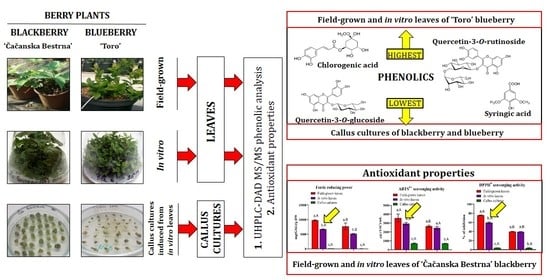
Phenolic Compounds and Antioxidant Properties of Field-Grown and In Vitro Leaves, and Calluses in Blackberry and Blueberry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. In Vitro Shoot Cultivation and Callus Induction from Leaves
2.3. Preparation of Leaves and Callus Culture Extracts
2.4. Total Phenolic and Flavonoid Content
2.5. UHPLC-DAD MS/MS Analysis of Leaves and Calluses
2.6. Antioxidant Properties
2.6.1. Ferric Reducing Power (FRP)
2.6.2. ABTS•+ and DPPH• Radical Scavenging Activity
2.7. Statistical Analysis
3. Results and Discussion
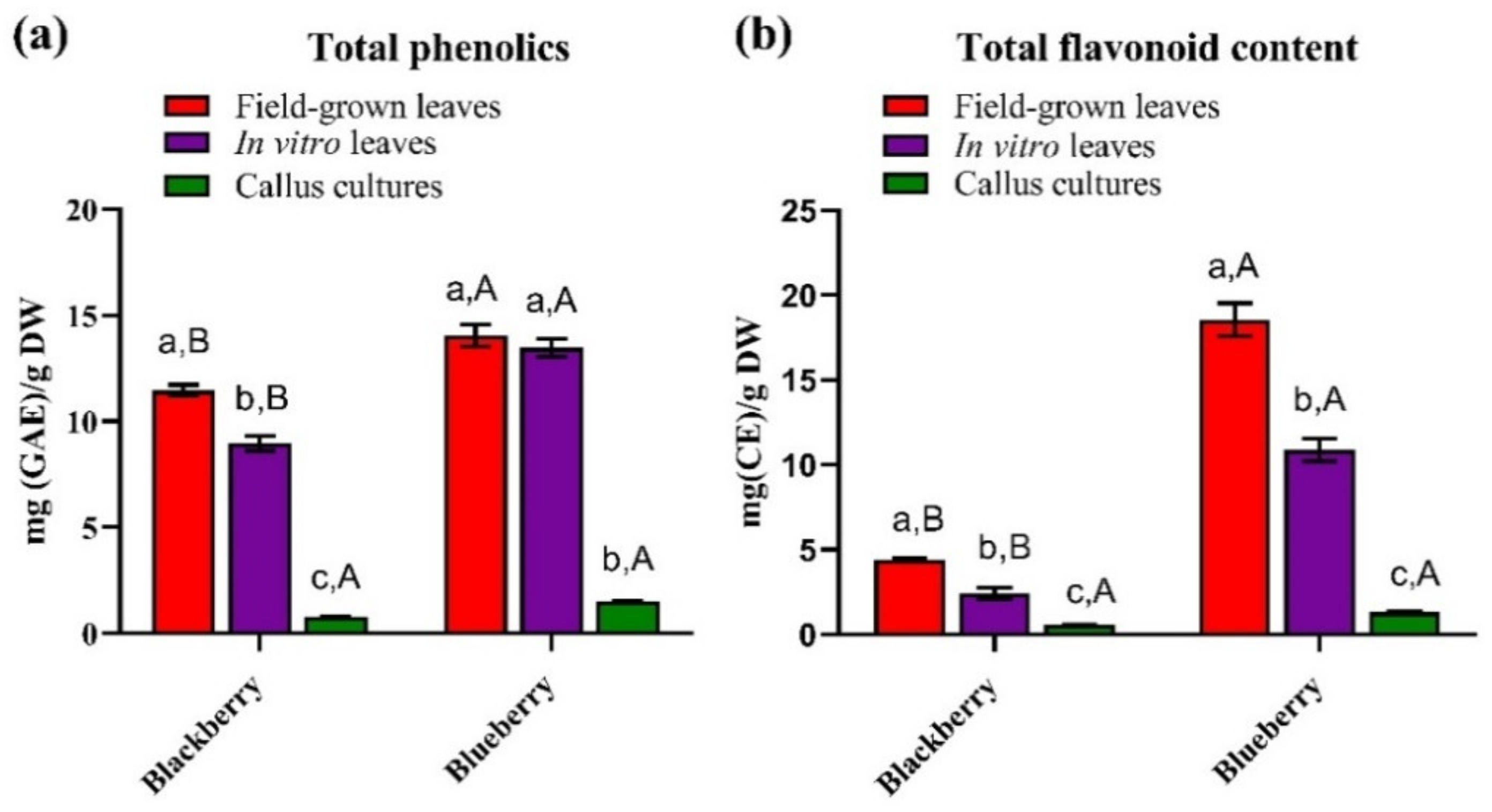
3.1. Total Phenolic and Flavonoid Content
3.2. UHPLC-DAD MS/MS Analysis of Blueberry and Blackberry Leaves and Callus Cultures
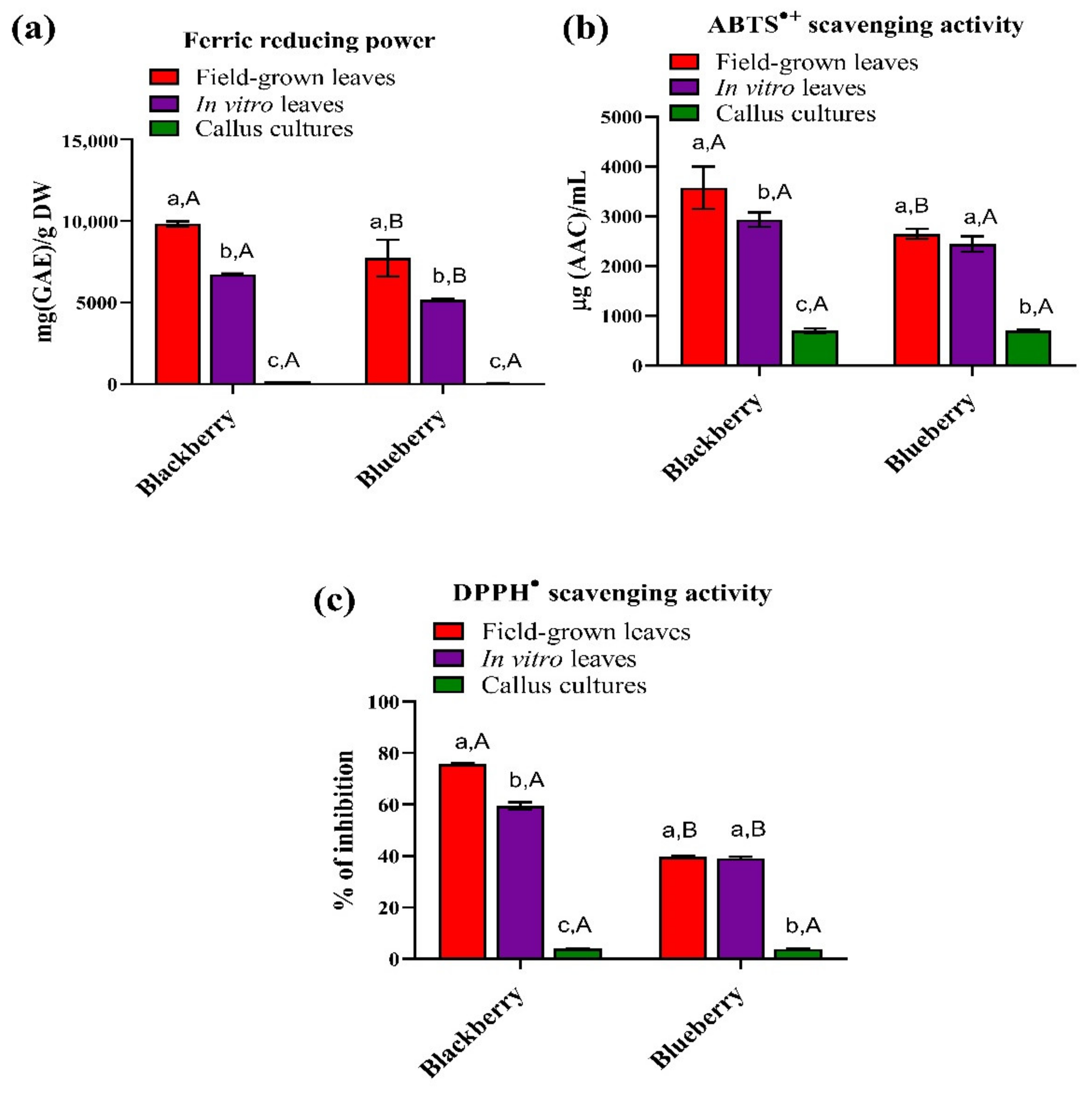
3.3. Antioxidant Properties
3.4. Ferric Reducing Power (FRP)
3.5. ABTS•+ and DPPH• Scavenging Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Petrović, S.; Leposavić, A.; Veljković, B. Blackberry and Blueberry, Technology of Production and Processing, 1st ed.; Fruit Research Institute Čačak: Čačak, Serbia; Ljekobilje doo Trebinje: Trebinje, Bosnia and Herzegovina, 2007. [Google Scholar]
- Diaconeasa, Z.; Florica, R.; Rugină, D.; Cuibus, L.; Socaciu, C. HPLC/PDA–ESI/MS Identification of Phenolic acids, flavonol glycosides and antioxidant potential in blueberry, blackberry, raspberries and cranberries. J. Food Nutr. Res. 2014, 2, 781–785. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Lamari, F.N. Berry leaves: An alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef]
- Pavlović, A.V.; Papetti, A.; Zagorac, D.Č.D.; Gašić, U.M.; Mišić, D.M.; Tešić, Ž.L.; Natić, M.M. Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind. Crops Prod. 2016, 87, 304–314. [Google Scholar] [CrossRef]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. The chemical and biological profiles of leaves from commercial blueberry varieties. Plants 2020, 9, 1193. [Google Scholar] [CrossRef]
- Gudej, J.; Tomczyk, M. Determination of flavonoids, tannins and ellagic acid in leaves from Rubus L. species. Arch. Pharm. Res. 2004, 27, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyło, A.; Gorzelany, J.; Kapusta, I. Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyło, A.; Nowicka, P.; Teleszko, M.; Cebulak, T.; Wolanin, M. Determination of phenolic compounds and antioxidant activity in leaves from wild Rubus L. species. Molecules 2015, 20, 4951–4966. [Google Scholar] [CrossRef] [PubMed]
- Riihinen, K.; Jaakola, L.; Kärenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and ‘Northblue’ blueberry (Vaccinium corymbosum x V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chai, Z.; Hutabarat, R.P.; Zeng, Q.; Niu, L.; Li, D.; Yu, H.; Huang, W. Blueberry leaves from 73 different cultivars in southeastern China as nutraceutical supplements rich in antioxidants. Food Res. Int. 2019, 122, 548–560. [Google Scholar] [CrossRef]
- Wang, L.-J.; Wu, J.; Wang, H.-X.; Li, S.-S.; Zheng, X.-C.; Du, H.; Xu, Y.-J.; Wang, L. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods 2015, 16, 295–304. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.; Pereira, M.; Costa, M.R.; Pintado, M. Evaluation of the antimicrobial activity of aqueous extracts from dry Vaccinium corymbosum extracts upon food microorganism. Food Control 2013, 34, 645–650. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Vojinović, U.D.; Kostić, A.; Pešić, M.B.; Špirović Trifunović, B.D.; Brkić, D.V.; Stević, M.; Kojić, M.O.; Stanisavljević, N.S. In vitro assessment of pesticide residues bioaccessibility in conventionally grown blueberries as affected by complex food matrix. Chemosphere 2020, 252, 126568. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.; Puente-Garza, C.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Matkowski, A. Plant in vitro culture for the production of antioxidants—A review. Biotechnol. Adv. 2008, 26, 548–560. [Google Scholar] [CrossRef]
- Smetanska, I. Sustainable Production of polyphenols and antioxidants by plant in vitro cultures. In Bioprocessing of Plant In Vitro Systems; Pavlov, A., Bley, T., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2018; pp. 1–45. [Google Scholar]
- Weathers, P.J.; Towler, M.J.; Xu, J. Bench to batch: Advances in plant cell culture for producing useful products. Appl. Microbiol. Biotechnol. 2010, 85, 1339–1351. [Google Scholar] [CrossRef]
- Dongliang, Q.; Xiangying, W.; Shufang, F.; Dawei, J.; Jianjun, C. Regeneration of blueberry cultivars through indirect shoot organogenesis. HortScience 2018, 53, 1045–1049. [Google Scholar]
- Fathy, H.; El-Leel, O.; Amin, M.; AbuEl-Leel, O. Micropropagation and biomass production of Rubus fruticosus L. (blackberry) plant. MEJAS 2020, 8, 1–14. [Google Scholar]
- Hunková, J.; Gajdošová, A.; Szabóová, M. Effect of mesos components(MgSO(4), CaCl(2), KH(2)PO(4)) on in vitro shoot growth of blackberry, blueberry, and saskatoon. Plants 2020, 9, 935. [Google Scholar] [CrossRef]
- Ružić, Đ.; Vujović, T.; Libiakova, G.; Cerović, R.; Gajdošova, A. Micropropagation in vitro of highbush blueberry (Vaccinium corymbosum L.). J. Berry Res. 2012, 2, 97–103. [Google Scholar] [CrossRef]
- Vujović, T.; Ružić, D.; Cerović, R.; Leposavić, A.; Žaklina, K.-S.; Mitrović, O.; Žurawicz, E. An assessment of the genetic integrity of micropropagated raspberry and blackberry plants. Sci. Hortic. 2017, 225, 454–461. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant callus: Mechanisms of induction and repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef] [PubMed]
- Sák, M.; Dokupilová, I.; Mihálik, D.; Lakatošová, J.; Gubišová, M.; Kraic, J. Elicitation phenolic compounds in cell culture of Vitis vinifera L. by Phaeomoniella chlamydospora. Nova Biotechnol. Chim. 2015, 13, 162–171. [Google Scholar] [CrossRef][Green Version]
- Saw, N.M.M.T.; Riedel, H.; Cai, Z.; Kütük, O.; Smetanska, I. Stimulation of anthocyanin synthesis in grape (Vitis vinifera) cell cultures by pulsed electric fields and ethephon. Plant Cell Tissue Organ Cult. (PCTOC) 2012, 108, 47–54. [Google Scholar] [CrossRef]
- Cai, Z.; Kastell, A.; Speiser, C.; Smetanska, I. Enhanced resveratrol production in Vitis vinifera cell suspension cultures by heavy metals without loss of cell viability. Appl. Biochem. Biotechnol. 2013, 171, 330–340. [Google Scholar] [CrossRef]
- Bonello, M.; Gašić, U.; Tešić, Ž.; Attard, E. Production of stilbenes in callus cultures of the Maltese indigenous grapevine variety, Ġellewża. Molecules 2019, 24, 2112. [Google Scholar] [CrossRef]
- Karaaslan, M.; Ozden, M.; Vardin, H.; Turkoglu, H. Phenolic fortification of yogurt using grape and callus extracts. LWT-Food Sci. Technol. 2011, 44, 1065–1072. [Google Scholar] [CrossRef]
- Ghosh, A.; Igamberdiev, A.U.; Debnath, S.C. Thidiazuron-induced somatic embryogenesis and changes of antioxidant properties in tissue cultures of half-high blueberry plants. Sci. Rep. 2018, 8, 16978. [Google Scholar] [CrossRef]
- Ramata-Stunda, A.; Valkovska, V.; Boroduske, A.; Silamikele, B.; Kaktina, E.; Rostoks, N.; Boroduškis, M.; Livkisa, D.; Pentjuss, A. Development of metabolic engineering approaches to regulate the content of total phenolics, antiradical activity and organic acids in callus cultures of the highbush blueberry (Vaccinium corymbosum L.). Agron. Res. 2020, 18, 1860–1872. [Google Scholar]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef]
- Zydlik, Z.; Cieśliński, S.; Mai, V.C.; Kafkas, N.E.; Morkunas, I. Soil Preparation, Running Highbush Blueberry (Vaccinium corymbosum L.) Plantation and Biological Properties of Fruits. In Modern Fruit Industry; Kahramanoglu, I., Kafkas, N.E., Küden, A., Çömlekçioğlu, S., Eds.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/69082 (accessed on 28 September 2021.).
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 2006, 15, 473–497. [Google Scholar] [CrossRef]
- Pavlović, A.V.; Dabić, D.; Momirović, N.M.; Dojčinović, B.P.; Milojković-Opsenica, D.M.; Tešić, Z.; Natić, M.M. Chemical composition of two different extracts of berries harvested in Serbia. J. Agric. Food Chem. 2013, 61, 4188–4194. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Gawron-Gzella, A.; Dudek-Makuch, M.; Matławska, I. Dpph Radical scavenging activity and phenolic compound content in different leaf extracts from selected blackberry species. Acta Biol. Crac. Ser. Bot. 2012, 54, 32–38. [Google Scholar] [CrossRef]
- Goyali, J.C.; Igamberdiev, A.U.; Debnath, S.C. Morphology, phenolic content and antioxidant capacity of lowbush blueberry (Vaccinium angustifolium Ait.) plants as affected by in vitro and ex vitro propagation methods. Can. J. Plant. Sci. 2013, 93, 1001–1008. [Google Scholar] [CrossRef]
- Noémi, K.; Éva, S.-B.; Enikő, P. Element Composition, total phenolics and antioxidant activity of wild and cultivated blackberry (Rubus fruticosus L.) fruits and leaves during the harvest time. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 563–569. [Google Scholar]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.V.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of cytotoxicity and antioxidant properties of berry leaves as by-products with potential application in cosmetic and pharmaceutical products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Gangrade, T.; Punasiya, R.; Ghulaxe, C. Rubus fruticosus (blackberry) use as an herbal medicine. Pharmacogn. Rev. 2014, 8, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shandi, K.; Alrawashdeh, A.; Al-Mazaideh, G.; Abu-Nameh, E.; Al-Amro, A.; Alsoufi, H.; Al-Ma’abreh, A.; Al-Dawdeyah, A. A novel strategy for the identification of the medicinal natural products in Rubus fruticosus plant by using GC/MS technique: A study on leaves, stems and roots of the plant. Adv. Anal. Chem. 2015, 5, 31–41. [Google Scholar]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.-O.; Dommes, J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009, 113, 1226–1233. [Google Scholar] [CrossRef]

| Genotype | Macro- Elements | Micro- Elements | Vitam. | Plant Growth Regulators (PGRs) | Sucrose | Agar | pH |
|---|---|---|---|---|---|---|---|
| Blackberry ‘Čačanska Bestrna’ | MS | MS | MS | 6-Benzylaminopurine (1 mg/L) Indole-3-butyric acid (0.1 mg/L) Gibberellic acid (0.1 mg/L) | 20 g/L | 7 g/L | 5.7–5.8 |
| Blueberry ‘Toro’ | MS ½ | MS | MS | Zeatin (2 mg/L) Indole-3-acetic acid (0.2 mg/L) | 20 g/L | 7 g/L | 4.5 |
| Genotype | Macro- Elements | Micro- Elements | Vitam. | Plant Growth Regulators (PGRs) | Sucrose | Agar | pH |
|---|---|---|---|---|---|---|---|
| Blackberry ‘Čačanska Bestrna’ | MS | MS | MS | 6-Benzylaminopurine (2 mg/L) 2,4-dichlorophenoxyacetic acid (2 mg/L) | 30 g/L | 7 g/L | 5.7–5.8 |
| Blueberry ‘Toro’ | MS ½ | MS | MS | 6-Benzylaminopurine (2 mg/L) 2,4-dichlorophenoxyacetic acid (2 mg/L) α-Naphthaleneacetic acid (1 mg/L) | 30 g/L | 7 g/L | 4.5 |
| Samples | Blueberry ‘Toro’ | Blackberry ‘Čačanska Bestrna’ | ||||
|---|---|---|---|---|---|---|
| Field-Grown Leaves | In Vitro Leaves | Callus Culture | Field-Grown Leaves | In Vitro Leaves | Callus Culture | |
| Phenolics (mg/kg) | ||||||
| Phenolic acids | ||||||
| Gallic acid | / | / | / | / | / | 0.202 ± 0.005 |
| Vanillic acid | / | / | 0.136 ± 0.009 | / | / | / |
| Ferulic acid | / | / | 0.330 ± 0.021 b | / | / | 0.154 ± 0.017 c |
| Syringic acid | / | / | / | 11.348 ± 1.125 a | 6.701 ± 0.243 b | / |
| Chlorogenic acid | 162.817 ± 11.251 b | 111.826 ± 3.174 c | / | 9.484 ± 0.591 d | 2.922 ± 0.041 e | / |
| Sum | 162.817 (41.3) | 111.826 (29.7) | 0.466 | 20.832 (56.4) | 9.623 (73.6) | 0.356 |
| Flavan-3-ols | ||||||
| Catechin | / | / | 0.567 ± 0.045 b | / | / | / |
| Catechin gallate | / | / | 0.051 ± 0.004 b | / | / | 0.048 ± 0.002 b |
| Gallocatechin | 17.077 ± 1.078 a | 56.239 ± 2.816 b | / | / | / | / |
| Sum | 17.077 (4.3) | 56.239 (14.9) | 0.618 | / | / | 0.048 |
| Flavonols | ||||||
| Quercetin | / | 17.304 ± 1.315 | / | / | / | / |
| Quercetin-3-O-rutinoside | 49.639 ± 1.526 c | 62.293 ± 4.403 d | 0.085 ± 0.006 b | 7.395 ± 0.197 e | 1.061 ± 0.079 f | / |
| Quercetin-3-O-glucoside | 160.113 ± 3.059 c | 115.598 ± 5.041 d | 0.390 ± 0.016 e | 5.483 ± 0.479 f | 1.697 ± 0.239 g | 0.183 ± 0.007 h |
| Quercetin-3-O-rhamnoside | / | 6.533 ± 0.528 | / | / | / | / |
| Isohramnetin-3-O-rutinoside | / | / | 0.034 ± 0.001 | / | / | / |
| Isohramnetin-3-O-glucoside | / | / | 0.093 ± 0.003 | / | / | / |
| Kaempferol-3-O-glucoside | 4.538 ± 0.207 b | 2.598 ± 0.249 c | / | 2.540 ± 0.100 c | 0.154 ± 0.015 d | / |
| Kaempferol | / | 1.435 ± 0.036 | / | / | / | / |
| Apigenin-7-O-glucoside | / | / | / | 0.284 ± 0.027 | / | / |
| Sum | 214.29 (54.3) | 205.761 (54.6) | 0.601 | 15.702 (42.3) | 2.912 (22.3) | 0.183 |
| Other detected phenols | ||||||
| Naringenin | 0.361 ± 0.028 | / | / | / | / | / |
| Aesculetin | / | 2.848 ± 0.181 a | / | 0.432 ± 0.024 b | 0.530 ± 0.028 c | / |
| Phlorizin | / | / | 0.068 ± 0.003 a | / | / | 0.058 ± 0.004 b |
| Sum | 0.361 | 2.848 | 0.068 | 0.432 | 0.530 | 0.058 |
| Total | 394.545 | 376.674 | 1.753 | 36.965 | 13.065 | 0.645 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolarević, T.; Milinčić, D.D.; Vujović, T.; Gašić, U.M.; Prokić, L.; Kostić, A.Ž.; Cerović, R.; Stanojevic, S.P.; Tešić, Ž.L.; Pešić, M.B. Phenolic Compounds and Antioxidant Properties of Field-Grown and In Vitro Leaves, and Calluses in Blackberry and Blueberry. Horticulturae 2021, 7, 420. https://doi.org/10.3390/horticulturae7110420
Kolarević T, Milinčić DD, Vujović T, Gašić UM, Prokić L, Kostić AŽ, Cerović R, Stanojevic SP, Tešić ŽL, Pešić MB. Phenolic Compounds and Antioxidant Properties of Field-Grown and In Vitro Leaves, and Calluses in Blackberry and Blueberry. Horticulturae. 2021; 7(11):420. https://doi.org/10.3390/horticulturae7110420
Chicago/Turabian StyleKolarević, Tijana, Danijel D. Milinčić, Tatjana Vujović, Uroš M. Gašić, Ljiljana Prokić, Aleksandar Ž. Kostić, Radosav Cerović, Sladjana P. Stanojevic, Živoslav Lj. Tešić, and Mirjana B. Pešić. 2021. "Phenolic Compounds and Antioxidant Properties of Field-Grown and In Vitro Leaves, and Calluses in Blackberry and Blueberry" Horticulturae 7, no. 11: 420. https://doi.org/10.3390/horticulturae7110420
APA StyleKolarević, T., Milinčić, D. D., Vujović, T., Gašić, U. M., Prokić, L., Kostić, A. Ž., Cerović, R., Stanojevic, S. P., Tešić, Ž. L., & Pešić, M. B. (2021). Phenolic Compounds and Antioxidant Properties of Field-Grown and In Vitro Leaves, and Calluses in Blackberry and Blueberry. Horticulturae, 7(11), 420. https://doi.org/10.3390/horticulturae7110420