Abstract
The biochemical changes that occur during the growth and ripening of fruit and vegetable tissues, especially for color and firmness, are the most important factors affecting the quality of fresh products. Cantaloupe (Cucumis melo, L.) is one of the main economically important fruits in the world and its quality parameters, e.g., sweetness, nutritional factors, and texture, influence consumer preferences. Hence, these two features, appearance and texture changes, were investigated in three different genotypes of netted melon, all characterized by an extended shelf life but with different ripening phases. In particular, in all melon cultivars, the cell wall-modifying enzymatic activities and indicators of softening as well as total polyphenols, ortho-diphenols, flavonoids, and tannins, and antioxidant activity were studied. One variety with excellent shelf-life displayed the best nutritional and healthy qualities, in the early stages of ripening, and the lowest degree of browning. The lytic enzyme activities were reduced in the initial stages and after they increased gradually until the overripe stage, with the same trend for all varieties under investigation. The antioxidant activities declined with increasing time of ripeness in all genotypes. The outcomes confirm that the activities of both classes examined, antioxidant and cell wall-modifying enzymes, may vary significantly during ripeness depending on the genotype, suggesting the involvement in determining the postharvest behavior of these fruits.
Keywords:
Cucumis melo; melon; antioxidant activity; polyphenols; ripening; softening enzymes; shelf-life 1. Introduction
Muskmelons (Cucumis melo L., Cucurbitaceae family) are largely cultivated and consumed throughout the world from tropical to sub-temperate zones, including numerous smooth-skinned and netted varieties. Among melons, cantaloupe (var. reticulatus) is of incredible economic worldwide diffusion due to its sweetness, juicy taste, pleasing flavor, and nutritional value of the pulp, which is an excellent source of vitamin A, vitamin C, and microelements, such as potassium and magnesium [1,2,3]. In recent years, it has been demonstrated that cantaloupe possesses useful medicinal properties, such as analgesic, anti-inflammatory, antioxidant, antiulcer, anticancer, antimicrobial, diuretic, and antidiabetic properties [1,4].
In the Mediterranean area, about 1.9 million tons of melon are harvested every year and the main European producers are Spain, Italy, and France, representing 35%, 32%, and 13%, respectively [5]. Important indicators of melon market quality and consumer preference are texture and the presence of phytochemicals with antioxidant activity [6]. In particular, antioxidants refer to a group of compounds that are able to delay or inhibit the oxidation of lipids or other biomolecules, thus preventing or repairing the damage from free radicals [7,8,9]. The demand for healthy foods rich in polyphenols has grown in recent years. In fact, the food industry is constantly looking for new natural sources of beneficial components with high nutritional value, appreciated for dietary, active cosmetic ingredients, and in pharmaceutical industries [10,11]. Melon fruit is considered a good source of antioxidants and, in particular, flavonoids and phenolic acids have been reported as the main polyphenols present in melon pulp [1,2,12].
Alongside nutritional factors, fruit ripening is another important parameter in the consumer’s choice of melon. This greatly organized process leads to color, firmness, taste, and flavor variation, which affect the appearance and the loss of textural quality of melon fruits. A more in-depth look at these changes has shown that modification in fruit color is due to degradation of chlorophyll and the accumulation of non-photosynthetic pigments; taste is an increase in sugar contents and decrease in organic acids; aroma variation is due to the production of volatile compounds; and texture is linked to softening processes by modification of the cell wall rigidity, which is enzymatically controlled.
Discoloration and browning processes may be considered as a result of oxidative phenomena, and superoxide dismutase SOD (SOD, EC 1.15.1.1) and catalase (CAT, EC 1.11.1.6) are the main antioxidant defense enzymes in plant cells. In this context, the enzymes SOD and CAT prevent the accumulation of superoxide radicals at toxic levels in the cells, a phenomenon that may trigger lipid peroxidation and other deteriorative reactions [13,14,15]. This antioxidant system is very complex because it includes other enzymes, such as the polyphenol oxidase (PPO, EC 1.14.18.1), which causes browning degradative reactions, and the peroxidase (POD, EC 1.11.1.7), which is considered an enzyme indicator of quality deterioration in fruits (e.g., the off-flavor). An interdependence between prevention of off-flavor development and inactivation of PPO and POD enzymes in frozen vegetables has been reported [16]. In fact, it has been demonstrated that inhibition of PPO and POD activities can reduce the browning process, preventing alteration of the appearance [17,18,19]. In particular, strictly related to ripening is the physiological process of differentiation from chloroplast to chromoplast; the transformation involves compositional, structural, and metabolic changes including the breakdown of chlorophyll and the accumulation of other pigments, above all phenolic compounds. Enzymatic browning is a consequence of oxidation of these phenolic compounds by means of PPO, which, in the presence of oxygen, catalyzes the hydroxylation of monophenols to ortho-diphenols and the following oxidation of ortho-diphenols to their corresponding ortho-quinones. The latter are polymerized to undesirable brown, red, or black pigments. The extent of the correlation between browning and polyphenols and PPO activity is genotype specific [20]. Closely related to PPO, POD, a thermostable enzyme that belongs to a group of oxidases that use H2O2 as a catalyst for oxidation of phenolic compounds, is considered relevant in enzymatic browning and promotes darkening in fruit and vegetable products [21]. Although POD activity is limited by the absence of electron compounds, such as superoxide radicals, hydrogen peroxide, and lipid peroxides, its involvement in the browning of various fruits and vegetables has been reported [18,21,22,23].
Texture is one of the most important quality parameters and it is partially responsible for consumer preferences of edible fruit, while fruit softening is a determining factor in the quality and postharvest life of fruit. Typically, the softening process is accompanied, alongside adjustment of the solute content in the apoplast, by degradation of the middle lamella and loss of cell adhesion and, in turn, derives from the solubilization of the cell wall pectin, by the action of cell wall hydrolytic enzymes, such as pectin methylesterase (PME, EC 3.1.1.11) and polygalacturonase (PG, EC 3.2.1.15). During fruit ripening, PME cleaves methyl esters from pectin polymer, producing methanol and pectin, which results in a low degree of esterification, and therefore greater susceptibility to degradation by endo-acting enzymes, like PG. The latter catalyzes the hydrolytic cleavage of α-(1,4)-galacturonan linkages, resulting in pectin undergoing final depolymerization [24,25,26].
It has been reported that high expression of endogenous enzymes involved in browning and softening may influence the postharvest life of vegetal products [17,27,28]. In fact, these two physiological changes could trigger quiescent/latent colonization by necrotrophic fungi in ripe melon fruits [29,30]. In melon farming, it is common to collect these climacteric fruits when they are not yet ripe, so they can be stored in special cold rooms (0–4 °C) with a modified atmosphere. Then, the ripening takes place successively, in an artificial mode, so that the fruit is ready to be marketed for human consumption. For this reason, current research efforts in agronomy have created numerous hybrids with upgraded storage and shelf-life properties [31,32]. Regarding postharvest features, melon cultivars may be grouped into three sets: traditional shelf-life (TSL), extended shelf-life (ESL), and long shelf-life (LSL). At commercial maturity, TSL melon types form an abscission layer at the peduncle attachment, whereas ESL and LSL varieties do not abscise [33].
To the best of our knowledge, little information has been provided on oxidative metabolism during the ripening of melon, looking mainly at the relationship that enzymatic systems have with their metabolic counterparts. Therefore, the aim of this study was to follow the biochemical changes that occur during fruit ripening in three diffused melon cultivars in Italy, harvested at five maturity stages. The melon genotypes in this study were all ESL but showed different ripening phases, thus indicating that overall fruit quality will be enhanced by investigation of the enzymatic activities (PPO, POD, SOD, CAT, PME, and PG), as well as the main biochemical attributes, for a phenotypic selection with improved commercial performance. In this view, it has to be highlighted that since cantaloupe melon is a climacteric fruit, and keeping in consideration the large melon market in Italy, the quality and safety of such products are an issue as these crops are more susceptible to spoilage by microorganisms of public health significance.
In this work, the relationship between enzymatic activities and phytonutrient variation gives strong evidence of their implications in postharvest technology ripening of cantaloupe cultivars. Further, this data can help to estimate the optimal harvesting date for the best fruit quality, and thus consumer acceptance, and on the other hand, set strategies for postharvest disease control by fungal pathogens.
2. Materials and Methods
2.1. Plant Materials
Three Italian cultivars of cantaloupe melon (C. melo L. var. reticulatus), named Eminenza, SV7881, and Iperione, were supplied by Enza Zaden Italia S.r.l. (Tarquinia—VT, S.S. Aurelia Km 96.71). Fruits were commercially grown in a greenhouse located at Villa Literno (Caserta, Italy; 41°0′33 N–14°4′34 E). They were all hand-harvested at the same five ripening stages, indicated as S0, S1, S2, S3, and S4, ranging from the unripe (S0) to overripe condition (S4). S0 features white flesh, whereas in S1, color change of the pulp occurs. S2 is characterized by the beginning of the abscess of the peduncle; S3 represents the commercial maturity condition, about 40–45 days from the time of fruit setting; and in S4, complete abscission of the peduncle occurs.
Pulps were collected upon arrival in the lab and randomly chosen pieces were utilized for the analyses. For the biochemical analysis, melon pulps were lyophilized for 24 h, powdered using a food mixer, and kept at −20 °C until extractions were performed. Freshly frozen pulps were employed for investigation of the enzymatic activity and physical-chemical properties. Three fruits for each ripening stage were considered for analysis.
2.2. Reagents and Standards
All analytical-grade solvents and reagents were purchased from Sigma Aldrich S.r.l. (Milan, Italy).
2.3. Physico-Chemical Properties
For each cultivar, during the five ripening stages, pulp was homogenized and filtered through cheesecloth. The obtained juice was used to measure the total soluble solids, pH, and titratable acidity. The total soluble solid (TSS) content was measured with a digital refractometer and expressed as °Brix at 20 °C. Titratable acidity (TA) was quantified by titrating 10 mL of melon juice with NaOH (0.1 N) to an endpoint of pH 8.1 and expressed as g/100 mL of acid citric [34]. Three replicates were carried out for all measurements.
2.4. Bioactive Compounds and Antioxidant Activity
Bioactive compound recovery was conducted using 200 mg of fine powder of cantaloupe pulp extracted with 10 mL of 95% ethanol (ratio 1:50 w/v) for 6 h at 50 °C in an ultrasound bath. The extracts were recovered by centrifugation at 13,000 g for 10 min at 4 °C and dried using a rotary evaporator, as previously reported by Vella et al. [35].
All extracts were analyzed in triplicates to evaluate changes in total polyphenol, ortho-diphenol, flavonoid, and tannin contents during the five ripening stages.
Total polyphenols were spectrophotometrically determined according to the Folin–Ciocalteu method [36]. In brief, 150 µL of extract were mixed with 750 µL of Folin–Ciocalteu reagent and 600 µL of 7.5% (w/v) Na2CO3. After incubation, the absorbance was read at 765 nm. The polyphenol amount was obtained using a calibration curve with gallic acid as the standard and the results are expressed as µg of gallic acid equivalents (GAE) per mg of extract.
The ortho-diphenol content was evaluated by Arnow assay [37]. Briefly, 400 µL of extract were mixed with 400 µL of 0.5 M HCl, 400 µL of 1.45 M NaNO2–0.4 M Na2MoO4, and 400 µL of 1 M NaOH. The absorbance was recorded at 500 nm and ortho-diphenols were determined by a calibration curve obtained using caffeic acid as the standard. The results are expressed as µg of caffeic acid equivalents (CAE) per mg of extract.
The flavonoid content in the extracts was determined according to the colorimetric method based on the formation of flavonoid-aluminum compounds [38]. In the assay, extract was mixed with distilled water and NaNO2. After 5 min, AlCl3 x 6H2O was added and the reaction was stopped by adding 1 M NaOH and distilled water. The absorbance was read at 510 nm and (+)−catechin was used to create the standard curve. The results are expressed as µg of catechin equivalents (CE) per mg of extract.
Total tannins were assessed as reported by Vella et al. [39], incubating extracts with BSA at 30 °C for 1 h. The supernatant, representing the non-tannin fraction, was collected by centrifugation at 13,000 g for 10 min at 4 °C and was analyzed using the Folin–Ciocalteu method. Tannins were determined by the difference in the amounts of polyphenols determined before and after BSA precipitation. Tannins are expressed as µg of gallic acid equivalents (GAE) per mg of extract.
The antioxidant activity of cantaloupe extracts was evaluated by means of two in vitro assays: the Ferric Reducing Antioxidant Power (FRAP) and the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical-scavenging activity. All extracts were analyzed in triplicates.
As reported by Benzie and Strain [40], freshly prepared FRAP reagent was added to the extract and the absorbance was recorded at 593 nm. The antioxidant activities were calculated from the calibration curve using various amounts of L-ascorbic acid. Results are expressed as µg of ascorbic acid equivalents (AAE) per mg of extract.
The free radical scavenging activity (RSA) of the extracts was assessed according to the procedure of Blois [41]. In brief, different concentrations of extracts were mixed with DPPH methanolic solution. The absorbance reduction at 517 nm of the DPPH was determined continuously. The RSA was calculated as a percentage of DPPH discoloration, using the following Equation (1):
where AS is the absorbance of the solution when the extract was added and ADPPH is the absorbance of the DPPH solution. The EC50 value was obtained from the graph of %RSA against the extract concentrations in mg/mL.
2.5. Enzymatic Activity
The following enzymatic activities, polyphenol oxidase (PPO), peroxidase (POD), catalase (CAT), superoxide dismutase (SOD), pectin methylesterase (PME), and polygalacturonase (PG), were evaluated in all cantaloupe cultivars. Fruit extracts were obtained using the method of Chisari et al. [25], with slight modification. For each sample, 7 g of melon pulp were cut and homogenized in 0.1 M phosphate buffer (pH 7.5) and 1 mM dithiothreitol (DTT). PME and PG activities were evaluated in the same mode with buffer containing 1 M NaCl. Mixtures were set at 4 °C for 1 h and then centrifuged at 4000 g for 20 min at 4 °C. Supernatants were filtered and so the crude extract was a result of the clarified supernatants.
All enzyme activity is expressed in enzyme units (U), defined as the quantity of enzyme required to produce 1 µmole of product per minute. Moreover, the specific activity is reported in U per µg protein. The protein content was determined on crude extract by following the method of Bradford [42] with BSA as the standard, measuring the absorbance at 595 nm.
The PPO assay was performed using 1.45 mL of 100 mM phosphate buffer pH 6.8 and 0.5 mL of 0.1 M freshly prepared catechol. The assay mixture was incubated for 3 min at room temperature and after 50 µL of crude extract were added. The reaction was monitored at 412 nm for 2 min at room temperature [43].
POD activity was determined on the following mixture: 2.7 mL of 100 mM phosphate buffer pH 6.8, 0.10 mL of 3% hydrogen peroxide, and 0.15 mL of 4% guaiacol, freshly prepared. After 3 min of incubation at room temperature, 50 µL of crude extract were added. The reaction was monitored at 470 nm for 2 min at room temperature [43].
CAT activity was determined by the addition of 200 µL of crude extract to 2.8 mL of 0.036% hydrogen peroxide in 50 mM phosphate buffer pH 7.0. The absorbance decrease at 240 nm was recorded for 2 min [44].
SOD activity was assayed at 450 nm utilizing a tetrazolium salt for the detection of superoxide radicals generated by xanthine oxidase and hypoxanthine (SOD activity assay kit Biovision—CA, USA). One unit of SOD was defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical.
PME activity was determined by a modified method of Hagerman and Austin [45], in which a pH increase of mixture was monitored spectrophotometrically. Briefly, an aliquot of crude extract (from 1 to 10 μL) was added to 1 mL of 1% pectin solution (from citrus peel; galacturonic acid >74%, methoxy groups >6.7%) with 0.1 g/L solution of bromothymol blue as the indicator. The reaction was started by adding the crude extract and an absorbance rate decrease was recorded at 620 nm. PME activity of 1 U was defined as an absorbance decrease of 0.1 per min.
The PG activity assay was based on the release of reducing groups produced by the enzyme and measured using the spectrophotometric method of dinitrosalicylic (DNS) acid reagent [46]. Briefly, crude extract was incubated with 100 µL of 0.5% polygalacturonic acid solution (MW 25,000–50,000) in 50 mM phosphate buffer pH 7.0. After 2 min of incubation at room temperature, the same volume of the DNS reagent was added to the test tube and the mixture was boiled for 5 min. The reaction was stopped in ice and the absorbance was measured at 546 nm.
2.6. Statistical Analysis
All samples were analyzed in triplicates and the results are expressed as mean ± standard deviation (SD). Means, SD, calibration curves, and linear regression analyses (R2) were determined using Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA). Correlation analyses were carried out by using CORREL function in Microsoft Excel 2013. Pearson correlation coefficients (r) were calculated and followed by t-Student test, with two-sample equal variance and two-tailed distribution. Differences at p < 0.05 were considered significant and p < 0.01 were highly significant.
Statistical comparisons were made with one-way ANOVA followed by the Tukey multiple comparison test with a level of significance of p < 0.05. All statistical calculation was performed using Microsoft Excel 2013.
3. Results and Discussion
All cantaloupe cultivars used in this study were characterized by an extended shelf life (ESL), with different behavior and rates of ripening phases. In particular, Eminenza has a limited storability due to rapid overripening, loss of firmness, and desiccation. SV7881 shows very good storability and lastly, Iperione has an excellent shelf-life with limited overripening and softening.
3.1. Physico-Chemical Characteristics, Soluble Solids, pH, and Organic Acids
Total soluble solid content (TSS, measured in °Brix) is adopted as a maturity index for fruits and represents the balance of sugars and acids present, which has a main impact on melon flavor [47,48]. The results of TSS for the three cultivars during ripening are reported in Table 1.

Table 1.
Total soluble solids, pH, and titratable acidity in Eminenza, SV7881, and Iperione at five ripening stages (S0–S4).
TSS reached the highest value in S4, which means that in this stage, cantaloupe is the richest in sugars. In particular, Eminenza had 17.65 ± 0.35 °Brix, SV7881 showed 15.90 ± 0.14 °Brix, and Iperione showed 16.35 ± 0.25 °Brix.
Eminenza and SV7881 had equivalent pH values recorded at S4 of 6.57 ± 0.02 and 6.68 ± 0.02, respectively (Table 1). Instead, Iperione reached a slightly higher pH value of 7.01 ± 0.01, always in the S4 stage. Since this parameter is related to the titratable acidity (TA), which is the total concentration of free protons that can be neutralized by a strong base, in the case of fruit, the acidity is due to the content of several organic acids, such as citric, malic, fumaric, acetic, ascorbic, and galacturonic [47,48]. The results revealed that the Eminenza and SV7881 genotypes showed lower values of TA in S4, being 0.068 ± 0.004 and 0.059 ± 0.002, respectively. Iperione reached a TA value of 0.040 ± 0.003, and the lower amount of organic acids found are in accordance with the higher value of pH shown.
Overall, the data are in harmony as in fact, during ripening, the titratable acidity decreases (due to catabolism of organic acids) and the sugar content (measured as the soluble solids content) increases. Postharvest determination of TSS, TA, and pH has been thoroughly discussed by other authors, reporting different values because of the cultivar, climatic conditions, and geographical origin [2,3,25,49].
3.2. Bioactive Compounds and Antioxidant Activity
Polyphenols are secondary metabolites commonly found in both edible and non-edible parts of fruits and vegetables. In plants, they possess important roles as biochemical protectors against attack of insects, pathogens, and microorganisms or as a reaction to environmental stresses, since they reduce the high levels of ROS molecules that are relayed during the above outbreaks. Phenolic phytochemicals in plants are associated with antioxidant activities against free radicals. Moreover, from a physiological view, they take an active part in determining the color, flavor, taste, and appearance of fruits and represent social signals useful in species propagation.
The bioactive characterization of the three genotypes, Eminenza, SV7881, and Iperione was carried out by means of evaluation of the total polyphenols, ortho-diphenols, flavonoids, and tannins content during the five ripening stages, as reported in Table 2. Eminenza reached the highest value of 2.80 ± 0.07 µg GAE/mg in S2, SV7881 in S3 with 3.68 ± 0.08 µg GAE/mg, while Iperione had the major value in S4, being 2.63 ± 0.02 µg GAE/mg. These results were about 2- and 20-fold higher than that stated by Ismail et al. [1] and Fundo et al. [3]. These variations could be attributed to several factors, including the cultivar, degree of ripening, and environmental issues, such as climatic conditions and geographical origin [2,49].

Table 2.
Polyphenols, ortho-diphenols, tannins, flavonoids, antioxidants power, and EC50 in Eminenza, SV7881, and Iperione during five ripening stages (S0–S4).
Among phenolics, ortho-diphenols are the most important because they are able to improve radical stability by forming an intra-molecular hydrogen bond between the hydrogen and phenoxyl radicals. The ortho-diphenol content in the three cultivars followed the same trend of the polyphenols, being higher in S2 for Eminenza (0.84 ± 0.01 µg CAE/mg), in S3 for SV7881 (0.73 ± 0.01 µg CAE/mg), and in S4 for Iperione (0.81 ± 0.02 µg CAE/mg). To our knowledge, no literature data until now have been presented on the evaluation of ortho-diphenols in cantaloupe pulps.
Considering flavonoids, the most common and widely distributed group of plant phenolics, Eminenza showed the highest content in S2, SV7881 in S3, and Iperione in S4, with 0.51 ± 0.01, 0.59 ± 0.01, and 0.48 ± 0.02 µg CE/mg, respectively. Even for this class of phytochemicals, the results were greater than those reported in the literature [1,2]. The differences could be attributed to the fact that flavonoids vary substantially among genotypes, degree of fruit maturation, plant physiology states, and seasons [2,49].
Tannins have been considered as health-promoting components in plant-derived foods and beverages, possessing anticarcinogenic and antimutagenic potential as well as antimicrobial, antioxidant, and antiradical properties [50,51,52]. In this study, the tannin content showed the same tendency of total polyphenols, ortho-diphenols, and flavonoids: in Eminenza, 0.68 ± 0.08 µg GAE/mg; in SV7881, 0.54 ± 0.08 µg GAE/mg; and in Iperione, 0.87 ± 0.03 µg GAE/mg in the S2, S3, and S4 stages, respectively. These results were approximately 10-fold higher than that reported by Maietti et al. [2], whose study was carried out on the analysis of condensed tannins.
The use of synthetic antioxidants is an old practice, and their safety is nowadays questioned by consumers. Alternative natural compounds with efficient antioxidant activity have been given increasing attention since free radicals and other reactive oxygen species (ROS) are molecules involved in many human diseases, including cancer, cardiovascular diseases, cataracts, asthma, hepatitis, liver injury, and immunodeficiency diseases [53].
The antioxidant properties of three Italian cantaloupe cultivars were estimated by means of the FRAP assay and by DPPH radical scavenging activity. The data obtained are reported in Table 2. The antioxidant power (FRAP), based on the reduction of a ferric 2,4,6-tripyridyl-s-triazine complex (Fe3+-TPTZ) to the ferrous form (Fe2+-TPTZ) in the presence of antioxidant [40], showed a similar drift to the result of the total polyphenols (r = 0.7786), ortho-diphenols (r = 0.9115), flavonoids (r = 0.7320), and tannins (r = 0.8324). These results indicated that the antioxidant power of cantaloupe extracts was significantly correlated (p < 0.05) with the amount of phenolic compounds, especially ortho-diphenols, which positively correlated with the antioxidant power in a highly significant way (p < 0.01). Particularly, the three cultivars, Eminenza, SV7881, and Iperione, displayed their highest FRAP of 1.40 ± 0.04, 1.48 ± 0.01, and 1.50 ± 0.03 µg AAE/mg in S2, S3, and S4, respectively.
The DPPH assay is based on the evaluation of the antiradical activity of extracts by reduction of DPPH radical to hydrazine. In this study, the amounts of all classes of phenolic compounds were negatively correlated with the EC50 and this result meant that an increase in these biomolecules corresponded to a lower extract concentration necessary to achieve 50% of DPPH inhibition, thus suggesting a higher antioxidant activity. In fact, for each cultivar, the maturation stage with the greatest polyphenol content possessed the lowest EC50: S2 for Eminenza (13.70 ± 0.94 mg/mL), S3 for SV7881 (17.94 ± 0.88 mg/mL), and S4 for Iperione (13.40 ± 0.67 mg/mL). These results were comparable with data reported by Ismail et al. [1].
Considering all data on the total polyphenol, ortho-diphenol, flavonoid, and tannin contents, as well as the antioxidant activity, it is possible to state that Eminenza displayed the best nutritional and healthy qualities in S2, SV7881 in S3, and Iperione in S4, suggesting a possible further improvement of this variety when it arrives on the market and to consumers.
3.3. Enzymatic Activity
Enzymatic browning during postharvest processing and storage affects the color, flavor, nutritional properties, and shelf life of fruits and vegetables [25,54].
Changes in PPO during ripening have been extensively reported as the main activity involved in the browning of several fruits, such as apples [54,55], peaches [56], bananas [57], strawberries [23], avocados [58], mangoes [59], and melons [22,25]. Moreover, during maturation, changes in the respiration rate and ethylene production lead to significant metabolic changes, among which the production of strongly reactive chemical species, such as superoxide and hydroxyl radicals, as well as hydrogen peroxide, can be utilized by POD as substrates. The PPO and POD activities of the three cantaloupe melons at the five ripening stages are reported in Figure 1A,B, respectively.
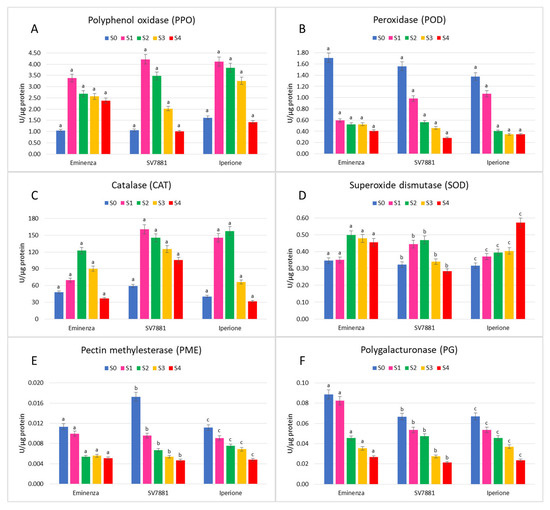
Figure 1.
Enzymatic activities of Eminenza, SV7881, and Iperione melons at five ripening stages (S0-S4): (A) polyphenol oxidase (PPO); (B) peroxidase (POD); (C) catalase (CAT); (D) superoxide dismutase (SOD); (E) pectin methylesterase (PME); (F) polygalacturonase (PG). Results are expressed as mean ± standard deviation. The values with different superscripts (a, b, or c) in the same graph are significantly different (Tukey test, p ≤ 0.05).
Among the three melon types, the PPO trend was similar: the highest activity was reached in the S2 stage, with a loss of 30% of the initial activity for Eminenza in S4. The same metabolic behavior was obtained for SV7881 and Iperione, which showed a significant decrease of 76% and 65%, respectively. Higher PPO activity at earlier maturity stages in melon is related to an accelerated metabolic activity and respiration rate as reported by Chisari et al. [25] and Menon and Ramana Rao [60]. In fact, fruits harvested at a younger immature age exhibit higher rates of ethylene production and chlorophyll degradation than those harvested at more advanced maturity stages [61,62]. Considering POD, the activity was initially high and declined gradually during ripening in all three cultivars. Chisari et al. [25] and Menon and Ramana Rao [60] observed that in melons, the highest level of this enzyme in S0 could be explained because peroxidase catalyzes the cross linking between the ferulic acid substituents, pectins. In turn, this leads to the synthesis of lignin and suberin [63,64], important polymers for the characteristic flesh firmness at the earlier stage of maturation.
CAT is one of the primary enzymatic defenses against oxidative stress induced by senescence [65]. Its activity showed an increased pattern in the early stages of ripening, with a maximum in S1 in SV788 and in S2 in Eminenza and Iperione, followed by a continuous decline up to the S4 stage (Figure 1C). Other authors have reported that cantaloupe melons exhibited a similar trend of CAT activity [60,66]. However, catalase activity increases continuously during ripening of tomato [67], whereas in saskatoon berries [68] and orange [15], it has been reported to decrease continuously.
The SOD activities of the three cantaloupe melons in the five ripening stages are reported in Figure 1D. Eminenza and SV7881 showed a similar tendency along the maturation, but Iperione showed a continuous increase in SOD activity, which reached the maximum in S4, a value 1.8-fold higher with respect to S0. Up to now, no literature data has been presented about the SOD activity in melon during maturation. In other fruits, such as banana, avocado, tomato, and apple, no changes in SOD activity with senescence of the fruits were reported [69]. However, due to a substantial increase in respiration, oxygen free radical production probably increases during the later stages of development. The concomitant increase in SOD activity in the S4 stage in Iperione would contribute to elimination of O2, thus effectively limiting the oxidative stress and the deterioration of this Italian cantaloupe cultivar with long storability. Further studies are needed to investigate whether this enzyme is involved in determining the shelf life of fruits and vegetables.
During ripening, the changes in the cell wall composition, which accompany fruit softening during ripening, and the progressive loss of firmness are the results of a gradual solubilization of pectin followed by de-polymerization and de-esterification as reported for many fruits [20,61]. Softening is frequently the major problem limiting fruits’ and vegetables’ shelf-life, and PME and PG are the main pectin-degrading enzymes normally involved during this process. PME and PG activities in Eminenza, SV7881, and Iperione during maturation are reported as U per µg protein in Figure 1E,F, respectively. In all three cantaloupe cultivars, a progressive PME decrease was found from the unripe to overripe condition, in accordance with results on melon given by Chisari et al. [25]. However, the activity of PME was shown to decrease, increase, or remain constant as reported by studies conducted on the ripening of different fruits [61,70,71].
During ripening-associated cell wall disassembly in melons, PME works together with other enzymes implicated in pectin depolymerization, such as PG. From the results obtained in PG activity during ripening of Eminenza, SV7881, and Iperione melons (Figure 1F), it emerged that there was a decreasing course of enzymatic activity, mainly in the latter ripening stage, losing 69.6%, 68.1%, and 64.9% of the initial activity, respectively. These results are comparable with those reported by Chisari et al. [25], suggesting that this enzyme, coupled with PME, plays a role in initiating the maturation process (S0-S2). Proceeding with ripening (S3-S4), the specific activity of PME and PG tend to decrease because the solubilization and depolymerization of pectin has already occurred.
4. Conclusions
This study provides valuable evidence about the best harvest period in which melons attain the highest nutritional and antioxidant potential, as well the appropriate texture for commercial purposes. Overall, the results suggest the highest suitability of one variety, Iperione, for use in long storage periods for both discoloration and softening performances, and in terms of a nutritionally balanced source of antioxidants. As far as we know, since simultaneous study of the phenolic and enzymatic changes during ripening of cantaloupe melons has not previously been reported, this article intends to thoroughly define the final fruit quality parameters to extend the shelf life and to minimize the risk of infection by postharvest microorganisms.
Author Contributions
Conceptualization, B.L.; Formal analysis, F.M.V. and B.L; Investigation, F.M.V.; software, R.C.; Writing—original draft, F.M.V. and B.L.; writing—review and editing, F.M.V. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Agostino Navarro for their helpful assistance during the sampling. Further thanks to Mario Noviello of Enza Zaden Italia S.r.l., and Gaetano Caliendo for providing the melon cultivars used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ismail, H.I.; Chan, K.W.; Mariod, A.A.; Ismail, M. Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chem. 2010, 119, 643–647. [Google Scholar] [CrossRef]
- Maietti, A.; Tedeschi, P.; Stagno, C.; Bordiga, M.; Travaglia, F.; Locatelli, M.; Arlorio, M.; Brandolini, V. Analytical traceability of melon (Cucumis melo var. reticulatus): Proximate composition, bioactive compounds, and antioxidant capacity in relation to cultivar, plant physiology state, and seasonal variability. J. Food Sci. 2012, 77, C646–C652. [Google Scholar] [PubMed]
- Fundo, J.F.; Miller, F.A.; Garcia, E.; Santos, J.R.; Silva, C.L.; Brandão, T.R. Physicochemical characteristics, bioactive compounds and antioxidant activity in juice, pulp, peel and seeds of cantaloupe melon. J. Food Meas. Charact. 2018, 12, 292–300. [Google Scholar]
- Vouldoukis, I.; Lacan, D.; Kamate, C.; Coste, P.; Calenda, A.; Mazier, D.; Conti, M.; Dugas, B. Antioxidant and anti-inflammatory properties of a Cucumis melo LC. extract rich in superoxide dismutase activity. J. Ethnopharmacol. 2004, 94, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Faostat, Food and Agriculture Organization of the United States. Available online: http://www.fao.org/home/en/ (accessed on 30 April 2020).
- Lester, G.E. Antioxidant, sugar, mineral, and phytonutrient concentrations across edible fruit tissues of orange-fleshed honeydew melon (Cucumis melo L.). J. Agric. Food Chem. 2008, 56, 3694–3698. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Tachakittirungrod, S.; Okonogi, S.; Chowwanapoonpohn, S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem. 2007, 103, 381–388. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzin, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Amaro, A.L.; Oliveira, A.; Almeida, D.P. Biologically active compounds in melon: Modulation by preharvest, post-harvest, and processing factors. In Processing and Impact on Active Components in Food; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015; pp. 165–171. [Google Scholar]
- Masia, A. Superoxide dismutase and catalase activities in apple fruit during ripening and post-harvest and with special reference to ethylene. Physiol. Plant. 1998, 104, 668–672. [Google Scholar] [CrossRef]
- Jimenez, A.; Creissen, G.; Kular, B.; Firmin, J.; Robinson, S.; Verhoeyen, M.; Mullineaux, P. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta 2002, 214, 751–758. [Google Scholar] [CrossRef]
- Huang, R.; Xia, R.; Hu, L.; Lu, Y.; Wang, M. Antioxidant activity and oxygen-scavenging system in orange pulp during fruit ripening and maturation. Sci. Hortic. 2007, 113, 166–172. [Google Scholar] [CrossRef]
- Yemenicioglu, A.; Özkan, M.; Cemeroglu, B. Some characteristics of polyphenol oxidase and peroxidase from taro (Colocasia antiquorum). Turk. J. Agric. For. 1999, 23, 425–430. [Google Scholar]
- Ioannou, I. Prevention of enzymatic browning in fruit and vegetables. Eur. Sci. J. 2013, 9, 30. [Google Scholar]
- Zhang, X.; Shao, X. Characterisation of polyphenol oxidase and peroxidase and the role in browning of loquat fruit. Czech. J. Food Sci. 2015, 33, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Chen, L.; Pang, L.; Chen, X.; Jia, X.; Li, X. Ultrasound treatment inhibits browning and improves antioxidant capacity of fresh-cut sweet potato during cold storage. RSC Adv. 2020, 10, 9193–9202. [Google Scholar] [CrossRef] [Green Version]
- Toivonen, P.M.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Tec. 2008, 48, 1–14. [Google Scholar]
- S2ingh, B.; Suri, K.; Shevkani, K.; Kaur, A.; Kaur, A.; Singh, N. Enzymatic browning of fruit and vegetables: A review. In Enzymes in Food Technology; Springer: Singapore, 2018; pp. 63–78. [Google Scholar]
- Chisari, M.; Barbagallo, R.N.; Spagna, G. Characterization and role of polyphenol oxidase and peroxidase in browning of fresh-cut melon. J. Agric. Food Chem. 2007, 56, 132–138. [Google Scholar] [CrossRef]
- Chisari, M.; Barbagallo, R.N.; Spagna, G. Characterization of polyphenol oxidase and peroxidase and influence on browning of cold stored strawberry fruit. J. Agric. Food Chem. 2007, 55, 3469–3476. [Google Scholar] [CrossRef]
- Huber, D.J. Polyuronide degradation and hemicellulose modifications in ripening tomato fruit. J. Am. Soc. Hortic. Sci. 1983, 108, 405–409. [Google Scholar]
- Chisari, M.; Silveira, A.C.; Barbagallo, R.N.; Spagna, G.; Artés, F. Ripening stage influenced the expression of polyphenol oxidase, peroxidase, pectin methylesterase and polygalacturonase in two melon cultivars. Int. J. Food Sci. Technol. 2009, 44, 940–946. [Google Scholar] [CrossRef]
- Menon, S.V.; Ramana Rao, T.V. Health-promoting components and related enzyme activities of muskmelon fruit during its development and ripening. J. Food Biochem. 2014, 38, 415–423. [Google Scholar] [CrossRef]
- Lamikanra, O. Enzymatic effects on flavor and texture of fresh-cut fruits and vegetables. In Fresh-Cut Fruits and Vegetables: Science, Technology and Market; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2002; pp. 125–186. [Google Scholar]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Prusky, D.; Lichter, A. Activation of quiescent infections by postharvest pathogens during transition from the biotrophic to the necrotrophic stage. FEMS Microbiol. Lett. 2007, 268, 1–8. [Google Scholar] [CrossRef]
- Alkan, N.; Fortes, A.M. Insights into molecular and metabolic events associated with fruit response to post-harvest fungal pathogens. Front. Plant. Sci. 2015, 6, 889. [Google Scholar] [CrossRef] [Green Version]
- Conesa, M.À.; Fullana-Pericàs, M.; Granell, A.; Galmés, J. Mediterranean long shelf-life landraces: An untapped genetic resource for tomato improvement. Front. Plant. Sci. 2020, 10, 1651. [Google Scholar] [CrossRef]
- Khabbazi, S.D.; Khabbazi, A.D.; Cevik, V.; Ergül, A. Genetic engineering of horticultural crops contributes to the improvement of crop nutritional quality and shelf life. In Transgenic Technology Based Value Addition in Plant Biotechnology; Elsevier: Amsterdam, The Netherlands, 2020; p. 247. [Google Scholar]
- Farcuh, M.; Copes, B.; Le-Navenec, G.; Marroquin, J.; Jaunet, T.; Chi-Ham, C.; Van Deynze, A. Texture diversity in melon (Cucumis melo L.): Sensory and physical assessments. Postharvest Biol. Technol. 2020, 159, 111024. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemist—Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemists: Virginia, VA, USA, 1984; pp. 414–422. [Google Scholar]
- Vella, F.M.; Cautela, D.; Laratta, B. Characterization of polyphenolic compounds in cantaloupe melon by-products. Foods 2019, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Arnow, L.E. Colorimetric determination of the components of 3,4-dihydroxyphenylalaninetyrosine mixtures. J. Biol. Chem. 1937, 118, 531–537. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoids contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Vella, F.M.; Laratta, B.; La Cara, F.; Morana, A. Recovery of bioactive molecules from chestnut (Castanea sativa Mill.) by-products through extraction by different solvents. Nat. Prod. Res. 2018, 32, 1022–1032. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Blois, M.S. Antioxidant determination by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Furumo, N.C.; Furutani, S. A simple method for assaying total protein, polyphenol oxidase and peroxidase activity from ‘Kaimana’ Litchi chinensis Sonn. J. Hawaii. Pac. Agric. 2008, 15, 1–7. [Google Scholar]
- Pinheiro, D.T.; Silva, A.L.D.; Silva, L.J.D.; Sekita, M.C.; Dias, D.C.F.D.S. Germination and antioxidant action in melon seeds exposed to salt stress. Pesqui. Agropecuária Trop. 2016, 46, 336–342. [Google Scholar] [CrossRef] [Green Version]
- Hagerman, A.E.; Austin, P.J. Continuous spectrophotometric assay for plant pectin methyl esterase. J. Agric. Food Chem. 1986, 34, 440–444. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Albuquerque, B.; Lidon, F.C.; Barreiro, M.G. A case study on the flavour properties of melon (Cucumis melo L.) cultivars. Fruits 2006, 61, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural products–A review. Sci. Hortic. 2015, 184, 179–192. [Google Scholar] [CrossRef]
- Tadmor, Y.; Burger, J.; Yaakov, I.; Feder, A.; Libhaber, S.E.; Portnoy, V.; Meir, A.; Tzuri, G.; Sa’ar, U.; Rogachev, I.; et al. Genetics of flavonoid, carotenoid, and chlorophyll pigments in melon fruit rinds. J. Agric. Food Chem. 2010, 58, 10722–10728. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds-nature, occurrence, dietary intake, and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini-Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef]
- Gülçin, I.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Koo, N.; Min, D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Holderbaum, D.F.; Kon, T.; Kudo, T.; Guerra, M.P. Enzymatic browning, polyphenol oxidase activity, and polyphenols in four apple cultivars: Dynamics during fruit development. HortScience 2010, 45, 1150–1154. [Google Scholar] [CrossRef] [Green Version]
- Murata, M.; Tsurutani, M.; Tomita, M.; Homma, S.; Kaneko, K. Relationship between apple ripening and browning: Changes in polyphenol content and polyphenol oxidase. J. Agric. Food Chem. 1995, 43, 1115–1121. [Google Scholar] [CrossRef]
- Brandelli, A.; Lopes, C.H. Polyphenoloxidase activity, browning potential and phenolic content of peaches during postharvest ripening. J. Food Biochem. 2005, 29, 624–637. [Google Scholar] [CrossRef]
- Chang, W.H.; Hwang, Y.J. Effect of ethylene treatment on the ripening, polyphenol oxidase activity and water-soluble tannin content of Taiwan northern banana at different maturity stages and the stability of banana polyphenol oxidase. Acta Hortic. 1990, 275, 603–610. [Google Scholar] [CrossRef]
- Weemaes, C.; Ludikhuyze, L.; Van den Broeck, I.; Hendrickx, M. Kinetic study of antibrowning agents and pressure inactivation of avocado polyphenoloxidase. J. Food Sci. 1999, 64, 823–827. [Google Scholar] [CrossRef]
- Cheema, S.; Sommerhalter, M. Characterization of polyphenol oxidase activity in Ataulfo mango. Food Chem. 2015, 171, 382–387. [Google Scholar] [CrossRef]
- Menon, S.V.; Ramana Rao, T.V. Nutritional quality of muskmelon fruit as revealed by its biochemical properties during different rates of ripening. Int. Food Res. J. 2012, 19, 1621–1628. [Google Scholar]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena—An overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef]
- Payasi, A.; Mishra, N.N.; Chaves, A.L.S.; Singh, R. Biochemistry of fruit softening: An overview. Physiol. Mol. Biol. Plants 2009, 15, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Quiroga, M.; Guerrero, C.; Botella, M.A.; Barceló, A.; Amaya, I.; Medina, M.I.; Alonso, F.J.; de Forchetti, S.M.; Tigier, H.; Valpuesta, V. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant. Physiol. 2000, 122, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Bernards, M.A.; Summerhurst, D.K.; Razem, F.A. Oxidases, peroxidases and hydrogen peroxide: The suberin connection. Phytochem. Rev. 2004, 3, 113–126. [Google Scholar] [CrossRef]
- Zimmermann, P.; Heinlein, C.; Orendi, G.; Zentgraf, U. Senescence specific regulation of catalase in Arabidopsis thaliana (L.) heynh. Plant. Cell Environ. 2006, 29, 1049–1060. [Google Scholar]
- Ben-Amor, B.; Latché, A.; Bouzayen, M.; Pech, J.C.; Romojaro, F. Inhibition of ethylene biosynthesis by antisense ACC oxidase RNA prevents chilling injury in Charentais cantaloupe melons. Plant. Cell Environ. 1999, 22, 1579–1586. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, N.S.; Malhotra, S.P.; Dhawan, K.; Singh, R. Antioxidant systems in ripening tomato fruits. Biol. Plant. 2004, 48, 49–53. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Kumar, G.N.M.; Knowles, N.R. Maturation and ripening of fruit of Amelanchier alnifolia Nutt. are accompanied by increasing oxidative stress. Ann. Bot. 1998, 81, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.E. Superoxide dismutase in ripening fruits. Plant. Physiol. 1976, 58, 644–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvaraj, Y.; Kumar, R. Studies on fruit softening enzymes and polyphenol oxidase activity in ripening mango (Mangifera indica L.) fruit. J. Food Sci. Technol. 1989, 26, 218–222. [Google Scholar]
- Prabha, T.N.; Yashoda, H.M.; Prasanna, V.; Jagadeesh, B.H.; Bimba Jain, M.V. Carbohydrate metabolism in relation to textural softening during fruit ripening. Trends Carbohyd. Chem. 2000, 6, 89–95. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).