Abstract
Poor and inconsistent quality is a major barrier to increasing produce consumption, and the lack of shelf-life after purchase is the quality issue at retail displays of produce. This research aimed to investigate and identify cooling techniques, namely vacuum cooling and package icing used in supply chains, and any resulting extension of broccoli shelf-life, maintenance of physicochemical quality, and delay in microbial growth at retail stores. Broccoli (Brassica oleracea var. italica cv. Montop) sustainably grown in the highlands of northern Thailand, Chiang Mai Province, under the Royal Project Foundation was experimented on vacuum cooling and package icing were selected to precool broccoli to 4 ± 1 °C. The effects of vacuum cooling using a final pressure of 0.6 kPa for 30 min and package icing using liner Styrofoam boxes (the best ratio of broccoli to crushed ice was 1:1 w/w) on physicochemical qualities, microbial growth, and shelf-life in simulated refrigerated retail displays were examined. The results illustrated that the shelf-life and quality of broccoli could be extended using both vacuum cooling and package icing. Both precooling techniques inhibited the yellowing of florets, provided high sensory scores, delayed microbial growth, and could be able to extend the shelf-life of broccoli. However, package icing offered greater potential for maintaining quality, especially retaining bioactive compounds, and extending shelf-life, thereby increasing the produce market window from 5 to 12 days at 8 ± 1 °C with 85% RH. Therefore, package icing was recommended in the supply chain for fresh broccoli cv. Montop grown in northern Thailand.
1. Introduction
Broccoli is popularly consumed throughout the world because of its potent nutritional value and related health benefits. Broccoli is a rich source of vitamins, minerals, fiber, and other bioactive compounds that are much higher in green vegetables [1,2,3]. Losses in the appreciable and nutritional quality of broccoli arise rapidly due to the yellowing after harvest caused by chlorophyll degradation, loss of texture along with off-odor development, and increased peroxidase activity [4] as well as nearly blooming flowers. This leads to a very short shelf-life after harvest and a reduction in commercial value.
The main factor in broccoli quality attributes is the temperature. Several techniques have been extensively investigated to extend shelf-life and enhance the visual or health value in broccoli, such as precooling systems. Precooling is known to be a good technique for the removal of field heat shortly after the harvest of a crop [5]. In general, there are many different methods to precool produce, including hydrocooling, forced air cooling, liquid ice-cooling, and vacuum cooling. The method that is the most suitable choice depends on various factors such as product characteristics, efficiency, scale, packaging, and economic viability [6]. Rapid precooling retards the physiological disorders and quality degradation of fruit and vegetables and extends the storage period or shelf-life by removing field heat [7]. A similar result was found in broccoli treated with hydrocooling, having effects on the reduced weight loss of broccoli after 21 days of storage compared with room-cooled broccoli [8]. Likewise, their results suggest that delaying precooling for 4–8 h increased the water loss of strawberries by about 50% and considerably decreased quality after arriving at the distribution center [9]. Similarly, [10] indicated that the potential of forced-air precooling helps to decrease weight loss and increase in sensory evaluation of green asparagus after 3 days of transportation. Nevertheless, vacuum cooling and package icing are the methods most suitable to apply with broccoli. Vacuum cooling is the fastest method, able to handle large volumes in a short processing time while being uniform and hygienic as well as independent of the packing method [11].
Vacuum cooling involves evaporating the moisture off the produce under vacuum conditions [12], and it is separated into three stages. In the first stage, the temperature will be stable, and no cooling takes place. When pressure drops into the saturation pressure consistent with the initial temperature of the product (flashpoint), the water inside the product starts to evaporate and continues to emit vapor. The cooling is initiated when the second stage begins. In the last stage, the air flows into the chamber, and after that, pressure returns to surrounding pressure [13]. Vacuum cooling can extend shelf-life and improve the quality and safety of the product [11]. Similar findings were reported by [14] where vacuum cooling of leafy greens quickly reduced the temperature, thus efficaciously extending the shelf-life. This was in concordance with the findings by [15] in which vacuum cooling was a clean and rapid method to maintain the high quality of fresh-cut vegetables during storage and the review by [16] about handling precooling after harvest, particularly in warm weather. Hydro cooling and vacuum cooling are precooling systems that can reduce the respiration rate and avoid water loss of baby spinach. Package icing is a traditional and effective method that involves adding crushed or flaked ice on top and then filling the voids around the harvested produce in the container. Generally, package icing is used for fresh produce that can tolerate water and wet conditions, for instance, green onions, asparagus, leafy greens, cauliflower, and broccoli [17]. Nevertheless, ice melts and cold water flowing through the fresh produce can help to cool down the product [18]. For this reason, ice needs to be carefully managed per safety requirements both as the ice is handled and as the melt water moves across surfaces due to increasing the risk of microorganism contamination on the produce. Ice is also very adaptable to precooling in the field as an essential part of the harvest operation. Residual ice in a mobile ice-based cooler can also help maintain the temperature of broccoli during transport. One finding from previous studies that tested broccoli treated by four cooling methods: room cooling, hydrocooling, forced-air cooling, and package icing, demonstrated that package icing and hydrocooling were better methods of cooling than room cooling and forced air precooling [19]. Similar findings were reported by [20] for package icing that replaces cooled water where crushed or slurry ice is used to quickly cool down the fresh produce. Therefore, it is necessary to remove the field heat by precooling after harvest, and keeping the optimum temperature throughout the supply chain is imperative to maintain quality and reduce losses [21]. In agreement with the study of [22] in the cold chain without precooling, 23% more quality loss was observed than in that with precooling.
Fresh produce of the Royal Project Foundation, Thailand, sold at retail outlets generally moves through a four-step supply chain consisting of farms (contract growers), the Royal Project development center, a packing house facility in Chiang Mai (distribution center), and retail stores in different provinces, as illustrated in Figure 1. Research had previously examined precooling techniques as important factors that could increase the retail freshness of broccoli as well as maintain the highest quality with the longest practical shelf-life. This work aimed to examine the most appropriate commercial cooling techniques for broccoli, including commercial vacuum cooling and package icing for the supply chain of the Royal Project Foundation in Thailand. The research focuses on the potential shelf-life of produce as it arrived at the retail stores. This research explored appropriate techniques to improve shelf-life, maintain quality, and delay microbial growth in the supply chain using commercial vacuum cooling and package icing. The effects of vacuum cooling and package icing on visual appearance, physicochemical, microbial growth, and shelf-life of fresh broccoli heads were evaluated. Furthermore, the inhibition of decay from microorganisms affected by commercial vacuum cooling and package icing was also investigated.
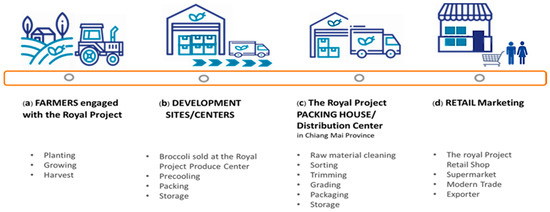
Figure 1.
The produces moved along the supply chain namely (a) at harvest sites (on farms), (b) at the Royal Project Development Center, (c) at the packing house facility in Chiang Mai Province (distribution center) (d) at the retail stores.
2. Materials and Methods
2.1. Plant Materials
Broccoli (Brassica oleracea var. italica cv. Montop) was planted in the field of the Mae Poon Luang Royal Project development center, Prao District, Chaing Mai, Thailand. Broccoli heads were hand-harvested early in the morning (6 am) at the commercial maturity stage. After harvest, broccoli heads were immediately transported to the Royal Project Development Center. Broccoli heads were sorted and selected for uniform color and size and the absence of visual defects. Heads of broccoli were trimmed with a sharp knife and packed in commercial plastic bags (perforated polyethylene, Ø 0.5 cm, with 18 holes). The broccoli heads were then randomly allocated to three treatments: vacuum cooling, package icing, and non-precooling (control), each with three replicates. Broccoli heads were precooled with commercial vacuum cooling and ice packaging. The broccoli heads from the control treatment were held outside in the ambient condition. Samples from all three treatments were transferred with a refrigerated car to the laboratory in the packing house facility in Chiang Mai and stored in a simulated refrigerated retail display. Physicochemical, sensory, and microbial quality, as well as shelf-life, were evaluated until the end of shelf-life.
2.2. Precooling, Processing, and Storage Conditions
Precooling processing was investigated and compared between vacuum cooling and ice cooling. Vacuum cooling systems are carried out using a vacuum chamber, vacuum pump, and vapor condenser. The experimental process of vacuum cooling started with placing broccoli approximately 500 kg (about 5 kg/crate) into 60 polypropylene crates (0.36 × 0.56 × 0.29 m3). Subsequently, plastic creates were placed inside the vacuum chamber. The initial weights and temperatures of the produce were measured by random selection. The final pressures were set at 6.0 millibar with a holding time for the vacuum stage of 30 min, and the system was ready to operate. On the removal, broccoli heads were reweighed to check weight loss percentages during vacuum cooling. Vacuum cooling is based on the reduction in the boiling point of water at reduced pressure. Vacuum cooling works by forcing a portion of the water found in the product to evaporate at low temperature, simply by lowering the atmospheric pressure around the produce. The energy required to evaporate the water comes from the product itself, resulting in evaporative cooling. The vapor from the chamber is removed by the vapor condenser outside the chamber. Throughout the operation, data including chamber pressure, chamber temperature, and product temperature at the center were recorded. Product temperature at the center was recorded every minute by a thermocouple probe from the beginning throughout the end of the cooling process. After completing the process, the weights of broccoli heads were evaluated immediately.
The package icing technique was accomplished by using crushed or flaked ice in the ratio of ice to broccoli of 1:1 (by packing broccoli and ice in a Styrofoam box, size 0.46 × 0.60 × 0.32 m). The temperature profile of the broccoli head at the center was recorded by a thermocouple probe, by drilling through a sealed Styrofoam box and measuring the temperature every minute. This process could rapidly cool the produce, maintain product temperature, and reduce moisture loss. This process requires a plastic liner to protect the samples because it helps to protect fresh broccoli from water, as water that melts from the ice could cause damage to the produce. Its advantages were that this technique could be used after transit and handling. Samples from both different precooling treatments were compared with the non-precooled samples. Samples were stored in a simulated refrigerated retail display at 8 ± 1 °C with 85% RH. The qualities in terms of physicochemical, sensory quality, and microbial growth were analyzed throughout the storage period.
2.3. Cooling Coefficient
The cooling process was evaluated by using the cooling coefficient, which represents the change of product temperature per cooling time unit in the middle of the product and environment [23]. The heterogeneous temperature of areas was conceived by plotting Equation (1). Dimensionless temperature (θ) is determined from product temperature (T), initial product temperature (Ti), and the medium of cooling temperature (Tm) as shown in the following equation.
θ = (T − Tm)/(Ti − Tm)
In general, the dimensional change of temperature over time (t) is expressed as an exponential equation, carried out with (c) as the cooling coefficient and (J) as the lag factor:
θ = J−ct
The half-cooling time (HCT) is the time required to reduce the temperature difference between the vegetable and the cooling by substituting θ = 0.5 into Equation (3).
HCT = [ln(2J)]/c
Similar to Equation (3), the seven-eighths cooling time (SECT) is given by substituting θ = 0.125 in Equation (4).
SECT = ([ln(8J)]/c
2.4. Weight Loss Percentage
Weight loss was assessed by weighing the sample daily and calculating according to the comparison of sample weight (control sample) during storage periods [24]. Weight loss percentage was calculated according to Equation (5).
where W is the initial weight of the broccoli sample (g), while Wi is the weight of the sample at the time of analysis (g).
Weight loss = (W − Wi)/W × 100%
2.5. Color Evaluation
Color change in broccoli is mostly due to pigment content. Yellowing is a symptom of senescence that occurs mainly in the buds of broccoli [3,25]. The values of L* (lightness), chroma, and hue angle (h°) were measured by a chromameter (KONICA MINOLTA, CR-400, Osaka, Japan). Five representative samples were obtained from each treatment. Every replicate was chosen at random, and each broccoli floret was taken at three different positions of the area by measuring at the same point throughout the analysis [26]. Then, the value was calculated as in Equations (6) and (7).
The hue angle (h°) was calculated as:
h° = tan−1 (b*/a*)
The chroma (saturation) was calculated as:
C* = (a* + b*) ½
2.6. Total Chlorophyll Content
Chlorophyll a (Chl a) and Chlorophyll b (Chl b) are the main pigments in the photosynthetic machinery in plants [27]. Chlorophyll degradation is a cause of senescence that leads to deterioration of the quality of broccoli heads. To prepare the samples, 10 g of broccoli was ground and added into 10 mL of 80% acetone and left until it became thoroughly green. The extract was filtered by Whatman filter paper No. 1. Chlorophyll content was measured at 663 and 645 nm by a spectrophotometer (Digital Spectrophotometer, Genesys 10S, Thermo Spectronic, West Palm Beach, USA). Chlorophyll a, chlorophyll b, and the total chlorophyll of broccoli were calculated according to the following equations [28]:
where V is the final volume of solute and W is a sample weight (g).
Chlorophyll a = [12.7(Abs. at 663 nm) − 2.69(Abs. at 645 nm)] × (V/1000 W)
Chlorophyll b = [22.9(Abs. at 645 nm) − 4.68(Abs. at 663 nm)] × (V/1000 W)
Total chlorophyll = [20.2(Abs. at 645 nm) + 8.02(Abs. at 663 nm)] × (V/1000 W)
2.7. Ascorbic Acid Content
Ascorbic acid was determined by titration with 2,6-dichlorophenolindophenol dye solution on 0.1% ascorbic acid for a standard curve. Ascorbic acid from a sample was extracted by 10 g of homogenized sample with 0.4% oxalic acid solution and adjusting the volume to 100 mL. The sample was thoroughly mixed by shaking the container for one hour and filtered with Whatman no. 1 filter paper. Ten milliliters of solution aliquots from each treatment was titrated. The titration continued until the endpoint of the titration was detected when the unreduced dye gave a rose-pink color that appeared for at least 15 s in acid solution. The results were represented as mg of ascorbic acid/100 g of fresh weight (FW) [29].
2.8. Total Phenolic Content
Total phenolic content was estimated by Folin-Ciocalteu’s assay as described by Tugli et al. [30] with slight modifications. The sample was extracted by 20 g of homogenized sample with 100% methanol and the sample was thoroughly mixed by shaking for one hour and filtered with Whatman no. 1 filter paper and a 0.45-micron nylon syringe filter. The sample (50 µL) was put into a small test tube and mixed with 50 µL of methanol, which was followed by 1000 µL of distilled water and 125 µL of Folin-Ciocalteu reagent. About 375 µL of 7.5% sodium carbonate was added into the mixture, which rested for 5 min and incubated for 120 min at room temperature. After that, the absorbance was measured at 765 nm by using a spectrophotometer (Digital Spectrophotometer, Genesys 10S, Thermo Spectronic, West Palm Beach, USA). The total phenol content was calculated and expressed as mg gallic acid equivalent (GAE)/g sample (FW).
2.9. Antioxidant Activity
Antioxidant activity was determined by the DPPH radical scavenging according to Khanam et al. [31], with slight modifications. The sample was extracted by 20 g of homogenized sample with 100% methanol and the sample was thoroughly mixed by shaking for one hour and filtered with Whatman no. 1 filter paper and a 0.45-micron nylon syringe filter. The absorbance of the DPPH solution was set at 515 nm and was performed with a spectrophotometer (Digital Spectrophotometer, Genesys 10S, Thermo Spectronic, West Palm Beach, USA). Extracts were added, and the mixture was then left at room temperature. The decrease in the intensity of the absorption was measured again at 515 nm. Trolox was used as a reference standard and the results were expressed as μg Trolox/g FW.
2.10. Microbiological Analysis
Twenty-five grams of broccoli was analyzed for microbiological evaluation as described by Mantilla et al. [32]. All samples were homogenized in 0.1% buffered peptone water (BPW). In the enumeration of each microbial group, there was a dilution series (10-fold) that was prepared in 9 mL of peptone water solution. Total aerobic bacteria were specified by using Petrifilm (3M™ Petrifilm™ Rapid Aerobic Count Plate) incubated at 35 ± 1 °C for 48 ± 3 h (AOAC 990.12). Yeast and mold were counted in Petrifilm (3M™ Petrifilm™ Rapid Yeast and Mold Count Plate) incubated at 20–25 °C for 5 d (AOAC 997.02). Lactic acid bacteria were enumerated with Petrifilm (3M™ Petrifilm™ Lactic Acid Bacteria Count Plate) incubated at 28–37 °C for 48 ± 3 h [33]. Three replicates from various treatments were analyzed in duplicate, and after incubation, colonies were enumerated and results were reported in log CFU/g of the sample.
2.11. Sensory Quality and SHELF-Life Evaluation
Sensory quality and shelf-life evaluation were terminated by a lack of consumer acceptability, which was caused by fresh weight loss (withering), yellowing of the florets, blooming, or decay which was performed by a trained panel throughout the storage period. All of the samples were presented in the same tray and assigned a code to prevent bias. Sensory quality and shelf-life evaluation were estimated in consideration of freshness, overall appearance (wilting, color, damage, and decay), and brightness following a 9-point hedonic scale where 9 = excellent, 7 = good, 5 = fair (limit of consumer acceptance), 3 = poor and 1 = extremely poor [34].
2.12. Statistical Analysis
Research experiment in a completely randomized design consisting of three treatments including a control (non-precooled) with three replicates was conducted. One-way analysis of variance (ANOVA) was used to analyze the data and Duncan’s multiple range test (DMRT) was used to separate the mean. Statistical analyses were performed by SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Evolution of the Temperature during Precooling
The cooling techniques on maintaining broccoli quality during storage in a simulated refrigerated retail display were investigated compared to non-precooled produce and focused on the effect of the rate of cooling on maintaining the quality of fresh broccoli. From the study, the temperature of broccoli decreased faster by commercial vacuum cooling compared to broccoli precooled using package icing. During the vacuum cooling process, heat transfer is accompanied by mass transfer as most of the cooling phenomenon is caused by water evaporation, as evaporation is the main determinant for the rapid cooling rate [35]. The cooling time using vacuum cooling was 4.63 times faster than package icing, as illustrated in Table 1 and Figure 2a,b. For the vacuum cooling system, pressure and temperature changed because the water of the broccoli boiled when the pressure in the chamber reached the saturation vapor pressure at the initial temperature of the broccoli. The vacuum pump reduced the chamber pressure from 951.70 to 7.40 mbar within 8 min, which was recognized as a flashpoint. As shown in Figure 2a, the chamber pressure and produce temperature continued to rapidly decline. When the pressure reached the target pressure of 6 kPa and 30 min of holding time, the broccoli had a final temperature of 4.06 °C. Consequently, the half and seven-eighths cooling times of vacuum-cooled samples were shorter than those were of package icing (Table 1). Meanwhile, the package icing works by the water of crushed ice flowing through the broccoli, thus exchanging heat with the broccoli while the air temperature increases. The obvious characteristics of phase transition cause the ice to maintain the temperature at a lower level and the logarithmic mean of temperature is greater [36]. Thus, the ice-packaging technique had many advantages, such as fast cooling capability as well as convenient transportation [37].

Table 1.
Cooling parameters of the precooling methods.
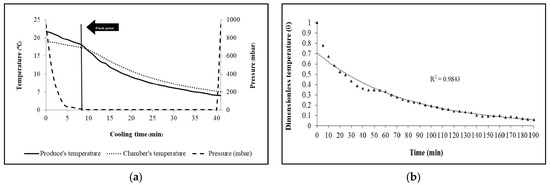
Figure 2.
(a) Broccoli cooling curve, chamber pressure and temperature changes during the vacuum precooling process. (b) Relationship between the dimensionless temperature and time of broccoli precooled by package icing (△).
From cooling coefficients that represented the rate of cooling of the produce, a small number for cooling coefficients meant reducing the temperature of the product to the desired temperature over a short time. Table 1 presents the cooling coefficient between the vacuum cooling and package icing, which were 0.03 min−1 and 0.06 min−1, respectively. Moreover, calculated lag factors of broccoli heads from the temperature data of both precooling methods were in the range of 1, especially the package-icing samples at 0.75, which demonstrated that broccoli heads had low internal resistance. Therefore, the vacuum cooling technique was superior to the ice packaging technique in cooling rates of 0.43 and 0.09 °C min−1, cooling coefficients of 0.03 and 0.06 min−1, respectively (Figure 2). In addition, vacuum-cooled and ice-cooled samples exhibited a weight loss percentage less than 2% (1.70% and 1.08%) after 5 days of storage. From the study, the results showed that fresh broccoli precooled by package icing lost fresh weight at a significantly lower rate than vacuum cooling. [38] also reported that package icing was a convenient process to maintain the low temperature with suitable relative humidity and reduce metabolic reactions and weight loss of fresh fruits and vegetables.
From the precooling process results, vacuum cooling was clearly faster than ice packaging, with a cooling rate approximately almost five times faster than that of ice-cooling (Table 1). Vacuum cooling could decline rapidly removing field heat by evaporating water directly from the produce [39]. Likewise, vacuum cooling was the most energy-efficient cooling method and resulted in firmer heads. The cooling rate by which broccoli heads were cooled to 4 ± 1 °C was likely to have impacts on shelf-life and quality.
3.2. Weight Loss Percentage and Sensory Quality
The weight loss percentage increased in every sample with storage duration and mostly in control samples, which exhibited faster quality deterioration. After harvest, the metabolic activities of vegetables, involving respiration and ripening, continue within cells until senescence and death. The higher the temperature at or after harvest directly affects respiration rate, resulting in reduced shelf-life and negative purchase decisions [40]. Besides the moisture loss from the precooling process, the respiration rate of broccoli was an important factor influencing shelf-life [41]. The effects of vacuum and package icing on the weight loss percentage of broccoli stored simulated retail refrigerated display are shown in Table 2 and Figure 3a. The results illustrated that the weight loss percentage of all samples increased; however, both precooled samples had a lower level of weight loss percentage than the control one. After 5 days of storage, the control samples showed rapid and significant weight loss percentages and had lower sensory scores (p ≤ 0.05) compared to the vacuum and ice-cooled samples. The results revealed that broccoli treated with package icing could retard the weight loss percentage and had a direct positive effect on the sensory quality (Table 2 and Figure 3a,b). Elansari et al. [19] also found that liquid ice cooling had a positive effect on the appearance throughout the supply chain; this method could keep the produce fresh for longer, including after reaching the end consumer.

Table 2.
Weight loss percentage and sensory quality of broccoli during storage period at 4 °C and 85% RH.
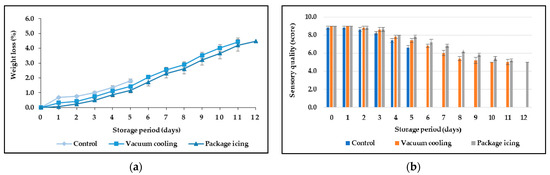
Figure 3.
Effect of precooling method on weight loss percentage (a) and sensory quality (b) of broccoli during storage period at 8 °C and 85% RH.
3.3. Color Evaluation
Color was one of the most important sensory properties [42]. This study demonstrated that changes in lightness (L*) of broccoli heads were observed during storage; the L* value tends to increase along with the storage period (Table 3). Control samples gave the significantly highest value of L* value compared with vacuum and ice-cooled samples (p ≤ 0.05). The results were in agreement with earlier findings that the precooling had the best quality with the lowest weight loss and yellowing along with better chlorophyll and ascorbic acid contents [43]. As shown in Table 3, the hue angle values of every sample gradually decreased with storage time, indicating that broccoli gradually changed colors from green to yellow. At the end of storage, all cooling sample delays gave the highest value of hue angle as compared with control samples, as described previously. Saad and EL SAYED [44] showed that the non-cooled samples gave lower hue angle values with marked de-greening or intense yellowing. Zhu et al. [45] revealed the effects of the vacuum cooling method on hue angle values of flowering cabbage decreased with storage time, particularly in the control treatment; the hue angle values decreased significantly at 15 days, while vacuum cooling treatment indicated lower hue angle values at 21 days of storage. The color of flowering cabbage turned yellow and became soft, leading to inedibility when the hue angle value fell to 130°. Additionally, the chroma was higher in all of the treatments and specifically in control samples compared with vacuum and ice-cooled samples (Table 3). After 5 days of storage, significant differences were observed among precooling treatments as described by DeEll and Toivonen [46]; at room temperature, the hue decreased rapidly and chroma increased.

Table 3.
Effect of precooling method on color changes of broccoli during storage at 8 °C with 85% RH.
The ∆E* was affected by precooling methods, which is shown in Table 3. The ∆E* values on stored heads of broccoli showed an increase in all treatments. The greatest color change occurred in the control samples of broccoli after 5 days of storage. The present findings are in agreement with the results reported by Kochhar and Kumar [8], who reported that the ∆E* value was highest for control samples and lowest for package iced samples, which is a consequence of the precooling treatments immediately after the harvest inhibiting the decay of broccoli color.
3.4. Total Chlorophyll Content
The green pigment is one of the main factors affecting the quality of broccoli ascribable to chlorophyll degradation, which is the first apparent sign of senescence, leads to customer rejection, and lowers the market price [47]. The chlorophyll contents of broccoli in the different treatments showed an overall downward trend according to Table 4 and Figure 4a, which was confirmed by Torales et al. [48], where the results involved degradation of chlorophyll caused by enzymatic reactions that occur mainly within the chloroplasts and vacuoles, which includes the interrelated expression of genes coding proteins involved in chlorophyll degradation [49]. After 5 days of storage, vacuum and ice-cooled samples significantly retarded the degradation of chlorophyll in broccoli [50]. This was consistent with the findings of An et al. [51], who reported that vacuum cooling with 50% hydrogen-rich water could effectively inhibit the degradation of chlorophyll, delaying the leaf yellowing of pakchoi. Similar work by TAO et al. [52] found that precooling methods delayed the decomposition of chlorophyll.

Table 4.
Total chlorophyll content, ascorbic acid content, antioxidant activity, and total phenolic content of broccoli during storage period at 8 °C and 85% RH.
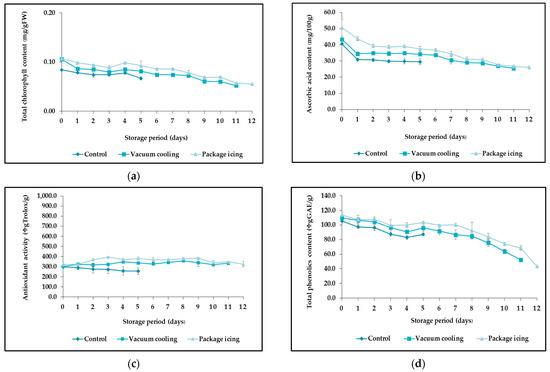
Figure 4.
Effect of precooling method on total chlorophyll content (a), ascorbic acid content (b), antioxidant activity (c), and total phenolic content (d) of broccoli during storage period at 8 °C and 85% RH.
3.5. Ascorbic Acid Content
Broccoli is a cruciferous vegetable rich in vitamin C; the levels vary over 4-fold in broccoli. Inevitably, the temperature has negative impacts on vegetables. Therefore, postharvest cooling rapidly removes the field heat and reduces the deterioration process of fresh products [53]. In addition, it results in temperature reduction to the target value suitable for transport and storage [35]. The large effects of vacuum cooling and package icing on the ascorbic acid content of broccoli in this present study were higher than in the control samples. Precooling can prevent the deterioration of broccoli heads and lead to maintained content of vitamin C, as confirmed by Li et al. [54], who found that the cooling delay after harvest may increase respiration rate and metabolic activity. Ascorbic acid showed a significant difference (p ≤ 0.05) between vacuum-cooled, ice-cooled, and control samples during the entire storage period (Table 4 and Figure 4b). These results were also in agreement with Kitazawa et al. [55]; the cooling was effective in preventing ascorbic acid degradation during storage, and the lowest values of ascorbic acid content resulted from the non-cooled treatment. Similar effects were also observed by Han et al. [26], who indicated a positive effect of vacuum cooling, which maintained greater ascorbic acid content of the lettuce.
3.6. Antioxidant Activity
Broccoli heads cooled with both precooling methods had significantly higher antioxidant activity than the control samples during storage (Table 4 and Figure 4c). After 5 days of storage, the antioxidant activities in vacuum and ice-cooled samples were significantly higher (p ≤ 0.05) than those in control samples. These results were in agreement with a study by Tao et al. [56], which indicated the highest expression of enzymatic antioxidant was found in mushrooms stored under modified atmosphere packaging (MAP) with vacuum cooling. Similar experimental trends have been reported by Li et al. [57], where the vacuum precooling treatment was an effective method for maintaining the antioxidant activities of blackberry fruit. Since phenolic compounds are important plant constituents with redox properties responsible for antioxidant activity, a strong correlation between the phenolic content and their antioxidant activities was shown in this study, ice-cooled samples were significantly higher than vacuum samples after 5 days of storage as well (p ≤ 0.05).
3.7. Total Phenolic Content
The total phenolic contents in both of precooling method and control samples decreased throughout the storage period. The results were shown in (Table 4 and Figure 4d), the phenolic content in the broccoli treated with vacuum and package icing was significantly (p ≤ 0.05) higher than control samples during storage. There were reports in the literature Zhu et al. [45] that vacuum cooling had a positive influence on oxidative metabolism, such as reducing respiration and regulating the activities of POD and CAT, thus delaying senescence of the samples. Moreover, the high levels of phenolic contents had a better correlation with antioxidant activity and vitamin C due to phenolic compounds playing an important role in antioxidant scavenging properties. In addition, vitamin C and carotenoids have antioxidant properties [58]. Furthermore, ice-cooled samples had significantly higher total phenolic contents after 5 days of storage than those precooled using vacuum cooling (p ≤ 0.05).
3.8. Microbiological Analysis
The initial microbiological counts of broccoli were higher than vacuum and package icing samples; counts of total aerobic bacteria after 4 days did not show significant differences among treatments during storage (Table 5). Vegetable microbiomes vary, and the quality and safety of vegetables involve a lot of factors, including soil, manure, water, the prevalence of animals in the field, and good management and sales. Controlling contamination with pathogenic and spoilage microorganisms is very difficult [59]. Some yeast and mold counts significantly increased in every treatment. Particularly in control samples, the amount of yeast and mold was higher than the samples treated with vacuum cooling and package icing. These results followed previous studies of Al-Dairi et al. [60], who reported that most of the microbial processes that cause produce quality deterioration are temperature dependent. The procedure without cooling resulted in decreased quality and increased decay of fresh produce [61]. This is in line with the findings of GOJIYA [62], where at the end of the storage period of guava fruit, the maximum amounts of yeast and mold were detected in the control sample at room temperature (16 × 104 CFU/g). These results could be due to precooling slowing down the deterioration and the rotting process by reducing the growth of microorganisms [63]. According to Zhu et al. [11], the moisture content of the surrounding air in the vacuum cooling process showed stronger effects on the microbial safety of the fresh produce, the low moisture content was accomplished by airflow. High moisture content in the surroundings could push the microbes into the guard cells and fissures around the epithelial cells, causing an increase in microorganisms. The interplay between the vacuum cooling method and moisture had an effect on microbial interaction with stomata; therefore, low moisture conditions during vacuum cooling should be avoided for microbial safety. Furthermore, the growth of lactic acid bacteria was higher in control samples compared to precooled treatments, which is probably due to both precooling techniques effectively reducing postharvest respiration, inhibiting microbial growth, and extending the storage period [64]. Likewise, applying vacuum cooling lengthened the shelf-life of mushrooms by reducing the water activity and inhibiting the growth of microorganisms [65]. Nevertheless, in the storage and transportation of fresh produce, there can be unforeseen circumstances that are not suitable conditions for the produce, which can result in stimulation of the ripening process as well as physiological and chemical deterioration due to the decomposition of vegetables.

Table 5.
Effect of precooling methods on microbiological growth of broccoli during storage at 8 °C with 85% RH.
3.9. Shelf-Life Evaluation
According to the results, vacuum cooling and package icing could extend the shelf-life of broccoli to 11 and 12 days, respectively, which were long enough to allow a market window of fresh broccoli in the supply chain of broccoli of the Royal Project Foundation. This is in contrast with the non-precooled samples (control treatment), where broccoli heads had a shelf-life of only 5 days and the end of shelf-life was due to wilting, less firmness, less freshness, floret yellowing, or the presence of rot in florets or stems. The study illustrated that the senescence process of non-precooled samples was faster than the precooled samples in both treatments since, at higher temperatures, broccoli deteriorate faster caused the control sample to have only 5 days of shelf-life. Furthermore, precooling with vacuum cooling and package icing were appropriate techniques to extend the shelf-life and maintain the quality of broccoli in terms of retaining firmness, color, vitamin C content, total phenolic content, and antioxidant activity, while subsequently maintaining the sensory quality of fresh broccoli. Similar results have been reported in baby cos lettuce of Kongwong et al. [23], which indicated that vacuum cooling was an efficient method for extending the shelf-life of baby cos lettuce from 9 days to 16 days. Additionally, Tian et al. [66] reported that in broccoli after 30 days of storage, the qualities of the broccoli treated with vacuum cooling and ice-water cooling method were significantly better than those were of the control (24 days), which illustrated that the precooling treatments maintained the sensory qualities and extended the shelf-life of broccoli during storage.
4. Discussion
The quality of broccoli purchased from the retail stores was significantly reduced by inconsistent quality and freshness. Supply chain studies indicated that broccoli precooled reasonably quickly after harvest. The precooling process can be beneficial to the reduced temperature inside produce or removing field heat from fruits and vegetables to increase shelf life and appearance quality. This paper will discuss the comparison of two precooling methods for broccoli in relation to improving shelf life, maintaining quality, and delaying microbial growth of broccoli in the supply chain.
In this work, the results indicate that both precooling methods retarded weight loss of broccoli, and samples had a lower level of weight loss percentage than the control sample. Especially, broccoli treated with package Icing had the lowest weight loss. This might be due to the reduction in the respiration, transpiration rate, and senescence related to metabolic processes by precooling and then storing under cold storage conditions [44,67], which probably maintained the sensory scores of produce. The most common damage to plants is from water loss which affects organs, tissues, and cellular components [68] causing the produce to wither and directly affects to the sensory quality.
Further results from weight loss of broccoli showed that the vacuum cooling and package icing method delayed the head of broccoli yellowing resulting in differences in chroma that could be involved in the water content of the produce as the more moist specimens showed higher color vividness [69].
From this study, the total chlorophyll contents in control samples were lower than in the precooled samples. This might be because the control sample was not cooled down, thereby resulting in a large amount of heat accumulation, which stimulated the process of respiration. Increased respiration after harvest may lead to a decrease in the chlorophyll content initially, after which it increases again as broccoli adapts to storage conditions. Chlorophyll was gradually depleted due to nutritional deficiencies and prolonged storage time, revealing associated carotenoids [3]. Some studies had also proposed that chloroplasts lose their integrity, destroying the membrane compartment structure segregating chlorophyll and chlorophyll-degrading enzymes, thus releasing enzymes in the chloroplast membrane to enzymatically degrade chlorophyll [70].
It is generally accepted that precooling the broccoli results in slowing down the ascorbic acid content, antioxidant activity, and total phenolic content; these correlations were related to broccoli samples in the precooled treatment that remained higher in ascorbic acid, antioxidant activity, and polyphenol contents than the control samples throughout the store. This again showed that both precooling methods could maintain a high level of antioxidants during storage. The major antioxidants present in broccoli were associated with polyphenols, which prevented the generation of free radicals [71].
However, in our case, the possible remaining water on the head surface of broccoli in vacuum cooling and package icing method did not promote microbial growth or decay, which this result is clearly different from the control sample. This result showed that the management of fresh produce to prevent contamination with pathogens of fruits and vegetables after harvest by precooling is an essential role critical for the success of fresh produce safety throughout the supply chain [72].
The two different cooling methods experimented with resulted in similar quality. In this study, few quality differences were observed between cooling treatments. Overall, both precooling treatments retained good quality during their subsequent shelf-life. However, ascorbic acid, chlorophyll, phenolic content, and antioxidant activity were significantly better in ice-cooled than in vacuum-cooled broccoli after 5 days of storage. The results are encouraging and show that the ice packaging technique improved the quality of broccoli.
5. Conclusions
The current study compared the effects of different precooling methods, namely on the quality of broccoli throughout the supply chain in terms of physicochemical and sensory quality as well as microbiological growth, along with the shelf-life. The study revealed that vacuum cooling and package icing could extend the shelf-life of broccoli from 5 to 11 and 12 days, respectively. The process of package icing was an appropriate technique to precool fresh broccoli, which is packed in Styrofoam boxes in the supply chain in Thailand, and suitable for domestic distribution since this process can cool the produce to the optimum temperature while in transport. The results of the study also confirmed that preventing moisture loss during the cooling process was another important factor for the acceptability of the consumers.
Author Contributions
Conceptualization, P.P.; methodology, P.D.; software, P.D.; validation, P.P. and D.B.; formal analysis, P.D.; investigation, P.P. and P.D.; resources, P.P.; data curation, P.P. and P.D.; writing by P.P. and P.D.; writing, review and editing, P.P. and D.B.; visualization, P.D.; supervision, P.P. and D.B.; project administration, P.P.; funding acquisition, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by Postharvest Technology Innovation Center, Ministry of Higher Education, Science, Research and Invention, Bangkok, 10400, Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The research was also supported by Food Science and Technology, Faculty of Agro-industry, Chiang Mai University. Moreover, the authors are grateful to the Royal Project Foundation for provision of laboratory works.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lv, J.; Wu, J.; Zuo, J.; Fan, L.; Shi, J.; Gao, L.; Li, M.; Wang, Q. Effect of Se treatment on the volatile compounds in broccoli. Food Chem. 2017, 216, 225–233. [Google Scholar] [CrossRef]
- Pezeshkpour, V.; Khosravani, S.A.; Ghaedi, M.; Dashtian, K.; Zare, F.; Sharifi, A.; Jannesar, R.; Zoladl, M. Ultrasound assisted extraction of phenolic acids from broccoli vegetable and using sonochemistry for preparation of MOF-5 nanocubes: Comparative study based on micro-dilution broth and plate count method for synergism antibacterial effect. Ultrason. Sonochem. 2018, 40, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Cheng, S.-C.; Cai, J.-H.; Wei, B.-D.; Zhou, X.; Zhou, Q.; Zhao, Y.-B.; Ji, S.-J. Chlorophyll degradation and carotenoid biosynthetic pathways: Gene expression and pigment content in broccoli during yellowing. Food Chem. 2019, 297, 124964. [Google Scholar] [CrossRef] [PubMed]
- Santana, J.C.C.; Araújo, S.A.; Alves, W.A.L.; Belan, P.A.; Jiangang, L.; Jianchu, C.; Dong-Hong, L. Optimization of vacuum cooling treatment of postharvest broccoli using response surface methodology combined with genetic algorithm technique. Comput. Electron. Agric. 2018, 144, 209–215. [Google Scholar] [CrossRef]
- Chiman, B.; Jayesh, D. Precooling techniques and applications for fruits and vegetables. Int. J. Process. Post Harvest Technol. 2016, 7, 141–150. [Google Scholar]
- Alibas, I.; Koksal, N. Forced-air, vacuum, and hydro precooling of cauliflower (Brassica oleracea L. var. botrytis cv. Freemont): Part I. determination of precooling parameters. Food Sci. Technol. 2014, 34, 730–737. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, G.-B.; Fawole, O.A.; Verboven, P.; Zhang, X.-R.; Wu, D.; Opara, U.L.; Nicolai, B.; Chen, K. Postharvest precooling of fruit and vegetables: A review. Trends Food Sci. Technol. 2020, 100, 278–291. [Google Scholar] [CrossRef]
- Kochhar, V. Effect of Different Pre-Cooling Methods on the Quality and Shelf Life of Broccoli. J. Food Process. Technol. 2015, 6, 424. [Google Scholar] [CrossRef]
- Pelletier, W.; Brecht, J.K.; Nunes, M.C.d.N.; Émond, J.-P. Quality of Strawberries Shipped by Truck from California to Florida as Influenced by Postharvest Temperature Management Practices. HortTechnology 2011, 21, 482–493. [Google Scholar] [CrossRef]
- Xiao-Ming, D.; Sheng, L.I.U.; JIA, L. Effect of Different Pre-cooling and Transportation Methods on the Quality of Green Asparagus. DEStech Trans. Environ. Energy Earth Sci. 2016, 486–490. [Google Scholar]
- Zhu, Z.; Geng, Y.; Sun, D.-W. Effects of operation processes and conditions on enhancing performances of vacuum cooling of foods: A review. Trends food Sci. Technol. 2019, 85, 67–77. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, S.; Tian, C.; Yan, G.; Wang, D. An overview of current status of cold chain in China. Int. J. Refrig. 2018, 88, 483–495. [Google Scholar] [CrossRef]
- Song, X.; Liu, B.; Jaganathan, G.K.; Chen, L. Mechanism of spillage and excessive boiling of water during vacuum cooling. Int. J. Refrig. 2015, 56, 37–42. [Google Scholar] [CrossRef]
- Ranjbaran, M.; Datta, A.K. Pressure-driven infiltration of water and bacteria into plant leaves during vacuum cooling: A mechanistic model. J. Food Eng. 2019, 246, 209–223. [Google Scholar] [CrossRef]
- Feng, C.; Drummond, L.; Zhang, Z.; Sun, D.-W.; Wang, Q. Vacuum cooling of meat products: Current state-of-the-art research advances. Crit. Rev. Food Sci. Nutr. 2012, 52, 1024–1038. [Google Scholar] [CrossRef]
- Garrido, Y.; Tudela, J.A.; Gil, M.I. Comparison of industrial precooling systems for minimally processed baby spinach. Postharvest Biol. Technol. 2015, 102, 1–8. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Domokos-Szabolcsy, É.; Abdalla, N.A.; Taha, H.S.; Fári, M. Postharvest Management of Fruits and Vegetables Storage. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 15, pp. 65–152. [Google Scholar]
- Behdani, B.; Fan, Y.; Bloemhof, J.M. Chapter 12—Cool Chain and Temperature-Controlled Transport: An Overview of Concepts, Challenges, and Technologies. In Sustainable Food Supply Chains; Accorsi, R., Manzini, R.B.T.-S.F.S.C., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 167–183. [Google Scholar]
- Elansari, A.M.; Fenton, D.L.; Callahan, C.W. Chapter 6—Precooling. In Postharvest Technology of Perishable Horticultural Commodities; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 161–207. [Google Scholar]
- Siegel, R.; Maté, J.; Watson, G.; Nosaka, K.; Laursen, P.B. Pre-cooling with ice slurry ingestion leads to similar run times to exhaustion in the heat as cold water immersion. J. Sports Sci. 2012, 30, 155–165. [Google Scholar] [CrossRef]
- Raut, R.D.; Gardas, B.B.; Narwane, V.S.; Narkhede, B.E. Improvement in the food losses in fruits and vegetable supply chain-a perspective of cold third-party logistics approach. Oper. Res. Perspect. 2019, 6, 100117. [Google Scholar] [CrossRef]
- Wu, W.; Defraeye, T. Identifying heterogeneities in cooling and quality evolution for a pallet of packed fresh fruit by using virtual cold chains. Appl. Therm. Eng. 2018, 133, 407–417. [Google Scholar] [CrossRef]
- Kongwong, P.; Boonyakiat, D.; Poonlarp, P. Extending the shelf life and qualities of baby cos lettuce using commercial precooling systems. Postharvest Biol. Technol. 2019, 150, 60–70. [Google Scholar] [CrossRef]
- Zhan, L.; Hu, J.; Ai, Z.; Pang, L.; Li, Y.; Zhu, M. Light exposure during storage preserving soluble sugar and L-ascorbic acid content of minimally processed romaine lettuce (Lactuca sativa L. var. longifolia). Food Chem. 2013, 136, 273–278. [Google Scholar] [CrossRef]
- Aiamla-or, S.; Kaewsuksaeng, S.; Shigyo, M.; Yamauchi, N. Impact of UV-B irradiation on chlorophyll degradation and chlorophyll-degrading enzyme activities in stored broccoli (Brassica oleracea L. Italica Group) florets. Food Chem. 2010, 120, 645–651. [Google Scholar] [CrossRef]
- Han, J.; Gomes-Feitosa, C.L.; Castell-Perez, E.; Moreira, R.G.; Silva, P.F. Quality of packaged romaine lettuce hearts exposed to low-dose electron beam irradiation. LWT Food Sci. Technol. 2004, 37, 705–715. [Google Scholar] [CrossRef]
- Khyasudeen, M.F.; Nowakowski, P.J.; Nguyen, H.L.; Sim, J.H.N.; Do, T.N.; Tan, H.-S. Studying the spectral diffusion dynamics of chlorophyll a and chlorophyll b using two-dimensional electronic spectroscopy. Chem. Phys. 2019, 527, 110480. [Google Scholar] [CrossRef]
- Witham, F.H.; Blaydes, D.F.; Devlin, R.M. Experiments in Plant Physiology; Van Nostrand-Reinhold: New York, NY, USA, 1971. [Google Scholar]
- Ranganna, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products; Tata McGraw-Hill Education: New Delhi, India, 1986; ISBN 0074518518. [Google Scholar]
- Tugli, L.S.; Essuman, E.K.; Kortei, N.K.; Nsor-Atindana, J.; Nartey, E.B.; Ofori-Amoah, J. Bioactive constituents of waakye; a local Ghanaian dish prepared with Sorghum bicolor (L.) Moench leaf sheaths. Sci. Afr. 2019, 3, e00049. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Mantilla, N.; Castell-Perez, M.E.; Gomes, C.; Moreira, R.G. Multilayered antimicrobial edible coating and its effect on quality and shelf-life of fresh-cut pineapple (Ananas comosus). LWT Food Sci. Technol. 2013, 51, 37–43. [Google Scholar] [CrossRef]
- Xu, X.; Luo, D.; Bao, Y.; Liao, X.; Wu, J. Characterization of diversity and probiotic efficiency of the autochthonous lactic acid bacteria in the fermentation of selected raw fruit and vegetable juices. Front. Microbiol. 2018, 9, 2539. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Carr, B.T.; Civille, G. V Sensory Evaluation Techniques, 3rd ed.; Taylor & Francis: New York, NY, USA, 1999; ISBN 9781439832271. [Google Scholar]
- Wang, N.; Kan, A.; Huang, Z.; Lu, J. CFD simulation of heat and mass transfer through cylindrical Zizania latifolia during vacuum cooling. Heat Mass Transf. 2020, 56, 627–637. [Google Scholar] [CrossRef]
- Mi, S.; Cai, L.; Ma, K.; Liu, Z. Investigation on flow and heat transfer characteristics of ice slurry without additives in a plate heat exchanger. Int. J. Heat Mass Transf. 2018, 127, 11–20. [Google Scholar] [CrossRef]
- Han, Z.; Hua, L.; Fang, Y.; Ma, Q.; Li, Y.; Wang, J.X. Innovative research on refrigeration technology of cold chain logistics. IOP Conf. Ser. Earth Environ. Sci. 2020, 474, 52105. [Google Scholar] [CrossRef]
- Rajapaksha, L.; Gunathilake, D.; Pathirana, S.M. Reducing post-harvest losses in fruits and vegetables for ensuring food security–Case of Sri Lanka. MOJ Food Process Technols 2021, 9, 7–16. [Google Scholar] [CrossRef]
- Song, X.; Liu, B.; Jaganathan, G.K. Temperature distribution pattern of Brassica chinensis during vacuum cooling. J. Food Process. 2016, 2016, 8247085. [Google Scholar] [CrossRef]
- Hussein, Z.; Caleb, O.J.; Opara, U.L. Perforation-mediated modified atmosphere packaging of fresh and minimally processed produce—A review. Food Packag. Shelf Life 2015, 6, 7–20. [Google Scholar] [CrossRef]
- Falagán, N.; Terry, L.A. Recent Advances in Controlled and Modified Atmosphere of Fresh Produce. Johnson Matthey Technol. Rev. 2018, 62, 107–117. [Google Scholar] [CrossRef]
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Yan, L.; Liu, S. Effect of different pre-cooling, packaging and cold storage treatments on quality of broccoli. In Proceedings of the 28th International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on Postharvest Technology in the Global Market, Lisbon, Portugal, 22–27 August 2010. [Google Scholar]
- Saad, M.; EL SAYED, M. Effect of Cooling Delays on Quality Attribute of Globe Artichoke during Cold Storage. Ann. Agric. Sci. Moshtohor 2019, 57, 105–112. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, X.; Geng, Y.; Sun, D.-W.; Chen, H.; Zhao, Y.; Zhou, W.; Li, X.; Pan, H. Effects of modified atmosphere vacuum cooling (MAVC) on the quality of three different leafy cabbages. LWT 2018, 94, 190–197. [Google Scholar] [CrossRef]
- De Ell, J.R.; Toivonen, P.M.A. Chlorophyll fluorescence as an indicator of physiological changes in cold-stored broccoli after transfer to room temperature. J. Food Sci. 1999, 64, 501–503. [Google Scholar] [CrossRef]
- Du, Y.; Jin, T.; Zhao, H.; Han, C.; Sun, F.; Chen, Q.; Yue, F.; Luo, Z.; Fu, M. Synergistic inhibitory effect of 1-methylcyclopropene (1-MCP) and chlorine dioxide (ClO2) treatment on chlorophyll degradation of green pepper fruit during storage. Postharvest Biol. Technol. 2021, 171, 111363. [Google Scholar] [CrossRef]
- Torales, A.C.; Gutiérrez, D.R.; Rodríguez, S.d.C. Influence of passive and active modified atmosphere packaging on yellowing and chlorophyll degrading enzymes activity in fresh-cut rocket leaves. Food Packag. Shelf Life 2020, 26, 100569. [Google Scholar] [CrossRef]
- Shi, J.; Gao, L.; Zuo, J.; Wang, Q.; Wang, Q.; Fan, L. Exogenous sodium nitroprusside treatment of broccoli florets extends shelf life, enhances antioxidant enzyme activity, and inhibits chlorophyll-degradation. Postharvest Biol. Technol. 2016, 116, 98–104. [Google Scholar] [CrossRef]
- Alanís-Garza, P.A.; Becerra-Moreno, A.; Mora-Nieves, J.L.; Mora-Mora, J.P.; Jacobo-Velázquez, D.A. Effect of industrial freezing on the stability of chemopreventive compounds in broccoli. Int. J. Food Sci. Nutr. 2015, 66, 282–288. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Luo, S.; Zhou, H.; Zhang, Y.; Zhang, L.; Hu, H.; Li, P. Effects of hydrogen-rich water combined with vacuum precooling on the senescence and antioxidant capacity of pakchoi (Brassica rapa subsp. Chinensis). Sci. Hortic. 2021, 289, 110469. [Google Scholar] [CrossRef]
- Tao, J.; Xie, J.; Wang, Y.; Gao, L.; Xiong, Y.; Sheng, D. Effect of different pre-cooling methods on quality of cutting greengrocery under low temperature storage. Food Ind. Technol. 2015, 337–340. [Google Scholar]
- Brat, P.; Bugaud, C.; Guillermet, C.; Salmon, F. Review of banana green life throughout the food chain: From auto-catalytic induction to the optimisation of shipping and storage conditions. Sci. Hortic. 2020, 262, 109054. [Google Scholar] [CrossRef]
- Li, L.; Lichter, A.; Kenigsbuch, D.; Porat, R. Effects of cooling delays at the wholesale market on the quality of fruit and vegetables after retail marketing. J. Food Process. Preserv. 2015, 39, 2533–2547. [Google Scholar] [CrossRef]
- Kitazawa, H.; Sato, T.; Nakamura, N.; Motoki, S. Effects of post-harvest cooling delay on weight loss, soluble solid and ascorbic acid contents of strawberry fruit. J. Food Agric. Environ. 2013, 11, 372–376. [Google Scholar]
- Tao, F.; Zhang, M.; Yu, H. Effect of vacuum cooling on physiological changes in the antioxidant system of mushroom under different storage conditions. J. Food Eng. 2007, 79, 1302–1309. [Google Scholar] [CrossRef]
- Li, J.; Ma, G.; Ma, L.; Bao, X.; Li, L.; Zhao, Q.; Wang, Y. Multivariate analysis of fruit antioxidant activities of blackberry treated with 1-methylcyclopropene or vacuum precooling. Int. J. Anal. Chem. 2018, 2018, 2416461. [Google Scholar] [CrossRef]
- López, A.; Javier, G.-A.; Fenoll, J.; Hellín, P.; Flores, P. Chemical composition and antioxidant capacity of lettuce: Comparative study of regular-sized (Romaine) and baby-sized (Little Gem and Mini Romaine) types. J. Food Compos. Anal. 2014, 33, 39–48. [Google Scholar] [CrossRef]
- de Oliveira, M.A.; Maciel de Souza, V.; Morato Bergamini, A.M.; De Martinis, E.C.P. Microbiological quality of ready-to-eat minimally processed vegetables consumed in Brazil. Food Control 2011, 22, 1400–1403. [Google Scholar] [CrossRef]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R. Effect of Postharvest Transport and Storage on Color and Firmness Quality of Tomato. Horticulturae 2021, 7, 163. [Google Scholar] [CrossRef]
- Rattanakaran, J.; Saengrayap, R.; Prahsarn, C.; Kitazawa, H.; Chaiwong, S. Application of Room Cooling and Thermal Insulation Materials to Maintain Quality of Okra during Storage and Transportation. Horticulturae 2021, 7, 188. [Google Scholar] [CrossRef]
- GOJIYA, D.K. Effect of pre-treatments on biochemical and microbial parameters of guava fruit during storage. Int. J. Nutr. Sci. Food Tech. 2017, 3, 32–37. [Google Scholar] [CrossRef][Green Version]
- Han, Q.; Gao, H.; Chen, H.; Fang, X.; Wu, W. Precooling and ozone treatments affects postharvest quality of black mulberry (Morus nigra) fruits. Food Chem. 2017, 221, 1947–1953. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Vigneault, C.; Delele, M.A.; Al-Said, F.A.-J. Design of packaging vents for cooling fresh horticultural produce. Food Bioprocess Technol. 2012, 5, 2031–2045. [Google Scholar] [CrossRef]
- Zhang, K.-X.; Pu, Y.-Y.; Sun, D.-W. Effective postharvest preservation methods for mushroom (Agricus bisporus): A brief review. Biosyst. Food Eng. Res. Rev. 2017, 22, 1. [Google Scholar]
- Tian, D.; Fen, L.; Jiangang, L.; Mengli, K.; Jingfen, Y.; Xingqian, Y.; Donghong, L. Comparison of different cooling methods for extending shelf life of postharvest broccoli. Int. J. Agric. Biol. Eng. 2016, 9, 178–185. [Google Scholar]
- Patel, B.; Sutar, R.; Javed, M.; Joshi, D. Respiration behaviour and heat of respiration of mango (cv. Langdo) under different storage conditions. Int. J. Agric. Environ. Biotechnol. 2016, 9, 855–859. [Google Scholar] [CrossRef]
- Charuvi, D.; Nevo, R.; Aviv-Sharon, E.; Gal, A.; Kiss, V.; Shimoni, E.; Farrant, J.M.; Kirchhoff, H.; Reich, Z. Chloroplast breakdown during dehydration of a homoiochlorophyllous resurrection plant proceeds via senescence-like processes. Environ. Exp. Bot. 2019, 157, 100–111. [Google Scholar] [CrossRef]
- Garrido, Y.; Tudela, J.A.; Marín, A.; Mestre, T.; Martínez, V.; Gil, M.I. Physiological, phytochemical and structural changes of multi-leaf lettuce caused by salt stress. J. Sci. Food Agric. 2014, 94, 1592–1599. [Google Scholar] [CrossRef]
- Sun, B.; Yan, H.; Liu, N.; Wei, J.; Wang, Q. Effect of 1-MCP treatment on postharvest quality characters, antioxidants and glucosinolates of Chinese kale. Food Chem. 2012, 131, 519–526. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Aworh, O.C. Food safety issues in fresh produce supply chain with particular reference to sub-Saharan Africa. Food Control 2021, 123, 107737. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).