Abstract
Sweet basil (Ocimum basilicum L.) is one of the most well-known aromatic herbs, which are economically important for food and pharmaceutical purposes. In vitro regeneration protocols are a fundamental part of molecular approaches, such as genome editing, which are used to enhance crop quality and pathogen resistance. In this research, in vitro regeneration methods were developed to examine the morphogenic aptitude of four different explant types from five commercial cvs of Ocimum basilicum L. (‘Prospera’, ‘Paoletto’, ‘Italiko FT’, ‘Dark opal’, and ‘Bolloso napoletano’). ‘Prospera’ showed the highest direct regeneration efficiency in all of the explant types (100% in the roots, 36% ± 0.02 in the cotyledons, 7.5% ± 0.2 in the hypocotyls, and 50% ± 0.04 in the cotyledonary nodes). The roots were found to be the most effective explant type, producing nodule-like meristems (100% in ‘Prospera’ and ‘FT Italiko’, 95.24% ± 0.01 in ‘Bolloso napoletano’), as precursors of shoots. Histological analysis was confirmed to be a suitable method to detect meristematic activity during the early morphogenic process and to evaluate the explants’ regeneration potential.
1. Introduction
The genus Ocimum includes 150 species that differ mainly in terms of their organoleptic characteristics and morphology. This genus presents great variability both in regard to its agronomic characteristics, which includes the different cultivation systems used, and in regard to the contents of essential oils and chemical compounds found within its species [1].
Belonging to the Lamiaceae family, Ocimum species are commonly known as basil and can differ in terms of their size, use, and chemical composition: Ocimum americanum L., for example, has a particular aroma like camphor, due to its high methyl cinnamate content, while Ocimum sanctum L. is largely used in oriental countries, due to its production of secondary metabolites, for phytotherapic purposes [2]. These compounds, such as essential oils and phenolic molecules, are extracted, especially from leaves and inflorescences, and can have different biological activities, including antioxidant, antimicrobial, anticancer, and anti-inflammatory properties [3].
O. basilicum L. is a perennial herbaceous plant that reaches up to 50–60 cm tall. It has opposite leaves that are petiolate and oval lanceolate in shape, which can show different colors, from dark green to purple, depending on the cvs. Flowers are typically hermaphrodite, small and white, with a corolla formed by five irregular petals, grouped into axillary inflorescences. It is a species that adapts well to all types of cultivation, such as pots, open fields, greenhouses, and soilless systems. However, it is susceptible to pathologies, such as Peronospora spp. and Fusarium spp. [4].
It is known as “sweet basil” and, although it originates from oriental countries (e.g., India, Pakistan, Thailand), the primary regions for the cultivation of the species and cvs for food purposes are now Europe and America [2].
One of the best-known sweet basil cvs is “Genovese-type” basil, cultivated especially in the Ligurian basin (Italy) for its fresh leaves to produce ‘’pesto sauce’’, a product certified as a Protected Denomination of Origin product (DOP) [5].
Within the O. basilicum species, 60 cvs have been identified, differing in terms of their aromatic, morphologic, and chemical features [2]. Due to the increased demand for fresh biomass, biotechnological approaches can be used to improve plant productivity by enhancing the quantity and quality of plant biomass [6].
One of the biggest problems in regard to basil cultivation is the variability from plant to plant and the genetic heterogeneity among the species. This heterogeneity in basil can also be observed in regard to its chemotype diversity that contributes to the variability of the species, reinforcing the cultivar-dependent response to in vitro culture protocols [7,8]. In recent decades, an increasing interest in crop genetic improvement using NGTs (new genomic techniques) has been noticed.
Recently, TEAs (Assisted Evolution Techniques), based on CRISPR/Cas9 technology, have been used for genetic improvement purposes to induce resistance to Peronospora belbahrii, a fungal parasite that causes extensive damage, with consequent negative economic implications [5].
The use of an in vitro regeneration protocol is the first step to perform genome editing experiments that are aimed, for example, to induce resistance to biotic and abiotic stresses [5], but often represents a bottleneck. Setting up in vitro protocols involves technical complexity and many species or tissues can be difficult to culture; nevertheless, in vitro culture is often the only way to maintain or optimize the survival rate of the species. Despite the fact that in vitro cultures have many advantages, such protocols need to be adapted for different cvs to avoid genetic mutations that can accumulate in long-term cultures [9]. Organogenesis is a pathway that could enable, directly or indirectly, the obtaining of new plants using Plant Growth Regulators (PGRs). As reported by [10,11,12,13,14], different PGRs, in factorial concentrations, can be used to improve neo-organogenesis in Ocimum spp., but it is also known that in vitro neo-organogenesis or regeneration is often cultivar dependent [15].
Histological analysis has a fundamental role in regard to regeneration protocols, offering the possibility to better understand the plant structures and basic mechanisms involved in the acquisition of competence of tissues, which is useful to optimize culture conditions and productivity [16].
In this work, five cvs of Ocimum basilicum L. (‘Prospera’, ‘Paoletto’, ‘Italiko FT’, ‘Dark opal’, and ‘Bolloso napoletano’) were evaluated. The morphogenetic aptitude related to neo-organogenesis has been analyzed through a histological examination, which enables the plant to exhibit a rapid response to hormonal stimuli, thereby accelerating the search for efficient protocols. This research will enrich the knowledge on the in vitro regeneration phase of Ocimum basilicum, related to the efficiency of different PGRs/concentrations, combined with the explant type, in order to obtain reliable and repeatable neo-organogenesis protocols.
2. Materials and Methods
2.1. Plant Material, Sterilization Protocol, and In Vitro Germination
The seeds of five selected cultivars of Ocimum basilicum L., i.e., ‘Italiko FT’ and ‘Paoletto FT’ (La Semiorto Sementi®, Sarno, Italy), ‘Prospera’ (Fenix Seeds®, Belpasso, Italy), ‘Bolloso napoletano’, and ‘Dark opal’ (Franchi Sementi®, Milan, Italy), were selected to obtain different explants to be used as starting plant materials for the experiments. In particular, the first 3 cvs are classified as “Genovese type” and are used for commercial purposes for their economic value in the food sector and for their resistance to biotic agents, while the last 2 were chosen for their aesthetic ornamental value and also for their high compound levels of secondary metabolites [6]. A total of 120 seeds of each cultivar were surface sterilized, according to the method outlined by Forti et al. [15]. The modified protocol involved pre-treated for 30 s in an ethanol solution (70% v/v) and then sterilization with a sodium hypochlorite solution (1.5% active chlorine) and 2–3 drops of Tween 20 (Duchefa Biochemie, Haarlem, The Netherlands) for 20 min at low agitation, and then the seeds were rinsed twice with sterile Milli-Q® water (Merck, Darmstadt, Germany). For each cultivar, 6 replications of 20 seeds/Petri dishes were used (FALCON® 60 × 15 mm, Glendale, AZ, USA) and seeds were cultured on an MS [17] standard medium, supplemented with 30 g/L sucrose and 6 g/L agar (Duchefa Biochemie, Haarlem, The Netherlands), at pH 5.7. The germination phase was performed in dark conditions at 23 ± 1 °C for the first two days, then the plates were transferred into a growth chamber at 23 ± 1 °C, for a 16 h photoperiod (PPFD 30 µE m−2 s−1). Macroscopical observations were carried out every two days, for 30 days, taking into consideration different parameters, such as the presence/absence of contamination, the germination rate (%G), and the average germination time (A.G.T), according to the formula by Ellis and Roberts [18].
2.2. Establishment of Cultures for In Vitro Regeneration
In vitro direct shoot regeneration was tested in the roots, hypocotyl segments, and cotyledons from 14-day-old seedlings and in the cotyledonary nodes (CNs) from 1-month-old seedlings of the 5 cvs. The four different explant types were cultured under different hormonal induction conditions, added to an MS basal medium, according to the best results reported in the literature [10,11,12,13,14] for the in vitro regeneration of each explant type considered (Table 1, Figure S1); each explant type was also cultured in a PGR-free medium, representing the control for the trials. The replication/explant types were set up as reported in Table 1. All the samples were cultured at 23 ± 1 °C, with a 16 h photoperiod (PPFD 30 µE m−2 s−1) for 4 weeks. Every week, macroscopical qualitative observations (necrosis, callogenesis and rhizogenesis events, pigmentation loss) and quantitative results (regeneration aptitude and percentage) were recorded for the 4 different explant types of the 5 cvs tested.

Table 1.
PGRs applied to the 4 explant types, according to the relevant references.
2.3. Histological Analysis
Roots and cotyledons of three selected cvs (‘Prospera’, ‘Paoletto’, and ‘Italiko FT’) were considered. The 3 selected cvs were chosen for their economic and commercial interest and it was a priority to investigate and better understand the regeneration process via histological organization. Moreover, 20-day-old explants, with or without (control) PGRs (Figure S2), were selected and fixed overnight in a paraformaldehyde solution (4% w/v) and phosphate buffer 0.1 M (pH 7.0), washed with the same buffer, dehydrated in an ethanol series (50%, 70%, 100%) and embedded in paraffin (Paraplast®, Merck KGaA, Darmstadt, Germany). Paraffin blocks were sectioned with a rotative microtome (Leica RM2265) to obtain 12 μm sections and the slides were stained with toluidine blue 0.25 M and mounted with DPX mountant for the histological analysis (Sigma-Aldrich Co., St. Louis, MO, USA) [19]. Microscopic analysis was performed with a DM 4000B optical microscope (Leica Microsystems, Wetzlar, Germany), equipped with a ToupCam Industrial Digital Camera (ToupTek Photonics, Hangzhou, Zhejiang, China).
2.4. Statistical Analysis
The collected data were statistically analyzed using RStudio software (RStudio 2022.12.0 Build 353© 2009–2022 Posit Software PBC, Boston, USA), which included an analysis of variance (ANOVA; p < 0.05) and a Siegel–Tukey test (Tukey HSD; p < 0.05). The percentage of germination was calculated as the ratio between the number of seeds germinated out of the total number of seeds subject to the test, while the average germination time rate (A.G.T.) was calculated according to the formula by Ellis and Roberts [18]. The regeneration percentage (%) was defined as the number of shoots regenerated on the different explant types considered and, for the roots, as the number of nodule-like meristems (NLMs) acting as de novo regeneration precursors. The results are presented as the mean ± standard error (SE) (the number of repetitions considered is reported in Table 1).
3. Results and Discussion
3.1. In Vitro Germination
The sterilization protocol ensured 100% of the seeds were sterile for all of the cvs tested, without interfering with their germination aptitude; in fact, all of the cvs were able to germinate with high efficiency on the medium and in the cultural conditions applied, with different cv-related responses. As shown in Table 2, the highest germination percentage (96%) combined with the lowest A.G.T. (0.65) was recorded for ‘Prospera’. The lowest germination percentage (83%) was recorded for ‘Bolloso napoletano’ combined with the highest A.G.T. (4.74), which is nevertheless a very appreciable result. No contamination was detected. A cultivar-related response was also reported by Zagoto et al. [20], who registered similar germination percentages for six different varieties, cultured in semi-vitro conditions.

Table 2.
Germination percentage and average germination time (A.G.T.) recorded for 5 cultivars of O. basilicum. Different letters indicate significant differences among the cultivars (p ≤ 0.05, Tukey HSD test).
The data analysis showed statistically significant differences among the cvs, both for the germination percentage and A.G.T. (Table 2).
3.2. In Vitro Regeneration
In Table S1, the macroscopical qualitative data are reported. After one month of culture, browning areas were observed, particularly on primary roots. However, this phenomenon did not affect the growth and the viability of adventitious roots across all of the cvs that, after their induction with PGRs, produced NLMs. Root culture did not show callus formation, but it was possible to observe it in different amounts on the other three starting explant types. In particular, in all the five cvs tested, callus induction was detected on the surface of the cotyledons and close to the cut area of the hypocotyls and CNs. This observation confirms that wounding can induce callus formation to protect damaged sites from pathogens or to regenerate new organs [21].
Callus induction can be achieved through the application of cytokinins; as reported by Bennur et al. [22], the influence of cytokinins during micropropagation can lead to direct or indirect regeneration, using the appropriate concentration. In this research, it was possible to observe that, in sweet basil, callus formation could be simultaneous with the presence of de novo shoots at the same PGR concentration, confirming that two morphogenic pathways (callogenesis vs. organogenesis) could be present, but remain strictly distinct; this was particularly evident in ‘Paoletto’ cotyledons (Figure 1).

Figure 1.
Example of NLMs (from root explants (A,E,I,M,Q)) or shoot formation in hypocotyls (B,F,J,N,R), in cotyledons (C,G,K,O,S), and on CNs (D,H,L,P,T) obtained after 4 weeks of regeneration induction, for all 5 cvs of O. basilicum ((A–D) ‘Prospera’; (E–H) ‘Paoletto’; (I–L) ‘FT Italiko’; (M–P) ‘Dark opal’; (Q–T) ‘Bolloso napoletano’ (scale bar 2 mm)).
It was observed that all of the cultivars showed the ability to form adventitious roots, especially from cotyledon segments. During PGR induction, hypocotyls and cotyledons showed a loss of pigmentation on the surface (Table S1). The total percentage of neo-formed structures for each explant type (all five cvs) was evaluated and the results are reported in Table 3. No regeneration events were observed in the controls in regard to any explant or cvs (Figure S3).

Table 3.
Total percentage of shoots or NLMs (only for root explants) for the 4 explant types in induction medium for the 5 cvs tested after 1 month of culture for specific PGR combinations. For each column, different letters indicate significant differences among the cultivars (p ≤ 0.05, Tukey HSD test).
The lowest regeneration percentage was observed in the hypocotyls of ‘Prospera’ and ‘Dark opal’, (7.5% ± 0.2 and 2.5% ± 0.01, respectively), while no response (0%) was observed in the other three cvs, confirming a strong correlation between genotypes and the explant type. A similar observation was reported by Barberini et al. [23], which showed a very low level of regeneration potential when hypocotyls were considered (9.18 ± 2.43). These results seem to differ from Verma et al. [24], who reported a promising direct regeneration rate starting from the hypocotyl. These observations can be related to the different competence of in vitro cultured cells or tissues, caused by many factors that can reduce or enhance the organogenic potential [25].
Both roots and CNs were suitable explants to induce regeneration in all of the five cvs tested. In our experience, roots are the best explant for obtaining de novo morphogenesis in all five cvs tested, particularly ‘Prospera’ and ‘FT Italiko’. Under 1.0 mg L−1 TDZ induction, structures known as NLMs developed. These structures possess high regeneration potential and genetic stability, rendering them a valuable alternative method for direct plant regeneration [26]. Fraj et al. [10] reported that NLMs can convert into shoots in PGR-free MS mediums in regard to the sweet basil cvs, ‘Grand Vert’, ‘Fin Vert’, and ‘Grand Vert Sweet’ basil (Table 4).

Table 4.
Number of shoots or NLMs (only for root explants) obtained on each explant type for the 5 cvs tested. For each column, different letters indicate significant differences among the cultivars (p ≤ 0.05, Tukey HSD test).
A cv-related response in regard to the regeneration percentage was also detected when cotyledons were used from ‘Prospera F1’ (36% ± 0.02) and ‘Paoletto’ (25.50% ± 0.03), which statistically differed from ‘FT Italiko’ (3.8% ± 0.01) and ‘Dark opal’ (10% ± 0.04). The morphogenic potential of cotyledonary tissue was improved by the PGR concentration compared to the results reported by Barberini et al. (19.7 ± 8.31 obtained from explants cultured on MS + 4 mgL−1 TDZ) [23]; the use of BAP was more efficient than TDZ induction in cotyledonary explants. ‘Bolloso napoletano’ did not show any direct regeneration events from cotyledonary explants, whilst this cv showed a strong ability to induce callus formation, which could be a suitable tissue to observe indirect morphogenesis (Table S1). As reported by Forti et al. [15] and Barberini et al. [23], cotyledon explants showed both callus formation (Table S1), especially on the cut surface, but also a good morphogenic aptitude (Table 3).
CNs were found to be the best explant for enhancing the direct shoot regeneration percentage, with 72.5 ± 0.04 for ‘Paoletto’. Strong CN regeneration potential was also reported by Laura et al. [5], reaching 2.6 regenerated shoots for each explant. ‘Prospera’ was the best performing cv in all of the tests, probably because it is a hybrid cv, and this observation is in accordance with the results reported by Forti et al. [15].
The total number of shoots and/or nodule-like meristems/explants for each of the cvs was reported (Table 4). The highest number of regeneration events, as NLMs, was observed in the roots of ‘Paoletto’ (7.37 ± 0.21), but it was not statistically different from ‘Prospera’ and ‘FT Italiko’ (5.96 ± 0.14 and 6.66 ± 0.14, respectively) (Table 4). A good number of shoots were observed in all of the cvs on the CN explants, compared to cotyledons and hypocotyls. Thus, it may be concluded, as also reported in the observations by Asghari et al. [12], that there is a strong effect between the type of explant source and the hormonal combinations on the direct regeneration of sweet basil plants.
3.3. Histological Detection
The histological analysis provided a good response in regard to the identification of early meristematic organization both in the roots (Figure 2) and cotyledons (Figure 3) grown in induction substrates (Figure 2 and Figure 3), compared to the control (Figure S4).
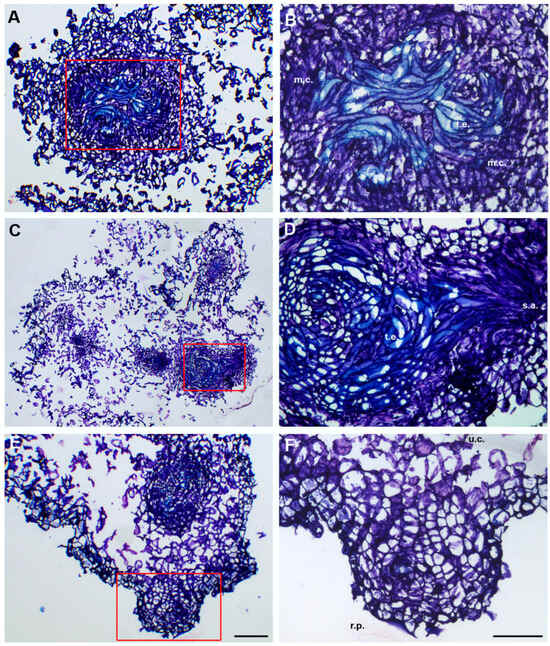
Figure 2.
Histological analysis of NLMs from root explants of O. basilicum cvs ‘Prospera’ (A,B), ‘Paoletto’ (C,D), and ‘FT Italiko’ (E,F). (A) NLM section of ‘Prospera’ at a high magnification; (B) particular meristematic centers (m.c.) and tracheary elements (t.e.) observed in ‘Prospera’ NLM, magnified from red rectangle in (A); (C) NLM section of ‘Paoletto’ at a high magnification; (D) presence of tracheary elements (t.e.) connected to shoot apex (s.a.), as early structure of shoot neo-formation, magnified from the red rectangle in (C); (E) NLM section of ‘FT Italiko’ at a high magnification; and (F) example of presence of undifferentiated cells (u.c.) in NLM section of ‘FT Italiko’ and presence of root primordia (r.p.), as early formation of new adventitious root. The section is magnified from the red rectangle in (E). Scale bar: 200 µm (A,C,E) and 100 µm (B,D,F).
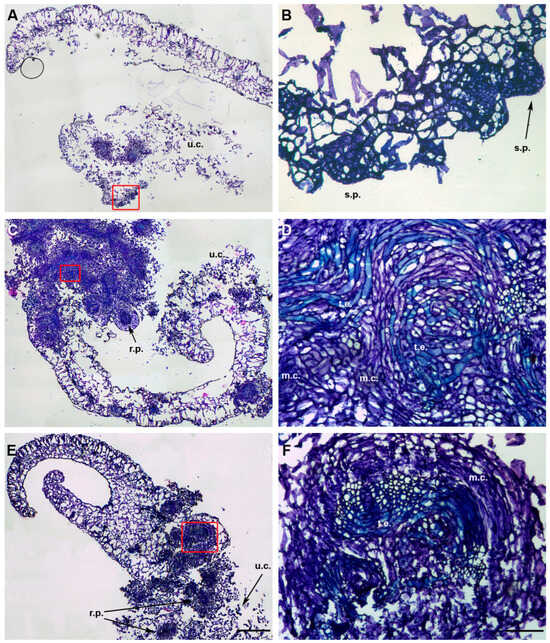
Figure 3.
Histological investigation of cotyledons of O. basilicum cvs ‘Prospera’ (A,B), ‘Paoletto’ (C,D), and ‘FT Italiko’ (E,F). (A) General view of ‘Prospera’ cotyledon section at a high magnification and, in particular, undifferentiated cells (u.c.) and organized meristematic zones, magnified from the red rectangle in (B); (B) magnification of (A): presence of shoot primordia (s.p.) as new regenerated tissue after PGR induction. (C) Section of ‘Paoletto’ cotyledon at a high magnification: presence, at the same time, of meristematic activity, magnified in (D) from the red rectangle, root primordia formation (r.p.) and undifferentiated cell zones (u.c.). (D) Magnification of (C) meristematic zone with examples of meristematic centers (m.c.) connected to tracheary elements (t.e.) as evidence of vascularization in the regenerated tissues. (E) General view of ‘FT Italiko’ cotyledon at a high magnification, with root primordia formation (r.p.), undifferentiated cells (u.c.), and meristematic activity zones, magnified in (F) from the red rectangle. (F) Magnified view of meristematic centers (m.c.) and tracheary elements (t.e.) in ‘FT Italiko’ cotyledon. Scale bar: 200 µm (A,C,E) and 100 µm (B,D,F).
Under the stimulus of PGRs, after 20 days, both explants of the three cvs tested were able to progress to the dedifferentiation process, showing undifferentiated cells (u.c.), but were also able to proliferate into a dense meristematic group of cells, characterized by a nucleus/cytoplasm ratio tending towards the nucleus, highlighted by an intense color in the cells. The meristematic centers (m.c.), dark blue in color, are often located close to the cut surface of the explants, confirming that the wounded stress-induced cellular re-organization process led to the formation of de novo sprouts [27].
The presence of a callus is also easily detected when cells with undefined borders and a loose structure, with small nuclei, were evident close to meristematic centers (m.c.) (Figure 3C) [27]; samples grown under the influence of cytokinin i (TDZ and BAP) showed early callus formation that could generate meristematic nodules and clumps, as shoot precursors.
Histological detection allowed us to observe the initial formation of parenchyma tissue, which began to differentiate into tracheary elements (t.e.) (Figure 2B and Figure 3D). These elements form a vascular connection with closer tissues, improving the uptake of water and nutrients, as reported by Dang et al. [28] in Dioscorea nipponica by conducting a histological analysis. Their presence indicated that morphogenesis events were in progress in order to turn the relevant tissue into new structures.
Among the O. basilicum cvs analyzed, different levels of intensity of meristematic activity were detected. These findings are evident both in the histological sections of roots (Figure 2C) under TDZ stimuli, and of the cotyledons (Figure 2C,E) under BAP induction.
The roots showed less meristematic activity, but it was possible to observe well-formed structures in the shoots, which had a vascular system (Figure 2D).
Cotyledonary sections showed a marked tendency toward the formation of preliminary meristematic cell organization zones, before the neo-organogenesis toward shoots; in these sections, root primordia (r.p.) were also detected. Microscopical analysis is crucial for developing a better understanding of the response of plant tissues to hormonal stimuli, confirming the macroscopic results obtained on in vitro regeneration.
Through the progressive observation of the sections, it was possible to highlight meristematic structures (s.p. and s.a.) that may develop into neo-formed shoots; in Figure 2D, it was possible to distinguish the early formation of the shoot apex as a dense structure, domed in shape. This observation is in accordance with the findings by Nassar et al. [29], who clearly performed histological and anatomical observations on Ocimum basilicum L. without PGR induction. This study confirms that PGR stimuli are a useful tool to enhance and speed up neo-shoot formation. In ‘Prospera’ cotyledons (Figure 3A,B), it was possible to detect meristematic nodule formation in the form of small protrusion during the first stages of shoot formation (s.p.). These structures could be considered as precursors in the early regeneration phase of the development of proper shoots [30,31], which means that they can be chosen as a promising explant type to obtain regeneration. Up to now, these results are the first to report on the histological observations of in vitro regeneration in sweet basil (Ocimum basilicum), as a tool that can improve the understanding of de novo morphogenesis and the regeneration process in in vitro culture.
4. Conclusions
This study enables the development of a better understanding of the mechanism of morphogenesis within the species Ocimum basilicum L., especially in cultivars with high commercial interest. From the results obtained, it was possible to underline a very high cultivar-dependent aptitude to in vitro regeneration, as already reported by previous studies on ‘Italiko FT’. Concerning the regenerative potential of the tested explants, roots showed the best aptitude in regard to organogenesis events (NLMs) in regard to all of the cvs examined, followed by cotyledons, cotyledonary nodes, and hypocotyls. The roots turn out to be the most suitable explant in order to perform a direct regeneration protocol; furthermore, the complete absence of callus formation in this explant could guarantee clonal propagation without the risk of somaclonal variability events, which could lead to genetic modifications within the variety itself. The role of cytokinins and their ability to induce organogenesis competence was demonstrated also in the basil species, during all of the trials. This study adds crucial information in order to manage the regeneration potential of an aromatic species with high economic importance that can undergo projects for its improvement using NGTs, according to which neo-organogenesis is a real bottleneck. It was possible to obtain a better understanding of micro and macro regeneration events that give rise to new structures, not only shoots, that, according to the literature, can develop entire plants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11091060/s1. Figure S1: Example of 4 different explant types (a: roots; b: hypocotyls; c: cotyledons; d: CNs), used as starting materials for the regeneration induction. Figure S2: Example of 2 different explant types (A,C,E: NLMs from roots; B,D,F: cotyledons), used as starting materials for the histological analysis of 3 cvs considered (‘Prospera’: A,B; ‘Paoletto: C,D; ‘FT Italiko’: E,F). Table S1: Detection of qualitative parameters (necrosis, callogenesis, rhizogenesis, and pigmentation loss) on 4 explant types (roots, hypocotyls, cotyledons, and CNs) in all 5 cvs of O. basilicum (‘Prospera’, ‘Paoletto’, ‘FT Italiko’, ‘Dark opal’, and ‘Bolloso napoletano’). Data related to the in vitro regeneration phase were collected according to the following range: absence (−), low (+), medium (++), and high (+++). Figure S3: Example of morphogenetic response observed in 4 different explant types (a: roots; b: hypocotyls; c: cotyledons; d: CNs) after 4 weeks without treatments (controls). Figure S4: Example of 20-day-old histological tissues of roots (A,C,E; c.c. = central cylinder) and cotyledon (B,D,F; adx.l. = adaxial lamina; abx.l. = abaxial lamina) without PGR induction (controls) in 3 cvs considered (‘Prospera’: A,B; ‘Paoletto: C,D; ‘FT Italiko’: E,F). Scale bar: 100 µm (A,C,E) and 200 µm (B,D,F).
Author Contributions
Conceptualization, M.M., A.C., L.P., M.S., and B.R.; methodology M.M., A.C., L.P., and M.S.; statistical analysis, M.M.; writing—original draft preparation, M.M. and M.S.; writing—review and editing, M.M., A.C., L.P., M.S., and B.R.; project administration and funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The funding support from the Italian Ministry of Agriculture, Food and Forestry Policies (MiPAAF), re-named in 2022 as “Ministero dell’agricoltura, della sovranità alimentare e delle foreste” (Masaf)—Project “Biotech” (grant number D.M. n. 15947 del 18/05/18), is acknowledged.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We sincerely thank Alberto Pardossi for providing seeds from the University of Pisa collection and Fernando Monroy for helping with the statistical analysis in RStudio.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| abx.l. | abaxial lamina |
| adx.l. | adaxial lamina |
| A.G.T. | average germination time |
| BAP | 6-Benzylaminopurine |
| c.c. | central cylinder |
| CNs | cotyledonary nodes |
| cvs | Cultivars |
| DOP | Denominazione di Origine Protetta (Protected Designation of Origin) |
| IAA | Indole-3-acetic acid |
| IBA | Indole-3-butyric acid |
| MS | Murashige and Skoog (1962) medium |
| m.c. | meristematic centers |
| NLMs | nodule-like meristems |
| NGTs | New Genomic Techniques |
| PGRs | Plant Growth Regulators |
| r.p. | root primordia |
| s.a. | shoot apex |
| s.p. | shoot primordia |
| t.e. | tracheary elements |
| TEAs | Assisted Evolution Techniques |
| TDZ | Thidiazuron |
| u.c. | undifferentiated cells |
References
- Pimentel, A.G.F.; Altenhofen da Silva, M.; Sartorio de Medeiros, S.D.; Queiroz Luz, J.M.; Sala, F.C. Agronomic, sensory and essential oil characterization of basil (Ocimum basilicum L.) accessions. Horticulturae 2023, 9, 831. [Google Scholar] [CrossRef]
- Makri, O.; Kintzios, S. Ocimum sp. (basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Bhattacharjya, D.; Adhikari, S.; Biswas, A.; Bhuimali, A.; Ghosh, P.; Saha, S. Ocimum phytochemicals and their potential impact on human health. In Phytochemicals in Human Health; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Vetrano, F.; D’Anna, F. First results on yield and quality response of basil (Ocimum basilicum L.) grown in a floating system. Acta Hortic. 2003, 609, 377–381. [Google Scholar] [CrossRef]
- Laura, M.; Forti, C.; Barberini, S.; Ciorba, R.; Mascarello, C.; Giovannini, A.; Pistelli, L.; Pieracci, Y.; Lanteri, A.P.; Ronca, A.; et al. Highly efficient CRISPR/Cas9 mediated gene editing in Ocimum basilicum ‘FT Italiko’ to induce resistance to Peronospora belbahrii. Plants 2023, 12, 2395. [Google Scholar] [CrossRef]
- Kiferle, C.; Lucchesini, M.; Mensuali-Sodi, A.; Maggini, R.; Raffaelli, A.; Pardossi, A. Rosmarinic acid content in basil plants grown in vitro and in hydroponics. Open Life Sci. 2011, 6, 946–957. [Google Scholar] [CrossRef]
- Bajomo, E.; Aing, M.; Ford, L.; Niemeyer, E. Chemotyping of commercially available basil (Ocimum basilicum L.) varieties: Cultivar and morphotype influence phenolic acid composition and antioxidant properties. NFS J. 2022, 26, 1–9. [Google Scholar] [CrossRef]
- Branca, F.; Treccarichi, S.; Ruberto, G.; Renda, A.; Argento, S. Comprehensive Morphometric and Biochemical Characterization of Seven Basil (Ocimum basilicum L.) Genotypes: Focus on Light Use Efficiency. Agronomy 2024, 14, 224. [Google Scholar] [CrossRef]
- Bekalu, Z.E.; Panting, M.; Holme, I.B.; Brinch-Pedersen, H. Opportunities and Challenges of In Vitro Tissue Culture Systems in the Era of Crop Genome Editing. Int. J. Mol. Sci. 2023, 24, 11920. [Google Scholar] [CrossRef]
- Fraj, H.; Hannachi, C.; Werbrouck, S.P.O. Efficient adventitious shoot organogenesis on root explants of Ocimum basilicum L. Acta Hortic. 2017, 1187, 89–92. [Google Scholar] [CrossRef]
- Ekmekci, H.; Aasim, M. In vitro plant regeneration of turkish sweet basil (Ocimum basilicum L.). Plant Sci. 2014, 24, 1758–1765. [Google Scholar]
- Asghari, F.; Hossieni, B.; Hassani, A.; Shirzad, H. Effect of explants source and different hormonal combinations on direct regeneration of basil plants (Ocimum basilicum L.). Aust. J. Agric. Eng. 2012, 3, 12–17. [Google Scholar]
- Gopi, C.; Sekhar, Y.N.; Ponmurugan, P. In vitro multiplication of Ocimum gratissimum L. through direct regeneration. Afr. J. Biotechnol. 2006, 5, 723–726. [Google Scholar]
- Jakovljević, D.; Stanković, M.; Warchoł, M.; Skrzypek, E. Basil (Ocimum L.) cell organ culture for the secondary metabolites production: A review. Plant Cell Tissue Organ Cult. 2022, 149, 61–79. [Google Scholar] [CrossRef]
- Forti, C.; Barberini, S.; Laura, M.; Ciorba, R.; Mascarello, C.; Giovannini, A.; Ruffoni, B.; Savona, M. Messa a Punto di Protocolli di Rigenerazione In Vitro in Ocimum Basilicum cv FT Italiko, Finalizzati al Miglioramento Genetico Via Genome Editing; Atti Giornate Scientifiche SOI: Bari, Italy, 2024. [Google Scholar]
- Rocha, D.I.; Kurczyńska, E.; Potocka, I.; Steinmacher, D.A.; Otoni, W.C. Histology and Histochemistry of Somatic Embryogenesis. In Somatic Embryogenesis: Fundamental Aspects and Applications; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- alMurashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. Improved equations for the prediction of seed longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar] [CrossRef]
- Jensen, W.A. Botanical Histochemistry, Principles and Practice; Freeman: San Francisco, CA, USA, 1962; p. 408. [Google Scholar]
- Zagoto, M.; De Freitas, P.S.L.; Contiero, R.L.; Da Rocha, E.M.T.; Cardia, G.F.E.; Mourão, K.S.M.; Filho, S.E.S.; Batistela, V.R.; Janeiro, V.; Cuman, R.K.N. Performance of seed sermination of six different varieties of basil (Ocimum basilicum spp.). Res. Soc. Dev. 2022, 16, e590111638517. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Iwase, A.; Rymen, B.; Lambolez, A.; Kojima, M.; Takebayashi, Y.; Heyman, J.; Watanabe, S.; Seo, M.; De Veylder, L. Wounding triggers callus formation via dynamic hormonal and transcriptional changes. Plant Physiol. 2017, 175, 1158–1174. [Google Scholar] [CrossRef]
- Bennur, P.L.; O’Brien, M.; Fernando, S.C.; Doblin, M.S. Improving transformation and regeneration efficiency in medicinal plants: Insights from other recalcitrant species. J. Exp. Bot. 2025, 76, 52–75. [Google Scholar] [CrossRef]
- Barberini, S.; Forti, C.; Laura, M.; Ciorba, R.; Mascarello, C.; Giovannini, A.; Ruffoni, B.; Savona, M. An optimized protocol for in vitro regeneration of Ocimum basilicum cv. FT Italiko. Horticulturae 2023, 9, 407. [Google Scholar] [CrossRef]
- Verma, S.K.; Sahin, G.; Das, A.K.; Gurel, E. In vitro plant regeneration of Ocimum basilicum L. is accelerated by zinc sulfate. In Vitro Cell. Dev. Biol.-Plant 2016, 52, 20–27. [Google Scholar] [CrossRef]
- Karami, O.; Aghavaisi, B.; Mahmoudi Pour, A. Molecular aspects of somatic-to-embryogenic transition in plants. J. Chem. Biol. 2009, 2, 177–190. [Google Scholar] [CrossRef]
- McCown, B.H.; Zeldin, E.L.; Pinkalla, H.A.; Dedolph, R. Nodule culture: A developmental pathway with high potential for regeneration, automated micropropagation, and plant metabolite production from woody plants. In Genetic Manipulation of Woody Plants; Springer: Berlin/Heidelberg, Germany, 1988; pp. 149–166. [Google Scholar]
- Altamura, M.M.; Biondi, S.; Colombo, L.; Guzzo, F. Elementi di Biologia Dello Sviluppo Delle Piante; Edises: Napoli, Italy, 2007; pp. 1–195. [Google Scholar]
- Dang, S.; Gao, R.; Zhang, Y.; Feng, Y. In vitro regeneration and its histological characteristics of Dioscorea nipponica Makino. Sci. Rep. 2022, 12, 18436. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.A.A.; El-Segai, M.U.; Azoz, S.N. Anatomical and Phytochemical Studies on Ocimum basilicum L. Plant (Lamiaceae). Int. J. Adv. Res. 2014, 2, 204–226. [Google Scholar]
- Bansal, S.; Sharma, M.K.; Singh, S.; Joshi, P.; Pathania, P.; Malhotra, E.V.; Rajkumar, S.; Misra, P. Histological and molecular insights into in vitro regeneration pattern of Xanthosoma sagittifolium. Sci. Rep. 2023, 13, 5806. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cheng, F.; Zhong, Y. Efficient plant regeneration via meristematic nodule culture in Paeonia ostii ‘Feng Dan’. Plant Cell Tissue Organ Cult. 2022, 149, 599–608. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).