduring Petunia inflorescence development. Development 1998, 125, 733–742. [Google Scholar] [CrossRef]
- Benlloch, R.; Berbel, A.; Ali, L.; Gohari, G.; Millán, T.; Madueño, F. Genetic control of inflorescence architecture in legumes. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Newton, L.A.; Runkle, E.S. Effects of benzyladenine on vegetative growth and flowering of potted Miltoniopsis orchids. Acta Hort. 2015, 1078, 121–127. [Google Scholar] [CrossRef]
- Subramanian, S.; Xavier, R.; Poobathy, R.; Sinniah, U.R. Establishement of in vitro Phalaenopsis violacea plant culture from flower-stalk cuttings. Adv. Nat. Appl. Sci. 2009, 3, 432–437. [Google Scholar]
- Tokuhara, K.; Mii, M. Induction of embryogenic callus and cell suspension culture from shoot tips excised from flower stalk buds of Phalaenopsis (Orchidaceae). Cell. Dev. Biol. Plant 2001, 37, 457–461. [Google Scholar] [CrossRef]
- Balilashaki, K.; Vahedi, M.; Karimi, R. In vitro Direct Regeneration from Node and Leaf Explants of Phalaenopsis cv. ‘Surabaya’. Plant Tissue Cult. Biotechnol. 2016, 25, 193–205. [Google Scholar] [CrossRef][Green Version]
- Datta, A.; Zahara, M.; Boonkorkew, P.; Mishra, A. Effect of plant growth regulators on the growth and direct shoot formation from leaf explants of the hybrid Phalaenopsis ‘Pink’. Acta Agric. Slov. 2018, 111, 5–16. [Google Scholar] [CrossRef]
- Tanaka, M.; Kumura, M.; Goi, M. Surface-sterilization for in vitro culture of Phalaenopsis flower-stalk cuttings using antimicrobials. Acta Hortic. 1983, 131, 321–328. [Google Scholar] [CrossRef]
- Thomas, P.; Prakash, G.S. Sanitizing long-term micropropagated grapes from covert and endophytic bacteria and preliminar field testing of plants after 8 years in vitro. Cell. Dev. Biol. Plant 2004, 40, 603–607. [Google Scholar] [CrossRef]
- Thomas, P.; Prabhakara, B.S.; Pitchaimuthu, M. Cleansing the long-term micropropagated triploid watermelon cultures from covert bacteria and field testing the plants for clonal fidelity and fertility during the 7–10 years period in vitro. Plant Cell Tissue Organ Cult. 2006, 85, 317–329. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Razdan, M.K. Plant Tissue Culture: Theory and Practice; a revised edition; Elsevier: Amsterdan, The Netherlands, 1996; 767p. [Google Scholar]
- Jana, S.; Sivanesan, I.; Jeong, B.R. Effect of cytokinins on in vitro multiplication of Sophoraton kinensis. Asian Pac. J. Trop. Biomed. 2013, 3, 549–553. [Google Scholar] [CrossRef]
- Erig, A.C.; Schuch, M.W. Ação da 6-benzilaminopurina e da qualidade da luz na multiplicação in vitro de macieira (Malus domestica BORKH.) cvs. Galaxy e Mastergala. Rev. Brasil. Agrociência 2006, 12, 151–155. [Google Scholar]
- Reis, I.N.R.S.; Lameira, O.A.; Cordeiro, I.M.C.C.; Carneiro, A.G.; Ferreira, S.F. In vitro shoot induction in paricá (Schizolobium parahyba var. amazonicum). Plant Cell Cult. Microprop. 2008, 4, 15–20. [Google Scholar]
- Nikolić, R.; Mitić, N.; Ninković, S.; Vinterhalter, B.; Korać, S.; Nesković, M. Gibberellic acid promotes in vitro regeneration and shoot multiplication in Lotus corniculatus L. Plant Growth Regul. 2010, 62, 181–188. [Google Scholar] [CrossRef]
- Baghel, S.; Bansal, Y.K. Synergistic effect of BAP and GA3 on in vitro flowering of Guizotia abyssinica Cass. a multipurpose oil crop. Physiol. Mol. Biol. Plants 2014, 20, 241–247. [Google Scholar] [CrossRef][Green Version]
- Zhou, W.; Tan, R.; Xu, C.; Lai, Y.Y.; Chen, D.Y.; Li, L. Gibberellic acid inhibits Browning, enzyme activity and gene expression of Phenylalanine ammonia-liase in Phalaenopsis leaf explants. Genes Genomes Genom. 2009, 3, 68–71. [Google Scholar]
- Xu, C.J.; Li, L. Changes of total phenol content and the activities of PPO, POD and PAL during the browning in Phalaenopsis explant in vitro. Acta Hortic. Sin. 2006, 33, 671–674. [Google Scholar]
- Xu, C.; Ru, Z.; Li, L.; Zeng, B.; Huang, J.; Huang, W.; Hu, O. The Effects of Polyphenol Oxidase and Cycloheximide on the Early Stage of Browning in Phalaenopsis Explants. Hort. Plant J. 2015, 1, 172–180. [Google Scholar] [CrossRef]
- Minamiguchi, J.; Machado Neto, N.B. Embriogênese somática direta em folhas de Phalaenopsis: Orchidaceae. Colloq. Agrar. 2007, 3, 7–13. [Google Scholar] [CrossRef]
- Chen, Y.; Piluek, C. Effects of thidiazuron and N6-benzylaminopurine on shoot regeneration of Phalaenopsis. Plant Growth Regul. 1995, 16, 99–101. [Google Scholar] [CrossRef]
- Farrokhzad, Y.; Babaei, A.; Yadollahi, A.; Kashkooli, A.B.; Mokhtassi-Bidgoli, A.; Hessami, S. Development of lighting intensity approach for shoot proliferation in Phalaenopsis amabilis through combination with silver nanoparticles. Sci. Hortic. 2022, 292, 110582. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Kumaria, S.; Tandon, P. High frequency regeneration protocol for Dendrobium nobile: A model tissue culture approach for propagation of medicinally important orchid species. S. Afr. J. Bot. 2016, 104, 232–243. [Google Scholar] [CrossRef]
- Raynalta, E.; Elina, J.; Sudarsono, S.; Sukma, D. Clonal Fidelity of Micro-propagated Phalaenopsis Plantlets Based on Assessment Using Eighteen Ph-Pto SNAP Marker Loci. AGRIVITA J. Agric. Sci. 2018, 40, 390–402. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Nurmansyah, N.Y.; Teixeira da Silva, J.A. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Rep. 2018, 37, 1451–1470. [Google Scholar] [CrossRef]
- Podwyszynska, M. Rooting of Micropropagated Shoots. In Encyclopedia of Rose Science; Andrew, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 66–76. [Google Scholar]
- Iiyama, C.M.; Cardoso, J.C. Micropropagation of Melaleuca alternifolia by shoot proliferation from apical segments. Trees 2021, 35, 1497–1509. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Hossain, M.M.; Sharma, M.; Dobránszki, J.; Cardoso, J.C.; Zeng, S. Acclimatization of in Vitro-derived Dendrobium. Hortic. Plant J. 2017, 3, 110–124. [Google Scholar] [CrossRef]
- Venturieri, G.A.; Arbieto, E.A.M. Ex-vitro establishment of Phalaenopsis amabilis seedlings in different substrates. Acta Sci. 2011, 33, 495–501. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Resende, C.F.; Pacheco, V.S.; Dornellas, F.F.; Oliveira, A.M.S.; Freitas, J.C.E.; Peixoto, P.H.P. Responses of antioxidant enzymes, photosynthetic pigments and carbohydrates in micropropagated Pitcairnia encholirioides L.B. Sm. (Bromeliaceae) under ex vitro water deficit and after rehydration. Braz. J. Biol. 2018, 79, 52–62. [Google Scholar] [CrossRef]
- Feng, J.H.; Chen, J.T. A novel in vitro protocol for inducing direct somatic embryogenesis in Phalaenopsis aphrodite without taking explants. Sci. World J. 2014, 2014, 1–7. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).