Abstract
Lime is an important commercial product in tropical and subtropical regions, where drought stress is becoming one of the most severe environmental challenges in the agricultural sector. Melatonin is an antioxidant molecule that helps plants regulate their development and respond to a variety of stresses. In this research, the effects of exogenous melatonin treatments were evaluated at different concentrations (0, 50, 100, and 150 μM) on biochemical aspects and gene expression in two species of lime plants (“Mexican lime” and “Persian lime”) under normal (100% field capacity (FC)) and drought stress conditions (75% and 40% FC). The experiments were factorial and based on a completely randomized design (CRD) with four replicates. Drought stress caused electrolyte leakage (EL) as well as accumulations of hydrogen peroxide (H2O2) and malondialdehyde (MDA), indicating the occurrence of damage to cellular membranes. In contrast, the melatonin pretreatment at various concentrations reduced the levels of EL, H2O2 and MDA while mitigating the negative effects of drought stress on the two lime species. The application of melatonin (100-μM) significantly increased the level of proline content and activity of antioxidant enzymes in plants under drought stress compared to control plants. According to real-time PCR analysis, drought stress and melatonin treatment enhanced the expression of genes involved in ROS scavenging, proline biosynthesis, and cell redox regulation in both species, as compared to their respective controls. According to these findings, melatonin is able to detoxify ROS and regulate antioxidant systems, thereby protecting lime plants from drought stress-induced damages.
1. Introduction
Limes are the smallest citrus fruits in terms of size, and are one of the most important commercial products in the tropics and subtropics. In these regions, drought is a major environmental problem that can cause a substantial decrease in their production [1]. There are two types of acidic lime species: “Persian lime” (Citrus latifolia Tanaka) and “Mexican lime” (Citrus aurantifolia (Christm) Swingle) [2,3]. While drought acts as a severe environmental restriction and prevents plants from growing or developing properly, it disrupts the morphological, physiological, and anatomical structures of plants in a variety of ways [4,5,6,7]. Drought stress generates an overabundance of reactive oxygen species (ROS) in plants and instigates membrane lipid peroxidation as well as electron leakage, thereby disrupting cell membrane structures [8,9]. Plants collect significant amounts of osmolytes that are characterized by low molecular weights and are highly soluble, non-toxic compounds, thereby maintaining plant water content under drought stress [10].
Proline is the most common plant-compatible osmolyte that can reduce membrane permeability and assist plants in maintaining water balance when they are under drought stress. In the production cycle of proline, an increase in proline accumulation in the cell can occur by the stimulation of enzyme production, especially delta 1 proline 5 carboxylate synthetase (P5CS) and proline 5 carboxylate reductase (P5CR) [11]. Protective mechanisms under osmotic stress involve direct ROS scavenging, balancing intracellular redox homeostasis, and cellular signaling enhancement [7]. In this regard, several plants have been studied, including arabidopsis (Arabidopsis thaliana) [12], walnut (Juglans regia) [13], pistachio (Pistacia vera) [14], three grapevine cultivars (Vitis vinifera L.) [15], and two olive cultivars (Olea europaea L., Arbequina and Empeltre cvs.) [16].
Plant metabolism may be severely harmed ROS, and these can cause permanent damage to vital macromolecules [17,18,19]. Hydrogen peroxide (H2O2), as the most important ROS, is involved in a number of activities related to plant growth. The production of ROS is considered a hazard to cellular metabolism since it generally results in electron leakage, speeds up cell membrane liposuction and generates the harmful chemical malondialdehyde (MDA), thereby making membranes more permeable and compromising their structural integrity [20]. Plants, on the other hand, have evolved a variety of strategies to combat the detrimental consequences of drought and to protect themselves from the harmful effects of high ROS levels. The antioxidant defense system, which is made up of both enzymatic and non-enzymatic components, may effectively scavenge ROS. Superoxide dismutase (SOD), ascorbate peroxidase (APX), peroxidase (POD), and catalase (CAT) are enzyme antioxidants found in plants. A positive correlation between antioxidant enzyme activity, production of osmolytes, and their gene expression has been reported in previous studies [21,22]. The depth-insights to abiotic stress tolerance in plants can be improved by studying the regulation of gene expression. It is plausible that over-expression of genes related to antioxidant enzymes, cellular redox regulation, and proline synthesis may enhance abiotic stress tolerance in plants. For instance, the increased SOD activity helps plants to show resistance to salinity and drought stresses in Brassica juncea plants [23]. Moreover, abscisic acid and melatonin application modulated the expression profile of the Cu/Zn superoxide dismutase (Cu/Zn SOD) gene in cotton (Gossypium hirsutum L.) plants under drought stress [24]. Additionally, the overexpression of Alternative oxidase (AOX) enhanced Arabidopsis thaliana tolerance to drought and salinity stresses [25]. Nonetheless, chronic high-stress exposure can result in severe damage and cell death [26,27]. As a result, several cases of research have aimed to increase crop resistance and reduce the destructive effects of various stressors, including drought stress. While several methods have been used for achieving this goal, plant bio-stimulators are one of the new techniques for improving plant adaptation and protection against unfavorable environmental conditions [28,29,30,31]. Findings in recent years have clearly suggested that melatonin may be a very effective substance for reducing stressors, particularly the type of stress that results from water deficits [32,33].
Melatonin reduces oxidative damage by directly scavenging ROS or by modulating the activity and production of enzymes and non-enzyme antioxidants [34]. Melatonin is reportedly able to reduce oxidative damage caused by water deficit in alfalfa (Medicago sativa L.) [35], apple [36], soybean plants [37,38], tobacco seedlings [29], and maize (Zea mays L.) [33]. Several cases of research have demonstrated that phytomelatonin can exert effects by modulating various components of the redox network or by interfering with the activity of other phytohormones even as they denote structural similarities between melatonin and indole-3-acetic acid (IAA) [39,40]. In response to its amphiphilic nature, melatonin may readily cross cellular membranes with ease, unlike other antioxidants. Several anti-stress-related genes are up-regulated in plants treated with exogenous melatonin [41]. Melatonin functions as a potent antioxidant agent while depending on its direct ability to scavenge ROS or to stimulate the activity of antioxidant enzymes. It also acts through its ability to produce a highly effective cascade of free radical scavengers among its metabolites, including N-acetyl-N-formyl 5-methoxyknuramine (AFMK) and N-acetyl-5-methoxykynuramine (AMK). Accordingly, melatonin is usually regarded as one of the most powerful antioxidants in the world when compared to other known chemicals [42].
Despite the fact that previous research has extensively focused on the impacts of this multifunctional molecule on plants, especially when plants are under abiotic stress, more research is needed to update and uncover the functions of melatonin. In fact, a complete image of melatonin-mediated drought tolerance has yet to be pictured. Melatonin acts as a protector against stress by regulating various elements of the redox network so that the expression of many stressor genes can be upheld. The current study aims at the protective functions of melatonin-induced drought-stress tolerance, including biochemical and molecular alterations, and its essential role in regulating the antioxidant system of the two lime species. The results of the current study can help growers to tackle the negative effects of drought stress on lime species through foliar application of melatonin.
2. Materials and Methods
2.1. Plant Material, Experimental Design and Treatments
One-year-old “Persian lime” (Citrus latifolia Tanaka) and “Mexican lime” (Citrus aurantifolia (Christm) Swingle) seedlings were considered as samples. All plants were grown in plastic pots (5 kg each, 33 cm in diameter and 36 cm in height). The pots were filled with soil and leaf litter (3:2 w/w). The field experiment was carried out in a greenhouse at Shiraz University, College of Agriculture in Shiraz, Iran, in September 2019. The plants were placed in a chamber with a mean temperature of 25 ± 2 °C, a relative humidity of 80%, and a day/night cycle of 14/10 h. Before initiating the experiments, all seedlings were thoroughly watered every day. A supplement of 1/2 Hoagland’s solution (pH 7.0) was given once a week. Healthy and uniform plants were selected for three watering regimes after three months of growth. The three watering regimes were, namely, (i) well-watered (100% field capacity) (FC), (ii) mild drought-stress (75% FC) and (iii) severe drought-stress (40% FC). Evaporative water loss was assessed by weighing each pot and measuring weight-related variations between each watering episode. Ethanol was used for dissolving melatonin (Sigma-Aldrich Chemie, Steinheim, Germany) and preparing different concentrations of melatonin. Additionally, Tween-20 (0.1%) as a surfactant was applied for the foliar application of melatonin. Exogenous melatonin was given to all plants in the well-watered and drought treatments, at concentrations of 50, 100, and 150 μM. As a result, four experimental groups were investigated: (i) non-melatonin treatment with well-watered conditions (i.e., well-watered control), (ii) non-melatonin treatment with drought treatments (i.e., moderate and severe stress), (iii) 50, 100, and 150 μM melatonin treatments with well-watered conditions, and (iv) 50, 100, and 150 μM melatonin treatments with drought treatments. The treatments were stopped after two months in the greenhouse. Fully young expanded leaves, from the middle part of the plant, in each treatment were sampled on day 60 for electrolyte leakage index, lipid peroxidation, proline content, antioxidant enzyme activity assessments, and RNA extraction for carrying out a relative gene expression analysis.
2.2. Estimation of Proline Content
According to the method developed by Bates et al. [43], proline content was determined in freshly collected leaves. Accordingly, 10 mL of sulfosalicylic acid (3%) (w/v) (Merck KGaA, Darmstadt, Germany) was used for homogenizing 0.5 g of fresh leaves. The extract was filtered and combined with an equal ratio of glacial acetic acid and acid-ninhydrin reagent (2 mL) (Merck KGaA, Darmstadt, Germany). The samples were incubated in boiling water for 40 min, and the reactions were then halted by an ice bath. The solution was thoroughly mixed after adding 4 mL of toluene. On a spectrophotometer (Jenway-7315, Staffordshire, UK), the light absorbance of the toluene phase was measured at 520 nm, and the proline content was evaluated using a standard proline curve. The concentration of proline was estimated in micromoles per gram of fresh weight (μmol g−1 FW).
2.3. Measurement of Electrolyte Leakage (EL)
The electrolyte leakage was measured using the Gulen and Eris [44] method. Leaf samples were sliced into one-centimeter pieces. The samples were put in test tubes and incubated in the dark for 24 h with 45 mL of distilled water (23 °C). The test tubes were stored at 25 °C. A conductivity meter was used to measure the electrical conductivity (EC1) of the electrolytes after shaking the test tubes vigorously (Hanna, HI8633, North Smithfield, RI). After the EC1 measurements, all samples were autoclaved for 15 min at 121 °C. Then, the samples were cooled (25 °C) and their electrical conductivity (EC2) was measured once more. The electrolyte leakage was calculated using the following formula:
Electrolyte leakage (%) = (EC1/EC2) × 100
2.4. Measurement of Lipid Peroxidation and Hydrogen Peroxide (H2O2) Concentration in Leaves
When malondialdehyde (MDA) was generated by the thiobarbituric acid reaction, its quantity was measured by evaluating lipid peroxidation. Accordingly, 100 mg of leaves were homogenized in 2 mL of trichloroacetic acid (0.1%) (TCA) solution (Merck KGaA, Darmstadt, Germany) and then centrifuged at 12,000 rpm for approximately 25 min at 4 °C (SIGMA 1-14 k, Osterode, Germany). The supernatant was then treated with 4 mL of thiobarbituric acid (0.5%) (TBA) in TCA (10%) (Merck KGaA, Darmstadt, Germany). The solution was centrifuged at 10,000 rpm for approximately 10 min (SIGMA 14 k, Osterode, Germany). The absorbance of the mixture was measured at 532 and 600 nm [45]. The concentration of MDA was determined using a correction factor of 0.155 (mol−1 cm−1) and was represented in micromoles per gram of fresh weight (μmol g−1 FW).
The amount of hydrogen peroxide was calculated using the method developed by Alexieva et al. [46], which involved reacting H2O2 with potassium iodide. In this technique, 0.5 g of fresh leaf tissue was crushed with 0.1% trichloroacetic acid (TCA). The resultant extract was centrifuged at 12,000 rpm for 15 min. Then, 2 mL of 1 M potassium iodide were added to 500 μL of supernatant and 500 μL of 100 mM potassium phosphate buffer (pH 7.0). The reaction mixture was kept at room temperature (25 °C) for one hour before the samples’ absorbance was measured at 390 nm.
2.5. Antioxidant Enzymes Activity
Extraction and measurement of antioxidant enzyme activity were performed using Ozden et al. [47] method. To prepare the extracts, 0.5 g of fresh leaf tissue was homogenized in 5 mL potassium phosphate buffer (50 mM) (pH 7.0) (Merck KGaA, Darmstadt, Germany) along with 2 mM ethylene diamine tetraacetic acid (EDTA) (Merck KGaA, Darmstadt, Germany) and 1% polyvinylpyrrolidone (PVP) (as extraction buffer) (Merck KGaA, Darmstadt, Germany). The resultant homogeneous mixture was centrifuged (15,000 rpm) for 15 min at 4 °C using a SIGMA 3-16PK refrigerated centrifuge (SIGMA 1-14 k, Osterode, Germany). The resultant supernatant was then stored at −84 °C until relevant experiments were performed. Using a spectrophotometer (Jenway 7315, Staffordshire, UK) to detect the increase in light absorption induced by the oxidation of guaiacol in the presence of H2O2 at a wavelength of 470 nm, the activity of the POD enzyme was calculated based on Hemeda and Klein [48]. The POD enzyme activity was determined in terms of oxidized μM of guaiacol at a rate of one min per gram of fresh sample weight using a quenching coefficient of 26.6 mM cm−1.
SOD activity was evaluated by measuring the decrease in light absorption of the nitroblutetrazolium chloride (NBT) complex [49]. In this method, the reaction mixture (3 mL) contained 50 μL extracted enzyme extract, 50 mM potassium phosphate buffer (pH 7.0), 13 mM L-methionine, 75 μM NBT, 0.1 mM EDTA, and 4 μM riboflavin. To perform the reaction, the mixtures were situated in a light chamber, which was sourced with four 20-watt fluorescent lamps. The samples were placed under the lamps for 15 min. The reaction was then stopped by turning off the lamps and placing the samples in the dark. The absorbance of each sample was read at a wavelength of 560 nm. The enzyme activity was reported as units per mg of fresh sample weight.
Dhindsa et al. [50]’s method was used for measuring the activity of CAT. According to this method, 50 μL of the extract was mixed with 1 mL of catalase-measuring solution, which contained potassium phosphate buffer (50 mM) (pH 7.0) and H2O2 (10 mM). Then, the adsorption of the solution was measured at 240 nm for one minute with a spectrophotometer (JENWAY-7315, Staffordshire, UK). The extinction coefficient for the activity of this enzyme is usually 39.4 mM−1 cm−1, and an enzyme unit (CAT) equal to the decomposition of one mM of H2O2 per minute was considered.
To measure the activity of APX, the reaction mixture consisted of 150 μL enzyme extract, 50 mM phosphate buffer, 1.2 mM oxygenated water, 0.5 mM ascorbic acid, and 0.1 mM EDTA. Due to ascorbic acid peroxidation, the decrease in light absorption was read at 290 nm with a spectrophotometer. The extinction coefficient for enzyme activity is usually 2.8 mM−1 cm−1. Absorption changes per minute, per gram of fresh sample weight, were used for calculating enzyme activity [51].
The activity of glutathione reductase (GR) was measured using the Foyer and Halliwell [52] technique in a reaction mixture containing 25 mM sodium phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.5 mM oxidized glutathione (GSSG), 0.12 mM NADPH, and 100 μL of enzyme extract. In accordance with NADPH oxidation, an extinction coefficient of 6.2 mM−1 cm−1 was used for evaluating GR activity by monitoring the reduction in absorbance at 340 nm. As a result, GR activity was expressed as specific activity units per mg−1 protein.
2.6. RNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR
RNA was extracted according to the manufacturer’s protocol using Iraizol kit (RNA Biotechnology Co., Isfahan, Iran). The total RNA concentration and its quality were measured using a Nano-Drop (ND) 1000 spectrophotometer (Implen Nano Photometer, NPOS 3.0 version 12984, Westlake Village, CA, USA). Agarose gel electrophoresis (1.2% w/v) was also used for evaluating total RNA integrity. DNaseI (Promega, Madison, WI, USA) was employed to eliminate genomic DNA before cDNA synthesis. Following the manufacturer’s instructions, cDNA was synthetized from 1 µg of total RNA using the cDNA synthesis kit (RNA Biotechnology Co., Isfahan, Iran). Primers for genes were involved in ROS scavenging (cytosolic ascorbate peroxidase, cAPX; Cu/Zn superoxide dismutase, Cu/Zn SOD; Fe superoxide dismutase, Fe SOD), cellular redox regulation (alternative oxidase, AOX; NADH dehydrogenase, NaDde), and proline synthesis (Pyrroline-5-carboxylate synthase, P5CS; pyrroline-5-carboxylate reductase, P5CR). The sequences of the primers (Table 1) were designed based on the conserved sequences in Rutaceae found in the NCBI GenBank database. The ABI Step One (Applied Biosystems, Waltham, MA, USA) was used for running the real-time PCR test in a 10 μL reaction mixture. Each reaction included 5 μL SYBR Green (2X RealQ Plus Master Mix Green, AMPLIQON, Odense, Denmark), 0.1 μL of each primer (10 µM), and 1 μL of five times diluted cDNA template, and deionized double distilled water up to 10 µL.

Table 1.
Primer sequences used for Real Time PCR analysis in “Mexican lime” (Citrus aurantifolia (Christm) Swingle) and “Persian lime” (Citrus latifolia Tanaka).
The amplification conditions had several steps, including initial denaturation at 95°C for 10 min, followed by 40 cycles of 95 °C for 15 s, appropriate annealing temperature (Table 1) for 30 s, and finally, 72 °C for 45 s. To confirm the purity of the amplified products, a melting curve was generated for each sample when each cycle ended. The 2−ΔΔCt technique was used for calculating relative gene expression levels [53] using the Actin gene (ACT) as an internal reference gene. The experiment on gene expression was conducted with three replicates in both technical and biological samples.
2.7. Statistical Analysis
The experiment was performed as a CRD in a factorial arrangement with three factors, including four concentrations of melatonin, three levels of drought stress, and two Citrus species. The experiment had 24 treatments with four replications (96 experimental units). The SAS software (SAS Institute, Cary, NC, USA, V.9.1) was used for carrying out the analysis of variance (ANOVA). The LSD test was used to compare mean values at a 5% probability level (p ≤ 0.05).
3. Results
3.1. Proline Content
Based on the results of comparing the mean effects of treatments, drought stress increased proline concentration in leaves, and a significant difference was observed between species. Comparison of means at different levels of stress showed that Mexican lime control plants with (55.5 μmol g−1 FW) had the lowest and Mexican lime and Persian lime species under severe drought stress and 100 μM melatonin foliar application with 155.3 and 204.3 μmol g−1 FW had the highest proline concentration, respectively. Proline levels in both species of lime under drought treatment showed a significant increase compared to the control. Overall, treatments of 100 and 50 μM melatonin significantly increased proline levels, and at the highest concentration (150 μM) this increase was less (Table 2).

Table 2.
Effect of melatonin concentrations, lime species, and percentage of drought stress on biochemical responses including proline content, percentage electrolyte leakage (%EL), hydrogen peroxidase (H2O2) and malondialdehyde (MDA).
3.2. Electrolyte Leakage
Drought stress caused an increase in EL into the intercellular space. This increase was 59.4% in “Mexican lime” and 33.4% in “Persian lime” in response to 40% FC, compared to the control (100% FC), implying that drought stress impaired cellular membrane integrity and fluidity. Exogenous melatonin, on the other hand, significantly controlled the rise of EL under drought conditions and ultimately led to a decrease of approximately 20% in the EL in plants sprayed with a concentration of 100 μM melatonin, compared to the control group under severe drought stress (Table 2). The foliar application of 100 μM melatonin was the most efficient treatment in preserving cellular membrane integrity.
3.3. Hydrogen Peroxide and Malondialdehyde Contents in Leaf Extracts
As important indicators of stress-induced ROS levels and oxidative damage, H2O2 and MDA levels were measured in the control and melatonin-pretreated plants during drought stress treatments. Melatonin had no influence on the levels of H2O2 or MDA in the control group. When drought stress was applied, melatonin-pretreated lime plants had significantly lower levels of H2O2 and MDA, compared to non-treated lime plants, indicating less oxidative damage. These findings suggest that exogenous melatonin treatment can reduce abiotic stress-induced ROS production and mitigate oxidative damage in both lime species. H2O2 and MDA accumulated extensively in plant leaves after the drought treatment, but this accumulation was partially mitigated by melatonin application. In plants treated with drought and melatonin, the leaf H2O2 concentration was lower than in control plants, although drought stress treatment alone raised the H2O2 content in “Mexican lime” and “Persian lime” by 4.5 and 7.8 μmol g−1 FW, respectively. Similarly, drought stress alone increased the leaf MDA content, whereas applying 100 μM melatonin caused a maximum decline in MDA content, regardless of the level of drought stress (Table 2).
3.4. Changes in the Activities of Antioxidant Enzymes
Drought stress affected SOD, POD, GR, and APX levels in the leaves of both lime species, either with or without melatonin treatment. The results demonstrated that severe drought-stressed plants (particularly at 40% FC) had higher SOD, GR, and APX activity, compared to the control plants. CAT activity showed a different trend than other enzymes. The highest activity of this enzyme was observed in Persian lime species under moderate drought stress (75% FC) and foliar application with 100 μM melatonin, which was not significantly different from plants under severe drought stress at the same concentration. The application of 100 μM melatonin caused plants to have higher antioxidant enzyme activity than plants that had been exposed to severe drought stress (Figure 1).
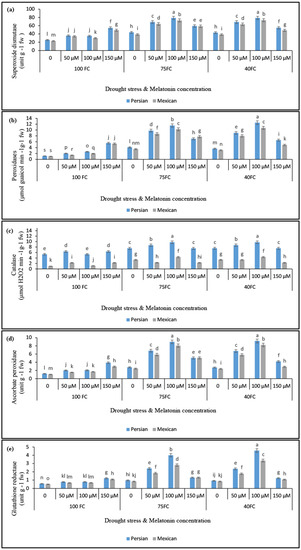
Figure 1.
The effects of different concentrations of exogenous melatonin, Citrus species, and various levels of drought stress on the activities of antioxidant enzymes including (a) superoxide dismutase, (b) peroxidase, (c) catalase, (d) ascorbate peroxidase, and (e) glutathione reductase. Based on the LSD test, columns with similar letters are not significant at the 5% probability level.
Both species showed significantly enhanced activities of SOD under stress, and those activities were further increased by exposure to melatonin. Generally, the results of this experiment showed that 100 μM melatonin under severe drought stress (40% FC) significantly enhanced the SOD activities in both Persian lime (78.8 units g−1 FW) and Mexican lime (73.5 units g−1 FW). Additionally, in moderate drought stress (75% FC), a similar trend was observed and melatonin foliar application showed a positive effect on increasing the activity of this enzyme. The lowest activities of SOD were observed in both species under unstressed condition without melatonin foliar application. According to these findings, there was a significant difference in POD activity caused by the different melatonin concentrations under normal and drought-stress conditions. The 100 μM melatonin treatment led to the increase in POD activity in “Mexican lime” (10.6 μmol guaicol min−1 g−1 FW) and “Persian lime” (11.9 μmol guaicol min−1 g−1 FW). Without using melatonin, the lowest POD activity (1.2 μmol guaicol min−1 g−1 FW) was obtained in the unstressed “Mexican lime” (Figure 1b). In general, the findings of this experiment demonstrated that 100 μM melatonin substantially increased CAT activity in both “Persian lime” (9.7 μmol H2O2 min−1 g−1 FW) and “Mexican lime” (4.3 μmol H2O2 min−1 g−1 FW) under severe drought stress, compared to the unstressed condition. At moderate drought stress (75% FC) similar results were obtained compared to severe drought stress (40% FC) and the application of 50 and 100 μM melatonin increased CAT activity. The lowest level of CAT activity in both species was observed in control plants (Figure 1c).
The findings showed that drought treatment at 40% FC increased APX activity in Mexican lime (9.2 units g−1 FW) and Persian lime (8.2 units g−1 FW), as compared to the control. Under moderate and severe drought conditions, treating lime plants with 100 μM melatonin increased the activity of this enzyme (Figure 1d). In drought-stressed plants, GR activity increased in a manner similar to other enzyme activities. During severe drought stress, using 100 µM melatonin increased the activity of GR in “Persian lime” and “Mexican lime” (4.5 and 2.8 units g−1 FW, respectively). Compared to the non-drought controls treated with different melatonin dosages, drought-stressed plants with the same melatonin treatments showed substantial GR activity in leaf extracts (Figure 1e).
3.5. Relative Gene Expression
The formation of 28srRNA and 18srRNA bands on the gel electrophoresis indicated no fracture or damage to the RNA structure. Additionally, the PCR products confirmed the length of the amplified fragments on a 1.2% agarose gel. It showed that all primers were able to amplify the expected fragments well (Figure 2).
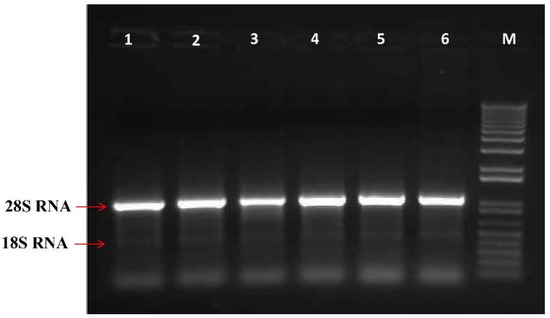
Figure 2.
Agarose gel electrophoresis shows total RNA extracted from Persian lime leaves in some treatments (1: 50 μM melatonin under mild drought-stress; 2: 100 μM melatonin under mild drought-stress; 3: 150 μM melatonin under mild drought-stress; 4: 50 μM melatonin under severe drought-stress; 5: 100 μM melatonin under severe drought-stress; 6: 150 μM melatonin under severe drought-stress; M: Marker III).
The results of the real-time PCR reaction in the “Mexican lime” revealed that the relative expression of SOD Cu/Zn and Fe SOD genes increased by about 19.04 and 27.412-fold in response to severe drought stress and foliar application (100 µM melatonin), respectively. Additionally, the highest expression of these genes was observed in Persian lime species in response to mild drought stress and foliar application (100 μM melatonin), which caused these genes to increase their expression by about 20.066 and 28.147-fold, respectively, compared to the control sample (Figure 3a,b).
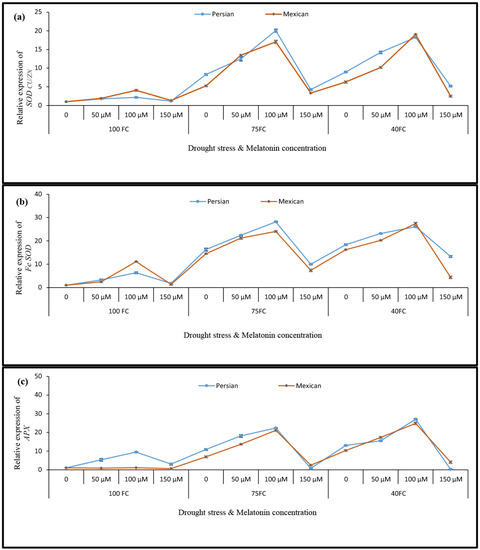
Figure 3.
The effect of different concentrations of exogenous melatonin, Citrus species, and various levels of drought stress on the expression of ROS scavenging genes including (a) Cu/Zn superoxide dismutase, Cu/Zn SOD, (b) Fe superoxide dismutase, Fe SOD, and (c) cytosolic ascorbate peroxidase, cAPX. Data describe the means of three biological replicates with three technical replicates each. Error bars represent standard deviation (SD).
The results of APX gene expression showed an increase in the expression of this gene in both Mexican and Persian lime species. Under severe drought stress and foliar application of 100 µM melatonin, the gene expression of APX increased in each species, as compared to the corresponding control. The largest increase in the transcript level occurred in the Mexican lime (24.86-fold higher) and in the Persian lime (27.04-fold higher) (Figure 3c). In drought-stressed plants, the P5CS and P5CR genes displayed two similar patterns of expression. Plants that were exposed to drought quickly responded by increasing the P5CR (Figure 4a) and P5CS expression (Figure 4b) in both lime species. When plants under drought stress were sprayed with 100 μM melatonin, the expression of the P5CR gene increased in both species. In response to severe drought stress and foliar application (100 μM melatonin), the expression of the P5CR gene in “Persian lime” and “Mexican lime” became 31.07 and 28.25 times higher than in the control plants, respectively. Furthermore, the transcript level in Persian lime was higher than in Mexican lime in each treatment.
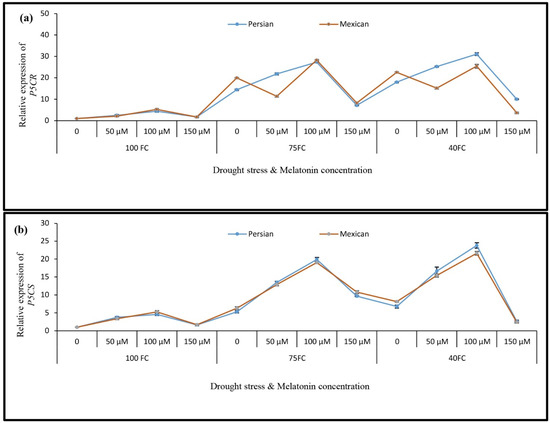
Figure 4.
The effect of different concentrations of exogenous melatonin, Citrus species, and various levels of drought stress on the expression of proline biosynthesis genes including (a) pyrroline-5-carboxylate reductase, P5CR and (b) pyrroline-5-carboxylate synthase, P5CS. Data describe the means of three biological replicates with three technical replicates each. Error bars represent standard deviation (SD).
As shown in Figure 5a, the AOX gene was upregulated after drought treatments. When plants under drought stress were treated with melatonin foliar spray, AOX gene expression was upregulated, compared to the expression in control plants. However, the foliar application of melatonin alone had no effect on increasing the AOX gene expression. The highest levels of gene expression in both Persian lime and Mexican lime were observed in response to the 100 μM melatonin treatment and severe drought stress. These expression levels were about 35.235 and 41.798-fold higher than in the control plants, respectively. While drought stress increased NaDde gene expression levels, pretreating the plants with melatonin also resulted in a substantial upregulation of NaDde gene expression. Its highest level of expression was observed in “Persian lime” in response to the 100 μM melatonin treatment and severe drought stress. The expression was 10.452-fold higher than in control plants. In “Mexican lime”, the highest level of gene expression (9.600-fold higher than the control) was observed in response to 100 μM melatonin and mild drought stress (Figure 5b).
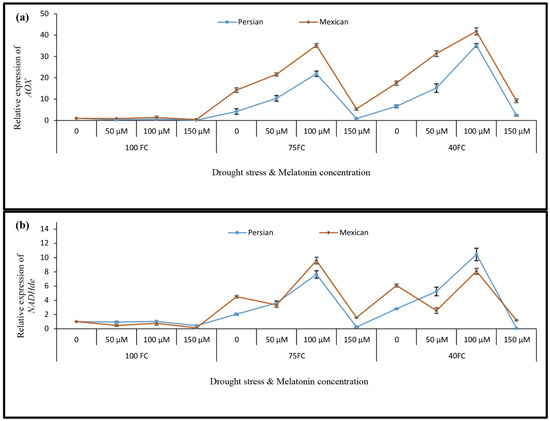
Figure 5.
The effect of different concentrations of exogenous melatonin, Citrus species, and various levels of drought stress on the expression of cellular redox regulation genes including (a) alternative oxidase, AOX and (b) NADH dehydrogenase, NADHde. Data describe the means of three biological replicates with three technical replicates each. Error bars represent standard deviation (SD).
4. Discussion
Excessive production of ROS under stress conditions damages the plant structure and disrupts the plant’s natural metabolism [54,55]. Plants prevent these disorders by regulating the expression of stress-related genes, physiological and biochemical responses, and the antioxidant defense system [56,57]. Plants can be safeguarded against stress by osmoprotectants, such as soluble proteins, proline, and total free amino acids, because these assist with cellular osmotic adjustment, enzyme stability, and protein detoxification of ROS, all of which contribute to the maintenance of membrane integrity [58]. As an osmotic protectant, proline aids in the preservation of turgor pressure in stressed cells and allows key proteins to be produced for a more efficient response to stress [59]. Melatonin treatments significantly increased proline content in lime seedlings under drought stress, indicating how melatonin can potentially assist plants in coping with drought stress (Table 2). These findings are in accordance with earlier studies that proline content reportedly increased in melatonin-treated plants [60,61,62,63,64]. Melatonin may have increased proline levels by inducing the expression of pyrroline-5-carboxylate synthetase 1 (P5CS1), an enzyme involved in proline biosynthesis [65]. The proline content may also increase in response to a decrease of proline oxidase activity during drought stress. When it is present at a proper concentration, melatonin can modulate osmotic metabolism and improve plant tolerance to stress by increasing proline accumulation [60].
Reactive oxygen species interact with phospholipids and fatty acids to accelerate cell membrane liposuction, producing malondialdehyde toxins, thereby increasing membrane permeability in plants and damaging membrane structural integrity [66,67]. Melatonin is thought to enhance the redox state of cells, reducing ROS and reactive nitrogen species levels and stabilizing biological membranes in plant cells [28]. The findings of this study showed that melatonin treatment significantly reduced MDA levels and membrane leakage in plants under drought stress. In melatonin-treated plants, this is accompanied by a decrease in H2O2 concentration. It is assumed that melatonin was responsible for the decrease in oxidative stress in both lime species. Melatonin is reportedly able to situate itself between the polar heads of polyunsaturated fatty acids in cell membranes, thereby lowering the level of lipid peroxidation and maintaining natural membrane fluidity [68]. According to several cases of research, melatonin plays a crucial role in preventing lipid peroxidation through its ability to react with lipid peroxyl (LOO•) and lipid alcoxyl (LO•) radicals so that the peroxidation cycle is interrupted and stopped [66,69]. Melatonin is reportedly capable of supporting membrane integrity while limiting lipid peroxidation products and electrolyte leakage in drought-stressed cucumber seedlings [70]. These findings demonstrate that the melatonin treatment can reduce MDA, EL, and H2O2 levels, and this observation appears to be consistent with prior drought-related research [71,72]. According to Meng et al. [73], under drought stress, melatonin-treated cuttings of grapevine accumulated smaller amounts of MDA in their leaves and had lower levels of relative electrolytic leakage, compared to grapevine cuttings with no melatonin treatment.
In addition to its direct interaction with ROS, melatonin increases antioxidant enzymes in plants. Plants have evolved defense systems, enzymatic and non-enzymatic, to limit ROS production [74,75]. In the current study, drought stress caused an increase in the activities of CAT, POD, SOD, APX, and GR enzymes in plants. Additionally, the exogenous application of melatonin further increased the activity of these antioxidant enzymes (Figure 1). Increased plant tolerance to stress using melatonin foliar application is due to the antioxidant properties of melatonin, which directly counteracts the harmful effects of reactive oxygen species by stimulating increased production of antioxidant enzymes, and thus improving plant antioxidant capacity [63,76,77]. Overall, melatonin is a broad-spectrum antioxidant and a receptor-independent free-radical scavenger that can increase the activity of antioxidant enzymes and other antioxidants to protect plant tissues from oxidative damage [78,79]. After treating the plants with 100 μM melatonin, the activities of SOD, POD, and APX increased significantly. In this regard, Gantait and Mukherjee [80] demonstrated that melatonin usually serves as a potent long-distance signal and has the capacity to be translocated via vascular bundles from treated leaves or roots to distant tissues, ultimately causing a systemic induction of various abiotic tolerances. According to Li et al. [81], exogenous melatonin is able to reduce stress-induced oxidative damage in Malus hupehensis by directly scavenging H2O2 and increasing antioxidant enzyme activity. Previous research by Wei et al. [82], Xia et al. [83], and Arnao and Hernández-Ruiz [41] showed that melatonin treatment increased antioxidant enzyme activity, but reduced ROS content in apple and kiwifruit under abiotic stress. These findings suggest that melatonin therapy reduces the negative effects of drought stress on lime seedlings and, thus, increases their tolerance.
Drought significantly increased the expression of genes involved in ROS scavenging, cellular redox regulation, and proline production. Melatonin increased plant tolerance by further inducing the expression of genes associated with the antioxidant system in response to stress [84,85,86]. Melatonin-mediated gene regulation during drought plays an important role in controlling cellular signaling pathways [87]. The activities of antioxidant enzymes are connected to the control of important genes that are involved in antioxidant expression. In fact, this supports the redox balance in cells that are under stressful conditions [88]. Plants activate ROS-scavenging gene families when they are under stress. SODs are the first line of defense against ROS in plants, and they are categorized by the metal ions bound to their active sites, such as copper/zinc (Cu/Zn SOD) and iron (Fe SOD). In drought-stressed plants, SODs defend the photosynthetic apparatus against ROS [89]. In a previous case of research, it was shown that increasing the expression of the SOD Cu/Zn gene in Poncirus trifoliata caused enhancements in antioxidant capacity and assisted in plant tolerance to stress [90]. Exogenous melatonin may promote the production of endogenous nitric oxide as an essential signaling molecule in plants, thereby activating ROS scavenging enzymes under drought conditions [32]. Meanwhile, melatonin can reduce the expression of miR398s and, thus, increase the production of ROS-scavenging enzyme genes such as Cu/Zn SOD and Fe SOD [91].
APX plays a role in the first cyclic phase of AsA-GSH. In fact, AsA-GSH scavenges ROS and protects plants from stress. H2O2 is scavenged by this heme enzyme through the AsA-GSH cycle, which converts H2O2 to water and dehydroascorbate DHA [92,93]. Meanwhile, APX activity usually increases in response to various abiotic stressors. The APX gene is capable of many isoforms that have been discovered in the genome. Under abiotic stress, the overexpression of APX2 enhances APX activity and leads to a decrease in H2O2 and MDA content [94]. According to ElSayed et al. [95], both APX3 and APX4 genes are involved in drought tolerance. At times of drought stress, melatonin usually upregulates genes, such as APX4, that are involved in ascorbate metabolism. Ascorbate biosynthesis is regulated by the APX3 gene, whereas H2O2 reduction is regulated by the APX4 gene [94]. Hydrogen peroxide helped apple seedlings resist oxidative stress more when the expression of APX was increased [42].
The regulation of proline metabolic genes by drought is a matter of frequent scientific report. It is generally known that dehydration causes an increase in gene expression, resulting in proline production [96]. As a major response to drought stress, the activities of P5CS and P5CR genes reportedly increased [97]. The P5CS gene, which codes for proline, is known to play a key role in the stress response. It brings an accumulation of proline at times of abiotic stress. The accumulation of proline is one of the primary reasons for an increase in osmotic pressure, which improves plant capacity for water retention [98]. P5CS expression was reportedly upregulated in Arabidopsis, oil palm, and three wheat cultivars under osmotic stress [99,100]. Another study considered the expression of P5CS and P5CR genes in two rose cultivars during drought stress. There was no significant increase after the stress period, and P5CR gene expression was very low when compared to P5CS gene expression. These findings suggested that the P5CS gene plays a more important role in proline accumulation in roses under drought stress, as compared to the role of the P5CR gene [101]. We observed appreciably enhanced accumulation of proline, accompanied by a 20-fold increase in P5CS1 transcript levels and P5CR in lime plants under melatonin-treated compared to control plants. This implies that melatonin positively influences proline accumulation during drought stress in higher plants. These findings are consistent with previous studies showing that melatonin use increases proline and P5CR activity in stressed plants compared with plants not treated with melatonin [102,103].
In plant cells, the mitochondrial transport chain is the prominent site of ROS production. It contains a number of enzymes such as AOX and NADHde that are involved in the detoxification of ROS [104]. The AOX intermittent pathway supposedly plays a role in modulating the production of ROS that are produced during the mitochondrial electron transfer chain [105]. In one study, it was stated that mild-to-moderate drought stress led to a gradual increase in AOX1 and in various components that contribute to ROS inhibition in tobacco [106]. In another study, the use of melatonin (10 μM) in alfalfa increased the expression of NADHde and AOX genes under stress conditions. Overall, the recent results showed how melatonin can be a promising agent for enhancements of plant tolerance to drought in ways that involve regulating nitro oxidative homeostasis and protecting plant structures [107].
5. Conclusions
The foliar application of melatonin to lime plants, especially at a concentration of 100 μM, substantially improved drought stress damage in both types of lime. This occurred by inhibiting membrane damage and reducing the concentration of malondialdehyde and hydrogen peroxide, despite moderate and severe drought stress. These effects were probably achieved by regulating the activity of antioxidant enzymes. Melatonin induced the accumulation of compatible osmolytes such as proline, facilitating its synthesis and thus instilling drought tolerance in plants. In addition, there were increases in gene expressions involved in ROS scavenging, cellular redox regulation, and proline biosynthesis genes, as a result of melatonin treatment on lime leaves. This suggests that exogenous melatonin is an effective protectant that improves drought tolerance in lime seedlings by enhancing antioxidant enzymes and reducing oxidative damage. The use of melatonin can be considered a promising method to reduce the negative impacts of drought stress, although the mechanism by which melatonin aids in drought tolerance would require further research.
Author Contributions
Conceptualization, M.J., A.R.S. and M.T.; methodology, M.J., A.R.S. and M.T.; software, M.J.; validation, M.J., A.R.S. and M.T.; formal analysis, M.J.; investigation, M.J.; resources, M.J. and A.R.S.; data curation, M.J., A.R.S. and M.T.; writing—original draft preparation, M.J.; writing—review and editing, M.J., A.R.S., M.T. and M.H.; visualization, M.J. and M.H.; supervision, A.R.S.; project administration, A.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
There were no available funding resources for this study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their gratitude to the College of Agriculture at Shiraz University and the Department of Biotechnology at Isfahan University of Technology for their cooperation during this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Jafari, M.; Shahsavar, A. The effect of foliar application of melatonin on changes in secondary metabolite contents in two Citrus species under drought stress conditions. Front. Plant Sci. 2021, 12, 692735. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh Khankahdani, H.; Rastegar, S.; Golein, B.; Golmohammadi, M.; Aboutalebi Jahromi, A. Relationship among vegetative growth and nutrient elements in the scion of different Persian lime accessions and its effect on WBDL phytoplasma. J. Plant Dis. Prot. 2022, 129, 145–154. [Google Scholar] [CrossRef]
- Jafari, M.; Shahsavar, A. The application of artificial neural networks in modeling and predicting the effects of melatonin on morphological responses of citrus to drought stress. PLoS ONE 2020, 15, e0240427. [Google Scholar] [CrossRef] [PubMed]
- Yoosefzadeh Najafabadi, M.; Soltani, F.; Noory, H.; Díaz-Pérez, J.C. Growth, Yield and Enzyme Activity Response of Watermelon Accessions Exposed to Irrigation Water Deficit. Int. J. Veg. Sci. 2018, 24, 323–337. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Jafari, M.; Shahsavar, A.R. Sodium nitroprusside: Its beneficial role in drought stress tolerance of “Mexican lime” (Citrus aurantifolia (Christ.) Swingle) under in vitro conditions. Vitr. Cell. Dev. Biol.-Plant 2022, 58, 155–168. [Google Scholar] [CrossRef]
- Shi, Y.; Chang, Y.-L.; Wu, H.-T.; Shalmani, A.; Liu, W.-T.; Li, W.-Q.; Xu, J.-W.; Chen, K.-M. OsRbohB-mediated ROS production plays a crucial role in drought stress tolerance of rice. Plant Cell Rep. 2020, 39, 1767–1784. [Google Scholar] [CrossRef] [PubMed]
- Jothimani, K.; Arulbalachandran, D. Physiological and biochemical studies of black gram (Vigna mungo (L.) Hepper) under polyethylene glycol induced drought stress. Biocatal. Agric. Biotechnol. 2020, 29, 101777. [Google Scholar] [CrossRef]
- Kaczmarek, P.; Rapp, M.; Koroniak, H. Pyrrolidine and oxazolidine ring transformations in proline and serine derivatives of α-hydroxyphosphonates induced by deoxyfluorinating reagents. RSC Adv. 2018, 8, 24444–24457. [Google Scholar] [CrossRef] [Green Version]
- Hesami, M.; Tohidfar, M.; Alizadeh, M.; Daneshvar, M.H. Effects of sodium nitroprusside on callus browning of Ficus religiosa: An important medicinal plant. J. For. Res. 2020, 31, 789–796. [Google Scholar] [CrossRef]
- Weng, Y.; Ge, L.; Jia, S.; Mao, P.; Ma, X. Cyclophilin AtROC1S58F confers Arabidopsis cold tolerance by modulating jasmonic acid signaling and antioxidant metabolism. Plant Physiol. Biochem. 2020, 152, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jing, D.; Liu, F.; Ma, H.; Liu, X.; Peng, L. Serendipita indica alleviates drought stress responses in walnut (Juglans regia L.) seedlings by stimulating osmotic adjustment and antioxidant defense system. Appl. Microbiol. Biotechnol. 2021, 105, 8951–8968. [Google Scholar] [CrossRef] [PubMed]
- Reyhani Haghighi, S.; Hosseininaveh, V.; Maali-Amiri, R.; Talebi, K.; Irani, S. Improving the drought tolerance in pistachio (Pistacia vera) seedlings by foliar application of salicylic acid. Gesunde Pflanz. 2021, 73, 495–507. [Google Scholar] [CrossRef]
- Aazami, M.A.; Shamsinow, E.; Hasan Poor Aghdam, M.B. Evaluation some of the physiological and biochemical changes in three grapevine cultivars (Vitis vinifera L.) in response to drought stress. Appl. Biol. 2021, 34, 58–81. [Google Scholar] [CrossRef]
- Melaouhi, A.; Baraza, E.; Escalona, J.M.; El-AouOuad, H.; Mahjoub, I.; Bchir, A.; Braham, M.; Bota, J. Physiological and biochemical responses to water deficit and recovery of two olive cultivars (Olea europaea L., Arbequina and Empeltre cvs.) under Mediterranean conditions. Theor. Exp. Plant Physiol. 2021, 33, 369–383. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Song, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic acid and hydrogen peroxide are involved in drought priming-induced drought tolerance in wheat (Triticum aestivum L.). Plant Biol. 2020, 22, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-X.; Xu, N.-W.; Yang, M.; Li, X.-L.; Han, J.-L.; Lin, X.-H.; Yang, Q.; Lv, G.-H.; Wang, J. Responses of photosynthesis, antioxidant enzymes, and related gene expression to nicosulfuron stress in sweet maize (Zea mays L.). Environ. Sci. Pollut. Res. 2022, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Uddin, M.J.; Hossain, M.A.; Henry, R.; Begum, M.K.; Sohel, M.A.T.; Mou, M.A.; Ahn, J.; Cheong, E.J.; Lim, Y.-S. Exogenous putrescine attenuates the negative impact of drought stress by modulating physio-biochemical traits and gene expression in sugar beet (Beta vulgaris L.). PLoS ONE 2022, 17, e0262099. [Google Scholar] [CrossRef] [PubMed]
- Alamri, S.; Hu, Y.; Mukherjee, S.; Aftab, T.; Fahad, S.; Raza, A.; Ahmad, M.; Siddiqui, M.H. Silicon-induced postponement of leaf senescence is accompanied by modulation of antioxidative defense and ion homeostasis in mustard (Brassica juncea) seedlings exposed to salinity and drought stress. Plant Physiol. Biochem. 2020, 157, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, J.; Yan, K.; Zhou, Z.; Zhao, W.; Zhang, X.; Pu, Y.; Yu, R. Beneficial effects of abscisic acid and melatonin in overcoming drought stress in cotton (Gossypium hirsutum L.). Physiol. Plant. 2021, 173, 2041–2054. [Google Scholar] [CrossRef]
- Manbir; Singh, P.; Kumari, A.; Gupta, K.J. Alternative oxidase plays a role in minimizing ROS and RNS produced under salinity stress in Arabidopsis thaliana. Physiol. Plant. 2022, 174, e13649. [Google Scholar] [CrossRef]
- Zhanassova, K.; Kurmanbayeva, A.; Gadilgereyeva, B.; Yermukhambetova, R.; Iksat, N.; Amanbayeva, U.; Bekturova, A.; Tleukulova, Z.; Omarov, R.; Masalimov, Z. ROS status and antioxidant enzyme activities in response to combined temperature and drought stresses in barley. Acta Physiol. Plant. 2021, 43, 114. [Google Scholar] [CrossRef]
- Boy, R.; Indradewa, D.; Putra, E.T.S.; Kurniasih, B. Drought-induced production of reactive oxygen species and antioxidants activity of four local upland rice cultivars in Central Sulawesi, Indonesia. Biodivers. J. Biol. Divers. 2020, 21, 2555–2565. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Pan, Y.; Yun, Z.; Zhang, Z.; Jiang, G.; Jiang, Y. Endogenous melatonin generation plays a positive role in chilling tolerance in relation to redox homeostasis in litchi fruit during refrigeration. Postharvest Biol. Technol. 2021, 178, 111554. [Google Scholar] [CrossRef]
- Liu, L.; Li, D.; Ma, Y.; Shen, H.; Zhao, S.; Wang, Y. Combined application of arbuscular mycorrhizal fungi and exogenous melatonin alleviates drought stress and improves plant growth in tobacco seedlings. J. Plant Growth Regul. 2021, 40, 1074–1087. [Google Scholar] [CrossRef]
- Azmat, A.; Yasmin, H.; Hassan, M.N.; Nosheen, A.; Naz, R.; Sajjad, M.; Ilyas, N.; Akhtar, M.N. Co-application of bio-fertilizer and salicylic acid improves growth, photosynthetic pigments and stress tolerance in wheat under drought stress. PeerJ 2020, 8, e9960. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.-S.E.; Abu-Elsaoud, A.M.; Hafez, Y.M. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Shazad, R.; Bilal, S.; Imran, Q.M.; Khan, M.; Kang, S.-M.; Khan, A.L.; Yun, B.-W.; Lee, I.-J. Exogenous Melatonin mediates the regulation of endogenous nitric oxide in Glycine max L. to reduce effects of drought stress. Environ. Exp. Bot. 2021, 188, 104511. [Google Scholar] [CrossRef]
- Ren, J.; Yang, X.; Ma, C.; Wang, Y.; Zhao, J. Melatonin enhances drought stress tolerance in maize through coordinated regulation of carbon and nitrogen assimilation. Plant Physiol. Biochem. 2021, 167, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, D.-X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.; Niu, J.; Irshad, A.; Kareem, H.A.; Hassan, M.U.; Xu, N.; Sui, X.; Guo, Z.; Amo, A.; Wang, Q. Exogenous melatonin protects alfalfa (Medicago sativa L.) seedlings from drought-induced damage by modulating reactive oxygen species metabolism, mineral balance and photosynthetic efficiency. Plant Stress 2021, 2, 100044. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Y.; Hu, L.; Zhang, J.; Yue, H.; Yang, S.; Liu, Y.; Gong, X.; Ma, F. Overexpression of MdASMT9, an N-acetylserotonin methyltransferase gene, increases melatonin biosynthesis and improves water-use efficiency in transgenic apple. Tree Physiol. 2021, 157, 1–13. [Google Scholar] [CrossRef]
- Zou, J.-N.; Yu, Q.; Jin, X.-J.; Wang, M.-Y.; Qin, B.; Ren, C.-Y.; Wang, M.-X.; Zhang, Y.-X. Effects of exogenous melatonin on physiology and yield of soybean during seed filling stage under drought stress. Acta Agron. Sin. 2020, 46, 745–758. [Google Scholar] [CrossRef]
- Cao, L.; Qin, B.; Zhang, Y.X. Exogenous application of melatonin may contribute to enhancement of soybean drought tolerance via its effects on glucose metabolism. Biotechnol. Biotechnol. Equip. 2021, 35, 964–976. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Mukherjee, S.; Flores, F.B.; Arnao, M.B.; Luo, Z.; Corpas, F.J. Functions of melatonin during postharvest of horticultural crops. Plant Cell Physiol. 2021, pcab175, 1–23. [Google Scholar] [CrossRef]
- Shafi, A.; Singh, A.K.; Zahoor, I. Melatonin: Role in abiotic stress resistance and tolerance. In Plant Growth Regulators; Aftab, T., KR, H., Eds.; Springer: Cham, Switzerland, 2021; pp. 239–273. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a plant biostimulant in crops and during post-harvest: A new approach is needed. J. Sci. Food Agric. 2021, 101, 5297–5304. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gulen, H.; Eris, A. Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci. 2004, 166, 739–744. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Ozden, M.; Demirel, U.; Kahraman, A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 2009, 119, 163–168. [Google Scholar] [CrossRef]
- Hemeda, H.M.; Klein, B.P. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J. Food Sci. 1990, 55, 184–185. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tavanti, T.R.; Melo, A.A.R.d.; Moreira, L.D.K.; Sanchez, D.E.J.; Silva, R.d.S.; Silva, R.M.d.; Reis, A.R.d. Micronutrient fertilization enhances ROS scavenging system for alleviation of abiotic stresses in plants. Plant Physiol. Biochem. 2021, 160, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Gholamin, R.; Khayatnezhad, M. Study of bread wheat genotype physiological and biochemical responses to drought stress. Helix 2020, 10, 87–92. [Google Scholar] [CrossRef]
- Hesami, M.; Daneshvar, M.H.; Yoosefzadeh-Najafabadi, M. An efficient in vitro shoot regeneration through direct organogenesis from seedling-derived petiole and leaf segments and acclimatization of Ficus religiosa. J. For. Res. 2019, 30, 807–815. [Google Scholar] [CrossRef]
- Zheng, X.; Tan, D.X.; Allan, A.C.; Zuo, B.; Zhao, Y.; Reiter, R.J.; Wang, L.; Wang, Z.; Guo, Y.; Zhou, J.; et al. Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci. Rep. 2017, 7, 41236. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadak, M.S.; Bakry, B.A. Alleviation of drought stress by melatonin foliar treatment on two flax varieties under sandy soil. Physiol. Mol. Biol. Plants 2020, 26, 907–919. [Google Scholar] [CrossRef]
- Madebo, M.P.; Luo, S.-m.; Wang, L.; Zheng, Y.-h.; Jin, P. Melatonin treatment induces chilling tolerance by regulating the contents of polyamine, γ-aminobutyric acid, and proline in cucumber fruit. J. Integr. Agric. 2021, 20, 3060–3074. [Google Scholar] [CrossRef]
- Silalert, P.; Pattanagul, W. Foliar application of melatonin alleviates the effects of drought stress in rice (Oryza sativa L.) seedlings. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12417. [Google Scholar] [CrossRef]
- Balci, G. Effects of melatonin applications on certain biochemical characteristics of strawberry seedlings in lime stress conditions. Turk. J. Agric. For. 2021, 45, 285–289. [Google Scholar] [CrossRef]
- Alyammahi, O.; Gururani, M.A. Chlorophyll-a fluorescence analysis reveals differential response of photosynthetic machinery in melatonin-treated oat plants exposed to osmotic stress. Agronomy 2020, 10, 1520. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [Green Version]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Chattopadhyay, A.; Bandyopadhyay, D. Melatonin and biological membrane bilayers: A never ending amity. Melatonin Res. 2021, 4, 232–252. [Google Scholar] [CrossRef]
- Malmir, M.; Naderi Noreini, S.; Ghafarizadeh, A.; Faraji, T.; Asali, Z. Ameliorative effect of melatonin on apoptosis, DNA fragmentation, membrane integrity and lipid peroxidation of spermatozoa in the idiopathic asthenoteratospermic men: In vitro. Andrologia 2021, 53, e13944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.M.; Tian, G.; Li, X.H.; Zhang, Z.Z.; Liu, J.; Li, Y.H.; Xie, J.F.; Wang, P.F. ROS Produced via BsRBOHD Plays an Important Role in Low Temperature-Induced Anthocyanin Biosynthesis in Begonia semperflorens. Russ. J. Plant Physiol. 2020, 67, 250–258. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin Inhibits Cadmium Translocation and Enhances Plant Tolerance by Regulating Sulfur Uptake and Assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Mao, Q.; Yan, Y.; Wu, M.; Wang, Y.; Ren, J.; Guo, P.; Liu, A.; Chen, S. Role of Melatonin in Arbuscular Mycorrhizal Fungi-Induced Resistance to Fusarium Wilt in Cucumber. Phytopathology 2020, 110, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.-F.; Xu, T.-F.; Wang, Z.-Z.; Fang, Y.-L.; Xi, Z.-M.; Zhang, Z.-W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, Y.; Rui, C.; Zhang, H.; Xu, N.; Dai, M.; Chen, X.; Lu, X.; Wang, D.; Wang, J.; et al. Melatonin Improves Cotton Salt Tolerance by Regulating ROS Scavenging System and Ca2+ Signal Transduction. Front. Plant Sci. 2021, 12, 693690. [Google Scholar] [CrossRef]
- Maleki, M.; Shojaeiyan, A.; Mokhtassi-Bidgoli, A. Genotypic variation in biochemical and physiological responses of fenugreek (Trigonella foenum-graecum L.) landraces to prolonged drought stress and subsequent rewatering. Sci. Hortic. 2021, 287, 110224. [Google Scholar] [CrossRef]
- Ahmad, S.; Su, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Javed, T.; Han, Q. Foliar application of melatonin delay leaf senescence in maize by improving the antioxidant defense system and enhancing photosynthetic capacity under semi-arid regions. Protoplasma 2020, 257, 1079–1092. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Fahadi Hoveizeh, N.; Gholami, R.; Abdelrahman, M.; Tran, L.-S.P. Exogenous melatonin mitigates salinity-induced damage in olive seedlings by modulating ion homeostasis, antioxidant defense, and phytohormone balance. Physiol. Plant. 2021, 173, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.K.; Howlader, P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 2020, 176, 104063. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Altaf, M.M.; Khan, L.U.; Shahid, S.; Jahan, M.S. Melatonin alleviates salt damage in tomato seedling: A root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Sci. Hortic. 2021, 285, 110145. [Google Scholar] [CrossRef]
- Gantait, S.; Mukherjee, E. Hairy root culture technology: Applications, constraints and prospect. Appl. Microbiol. Biotechnol. 2021, 105, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, P.; Wei, Z.; Liang, D.; Liu, C.; Yin, L.; Jia, D.; Fu, M.; Ma, F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Gao, T.; Liang, B.; Zhao, Q.; Ma, F.; Li, C. Effects of exogenous melatonin on methyl viologen-mediated oxidative stress in apple leaf. Int. J. Mol. Sci. 2018, 19, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.; Ni, Z.; Hu, R.; Lin, L.; Deng, H.; Wang, J.; Tang, Y.; Sun, G.; Wang, X.; Li, H.; et al. Melatonin alleviates drought stress by a non-enzymatic and enzymatic antioxidative system in kiwifruit seedlings. Int. J. Mol. Sci. 2020, 21, 852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Omran, V.O.; Ghorbani, A.; Sajjadi-Otaghsara, S.A. Melatonin alleviates NaCl-induced damage by regulating ionic homeostasis, antioxidant system, redox homeostasis, and expression of steviol glycosides-related biosynthetic genes in in vitro cultured Stevia rebaudiana Bertoni. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 319–331. [Google Scholar] [CrossRef]
- Li, Z.; Su, X.; Chen, Y.; Fan, X.; He, L.; Guo, J.; Wang, Y.; Yang, Q. Melatonin Improves Drought Resistance in Maize Seedlings by Enhancing the Antioxidant System and Regulating Abscisic Acid Metabolism to Maintain Stomatal Opening under PEG-Induced Drought. J. Plant Biol. 2021, 64, 299–312. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Effects of melatonin on antioxidant capacity in naked oat seedlings under drought stress. Molecules 2018, 23, 1580. [Google Scholar] [CrossRef] [Green Version]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Río, L.A.d.; Corpas, F.J.; López-Huertas, E.; Palma, J.M. Plant superoxide dismutases: Function under abiotic stress conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar]
- He, J.-D.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Sci. Hortic. 2020, 262, 108745. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.; Yu, X.; Cui, W.; Pan, J.; Zhao, G.; Xu, S.; Wang, R.; Shen, W. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef]
- Pandey, P.; Singh, J.; Achary, V.M.M.; Reddy, M.K. Redox homeostasis via gene families of ascorbate-glutathione pathway. Front. Environ. Sci. 2015, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Panchuk, I.I.; Zentgraf, U.; Volkov, R.A. Expression of the Apx gene family during leaf senescence of Arabidopsis thaliana. Planta 2005, 222, 926–932. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, A.I.; Rafudeen, M.S.; Gomaa, A.M.; Hasanuzzaman, M. Exogenous melatonin enhances the reactive oxygen species metabolism, antioxidant defense-related gene expression, and photosynthetic capacity of Phaseolus vulgaris L. to confer salt stress tolerance. Physiol. Plant. 2021, 173, 1369–1381. [Google Scholar] [CrossRef]
- Surender Reddy, P.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kavi Kishor, P.B. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iskandar, H.M.; Widyaningrum, D.; Suhandono, S. Cloning and characterization of P5CS1 and P5CS2 genes from Saccharum officinarum L. under drought stress. J. Trop. Crop Sci. 2014, 1, 23–30. [Google Scholar] [CrossRef]
- De Carvalho, K.; de Campos, M.K.F.; Domingues, D.S.; Pereira, L.F.P.; Vieira, L.G.E. The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol. Biol. Rep. 2013, 40, 3269–3279. [Google Scholar] [CrossRef] [PubMed]
- Funck, D.; Baumgarten, L.; Stift, M.; von Wirén, N.; Schönemann, L. Differential Contribution of P5CS Isoforms to Stress Tolerance in Arabidopsis. Front. Plant Sci. 2020, 11, 565134. [Google Scholar] [CrossRef]
- Ghaderi, A.; Jahanbakhsh Godehkahriz, S.; Raeisi Sadati, S.Y. Study of total protein content, soluble sugar, proline content and P5CS gene expression in leaves of three wheat cultivars under drought stress. Agric. Biotechnol. J. 2021, 12, 122–141. [Google Scholar] [CrossRef]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H.; Razi, H.; Karami, A.; Moghadam, A. Metabolic and genes expression analyses involved in proline metabolism of two rose species under drought stress. Plant Physiol. Biochem. 2020, 155, 105–113. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Sheikh-Assadi, M.; Sharafi, Y.; Farmani, B.; Fard, J.R.; Razavi, F. Employing exogenous melatonin applying confers chilling tolerance in tomato fruits by upregulating ZAT2/6/12 giving rise to promoting endogenous polyamines, proline, and nitric oxide accumulation by triggering arginine pathway activity. Food Chem. 2019, 275, 549–556. [Google Scholar] [CrossRef]
- Sharafi, Y.; Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Razavi, F.; Fard, J.R.; Farmani, B. Melatonin treatment promotes endogenous melatonin accumulation and triggers GABA shunt pathway activity in tomato fruits during cold storage. Sci. Hortic. 2019, 254, 222–227. [Google Scholar] [CrossRef]
- Wang, J.; Rajakulendran, N.; Amirsadeghi, S.; Vanlerberghe, G.C. Impact of mitochondrial alternative oxidase expression on the response of Nicotiana tabacum to cold temperature. Physiol. Plant. 2011, 142, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Vanlerberghe, G.C. Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef]
- Wang, J.; Vanlerberghe, G.C. A lack of mitochondrial alternative oxidase compromises capacity to recover from severe drought stress. Physiol. Plant. 2013, 149, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).