Abstract
A full diallel mating design was used to hybridize seven pumpkin inbred parental lines, yielding 42 F1 hybrids, including reciprocals. The generated F1 hybrids, parental lines and commercial check hybrid were evaluated in two environments to investigate the per se performances, combining ability effects and magnitude of heterosis over mid-parent, better-parent and commercial check hybrids for yield, yield attributed, sweetness, total carotenoid and antioxidant traits, using a randomized complete block design with three replications. The analysis of variance for most of the examined traits revealed highly significant differences (p ≤ 0.01) for GCA, SCA, reciprocal, maternal and non-maternal variances and their interaction with the environment. Since the inheritance of yield and its contributing fruit quality, and antioxidant traits is governed by non-additive gene action, it suggests heterosis breeding would be useful in obtaining further improvements in pumpkin. From the experiment, it was found that the paternal lines P1 (Gold Butter 315) for dry matter content and DPPH (%), P4 (Asian pumpkin) for total carotenoid content, P6 (Sarawak) for fruit number per plant and P7 (Australia-1) for single fruit weight, fruit flesh thickness, yield per plant and total soluble solid were shown to be good general combiners. In respect to per se performance, combining ability effects and magnitude of heterosis over mid-parent, better-parent and commercial check, the cross P2 (928 Fuxiang) × P1 (Gold Butter 315) and P4 (Asian pumpkin) × P5 (Sarawak) for single fruit weight and yield per plant, the cross P5 (Sarawak) × P2 (928 Fuxiang) for fruits number per plant, P1 (Gold Butter 315) × P7 (Australia-1) and P1 (Gold Butter 315) × P6 (Sarawak) for total soluble solid and dry matter content, P7 (Australia-1) × P2 (928 Fuxiang) for total carotenoid content and P3 (Ser Bajadi) × P1 (Gold Butter 315) for DPPH (antioxidant) were identified to be highly significant positive specific combiners and the highest performers, and these crosses may be exploited as commercial hybrids.
1. Introduction
The pumpkin, Cucurbita spp. (2 n = 2 x = 40) is one of the most important herbaceous, annual and sexually reproduced vegetable crops in the family Cucurbitaceae. Pumpkin is cultivated worldwide due to its excellent rewards to farmers and outstanding nutritional and pharmacological value [1]. The crop was first introduced to North America, Asia, and Europe. It is widely cultivated in nearly every corner of the world, regardless of altitude [2]. Pumpkin is considered an important herbaceous vegetable and a revolutionary-era crop that provides balanced nutrition and is better suited to different atmospheric conditions than other prominent crops [3]. Pumpkin flesh is abundant with vitamins, carotenoids, lutein, zeaxanthins and micro and macro minerals [4]. The most important nutrients are lutein and alpha- and β-carotene, with the latter generating Vitamin A in the body [5]. Phytochemicals, including polyphenols, flavonoids, carotenoids and antioxidant properties, are most abundant in pumpkin flesh, skin and seeds [6] The synthesis of carotenoids is affected by environmental factors such as drought and temperature stress, which lowers the breeding efficiency of selection [7]. Pumpkin seeds are an excellent source of protein and crucial omega-3 and omega-6 fatty acids. Due to these important nutritional components, it is often used as a constituent of baked goods, salami, sausage, mayonnaise and several other food crops [8,9,10]. Pharmacologically, pumpkin is used as an anti-hypertensive, antihypercholesterolemia, anti-inflammatory, antitumour, antiparasitic, antioxidant, anticarcinogenic, antidiabetic and antibacterial [11]. Due to the fast expansion of the global population, rising demand for vegetables and the limited quantity of cultivable area, improved vegetable varieties are needed to boost yields and meet consumer demands [12]. The use of heterosis breeding offers the possibility of improving the quantity, quality and productivity of any crop. Identifying the best combiner employed in crosses to generate desirable segregates and accumulate fixable genes or exploit heterosis requires the employment of a strong tool that considers both the combiner’s abilities and its heterosis [13]. This is a pre-requisite to understanding the genetic architecture of various characteristics, allowing the breeder to build an effective breeding strategy for available germplasm. Breeders may also find the information beneficial for the genetic enhancement of existing genotypes based on how well they perform in various hybrid combinations. Heterosis is more obvious in cross-pollinated crops than in self-pollinated [13]. The first filial generation of hybrids (F1) results from crossing two pure lines [14]. Pure lines are a crucial component in the production of high-quality hybrids. It is a homozygous genotype developed over several generations by repeated selfing with selection [15]. Compared to normal fertilization, hybrids have a 15–25% increased production potential [16]. Identification of the potential coupling of two or more parental genotypes to maximize variation in a population to produce superior transgressive segregants of a segregated group of hybrids can be achieved through hybridization [17]. In real crop breeding strategies, combining ability study has been utilized to assess the relative value of general combining ability (GCA) and specific combining ability (SCA), as well as outstanding parents for mating in F1 hybrid breeding [18]. GCA and SCA are computational genetics approaches for assessing the characteristics of gene action involved in the progeny of commercially suitable plant attributes and the potential of parents to merge when they are hybridized [19]. The selection of paternal materials and proper breeding designs in conventional crop breeding are criteria for a successful plant breeding program. To develop superior varieties, plant breeders combined all the breeding strategies to identify appropriate parents and breeding techniques [20]. The diallel approach (the full diallel design) was used to determine the general combining ability (GCA) of parents and the specific combining ability (SCA) of their F1 hybrids. Non-additive gene action effects specific combining ability, whereas the impact of general combining ability causes additive gene action. The exhibition of additive and non-additive gene action offered by heterosis breeding can improve genotypes [21]. The estimation of general combining ability (GCA), specific combining ability (SCA), broad and narrow senses heritability and additive and non-additive gene action were all addressed by the diallel breeding model [22]. The main purpose of this study was to evaluate the effects of combining ability and heterosis, as well as gene action that affects yield, yield-related and antioxidant properties for identifying the most suitable general and specific parent combining abilities for producing profitable hybrid pumpkins with higher yield with improved quality.
2. Materials and Methods
2.1. Experiment Materials, Design and Location
Seven promising diverse developed inbred parental lines of pumpkin (Cucurbita moschata Duch. ex Poir.); Gold Butter 315 (P1), 928 Fuxiang (P2), Ser Bajadi (P3), Asian pumpkin (P4), Sarawak (P5), F3-2 (P6) and Australia-1 (P7) (Table A1), collected from the Institute of Tropical Agriculture and Food Security, University Putra Malaysia gene bank, were used in this study. The inbred lines were crossed in all possible combinations in a complete diallel mating design, including reciprocals, to produce 42 (21 F1 and 21 F1 r) hybrids. For the resulting 42 F1 hybrids, 7 parental lines and one commercial check hybrid were evaluated for two growing seasons (October 2020 to January 2021 and March 2021 to June 2021) at field-10, which is located at latitude 2°59′ north and longitude 101°42′ east, 45 m above sea level. The growing conditions throughout the two seasons are presented in Table 1. The two experiments were set up using a randomized complete block design (RCBD) with three replications, and each of the seven plants were spaced 1.5 m apart in each genotype.

Table 1.
The growing conditions at the experimental area throughout the research study.
2.2. Hybridization to Generate 42 F1 Hybrids and 7 Inbred Parental Lines
For the crossing, the conventional hybridization process was employed. The day before flowering, the male and female flowers with yellow tips on their petals were chosen for pollination. The selected male and female flowers expected to open the next morning were enclosed with butter paper clips before the flowers opened to avoid unwanted cross-pollination. The pollens from the selected male flower of the desired plant were dusted on the selected female flower from different parental lines and enclosed again until the fruit was set. A label with the parents’ names and the pollination date was affixed. Selfing of each of the 7 parental lines was performed to get inbred parental lines to retain genetic purity. Pollen from the selected male flower was collected and dusted on the selected female flower of the same plant, which was again covered until the fruit was set. F1 hybrid and inbred seeds were extracted from physiologically mature fruits for the subsequent study.
2.3. Agronomic Practices and Plant Protection
The seeds from each accession were sown in germination trays filled with peat moss soil and were later transplanted to the main field after 14 days in the nursery. The field was instantly irrigated after transplanting for easy establishment of roots into the soil, and was then irrigated twice daily. Subsequently, 24 g of NPK green fertilizer (15:15:15) was given per plant one week after transplanting. After four weeks, the same dose of NPK blue fertilizer (12:12:17 + trace elements) was given. Other cultural practices, such as manual weeding, diseases and pests, were managed using different insecticides, such as inorganic and organic (neem and garlic), per the prescribed dose for keeping plants healthy.
2.4. Data Collection
2.4.1. Data Collection for Yield and Yield Contributing Traits
Data were collected from five plants per genotype in each replication. Data on morphological observations and measurements were collected following the cucurbit descriptor IPGRI, 2003 [23], as presented in Table 2.

Table 2.
List of evaluating of yield and its contributing traits with method of assessment.
2.4.2. Data Collection on Fruit Quality Traits
Total Soluble Solid (Sweetness) (°Brix)
Hand refractometer PAL-1 (ATAGO, Kyoto, Japan, scale of 0–53°) was used to measure a homogenized sample of flesh from the middle and the polar part of the fruit [24].
Dry Matter Content (%)
A sample of 100 g of fresh pumpkin was cut into small pieces and then dried in a hot air oven at 60 °C until constant weight. Dry matter content (%) was determined by following the formula of Kumar et al. [24]:
Total Carotenoid Content Estimation
Total carotenoid estimation was performed following the modified method of Lee & Castle [25]. Reagents used for this analysis were ethanol (95%) and hexane (HPLC grade, 98.5%). Ethanol and hexane were mixed in a ratio of 1:1 and shacked strongly. Then, 20 mL of solvent was added into the 2 g of homogenized pumpkin flesh tissue in a 50 mL centrifuge tube and run at 13,000 rpm for 20 min at 20 °C to form a homogenous sample. It was let to stand for 30 min before being divided into two phases. After that, the supernatant was poured into another 50 mL centrifuge tube (extraction pool). To keep just the solid, water was drained, and 20 mL of solvent was added. The mixture was vortexed until homogeneous. Each tube was then filled with 20 mL of deionized water, which was vortexed for 30 s. After that, phase separation was given five minutes. The extraction pool (tube) was once again filled with 20 mL of deionized water, vortexed for 30 s, and then stored in the freezer for an hour. After that, samples were read using a spectrophotometer (Shimadzu, Kyoto, Japan) absorbance at 470 nm [26]. Each analysis was carried out three times. Total carotenoid content was calculated using the following formula by Talcott & Howard [26].
where, A = absorbance at 470 nm, V = total volume of extract, g = sample weight and A1% = the extinction coefficient for a mixture of solvents arbitrary set at 2500. Then the value was converted to mg 100 g−1.
Total carotenoid content (µg g−1) = (A × V × 104)/(A1% × g)
Methanolic Extraction and Estimation of Antioxidant (DPPH%)
Methanolic extraction was performed using the modified method of Addai et al. [27]. First, 2 g fresh fruit sample was ground with liquid nitrogen using a pestle and mortar. Then, the sample was mixed with 20 mL of 80% methanol (HPLA grade, sigma, USA) in a test tube and wrapped with aluminium foil. The mixture was placed in an orbital shaker for 1 h at 180 rpm and centrifuged at 13,000 rpm for 20 min at 20 °C. The supernatant was collected in 20 mL dark bottles with cork closures and refrigerated until use. The extract was ready for analysis or kept at −20 °C. DPPH (2,2-diphenyl-1-picrylhydrazyl) Radical Scavenging Assay (%) content of pumpkin flesh was analysed following the modified method of Addai et al. [27].The stock solution of 1 Mm DPPH was prepared by dissolving 40 mg of DPPH in 100 mL of 80% Methanol and kept at −20 °C until used. DPPH Radical Scavenging Assay was analysed. Firstly, 100 µL of extract was taken and 1 mL DPPH was added and the mixture was mixed thoroughly and then kept in dark for 30 min at room temperature, 25 °C [28]. The absorbance was measured using a spectrophotometer (Shimadzu, Kyoto, Japan) at 516 nm. Each of the analyses was repeated three times. DPPH scavenging activity (%) was calculated by the following method used by Addai et al. [27].
DPPH Scavenging Activity (%) = (Absorbance of control − Absorbance of extract)/(Absorbance of control) × 100
2.5. Statistical Analysis
SAS (Statistical Analysis Software, SAS Institute Inc., Cary, NC, USA) version 9.4 was used for pooled analysis of variance (ANOVA) and the Duncan Multiple Range Test (DMRT) with a 5% level of significance to compare the means. GCA, SCA and reciprocal effects were analysed using Griffing’s method 1, model 1 with SAS [21,29].
2.5.1. Calculating GCA, SCA and Reciprocal Variances
GCA, SCA, reciprocal variances (Fixed model) were estimated following the formula used by Singh & Chaudhary [30].
where, MSg = Mean square of GCA, MSe = Mean square of error, n = Number of parents.
GCA variances due to GCA mean squares
σ2g = (MSg − MSe)/2 n
SCA variances due to SCA mean squares
where, MSs = Mean square of SCA, MSe = Mean square of error.
σ2s = MSs − MSe,
Reciprocal variances due to reciprocals mean squares
where, MSr = Mean square of reciprocals, MSe = Mean square of error.
σ2r = (MSr − MSe)/2,
2.5.2. Estimates of Additive and Non-Additive Variances
Estimates of additive and non-additive genes were performed following the formula used by Singh & Chaudhary [30].
Variances due to additive genes
where, σ2A = Additive variances, σ2g = GCA variances.
σ2A = 2 × σ2g,
Variances due to dominance genes
where, σ2D = Dominance variances, σ2s = SCA variances.
σ2D = σ2s,
2.5.3. Estimation of Heritability (%)
Heritability of narrow sense (h2N) and broad sense (h2B) were calculated using the below formula used by Feyzian et al. [31].
where, r = number of replications.
where, r = number of replications.
Heritability of narrow sense (h2N) = Additive variance/{Additive variance + dominance variance + (Error mean square)/r},
Heritability of broad sense (h2B) = Additive variance + Dominance variance/{Additive variance + dominance variance + (Error mean square)/r},
2.5.4. Genetic Ratio or Baker Ratio (BR)
The predictability ratio was estimated using the below formula by Baker [32].
where, σ2g = GCA variances, σ2s = SCA variances.
Baker ratio (BR) = 2 × σ2g/(2 × σ2g + σ2s),
2.5.5. Magnitude of Heterosis
The magnitude of heterosis for all the hybrids was estimated over mid-parent, better-parent and commercial hybrids as given below. All the types of heterosis were expressed as percentages [33].
Relative Heterosis or Mid-Parent Heterosis (MPH)
The deviation of F1 over mid-parental value was estimated as follows.
where, F1 = Mean value of hybrid, MP = Mean value of mid-parent.
MPH = (F1 − MP)/MP × 100
Heterobeltiosis or Better Parent Heterosis (BPH)
The deviation of F1 over better-parent was estimated as follows:
where, F1 = Mean value of hybrid, BP = Mean value of better-parent.
BPH = (F1 − BP)/BP × 100
Control or Standard Heterosis (SH)
The deviation of F1 over commercial hybrid was estimated as follows:
where, F1 = Mean value of hybrid, CH = Mean value of commercial check hybrid.
SH = (F1 − CH)/CH × 100
2.5.6. Test of Significance of Heterosis Magnitude
Test of significance for heterosis magnitude performed by critical differences (CD) following the formula of Ene et al. [33].
where, MSE = Error Mean Square, SE = Standard Error, r = Number of replications.
CD = SE × t value of 192 (error df) at 5% and 1% level of significance
SE = sqrt (2 MSE/r)
3. Results
3.1. Pooled ANOVA of All the Studied Traits Grown across Two Environments
The pooled analysis of variance for yield, yield components and fruit quality traits are presented in Table 3. Highly significant differences (p ≤ 0.01) were observed among the genotypes (parental lines, commercial check and F1 hybrids) and genotypes by environments (G × E) for all the evaluated traits. Similarly, highly significant (p ≤ 0.01) differences were observed in environments for all the studied traits except fruit flesh thickness. For replication within environments, there were no significant differences for the traits under study. The significance of genotypes means squared revealed that the expected comparisons for identifying the pattern of variation and determining the level of heterosis for these variables were valid and could be implemented.

Table 3.
Pooled ANOVA of studied traits grown across two environments.
3.2. Mean Performance of F1 Hybrids with Their Parental Lines
The per se performance of all parents and their F1 hybrids revealed that the parents in this study were genetically different and had potential breeding values. The mean performance of 42 F1 hybrids with their parental lines and commercial check hybrid are presented in Table 4. Amongst the parental genotypes, number of fruits per plant diverged from 2.34 (P2) to 3.56 (P6), while the hybrids diverged from 1.94 (P3 × P1) to 5.22 (P5 × P2). On the other hand, fruit flesh thickness ranged from 18.50 mm (P6) to 29.68 mm (P4) for the parents, whereas the F1 hybrids ranged from 21.90 mm (P4 × P2) to 39.80 mm (P2 × P5). Single fruit weight ranged between 1.00 kg (P6) and 1.97 kg (P7) among the parental genotypes, whereas the F1 hybrids ranged from 0.98 kg (P4 × P2) to 2.85 kg (P2 × P1). However, yield per plant varied from 3.34 kg (P6) to 4.77 kg (P7) amid the parents, whereas the hybrids varied from 2.45 kg (P4 × P2) to 11.29 kg (P2 × P1) (Table 4 and Figure 1). The number of seeds per fruit showed a wide range of variation, from 304.13 (P4) to 474.43 (P7) among the parental genotypes, but the F1 hybrids ranged from 311.80 (P1 × P3) to 702.13 (P4 × P5). For fruit quality assessment, total soluble solids varied from 11.59 °Brix (P3) to 13.92 °brix (P1) in case of parents, while the hybrids varied from 9.33 °Brix (P3 × P5) to 16.23 °Brix (P1 × P7) (Table 4 and Figure 2). The dry matter content ranged from 10.45% (P6) to 23.36% (P1) among the parental genotypes, whereas the hybrids ranged from 8.66% (P5 × P4) to 21.13% (P1 × P7). Total carotenoid content showed diverse range, from 1.86 mg 100 g−1 (P3) to 2.70 mg 100 g−1 (P4) for the parents, while the hybrids varied from 1.42 mg 100 g−1 (P3 × P1) to 2.82 mg 100 g−1 (P5 × P3). DPPH (antioxidant) levels diverged from 64.16% (P4) to 78.53% (P7) amongst the parental genotypes, whereas the hybrids ranged from 62.88% (P7 × P3) to 92.94% (P5 × P4). Overall, regarding mean performance, the study revealed that the hybrid mean is higher than the commercial check and parental lines’ mean for number of fruits per plant, single fruit weight, yield per plant, total carotenoid content and DPPH (%).

Table 4.
Mean performances of 7 parents, 42 F1 hybrids and 1 commercial check hybrid variety for the evaluated traits.
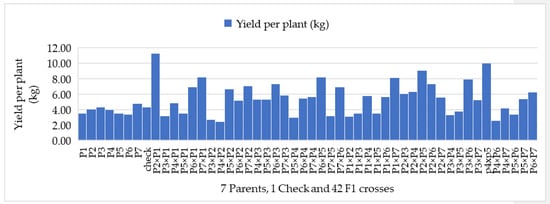
Figure 1.
Mean comparisons of parents, commercial check and 42 F1 crosses for yield per plant (kg), (Note—P1—Gold Butter 315, P2—928 Fuxiang, P3—Ser Bajadi, P4—Asian pumpkin, P5—Sarawak, P6—F3-2 and P7—Australia-1).
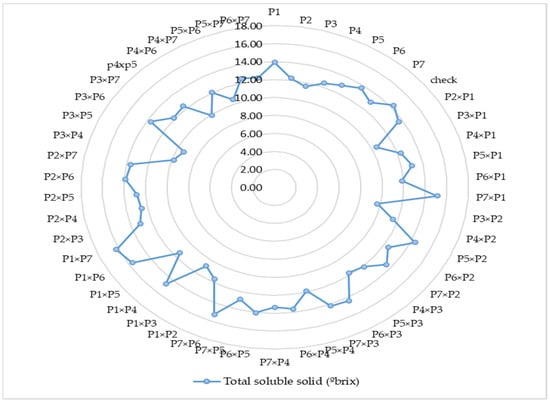
Figure 2.
Mean comparisons of 7 parents, 1 commercial check and 42 F1 crosses for total soluble solids (°Brix), (Note—P1—Gold Butter 315, P2—928 Fuxiang, P3—Ser Bajadi, P4—Asian pumpkin, P5—Sarawak, P6—F3-2 and P7—Australia-1).
3.3. ANOVA for Combining Ability, Maternal and Non-Maternal Variances
The analysis of variance for the combining ability of the studied characteristics is presented in Table 5. It was observed that the mean square of general combining ability (GCA), specific combining ability (SCA) and reciprocal variances showed highly significant differences (p ≤ 0.01) for all the studied traits, demonstrating the significance of the non-additive and additive factors. The interaction between environments and GCA, SCA, reciprocal, maternal and non-maternal variances showed highly significant differences except seed number per fruit for GCA variance. Maternal (general reciprocals) and non-maternal (specific reciprocals) effects were computed from reciprocal effects and showed highly significant differences (p ≤ 0.01) for all the traits. The interaction of environments with maternal and non-maternal variances also showed highly significant differences for all the studied traits.

Table 5.
Analysis of variance for mean square due to combining ability of parents, crosses and reciprocals for studied traits across two environments.
3.4. Genetic Variability Parameters and Gene Action
Genetic variability parameters, due to combining ability for the yield, yield-related traits and fruit quality are presented in Table 6. The broad-sense heritability ranged from 99.33% (total soluble solid) to 99.95% (DPPH%). Narrow-sense heritability ranging from 8.30 to 34.80% was observed for the studied traits. The lower value of narrow-sense heritability (h2N) was observed for yield per plant, DPPH, single fruit weight and seed number per fruit, while moderate narrow sense heritability was recorded for total carotenoid content, total soluble solid, dry matter content, fruit flesh thickness and number of fruits per plant. In this study, the narrow sense heritability was recorded as smaller than the corresponding values of broad sense heritability for all the studied traits. The GCA/SCA predictability or Baker ratio in this study ranged from 0.08 to 0.35, with the analyzed features’ values not being remarkably close to unity. All of the analyzed parameters exhibited genetic or Baker ratios (GCA/SCA) that were less than unity, indicating the involvement of dominant gene action in the inheritance of such traits.

Table 6.
Genetic variability parameters due to combining ability for the studied traits.
3.5. General Combining Ability (GCA) Effect of Parents and Specific Combining Ability (SCA) Effect of F1 Hybrids
General combining ability (GCA) effect of parents and specific combining ability (SCA) effect of F1 hybrids for all the examined traits are presented in Table 7. For the number of fruits per plant, the GCA effect ranged from −0.30 (P1) to 0.63 (P6), here, the parents P6 followed by P2 were the highly significant positive general combiners for this characteristic. On the other hand, the estimated SCA effect for this characteristic ranged from –1.42 (P5 × P6) to 1.01 (P5 × P2). Twelve crosses had highly significant positive SCA effects and the cross P5 × P2 was found as the best specific combiner, followed by the reciprocal cross P4 × P5, for the number of fruits per plant. The GCA effect on fruit flesh thickness varied from −2.76 (P6) to 2.70 (P7) and the parents P7, followed by P1 and P2, were shown to be highly positive significant general combiners. Moreover, sixteen crosses showed extremely positive significant SCA results for this trait, which ranged from −4.66 (P7 × P5) to 6.57 (P2 × P5). The reciprocal cross P2 × P5, followed by the cross P7 × P1 (5.80), were shown to be the best specific combiners for the attributes. The GCA impact varied from −0.15 (P6) to 0.16 (P7) for single fruit weight. For this characteristic, the parents P7, followed by P1 and P2, had a highly significant positive GCA effect and were considered as good general combiners. Meanwhile, the estimated SCA effect for the traits ranged from −0.69 (P1 × P2) to 0.76 (P4 × P5) and fifteen crosses expressed highly significant positive values of SCA effect for this trait. The reciprocal cross P4 × P5, followed by the cross P2 × P5, were found as the best specific combiners for the traits. The parents P2, P6 and P7 all demonstrated highly significant positive GCA impacts, with the GCA effect on yield per plant ranging from −0.57 to 0.46. The attributes’ estimated SCA effects ranged from −4.11 (P1 × P2) to 3.51 (P4 × P5) and the reciprocal cross P4 × P5, followed by the cross P6 × P3, were identified as the top specific combiners for yield per plant. Thirteen crosses exhibited extremely significant positive SCA impacts for yield per plant. The GCA effect for seed number per fruit varied from −28.19 (P3) to 57.72 (P7) and the parent P7, followed by the parents P6 and P2, showed highly positive significance. The estimated SCA effect for this trait ranged from −99.98 (P1 × P4) to 155.92 (P5 × P3) and seventeen crosses expressed highly significant positive values. Regarding SCA effect, the cross P5 × P3 and reciprocal cross P4 × P5 were found as the top two reciprocal combiners for seed number per fruit. The range of the GCA impact for total soluble solid was from −0.94 (P3) to 0.80 (P7), whereas considerable GCA impact was significantly beneficial for the parents P7, followed by P1 and P6. The estimated SCA impact for this trait varied from −1.86 (P4 × P6) to 1.91 (P1 × P6) and nine of the forty-two hybrids showed extremely significant positive SCA effects. The reciprocal cross P1 × P6, followed by the cross P6 × P3, were revealed as the best specific combiners for total soluble solids. For dry matter content, the GCA effect varied from −1.11 (P6) to 2.75 (P1) and only the parent P1 shown a significant GCA effect, whereas the SCA impact for this characteristic varied from −3.57 (P5 × P4) to 3.49 (P1 × P6), and seventeen crosses exhibited positive SCA effects. The reciprocal cross P1 × P6 and the cross P5 × P3 performed as good specific combiners for dry matter content among the crosses. The range of the GCA impact on the total carotenoid content was from −0.16 (P1) to 0.19 (P4). In the desired orientation, the parental lines P4, followed by the parent P5, P7 and P6, exhibited positively high GCA effects. The SCA impact of the traits was estimated from −0.53 (P5 × P6) to 0.45 (P7 × P2). The cross P7 × P2, followed by the reciprocal cross P2 × P3, were noted as the greatest specific combiners, and fifteen crosses demonstrated highly substantial positive SCA effects in the preferred direction for total carotenoid content. The GCA effect for DPPH (%) varied from −2.28 (P4) to 2.38 (P1) and the parents P1, P5 and P3 showed highly positive significant effects. The calculated SCA effect for the trait ranged from −12.76 (P2 × P3) to 13.87 (P3 × P1). Among the forty-two crosses, twenty-two crosses expressed highly significant positive SCA effects for this trait, and the cross P3 × P1, followed by the reciprocal cross P1 × P5, were identified as the top specific combiners for DPPH (%). (Table 7).

Table 7.
General combining ability effect of parents and specific combining ability effect of 42 F1 hybrids, including reciprocals for the studied traits.
3.6. Magnitude of Heterosis over Mid-Parent, Better-Parent and Commercial Hybrids
Heterosis was estimated for each characteristic as a percentage of increase in F1 hybrid over mid-parent (average or relative heterosis), better-parent (heterobeltiosis) and commercial check hybrids (standard heterosis) and is presented in Table 8. For the number of fruits per plant, mid-parent, better-parent and standard heterosis ranged from – 30.98 to 95.81%, −39.05 to 74.11% and −30.05% to 97.96%, respectively. Out of forty-two F1 hybrids, twenty-nine crosses for mid-parent, twenty-seven crosses for better-parent and twenty-eight crosses for standard heterosis were highly significant. The cross P5 × P2 for mid-parent (95.81%) and better-parent (74.11%) and P6 × P5 for commercial check (97.96%) were the top three ranking hybrids with effects in the desirable direction for number of fruits per plant (Table 8). In the case of fruit flesh thickness, a varied range of heterosis was found in mid-parent (−23.90 to 47.23%), better-parent (−26.22 to 43.00%) and standard heterosis (−36.06% to 16.20%). Amid the F1 hybrids, twenty-two crosses for mid-parent, fifteen crosses for better-parent and five crosses for standard heterosis were positively significant. At the same time, the reciprocal cross P2 × P5 was in the top ranking considering for the mid-parent, better-parent and standard heterosis. A wide range in mid-parent (−39.29 to 108.59%), better-parent (−42.80 to 86.51%) and standard heterosis (−38.36 to 78.93%) were observed for single fruit weight. Among all the F1, twenty-four crosses for mid-parent, nineteen crosses for better-parent and twenty-one crosses for standard heterosis were positively highly significant. The reciprocal cross P4 × P5 for mid-parent and better-parent and the cross P2 × P1 for standard heterosis were the top-ranking hybrids for single fruit weight. Amongst all hybrids, twenty-nine crosses for mid-parent, twenty-seven crosses for better-parent and twenty-eight crosses for standard heterosis showed positively highly significant results. For yield per plant, the cross P2 × P1 for mid-parent and better-parent and standard heterosis was found as the top performing hybrid (Figure 3). For the number of seeds per fruit, mid-parent, better-parent and standard heterosis ranged from -18.41 to 116.71%, −27.67 to 104.18% and −49.91 to 12.79%, respectively. Two crosses for mid-parent and two crosses for better-parent and no single cross for standard heterosis were highly significant for this trait. The reciprocal cross P4 × P5 over mid-parent and better-parent was shown to be a positive highly significant performer and a positive and non-significant performer for standard heterosis (Figure 4). A diverse range of heterosis was noted for mid-parent (−24.21 to 20.60%), better-parent (−28.02 to 27.37%) and standard heterosis (−34.94 to 13.26%) in case of total soluble solid (ºbrix). Eighteen crosses for mid-parent, sixteen crosses for better-parent and five crosses for economic check were positive and highly significant for this trait. The reciprocal cross P1 × P7 for mid-parent and standard heterosis and P1 × P4 for better-parent showed positively highly significant performance for total soluble solid. For dry matter content, a diverse range of heterosis was noted for mid-parent (−44.34 to 51.57%), better-parent (−54.34 to 41.83%) and standard heterosis (-58.49 to 1.31%) for dry matter content. Twenty crosses over mid-parent and fourteen crosses over better-parent heterosis were shown to be highly significant positive performers and only one cross performed positive non-significant standard heterosis for dry matter content. The reciprocal cross P7 × P6 for mid-parent and better-parent and P1 × P7 for standard heterosis were the top-ranking hybrids in desirable directions. For total carotenoid content, a diverged ranged of mid-parent (−34.55 to 37.75%), better-parent (−43.70 to 25.87%) and standard heterosis (−30.38 to 38.66%) was identified among the F1 hybrids, and eleven crosses for mid-parent, seven crosses for better-parent and twenty-one crosses for the commercial check were positively highly significant. The cross P5 × P3 is regarded as the highest ranked hybrid in mid-parent, better-parent, and standard heterosis for total carotenoid content (Figure 5). A diverse range of heterosis also noted for mid-parent (−16.14 to 33.54%), better-parent (−19.93 to 29.73%) and standard heterosis (−7.61 to 36.56%) in case of DPPH (%). Fifteen crosses for mid-parent, ten crosses for better-parent, and twenty-six crosses for standard heterosis were positively highly significant for DPPH (%). The cross P5 × P4 for mid-parent and standard heterosis and the cross P3 × P2 for better-parent heterosis were identified as the uppermost scoring hybrids for DPPH (%) (Figure 6).

Table 8.
Magnitude of heterosis over mid-parent, better-parent and commercial check hybrid for the evaluated traits.
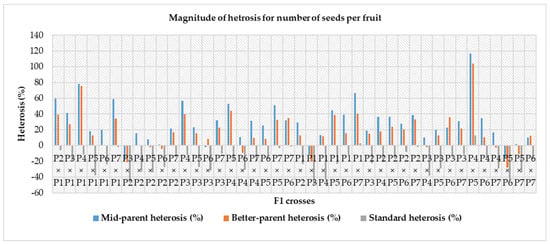
Figure 3.
Magnitude of heterosis over mid-parent, better-parent and commercial hybrids for yield per plant (Note—P1—Gold Butter 315, P2—928 Fuxiang, P3—Ser Bajadi, P4—Asian pumpkin, P5—Sarawak, P6—F3-2 and P7—Australia-1).
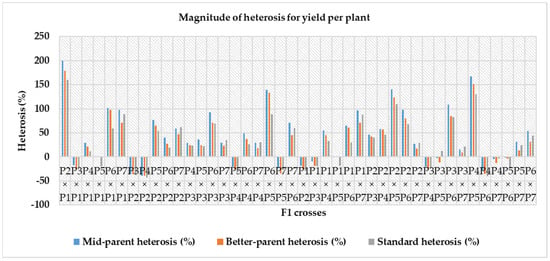
Figure 4.
Magnitude of heterosis over mid-parent, better-parent and commercial hybrids for number of seeds per fruit (Note—P1—Gold Butter 315, P2—928 Fuxiang, P3—Ser Bajadi, P4—Asian pumpkin, P5—Sarawak, P6—F3-2 and P7—Australia-1).
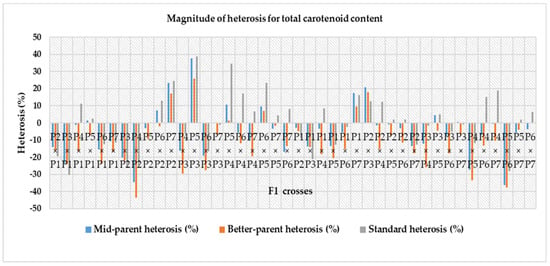
Figure 5.
Magnitude of heterosis over mid-parent, better-parent and commercial check hybrids for total carotenoid content (Note—P1—Gold Butter 315, P2—928 Fuxiang, P3—Ser Bajadi, P4—Asian pumpkin, P5—Sarawak, P6—F3-2 and P7—Australia-1).
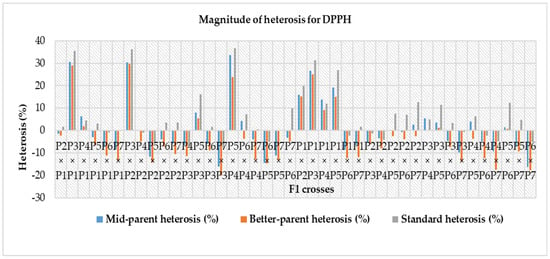
Figure 6.
Magnitude of heterosis over mid-parent, better-parent and commercial check hybrids for DPPH (Note—P1—Gold Butter 315, P2—928 Fuxiang, P3—Ser Bajadi, P4—Asian pumpkin, P5—Sarawak, P6—F3-2 and P7—Australia-1).
4. Discussions
4.1. Pooled ANOVA for the Studied Traits Grown across Two Environments
According to the findings of the pooled ANOVA (Table 3), the mean square for the genotypes and interactions between environments and genotypes for all the examined variables showed enormously significant differences, viewing that the progenies and their parental lines varied from each other. These findings provided evidence for the existence of a high and large degree of genetic variation among the hybrids and their parental accessions, which may promote genetic improvement, utilizing such genetic pools as Cucurbita accessions. As a result, the comprehensive study of combining ability and the kind of gene action was suitable for the purpose of determining the characteristics that had significant variance between genotypes. This study was supported by the findings of Mohsin et al. [34], Darrudi et al. [35] and Kumar et al. [36], who also found highly significant differences for the means squared of the studied traits in F1 hybrids of pumpkin genotypes (parental lines, commercial check and hybrids), as well as interactions between genotypes and environments.
4.2. Mean Performance for the Studied Traits
In this study, significant differences in characteristics were noticed among the parents and hybrids. The best performing hybrids, P5 × P2 (5.22), produce significantly higher fruits number per plant than the best performing parents, P2 (3.56) (Table 4). These results were more or less relevant to the reported values of Kumar et al. [36] and mentioned that the range of fruits number per plant in F1 hybrids was from 1.70 to 5.21, which was a higher positive value than the parental lines. These might be due to environmental and genetic constitutes of the genotypes. Fruits with thicker flesh are simpler to carry and keep their freshness for a longer period of time. The best hybrids, P2 × P5 (39.80 mm), generated much thicker flesh than the best parents, P4 (29.68 mm). Abdein et al. [22] also explored the range of fruit flesh thickness from 27.05 to 53.50 mm, which was somewhat consistent with this experiment. The yield potential is directly correlated with single fruit weight and considered as the most important factor in a hybridization program. In this study, the parent P7 is the top performer for single fruit weight (1.97 kg) and yield per plant (4.77 kg) and the P2 × P1 hybrids produced significantly higher value for single fruit weight (2.85 kg) and yield per plant (11.29 kg) for the hybrids. Pumpkin hybrids with single fruit weight and yield per plant ranging from 1.47 to 4.34 kg and 1.62 to 12.33 kg, respectively, were reported by Hatwal et al. [37] and Marxmathi & Krishnamoorthy [38] and results were revealed to be better than the present investigation and these might be due to varietal differences. In this study, for seed number per fruit best hybrids, P4 × P5 (702.13), produced enhanced value compared to the highest performing parent, P7 (456.17). Darrudi et al. [35] reported that seed number in pumpkin genotypes ranged 216.33 to 571.58 and these results were found as close in proximity to F1 hybrids compared to the parental value in pumpkin accessions. On the other hand, chemical constituents play important roles regarding the nutritional quality of pumpkin as a fruity vegetable. Amount of sugar in the palatable portion of a pumpkin is a sum of total soluble compounds and is a key factor in determining sweet taste. In the present study, the best hybrids, P1 × P7 (16.23 °Brix), produced significantly higher TSS value than the best parents, P1 (13.92 °Brix). The uniformity findings of total soluble solid from 8.20 to 15.00 °Brix was noted by Du et al. [39] and dry matter content (%) from 4.00 to 25.10% was found by Seroczynska et al. [40] in pumpkin genotypes. In this experiment, the hybrid P5 × P3 showed a significantly higher value of total carotenoid content (2.82 mg 100 g−1) than the top scorer parent, P4 (2.70 mg 100 g−1), while Selvi et al. [41] found the range from 0.51 to 3.07 mg 100 g−1 total carotenoid content in pumpkin. For DPPH (%), the cross P5 × P4 (92.94%) expressed higher concentration then the best performing parents P7 (78.53%) in this study whereas, Sharma & Bhat [42] found the range of DPPH (%) from 50.61 to 91.55% in pumpkin genotypes. The biochemical constituents, total carotenoid content and antioxidant scavenging activity (DPPH) of fruits, might be influenced by temperature, fruit harvesting stage and also genetic factors [7]. Overall, the study found that for the traits of number of fruits per plant, fruit flesh thickness, single fruit weight, yield per plant, total soluble solid, total carotenoid content, and DPPH (antioxidant), the hybrid mean performance is higher than the commercial check hybrids and parental means and may be due to presence of heterosis. These results indicated that hybrids were identified as better genetic resources for pumpkin genotype improvements. Heterosis is the superior performance of F1 hybrids over the superior parent or the parents’ mean or the typical check, depending on the gathering of favorable dominant gene to the F1 hybrid population that came from both female and male parents (36).
4.3. ANOVA Due to Combining Ability
Highly significant differences were observed for the general combining ability, specific combining ability, reciprocal, maternal, non-maternal variances and their interaction with the environment for most of the traits under investigation (Table 5). The highly significant general and specific combining ability means squared recorded in all variables imply that both non-additive and additive components of heritable variance are important. These components are accountable for the variation observed in these traits under study. These results are substantiated with El-Tahawey et al. [43] and Hussien & Hamed [44]. Darrudi et al. [34], Gvozdanovi-Varga et al. [45] and Bahari et al. [46] also observed significant means squared of GCA, SCA, reciprocal, maternal, non-maternal and environmental interaction variances in cucurbits. GCA means squared were smaller than SCA means squared for yield and its constituent traits, indicating that hybridization might improve such features due to the preponderance of non-additive gene action. The primary causes of reciprocal variations are maternal and non-maternal effects, having maternal effects induced by cytoplasmic genetic factors as well as non-maternal effects described by the combination of nuclear genes as well as cytoplasmic gene impacts [47]. According to Griffing’s study, all of the examined traits had substantial reciprocal effects in F1 diallel crosses. These findings imply that extra-nuclear genes are critical regulators of all the phenotypes under investigation. As a result, developing cytoplasmic male sterility (CMS) lines might make it simpler to transmit on favorable traits. Similar to this, reciprocal crossings may help achieve some specific objectives in the development of hybrid seeds [48].
4.4. Genetic Variability Parameters and Gene Action
The genetic ratio, or Baker ratio, for all the studied yields and their attributes and fruit quality traits showed less than unity (Table 6), suggesting that non-additive gene action predominated in the inheritance of those features. Baker [32] concluded a similar finding, confirming that when the GCA/SCA ratio was near unity, additive gene action predominated in the inheritance of those features, and when it was less than one, non-additive gene action predominated. Moreover, a breeder should pay attention to a trait’s heritability, as it indicates the scope and opportunity for improvement that selection affords. As it assesses the relationship between parents and offspring, it also suggests the direction of selection potential to be employed for a trait during selection. The analyzed characteristics’ broad-sense heritability (h2B) values were found to be more than twice as high as the corresponding narrow-sense heritability (h2N) values (Table 6). These findings indisputably showed that the non-additive gene effect, as compared to the additive gene effect, has a bigger impact on the inheritance of these traits and, thus, on their total performance. This finding suggested that it has the adequate scope of heterosis breeding for these parameters’ improvement. This conclusion is in line with that of Abdein et al. [22], who found that the traits of flesh thickness, average fruit weight, number of fruits per plant, yield per plant, and total soluble solid had low narrow sense heritability in pumpkin. Our broad and narrow sense heritability findings also coincided with the findings of Golabadi et al. [48], regarding the number of fruits per plant and yield per plant in greenhouse cucumber (Cucumis sativus L.).
4.5. General Combining Ability (GCA) and Specific Combining Ability (SCA) Effect
In this context, finding all desirable plant economic characteristics in a single unit is rare. As a result, breeders typically select parents and crosses when developing high-yielding cultivars. A parent is regarded as a good general combiner if it has higher positive substantial GCA effects [49]. The additive genetic variation often causes the GCA factor and choices made by each parent that are significantly influenced by the GCA variation. In this experiment, the parent P6 presented the highest GCA value for number of fruits per plant and the parental line P7 showed maximum significantly positive GCA values across two environments for fruit flesh thickness, single fruit weight, yield per plant, seed number per fruit and total soluble solid (Table 7). This finding showed that P7 is responsible for increased sweetness, fruit flesh thickness and yield per plant. On the other hand, the parental line P4 fashioned a higher GCA value for total carotenoid content, and the parental line P1 performed with highest GCA value on antioxidant activity (DPPH%) and dry matter content. These results suggested that the parental line P1 is the best to improve dry matter content and free radical scavenging activity (DPPH%). According to our results, the highest GCA value was distributed in the parental lines P1, P4, P6 and P7, indicating that none of the parental lines were the best general combiner for all the studied traits and could therefore be used as parental breeding materials for pumpkin varietal development. Similar findings were found by El-Gazzar et al. [50], who investigated 6 F1 hybrids with reciprocal and inbred lines and revealed that no paternal line outperformed for all of the qualities studied. According to our SCA estimates effects, the cross P5 × P2 (low × high combiner) for number of fruits per plant, P2 × P5 (high × low combiner) for fruit flesh thickness, P1 × P6 (high × low combiner) for dry matter content and P7 × P2 (high × low combiner) for total carotenoid content had the positively highest significant SCA values, which may be due to the epistatic × additive or additive × epistatic mode of gene action. However, the cross P4 × P5 (low × low combiner) presented the highest SCA effects for single fruit weight and yield per plant and may be ascribed as dominant × dominant gene action and showed a non-fixable overdominance interaction. Moreover, the cross P1 × P6 (high × high combiner) for total soluble solid, and the cross P3 × P1 (high × high combiner) for DPPH, showed that the highest positively significant SCA effect may be caused by additive × additive gene action. The development of high-performing hybrids required crossings with higher specific combining ability effects. This could be due to the fact that they comprised parents who had high × high, high × low and low × low general combining ability effects, respectively, which suggested the existence of additive, dominance and epistatic gene impacts for regulating the traits. Additionally, the genetic variation of the parents, as measured by the number of heterozygous loci of the parents engaged in the cross combinations, may be responsible for the supremacy of cross combinations using high × low, or low × low general combiners as parents [33]. Yadav et al. [1], Hatwal et al. [37], El-hadi et al. [51] and Marxmathi et al. [52] noticed similar significant findings for number of fruits per plant, single fruit weight, fruit fresh thickness, fruit yield per plant and seeds number per fruit in pumpkin genotypes.Identical findings were noted for total soluble solid (sweetness) by Kaur et al. [53] and for total carotenoid content and DPPH (%) by Irshad et al. [54] and Karmakar et al. [55] in pumpkin accessions. According to our results, few hybrids produced poor SCA effects from parents with high GCA effects in some of the variables under investigation. This might be a result of the parental genes not being sufficiently complemented. On the contrary, parents who had poor GCA effects generated hybrids with high SCA effects, which could be attributed to the complementary gene influence. Similar findings were found by Patil et al. [56] and Singh et al. [57] in bitter gourd. High × low general combining ability couples are appropriate for heterosis breeding, whereas high general combining ability combinations can be taken into consideration for creating superior varieties using the pedigree technique, according to Singh et al. [57] and Gharib et al. [58]. Thus, the cross P4 × P5 for yield per plant, P7 × P2 for total carotenoid content, P1 × P6 for total soluble solid and the cross P3 × P1 for DPPH are the best cross-combinations that may be used as hybrids with maximum vigor for yield per plant and improved fruit qualities.
4.6. Magnitude of Heterosis over Mid-Parent, Better-Parent and Commercial Check Hybrid
The advancement of commercial applications for the heterosis trait is one of the most important contributions to plant breeding. The genetic variation and breeding value of the parents utilized in the cross, as well as the environmental conditions in which the hybrid was grown, largely determine the degree of heterotic response displayed by the F1 hybrid. Heterosis is the enhanced performance of the F1 population over the better-parent, mid-parent, or the standard check after the accumulation of favorable dominant genes in the F1 population [59,60]. According to the finding of heterosis in this study, the hybrid P2 × P5 produced significantly higher fruit flesh thickness than mid-parent, better-parent and commercial check, whereas P5 × P2 produced a greater number of fruits per plant than the mid-parent and better-parent, but the cross P6 × P2 gave a greater number of fruits per plant than the commercial check hybrid (Table 8). In respect of yield per plant, P2 × P1 and the reciprocal hybrid P4 × P5 produced highly significant and higher amounts of yield than the mid-parent, better-parent and the commercial check hybrid. For fruit quality parameters, the hybrids P1 × P7, P5 × P3 and P5 × P4, for total soluble solids, total carotenoid and DPPH%, had highly significant and greater extents of heterosis in the desired direction over mid-parent, better-parent and commercial check hybrid. Thus, such hybrids can be used commercially for greater production of fruit yield and for improved fruit qualities. This result was in line with Kumar et al. [61], who revealed considerable significant heterosis in pumpkin over standard and better parents for fruit flesh thickness, yield per plant, dry matter content and total soluble solid. The significant heterosis was noted over the mid-parent and better-parent for single fruit weight and mid-parent heterosis for fruits number per plant, plant yield and carotenoid content by Jansi et al. [62] in pumpkin. The extent of heterosis is influenced by both the parents’ genetic make-up and the environment’s effects on the parents [7]. El-Hadi et al. [63] worked on pumpkin F1 hybrids and the uniformity results revealed that average fruit weight, fruit number per plants, plant fruit yield and the maximum significant desirable heterosis over mid-parent and better-parent. Hussien & Hamed [44] noted positively significant heterosis in required dimensions for plant yield, followed by total yield per plant, number of fruits per plant and weight of average. Heterosis (hybrid vigor) or supremacy of traits accomplished in F1 hybrids may be due to the dominant alleles suppressing the expression of harmful recessives alleles. Therefore, the effect of dominant positive genes covering undesired recessive ones is the explanation for a hybrid’s superiority [64].
5. Conclusions
The overall goal of the present study was to improve yield and fruit quality parameters; sweetness, total carotenoid and antioxidant content of pumpkin F1 hybrids through a complete diallel mating design. To achieve these objectives, 42 F1 hybrids with seven parental lines and one commercial check hybrid were evaluated in two environments to estimate combining ability effects and amount of heterosis. The analysis of variances offered that GCA, SCA, reciprocal, maternal and non-maternal variances and their interaction with environments showed highly significant differences (p ≤ 0.01) for all the evaluated traits of parental lines and F1 hybrids. The narrow-sense heritability (h2N) values were found to be lower to intermediate and the Baker or genetic ratio showed less than unity for all the studied traits. The fruit yield and quality characteristics showed that there was a prevalence of non-additive gene action on the inheritance for all the examined traits, which suggested that heterosis breeding would be advantageous to achieve improvements in the pumpkin’s genotypes. In terms of per se performances of the parental lines, Gold Butter 315 (P1), for total soluble solid and dry matter content, Asian pumpkin (P4), for total carotenoid content, Sarawak (P5), for number of fruits per plant and Australia-1 (P7), for single fruit weight, seed number per fruit, yield per plant and DPPH, were noted as the highest desirable performers. In respect to general combining ability (GCA) effects of the parental lines, the parent Gold Butter 315 (P1), for dry matter content and DPPH, Asian pumpkin (P4), for total carotenoid content, F3-2 (P6), for number of fruits per plant and Australia-1 (P7), for fruit flesh thickness, single fruit weight, yield per plant and total soluble solid, were noted as the best general combiners. In terms of per se performances and heterosis magnitude of F1 hybrids, the crosses 928 Fuxiang (P2) × Gold Butter 315 (P1), for yield per plant, Sarawak (P5) × 928 Fuxiang (P2), for fruits number per plant, Sarawak (P5) × Bajadi (P3), for number of seeds per fruit and total carotenoid, Sarawak (P5) × Asian pumpkin (P4), for DPPH, Australia-1 (P7) × F3-2 (P6), for dry matter content, the reciprocal cross Gold Butter 315 (P1) × Australia-1 (P7), for total soluble solid, 928 Fuxiang (P2) × Sarawak (P5), for fruit flesh thickness and Asian pumpkin (P4) × Sarawak (P5), for number of seeds per fruit and single fruit weight, were identified as the best performers over mid-parent, better-parent and standard heterosis. In relation to SCA effects, the cross Sarawak (P5) × 928 Fuxiang (P2), for fruits number per plant, Sarawak (P5) × Bajadi (P3), for number of seeds per fruit, 928 Fuxiang (P2) × Sarawak (P5), for flesh thickness, Asian pumpkin (P4) × Sarawak (P5), for single fruit weight and yield per plant, Gold Butter 315 (P1) × F3-2 (P6), for total soluble solid and dry matter content, Australia-1 (P7) × 928 Fuxiang (P2), for total carotene content and Bajadi (P3) × Gold Butter 315 (P1), for DPPH (antioxidant), were identified as the best specific combiners. Based on the findings, it can be concluded that such hybrids and parental lines of pumpkins would make great prospects for commercial application.
Author Contributions
Conceptualization, M.H. and M.Y.R.; Data curation, M.F.N.C.; Formal analysis, M.H., K.M.R.K. and J.H.; Funding acquisition, M.Y.R.; Investigation, M.Y.R., N.M. and M.J.; Methodology, M.Y.R.; Writing—original draft, M.H. and O.Y.; Writing—review & editing, M.H., K.M.R.K., O.Y., R.R., K.M.R.K., M.F.I. and J.H. All authors offered suggestions on various drafts of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the research grant title evaluation and selection of crop varieties for utilization to increase yield and production with vot number 6383900, Universiti Putra Malaysia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the manuscript.
Acknowledgments
The authors are thankful to the Ministry of Agriculture (MoA), Bangabandhu science and technology fellowship trust of the People’s Republic of Bangladesh and Universiti Putra Malaysia. (UPM).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Seven inbred parental lines’ descriptive characteristics were used in the hybridization programme.
Table A1.
Seven inbred parental lines’ descriptive characteristics were used in the hybridization programme.
| Parental Lines | Experimental Code | Fruit Shape and Colour | Reference |
|---|---|---|---|
| Gold Butter 315 | P1 | Flattened and brown |  |
| 928 Fuxiang | P2 | Flattened and brown |  |
| Ser Bajadi | P3 | Flattened and black |  |
| Asian pumpkin | P4 | Flattened and brown |  |
| Sarawak | P5 | Pyriform and green |  |
| F3-2 | P6 | Pyriform and brown |  |
| Australia-1 | P7 | Flattened and green |  |
References
- Yadav, M.K.; Singh, D.P.; Rajiv, V.P.S.; Kumar, S. Combining ability analysis of yield and yields attributing traits of pumpkin (Cucurbita moschata Duch ex Poir). Pharma Innov. J. 2021, 10, 250–254. [Google Scholar]
- Yadav, M.; Jain, S.; Tomar, R.; Prasad, G.; Yadav, H. Medicinal and biological potential of pumpkin: An updated review. Nutr. Res. Rev. 2010, 23, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Hosen, M.; Rafii, M.Y.; Mazlan, N.; Jusoh, M.; Oladosu, Y.; Chowdhury, M.; Nazneen, F.; Muhammad, I.; Khan, M.M.H. Pumpkin (Cucurbita spp.): A Crop to Mitigate Food and Nutritional Challenges. Horticulturae 2021, 7, 352. [Google Scholar] [CrossRef]
- Adubofuor, J.; Anomah, J.W.; Amoah, I. Anti-nutritional factors and mineral composition of pumpkin pulp and functional properties of pumpkin-wheat composite flour for bread preparation. Int. J. Food Sci. Technol. 2018, 1, 1–9. [Google Scholar] [CrossRef]
- Kakamari, G.S.; Jagadeesha, R.C. Estimation of combining ability for growth, yield and its components in pumpkin (Cucurbita moschata Duch. Ex. Poir). Res. Environ. Life Sci. 2017, 10, 280–283. [Google Scholar]
- Hashash, M.M.; El-Sayed, M.M.; Abdel-Hady, A.A.; Hady, H.A.; Morsi, E.A. Nutritional potential, mineral composition and antioxidant activity squash (Curcurbita pepo L.) fruits grown in Egypt. Inflammation 2017, 9, 11–12. [Google Scholar]
- Nemeskéri, E. Breeding strategy for improvement of colour quality and carotenoid levels in dry pea seeds. Commun. Biometry Crop Sci. 2006, 1, 49–55. [Google Scholar]
- Seymen, M.; Uslu, N.; Türkmen, Ö.; Al Juhaimi, F.; Özcan, M.M. Chemical compositions and mineral contents of some hull-less pumpkin seed and oils. J. Am. Oil Chem. Soc. 2016, 93, 1095–1099. [Google Scholar] [CrossRef]
- Rani, R.; Kumar, S.; Yadav, S. Pumpkin and chia seed as dietary fibre source in meat products: A review. J. Pharm. Innov. 2021, 10, 477–485. [Google Scholar]
- Seymen, M. Seed Yield and Characteristics in a Half-Diallel Pumpkin Population. Selcuk J. Agric. Food Sci. 2020, 34, 200–206. [Google Scholar] [CrossRef]
- Caili, F.U.; Huan, S.; Quanhong, L.I. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum. Nutr. 2006, 61, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Nisha, S.K.; Veeraragavathatham, D. Heterosis and combining ability for fruit yield and its component traits in pumpkin (Cucurbita moschata Duch. ex Poir.). Adv. Appl. Sci. Res. 2014, 6, 158. [Google Scholar] [CrossRef]
- Norman, A.; Taylor, J.; Edwards, J.; Kuchel, H. Optimising genomic selection in wheat: Effect of marker density, population size and population structure on prediction accuracy. G3 Genes Genomes Genet. 2018, 8, 2889–2899. [Google Scholar] [CrossRef]
- Falconer, D.S. Introduction to Quantitative Genetics; Pearson Education India: Noida, India, 1996; ISBN 8131727408. [Google Scholar]
- Virmani, S.S. Heterosis in Rice. In Monogr. Theoretical and Applied Genetics; Virmani, S.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Fasahat, P.; Rajabi, A.; Rad, J.M.; Derera, J. Principles and utilization of combining ability in plant breeding. Biom. Biostat. Int. J. 2016, 4, 1–24. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Sugiyama, M.; Sakata, Y. Combining ability analysis of fruit texture traits in cucumber by mechanical measurement. Breed. Sci. 2010, 60, 65–70. [Google Scholar] [CrossRef]
- Ferreira, M.G.; de Almeida, G.Q.; Pessoa, H.P.; Dariva, F.D.; Dias, F.d.O.; Nick, C. Selection of squash “Menina Brasileira” carrying the allele “Bush” with high yield potential. Hortic. Bras. 2019, 37, 35–39. [Google Scholar] [CrossRef]
- Kearsey, M.J.; Pooni, H. Genetical Analysis of Quantitative Traits; Garland Science: New York, NY, USA, 2020; ISBN 1000144178. [Google Scholar]
- Griffing, B. Concept of general and specific combining ability in relation to diallel crossing systems. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef]
- Abdein, M.A.E.; Hassan, H.M.F.; Dalia, H.M. General performance, combining abilities and heritability of yield and yield component traits in pumpkin (Cucurbita moschata poir.) at different conditions. Curr. Appl. Sci. Technol. 2017, 17, 121–129. [Google Scholar]
- Díez, M.J.; Dooijeweert, W.; Maggioni, L.; Lipman, E. Minimum Descriptor lists for Cucurbit; cucumber, melon and watermelon. In Proceedings of the Peport of a Working Group on Cucurbits First Meeting, Plovdiv, Bulgaria, 1–2 September 2005; pp. 10–11. [Google Scholar]
- Kumar, V.; Mishra, D.; Yadav, G.; Yadav, S.; Kumar, S. Determining relationships between yield and biochemical traits in pumpkin. Pharm. Innovation. 2018, 7, 14–18. [Google Scholar]
- Lee, H.S.; Castle, W.S. Seasonal changes of carotenoid pigments and color in Hamlin, Earlygold, and Budd Blood orange juices. J. Agric. Food Chem. 2001, 49, 877–882. [Google Scholar] [CrossRef]
- Talcott, S.T.; Howard, L.R. Phenolic autoxidation is responsible for color degradation in processed carrot puree. J. Agric. Food Chem. 1999, 47, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Addai, Z.R.; Abdullah, A.; Mutalib, S.A. Influence of ripening stages on antioxidant properties of papaya fruit (Carica papaya L.). In Proceedings of the American Institute of Physics Proceedings; American Institute of Physics: College Park, MD, USA, 2013; Volume 1571, pp. 696–701. [Google Scholar]
- Musa, K.H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant activity of pink-flesh guava (Psidium guajava L.): Effect of extraction techniques and solvents. Food Anal. Methods 2011, 4, 100–107. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, M.S.; Lamkey, K.R. DIALLEL-SAS05: A comprehensive program for Griffing’s and Gardner–Eberhart analyses. Agron. J. 2005, 97, 1097–1106. [Google Scholar] [CrossRef]
- Singh, R.K.; Chaudhary, B.D. Biometrical Method in Quantitative Genetic Analysis; Kalyani Publishers: New Delhi, India, 1985; pp. 127–140. [Google Scholar]
- Feyzian, E.; Dehghani, H.; Rezai, A.M.; Javaran, M.J. Diallel cross analysis for maturity and yield-related traits in melon (Cucumis melo L.). Euphytica 2009, 168, 215–223. [Google Scholar] [CrossRef]
- Baker, R.J. Issues in diallel analysis. Crop Sci. 1978, 18, 533–536. [Google Scholar] [CrossRef]
- Ene, C.O.; Ogbonna, P.E.; Agbo, C.U.; Chukwudi, U.P. Heterosis and combining ability in cucumber (Cucumis sativus L.). Inf. Process. Agric. 2019, 6, 150–157. [Google Scholar] [CrossRef]
- Mohsin, G.M.; Doullah, M.A.U.; Hasanuzzaman, M.; Biswas, B.K.; Islam, M.S.; Rahman, S.; Islam, A.; Dinajpur, B. Combining ability analysis in pumpkin (Cucurbita moschata Duch Ex Poir). Contemp. Res. India 2017, 7, 176–181. [Google Scholar]
- Darrudi, R.; Nazeri, V.; Soltani, F.; Shokrpour, M.; Ercolano, M.R. Evaluation of combining ability in Cucurbita pepo L. and Cucurbita moschata Duchesne accessions for fruit and seed quantitative traits. J. Appl. Res. Med. Aromat. Plants 2018, 9, 70–77. [Google Scholar] [CrossRef]
- Kumar, R.; Rajasree, V.; Praneetha, S.; Rajeswari, S.; Khuntia, S. Heterosis breeding in pumpkin (Cucurbita moschata Duch. ex Poir.) for small size, thick flesh with high yield and β-carotene. Int. J. Chem. Stud. 2018, 6, 81–85. [Google Scholar]
- Hatwal, P.K.; Yadav, V.S.; Thakur, R.; Mahawar, A.K. Estimation of heterosis in relation to combining ability for earliness, yield and quality attributes in pumpkin (Cucurbita moschata Duch. ex Poir). Indian J. Agric. Res. 2018, 52, 548–553. [Google Scholar]
- Marxmathi, P.; Krishnamoorthy, V. Per se performance of pumkin (Cucurbita moschata Duch ex Poir) hybrids for yield and quality. Asian J. Hortic. 2017, 12, 260–266. [Google Scholar] [CrossRef]
- Du, X.; Sun, Y.; Li, X.; Zhou, J.; Li, X. Genetic divergence among inbred lines in Cucurbita moschata from China. Sci. Hortic. (Amst.) 2011, 127, 207–213. [Google Scholar] [CrossRef]
- Seroczynska, A.; Antczak, A.; Korytowska, M.; Kaminska, K.; Radomski, A.; Korzeniewska, A.; Zawadzki, J.; Niemiriwicz-Szczytt, K. Evaluation of the selected forms of winter squash (Cucurbita maxima Duch.) for the content of free sugars and polysaccharides. Pol. J. Agron. 2014, 16, 69–73. [Google Scholar]
- Selvi, N.A.T.; Jansirani, P.; Pugalendhi, L. Studies on heterosis in pumpkin (Cucurbita moschata Duch. ex Poir). J. Hortic. Sci. 2014, 9, 131–140. [Google Scholar]
- Sharma, M.; Bhat, R. Extraction of carotenoids from pumpkin peel and pulp: Comparison between innovative green extraction technologies (ultrasonic and microwave-assisted extractions using corn oil). Foods 2021, 10, 787. [Google Scholar] [CrossRef]
- El-Tahawey, M.; Kandeel, A.M.; Youssef, S.M.S.; Abd El-Salam, M.M.M. Heterosis, potence ratio, combining ability and correlation of some economic traits in diallel crosses of pumpkins. Egypt. J. Plant Breed. 2015, 19, 419–439. [Google Scholar] [CrossRef]
- Hussien, A.; Hamed, A. Diallel Analysis for Studying Heterosis and Combining Ability of Some Economical Yield Traits in Pumpkin. Int. J. Plant Prod. 2015, 6, 261–270. [Google Scholar] [CrossRef]
- Gvozdanović-Varga, J.; Vasić, M.; Milić, D.; Červenski, J. Diallel cross analysis for fruit traits in watermelon. Genetika 2011, 43, 163–174. [Google Scholar] [CrossRef]
- Bahari, M.; Rafii, M.Y.; Saleh, G.B.; Latif, M.A. Combining ability analysis in complete diallel cross of watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai). Sci. World J. 2012, 543158, 6. [Google Scholar]
- Kundu, S.; Mandal, A.R.; Mukherjee, D.; Banerjee, S.; Ghosh, T.; Mandal, A.K.; Chattopadhyay, A. Breeding bitter gourd (Momordica charantia L.) for simultaneous improvement in yield, nutritional quality and downy mildew disease tolerance. Ann. Plant Soil Res. 2022, 24, 250–258. [Google Scholar]
- Golabadi, M.; Golkar, P.; Eghtedary, A. Combining ability analysis of fruit yield and morphological traits in greenhouse cucumber (Cucumis sativus L.). Can. J. Plant Sci. 2015, 95, 377–385. [Google Scholar] [CrossRef]
- Shafin, M.S.; Haque, M.E.; Parvin, M.S.; Akhter, F. Heterosis and Combining Ability in Pumpkin Inbreds (Cucurbita moschata Duch. ex Poir.). bioRxiv 2022, 9, 37–56. [Google Scholar]
- El-Gazzar, T.M.; Tartoura, E.A.; Nada, M.M. Evaluation of new inbred lines and their hybrids in balady squash variety (Cucurbita pepo L.). Int. J. Plant Prod. 2015, 6, 135–143. [Google Scholar] [CrossRef]
- El-hadi, A.H.A.; Fathy, M.; Abdein, M.A. Manifestation of heterosis and the role of the genetic parameters associated with it for some vegetative traits in squash (Cucurbita pepo, L.). Alexandria Sci. Exch. J. 2014, 35, 190–202. [Google Scholar]
- Marxmathi, P.; Krishnamoorthy, V.; Thankaraj, P. Combining ability studies in pumpkin (Cucurbita moschata Duch ex Poir). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3033–3039. [Google Scholar] [CrossRef][Green Version]
- Kaur, S.; Sharma, S.P.; Sarao, N.K.; Deol, J.K.; Gill, R.; Abd-Elsalam, K.A.; Alghuthaymi, M.A.; Hassan, M.M.; Chawla, N. Heterosis and Combining Ability for Fruit Yield, Sweetness, β-Carotene, Ascorbic Acid, Firmness and Fusarium Wilt Resistance in Muskmelon (Cucumis melo L.) Involving Genetic Male Sterile Lines. Horticulturae 2022, 8, 82. [Google Scholar] [CrossRef]
- Irshad, M.; Ahmad, I.; Mehdi, S.J.; Goel, H.C.; Rizvi, M.M.A. Antioxidant capacity and phenolic content of the aqueous extract of commonly consumed cucurbits. Int. J. Food Prop. 2014, 17, 179–186. [Google Scholar] [CrossRef]
- Karmakar, P.; Munshi, A.; Behera, T.; Kumar, R.; Kaur, C.; Singh, B. Hermaphrodite inbreds with better combining ability improve antioxidant properties in ridge gourd [Luffa acutangula (Roxb.) L.]. Euphytica 2013, 191, 75–84. [Google Scholar] [CrossRef]
- Patil, S.A.; Salimath, P.M.; Dharmatti, P.R.; Byadgi, A.S.; Nirmala, Y. Heterosis and combining ability analysis for productivity traits in bitter gourd (Momordica charantia L.). Karnataka J. Agric. Sci. 2012, 25, 9–13. [Google Scholar]
- Singh, A.K.; Pan, R.S.; Bhavana, P. Heterosis and combining ability analysis in bittergourd (Momordica charantia L.). Bioscan 2013, 8, 1533–1536. [Google Scholar]
- Gharib, A.H.A.M.; El Sayed, A.A.; El Tahawey, M.A.; Khafagi, E.Y. Breeding for fusarium wilt resistance and some economic characters in cucumber. J. Appl. Hortic. 2020, 22, 255–264. [Google Scholar]
- Hayes, J.D.; Foster, C.A. Heterosis in self pollinated crops with particular reference to barley, Heterosis in plant breeding. In Proceedings of the 7th Congress, European Association for Research on Plant Breeding, Budapest, Hungary, 24–29 June 1976; pp. 239–256. [Google Scholar]
- Khattak, G.S.S.; Ashraf, M.; Zamir, R. Gene action for synchrony in pod maturity and indeterminate growth habit in mungbean (Vigna radiata (L.) Wilczek). Pak. J. Bot. 2004, 36, 589–594. [Google Scholar]
- Kumar, V.; Mishra, D.P.; Yadav, G.C.; Yadav, S. Exploitation of heterobeltiosis and economic heterosis for horticultural yield, and its attributes and biochemical traits in pumpkin (Cucurbita moschata Duch. ex. Poir) under salt affected soil. Curr. Sci. 2018, 115, 1550–1556. [Google Scholar] [CrossRef]
- Jansi, V. Heterosis and inbreeding depression studies in pumpkin (Cucurbita moschata Duch. ex Poir.). Electron. J. Plant Breed. 2018, 9, 1031–1037. [Google Scholar] [CrossRef]
- El-Hadi, A.; El-Aziz, A.; Abd Alla, M.A.; Ashak, M.G. Genetic Evalution of some Economical Traits in Summer Squash. J. Agric. Chem. Biotechnol. 2020, 11, 147–153. [Google Scholar] [CrossRef]
- Bernardo, R. Breeding for quantitative traits in plants. In Woodbury; Stemma Press: Woodbury, MN, USA, 2014; Volume 1, p. 369. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).