Abstract
Boron (B) is a crucial micronutrient for the initial formation, development, and final quality of fruits, as it affects their physical and chemical properties and helps prevent various functional disorders. Recently, numerous physiological disorders in fruits have been reported, which have been linked to B deficiency. However, there is still uncertainty about whether these issues are directly related to B, other nutrients, their combinations, or environmental conditions. This review aims to compile current and accurate information on how B is absorbed by plants, its role in the cell wall and membrane, its impact on flowering and fruit set, and its influence on physical and chemical properties, as well as its role in preventing physiological disorders. This review examines the latest studies on B published in major scientific journals (Elsevier, Springer, MDPI, Frontiers, Hindawi, Wiley, and SciELO). Boron is mobile in the xylem and slightly mobile in the phloem, and it plays a crucial role in pollination and fruit set. It reduces mass loss, maintains firmness, improves color, and results in larger, heavier fruits. Also, boron increases soluble solids, regulates total titratable acidity and pH, decreases respiration rate, and stabilizes ascorbic acid by delaying its breakdown. It also helps prevent disorders such as splitting, cork spots, internal rot, shot berry in grapes, blossom end rot, and segment drying in citrus. Foliar or soil application of B enhances fruit yield and post-harvest quality.
1. Introduction
Improper fertilization management can lead to physiological problems in plants, resulting in disease processes that impact fruit quality []. In this context, boron (B) is involved in forming borate–pectin complexes that strengthen cell walls, improve chemical properties, and enhance the sensory attributes of fruits []. However, boron content in tropical soils is generally low because the primary source of the element is organic matter, and its deficiency in plants limits agricultural production [].
Boron deficiency is associated with various problems in fruits, directly affecting physical and chemical properties and ultimately reducing commercial quality []. Boron has a structural role and is critical for the architecture of fruit walls and membranes. Thus, its deficiency weakens cell walls by limiting the formation of dRG-II-B complexes (rhamnogalacturonan II-borate dimers) [], leading to cracking, deformities, reduced mechanical resistance, and faster deterioration, which decreases post-harvest storage. From a physiological standpoint, B deficiency causes conditions such as corky spots (abnormal suberization), internal rot (activation of enzymes that degrade the cell wall), blossom end rot (involvement in calcium mobility), and segment drying in citrus fruits (dehydrated vesicles) []. Additionally, metabolically, the absence of B reduces sugar transport, alters acidity and pH, raises respiration rates, accelerates the degradation of ascorbic acid, and diminishes fruit color intensity []. It also results in decreased fruit mass, size, and overall yield due to its role in pollen germination and pollen tube extension [].
Applying B significantly enhances fruit quality by reinforcing cell walls and stabilizing lipid bilayers through complexes with glycoproteins, which regulate cell permeability and signaling. Consequently, it reduces fruit weight loss, prevents deformation, and increases firmness by inhibiting enzymes such as polygalacturonase (PG) and pectin methyl esterase (PME) []. Furthermore, it modulates the synthesis of anthocyanins and offers protection against oxidative stress, improving the fruits’ color intensity []. B also raises soluble solids in fruits by facilitating efficient carbohydrate transport []. Adequate B levels decrease fruit respiration, prolonging energy reserves and limiting mass loss []. Studies on pear, strawberry, and avocado indicate that foliar B applications improve pollen germination and enhance fruit set and retention, leading to higher yields [,,].
Applying B shows great potential for enhancing fruit quality in various crops; however, a detailed review of its specific effects on fruit quality is still needed. Therefore, this study aims to gather information about the effects of B on fruit production and quality.
2. Plant Uptake of Boron
Boron is the most mobile micronutrient in soil. It moves via passive diffusion without the need for protein catalysis or energy use [], making it highly prone to leaching (pH below 5.0) and one of the most deficient elements in soil []. However, the optimal concentration is 2 mg kg−1 [], because higher levels are considered toxic to plants []. Boron in soil exists as undissociated boric acid [B(OH)3] in acidic and neutral soils (pH < 7.0) and as borate anion [B(OH)4]− in alkaline soils (pH > 8.5). Therefore, its availability depends on pH, being less available at alkaline pH levels [].
Plants absorb B as boric acid through plasma membrane channels, and it is then transported via specific carriers in the form of [B(OH)4]− []. In this context, two types of B transporters have been identified as follows: one called BOR1, which is crucial for moving B through the xylem and to younger plant tissues, and another made up of a subgroup of aquaporins known as nodulin-26-like intrinsic proteins (NIPs), which act as channels for transporting boric acid, essential for plant growth and delivering B to new shoots [] (Figure 1).
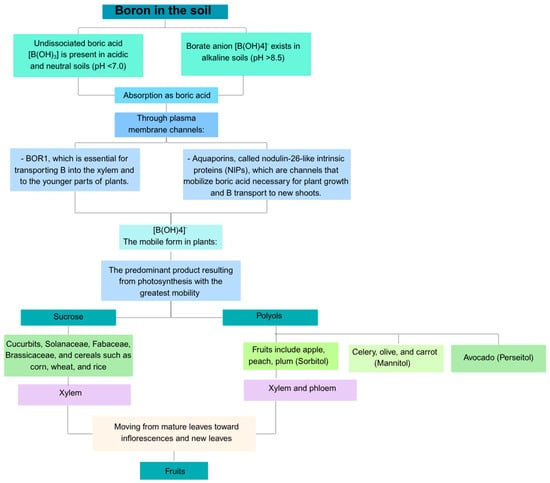
Figure 1.
Boron transfer from the soil to the fruits.
Boron mobilization and distribution in both xylem and phloem depend on the dominant photosynthesis product, which has the highest mobility in each species. When sucrose is the primary carbohydrate transported—such as in cucurbits, solanaceae, fabaceae, brassicaceae, and cereals like corn, wheat, and rice—B primarily moves within the xylem, relying on the transpiratory flow because B binds to sucrose and forms insoluble complexes []. Conversely, B is mobile in both xylem and phloem in species that transport primary products like polyols, including apple, peach, and plum (sorbitol), as well as celery, olive, and carrot (mannitol). In these plants, B forms stable complexes with polyols, such as boropentahydroxyl sorbitol, which enables its movement through the phloem and redistribution to younger tissues []. For example, in avocado, Minchin et al. [] reported that B forms a complex with perseitol, the polyol form of D-mannoheptulose, and is transported via the phloem towards new growth organs such as shoots and new leaves.
It has been observed that, depending on the phenological state of the plant, species vary in their polyol production, which causes changes in B mobility within the phloem. In this regard, during reproduction and fruit filling, low polyol production occurs, affecting the B transporters that supply the reproductive shoots [].
3. Boron in the Cell Wall and Membrane
Boron in plants is mainly stored in the cell wall, where it acts as a borate bridge that links with the polysaccharide rhamnogalacturonan II (RG-II) to form the dimer RG-II bound to B (dRG-II-B) and contributes to pectin formation []. Under B deficiency, the demethylation of new pectin is hindered [], with only 10% to 20% being incorporated into the cell wall as dRG-II-B, whereas more than 80% of B remains in its dimeric form. Therefore, the ratio of dimers can serve as an indicator for diagnosing B deficiency in plants []. Similarly, the amount of B correlates with the development and lignification of secondary walls []. B is crucial for cell wall adhesion because it forms a complex with RG-II and glycosyl inositol phosphoryl ceramides (GIPC) in the cell membrane, confirming its role in the pectin matrix formed by cellulose, hemicellulose, and proteins. This helps maintain the wall’s rigidity and thickness and is vital for proper assembly, ensuring the structure of the cell wall and its functional stability [].
B plays a structural role in the membrane by forming cis-diol complexes with glycoproteins, which improves the stability and function of the lipid bilayer []. These complexes form through boron’s interaction with hydroxyl groups in the cis configuration of mobile sugar residues, such as apiose, found in glycoproteins and glycolipids, thereby stabilizing their structure and facilitating their involvement in cell signaling and adhesion. This interaction is also crucial for maintaining the integrity, fluidity, and permeability of the cell membrane [].
Another role of B in the cell membrane is regulating electron transport []. In this context, Malavé [] explained that the auto-inducer AI-2 is made of a furanosyl borate diester, which not only acts as a bacterial signal but also transports B into and out of the cell. Similarly, it was observed that as water stress increases, these channels reduce their activity, limiting B transport and causing B deficiency []. Likewise, the activity of the B transporter (AtBOR1-1) heavily depends on the B level in the soil [].
B deficiency impacts the membrane potential because it decreases ATPase activity in proton pumping, alters the electrochemical gradient across the membrane, and reduces the activity of enzymes like Fe reductase, superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and antioxidants such as ascorbic acid (ASA) []. Additionally, under stress conditions and B deficiency, levels of phenolic compounds and other derivatives, such as quinones, increase, which can be oxidized and accelerate reactive oxygen species (ROS) production [].
4. The Effect of Boron on Flowering and Fruit Set
Boron is a vital element during flowering and fruiting in crops, and its deficiency is linked to low fruit set. This leads to underdeveloped pistils, reduced pollen viability, smaller flowers, and floral abortion. These issues are connected to decreased carbohydrate translocation and lower starch content in these structures [,]. In this context, Botelho et al. [] noted that B is particularly immobile in most plant tissues of particular species and faces difficulty translocating from source organs to young buds, where it is essential for pollen production, pollen tube growth, and other reproductive processes.
The role of B in the cell wall and membrane is evident in the growth and development of the pollen tube, because the gene responsible for borate binding to RG-II is highly active in pollen tubes, influencing flower fertility and the development of reproductive tissues []. In this regard, it was observed that once pollen germinates, pollen tubes extend through apical growth via secretory vesicles transported to the tip by cytoplasmic flow []. These vesicles bind to the plasma membrane of the tube and release polysaccharides and pectins into the exterior, where they become part of the cell wall. This ongoing activity enables the elongation of the pollen tube, a structure that grows from its apex and follows a polarized pattern [].
In this regard, the stigma or style must supply B in flowers to ensure proper pollen tube growth and is essential for the development of female reproductive organs []. In this sense, applying 300 mg L−1 of boric acid in Pyrus pyrifolia had a significant effect on pollen germination and pollen tube growth []. They also observed an increased weight of the anthers and pollen, and higher B concentration in the buds. Similarly, in strawberry, Sarıdaş et al. [] achieved significant increases in pollen viability, up to 71.8%, with a foliar (5 g per 100 L−1 of Tween 20) and soil (0.5 kg ha−1) application of B. Likewise, Mir et al. [] (2025) found that applying the sugar–borate complex has a greater translocation capacity than non-ionized or uncomplexed sugars, which enhances the efficiency of carbohydrate transport during pollen germination.
Similarly, in avocado flower styles, the concentration of B has a strong positive correlation with initial fruit set []. These authors [] also stated that adding B improves fruit set after self-pollination because self-pollination tubes generally grow more slowly than cross-pollination tubes, which reduces the chances of self-fertilization and fruit set. Furthermore, B is transported in small amounts to flowers and fruits []. They mentioned that in the ‘Hass’ variety, applying 30 g per tree resulted in higher B levels in the flowers compared to the control. In this regard, it was suggested that the higher demand for B in reproductive tissues is due to the limited number of vascular bundles that facilitate B movement through transpiration []. This necessitates the application of additional foliar B to the developing reproductive tissues, which promotes the crop’s reproductive success even when soil B levels are adequate.
Similarly, foliar application of B has been shown to increase B levels in flower buds, which enhances initial fruit set, retention, and final fruit set. This has been documented in species such as Persea americana [] and Punica granatum []. In this context, in Macadamia integrifolia, applying 15 g of B before flowering improved fruit set three weeks after peak anthesis. Likewise, a dose of 30 g of B elevated foliar B levels during peak anthesis, contributing to an increase in total yield []. Additionally, foliar B applications in gulupa (Pasiflora edulis f. edulis Sims) increased flower bud emission, the number of fruits, and the percentage of fruit set with the application of 0.6 kg ha−1 of B []. They also noted that B requirements are higher in reproductive tissues than in vegetative tissues. In sweet cherry (Prunus avium L.), B applications nine days before flowering increased endogenous B content and fruit set []. Similarly, in mango, B application reduces the time to fruit set, which is linked to B’s role in regulating RNA synthesis, an essential factor in enhancing physiological activity and enabling earlier flowering []. Likewise, applying 1% B to pomegranate fruits increased fruit set and retention while decreasing fruit drop compared to the control [].
With B deficiency, pollen tube secretory activity is impaired due to abnormal swelling or rupture of the apical region. The latter occurs due to cell wall weakness caused by B deficiency, which explains the slow growth of pollen tubes []. Additionally, B deficiency can disrupt key processes, such as microsporogenesis, leading to decreased pollen production, size, and viability. On the other hand, adequate or high levels of boron in the pistils can promote pollen germination and pollen tube growth, which benefits pollination, fertilization, and, ultimately, fruit setting and yield []. However, in a study with apple pollen (Malus domestica), high B concentration (0.02%) inhibited both pollen germination and pollen tube growth, accompanied by morphological abnormalities such as short pollen tubes with swollen ends and larger-than-usual diameters []. Excessively high doses (0.2%) significantly decreased pollen germination, reducing the percentage by up to 12.87%.
5. The Effect of B on the Physical Properties of the Fruit
Boron is a micronutrient that significantly influences the physical properties of the fruit, as it improves fruit firmness, size, and uniformity, which promotes the development of strong and well-organized cellular structures (Figure 2). Likewise, B is involved in cell wall synthesis, which directly affects fruit quality []. In this sense, B deficiency causes deformation, cracking, and reduced mechanical strength, which limits the marketability and post-harvest life of the fruit [,].
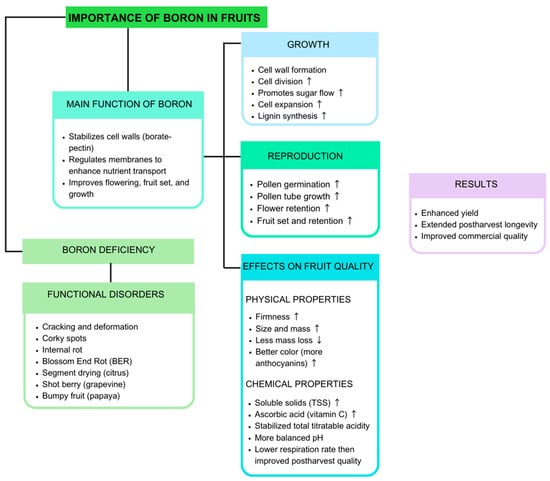
Figure 2.
Importance of boron in fruits. ↑ increase; ↓ decrease.
5.1. Mass Loss
B plays a vital role in preventing dehydration and loss of fruit mass during storage []. In this context, B deficiency often causes abnormal, thick, and fragile cell walls with altered porosity and mechanical strength []. Boron is also observed to form a complex with glycosyl inositol phosphoryl ceramides (GIPC) in the cell membrane [], which provides cell integrity and reduces fruit mass loss. Additionally, B deficiency can inhibit the activity of oxidoreductase, an enzyme bound to the plasma membrane involved in increasing the output of potassium and organic compounds, leading to faster water loss in deficient fruits []. Adequate B supply is essential for minimizing fruit mass loss, as observed in a study on peaches where immersing fruits in 6 μg mL−1 of Power B reduced mass loss to only 6%, compared to 18% in the control []. Similarly, applying 3% boric acid to pear fruits resulted in a significant reduction in mass loss, with only 3.2% compared to 5.4% in control fruits, demonstrating the effectiveness of boric acid in decreasing post-harvest dehydration and preserving fruit quality during storage []. Moreover, spraying mangoes with 1 mL L−1 of Ca-B delayed mass loss, while also increasing cell wall thickness and decreasing intercellular space in the exocarp []. Likewise, a 10.18% reduction in mass loss was observed with the application of 0.1% boric acid combined with 0.2% calcium [].
5.2. Firmness
Boron is crucial for maintaining fruit firmness, as it promotes fruit mechanical strength by affecting cell wall structure and key chemical elements []. In this context, boron application delays fruit softening during post-harvest by reducing the activity of enzymes such as cellulose, polygalacturonase (PG), pectin methylesterase (PME), β-galactosidase (β-Gal), pectate lyase (PL), and α-L-arabinofuranosidase (AFase) []. Additionally, these authors [] found that applying B to strawberry fruits significantly lowered the activity of PG (20.2%) and PL (38.1%), while increasing PME activity by 18.9%. This process reduces pectin breakdown, promotes pectin demethylation, and slows fruit softening []. Moreover, during fruit softening and ripening, B bound to the cell wall is transferred to the protoplast and the apoplast, leading to fruit decomposition. However, with the B application, this transfer is decreased, which further helps keep the cell wall firm and extends the fruit’s post-harvest life [].
The effect of B application on fruit firmness has been documented by various researchers (Figure 3). For example, strawberries harvested from plants treated with B showed 11% greater firmness than control fruits [,]. In strawberries, foliar spraying with 0.1% H3BO3 resulted in 45.7% higher yields and fruits that were 25.6% firmer than the control []. Similarly, peach fruits of the ‘Andross’ cultivar immersed in 6 μg mL−1 of powdered B exhibited 36.9% more firmness than control fruits []. Additionally, applying a 1 mL L−1 calcium and B solution to mangoes significantly delayed fruit pulp softening for up to 21 days after harvest, which was linked to a lower activity of PME and PG []. Likewise, in the blueberry variety ‘Brigitta’, spraying plants with 400 mg L−1 B increased fruit firmness by 25%, whereas in the ‘Legacy’ variety, a dose of 200 mg L−1 increased firmness by 48%. However, higher doses than those mentioned decreased firmness values [].
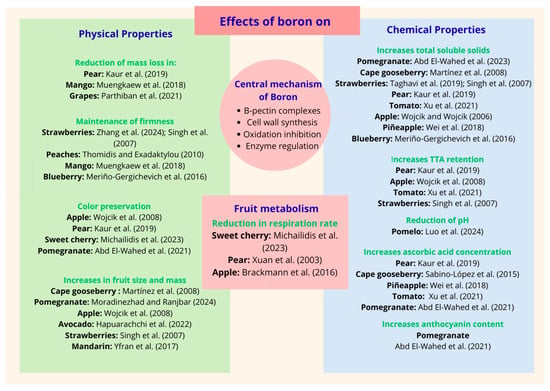
Figure 3.
The effects of boron on the physicochemical properties of fruits. Kaur et al. (2019) []; Muengkaew et al. (2018) []; Parthiban et al. (2021) []; Zhang et al. (2024) []; Singh et al. (2007) []; Thomidis and Exadaktylou (2010) []; Meriño-Gergichevich et al. (2016) []; Wojcik et al. (2008) []; Michailidis et al. (2023) []; Abd El-Wahed et al. (2021) []; Martinez et al. (2008) []; Moradinezhad and Ranjbar (2024) []; Hapuarachchi et al. (2022) []; Yfran et al. (2017) []; Xuan et al. (2003) []; Brackmann et al. (2016) []; Abd El-Wahed et al. (2023) []; Taghavi et al. (2019) []; Xu et al. (2021) []; Wojcik and Wojcik (2006) []; Wei et al. (2018) []; Xu et al. (2021) []; Luo et al. (2024) []; Sabino-López et al. (2015) [].
5.3. Color
The borate–pectin complex prevents phenols from leaking into the apoplast and being oxidized by polyphenol oxidases (PPO), thereby reducing browning [,]. Similarly, B influences the activity of phenylalanine ammonia-lyase (PAL), and its deficiency suppresses PAL expression, limiting the production of lignin precursors, flavonoids, and anthocyanins, the latter of which are responsible for red, purple, and blue colors []. B deficiency is linked to decreased photosynthetic efficiency, which hampers the production and transport of sugars needed for pigment formation [] and compromises cell membrane stability, leading to increased reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage. These effects accelerate the degradation of chlorophylls and carotenoids [] and cause fruit discoloration [].
In this regard, it was found that soil B fertilization in apple trees improved fruit color intensity compared to the control, which is attributed to increased photosynthesis and the enhanced transport of photo-assimilates from leaves to fruits []. Likewise, the post-harvest application of boric acid on pear fruits maintained more negative values in the green–red direction (a*) for a more extended period, indicating a greater retention of green color. At the same time, luminosity (L*) and variation along the blue–yellow axis (b*) increased to a lesser extent in treated fruits, indicating that B application preserves chlorophyll pigments and delays carotenoid synthesis []. However, pre-harvest B application did not significantly affect the external color of strawberry fruits; nevertheless, when combined with calcium, fruits showed higher luminosity, a*, and b* values, suggesting that B, in combination with Ca, contributes to the external appearance of the strawberry []. Additionally, in ‘Buttner’s Red’ sweet cherry trees, B fertilization increased anthocyanin content, which improved color intensity []). In pomegranate fruits, coloration increased with B application []. This is attributed to the role of B in the metabolism of phenolic compounds, which promotes the synthesis of pigments that enhance skin color and improve its commercial appeal [].
5.4. Fruit Size and Mass
Boron is essential for determining fruit size and weight. Therefore, a deficiency reduces tissue elasticity and extensibility, limiting cell expansion during growth, which results in smaller and misshapen fruits []. Additionally, B helps regulate sugar transport via the phloem and plays a role in synthesizing auxins, which are vital for cell division. As a result, a deficiency in this element decreases energy supply and inhibits mitosis in meristems and ovaries []. This affects pollination and protein synthesis, reduces dry matter accumulation, and produces fruits with a lower weight and insufficient size for marketability. In this context, under limited B conditions, B application helps stimulate new cell wall synthesis and promotes optimal fruit growth by maintaining physical pressure inside and outside the fruit [].
Boron deficiency reduced the diameter and mass of cape gooseberry fruits by 56% and 86% at harvest []. This occurs because the lack of B disrupts the translocation of photosynthates to the fruits, affecting their development and dry mass accumulation. Furthermore, B deficiency in fruits causes reduced size, deformities, and severe tissue damage from rot in the stem insertion area, as well as calyx deformities []. Similarly, it was observed that foliar application of a B nano-fertilizer before flowering increased yield in Punica granatum, likely due to high B absorption by flowers, which enhanced metabolic processes like carbohydrate transport []. These authors also stated that the yield increase may be due to the role of B in pollen germination, pollen tube elongation, and fruit formation, similar to findings in apples, where production per tree increased by 38% and 119% with soil and foliar B applications, respectively []. Likewise, both foliar and soil B applications increased fruit size in avocado trees, noting that fruits with higher B levels have better post-harvest quality and less browning [].
The effects of B have also been seen in the ‘Chandler’ strawberry variety, where combining it with calcium increased yield per plant by 20%. This was due to less fruit loss caused by poor quality. However, individual fruit weight was not affected by separate applications [], similar to findings for cape gooseberry fruit []. Likewise, in mandarin, a reduction in dropped fruits was observed when a 0.6% Ca-B mixture was applied, which helped to increase the production [].
6. The Effect of B on the Chemical Properties of the Fruit
Adequate B availability improves sugar transport, which increases total soluble solids (TSS) content, and is associated with fruits that exhibit greater sweetness and better organoleptic quality. Furthermore, B plays a role in balancing organic acids, which can alter pH and ATT, enhancing flavor perception and the chemical stability of the fruit. Additionally, this micronutrient is essential in regulating enzymes involved in ripening and cellular metabolism, such as polyphenol oxidase and peroxidase. A deficiency can disrupt these processes, reducing the nutritional and commercial quality of the fruit.
6.1. Total Soluble Solids
Boron helps transport photo-assimilates from leaves to fruits and is closely linked to sugar movement in the phloem and carbohydrate metabolism, which affects the TSS of fruits []. Additionally, B helps to regulate enzymes such as invertases and sucrose synthases, which are essential for the hydrolysis and accumulation of sugars in vacuoles []. Moreover, a deficiency of this element reduces photosynthetic activity in leaves (resulting in fewer photo-assimilates for fruit filling). It increases cellular respiration in fruits, indicating a loss of accumulated substrates []. At the same time, B influences pectin synthesis, decreasing osmotic potential and causing water imbalances that dilute TSS, resulting in fruits with lower °Brix and imbalances in the maturity ratio (MR), which affect their organoleptic quality.
In this regard, Moradinezhad and Ranjbar [] found that foliar application of B significantly increased leaf chlorophyll, soluble carbohydrates, relative water content, and proline in Punica granatum fruits []. Likewise, the absence of B fertilization decreased the °Brix concentration in cape gooseberry fruits by 40% compared to the control, which received all nutrients [], similar to findings that B deficiency results in fruits with poor TSS and ascorbic acid accumulation []. Similarly, pear fruits treated with 3% boric acid during the first 60 days of storage had a delayed increase in TSS, while at the end of post-harvest, fruits with B application had higher TSS content and maintained eating quality longer [].
Moreover, the TSS of strawberry fruits from plants treated with B during storage for 5 days at 10 °C showed 8.2 °Brix, while the control had higher values (8.5 °Brix) []. This difference is attributed to the effect of B, which reduces respiration rate and helps maintain post-harvest fruit quality by modulating TSS content, delaying softening, and lowering the incidence of pathogens and physiological disorders. Additionally, Xu et al. [] found that tomato fruits treated with 5.7 mg L−1 of H3BO3 achieved greater TSS accumulation (5.13 °Brix) compared to the control (4.63 °Brix). Likewise, in paprika, increases in TSS were observed with doses of 2 g L−1 applied to both soil and foliage []. Moreover, the application of 1 and 2 mL L−1 of Ca-B resulted in the slower growth of RM values, enhanced post-harvest stability, and better preservation of vitamin C, indicating fruits with higher nutritional and antioxidant value []. Additionally, apple fruits from trees fertilized with foliar and soil B contained 7.5% higher TSS than the control []. On the other hand, the application of B at doses of 0.5 and 1 mg kg−1 increased TSS by 6.3 and 7.5%, respectively []. Still, doses of 4 mg kg−1 reduced TSS and negatively affected pineapple quality []. Similarly, in blueberries, applying 200 mg L−1 of B increased TSS, while higher doses decreased it [].
6.2. Total Titratable Acidity (TTA)
Boron affects the synthesis, storage, and breakdown of organic acids by promoting vacuolar compartmentalization, which prevents acids like malic and citric from leaking into the cytosol []. In this context, B deficiency blocks PEP carboxylase activity, thereby decreasing malate production and over-activating NADP-malate dehydrogenase, which accelerates acid breakdown and acid loss through respiration []. B deficiency also impacts processes like photosynthesis, limiting the supply of carboxylic precursors and causing oxidative stress that triggers fermentative pathways []. This results in unstable TTA, with lower TTA levels in unripe fruits and abnormally high TTA levels in ripe fruits, which impacts taste and reduces shelf life.
In this regard, applying B to pear fruits increased TTA retention by 22.8% to 40% compared to the control []. Similarly, foliar and soil fertilization with B in apple trees increased fruit TTA values by 14.2% and 3.1%, respectively, compared to the control, which is attributed to the effect of B on a higher photosynthetic rate and, possibly, greater accumulation of organic acids []. Also, the application of foliar and soil B at doses ranging from 1.9 to 5.7 mg L−1 of H3BO3 resulted in average increases in the TTA of tomato fruits of up to 8.9% []. Likewise, increases in TTA were observed in bell pepper fruits with the application of 2 g L−1 of both foliar and soil []. Similarly, the foliar application of B in strawberries increased TTA by 3% compared to the control []. In contrast, no significant differences in the TTA of pineapple fruits with varying doses of B, ranging between 0.5 and 4 mg kg−1, were found []. Conversely, applying B at toxic levels (borax: 100 g per plant of Na2B4O7·10H2O) showed a significant increase in TTA in Citrus grandis fruits [].
6.3. pH
B deficiency affects the synthesis of malic and citric acids and weakens cell membrane and wall integrity, which allows protons (H+) to leak from vacuoles into the cytosol. This leads to an increased pH during the early stages of fruit growth []. However, during ripening, low B levels cause metabolic dysfunction that accelerates the oxidative breakdown of sugars and nitrogenous compounds [], resulting in the production of acetic and succinic acids, as well as amines, which lower the pH by acidifying the medium []. Simultaneously, the inhibition of proton pumps (H+-ATPases) and changes in tissue buffering capacity [] result in abnormal pH fluctuations, affecting the fruit’s sensory qualities and biochemical stability. It has also been reported that B does not significantly influence the pH values of cape gooseberry fruits [,]. Conversely, applying borax at 100 g per plant significantly lowers pH values in Citrus grandis fruits [].
6.4. Respiration Rate (RR)
Boron plays a vital role in the structure of cellular and mitochondrial membranes, affecting their permeability and the efficiency of the electron transport chain []. B deficiency increases O2 consumption and promotes uncontrolled substrate oxidation, which raises respiratory activity in fruits. Likewise, B deficiency influences the activity of citrate synthase and pyruvate dehydrogenase, limiting efficient ATP production []. This results in the rapid depletion of fruit acids and carbohydrate reserves, thereby accelerating the senescence process.
In this regard, foliar application of 0.2% boric acid to cherry trees (Prunus avium L.) decreased the fruit respiration rate during growth stages from day 12 to 46 after full bloom, compared to the control []; however, after this period, no differences were observed between treatments. Similarly, ‘Conference’ pear fruits treated with B showed lower CO2 emissions during five months of refrigerated storage []. Furthermore, after applying 1.5 kg ha−1 of B to apple trees via leaves, the fruits exhibited a lower respiration rate during the first two days of storage; subsequently, the control treatment showed the highest values []. In this context, Dar [] mentioned that applying B slightly decreases respiratory activity and affects the activity of enzymes involved in respiration.
6.5. Ascorbic Acid (AsA)
Although B does not directly influence the synthesis of ascorbic acid (AsA), adequate levels of the element are essential to prevent its breakdown and maintain a balanced redox potential []. However, B is involved in the regulation of the activity of L-galactono-1,4-lactone dehydrogenase, a key enzyme in AsA biosynthesis, by limiting the conversion of sugar precursors into vitamin C []. Similarly, B deficiency causes oxidative damage to cell membranes due to the excessive accumulation of reactive oxygen species [], which exposes AsA to enzymes like ascorbate oxidase and catalytic metals (Fe2+/Cu+), thereby accelerating its conversion to dehydroascorbic acid. This lowers AsA levels and reduces the nutritional quality and antioxidant defense of the fruits [].
In this context, applying 3% boric acid to fruits before storage kept 48% higher AsA levels in ‘Patharnakh’ pears compared to the control []. This is due to the role of B in suppressing PPO, thereby delaying oxidative deterioration by reducing the oxidation of phenolic compounds and ascorbate. Similarly, cape gooseberry fruits from plants that received foliar spraying of 3 mg L−1 of B showed 21.2% more AsA compared to the control treatment []. Likewise, Wei et al. [] demonstrated that applying B to pineapple fruits had a positive effect on vitamin C content. In tomato, the soil application of B increased AsA content by 185.2% compared to the control []. In pomegranate fruits, it was observed that applying B increased vitamin C and anthocyanin levels []. However, treating strawberry fruits with Boron did not significantly affect the AsA content [].
7. Boron in Fruit Functional Disorders
Fruit production and quality depend on proper plant nutrition. Since B is relatively immobile within the plant, its deficiency first appears in the youngest tissues, impacting crop yields []. This mainly occurs in arid or water-scarce areas where sandy soils contain soluble B levels below 0.5 mg kg−1 []. In such cases, B-deficient plants show reduced activity of the enzyme indoleacetic acid (IAA) oxidase due to increased accumulation of auxin [] and IAA [], leading to short, thick stems and rosette formation. This also results in wrinkled leaves and slower leaf growth, ultimately causing fruit issues and a decline in quality.
7.1. Fruit Deformation and Cracking
Fruit deformation and cracking occur due to poor cell division, among other causes, caused by boron deficiency (Figure 4a–c) [], which weakens the structural integrity of the cells []. It also plays a role in regulating indole-3-acetic acid (IAA) and enhancing water absorption, both of which support fruit structure and reduce susceptibility to cracking []. Foliar application of B reduces cracking symptoms by improving carbohydrate transport, overall metabolism, and ascorbic acid levels, thereby enhancing fruit quality and post-harvest shelf life []. In this context, B deficiency causes flower and fruit deformation, resulting in a decrease in their size and quantity []. Fertilizing plants with B results in fewer deformed fruits, which correlates with a higher concentration of B in fruits and leaves [].

Figure 4.
Possible boron deficiencies in fruits: (a) cracking in feijoa, (b) cracking in Passiflora edulis, (c) cracking in chirimoya, (d) fruit deformation and cracking in cape gooseberry, and (e) normal tangerine vesicles and (f) tangerine ‘Arrayana’ vesicles with segment drying.
The absence of B in the nutrient solution of cape gooseberry plants resulted in a 5.5% to 13% increase in the incidence of cracked fruits [] (Figure 4d). Therefore, B is essential for maintaining the integrity of the epidermis []. These authors also state that during the early growth stage of cape gooseberry fruit, the leaf area proportion exceeds that of the fruit, which could lead to competition for assimilates, causing B deficiency and contributing to cracking []. In this regard, applying 0.4% borax to pomegranate fruit reduced cracking by 12% to 20.4% []. Also, it was found that pre-harvest foliar application of borax reduced cracking and increased resistance to salt stress in pomegranate fruit []. Likewise, borax application decreased fruit cracking and increased sugar, anthocyanin, and vitamin C content [], consistent with the findings that borax treatment reduced cracking and sunburn in the same fruit [].
Similarly, the application of 0.1% boric acid + 0.2% calcium reduced grapefruit cracking and fruit drop by 3.46% and 2.42%, respectively [], while B + putrescine decreased cracking in Asian pears (Pyrus pyrifolia), and it was noted that B applied before flowering and after harvest extended the fruit’s shelf life []. In Aegle marmelos (Rutaceae), ripe fruits exhibit cracks due to B deficiency []. In grapefruit, applying B decreased the percentage of fruit cracking; however, adding Ca and Ca + B further reduced cracking, indicating that combined applications significantly improve crack prevention [].
7.2. External Corking
Boron deficiency leads to weakened cell adhesion and causes microtears in the hypodermis []. These lesions trigger a repair process in which damaged cells accumulate phenolic compounds and activate an overexpression of enzymes such as polyphenol oxidase, which generates quinones that cause oxidation []. This stimulates uncontrolled lignification and suberization, resulting in rigid, rough patches (corky spots) that ultimately lead to depressed, necrotic areas, reducing fruit quality.
In this regard, Khan and Ahmed [] mentioned that inadequate B supply causes corky spots both internally and externally in mango fruits. They also state that foliar application of B is more effective than soil application and that it improves fruit quality. Similarly, it was noted that B deficiency causes hardening of the fruit’s internal tissue, giving it a dry, corky appearance []. They report that the incidence of bitter spots averaged 13% across different varieties []. They also found that native Indian cultivars are more susceptible to physiological disorders than exotic cultivars, and that fruits with these disorders have lower concentrations of B and Ca []. At the same time, other nutrients remained at similar levels compared to healthy fruits.
The application of 1.5 g L−1 of B resulted in a 65% reduction in bitter spots (bitter pit in apples) and increased yield by 60.8% []. However, they noted that applying 2.5 g L−1 of Ca plus 2 g L−1 of B successfully reduced bitter spots by 90.4% and increased yield by 107% []. Thus, they conclude that the individual use of B and Ca has positive effects, but combining these two elements further enhances the benefits to the fruits [].
7.3. Internal Rot (Brown Rot)
Boron enhances the structural integrity and biochemical resistance of tissues. Therefore, a deficiency of this element causes cell walls to weaken []. This facilitates the rupture of parenchymal cells, creating cavities that fill with sugars and nutrients. Along with damage to cell membranes, this releases enzymes such as polygalacturonase that break down the pectic matrix, resulting in watery, brown, and foul-smelling areas. This promotes the growth of endophytic fungi (Fusarium, Botrytis, Rhizopus) and bacteria, which accelerate tissue decay [].
Similarly, B deficiency decreases the synthesis of phytoalexins and polyphenols involved in plant defense by disrupting the phenylpropanoid pathway and inhibiting lignification in vascular tissues, which allows pathogens to grow and cause internal fruit decay. In this context, applying B to peach fruit reduced internal rot from 53.2% to 5.2% []. Likewise, in mango fruits, internal rot is linked to B deficiencies and excess nitrogen, and it is noted that applying 500 g of borax to the soil and 1% foliar B significantly reduces internal necrosis []. Additionally, in amla (Indian gooseberry), internal necrosis in fruits has been reported, and bi-weekly foliar application of 0.6% borax resulted in a notable increase in B levels in leaves and fruits, along with a reduction in internal browning [].
7.4. Blossom End Rot (BER)
Boron plays a crucial role in the disease known as blossom end rot (BER), as it facilitates efficient calcium mobilization and promotes cell wall integrity []. Boron deficiency disrupts calcium pectate formation, which weakens cell cohesion at the fruit apex []. Similarly, B regulates the expression of ATPases, so its deficiency reduces the flow of Ca2+ into the phloem, limiting its role in membrane stability and cell signaling []. This triggers a loss of turgor and selective permeability, resulting in watery, sunken, and brown lesions characteristic of BER in tomatoes or bell peppers. In this regard, the application of B to tomato fruit increases symplast Ca levels and firmness, while decreasing PME activity and the incidence of BER in the fruit [].
7.5. Shot Berry
Shot berry is also known as Millerandage disease. In this disease, grapes in the same bunch are found that are significantly smaller and less ripe than the other berries. This disease is attributed to a B deficiency and results in a decline in quality and an appearance that affects the marketability of the product [].
In this regard, the concentration of B in floral tissues affects the formation of parthenocarpic fruits in grapevines, which shows a high correlation with the amount of abnormal pollen, where defective grains do not germinate or develop pollen tubes, leading to the presence of shot berries []. Likewise, the exogenous application of gibberellins increased the incidence of shot berries and reduced both the B content in flowers and the expression of the VvBOR3 and VvBOR4 genes, which are responsible for transporting B to reproductive tissues, limiting their mobility and increasing the occurrence of parthenocarpic fruits []. Additionally, it was found that foliar application of 0.5 g L−1 of B reduced the number of berries with shot berries compared to the control, with a much greater decrease when B was combined with zinc and epibrassinolide []. These treatments reduced the number of seedless berries, attributed to the effect of B on pollen germination and pollen tube growth, which promotes successful fertilization. Likewise, the application of 0.05% boric acid decreased the incidence of shot berries in grapes; however, its effect was more pronounced when combined with glutamic acid, reducing shot berries by 6.5% and increasing berry weight, size, and overall quality.
Furthermore, the incidence of shot berry has been reported in olive trees. It is reported that foliar application of different doses of B improved fruit set. However, it did not affect the proportion of parthenocarpic fruits, and shot berry production was similar to that of the control. These factors are attributed to inadequate fertilization management rather than B deficiencies [].
7.6. Segment Drying
Segment drying is a physiological disorder that affects citrus fruits during the pre- and post-harvest stages. This condition leads to dehydration, flavor loss, and a hardened texture, impacting the fruit’s organoleptic quality [] (Figure 4e). In some regions, it is called “fruit hardening” or “vesicle thickening,” while in China, it is known as “kushui,” as the fruits appear dull and dry, with a grayish color, dry vesicles, a bland flavor, a grainy texture, and a juice content reduction of up to 70% in mandarin, grapefruit, and orange []. In citrus fruits, it was found that B deficiency causes low sugar levels and thickening of the peel, due to the role of B in sugar translocation, indicating a strong link between B fertilization and grapefruit granulation []. Also, dry juice vesicles in citrus were attributed to low B and P levels, high temperatures, and high relative humidity [].
7.7. Bumpy Fruit
Bumpy fruit is a disease that affects papaya fruits and is caused by a B deficiency []. The deformation begins in young fruits, worsens during ripening, and is marked by the exudation of milky latex from the fruit surface. The infection spreads, forming a ball-shaped bump at the ends of the tissue. Its incidence has been reduced by applying 0.25% borax to the leaves and 1 to 3 kg ha−1 of B to the soil, which has increased B concentrations in the petioles and decreased the occurrence of this defect.
8. Conclusions
Adequate levels of B are crucial for good fruit quality because it serves multiple functions. Its role in forming borate–pectin complexes helps maintain cellular integrity, which reduces fruit mass loss. B also aids in the efficient transport of sugars and the compartmentalization of organic acids, while preventing the degradation of antioxidants like ascorbic acid. Additionally, B shortens anthesis time in fruit trees, increases the number of flowers per shoot, reduces flower drop, and boosts fruit set. It also decreases cork spots and deformed fruits while increasing overall production and yield. Fruits from plants treated with B have a lower respiration rate, which helps preserve post-harvest quality by maintaining soluble solids content, delaying softening, and reducing the incidence of pathogens and physiological issues such as cracking, cork spots, internal rot, blossom end rot, and segment drying. Foliar application of B is more effective than soil application. Future research should focus on the application of boron nano-fertilizers to improve their efficiency in plant uptake and their impact on fruit functional disorders. Likewise, the effect of boron on the incidence of post-harvest diseases should be evaluated. The synergistic or antagonistic effects of boron with other nutrients on final fruit quality should also be assessed. The way boron affects the quality of fruits from plants under conditions of drought, salinity, or extreme temperatures should also be studied.
Author Contributions
Conceptualization, G.F. and J.G.Á.-H.; methodology, J.G.Á.-H. and M.J.-G.; software, M.J.-G.; validation, J.G.Á.-H., M.J.-G. and G.F.; formal analysis, J.G.Á.-H.; investigation, M.J.-G.; resources, J.G.Á.-H.; data curation, J.G.Á.-H. and M.J.-G.; writing—original draft preparation, J.G.Á.-H. and M.J.-G.; writing—review and editing, G.F.; visualization, J.G.Á.-H. and M.J.-G.; supervision, J.G.Á.-H. and G.F.; project administration, J.G.Á.-H.; funding acquisition, J.G.Á.-H. and M.J.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Directorate (DIN) of the Universidad Pedagógica y Tecnológica de Colombia, through project SGI 3903.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data will be made available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fischer, G.; Balaguera-López, H.E.; Álvarez-Herrera, J. Causes of fruit cracking in the era of climate change. A review. Agron. Colomb. 2021, 39, 196–207. [Google Scholar] [CrossRef]
- Long, Y.; Peng, J. Interaction between boron and other elements in plants. Genes 2023, 14, 130. [Google Scholar] [CrossRef]
- Brdar-Jokanović, M. Boron toxicity and deficiency in agricultural plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef]
- Thakur, S.; Sinha, A.; Ghosh Bag, A. Boron—A critical element for fruit nutrition. Commun. Soil Sci. Plant Anal. 2023, 54, 2899–2914. [Google Scholar] [CrossRef]
- Vera-Maldonado, P.; Aquea, F.; Reyes-Díaz, M.; Cárcamo-Fincheira, P.; Soto-Cerda, B.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Role of boron and its interaction with other elements in plants. Front. Plant Sci. 2024, 15, 1332459. [Google Scholar] [CrossRef] [PubMed]
- Haleema, B.; Shah, S.T.; Basit, A.; Hikal, W.M.; Arif, M.; Khan, W.; Fhatuwani, M. Comparative effects of calcium, boron, and zinc inhibiting physiological disorders, improving yield and quality of Solanum lycopersicum. Biology 2024, 13, 766. [Google Scholar] [CrossRef] [PubMed]
- Hapuarachchi, N.S.; Kämper, W.; Wallace, H.M.; Hosseini Bai, S.; Ogbourne, S.M.; Nichols, J.; Trueman, S.J. Boron effects on fruit set, yield, quality and paternity of Hass avocado. Agronomy 2022, 12, 1479. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, C.; Tian, H.; Xu, J.; Wu, X. Foliar spraying of boron prolongs preservation period of strawberry fruits by altering boron form and boron distribution in cell. Front. Plant Sci. 2024, 15, 1457694. [Google Scholar] [CrossRef]
- Dong, X.; Jiang, C.; Wei, S.; Jiao, H.; Ran, K.; Dong, R.; Wang, S. The regulation of plant lignin biosynthesis under boron deficiency conditions. Physiol. Plant. 2022, 174, e13815. [Google Scholar] [CrossRef] [PubMed]
- Moradinezhad, F.; Ranjbar, A. Foliar application of fertilizers and plant growth regulators on pomegranate fruit yield and quality: A review. J. Plant Nutr. 2024, 47, 797–821. [Google Scholar] [CrossRef]
- Lu, Y.B.; Yang, L.T.; Li, Y.; Xu, J.; Liao, T.T.; Chen, Y.B.; Chen, L.S. Effects of boron deficiency on major metabolites, key enzymes and gas exchange in leaves and roots of Citrus sinensis seedlings. Tree Physiol. 2014, 34, 608–618. [Google Scholar] [CrossRef]
- Kaur, A.; Gill, P.P.S.; Jawandha, S.K.; Singh, M. Pre-storage exogenous application of boric acid extends storability and maintains quality of pear fruits. Sci. Hortic. 2019, 256, 108616. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, R.R.; Tyagi, S.K. Pre-harvest foliar application of calcium and boron influences physiological disorders, fruit yield and quality of strawberry (Fragaria× ananassa Duch.). Sci. Hortic. 2007, 112, 215–220. [Google Scholar] [CrossRef]
- Hrmova, M.; Gilliham, M.; Tyerman, S.D. Plant transporters involved in combating boron toxicity: Beyond 3D structures. Biochem. Soc. Trans. 2020, 48, 1683–1696. [Google Scholar] [CrossRef]
- Li, S.; Yan, L.; Venuste, M.; Xu, F.; Shi, L.; White, P.J.; Ding, G. A critical review of plant adaptation to environmental boron stress: Uptake, utilization, and interplay with other abiotic and biotic factors. Chemosphere 2023, 338, 139474. [Google Scholar] [CrossRef]
- Zhao, S.; Huq, M.E.; Fahad, S.; Kamran, M.; Riaz, M. Boron toxicity in plants: Understanding mechanisms and developing coping strategies; a review. Plant Cell Rep. 2024, 43, 238. [Google Scholar] [CrossRef]
- Zhou, X.X.; Yang, L.T.; Qi, Y.P.; Guo, P.; Chen, L.S. Mechanisms on boron-induced alleviation of aluminum-toxicity in Citrus grandis seedlings at a transcriptional level revealed by cDNA-AFLP analysis. PLoS ONE 2015, 10, e0115485. [Google Scholar] [CrossRef]
- Martinelli, J.; Campos, M.D.; Costa, C.A.C.; Garcia, A.; Tarumoto, M.B.; Ferraz, G.; Brown, P.H.; Costa, C.A. Adequate boron supply modulates carbohydrate synthesis and allocation in sugarcane. Plants 2025, 14, 657. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.L.; Siqueira, J.A.; Batista-Silva, W.; Cardoso, F.B.; Nunes-Nesi, A.; Araújo, W.L. Boron: More than an essential element for land plants? Front. Plant Sci. 2021, 11, 610307. [Google Scholar] [CrossRef] [PubMed]
- Minchin, P.E.H.; Thorp, T.G.; Boldingh, H.L.; Gould, N.; Cooney, J.M.; Negm, F.B.; Brown, P. A possible mechanism for phloem transport of boron in ‘Hass’ avocado (Persea americana Mill.) trees. J. Hortic. Sci. Biotechnol. 2012, 87, 23–28. [Google Scholar] [CrossRef]
- Diehn, T.A.; Bienert, M.D.; Pommerrenig, B.; Liu, Z.; Spitzer, C.; Bernhardt, N.; Bienert, G.P. Boron demanding tissues of Brassica napus express specific sets of functional Nodulin26-like intrinsic proteins and BOR1 transporters. Plant J. 2019, 100, 68–82. [Google Scholar] [CrossRef]
- Yan, L.; Riaz, M.; Wu, X.; Du, C.; Liu, Y.; Jiang, C. Ameliorative effects of boron on aluminum induced variations of cell wall cellulose and pectin components in trifoliate orange (Poncirus trifoliate (L.) Raf.) rootstock. Environ. Pollut. 2018, 240, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sun, X.; Zhang, L.; Zeng, X.; Liu, G.; Sheng, O. Lignin metabolism plays an essential role in the formation of corky split vein caused by boron deficiency in ‘Newhall’ navel orange (Citrus sinensis Osb.). Sci. Hortic. 2022, 294, 110763. [Google Scholar] [CrossRef]
- Dong, X.; Lu, X.; Wu, X.; Liu, G.; Yan, L.; Muhammad, R.; Jiang, C. Changes in chemical composition and structure of root cell wall of citrus rootstock seedlings in response to boron deficiency by FTIR spectroscopy. J. Hortic. Sci. Biotechnol. 2018, 93, 150–158. [Google Scholar] [CrossRef]
- Bolaños, L.; Abreu, I.; Bonilla, I.; Camacho-Cristóbal, J.J.; Reguera, M. What can boron deficiency symptoms tell us about its function and regulation? Plants 2023, 12, 777. [Google Scholar] [CrossRef]
- Malavé, A.C.; Carrero, P.E. Desempeño funcional del boro en las plantas. Rev. Cient. UDO Agríc. 2007, 7, 1–14. [Google Scholar]
- Wimmer, A.; Eichert, T. Mechanisms for boron deficiency-mediated changes in plant water relations. Plant Sci. 2013, 203, 25–32. [Google Scholar] [CrossRef]
- Quiroga-Ramos, I.A.; Fischer, G.; Melgarejo, L.M. Efecto de la aplicación foliar de boro en el desarrollo fenológico y cuajado de fruto de gulupa (Passiflora edulis f. edulis Sims). Rev. Colomb. Cienc. Hortic. 2018, 12, 20–30. [Google Scholar] [CrossRef]
- Rerkasem, B.; Jamjod, S.; Pusadee, T. Productivity limiting impacts of boron deficiency, a review. Plant Soil 2020, 455, 2340. [Google Scholar] [CrossRef]
- Botelho, R.V.; Müller, M.M.L.; Umburanas, R.C.; Laconski, J.M.O.; Terra, M.M. Boron in fruit crops: Plant physiology, deficiency, toxicity, and sources for fertilization. In Boron in Plants and Agriculture: Exploring the Physiology of Boron and Its Impact on Plant Growth; Academic Press: Cambridge, MA, USA, 2022; pp. 29–50. [Google Scholar] [CrossRef]
- Cakmak, I.; Brown, P.; Colmenero-Flores, J.M.; Husted, S.; Kutman, B.Y.; Nikolic, M.; Zhao, F.J. Micronutrients. In Marschner’s Mineral Nutrition of Plants; Academic Press: London, UK, 2023; pp. 283–385. [Google Scholar] [CrossRef]
- Fang, K.; Zhang, W.; Xing, Y.; Zhang, Q.; Yang, L.; Cao, Q.; Qin, L. Boron toxicity causes multiple effects on Malus domestica pollen tube growth. Front. Plant Sci. 2016, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.F.; Du, B.S.; Zhang, Q.; Xing, Y.; Cao, Q.Q.; Qin, L. Boron deficiency alters cytosolic Ca2+ concentration and affects the cell wall components of pollen tubes in Malus domestica. Plant Biol. 2019, 21, 343–351. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, W.S.; Han, T.H. Effects of post-harvest foliar boron and calcium applications on subsequent season’s pollen germination and pollen tube growth of pear (Pyrus pyrifolia). Sci. Hortic. 2009, 122, 77–82. [Google Scholar] [CrossRef]
- Sarıdaş, M.A.; Karabıyık, Ş.; Eti, S.; Paydaş Kargı, S. Boron applications and bee pollinators increase strawberry yields. Int. J. Fruit Sci. 2021, 21, 481–491. [Google Scholar] [CrossRef]
- Mir, M.M.; Mir, M.; Iqbal, U.; Mushtaq, I.; Rehman, M.U.; Iqbal, R.; Mehdi, Z. The impact of pollination requirements in Sweet Cherry: A systematic review. J. Plant Growth Regul. 2025, 44, 3425–3443. [Google Scholar] [CrossRef]
- Boldingh, H.L.; Alcaraz, M.L.; Thorp, T.G.; Minchin, P.E.H.; Gould, N.; Hormaza, J.I. Carbohydrate and boron content of styles of ‘Hass’ avocado (Persea americana Mill.) flowers at anthesis can affect final fruit set. Sci. Hortic. 2016, 198, 125–131. [Google Scholar] [CrossRef]
- Davarpanah, S.; Tehranifar, A.; Davarynejad, G.; Abadía, J.; Khorasani, R. Effects of foliar applications of zinc and boron nano-fertilizers on pomegranate (Punica granatum cv. Ardestani) fruit yield and quality. Sci. Hortic. 2016, 210, 57–64. [Google Scholar] [CrossRef]
- De Silva, A.L.; Kämper, W.; Wallace, H.M.; Ogbourne, S.M.; Hosseini Bai, S.; Nichols, J.; Trueman, S.J. Boron effects on fruit set, yield, quality and paternity of macadamia. Agronomy 2022, 12, 684. [Google Scholar] [CrossRef]
- Michailidis, M.; Bazakos, C.; Kollaros, M.; Adamakis, I.D.S.; Ganopoulos, I.; Molassiotis, A.; Tanou, G. Boron stimulates fruit formation and reprograms developmental metabolism in sweet cherry. Physiol. Plant. 2023, 175, e13946. [Google Scholar] [CrossRef]
- Pavithra, G.; Alila, P.; Maiti, C.S.; Sarkar, A.; Sahu, A.K. Effect of different sources of boron on flowering and yield of mango cv. Amrapali. Pharma Innov. J. 2022, 11, 909–913. [Google Scholar]
- Abd El-Wahed, A.N.; Abd-Alrazik, A.M.; Khalifa, S.M. Effect of some nutrients on growth, yield and fruit quality of “Wonderful” cultivar pomegranate. Al-Azhar J. Agric. Res. 2021, 46, 1–15. [Google Scholar] [CrossRef]
- Abou Seeda, M.A.; Abou El-Nour, E.A.A.; Yassen, A.A.; Hammad, S.A. Boron, structure, functions and its interaction with nutrients in plant physiology. A review. Middle East J. Agric. Res. 2021, 10, 117–179. [Google Scholar] [CrossRef]
- Wang, N.; Yang, C.; Pan, Z.; Liu, Y.; Peng, S.A. Boron deficiency in woody plants: Various responses and tolerance mechanisms. Front. Plant Sci. 2015, 6, 916. [Google Scholar] [CrossRef] [PubMed]
- Voxeur, A.; Fry, S.C. Glycosylinositol phosphorylceramides from Rosacell cultures are boron-bridged in the plasma membrane and form complexes with rhamnogalacturonan II. Plant J. 2014, 79, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Thomidis, T.; Exadaktylou, E. Effect of boron on the development of brown rot (Monilinia laxa) on peaches. Crop Prot. 2010, 29, 572–576. [Google Scholar] [CrossRef]
- Muengkaew, R.; Whangchai, K.; Chaiprasart, P. Application of calcium–boron improve fruit quality, cell characteristics, and effective softening enzyme activity after harvest in mango fruit (Mangifera indica L.). Hortic. Environ. Biotechnol. 2018, 59, 537–546. [Google Scholar] [CrossRef]
- Parthiban, S.; Indirani, R.; Subbiah, A.; Saraswathy, S.; Nireshkumar, N. Effect of calcium, boron and micronutrient formulations on berry cracking in grapes var. Muscat Hamburg. Madras Agric. J. 2021, 108, 332–339. [Google Scholar] [CrossRef]
- Montecchiarini, M.L.; Silva-Sanzana, C.; Valderramo, L.; Alemano, S.; Gollán, A.; Rivadeneira, M.F.; Tripodi, K.E.J. Biochemical differences in the skin of two blueberries (Vaccinium corymbosum) varieties with contrasting firmness: Implication of ions, metabolites and cell wall related proteins in two developmental stages. Plant Physiol. Biochem. 2021, 162, 483–495. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, R.R.; Moretti, C.L.; Kumar, A.; Gupta, R.K. Foliar application of calcium and boron influences physiological disorders, fruit yield and quality of strawberry (F.× ananassa Duch.). Acta Hortic. 2009, 842, 835–838. [Google Scholar] [CrossRef]
- Meriño-Gergichevich, C.; Pacheco, E.; Reyes-Díaz, M. The effect of foliar boron spraying on the fruit features of Brigitta and Legacy highbush blueberry (Vaccinium corymbosum) cultivars. Cienc. Investig. Agrar. 2016, 43, 452–463. [Google Scholar] [CrossRef]
- Wojcik, P.; Wojcik, M.; Klamkowski, K. Response of apple trees to boron fertilization under conditions of low soil boron availability. Sci. Hortic. 2008, 116, 58–64. [Google Scholar] [CrossRef]
- Martínez, F.E.; Sarmiento, J.; Fischer, G.; Jiménez, F. Efecto de la deficiencia de N, P, K, Ca, Mg y B en componentes de producción y calidad de la uchuva (Physalis peruviana L.). Agron. Colomb. 2008, 26, 389–398. [Google Scholar]
- Yfran, M.D.L.M.; Chabbal, M.D.; Píccoli, A.B.; Giménez, L.I.; Rodríguez, V.A.; Martínez, G.C. Fertilización foliar con potasio, calcio y boro. Incidencia sobre la nutrición y calidad de frutos en mandarino Nova. Cultivos Trop. 2017, 38, 22–29. [Google Scholar]
- Xuan, H.; Streif, J.; Saquet, A.A.; Bangerth, F. Boron application affects respiration and energy status of ‘Conference’ pears during CA-storage. Acta Hortic. 2003, 628, 167–174. [Google Scholar] [CrossRef]
- Brackmann, A.; Thewes, F.R.; Anese, R.D.O.; Linke, W. Preharvest boron application and its relation with the quality of ‘Galaxy’ apples after harvest and controlled atmosphere storage. Ciênc. Rural 2016, 46, 585–589. [Google Scholar] [CrossRef]
- Abd El-Wahed, M.H.; Eissa, M.A.; Almasoudi, N.M.; Abo-Elyousr, K.A. Macronutrient-rich biochar induces boron nanoparticles in improving the salt tolerance of pomegranate (Punica granatum L.) in arid degraded soils. Sci. Hortic. 2023, 313, 111908. [Google Scholar] [CrossRef]
- Taghavi, T.; Siddiqui, R.; Rutto, L.K. The effect of preharvest factors on fruit and nutritional quality in strawberry. In Strawberry—Pre- and Post-Harvest Management Techniques for Higher Fruit Quality; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Xu, W.; Wang, P.; Yuan, L.; Chen, X.; Hu, X. Effects of application methods of boron on tomato growth, fruit quality and flavor. Horticulturae 2021, 7, 223. [Google Scholar] [CrossRef]
- Wojcik, P.; Wojcik, M. Effect of boron fertilization on sweet cherry tree yield and fruit quality. J. Plant Nutr. 2006, 29, 1755–1766. [Google Scholar] [CrossRef]
- Wei, C.; Ma, Z.; Liu, Y.; Qiao, J.; Sun, G. Effect of boron on fruit quality in pineapple. AIP Conf. Proc. 2018, 1956, 020006. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, L.; Hu, W.; Wang, Y.; Tao, J.; Jia, Y.; Miao, R.; Chen, L.-S.; Guo, J. Excessive boron fertilization-induced toxicity is related to boron transport in field-grown pomelo trees. Front. Plant Sci. 2024, 15, 1438664. [Google Scholar] [CrossRef] [PubMed]
- Sabino-López, J.E.; Sandoval-Villa, M.; Alcántar-González, G.; Ortiz-Solorio, C.; Vargas-Hernández, M.; Colinas-León, M.T. Potassium, pruning, and boron on yield and quality of Physalis peruviana L. fruit grown in greenhouse. Wilfenia 2015, 22, 259–272. [Google Scholar]
- Wimmer, M.; Goldbach, H. Boron in the apoplast of higher plants. In The Apoplast of Higher Plants: Compartment of Storage, Transport and Reactions; Springer: Dordrecht, The Netherlands, 2007; pp. 19–32. [Google Scholar] [CrossRef]
- Han, S.; Chen, L.S.; Jiang, H.X.; Smith, B.R.; Yang, L.T.; Xie, C.Y. Boron deficiency decreases growth and photosynthesis, and increases starch and hexoses in leaves of citrus seedlings. J. Plant Physiol. 2008, 165, 1331–1341. [Google Scholar] [CrossRef]
- Yang, L.T.; Pan, J.F.; Hu, N.J.; Chen, H.H.; Jiang, H.X.; Lu, Y.B.; Chen, L.S. Citrus physiological and molecular response to boron stresses. Plants 2021, 11, 40. [Google Scholar] [CrossRef]
- Seo, H.-J.; Sawant, S.S.; Song, J. Fruit cracking in pears: Its cause and management—A review. Agronomy 2022, 12, 2437. [Google Scholar] [CrossRef]
- Martínez, F.E.; Sarmiento, J.; Fischer, G.; Jiménez, F. Síntomas de deficiencia de macronutrientes y boro en plantas de uchuva (Physalis peruviana L.). Agron. Colomb. 2009, 27, 169–178. [Google Scholar]
- Cooman, A.; Torres, C.; Fischer, G. Determinación de las causas del rajado del fruto de uchuva (Physalis peruviana L.) bajo cubierta. II. Efecto de la oferta de calcio, boro y cobre. Agron. Colomb. 2005, 23, 74–82. [Google Scholar]
- Ali, M.M.; Anwar, R.; Rehman, R.N.U.; Ejaz, S.; Ali, S.; Yousef, A.F.; Chen, F. Sugar and acid profile of loquat (Eriobotrya japonica Lindl.), enzymes assay and expression profiling of their metabolism-related genes as influenced by exogenously applied boron. Front. Plant Sci. 2022, 13, 1039360. [Google Scholar] [CrossRef] [PubMed]
- Pagard, A.; Zare-Bavani, M.R.; Eftekhari, S.A. Effects of fertigation and foliar application of boron on fruit yield and several physiological traits of bell pepper. Int. J. Hortic. Sci. Technol. 2024, 11, 423–436. [Google Scholar] [CrossRef]
- Brown, P.H.; Bellaloui, N.; Wimmer, M.A.; Bassil, E.S.; Ruiz, J.; Hu, H.; Römheld, V. Boron in plant biology. Plant Biol. 2002, 4, 205–223. [Google Scholar] [CrossRef]
- Han, J.; Lee, J.Y.; Kwon, H.; Yeo, J.S. Synthesis of wafer-scale hexagonal boron nitride monolayers free of aminoborane nanoparticles by chemical vapor deposition. Nanotechnology 2014, 25, 145604. [Google Scholar] [CrossRef]
- Ferrol, N.; Belver, A.; Roldan, M.; Rodriguez-Rosales, M.P.; Donaire, J.P. Effects of boron on proton transport and membrane properties of sunflower (Helianthus annuus L.) cell microsomes. Plant Physiol. 1993, 103, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Bie, Z. Boron: Functions and approaches to enhance its availability in plants for sustainable agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Vinokurova, N.G.; Yusupova, A.I.; Morgunov, I.G. Succinic acid production from n-alkanes. Eng. Life Sci. 2012, 12, 560–566. [Google Scholar] [CrossRef]
- Dar, G.A. Impact of boron nutrition in fruit crops. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 4145–4155. [Google Scholar] [CrossRef]
- Gad, A.A.M.; Sirko, A. L-gulono-γ-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis. Curr. Issues Mol. Biol. 2024, 46, 11057–11074. [Google Scholar] [CrossRef]
- Ahmad, W.; Zia, M.H.; Malhi, S.S.; Niaz, A.; Ullah, S. Boron deficiency in soils and crops: A review. Crop Plant 2012, 2012, 65–97. [Google Scholar] [CrossRef]
- Hegazi, E.S.; El-Motaium, R.A.; Yehia, T.A.; Hashim, M.E. Effect of foliar boron application on boron, chlorophyll, phenol, sugars and hormones concentration of olive (Olea europaea L.) buds, leaves, and fruits. J. Plant Nutr. 2018, 41, 749–765. [Google Scholar] [CrossRef]
- Singh, A.; Shukla, A.K.; Meghwal, P.R. Fruit cracking in pomegranate: Extent, cause, and management—A review. Int. J. Fruit Sci. 2020, 20 (Suppl. 3), S1234–S1253. [Google Scholar] [CrossRef]
- Singh, A.; Burman, U.; Saxena, A.; Meghwal, P.R. Interactive effects of micronutrients, kaolin and mulching under drip irrigation system in managing fruit cracking of pomegranate (Punica granatum). Acta Hortic. 2019, 1254_32, 213–218. [Google Scholar] [CrossRef]
- Kumar, S.; Preet, R.; Choudhary, U. Physiological Disorders of Horticultural Crops; NIPA: New Delhi, India, 2023. [Google Scholar]
- Du, K.; Lin, H.; Luo, Q.; Li, T.; Wu, H.; Wang, B.; She, W. Calcium and boron foliar fertilizer to relieve cracking of ‘Liuyuezao’ pummelos. Foods 2025, 14, 595. [Google Scholar] [CrossRef]
- Zou, H.; Xiao, Q.; Li, G.; Wei, X.; Tian, X.; Zhu, L.; Li, M. Revisiting the advancements in plant polyphenol oxidases research. Sci. Hortic. 2025, 341, 113960. [Google Scholar] [CrossRef]
- Khan, M.; Ahmed, N. Sustainable Management of Mango Nutrition for Better Yield and Quality. 2020. Available online: https://repository.iuls.ro/handle/20.500.12811/1164 (accessed on 1 May 2025).
- Sharma, R.R.; Singh, R. The fruit pitting disorder—A physiological anomaly in mango (Mangifera indica L.) due to deficiency of calcium and boron. Sci. Hortic. 2009, 119, 388–391. [Google Scholar] [CrossRef]
- Bhat, Z.A.; Rather, T.R.; Rather, G.H.; Javeed, K.; Itoo, H.U.; Sheikh, K.A.; Nisar, S.; Hegazi, E.S.; El-Motaium, R.A.; Yehia, T.A.; et al. Effect of calcium and boron application on fruit set, yield and quality of apple cv. Ambri. Int. J. Curr. Microbiol. App. Sci. 2020, 11, 4038–4042. [Google Scholar]
- Shin, Y.; Chane, A.; Jung, M.; Lee, Y. Recent advances in understanding the roles of pectin as an active participant in plant signaling networks. Plants 2021, 10, 1712. [Google Scholar] [CrossRef] [PubMed]
- Bonomelli, C.; Arredondo, G.; Nario, A.; Artacho, P.; Contreras, C. Calcium allocation to the tree canopy and the edible part of sweet cherry fruit is hindered by boron soil deficiency. Agronomy 2025, 15, 691. [Google Scholar] [CrossRef]
- Gholamnejad, S.; Haghighi, M.; Etemadi, N.; Pessarakli, M. Effects of boron on nutrient partitioning, Ca movement, and fruit quality of tomatoes. J. Plant Nutr. 2023, 46, 697–713. [Google Scholar] [CrossRef]
- Tello, J.; Ibáñez, J. What do we know about grapevine bunch compactness? A state-of-the-art review. Aust. J. Grape Wine Res. 2018, 24, 6–23. [Google Scholar] [CrossRef]
- Alva, O.; Roa-Roco, R.N.; Pérez-Díaz, R.; Yáñez, M.; Tapia, J.; Moreno, Y.; Ruiz-Lara, S.; González, E. Pollen morphology and boron concentration in floral tissues as factors triggering natural and GA-induced parthenocarpic fruit development in grapevine. PLoS ONE 2015, 10, e0139503. [Google Scholar] [CrossRef]
- Tadayon, M.S.; Moafpourian, G. Effects of exogenous epi-brassinolid, zinc and boron foliar nutrition on fruit development and ripening of grape (Vitis vinifera L. clv. ‘Khalili’). Sci. Hortic. 2019, 244, 94–101. [Google Scholar] [CrossRef]
- Spinardi, A.; Bassi, D. Olive fertility as affected by cross-pollination and boron. Sci. World J. 2012, 2012, 375631. [Google Scholar] [CrossRef]
- Huang, C.; Hou, J.; Huang, M.; Hu, M.; Deng, L.; Zeng, K.; Yao, S. A comprehensive review of segment drying (vesicle granulation and collapse) in citrus fruit: Current state and future directions. Sci. Hortic. 2023, 309, 111683. [Google Scholar] [CrossRef]
- Li, Q.; Yao, S.; Deng, L.; Zeng, K. Changes in biochemical properties and pectin nanostructures of juice sacs during the granulation process of pomelo fruit (Citrus grandis). Food Chem. 2022, 376, 131876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cheng, C.; Lin, W.; Li, X.; Zhu, D.; Lisong, C.; Guo, J.; Li, Y. Analysis of boron nutrition status in soils and trees and its relationship with fruit granulation in ‘Guanximiyou’ pomelo. J. Fruit Sci. 2019, 36, 468–475. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).