Abstract
Background: The implementation of advanced agronomical strategies, including the use of antitranspirant, in order to mitigate the negative effects of environmental stress, particularly heat stress on plants, has become a focal area of research in the Mediterranean basin. This region is characterized by hot and dry summer that affects plant physiology. Methods: The experiment was carried out in Sicily (South Italy) on 12-year-old avocado cv. Hass grafted onto Walter Hole rootstock. Two subplots each of forty homogenous trees were selected and treated (1) with calcium carbonate (DECCO Shield®) and (2) with water (control) at the following phenological phases: 711, 712 and 715 BBCH. The climatic parameters were recorded throughout the year. Physiological measurements (leaf transpiration, net photosynthesis, stomatal conductance, leaf water potential) were measured at 105, 131 and 168 days after full bloom. Fruit growth was monitored, and physico-chemical analyses were carried out at harvest. Results: The antitranspirant increased photosynthesis and stomatal conductance and reduced leaf transpiration (−26.1%). Fruit growth rate increased during summer, although no morphological and qualitative difference was observed at harvest. PCA highlighted the positive effect of the calcium carbonate on overall plant physiology. Conclusions: Antitranspirant foliar application reduced heat stress effects by improving physiological responses of avocado trees.
1. Introduction
Avocado (Persea americana Mill.) is increasingly popular in several countries, particularly in the Mediterranean basin, where its cultivation for trade is expanding. The fruit is mainly consumed fresh for its nutraceutical properties [1,2,3]. In fact, avocado is rich in biochemical compounds with high antioxidant value, but it also contains proteins and fat-soluble vitamins, in greater quantities than other fruits, such as vitamins A, B, D and E. Similarly to olive fruit, the pulp is rich in oil content that gives it a pleasant taste, but it is also widely used in the pharmaceutical and cosmetic industries [2].
Among the Mediterranean countries, avocado cultivation is expanding in Italy, widely grown in Sicily and in other southern Italian regions to a lesser extent. The main varieties are Hass, Bacon and Fuerte [4]. The avocado plant is a crop that adapts very well to tropical or subtropical climates. However, several areas of the Mediterranean basin may be less favorable for cultivation due to the hot summer. In addition, the limited availability of water during the summer months and potential salinity problems in irrigation water are among the abiotic factors that restrict avocado cultivation [4]. In this context, previous researchers have focused on several problems during fruit production associated with environmental conditions and abiotic stresses, especially high solar radiation, wind, and hot temperature. Therefore, the adoption of techniques to mitigate high temperatures, such as cultivation of avocados or other crops under shade nets or application of mineral particle films, is becoming an increasingly relevant topic [5,6,7,8,9,10].
Previous research has shown that the cultivation of avocados under shade nets results in a lower incidence of fruit sunburn due to excessive sun exposure. In addition, avocado plants may benefit from this practice for the better water status and productivity, although a delay in maturation was observed [6].
Lazare et al. [7] investigated the use of a canopy-cooling irrigation system in avocado orchards to regulate plant stress during hot days. This system was effective in reducing physiological stress in avocado trees during heat waves, improving yields and production. The effectiveness of the cooling system justified the cost of its implementation, considering that this agronomic technique led to an increase in yield of 200 kg per hectare. However, it can only be used if high-quality water is available for irrigation, since the scarcity of fresh water could lead to the risk of salt accumulation in the leaves of the avocado, which is very sensitive to salt [7].
Previous studies have demonstrated that the application of natural coatings in pre-harvest can reduce negative environmental impacts improving plant physiological responses. Notably, the use of antitranspirant agents play a pivotal role to mitigate the effects of drought and salt stress on several crops, including clementines [10], apples [11], and grapefruits [12] and grape [13]. In fact, sun protection coating forms a physical barrier that reduces the intensity of solar radiation and the temperature, mitigating plant and fruit damages. In particular, antitranspirants increased net photosynthesis, reduced transpiration rate, and increased stomatal conductance [14]. However, other studies reported that the kaolin application caused a reduction in photosynthesis in olive trees, demonstrating that efficacy of these treatments depends on the species and environmental condition [15,16].
There are some studies conducted on the use of shade nets and their influence on plant physiology and fruit quality in avocado cultivation [6,14,17]. Some studies focused on the post-harvest application of antitranspirant products on avocado fruits, while there is limited research available regarding their use in pre-harvest to mitigate damages caused by rising temperatures in the Mediterranean basin [18,19].
Climate change affects the duration and quality of plant growth and phenological stages, affecting especially hormone balance and plant physiology [20]. In particular, the increase in temperature and the reduced water availability influences the quantity and quality of production, negatively affecting the economic sustainability of avocado production in some producing regions, including the Mediterranean basin. For this reason, the use of sun protection techniques is one of the possible useful strategies for the management of abiotic stress in crops, i.e., high temperatures. Previous studies have shown that the use of adequate sun filters, such as kaolin, films, shading nets and reflective substances, can reduce the intensity of solar radiation and mitigate the thermal effect [14]. In this context, water availability and climatic conditions, especially high temperatures, strongly affect fruit and vegetative plant growth in avocado plants, also influencing qualitative fruit traits [21,22]. In particular, some agricultural areas in southern Italy may reach high temperature levels, even higher than 40 °C for weeks, due to the high solar irradiation which can cause sunburn damage. In this context, the use of plant growth regulators (PGRs) and protective particle films, including calcium carbonate, may play a key role in regulating the response to abiotic stress in different crops [8,23,24]. Photosynthesis, transpiration, and stomatal conductance are highly temperature-sensitive processes [25], and this tool may be effective for regulating plant physiology, maintaining a favorable plant water status [24].
In this context, the aim of this study is to investigate the physiological response of avocado plants cultivar Hass subjected to the foliar application of calcium carbonate.
2. Materials and Methods
2.1. Plant Material and Treatments
The experiment was carried out in Giarre (Catania, Italy), characterized by volcanic soil with sandy texture and low water field capacity. The orchard of 12-year-old avocado cv. Hass grafted onto Walter Hole rootstock had plants spaced 9 m x 9 m apart (37°41′08″ N; 15°10′45″ E) and was drip irrigated three times per week. A total of 80 plants were selected and divided into 2 subplots each of 40 homogenous trees that were treated at the following phenological phases—711, 712 and 715 BBCH—as follows: (1) with calcium carbonate (DECCO Shield®) (Figure 1) and (2) with tap water (control) [26]. Treatments were carried out with an atomizer at a pressure of 15 bar and a speed of 8 km/h to spray 15 L ha−1 of calcium carbonate. In order to characterize the climatic environment of the site, data of the maximum, mean, and minimum temperature (Figure S1) were recorded from January to December 2022. Data were provided by weather stations located near the cultivation site managed by Servizio Informativo Agrometeorologico Siciliano, SIAS (http://www.sias.regione.sicilia.it/ accessed on 4 December 2024).

Figure 1.
Leaves and fruit of avocado cultivar Hass treated with calcium carbonate (left) and field trial (right).
2.2. Physiological Measurements
Physiological parameters were measured at 105 DAFB (T1), 131 DAFB (T2) and 168 DAFB (T3). Leaf transpiration (E, mmol H2O m−2 s−1), net photosynthesis (A, μmol CO2 m−2 s−1) and stomatal conductance (gs, μmol CO2 m−2 s−1) were monitored with a portable infrared gas analyzer (LCi, ADC Bioscientific Ltd., Hoddesdon, UK). Measurements were made on clear days, between 8:00 h and 10:00 h (solar time) on fully developed leaves (5 leaves per 10 trees per treatment). Water use efficiency (WUE) was calculated by the ratio of the moles of carbon fixed by photosynthesis divided by the moles of water lost via transpiration. Chlorophyll fluorescence ratio (Fv/Fm) was measured using a portable modulated pulse fluorometer (Handy Pea, Hansatech Instruments Ltd. Narborough, United Kingdom) on the same leaves after dark acclimation for at least 30 min to inhibit all light-dependent reactions by completely oxidizing PSII electron acceptor molecules. The leaf water potential Ψ (MPa) was monitored using a Scholander pressure chamber (Model 600, PMS Instrument Company, Albany, OR, USA), as reported by Vanella et al. [27].
2.3. Morphological and Physico-Chemical Analyses
The diameter of 20 fruits for each treatment was measured from 66 to 363 days after full bloom (DAFB) using an electronic digital slide gauge (model CD-15 DC; Mitutoyo (UK) Ltd., Telford, UK) to within 0.01 mm accuracy. At harvest time (719 BBCH, 363 DAFB), 40 fruits (4 fruits per 10 plants) of both treatments were collected. Peel and flesh color were recorded on the equatorial region for each fruit using a Minolta CR-400 chromameter (Minolta Corp., Osaka, Japan) according to the methodology described by Modica et al. [28]. Samples of fresh mesocarp were dried in an oven, and dry matter content was calculated and expressed as a percentage. Fruit and seed height (mm) and equatorial diameter (mm) were measured using an electronic digital slide gauge (model CD-15 DC; Mitutoyo (UK) Ltd., Telford, UK) to within 0.01 mm accuracy. Individual fruit and seed weight (g) were measured using an electronic balance and rind thickness using an electronic digital gauge (model CD-15 DC, Mitutoyo Ltd., Telford, Shropshire, UK).
2.4. Statistical Analysis
Statistical analyses were performed using STATISTICA 6.0 (Statsoft Inc., Tulsa, OK, USA) and used to test the significance of each variable (p ≤ 0.05). A basic descriptive statistical analysis was followed by an analysis of variance test for mean comparisons. The method used to discriminate among the means (Multiple Range Test) was Fisher’s Least Significant Difference (LSD) procedure at a 95.0% confidence level. Principal component analysis (PCA) was performed using R software (v. 4.3.1) computing the “prcomp” function of the package ‘tidyverse’. The results are represented using the package ‘ggplot2′, as previously described by Modica et al. [29].
3. Results
3.1. Physiological Measurements and Climatic Site Characterization
The air temperature was monitored from January to December 2022, revealing that it exceeded 30 °C almost daily during the summer months (from 93 to 215 DAFB), even if it did not reach 40 °C. Additionally, it was observed that rainfall was mainly concentrated in March, April, October, November and December (Figure S1).
Physiological measurements were reported in Figure 2. Net photosynthesis showed an increase (+14.5%) compared to the control at the second sampling (T2). In detail, the value ranged from 7.14 μmol CO2 m−2 s−1 for the control to 8.2 μmol CO2 m−2 s−1 for the calcium carbonate treatment. At T3, no difference was observed in net photosynthesis (Figure 2A). Regarding the transpiration rate (Figure 2B), a significant decrease in treated trees was recorded at T2 (−31.5%) and T3 (−26.1%) compared to the control. Indeed, treated trees with calcium carbonate exhibited at T2 a higher stomatal conductance (0.06 μmol CO2 m−2 s−1) than the control (Figure 2C). The chlorophyll fluorescence monitored did not show any difference between the plants of control and the treated trees during the experiment (Figure 2D).
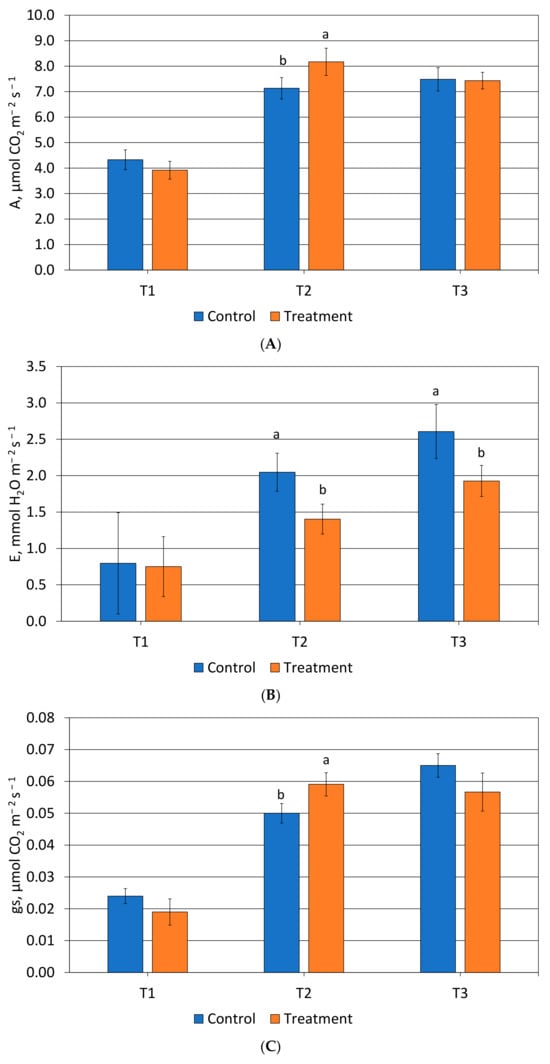
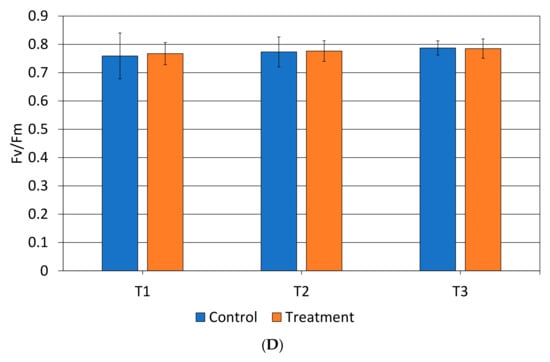
Figure 2.
(A) Net photosynthesis (A, μmol CO2 m−2 s−1), (B) leaf transpiration (E, mmol H2O m−2 s−1), (C) stomatal conductance (gs, μmol CO2 m−2 s−1) and (D) chlorophyll fluorescence (Fv/Fm) monitored in control and treated tress at T1, T2 and T3. Values without letters have no significant differences according to Fisherʼs LSD procedure at 95% confidence level.
Leaf water potential monitored on treated trees with calcium carbonate showed always less negative values than the control, although no significant difference was recorded (Figure 3).
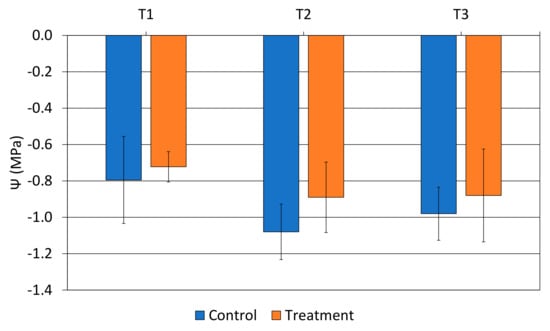
Figure 3.
Leaf water potential (Ψ, MPa) monitored in control and treated trees at T1, T2 and T3. Values without letters have no significant differences according to Fisherʼs LSD procedure at 95% confidence level.
Water use efficiency, that is the ratio between net photosynthetic rate (A) and transpiration (E), was evaluated. The results showed that treated trees with the antitranspirant improved the efficiency of water use compared to control trees at T2 and T3 (Table S1). In detail, a statistically significant increase in WUE was noted in plants treated with calcium carbonate at T2 and T3.
3.2. Morphological and Physico-Chemical Traits
During the monitoring period of fruit growth, it was observed that the calcium carbonate treatment determined an increase in fruit diameter compared to the control, even if it was not statistically different (Figure 4); in detail, the increment was recorded at 119 DAFB, maintaining this trend since the last measurement (363 DAFB). At harvest, the fruit diameter ranged from 53.7 mm for the control to 54.5 for the thesis (Table 1), and the calcium carbonate treatment determined the higher fruit weight (122 g) in comparison with the control (115 g). No statistical differences were noticed in relation to morphological traits of the seeds (Table 1), nor in dry matter of the flesh. Moreover, no difference was highlighted on peel and flesh color between the control and the treatment (Table S2).
Figure 4.
Average fruit diameter ± standard deviation monitored on the trees from day 66 to 363 after full bloom (DAFB).

Table 1.
Qualitative characteristics of the fruits of control and treatment. Values with letters within the same column are not significantly different according to Fisher’s LSD procedure at a 95% confidence level.
3.3. Principal Component Analysis
A principal component analysis (PCA) on the physiological (net photosynthesis, transpiration rate, stomatal conductance, chlorophyll fluorescence and leaf water potential) and morphological traits (fruit height and diameter) measured during the trial was carried out to assess and summarize the effects of the calcium carbonate on fruits of avocado Hass (Figure 5).
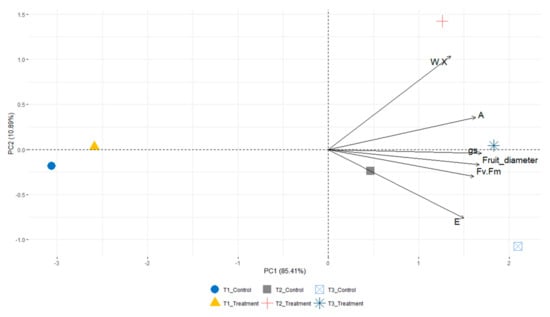
Figure 5.
Principal component analysis (PCA) showing the distribution of the plant of the control and the treatment. PCA of the parameters measured during the trial: leaf water potential (W.X), transpiration rate (E), stomatal conductance (gs), net photosynthesis (A), chlorophyll fluorescence (Fv/Fm) and fruit diameter.
The first two principal components (PCs) accounted for the 96.30% of total variance (PC1 = 85.41%; PC2 = 10.89%). The main variables contributing to PC1 were fruit diameter and stomatal conductance. PC2 was positively correlated with physiological analyses, specifically leaf water potential (0.750) and net photosynthesis (0.259), while it was negatively associated with transpiration rate (−0.553). The two were grouped in the same area of the graph at T1, while a separation between control and calcium carbonate treatment was recorded at T2 and T3. Specifically, at T2, treated trees were highly associated with PC1 and PC2 due to the higher values of stomatal conductance (Figure 2) and leaf water potential (Figure 3). Plants treated with calcium carbonate were positively correlated with PC1 at T3, while control trees were negatively associated with PC2 due to the higher values of transpiration rate (Figure 2).
4. Discussion
Overall, net photosynthesis values increased in treated trees with calcium carbonate, specifically at T2 (+14.5%), due to the higher stomatal conductance monitored, aligning with results reported by other researchers [24]. In addition, a decrease in transpiration was exhibited on avocado trees treated with calcium carbonate at T2 (−31.5%) and T3 (−26.1%). Boari et al. [10] reported that kaolin could have an antitranspirant effect, favoring plant adaptation in adverse conditions. This is because the application of kaolin reduces transpiration, allowing plants to resist dehydration. In fact, Brillante et al. [30] proposed that the stomatal opening narrows, but does not close completely, with the foliar application of kaolin. Furthermore, the presence of mineral particles, such as calcium carbonate, on the surface of leaves and fruits interferes with all physiological processes, mainly with thermal balance, light and gas exchanges. Several studies observed that mineral particles applied with foliar treatment and deposited on leaf and fruits showed a physical impact on vegetation development [31].
Generally, heat stress causes damage to PSII by reducing photochemical efficiency (Fv/Fm), as observed in other crops [32,33]. The maximum daily temperatures were 35 °C and calcium carbonate application did not cause any significant change in photochemical processes; thus, at this heat level, fluorescence measurements in avocado were not effective as an indicator for heat stress [3,34].
Previous studies reported that exposure to high temperatures triggered a reduction in physiological processes, an accumulation of photosynthetic pigments, and a decrement in water use efficiency in plants. In our investigation, the antitranspirant was effective in increasing the efficiency of water use, thus enhancing the physiological adaptation of avocado to unfavorable climatic conditions, confirming the higher tolerance to such stress observed on other crops [35,36,37,38]. Indeed, the results trigger the hypothesis that WUE optimization is due not only to the limited water loss via transpiration for the presence of the calcium carbonate particles, but also to the increased rate of photosynthetic carbon assimilation, probably due to the lower temperature of the treated leaves. In fact, the measurement of WUE is an important parameter for understanding the physiological efficiency of plants, being strictly related to stomatal conductance and environmental inputs of light, temperature, CO2 and atmospheric humidity. This explains why there is a linear response between stomatal opening, gs values and temperature increase [39].
A consistent result was highlighted by the higher leaf water potential recorded in trees where calcium carbonate was applied, although no significant differences were found, denoting their better water status, in accordance with previous studies [27,40]. However, a significant increase in WUE was recorded in plants treated with calcium carbonate at T2 and T3.
Abiotic stresses, including extreme temperatures, may interfere with the hormonal and nutritional balance of plants affecting avocado production in qualitative and quantitative terms [41,42,43]. The avocado tree, in fact, is particularly sensitive to heat stress that can also cause burns on leaves and fruits, compromising significantly the productivity of the tree and the commercial quality of the harvested products. This damage also affects economic profitability, especially in areas characterized by hot summers [14]. In this sense, the use of expensive agronomic techniques, i.e., the canopy-cooling irrigation system, reduces the negative effect of the heat waves, even if significant irrigation volumes and high installation costs are required. Overall, an increase in yield of avocado plants was recorded [7]. Therefore, the use of sun protection techniques or products may be an alternative strategy for the management of heat and light stresses that could compromise yield and qualitative traits of avocado [6,7,44,45]. In our trial, the only qualitative trait that was affected by the antitranspirant application in our trial was the fruit weight, as reported in other crops [31], the most important commercial parameter [46], corroborating the importance of the mitigation of heat stress [47]. Fruits treated with calcium carbonate showed the highest weight (122 g) compared to the control (115 g).
Avocado has a high nutritional and caloric value, due to the high content of fatty acids in the pulp. It is also rich in vitamins, ascorbic acid, β-carotene and potassium. The dry matter recorded in the flesh is closely related to the content of lipid and, for this reason, it represents a pivotal parameter for the quality of avocado fruits [48]. Our results highlighted a slight increase in the dry matter content on the treated plants, although no significant difference was found. Overall, the dry matter percentage measured was consistent with values recorded for the cultivar Hass [48].
The application of antitranspirant is considered an agronomic technique aimed at enhancing the physiological response of plants under abiotic stress [25], particularly affecting the photosynthetic process [49]. The principal component analysis (PCA) on the overall physiological performances explained the positive effects of the calcium carbonate on avocado Hass trees. In detail, PCA explained that, at T1 (15 days after the last treatment), there were no differences between treatments, while at T2 and T3, plants treated with calcium carbonate were correlated with transpiration rate, stomatal conductance, and leaf water potential. Confirming this, treated plants exhibited the less negative values of Ψ (−0.88 MPa) and the lowest transpiration rate (1.2 mmol H2O m−2 s−1) at T3.
5. Conclusions
The application of calcium carbonate significantly reduced temperature stress affecting the plant physiological response. Foliar treatment with the antitranspirant increased photosynthesis and stomatal conductance and reduced leaf transpiration, thus determining a higher water use efficiency. Fruit growth rate increased slightly during summer, and fruit weight at harvest was positively affected. Hence, sun protection products such as antitranspirants may be effective for regulating plant physiology, maintaining a favorable plant water status. In summary, our study offers new insights for farmers on the application of a foliar antitranspirant; this technique effectively reduced physiological stress, enhancing plant performance in terms of gas exchanges, including transpiration rate (−26.1%) and water use efficiency (+34.5%).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11080928/s1, Figure S1: Daily minimum (blue, °C), mean (gray, °C) and maximum (orange, °C) air temperature and rainfall (yellow, mm) recorded in the field from January to December 2022; Table S1: Water use efficiency (WUE, μmol CO2/mmol H2O) measured in control and treated plants at T1, T2 and T3; Table S2: Peel and flesh color of fruit of control and treatment. Values with letters within the same column are not significantly different according to Fisher’s LSD procedure at a 95% confidence level.
Author Contributions
Methodology, A.C.; validation, A.C., A.G. and S.L.M.; formal analysis, G.M. and F.A.; investigation, G.M.; writing—original draft preparation, G.M.; writing—review and editing A.C., S.L.M. and A.G.; supervision, A.C.; project administration, A.C.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from Project Superavocado-Avocado biologico siciliano: superfood per la valorizzazione delle aree ionico-tirreniche (CUP G79J2100558009); PSR Sicilia 2014–2020 Misura 16—Sottomisura 16.2.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Villa-Rodríguez, J.A.; Molina-Corral, F.J.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Effect of maturity stage on the content of fatty acids and antioxidant activity of ‘Hass’ avocado. Food Res. Int. 2011, 44, 1231–1237. [Google Scholar] [CrossRef]
- Duarte, P.F.; Chaves, M.A.; Borges, C.D.; Mendonça, C.R.B. Avocado: Characteristics, health benefits and uses. Ciciencia Rural 2016, 46, 747–754. [Google Scholar] [CrossRef]
- Stephen, J.; Radhakrishnan, M. Avocado (Persea americana Mill.) fruit: Nutritional value, handling and processing techniques, and health benefits. J. Food Process. Preserv. 2022, 46, e17207. [Google Scholar] [CrossRef]
- Kourgialas, N.N.; Dokou, Z. Water management and salinity adaptation approaches of Avocado trees: A review for hot-summer Mediterranean climate. Agric. Water Manag. 2021, 252, 106923. [Google Scholar] [CrossRef]
- Solomakhin, A.; Blanke, M. Can coloured hail nets improve taste (sugar, sugar: Acid ratio), consumer appeal (colouration) and nutritional value (anthocyanin, vitamin C) of apple fruit? LWT—Food Sci. Technol. 2010, 43, 1277–1284. [Google Scholar] [CrossRef]
- Tinyane, P.P.; Soundy, P.; Sivakumar, D. Growing ‘Hass’ avocado fruit under different coloured shade netting improves the marketable yield and affects fruit ripening. Sci. Hort. 2018, 230, 43–49. [Google Scholar] [CrossRef]
- Lazare, E.; Vitoshkin, S.; Alchanatis, H.; Reshef, V.; Ziv, G.; Simenski, D.; Dag, A.E. Canopy-cooling systems applied on avocado trees to mitigate heatwaves damages. Sci. Rep. 2022, 12, 12563. [Google Scholar] [CrossRef]
- Patanè, C.; Pellegrino, A.; Di Silvestro, I. Effects of calcium carbonate application on physiology, yield and quality of field-grown tomatoes in a semi-arid Mediterranean climate. Crop Pasture Sci. 2018, 69, 411–418. [Google Scholar] [CrossRef]
- Alon, E.; Shapira, O.; Azoulay-Shemer, T.; Rubinovich, L. Shading nets reduce canopy temperature and improve photosynthetic performance in ‘Pinkerton’ avocado trees during extreme heat events. Agronomy 2022, 12, 1360. [Google Scholar] [CrossRef]
- Boari, F.; Donadio, A.; Schiattone, M.I.; Cantore, V. Particle film technology: A supplemental tool to save water. Agric. Water Manag. 2015, 147, 154–162. [Google Scholar] [CrossRef]
- Glenn, D.M.; Erez, A.; Puterka, G.J.; Gundrum, P. Particle films affect carbon assimilation and yield in ‘Empire’ apple. J. Am. Soc. Hortic. Sci. 2003, 128, 356–362. [Google Scholar] [CrossRef]
- Jifon, J.L.; Syvertsen, J.P. Kaolin Particle Film Applications can increase photosynthesis and Water Use Efficiency of ‘Ruby Red’ Grape fruit Leaves. J. Am. Soc. Hortic. Sci. 2003, 128, 107–112. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Y.; Wang, Z.L.; Tian, X.L.; Han, X.; Wu, D.; Fei, Y.; Hui, M.; Li, H.; Wang, H. Effects of kaolin particle film coatings on the water-saving efficiency and fruit quality of Cabernet Sauvignon (Vitis vinifera L.) grape plants in the Ningxia region of China. Hortic. Environ. Biotechnol. 2023, 64, 421–435. [Google Scholar] [CrossRef]
- Domingues Neto, F.J.; Carneiro, D.C.D.S.; Silva, M.D.S.; Tecchio, M.A.; Leonel, S.; Pimentel Junior, A.; Ono, E.O.; Rodrigues, J.D. Sun Protection as a Strategy for Managing Heat Stress in Avocado Trees. Plants 2024, 13, 2854. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, A.; Bertazza, G.; Faccini, B.; Ferretti, G.; Morrone, L. Effect of different foliar particle films (kaolin and zeolitite) on chemical and sensory properties of olive oil. Agronomy 2022, 12, 3088. [Google Scholar] [CrossRef]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z. Shade netting on subtropical fruit: Effect on environmental conditions, tree physiology and fruit quality. Sci. Hort. 2019, 256, 108556. [Google Scholar] [CrossRef]
- Rensburg, E.V.; Enqelbrecht, A.H.P. Effect of calcium salts on susceptibility to browning of avocado fruit. J. Food Sci. 1986, 51, 1067–1068. [Google Scholar] [CrossRef]
- Roets, N.J.R.; De Meillon, S.; Kaiser, C.; Robbertse, P.J.; Owen, R.; Ehlers, R. Possible causes and measures to prevent excessive leaf abscission in the avocado (Persea americana Mill.) cultivar Ryan. SAAGA Yearb. 2006, 29, 21–36. [Google Scholar]
- Henao-Rojas, J.C.; Lopez, J.H.; Osorio, N.W.; Ramírez-Gil, J.G. Fruit quality in Hass avocado and its relationships with different growing areas under tropical zones. Rev. Ceres 2019, 66, 341–350. [Google Scholar] [CrossRef]
- Shapira, O.; Chernoivanov, S.; Neuberger, I.; Levy, S.; Rubinovich, L. Physiological characterization of young “hass” avocado plant leaves following exposure to high temperatures and low light intensity. Plants 2021, 10, 1562. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Patakas, A.; Kofidis, G.; Bosabalidis, A.; Nastou, A. Water stress affects leaf anatomy, gas exchange, water relations and growth of two avocado cultivars. Sci. Hortic. 2002, 95, 39–50. [Google Scholar] [CrossRef]
- Glenn, D.M.; Prado, E.; Erez, A.; McFerson, J.; Puterka, G.J. A reflective processed kaolin particle film affects fruit temperature, radiation reflection and solar injury in apple. J. Amer. Soc. Hort. Sci. 2002, 127, 188–193. [Google Scholar] [CrossRef]
- Glenn, D.M. Particle film mechanisms of action that reduce the effect of environmental stress in ‘Empire’ apple. Amer. Soc. Hort. Sci. 2009, 134, 314–321. [Google Scholar] [CrossRef]
- Gullo, G.; Dattola, A.; Vonella, V.; Zappia, R. Effects of two reflective materials on gas exchange, yield, and fruit quality of sweet orange tree Citrus sinensis (L.) Osb. Eur. J. Agron. 2020, 118, 126071. [Google Scholar] [CrossRef]
- Goreta, S.; Leskovar, D.I.; Jifon, J.L. Gas exchange, water status, and growth of pepper seedlings exposed to transient water deficit stress are differentially altered by antitranspirants. J. Am. Soc. Hortic. Sci. 2007, 132, 603–610. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Thorp, T.G.; Hormaza, J.I. Phenological growth stages of avocado (Persea americana) according to the BBCH scale. Sci. Hortic. 2013, 164, 434–439. [Google Scholar] [CrossRef]
- Vanella, D.; Consoli, S.; Continella, A.; Chinnici, G.; Milani, M.; Cirelli, G.L.; D’amico, M.; Maesano, G.; Gentile, A.; La Spada, P.; et al. Environmental and Agro-Economic Sustainability of Olive Orchards Irrigated with Reclaimed Water under Deficit Irrigation. Sustainability 2023, 15, 15101. [Google Scholar] [CrossRef]
- Modica, G.; Legua, P.; La Malfa, S.; Gentile, A.; Continella, A. Qualitative Traits and Antioxidant Properties of Blood Oranges Are Affected by the Genotype and the Climatic Conditions. Foods 2024, 13, 3137. [Google Scholar] [CrossRef]
- Modica, G.; Arcidiacono, F.; Puglisi, I.; Baglieri, A.; La Malfa, S.; Gentile, A.; Arbona, V.; Continella, A. Response to Water Stress of Eight Novel and Widely Spread Citrus Rootstocks. Plants 2025, 14, 773. [Google Scholar] [CrossRef]
- Brillante, L.; Belfiore, N.; Gaiotti, F.; Lovat, L.; Sansone, L.; Poni, S.; Tomasi, D. Comparing kaolin and pinolene to improve sustainable grapevine production during drought. PLoS ONE 2016, 11, e0156631. [Google Scholar] [CrossRef]
- Cantore, V.; Pace, B.; Albrizio, R. Kaolin-based particle film technology affects tomato physiology, yield and quality. Environ. Exp. Bot. 2009, 66, 279–288. [Google Scholar] [CrossRef]
- Allakhverdiev, I.S.; Kreslavski, V.D.; Klimov, V.V.; Los, A.D.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Van der Westhuizen, M.M.; Oosterhuis, D.M.; Berner, J.M.; Boogaers, N. Chlorophyll a fluorescence as an indicator of heat stress in cotton (Gossypium hirsutum L.). S. Afr. J. Plant Soil 2020, 37, 116–119. [Google Scholar] [CrossRef]
- Arief, M.A.A.; Kim, H.; Kurniawan, H.; Nugroho, A.P.; Kim, T.; Cho, B.K. Chlorophyll fluorescence imaging for early detection of drought and heat stress in strawberry plants. Plants 2023, 12, 1387. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.; Du, X.; Shellie, K.; Ross, C.; Qian, M.C. Volatile compounds and sensory attributes of wine from cv. Merlot (Vitis vinifera L.) grown under differential levels of water deficit with or without a kaolin-based, foliar reflectant particle film. J. Agric. Food Chem. 2010, 58, 12890–12898. [Google Scholar] [CrossRef]
- Denaxa, N.K.; Roussos, P.A.; Damvakaris, T.; Stournaras, V. Comparative effects of exogenous glycine betaine, kaolin clay particles and Ambiol on photosynthesis, leaf sclerophylly indexes and heat load of olive cv. Chondrolia Chalkidikis under drought. Sci. Hortic. 2012, 137, 87–94. [Google Scholar] [CrossRef]
- Barreales, D.; Capitão, S.; Bento, A.A.; Casquero, P.A.; Ribeiro, A.C. Adapting almond production to climate change through deficit irrigation and foliar kaolin application in a mediterranean climate. Atmosphere 2023, 14, 1593. [Google Scholar] [CrossRef]
- Teker, T. A study of kaolin effects on grapevine physiology and its ability to protect grape clusters from sunburn damage. Sci. Hort. 2023, 311, 111824. [Google Scholar] [CrossRef]
- Nguyen, T.B.A.; Lefoulon, C.; Nguyen, T.H.; Blatt, M.R.; Carroll, W. Engineering stomata for enhanced carbon capture and water-use efficiency. Trends Plant Sci. 2023, 28, 1290–1309. [Google Scholar] [CrossRef]
- Chung, S.W.; Rho, H.; Lim, C.K.; Jeon, M.K.; Kim, S.; Jang, Y.J.; An, H.J. Photosynthetic response and antioxidative activity of ‘Hass’ avocado cultivar treated with short-term low temperature. Sci. Rep. 2022, 12, 11593. [Google Scholar] [CrossRef]
- Garner, L.C.; Lovatt, C.J. Physiological factors affecting flower and fruit abscission of ‘Hass’ avocado. Sci. Hortic. 2016, 199, 32–40. [Google Scholar] [CrossRef]
- Al-Saif, A.M.; Mosa, W.F.; Saleh, A.A.; Ali, M.M.; Sas-Paszt, L.; Abada, H.S.; Abdel-Sattar, M. Yield and fruit quality response of pomegranate (Punica granatum) to foliar spray of potassium, calcium and kaolin. Horticulturae 2022, 8, 946. [Google Scholar] [CrossRef]
- Dattola, A.; Gullo, G. Effect of two reflective materials on the physiological and production behaviour of bergamot (Citrus bergamia Risso et Poiteau) plants. Sci. Hortic. 2024, 338, 113636. [Google Scholar] [CrossRef]
- Thomas, A.L.; Muller, M.E.; Dodson, B.R.; Ellersieck, M.R.; Kaps, M. A kaolin-based particle film suppresses certain insect and fungal pests while reducing heat stress in apples. J. Am. Pomol. Soc. 2004, 58, 42–51. [Google Scholar]
- Méndez Hernández, C.; Grycz, A.; Rios Mesa, D.; Rodríguez Galdón, B.; Rodríguez-Rodríguez, E.M. The Quality Evaluation of Avocado Fruits (Persea americana Mill.) of Hass Produced in Different Localities on the Island of Tenerife, Spain. Foods 2024, 13, 1058. [Google Scholar] [CrossRef]
- Sharma, S. Heat stress effects in fruit crops: A review. Agric. Rev 2020, 41, 73–78. [Google Scholar] [CrossRef]
- Ozdemir, F.; Topuz, A. Changes in dry matter, oil content and fatty acids composition of avocado during harvesting time and post-harvesting ripening period. Food Chem. 2004, 86, 79–83. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Rivero, R.M.; Martínez, V.; Gómez-Cadenas, A.; Arbona, V. Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol. 2016, 16, 105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).