Abstract
The Tuta absoluta species represents a significant threat to solanaceous crops globally and has developed resistance to conventional synthetic insecticides. This study investigated the insecticidal properties of essential oils (EOs) from Melaleuca alternifolia and Eucalyptus staigeriana against T. absoluta using the age-stage, two-sex life table methodology. Initially, the EOs of M. alternifolia and E. staigeriana were chemically characterized by gas chromatography (GC) techniques. In this analysis, we identified 19 compounds in M. alternifolia essential oil, with terpinen-4-ol, γ-terpinene, and α-terpinene as the predominant constituents. Eucalyptus staigeriana essential oil contained 25 identified compounds, predominantly limonene, terpinolene, geranial, and neral. Essential oils were dissolved in acetone and applied topically to larval stages. Both treatments significantly reduced pest longevity and adversely affected key demographic parameters. Melaleuca alternifolia treatment resulted in a substantial decrease in the intrinsic rate of population increase, indicating potential for population suppression. These findings support the potential application of M. alternifolia and E. staigeriana EOs as biological control agents against T. absoluta in integrated pest management programs.
1. Introduction
The South American tomato pinworm, Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae), is a highly invasive insect of Solanaceae crops and is recognized as one of the most significant pest to tomato (Solanum lycopersicum L.) production globally, having been reported in more than 90 countries [1,2]. In Asia, its occurrence has been confirmed in several regions, including South Korea, where its first detection was reported in Jeonbuk Province [3]. Due to its high fecundity, rapid generational turnover, and ability to complete several generations annually [4,5], infestations can result in substantial yield losses, ranging from 50% to total crop failure [6,7].
The global spread of T. absoluta in has led to increased reliance on synthetic insecticides [5]. However, the use of these synthetic insecticides has accelerated the development of resistance in T. absoluta populations to major insecticide classes including organophosphates, pyrethroids, and diamides [8,9,10,11]. Furthermore, the extensive use of synthetic insecticides contributes to ecological imbalances, environmental contamination, and the resurgence of secondary pests [12,13,14]. Considering the environmental contamination and health risks associated with conventional insecticides, there is a critical need to design, develop, and evaluate alternative pest management strategies that are both effective and ecologically sound [15,16,17].
Botanical insecticides, derived from plant materials such as dried and ground tissues, crude extracts, or secondary metabolites, have gained increasing attention as sustainable pest control options [17]. These products exert both lethal effects (e.g., mortality) and sublethal effects, and interfere with metabolic pathways in arthropods [17,18,19,20]. These insecticides are most often easily degradable and are considered environmentally friendly products [21,22]. In addition, the raw materials are available in large quantities and at low cost, and many plant derivatives have several mechanisms of action that are still unexplored [23]. Among the botanical insecticides, essential oils (EOs) have gained prominence in biopesticides due to their bioactivity, which refers to their capacity to interfere with the behavior, development, reproduction, or survival of insect pests [17].
The use of plant EOs represents a promising strategy within integrated pest management (IPM) programs, as they are rich in bioactive compounds and exhibit insecticidal, repellent, and oviposition-deterring properties [24,25,26]. In addition to their efficacy, EOs generally pose low environmental risks due to their volatility, rapid degradation, and minimal soil persistence, reducing the likelihood of resistance development in pest populations due to their multiple modes of action [25]. These oils are typically complex mixtures of terpenoids, whose biological effects often result from synergistic or additive interactions among constituents [26]. In T. absoluta, various EOs have been shown to induce adult and larval mortality [27], exhibit repellency [28], and inhibit oviposition behavior [29]. Despite these benefits, EOs derived from Myrtaceae species remain underexplored in the management of the South American tomato pinworm [15].
Recent studies have confirmed the fumigant toxicity and behavioral effects of selected plant EOs on T. absoluta while also highlighting their relative safety to beneficial predators such as Macrolophus pygmaeus (Rambor, 1839) (Hemiptera: Miridae) [30,31]. The EOs of Myrtaceae plants are volatile secondary metabolites composed predominantly of terpenes and aromatic compounds [32]. These plants are gaining increasing interest in IPM programs due to their ovicidal, larvicidal, and adulticidal action, repellent activity, and fumigant and contact toxicity [33].
The Eucalyptus staigeriana F. Muell. ex Bailey and Melaleuca alternifolia Cheel are among the species of the family Myrtaceae with insecticidal activity. The EOs from the leaves of plants of the genus Eucalyptus are commercially used in the pharmaceutical, cosmetic, and food industries [34]. The insecticidal activity of E. staigeriana EO has been documented for different insect orders. For example, in insects of the order Coleoptera, a harmful effect was observed on the oviposition and emergence of Zabrotes subfasciatus (Both, 1833) (Bruchidae) and Calosobruchus maculatus (Fabricius, 1775) (Chrysomelidae) [35] as well as repellency to C. maculatus [36] and Sitophilus zeamais (Mots, 1885) (Curculionidae) [37]. In addition, the survival of Lutzomyia longipalpis (Lutz and Neiva, 1912) (Diptera: Psychodidae) [38] and the reproductive parameters of Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae) were affected [39].
On the other hand, EOs obtained from the stems and leaves of M. alternifolia, which are rich in terpenes, have natural pharmacological properties [40]. In addition, in insect pests, these EOs have an inhibitory effect on feeding [41,42], a lethal effect, and on behavior [43] as well as potential in the control of stored grain pests due to their fumigant action [41].
Although essential oils from the Myrtaceae family have shown insecticidal potential against various pest species, their effects on T. absoluta, particularly in terms of both lethal and sublethal impacts, remain poorly understood. To our knowledge, no previous studies have systematically assessed the demographic consequences of exposure to the EOs of M. alternifolia and E. staigeriana on this pest. Understanding these effects is essential to determine their practical viability within integrated pest management strategies.
In this study, we assessed the insecticidal potential of E. staigeriana and M. alternifolia EOs against T. absoluta. Initially, both oils were chemically characterized to determine their main constituents. Then, their effect was inferred from the dose–response curves and life history parameters of T. absoluta. The results offer valuable insights into the lethal and sublethal effects of these EOs on T. absoluta, contributing to the understanding of their effects on pest population dynamics. This study provides relevant information on the applicability of the tested EOs to control a pest of global importance.
2. Materials and Methods
2.1. Biological Material and Experimental Conditions
Rearing was initiated with T. absoluta eggs and larvae collected from tomato plants at UFLA and Agroteste LTDA (21°12′ S, 45°03′ W), both with no history of insecticide application. After collection, the insects were transferred to acrylic cages (60 × 30 × 30 cm) containing tomato (S. lycopersicum cv. Santa Clara) branches for feeding.
After emergence, approximately 500 adults were transferred to a new cage containing tomato shoots that served as oviposition substrates. The adults were offered an aqueous solution of honey (1:1) soaked in moistened cotton wool as a food source. The branches containing the eggs were removed every four days and placed in acrylic cages for maintenance in the laboratory, aiming to provide insects for the bioassays. From the second generation onward, second-instar and same-generation caterpillars were used in the bioassays. The rearing of T. absoluta and the bioassays were conducted under controlled temperature (25 ± 2 °C), relative humidity (70 ± 10%), and photophase (12 h) conditions.
2.2. Obtention and Chemical Characterization of EOs
The EOs of M. alternifolia and E. staigeriana were purchased from Company Ferquima Indústria e Comércio Ltda., Vargem Grande Paulista, São Paulo-Brazil. The chromatographic conditions for analyzing the EOs of M. alternifolia and E. staigeriana followed previously established protocols [44]. All analyses were performed in triplicate. The analyte concentrations were expressed as the mean relative area percentage of the chromatographic peaks ± standard deviation (n = 3).
2.3. Bioassays with T. absoluta
2.3.1. Acute Toxicity of EOs in a Topical Application Trial
The EOs of M. alternifolia and E. staigeriana were previously diluted in acetone (µg of OE/µL of acetone) and applied topically to the dorsum of second-instar T. absoluta caterpillars at a dose of 120 µL of EO/caterpillar. Each caterpillar received 1 µL of the solution using a microsyringe (Hamilton® 25 µL). Untreated control insects were treated with acetone alone. A completely randomized design was used, with 60 caterpillars per treatment, each replicate consisting of a single treated caterpillar placed in a 5 cm diameter Petri dish containing a leaflet of tomato plant S. lycopersicum cv. Santa Clara to feed the insects. A piece of filter paper moistened with distilled water was placed under the leaflet to maintain its turgidity. The experiment was repeated twice on different days, totaling 120 replicates per treatment. The mortality evaluations of the caterpillars were performed at 6, 12, 24, 36, 48, and 72 h after the application of the treatments using a stereoscopic microscope (40×). A dead caterpillar was considered one that did not respond to touch with a soft-bristled brush, remaining still.
2.3.2. Dose–Response and Time–Response Bioassays of EOs
The solution of EO from M. alternifolia was applied to T. absoluta caterpillars at doses of 30, 40, 55, 74, and 100 µL of EO/caterpillar, and E. staigeriana EO was applied at doses of 50, 62, 78, 97, and 120 µL of EO/caterpillar. These doses were determined by means of previous tests and by arithmetic progression, aiming to obtain average percentages of mortality between 20 and 90% [45]. The negative control treatment was acetone. All treatments were applied topically to the dorsal region of the second-instar T. absoluta caterpillars as described in subitem 2.3.1. A completely randomized design was used, with 60 replicates per treatment, each represented by a treated caterpillar kept in a Petri dish containing one tomato leaflet. The assays were repeated twice.
2.3.3. Life History Table and Demographic Parameters of T. absoluta Caterpillar Treated with the DL50 of EOs of M. alternifolia and E. staigeriana
For the life table bioassay, approximately 200 adult couples of T. absoluta 72 h after emergence were kept in acrylic cages (60 × 30 × 30 cm) containing tomato plants cv. Santa Clara (15 cm tall) for 48 h to oviposition. The plants were observed daily to verify the appearance of second-instar caterpillars, which were then removed with the aid of a brush and subjected to the application of the treatments. The EOs were used at doses equivalent to the previously estimated LD50 (lethal dose capable of killing 50% of the insect population) (subitem 2.3.2). The treatments consisted of acetone (negative control), M. alternifolia (76.5 µL of EO/caterpillar), and E. staigeriana (78.5 µL of EO/caterpillar), which were applied to the dorsum of the caterpillars with the aid of a microsyringe (subitem 2.3.2). Then, the caterpillars were individualized in Petri dishes (2 cm high × 5 cm in diameter) and fed every 72 h with leaflets of tomato plants that contained their petioles wrapped in cotton moistened with distilled water and inserted into a microtube. All second-instar caterpillars were individually placed in Petri dishes sealed with a perforated PVC film to allow gas exchange and moisture stabilization. The experimental design used was completely randomized, with 3 treatments and 100 replicates, each consisting of a Petri dish with a treated second-instar caterpillar. The duration of the instars, larval and pupal survival, and duration of the larval and pupal stages of the insects were evaluated daily after the topical application of the EOs.
To evaluate the effects of the oils on the reproduction and longevity of adults from the treated caterpillars, couples were formed with newly emerged insects (48 h of age). Each pair was placed in a Petri dish (2 cm high × 15 cm diameter) covered with PVC plastic film with small holes made with an entomological pin to prevent the escape of insects and enable gas exchange. Each Petri dish contained a piece of cotton soaked in a 1:1 aqueous solution of a tomato plant (five 5 leaflets) with its stem wrapped in moistened cotton and placed in a microtube, which served as the substrate for oviposition. The oviposition period, survival, and longevity of the males and females were recorded. In addition, the number of eggs was recorded daily under a stereoscopic microscope to determine fecundity.
2.4. Statistical Analyses
Data on insect survival over time were analyzed using the nonparametric Kaplan–Meier estimator and subjected to the log-rank test using the survival package [45]. The survival curves were compared using the pairwise multiple comparison test. The median lethal time (LT50) (i.e., the time needed to cause 50% mortality in the population) was also estimated for each treatment. To determine the median lethal dose (LD50), the data were subjected to logit analysis using the drc package (log-logistic) [46]. These analyses were performed using the statistical program R® [47].
The processing of the data analysis for the preparation of the life tables was performed using the TWOSEX-MSChart program [48]. The means, variances, and standard errors of the parameters were compared in pairs between treatments by the bootstrap method with 100,000 replicates [48]. The life table considers the means of survival parameters, life expectancy, and fertility until age (x) and stage (j) are reached. Differences between treatments were analyzed using the paired bootstrap test with a significance level of 5%.
3. Results
3.1. Chemical Characterization of EOs
The chemical analysis of the EO of E. staigeriana indicated the presence of 25 compounds, and the main compounds were limonene, terpinolene, neral, and geranial, with amounts varying from 8.919% to 27.298%. The EO of M. alternifolia presented 19 chemical compounds; 4-terpineol, γ-terpinene, and α-terpinene were the major compounds with areas of 42.23%, 22.10%, and 10.44%, respectively (Table 1).

Table 1.
Chemical composition of the essential oils of Eucalyptus staigeriana and Melaleuca alternifolia.
3.2. Bioassays with T. absoluta
3.2.1. Acute Toxicity of EOs in a Topical Application Trial
The EOs of M. alternifolia and E. staigeriana caused 100% mortality of the T. absoluta caterpillars at a dose of 120 µL of EO/caterpillar 36 h after application (χ2 = 183; df = 2; p ≤ 0.001; Figure 1). Notably, after 6 h, both EOs caused mortality in 50% of the insects.
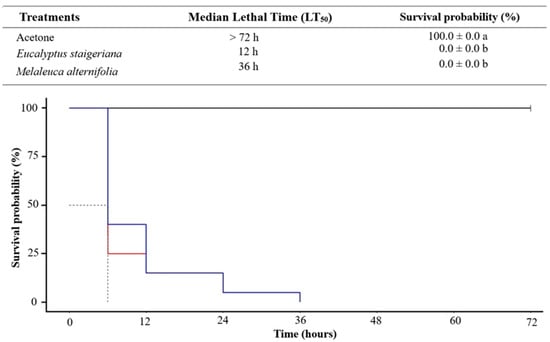
Figure 1.
Mortality of Tuta absoluta caterpillars, over time, treated topically with the essential oils of Eucalyptus staigeriana (red line) and Melaleuca alternifolia (blue line) at a dose of 120 µL of essential oil/caterpillar. Means in the same line followed by different letters differ from each other (p < 0.05).
3.2.2. Determination of Dose–Response and Time–Response Curves of EOs
After the application of the EOs on second-instar T. absoluta caterpillars, the LD50 and LD90 values of M. alternifolia and E. staigeriana EOs were estimated (Table 2).

Table 2.
Dose–response of Melaleuca alternifolia and Eucalyptus staigeriana for Tuta absoluta.
An increase in the mortality of T. absoluta caterpillars was observed with the increase in the tested doses of E. staigeriana EOs (χ2 = 242; df = 5; p < 0.001 and M. alternifolia (χ2 = 223; df = 5; p < 0.001). The lowest dose needed to reduce the probability of survival was 50 µL for E. staigeriana EO and 40 µL M. alternifolia EO. For the E. staigeriana EO, the dose of 97 µL of EO/caterpillar caused a probability of survival of 56.7% at the end of the evaluation period, while for M. alternifolia EO, the dose of 100 µL of EO/caterpillar was enough to cause a probability of survival of 6.67% (Figure 2).
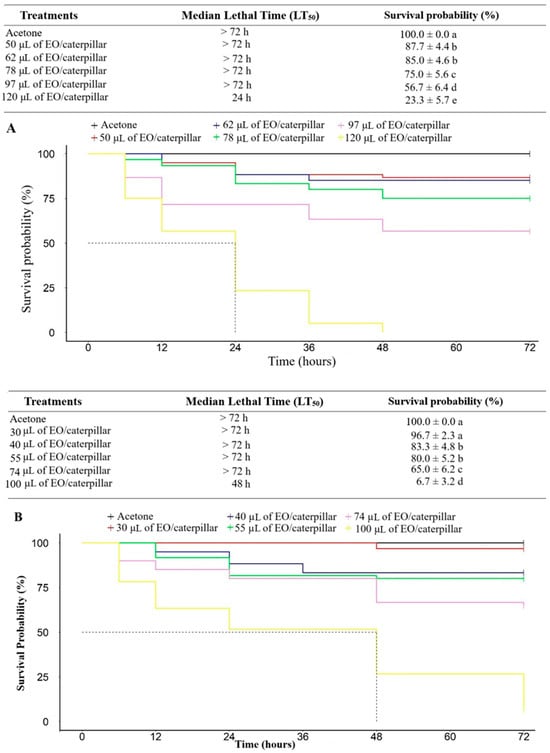
Figure 2.
Survival analysis, over time, of Tuta absoluta caterpillars that were topically treated with different doses of the essential oils of (A) Eucalyptus staigeriana, and (B) Melaleuca alternifolia. EO: essential oil. Means in the same line followed by different letters differ from each other (p < 0.05).
3.2.3. Life History Table and Demographic Parameters of T. absoluta Caterpillar Treated with the DL50 of EOs of M. alternifolia and E. staigeriana
It was observed that M. alternifolia EO reduced the development time of the third- and fourth-instars when compared with the other treatments and increased the duration of the pupal stage compared with the acetone control. The EOs of E. staigeriana and M. alternifolia reduced the longevity of adults. The EO of M. alternifolia decreased the life cycle of females, while that of E. staigeriana prolonged this biological parameter (Table 3).

Table 3.
Mean (±SE) of the development time of the life stages and longevity (days) of Tuta absoluta subjected to treatments with sublethal doses of essential oils of Melaleuca alternifolia (76.5 µL of essential oil/caterpillar) and Eucalyptus staigeriana (78.5 µL of essential oil/caterpillar).
Except for the oviposition period that increased in the treatment with E. staigeriana EO, the reproductive parameters of T. absoluta were not affected, whose second-instar caterpillars were treated with the EOs of M. alternifolia and E. staigeriana, as there was no difference compared with the treatment with acetone (Table 4).

Table 4.
Reproductive parameters of Tuta absoluta adults derived from second-instar caterpillars that survived exposure to Melaleuca alternifolia (76.5 µL of essential oil/caterpillar) and Eucalyptus staigeriana (78.5 µL of essential oil/caterpillar).
The age-specific survival rate (sxj) was affected by the treatments. Both EOs prolonged the fourth-instar and the pupal stage and reduced adult survival (males and females) (Figure 3).
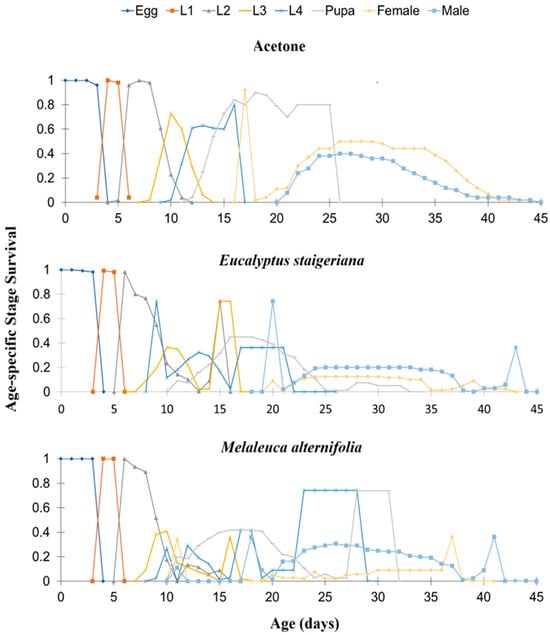
Figure 3.
Age-specific survival rate (sxj) of Tuta absoluta in the acetone treatments, Melaleuca alternifolia (76.5 µL of essential oil/caterpillar), and Eucalyptus staigeriana (78.5 µL of essential oil/caterpillar). L1 = first-instar caterpillar, L2 = second-instar caterpillar, L3 = third-instar, and L4 = fourth-instar caterpillar.
It was observed that life expectancy by age (exj) decreased consistently with time in the treatment with acetone. However, there were fluctuations throughout the cycle in the other treatments (Figure 4).
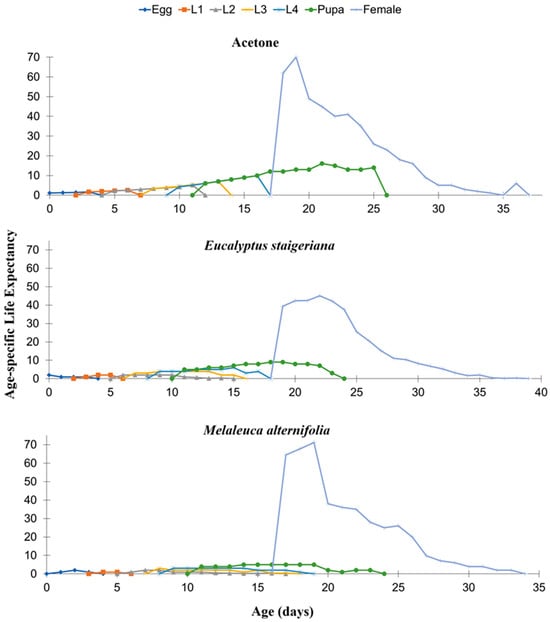
Figure 4.
Age-specific life expectancy (exj) of Tuta absoluta in the acetone, Melaleuca alternifolia (76.5 µL of essential oil/caterpillar), and Eucalyptus staigeriana (78.5 µL of essential oil/caterpillar) treatments. L1 = first-instar caterpillar, L2 = second-instar caterpillar, L3 = third-instar, and L4 = fourth-instar caterpillar.
The age-specific survival rate (lx) decreased from the 6th to 9th day of life of the insects in the treatments with EOs. However, in the treatment with acetone, this decrease was observed later, on the 28th day of the life cycle. There was a delay and a lower age-specific fecundity peak (fxj) in the treatment with E. staigeriana EO, with approximately 15 eggs on the 24th day, while in the other treatments, the fecundity peak occurred on the 19th day of the gestation cycle, with approximately 30 eggs (Figure 5).
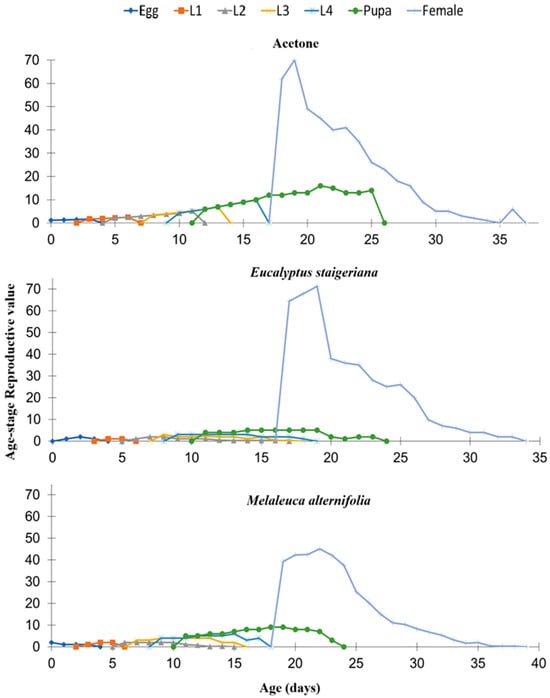
Figure 5.
Age-specific survival rate (lx), age-specific fertility, and stage of development (fx), age-specific fertility (mx) and age-specific maternity (lxmx) of Tuta absoluta in the acetone, Melaleuca alternifolia (76.5 µL of essential oil/caterpillar), and Eucalyptus staigeriana (78.5 µL of essential oil/caterpillar) treatments.
When analyzing the reproductive value by specific age (vxj), it was found that in the treatment with the EO of M. alternifolia, the females reached a lower peak, with approximately 40 eggs, while in the other treatments, the values reached approximately 70 eggs (Figure 6).
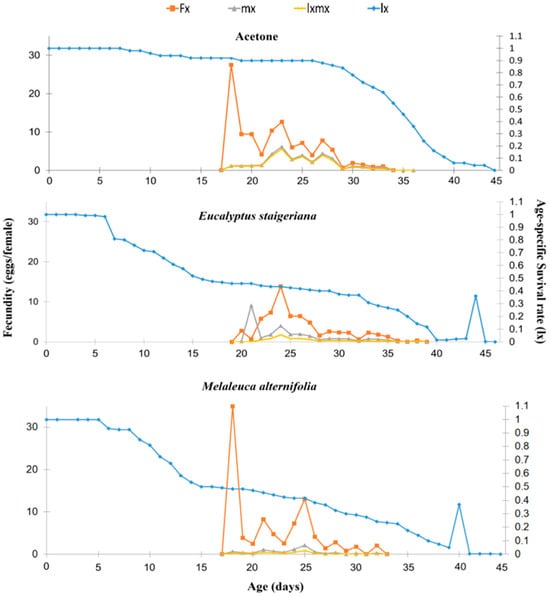
Figure 6.
Reproductive value by age (vxd) of Tuta absoluta in the acetone, Eucalyptus staigeriana (78.5 µL of essential oil/caterpillar), and Melaleuca alternifolia (76.5 µL of essential oil/caterpillar) treatments. L1 = first-instar caterpillar, L2 = second-instar caterpillar, L3 = third-instar, and L4 = fourth-instar caterpillar.
The intrinsic rate of increase (r) was reduced by 50% when the insects were treated with M. alternifolia EO. Both EOs caused lower values of the finite rate of increase (λ) and net reproductive rate (R0). Regarding the crude reproductive rate (GRR), only the treatment with M. alternifolia EO differed from that with acetone (Table 5).

Table 5.
Demographic parameters of Tuta absoluta treated with the EO of Melaleuca alternifolia (76.5 µL of essential oil/caterpillar) and Eucalyptus staigeriana (78.5 µL of essential oil/caterpillar).
4. Discussion
The EOs of aromatic plants and their main constituents are widely regarded as promising alternatives to conventional pesticides for the control of various arthropod pests [15,16]. The EOs of M. alternifolia and E. staigeriana have demonstrated insecticidal activity against important insect pests such as Lucilia cuprina (Wiedemann, 1830) (Diptera: Calliphoridae), C. maculatus, Paropsisterna tigrina (Olivier, 1807) (Coleoptera: Chrysomelidae) and Faex sp. (Coleoptera: Chrysomelidae), S. frugiperda, and S. zeamais [36,37,39,49,50]. In our study, both EOs caused 100% mortality of T. absoluta caterpillars under laboratory conditions, confirming their strong insecticidal potential against this pest. To the best of our knowledge, this is the first report of both lethal and sublethal effects of these specific EOs on T. absoluta. While other studies have reported the toxicity of Myrtaceae derived oils against T. absoluta [30,31], our results reinforce the potential of M. alternifolia and E. staigeriana EOs as candidate compounds for pest control strategies targeting this species.
The estimated LD50 for the EOs of M. alternifolia and E. staigeriana for T. absoluta was 76.5 µL of essential oil/caterpillar and 78.5 µL of essential oil/caterpillar, higher than the LD50 of 50.28 µL of essential oil/caterpillar of M. alternifolia EO for Helicoverpa armigera (Hübner, 1805) (Lepidoptera: Noctuidae), which also caused feeding deterrence [41]. Although high mortality is often sought in pest control, it should not be the sole objective of botanical insecticide use [17,18,19], as achieving such effects generally requires elevated doses and significant biomass. Thus, sublethal effects on life cycle, oviposition, reproduction, and demographic parameters are also highly desirable in integrated pest management (IPM) strategies.
The chemical composition of natural products plays a crucial role in their bioactivity [17,51]. In this study, GC/MS analysis identified terpinene-4-ol (42.23%) as the predominant compound in M. alternifolia EO. This compound has been found in several aromatic plant EOs [52,53,54,55], and its profile in this study was like that reported in the previous literature [39,41] with slight variations in γ-terpinene and α-terpinene contents. The EO of E. staigeriana also showed a composition consistent with prior studies [34], though natural variation was observed in the limonene, neral, and geranial concentrations, which is expected due to factors such as geographic origin, climate, soil, and extraction methods [56,57,58]. Essential oils may act through different physiological pathways. For instance, M. alternifolia EO has been shown to inhibit the enzymes acetylcholinesterase (AChE) and glutathione-S-transferase (GST) in H. armigera [41], while terpinene-4-ol inhibited Na+/K+-ATPase activity in Musca domestica (Linnaeus, 1758) (Diptera: Muscidae) [59] and affected multiple enzymes in Plutella xylostella (Linnaeus, 1758) (Lepidoptera: Plutellidae) [60]. Among the components of E. staigeriana, limonene, is known for its contact, fumigant, and ingestion toxicity, often associated with AChE inhibition, leading to neural overstimulation and death [61,62,63]. The physiological consequences of this disruption include ataxia and neuromuscular failure [64,65]. In addition, Na+/K+-ATPase, an essential ion pump involved in the maintenance of electrochemical gradients in nerve cells, has also been identified as a molecular target for botanical insecticides due to its central role in neuronal signaling and homeostasis [65,66].
In S. frugiperda, limonene caused hyperexcitation, paralysis, and mortality when applied topically, in addition to negative effects on nutrient levels and reproductive physiology [41]. Similarly, in our study, exposure to the LD50 of both EOs reduced the intrinsic rate of increase (r), finite rate of increase (λ), and net reproductive rate (R0) of T. absoluta, indicating a strong population-level impact. Life table analyses incorporating age and developmental stage provide a more comprehensive view of population dynamics than mortality alone [48,67,68].
The results showed differential impacts between EOs, which may be related to their distinct chemical compositions. The EO of M. alternifolia, rich in terpinen-4-ol, γ-terpinene, and α-terpinene, led to more pronounced reductions in r, female longevity, and reproductive value (vxj) and affected the duration of larval and pupal stages. These effects may possibly be associated with the biological activity of terpinen-4-ol [41,69,70,71] and its capacity to modulate oxidative stress [72,73]. However, we acknowledge that this interpretation is hypothetical and requires mechanistic confirmation in future studies.
Conversely, the EO of E. staigeriana, containing limonene, terpineole, neral, and geranial, produced more moderate effects on reproductive parameters. Notably, it increased the oviposition period and delayed the peak of fecundity (fxj), possibly reflecting the antifeedant effects known for monoterpenes like neral and geranial [74]. The prolongation of the oviposition period with E. staigeriana EO may indicate a compensatory mechanism of the pest against the effects of neral and geranial compounds, known for their anti-feeding activity. This adaptive response aims to maximize egg production despite the limitations imposed by the treatment. This suggests a more moderate interference in the physiological systems of T. absoluta, unlike the more intense lethal effect of M. alternifolia EO. Thus, the different chemical profiles of EOs can cause distinct biological responses, which is important for integrated pest management. Again, while these associations are plausible, they remain speculative and further biochemical and physiological studies are necessary to confirm these mechanisms.
Moreover, both EOs altered the age-specific survival patterns (sxj and lx), causing earlier and more severe declines than the control. The EO of M. alternifolia was particularly impactful, reinforcing its potential for population suppression under controlled conditions. The results obtained demonstrate the insecticidal potential of M. alternifolia and E. staigeriana EOs against T. absoluta under controlled laboratory conditions, with consistent lethal and sublethal effects. Among the available exposure methods for insecticide bioassays, including fumigation, ingestion, spraying, and topical application, topical application is widely used in preliminary toxicological assessments, particularly when the intrinsic toxicity of a substance is unknown [19,20,24,25,27,28,29,30,71]. This method ensures direct, standardized exposure and reduces variability related to environmental degradation, plant surface interactions, or insect behavior. Its use is well-established in studies involving T. absoluta and other lepidopterans [15,16,24,27,71], supporting its validity for initial screening.
However, given the limitations of topical application in simulating field conditions, further studies under semi-controlled and field environments are recommended. These should employ more representative exposure routes such as foliar spraying or residual contact to assess the practical efficacy and ecological relevance of these EOs in integrated pest management strategies. Furthermore, these studies should investigate the potential of these EOs to affect egg hatchability, a key parameter for assessing the long-term suppression of this insect. Such research would help determine whether EO exposure impairs fertility and offspring viability, contributing to a more complete understanding of their potential in population suppression strategies.
5. Conclusions
This study demonstrated that the EOs of M. alternifolia and E. staigeriana exhibit both lethal and sublethal effects on T. absoluta under controlled laboratory conditions. The distinct chemical compositions of these oils led to different biological responses, with M. alternifolia causing stronger disruptions to population growth parameters. These findings contribute to the understanding of Myrtaceae derived EOs as promising candidates for the management of this pest. While the topical application method used here enabled a standardized comparison of intrinsic toxicity, it does not replicate field conditions. Therefore, future studies should explore new exposure routes, such as foliar immersion, semi-field, and field trials, to evaluate the efficacy, persistence, and potential integration of these EOs into sustainable management programs for T. absoluta.
Author Contributions
Conceptualization, B.C.F.B., G.A.C. and D.S.A.; Methodology, B.C.F.B., A.F.L., K.G.F., J.A.C.O., S.K.V.B., G.A.C. and D.S.A.; Software, B.C.F.B. and K.G.F.; Validation, S.K.V.B., G.A.C. and D.S.A.; Investigation, B.C.F.B., A.F.L., K.G.F., J.A.C.O. and V.C.C.; Data curation, B.C.F.B., A.F.L., K.G.F., J.A.C.O. and D.S.A.; Writing—preparation of original draft, B.C.F.B., J.A.C.O., G.A.C. and D.S.A.; Writing—review and editing, B.C.F.B., J.A.C.O., G.A.C. and D.S.A.; Supervision, G.A.C. and D.S.A.; Project administration, G.A.C. and D.S.A.; Acquisition financing, G.A.C. and D.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
The authors thank the National Council for Scientific and Technological Development (CNPq—grant 408121/2021-1), Minas Gerais State Foundation (FAPEMIG), and the Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil for financial support and fellowship.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| EOs | Essential oils |
| EO | Essential oil |
References
- EPPO. Tuta absoluta (GNORAB). Available online: https://gd.eppo.int/taxon/GNORAB/distribution (accessed on 15 May 2023).
- Simoglou, K.Β.; Stavrakaki, M.; Alipranti, K.; Mylona, K.; Roditakis, E. Understanding Greenhouse Tomato (Solanum lycopersicum L.) Growers’ Perceptions for Optimal Phthorimaea absoluta (Meyrick) Management—A Survey in Greece. Agriculture 2024, 14, 2291. [Google Scholar] [CrossRef]
- Lee, M.H.; Jeong, D.; Lee, G.-S.; Paik, C. First report of Phthorimaea absoluta (Lepidoptera: Gelechiidae) in Korea. J. Integr. Pest Manag. 2024, 15, 36. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Picanço, M.C. The tomato borer Tuta absoluta in South America: Pest status, management and insecticide resistance. EPPO Bull. 2012, 42, 211–216. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.H.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Biratu, W. Review on the effect of climate change on tomato (Solanum lycopersicon) production in Africa and mitigation strategies. J. Nat. Sci. Res. 2018, 8, 62–70. [Google Scholar]
- Mahlangu, L.; Sibisi, P.; Nofemela, R.S.; Ngmenzuma, T.; Ntushelo, K. The differential effects of Tuta absoluta infestations on the physiological processes and growth of tomato, potato, and eggplant. Insects 2022, 13, 754. [Google Scholar] [CrossRef]
- Haddi, K.; Berger, M.; Bielza, P.; Rapisarda, C.; Williamson, M.S.; Moores, G.; Bass, C. Mutation in the ace-1 gene of the tomato leaf miner (Tuta absoluta) associated with organophosphates resistance. J. Appl. Entomol. 2017, 141, 612–619. [Google Scholar] [CrossRef]
- Haddi, K.; Berger, M.; Bielza, P.; Cifuentes, D.; Field, L.M.; Gorman, K.; Rapisarda, C.; Williamson, M.S.; Bass, C. Identification of mutations associated with pyrethroid resistance in the voltage-gated sodium channel of the tomato leaf miner (Tuta absoluta). Insect Biochem. Mol. Biol 2012, 42, 506–513. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Roditakis, E.; Campos, M.R.; Haddi, K.; Bielza, P.; Siqueira, H.A.A.; Tsagkarakou, A.; Vontas, J.; Nauen, R. Insecticide resistance in the tomato pinworm Tuta absoluta: Patterns, spread, mechanisms, management and outlook. J. Pest Sci. 2019, 92, 1329–1342. [Google Scholar] [CrossRef]
- Silva, G.A.; Picanço, M.C.; Bacci, L.; Crespo, A.L.B.; Rosado, J.F.; Guedes, R.N.C. Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag. Sci. 2011, 67, 913–920. [Google Scholar] [CrossRef]
- Biondi, A.; Zappalà, L.; Stark, J.D.; Desneux, N. Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 2013, 8, e76548. [Google Scholar] [CrossRef]
- Roditakis, E.; Skarmoutsou, C.; Staurakaki, M. Toxicity of insecticides to populations of tomato borer Tuta absoluta (Meyrick) from Greece. Pest Manag. Sci. 2013, 69, 834–840. [Google Scholar] [CrossRef]
- Sola, P.; Mvumi, B.M.; Ogendo, J.O.; Mponda, O.; Kamanula, J.F.; Nyirenda, S.P.; Belmain, S.R.; Stevenson, P.C. Botanical pesticide production, trade and regulatory mechanisms in Sub-Saharan Africa: Making a case for plant-based pesticidal products. Food Secur. 2014, 6, 369–384. [Google Scholar] [CrossRef]
- Soares, M.A.; Campos, M.R.; Passos, L.C.; Carvalho, G.A.; Haro, M.M.; Lavoir, A.V.; Biondi, A.; Zappalà, L.; Desneux, N. Botanical insecticide and natural enemies: A potential combination for pest management against Tuta absoluta. J. Pest Sci. 2019, 92, 1433–1443. [Google Scholar] [CrossRef]
- Salazar, A.M.; Arismendi, N.; López, M.D.; Vargas, M.; Schoebitz, M.; Palacio, D.A.; Becerra, J.; Cedeño, B.; Zapata, N. Stability of the oil-based nanoemulsion of Laureliopsis philippiana (Looser) and its insecticidal activity against tomato borer (Tuta absoluta Meyrick). Ind. Crops Prod. 2022, 188, 115635. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century-fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Tak, J.H.; Isman, M.B. Metabolism of citral, the major constituent of lemongrass oil, in the cabbage looper, Trichoplusia ni, and effects of enzyme inhibitors on toxicity and metabolism. Pestic. Biochem. Physiol. 2016, 133, 20–25. [Google Scholar] [CrossRef]
- Lima, A.F.; do Prado, R.L.; Gonçalves, G.L.P.; Maimone, N.M.; Gissi, D.S.; de Lira, S.P.; Vendramim, J.D. Searching for bioactive compounds from solanaceae: Lethal and sublethal toxicity to Spodoptera frugiperda and untargeted metabolomics approaches. J. Pest Sci. 2022, 95, 1317–1329. [Google Scholar] [CrossRef]
- Lima, A.F.; Ribeiro, L.P.; Lira, S.P.; Carvalho, G.A.; Vendramim, J.D. Growth inhibitory activities and feeding deterrence of solanaceae-based derivatives on fall armyworm. Agriculture 2023, 13, 420. [Google Scholar] [CrossRef]
- Mossa, A.-T.H. Green pesticides: Essential oils as biopesticides in insect-pest management. J. Environ. Sci. Technol. 2016, 9, 354–378. [Google Scholar] [CrossRef]
- Nollet, L.M.; Rathore, H.S. Green Pesticides Handbook: Essential Oils for Pest Contro; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and Prospects of Botanical Biopesticides in Europe and Mediterranean Countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Bibiano, C.S.; Alves, D.S.; Freire, B.C.; Bertolucci, S.K.V.; Carvalho, G.A. Toxicity of essential oils and pure compounds of lamiaceae species against Spodoptera frugiperda (Lepidoptera: Noctuidae) and their safety for the nontarget organism Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Crop Prot. 2022, 158, 106011. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Kedia, A.; Das, S.; Dubey, N.K. Essential oils and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: Prospects and retrospects. Environ. Sci. Pollut. Res. 2021, 15, 18918–18940. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.-H.; Isman, M.B. Enhanced cuticular penetration as the mechanism for synergy of insecticidal constituents of rosemary essential oil in Trichoplusia ni. Sci. Rep. 2015, 5, 12690. [Google Scholar] [CrossRef]
- Ngongang, M.D.T.; Eke, P.; Sameza, M.L.; Mback, M.N.L.N.; Lordon, C.D.; Boyom, F.F. Chemical constituents of essential oils from Thymus vulgaris and Cymbopogon citratus and their insecticidal potential against the tomato borer, Tuta absoluta (Lepidoptera: Gelechiidae). Int. J. Trop. Insect Sci. 2022, 42, 31–43. Available online: https://link.springer.com/article/10.1007/s42690-021-00514-7#citeas (accessed on 10 July 2025). [CrossRef]
- Essoung, F.R.E.; Tadjong, A.T.; Chhabra, S.C.; Mohamed, S.A.; Hassanali, A. Repellence and fumigant toxicity of essential oils of Ocimum gratissimum and Ocimum kilimandscharicum on Tuta absoluta (Lepidoptera: Gelechiidae). Environ. Sci. Pollut. Res. 2020, 27, 37963–37976. [Google Scholar] [CrossRef]
- Yarou, B.B.; Bawin, T.; Boullis, A.; Heukin, S.; Lognay, G.; Verheggen, F.J.; Francis, F. Oviposition deterrent activity of basil plants and their essentials oils against Tuta absoluta (Lepidoptera: Gelechiidae). Environ. Sci. Pollut. Res. 2018, 25, 29880–29888. [Google Scholar] [CrossRef]
- Cherif, A.; Mansour, R.; Ncibi, S.; WHached, W.; Grissa-Lebdi, K. Chemical composition and fumigant toxicity of five essential oils toward Tuta absoluta and its mirid predator Macrolophus pygmaeus. J. Plant Dis. Prot. 2025, 132, 34. [Google Scholar] [CrossRef]
- Lo Pinto, M.; Vella, L.; Agrò, A. Oviposition deterrence and repellent activities of selected essential oils against Tuta absoluta Meyrick (Lepidoptera: Gelechiidae): Laboratory and greenhouse investigations. Int. J. Trop. Insect Sci. 2022, 42, 3455–3464. [Google Scholar] [CrossRef]
- Nouri-Ganbalani, G.; Ebadollahi, A.; Nouri, A. Chemical composition of the essential oil of Eucalyptus procera Dehnh. and its insecticidal effects against two stored product insects. J. Essent. Oil Bear. Plants 2016, 19, 1234–1242. [Google Scholar] [CrossRef]
- Ebadollahi, A. Essential Oils Isolated from Myrtaceae Family as Natural Insecticides. Annu. Res. Rev. Biol. 2013, 3, 148–175. Available online: https://journalarrb.com/index.php/ARRB/article/view/1208 (accessed on 4 August 2025).
- Pedrotti, C.; Trentin, T.R.; Cavião, H.C.; Vilasboa, J.; Scariot, F.J.; Echeverrigaray, S.; Piemolini-Barreto, L.T.; Schwambach, J. Eucalyptus staigeriana essential oil in the control of postharvest fungal rots and on the sensory analysis of grapes. Pesqui. Agropec. Bras. 2022, 57, 02782. [Google Scholar] [CrossRef]
- Brito, J.P.; Baptistussi, R.C.; Funichello, M.; Oliveira, J.E.M.; de Bortoli, S.A. Efeito de óleos essenciais de Eucalyptus spp. sobre Zabrotes subfasciatus (Boh., 1833) (Coleoptera: Bruchidae) e Callosobruchus maculatus (Fabr., 1775) (Coleoptera: Bruchidae) em duas espécies de feijões. Bol. Sanid. Veg. 2006, 32, 573–580. [Google Scholar]
- Gusmão, N.M.S.; de Oliveira, J.V.; Navarro, D.M.A.F.; Dutra, K.A.; da Silva, W.A.; Wanderley, M.J.A. Contact and fumigant toxicity and repellency of Eucalyptus citriodora Hook., Eucalyptus staigeriana F., Cymbopogon winterianus Jowitt and Foeniculum vulgare Mill. essential oils in the management of Callosobruchus maculatus. J. Stored Prod. Res. 2013, 54, 41–47. [Google Scholar] [CrossRef]
- Araújo, A.M.N.; Oliveira, J.V.; França, S.M.; Navarro, D.M.A.F.; Barbosa, D.R.S.; Dutra, K.A. Toxicity and repellency of essential oils in the management of Sitophilus zeamais. Rev. Bras. Eng. Agríc. Ambient. 2019, 23, 372–377. [Google Scholar] [CrossRef]
- Maciel, M.V.; Morais, S.M.; Bevilaqua, C.M.L.; Silva, R.A.; Barros, R.S.; Sousa, R.N.; Sousa, L.C.; Brito, E.S.; Souza-Neto, M.A. Chemical composition of Eucalyptus spp. essential oils and their insecticidal effects on Lutzomyia longipalpis. Vet. Parasitol. 2010, 167, 1–7. [Google Scholar] [CrossRef]
- Cruz, G.S.; Wanderley-Teixeira, V.; Oliveira, J.V.; D’Assunção, C.G.; Cunha, F.M.; Teixeira, Á.A.C.; Guedes, C.A.; Dutra, K.M.; Barbosa, D.R.S.; Breda, M.O. Effect of trans-anethole, limonene and your combination in nutritional components and their reflection on reproductive parameters and testicular apoptosis in Spodoptera frugiperda (Lepidoptera: Noctuidae). Chem. Biol. Interact. 2017, 263, 74–80. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, S.; Lin, G.; Chen, J.; Li, H.; Xiao, Y.; Chen, X.; Chen, J.; Wu, Y.; Xiao, H.; et al. The chromosome-level Melaleuca alternifolia genome provides insights into the molecular mechanisms underlying terpenoids biosynthesis. Ind. Crops Prod. 2022, 189, 115819. [Google Scholar] [CrossRef]
- Liao, M.; Xiao, J.-J.; Zhou, L.-J.; Yao, X.; Tang, F.; Hua, R.-M.; Wu, X.-W.; Cao, H.-Q. Chemical composition, insecticidal and biochemical effects of Melaleuca alternifolia essential oil on the Helicoverpa armigera. J. Appl. Entomol. 2017, 141, 721–728. [Google Scholar] [CrossRef]
- Ismail, S.M.; Hassan, N.A.; Wahba, T.F.; Shaker, N. Chemical composition and bioactivities of Melaleuca alternifolia essential oil and its main constituents against Spodoptera littoralis (Boisaduval, 1833). Bull. Natl. Res. Cent. 2022, 46, 157. [Google Scholar] [CrossRef]
- Chohan, T.; Chohan, T.; Zhou, L.; Yang, Q.; Min, L.; Cao, H. Repellency, toxicity, gene expression profiling and in silico studies to explore insecticidal potential of Melaleuca alternifolia essential oil against Myzus persicae. Toxins 2018, 10, 425. [Google Scholar] [CrossRef]
- Oliveira, J.A.C.; Ferreira, L.S.; Garcia, I.P.; Santos, H.L.; Ferreira, G.S.; Rocha, J.P.M.; Nunes, S.A.; de Carvalho, A.A.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Eugenia uniflora, Melaleuca armillaris, and Schinus molle essential oils to manage larvae of the filarial vector Culex quinquefasciatus (Diptera: Culicidae). Environ. Sci. Pollut. Res. 2022, 29, 34749–34758. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Chi, H.; Güncan, A.; Kavousi, A.; Gharakhani, G.; Atlihan, R.; Özgökçe, M.S.; Shirazi, J.; Amir-Maafi, M.; Maroufpoor, M.; Taghizadeh, R. TWOSEX-MSChart: The key tool for life table research and education. Entomol. Gen. 2022, 42, 845–849. [Google Scholar] [CrossRef]
- Bustos-Segura, C.; Fornoni, J.; Núñez-Farfán, J. Evolutionary changes in plant tolerance against herbivory through a resurrection experiment. J. Evol. Biol. 2014, 27, 488–496. [Google Scholar] [CrossRef]
- Callander, J.T.; James, P.J. Insecticidal and repellent effects of tea tree (Melaleuca alternifolia) oil against Lucilia cuprina. Vet. Parasitol. 2012, 184, 271–278. [Google Scholar] [CrossRef]
- Isman, M.B.; Miresmailli, S.; MacHial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Nóbrega, F.F.F.; Salvadori, M.G.S.S.; Masson, C.J.; Mello, C.F.; Nascimento, T.S.; Leal-Cardoso, J.H.; de Sousa, D.P.; Almeida, R.N. Monoterpenoid terpinen-4-ol exhibits anticonvulsant activity in behavioural and electrophysiological studies. Oxid. Med. Cell Longev. 2014, 2014, 703848. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.P.; Abraham, Z. Chemical composition of essential oils obtained from plant parts of Alpinia calcarata Rosc. (Lesser Galangal) germplasm from South India. J. Essent. Oil Res. 2015, 27, 238–243. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Grando, T.H.; Souza, C.F.; Gressler, L.T.; Stefani, L.M.; da Silva, A.S.; Monteiro, S.G. In vitro and in vivo action of terpinen-4-ol, γ-terpinene, and α-terpinene against Trypanosoma evansi. Exp. Parasitol. 2016, 162, 43–48. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A Review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Khammassi, M.; Polito, F.; Amri, I.; Khedhri, S.; Hamrouni, L.; Nazzaro, F.; Fratianni, F.; de Feo, V. Chemical composition and phytotoxic, antibacterial and antibiofilm activity of the essential oils of Eucalyptus occidentalis, E. striaticalyx and E. stricklandii. Molecules 2022, 27, 5820. [Google Scholar] [CrossRef]
- Macedo, I.T.F.; Bevilaqua, C.M.L.; de Oliveira, L.M.B.; Camurça-Vasconcelos, A.L.F.; Vieira, L.S.; Oliveira, F.R.; Queiroz-Junior, E.M.; da, R. Tomé, A.; Nascimento, N.R.F. Anthelmintic effect of Eucalyptus staigeriana essential oil against goat gastrointestinal nematodes. Vet. Parasitol. 2010, 173, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, W.L.C.; Macedo, I.T.F.; dos Santos, J.M.L.; de Oliveira, E.F.; Camurça-Vasconcelos, A.L.F.; de Paula, H.C.B.; Bevilaqua, C.M.L. Activity of chitosan-encapsulated Eucalyptus staigeriana essential oil on Haemonchus contortus. Exp. Parasitol. 2013, 135, 24–29. [Google Scholar] [CrossRef]
- Zhi-Bo, G.; Zhi-Qing, M.; Jun-Tao, F.; Xing, Z. Inhibition of Na+,K+-ATPase in housefly (Musca domestica L.) by terpinen-4-ol and its ester derivatives. Agric. Sci. China 2009, 8, 1492–1497. [Google Scholar] [CrossRef]
- Huang, X.; Du, L.; Liu, T.; Ma, R.; Liu, X.; Yuan, H.; Liu, S. Insecticidal activity of a component, (-)-4-terpineol, isolated from the essential oil of Artemisia lavandulaefolia DC. against Plutella xylostella (L.). Insects 2022, 13, 1126. [Google Scholar] [CrossRef]
- Rajashekar, Y.; Raghavendra, A.; Bakthavatsalam, N. Acetylcholinesterase inhibition by biofumigant (coumaran) from leaves of Lantana camara in stored grain and household insect pests. Biomed. Res. Int. 2014, 2014, 187019. [Google Scholar] [CrossRef]
- Bezerra, C.S.; Pott, A.; Elifio-Esposito, S.; Dalarmi, L.; Fialho, K.N.; Burci, L.M.; de Oliveira, M.; de Fátima Gaspari Dias, J.; Zanin, S.M.W.; Miguel, O.G.; et al. Effect of donepezil, tacrine, galantamine and rivastigmine on acetylcholinesterase inhibition in Dugesia tigrina. Molecules 2016, 21, 53. [Google Scholar] [CrossRef]
- Johnson, T.O.; Abolaji, A.O.; Omale, S.; Longdet, I.Y.; Kutshik, R.J.; Oyetayo, B.O.; Adegboyega, A.E.; Sagay, A. Benzo[a]Pyrene and Benzo[a]Pyrene-7,8-Dihydrodiol-9,10-Epoxide induced locomotor and reproductive senescence and altered biochemical parameters of oxidative damage in canton-S Drosophila melanogaster. Toxicol. Rep. 2021, 8, 571–580. [Google Scholar] [CrossRef]
- Lu, Y.H.; He, Y.P.; Gao, X.W. Comparative studies on acetylcholinesterase characteristics between the aphids, Sitobion avenae and Rhopalosiphum padi. J. Insect Sci. 2013, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademosun, A.O.; Olumuyiwa, T.A.; Olasehinde, T.A.; Ademiluyi, A.O.; Adeyemo, A.C. Insecticidal activity of essential oil from orange peels (Citrus sinensis) against Tribolium confusum, Callosobruchus maculatus and Sitophilus oryzae and its inhibitory effects on acetylcholinesterase and Na+/K+-ATPase activities. Phytoparasitica 2017, 45, 501–508. [Google Scholar] [CrossRef]
- Cheng, D.; Feng, M.; Ji, Y.; Wu, W.; Hu, Z. Effects of Celangulin IV and V from Celastrus angulatus Maxim on Na+/K+-ATPase Activities of the Oriental Armyworm (Lepidoptera: Noctuidae). J. Insect Sci. 2016, 16, 59. [Google Scholar] [CrossRef]
- Stark, J.D.; Banks, J.E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 2003, 48, 505–519. [Google Scholar] [CrossRef]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Fang, F.; Candy, K.; Melloul, E. In vitro activity of ten essential oils against Sarcoptes scabiei. Parasites Vectors 2016, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Isman, M.B.; Tak, J.H. Insecticidal activity of 28 assential oils and a commercial product containing Cinnamomum cassia Bark essential oil against Sitophilus zeamais Motschulsky. Insects 2020, 11, 474. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Y.; Xing, R.; Liao, M.; Wu, H.; Huang, Y.; Gao, Q.; Cao, H. Heat shock protein mediates the fumigation activity of terpinen-4-ol and limonene against Tribolium confusum. Pest Manag. Sci. 2025, 81, 5250–5259. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, M.; Yang, Q.; Xiao, J.; Hu, Z.; Zhou, L.; Cao, H. Transcriptome profiling reveals differential gene expression of detoxification enzymes in Sitophilus zeamais responding to terpinen-4-ol fumigation. Pestic. Biochem. Physiol. 2018, 149, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Deen, J.I.; Zawad, A.N.M.S.; Uddin, M.; Chowdhury, M.A.H.; Araby, S.Q.A.; Rahman, M.A. Terpinen-4-ol, A volatile terpene molecule, extensively electrifies the biological systems against the oxidative stress-linked pathogenesis. Adv. Redox Res. 2023, 9, 100082. [Google Scholar] [CrossRef]
- Dancewicz, K.; Szumny, A.; Wawrzeńczyk, C.; Gabryś, B. Repellent and Antifeedant Activities of Citral-Derived Lactones against the Peach Potato Aphid. Int. J. Mol. Sci. 2020, 21, 8029. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).