Abstract
Agave species are plants with great economic value and multiple possibilities of use as ornamentals, medicinal plants, and fibers, as well as being significant sources of bioethanol. However, their long life cycles hinder their conventional breeding. Therefore, biotechnology tools are the most effective means for clonal propagation and genetic improvement. In vitro micropropagation of A. sisalana via axillary shoot proliferation from bulbil explants was attained using Murashige and Skoog medium (MS) supplemented with cytokinins (CKs), such as 6-benzyladenine (BA), kinetin (KIN), or thidiazuron (TDZ). The optimum significant shoot proliferation (14.67 shoots/explant) was achieved on 1.0 mg L−1 TDZ. The carry-over effect of CKs on subsequent rooting could be detected. Control and KIN treatments could enhance the rooting of shoots on shoot proliferation media. The regenerated plantlets were acclimatized directly with 100% survival. To mitigate this carry-over effect, that causes hindering further root growth and development, and promote healthy growth of roots, subculturing shoots onto a CK-free medium is a recommended practice. The shoots induced on all BA treatments, and TDZ at 0.5 and 1.0 mg L−1 could be rooted after two subcultures on CK-free medium, then they were acclimatized with 100% survival. However, the higher concentrations of TDZ inhibited in vitro rooting even after two subcultures on CK-free medium, and the acclimatization percentage was reduced by increasing the TDZ concentration recorded from 10 to 0%.
Keywords:
acclimatization; Agave sisalana; bulbils; cytokinins; in vitro propagation; KIN; BA; TDZ; shoot; root 1. Introduction
Agaves are a large group of succulent and perennial plants belonging to the Agavaceae (Asparagaceae) family, and are cultivated in arid and semi-arid areas [1]. Agaves have attracted interest because they have extensive applications in the ornamental, pharmaceutical, natural fibers, and functional food industries [2]. In addition, they have been recognized as highly promising bioenergy crops for biofuel production [3]. The potential of agaves for human health, nutrition, and food security comes from their content of bioactive phytochemicals such as fructans, inulin, antioxidants, phenolic compounds, amino acids, dietary fiber, minerals, and steroidal glycosides [2,4]. Moreover, they are a good source of the saponins and flavonoids [5], which have been reported to possess physiological, immunological, and therapeutic properties that provide antiseptic, anticancer, anti-inflammatory, antibacterial, and antifungal health benefits [6] for treating of various pathologies as dysentery, sores, and syphilis, and they can help in lowering blood pressure, improving lipid balance [7,8].
Agave sisalana Perr. Syn, also known as sisal, is an herbaceous monocotyledonous plant originating from Mexico. It is cultivated mainly for its fibers. Sisal fibers have received a lot of attention, as they are natural, highly resistant, renewable, biodegradable, and low-cost fibers, and they could be used as a sustainable alternative to traditional materials like cotton, particularly in water-scarce areas to make health and hygiene products [9,10]. They are used in manufacturing rope, paper, cloth, carpets, blankets, and musical instruments [5]. Sisal leaves are used for decortication, roots are applied for making alcoholic drinks, and the new shoots are known as an edible vegetable [11].
Conventional reproduction of A. sisalana could be sexually by seeds or asexually via offshoots from rhizomes and bulbils from the flowering stalk [12,13]. However, despite their potential properties, due to morphology, slow growth, low seed viability, long lifecycle of juvenile stage till maturity at flowering (reproductive) stage, and monocarpic nature of Agave plants, there are few attempts to improve and select for characteristics of interest [14,15,16]. Over-harvesting of Agave plants driven by the growing demands for alcoholic beverages, fiber-based products, herbal medicine, therapeutic agents, and bioethanol industries is causing severe seed shortage, ecological damage, significant harm to Agave populations and their natural habitats. As a result of overexploitation of these natural resources, many Agave species are endangered and are in danger of extinction and have not been intensively bred [17,18]. This situation highlights the crucial need for sustainable, mechanized, and large-scale cultivation. The biotechnological alternatives are urgently required for sustainable cultural practices, biodiversity management, long-term conservation, and reforestation of these significant species and their ecosystems [19,20,21,22].
Micropropagation is a viable tool to traditional vegetative propagation methods used for improving of agaves, achieving massive clonal propagation for obtaining large populations with desirable characteristics and stable genetic backgrounds, optimizing field production, cloning of selected elite high-yielding agaves for future breeding programs, induction of somaclonal variation to enhance agronomic traits of interest, conservation of specific genotypes, and preservation of biodiversity, producing of genetically modified plant materials for stress-tolerant development, and for overcoming obstacles associated with traditional propagation [16,23,24,25,26,27,28]. Micropropagation and regeneration systems through either organogenesis [14,23,29,30] or somatic embryogenesis [17,30,31], or bioreactors [32,33], have already been established for a few Agave species, hybrids, and for A. sisalana from different explants [3,16,34].
This work aimed to establish an efficient micropropagation protocol with a high-frequency shoot multiplication rate, improve subsequent rooting capacity then later a successful acclimatization for A. sisalana. In this context, we evaluated and compared the potential of three different cytokinins (i.e., BA, KIN, and TDZ) on shoot proliferation, shoot and root development, and acclimatization of A. sisalana
2. Materials and Methods
This research was performed in 2022–2023 at the Physiology and Breeding of Horticultural Crops Laboratory, Horticulture Department, Faculty of Agriculture, Kafrelsheikh University, Egypt.
2.1. Plant Materials
A 4-year-old A. sisalana; grown on the Horticulture Department farm, Faculty of Agriculture, Kafrelsheikh University, was used as a source of the explants. Small bulbils (plantlets that occur on the flowering stalks of Agave plants) were the explants used in the current study (Figure 1(1–3)).
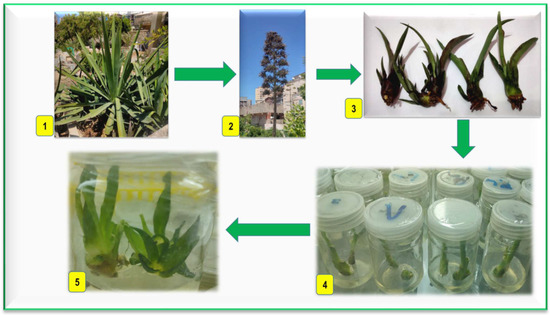
Figure 1.
Plant material and induction of axillary shoots; (1) sisal at vegetative stage; (2) sisal at flowering stage with bulbils; (3) bulbils explants; (4) bulbils after 4 weeks on MS-free medium at light; (5) split bulbils after 4 weeks on MS-free medium at light for shoots induction.
2.2. Establishment of Aseptic Cultures and Induction of Axillary Shoots
The bulbils were washed with tap water and some drops of liquid detergent for 20 min. Then, they were rinsed with distilled water twice. Under sterile conditions in the Laminar Air Flow, the bulbil explants were surface sterilized with 70% ethanol for one min, then immersed in a mercuric chloride solution (0.1% w/v) containing three drops of Tween-20 for 12 min (Loba Chemical Company, Mumbai, India). After sterilization, bulbil explants were rinsed four times with sterilized distilled water, 5 min each. The leaves of the bulbils were removed, leaving the lower part that includes the leaf bases and buds, and then cultured in a cylindrical glass jar with a capacity of 375 mL containing 50 mL of full MS medium without growth regulators, 3% (w/v) sucrose, and solidified with 6.0 g L−1 agar. The cultures were incubated in total darkness conditions for 2 weeks to control the browning phenomenon, which resulted from the oxidation of phenolic compounds and can damage the in vitro cultured tissues, and hamper the successful in vitro propagation of plants. Cultures are often initially incubated in the dark for a certain period, and frequently sub-culturing explants on fresh media could reduce this problem [35,36]. The explants were subcultured on the same medium and kept in light at 40 µmol m−2 s−1 photosynthetic photon flux density (PPFD) for 4 weeks (Figure 1(4)). After that, the bulbil explants were split into two halves to break the apical dominance of buds and enhance lateral branching. Then they were cultured on the same medium in a cylindrical glass jar of 150 mL capacity, containing 40 mL medium, then incubated for 4 weeks at 25 °C under 16/8 photoperiod by cool-white fluorescent light at 40 µmol m−2 s−1 PPFD for axillary shoots induction (Figure 1(5)).
2.3. Shoot Proliferation from Axillary Buds of Bulbil Explants
The split bulbil explants of A. sisalana were transferred from MS medium without growth regulators, then cultured on MS medium containing 3% (w/v) sucrose, and solidified with 6 g L−1 agar. The media were supplemented with different cytokinins (i.e., BA and KIN at concentrations of 2.0, 4.0, 6.0, 8.0, and 10 mg L−1; and TDZ at 0.5, 1.0, 1.5, 2.0, and 2.5 mg L−1). The three cytokinins were added separately to MS medium. MS basal medium without CKs served as a control. The media were poured into the cylindrical glass jar of 375 mL capacity, containing 50 mL medium. The cultures were incubated for 4 weeks at 25 °C under a photoperiod of 16/8 h with cool-white fluorescent light at 40 µmol m−2 s−1 PPFD for shoot multiplication. After 4 weeks of incubation, the bulbils with shoot clusters were subcultured on the same media twice, 4 weeks each. The number of shoots/explant, shoot length (cm), shoot fresh weight (g), number of leaves/shoot, number of roots/shoot, and root length (cm) were estimated after 12 weeks of culturing on cytokinins-supplemented MS media according to the following equation:
Number of shoots/explant = [(no. of shoot for the 1st explant + no. of shoot for the 2nd explant in the 1st replicate) + (no. of shoot for the 1st explant + no. of shoot for the 2nd explant in the 2nd replicate) + (no. of shoot for the 1st explant + no. of shoot for the 2nd explant in the 3th replicate)] ÷ 6
Or = Total number of shoots/total number of explants
Or = [no. of shoots for the 1st replicate + no. of shoots for the 2nd replicate + no. of shoots for the 3rd replicate…etc.]/no. of replicates
This equation could be applied to calculate the no. of leaves/shoot and the no. of roots/shoot.
Note: the explants were split bulbils in the shoot multiplication experiment and shoot clusters in the rooting experiment
2.4. Shoot Growth, Development, Elongation, and In Vitro Rooting
A. sisalana shoots clusters, from treatments that did not give roots (which produced on BA and TDZ-supplemented media), were separated into small clusters containing 3–4 shoots, then cultured on CK-free medium for their subsequent growth and elongation, development of new shoots from the buds, as well as for root formation. Shoot clusters cultured in a cylindrical glass jar of 375 mL capacity containing 50 mL medium. The cultures were kept at 25 °C and 40 μmol m−2 s−1 PPF at 16 h/d for 4 weeks. Shoots were subcultured on the same culture media. The number of shoots/explant, shoot length (cm), shoot fresh weight (g), number of leaves/shoot, number of roots/shoot, and root length (cm) were estimated after 8 weeks of culturing.
The pH of the media used in the study was set to 5.8 before autoclaving, and then the media were autoclaved at 121 °C and 105 Pa for 20 min.
2.5. Ex Vitro Acclimatization
Plantlets (produced on all treatments, shootlets which rooted directly on KIN-supplemented and MS basal media, and the shootlets produced on BA and TDZ-supplemented media and then required its transfer to MS-free medium for rooting induction), at the 3–4 leaf stage, were carefully removed from the medium, were rinsed well by tap water, then were dipped for 2 min in a fungicide solution (1 g L−1 Double 56% WP which contains 16% w/w 5-methylisoxazol-3-ol and 40% w/w dimethyl 4,4-(o-phenylene) bis (3-thioallophanate), StarChem Company, Giza, Egypt).
Plantlets were transplanted into culture black plastic pots (12 cm) filled with sterilized peat moss and vermiculite (2:1, v:v) treated with fungicide (1 g L−1 Moncut 25% WP of triflucro-3-isopropoxy-o-toluanilide), Shoura Chemical Company, Giza, Egypt). The plantlets were covered with a clear plastic (50 µ) for the first 30 days of culture in the greenhouse. The environmental condition in the shaded greenhouse was 25 ± 2 °C air temperature, relative humidity (60–70%), and 300 mol m−2 s−1 PPF. The plantlets were regularly fertigated using a fertilizer solution (N:P:K at 19:19:19) (Rosasol; Rosier, Moustier, Belgium) at 0.5 g L−1. After 40 days, the acclimatization percentage was recorded according to the equation:
Acclimatization percentage (%) = number of survival plants /number of acclimatized plants × 100
2.6. Experimental Design and Statistical Analyses
An experiment of multiplication was set up in a completely randomized design, and each cytokinin treatment had three replicates. Each replicate was represented by a culture jar containing two split bulbils. Data were subjected to analysis of variance using the CoStat program (Version 6.303, PMB 320, Monterey, CA, USA). The mean separations were done using Duncan’s multiple range test, which was determined at p ≤ 0.05. An experiment on rooting was set up in a completely randomized design with one treatment. The mean separations were carried out using Duncan’s multiple range test, which was determined at p ≤ 0.05.
3. Results
3.1. Effect of Cytokinins on Shoot Proliferation from Axillary Buds of Bulbils
The data shown in Table 1 indicated highly significant differences among cytokinin treatments for all recorded parameters. In vitro shoot multiplication of A. sisalana was significantly influenced by the type and concentration of cytokinins applied to MS medium. 1.0 mg L−1 TDZ produced a maximum significant number of proliferated shoots (14.67 shoots per explant), followed by BA then KIN at 4.0 and 8.0 mg L−1 recording 12.0 and 10.0 shoots/explant, respectively (Figure 2(1–3)). In contrast, 10.0 mg L−1 BA and CK-free medium recorded the lowest number of shoots (1.67 and 2.0 shoots, respectively) without significant differences from each other. The proliferated shoots on all KIN treatments were simultaneously rooted directly on the same proliferation media (Figure 3(1)). However, KIN induced a moderate number of shoots for most concentrations except 8.0 mg L−1 (10.0 shoots). While the other examined cytokinins (BA and TDZ) induced better shoot multiplication than KIN, they inhibited root formation on the shoot proliferation media. The maximum values for root and shoot length (3.83 and 14.03 cm, respectively) were observed on 4.0 mg L−1 KIN. On the other hand, the number of leaves/shoot was maximum on 8.0 mg L−1 KIN (7.67). CK-free treatment gave the highest value (3.67) of shoot weight. The optimum number of roots/shoot (12.0) was recorded for 4.0 mg L−1 KIN, followed by 2.0 and 6.0 mg L−1 KIN (10.0 and 10.3, respectively) without significant differences among the three treatments.

Table 1.
Effect of different cytokinins on shoot proliferation of A. sisalana from axillary buds of bulbils after 12 weeks of culturing.

Figure 2.
Effect of different cytokinins on shoot proliferation of Agave sisalana; (1) shoot proliferation on CK-free medium (left) and 8.0 mg L−1 KIN (right) after 12 weeks; (2) and (3) shoot proliferation on 4.0 mg L−1 BA and 1.0 mg L−1 TDZ after 12 weeks, respectively.

Figure 3.
In vitro rooting and acclimatization of Agave sisalana; (1) rooted plantlets on CK-free (left) and 8.0 mg L−1 KIN (right) containing medium after 12 weeks (subjected directly to acclimatization); (2) and (3) rooted plantlets propagated on media with 4.0 mg L−1 BA and 1.0 mg L−1 TDZ, respectively, after 8 weeks on CK-free medium for root induction; (4), (5) and (6) acclimatized plants after 4 weeks, 4 months, and one year, respectively.
3.2. Effect of CK-Free Medium on Shoot Growth, Elongation, and In Vitro Rooting
After three subcultures, 4 weeks each (12 weeks) on shoot proliferation media containing cytokinins of BA and TDZ, clusters of microshoots were transferred to CK-free medium. Growth of microshoots was more rapid, and the shoot multiplication rate was increased, compared to that induced on shoot proliferation media. Data shown in Table 2 presented highly significant differences for all treatments on the recorded measurements. It was noticed that shoot multiplication could be enhanced from the small buds on the shoot clusters produced previously on both BA and TDZ-supplemented media. The optimum number of shoots/explant (19.67) was recorded for 4.0 mg L−1 BA, followed by 1.0 mg L−1 TDZ (18.67) without significant differences. Maximum values of number of leaves/shoot and shoot length (8.67 and 17.17) were observed for 2.0 mg L−1 BA, while those for shoot weight (1.93) were observed for 10.0 mg L−1 BA. All shoots rooted in all treatments of BA, while only the first two treatments of TDZ (0.5 and 1.0 mg L−1) produced roots on the shoots (Figure 3(2,3)). However, the high concentrations of TDZ (1.5, 2.0, and 2.5 mg L−1) did not result in root formation. It could be concluded that BA at all examined concentrations and TDZ at low concentrations up to 1.0 mg L−1 enhanced shoot multiplication, growth, and elongation as well as root induction after placing shoots on CK-free medium. On the other hand, at the higher concentrations of TDZ (1.5–2.5 mg L−1), the number of multiplied shoots and shoot length decreased as the concentration of TDZ increased, and root formation was also completely inhibited on the shoots.

Table 2.
Effect of CK-free medium on shoot growth and root formation of Agave sisalana after 8 weeks of culturing.
3.3. Ex Vitro Acclimatization of Agave Sisalana In Vitro Plantlets
As shown in Table 3, all plantlets rooted directly on CK-free medium (control), and KIN-supplemented media during shoot proliferation could be acclimatized successfully, with a percentage of 100%. All shoots produced on BA containing media, as well as on media containing TDZ at 0.5 and 1.0 mg L−1, and then rooted on CK-free medium could also be acclimatized with 100% success (Figure 3(4–6)). Only 10% of shoots that were induced on 1.5 mg L−1 TDZ could be acclimatized. In contrast, all non-rooted shoots produced on the two higher concentrations of TDZ (2.0 and 2.5 mg L−1) failed to become acclimatized, recording 0% survival and finally died.

Table 3.
Acclimatization percentage of Agave sisalana in vitro plantlets after 40 days.
4. Discussion
Agave species are highly valued plants as they possess economic, cultural, gastronomic, and ecological significance with diverse industrial applications [37,38]. Since the world faces increasing global warming and climate changes resulting from consuming non-renewable resources, the production of eco-friendly natural plant fibers such as flax, cotton, jute, and sisal is required to provide a sustainable substitute for synthetic fibers [39]. Because of the great challenges and difficulties that face the conventional propagation of A. sisalana, there is an urgent need for micropropagation of this economically important plant for commercial production on a large scale to satisfy the growing global demand, especially for the fiber industry [20].
Plant tissue culture (PTC) is one of the biotechnology approaches with a wide range of applications. The most efficient technique for plant propagation, especially for endangered species, is enhanced shoot proliferation from axillary buds [40,41]. Shoot multiplication is a decisive stage that determines the economic efficiency of the micropropagation protocol; it directly affects the overall success of micropropagation of a certain species [42]. CKs are adenine-derived molecules involved in regulating various aspects of plant physiology and development, such as cell division, shoot proliferation, shoot and root development, embryo and seed development, organogenesis, metabolism, leaf senescence, chloroplast development, vascular differentiation, and influencing the response to environmental stimuli [43,44]. CKs significantly affect and regulate in vitro morphogenic responses and play a critical role during shoot organogenesis and regeneration [42,45]. CKs could stimulate the proliferation of axillary shoots by reducing apical dominance and promoting the growth of axillary buds from pre-existing meristems [46].
Earlier attempts were made to improve the in vitro multiplication rate of A. sisalana via direct shoot organogenesis [47], indirect organogenesis [48], or somatic embryogenesis [49]. Cytokinin was reported to be the essential factor for the micropropagation of Agave species. The highest multiplication rates of A. sisalana through direct or indirect organogenesis were obtained when BA was added alone to the culture medium. It was reported that the optimum shoot proliferation (18.50) from the basal stem of A. americana occurred on 13.32 µM BA (≈3.0 mg L−1) supplemented to half MS medium [3]. In contrast, the highest number of shoots (25.3) regenerated from callus culture of A. sisalana was obtained on 26.6 µM BA (≈6.0 mg L−1) added solely to the culture medium [48]. The current study reported that TDZ induced a significant increase in the shoot multiplication rate of A. sisalana rather than BA and KIN. Also, BA was more efficient than KIN in promoting shoot bud formation as reported for Quercus robur L. [50]. Similar results were reported on Aglaonema ‘Valentine’ [51]. However, it was reported that KIN at a concentration of 2.32 µM (≈0.5 mg L−1) is required to induce direct organogenesis in A. sisalana, and this concentration varies depending on the species [16]. Previous studies stated that TDZ significantly enhanced shoot multiplication in strawberries and other plant species, such as Capsicum species [52], Rauvolfia tetraphylla [53], and Cassia sophera [54].
The mechanism by which CKs could regulate in vitro regeneration of the explants should be addressed. Signaling pathways related to the effect of CKs are one of the important issues to increase the regenerative capacity of in vitro cultures. Histidine-phosphotransfer (HPt) protein has been reported to be responsible for transferring the phosphate signal that regulates the expression of the genes involved in CKs regulation to dominate the recalcitrance of in vitro cultured plants, and then enhance cell differentiation, proliferation, multiplication, and regeneration [55]. Cytokinin signaling is initiated by membrane-associated histidine kinase receptors and transduced through a phosphorelay system [56]. TDZ often has better cytokinin activity for promoting shoot multiplication than other adenine-type cytokinins, as it causes a high level of expression in BrCKX and BrIP genes [57]. TDZ promoted axillary bud proliferation in strawberry by enhancing cytokinin signaling transduction, which is crucial for cell division and shoot initiation. Moreover, the expression level of CRE1, AHP, and type-A ARRs genes was significantly increased after TDZ application [58].
A successful in vitro propagation protocol requires the optimal conditions for the development of regenerated shoots and root induction [59]. In addition to their significant role in shoot multiplication, CKs have also been implicated in different aspects of root initiation as they mainly promote the differentiation of meristematic roots, which is essential for root induction and development [43]. CKs signaling and perception are essential for root development [60]. However, different cytokinins can influence root development, with some promoting root growth while others inhibit it [61]. They are necessary for adventitious root (AR) formation in Jork 9 apple rootstock [62]. On the other hand, BA or TDZ can have serious side effects both during the shoot development, and as harmful post-effects during either the next propagation cycle (axillary or adventitious), or subsequent rooting and acclimatization processes. These side effects depend both on the type and on the concentration of cytokinins applied to shoot proliferation medium [63], and they can be different in different plant species or even genotypes and in different explant types. They, as a post-effect, can be harmful and manifested in difficult rooting or acclimatization [46]. The literature has investigated the post-effect of cytokinins (type and concentration) used in the proliferation medium on the subsequent rooting ability of ‘Red Fuji’ apple microshoots [64]. Also, CK content of the shoot regeneration media influenced the rooting features of in vitro shoots of ‘Royal Gala’ apple even after more subcultures on PGRs-free medium [65]. It was observed that TDZ or BA can negatively affect the rooting stage, potentially reducing or inhibiting the formation of roots in many plant species [61]. BA at 2.22 μmol L–1 (≈0.5 mg L−1) completely inhibited AR formation in GL-3 apple microshoots, whereas the control developed AR [62]. It was found that BA has prevented the root formation on A. americana adventitious shoots that were produced via indirect organogenesis at concentrations ranging between 0–10 mg L−1 [66]. The results showed that TDZ and BA had a carry-over effect that enabled shoots to continue proliferating on CK-free medium. This carry-over effect could be beneficial, promoting continued shoot proliferation and elongation, but it should be carefully managed. TDZ had a stronger carry-over effect, which causes hindering rooting in A. sisalana, than BA, and the latter allowed rooting after two subcultures on CK-free medium while TDZ needed more subcultures to enable rooting. The higher concentration of CKs inhibits in vitro rooting, root growth, or delays the formation of roots [67]. TDZ reduced rooting frequency in Tamarindus indica [68]. Rooting strongly inhibited in Leucaena leucocephala as TDZ level increased in shoot multiplication media [69]. The high carry-over effect of TDZ, especially at high concentrations, suggests subculturing the shoots to a hormone-free medium [70]. These results have been proven earlier by Huetteman and Preece [71] who stated that cytokinins inhibit root formation, especially when used in shoot proliferation medium at high concentrations or extended periods, hence subsequent rooting of microshoots may be slightly inhibited by prior exposure to TDZ due to the ‘carry-over’ effect of cytokinins in shoot induction medium. To avoid the bad side effects that might be caused by TDZ in in vitro plants such as difficulties in rooting following in vitro shoot multiplication [72], it should be added at very low or pulse concentrations, and the period of exposure must be shortened as well [59].
To our knowledge, there are no in vitro studies carried out on A. sisalana in Egypt, and there are limited and not recent studies on this plant species all over the world [5,34,48,49,73]. Wide-scale use of micropropagated A. sisalana would be the source that supplies us with adequate plant materials needed for large-scale plantations for economical purposes, reforestation, or to carry out studies on this species to assess its full potential, avoiding exploitation of wild plants [18].
5. Conclusions
Due to difficulties in establishing propagules using traditional methods, most ornamental, medicinal, and fruit plants urgently need to be propagated in vitro. The ability of CKs to regulate growth and key developmental processes in plants has remained important and offers new insights into their potential to achieve large-scale in vitro mass propagation. Even though 1.0 mg L−1 TDZ gave the highest significant number of shoots, it failed to support rooting. So, we recommended using BA at 4.0 mg L−1. It would be superior to develop an efficient in vitro propagation protocol for A. sisalana so that it can be established in the soil for large-scale production. For an effective micropropagation protocol of a certain species, the effects and after-effects of CKs should be addressed and managed carefully.
We studied the singular effect of one cytokinin added separately to MS medium on the in vitro propagation of A. sisalana. Further in vitro studies should be carried out to investigate the dual effect of cytokinins, and their interactions with auxins and/or gibberellins for the establishment of A. sisalana in Egypt as a fiber manufacturing, a new energy source, and for medicinal applications. Moreover, advanced plant breeding technologies, such as genetic transformation and genome editing, are crucial for the future improvement of this highly valuable plant.
Author Contributions
Conceptualization, M.E.E.-M.; methodology, M.K.S., N.A., and M.E.E.-M.; software, M.E.E.-M., and M.K.S.; validation, M.E.E.-M., and N.A.; formal analysis, M.E.E.-M.; investigation, M.K.S.; resources, M.E.E.-M.; data curration, M.E.E.-M. and N.A.; writing—original draft preparation, M.E.E.-M. and N.A. writing—review and editing, N.A., M.K.S., and M.E.E.-M.; visualization, M.E.E.-M., and N.A.; funding acquisition, M.E.E.-M. and N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank the staff members of the Physiology and Breeding of Horticultural Crops Laboratory, Department of Horticulture, Faculty of Agriculture, Kafrelsheikh University, Kafr El-Sheikh, Egypt, for supporting the completion of this work. The authors greatly thank Judit Dobránszki, University of Debrecen, Hungary, for editing and revising the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ávila-Reyes, S.V.; Jiménez-Aparicio, A.R.; Melgar-Lalanne, G.; Fajardo-Espinoza, F.S.; Hernández-Sánchez, H. Mezcal: A Review of Chemistry, Processing, and Potential Health Benefits. Foods 2025, 14, 1408. [Google Scholar] [CrossRef]
- Santiago-Martínez, A.; Pérez-Herrera, A.; Martínez-Gutiérrez, G.A.; Meneses, M.E. Contributions of agaves to human health and nutrition. Food Biosci. 2023, 53, 102753. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Hu, F.; Yang, H.; Yue, L.; Trigiano, R.N.; Cheng, Z.M. Micropropagation of Agave americana. HortScience 2014, 49, 320–327. [Google Scholar] [CrossRef]
- Rawat, M.; Varshney, A.; Kandpal, R.; Choudhary, A.; Gupta, A.K.; Pratiksha; Naik, B.; Kumar, V.; Kumar, A.; Kheto, A.; et al. Exploration of compositional, functional, nutraceutical, and metabolites of Ram kandmool (Agave sisalana Perrine) for potential application in food systems. Int. J. Biol. Macromol. 2025, 307, 142095. [Google Scholar] [CrossRef] [PubMed]
- Debnath, M.; Pandey, M.; Sharma, R.; Thakur, G.S.; Lal, P. Biotechnological intervention of Agave sisalana: A unique fiber yielding plant with medicinal property. J. Med. Plants Res. 2010, 4, 177–187. [Google Scholar]
- Costa, L.T.S.d.; Fracasso, J.A.R.; Guarnier, L.P.; Brito, G.R.d.; Fumis, D.B.; Camargo Bittencourt, R.A.d.; Guiotti, A.M.; Barros Barbosa, D.d.; Camargo, I.C.C.; Souza, E.B.d.; et al. Toxicity and Anti-Inflammatory Effects of Agave sisalana Extract Derived from Agroindustrial Residue. Plants 2023, 12, 1523. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; Gutiérrez-Mora, A.; García-Lara, S. Micropropagation of Agave salmiana: Means to Production of Antioxidant and Bioactive Principles. Front. Plant Sci. 2015, 6, 1026. [Google Scholar] [CrossRef]
- Chege, B.M.; Nyaga, N.M.; Kaur, P.S.; Misigo, W.O.; Khan, N.; Wanyonyi, W.C.; Mwangi, P.W. The significant antidyslipidemic, hypoglycemic, antihyperglycemic, and antiobesity activities of the aqueous extracts of Agave Sisalana juice are partly mediated via modulation of calcium signaling pathways. Heliyon 2023, 9, e12400. [Google Scholar] [CrossRef]
- Sari, N.H.; Suteja, S.; Hidayatullah, S.; Al-Farizi, F.H.; Lokantara, I.P. Performance evaluation of hybrid sisalana Agave fiber and carbon powder in polyester composites: A study on mechanical, thermal, and microstructural characteristics. Case Stud. Chem. Environ. Eng. 2025, 11, 101215. [Google Scholar] [CrossRef]
- Molina, A.; Kothari, A.; Odundo, A.; Prakash, M. Agave sisalana: Towards distributed manufacturing of absorbent media for menstrual pads in semi-arid regions. Commun. Eng. 2023, 2, 81. [Google Scholar] [CrossRef]
- Tewari, D.; Tripathi, Y.C.; Anjum, N. Agave sisalana: A Plant with High Chemical Diversity and Medicinal Importance. World J. Pharm. Res. 2014, 3, 238–249. [Google Scholar]
- Arizaga, S.; Ezcurra, E. Insurance against reproductive failure in a semelparous plant: Bulbil formation in Agave macroacantha flowering stalks. Oecologia 1995, 101, 329–334. [Google Scholar] [CrossRef]
- Arizaga, S.; Ezcurra, E. Propagation mechanisms in Agave macroacantha (Agavaceae), a tropical arid-land succulent rosette. Am. J. Bot. 2002, 89, 632–641. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Li, X.; Chen, Z.; Li, J.-F.; Lu, J.-Y.; Zhou, W.-Z. Shoot organogenesis and plant regeneration in Agave hybrid, No. 11648. Sci. Hortic. 2013, 161, 30–34. [Google Scholar] [CrossRef]
- Cruz García, H.; Campos Ángeles, G.V.; Enríquez del Valle, J.R.; Rodríguez Ortiz, G.; Velasco Velasco, V.A. Development of micropropagated plants of Agave americana var. Oaxacensis during greenhouse acclimatization. Rev. Mex. De Cienc. Agric. 2019, 10, 1491–1503. [Google Scholar]
- Bautista-Montes, E.; Hernández-Soriano, L.; Simpson, J. Advances in the Micropropagation and Genetic Transformation of Agave Species. Plants 2022, 11, 1757. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Aceves, L.; González-Arnao, M.T.; Santacruz-Ruvalcaba, F.; Folgado, R.; Portillo, L. Indirect Somatic Embryogenesis and Cryopreservation of Agave tequilana Weber Cultivar ‘Chato’. Plants 2021, 10, 249. [Google Scholar] [CrossRef]
- Chávez Ortiz, L.I.; Balch, P.M.E. In Vitro Propagation of Threatened Agave Species Using a Two-Stage System. Methods Mol. Biol. 2024, 2827, 165–178. [Google Scholar] [CrossRef]
- Singh, V.; Maiti, R.K. A review on in-vitro micropropagation of agave and other plants. Farm. Manag. 2020, 5, 108–114. [Google Scholar] [CrossRef]
- del Rosario, M.-H.M.; Abel, L.-B.J.; Karen, S.-F.M.; Adriana, C.-O.; Jabín, B.-B.J. Arbuscular mycorrhizal fungi improve the growth, nutrient uptake and survival of micropropagated agave (Agave marmorata Roezl) plantlets during acclimatization. J. Arid Environ. 2025, 228, 105330. [Google Scholar] [CrossRef]
- Santacruz-Ruvalcaba, F.; Castañeda-Nava, J.J.; Villanueva-Gónzalez, J.P.; García-Sahagún, M.L.; Portillo, L.; Contreras-Pacheco, M.L. Micropropagation of Agave maximiliana Baker by axillary bud proliferation. Polibotánica 2022, 54, 139–151. [Google Scholar] [CrossRef]
- Rieger, I.A. “Rewilding” the Mezcal Market: Cultural Practices and the Conservation of Agaves in Oaxaca, Mexico. Wild 2025, 2, 20. [Google Scholar] [CrossRef]
- Angeles-Vázquez, B.V.; Alvarez-Cervantes, J.; Tovar-Jiménez, X.; Rodríguez-Garay, B. Plant regeneration from indirect somatic embryogenesis of Agave salmiana 0tto ex Salm-Dyck subsp. salmiana using zygotic embryo obtained by in-casa pollination as explants. Polibotánica 2023, 56, 171–182. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; Espinosa-Leal, C.A.; García-Lara, S. Effects of saline elicitors on saponin production in Agave salmiana plants grown in vitro. Plant Physiol. Biochem. 2021, 162, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Reyna-Morales, C. Micropropagation of Agave montium-sancticaroli Shoots from Apical Buds. Master’s Thesis, University of Nuevo LeÓn, Faculty of Agronomy, San Nicolás de los Garza, Mexico, 2020. Available online: http://eprints.uanl.mx/20218/1/1080314060.pdf (accessed on 27 May 2025). (In Spanish).
- Robert, M.-L.; Herrera-Herrera, J.-L.; Castillo, E.; Ojeda, G.; Herrera-Alamillo, M.-A. An Efficient Method for the Micropropagation of Agave Species. Plant Cell Cult. Protocol. 2005, 318, 165–178. [Google Scholar]
- Wijerathna-Yapa, A.; Hiti-Bandaralage, J. Tissue Culture—A Sustainable Approach to Explore Plant Stresses. Life 2023, 13, 780. [Google Scholar] [CrossRef]
- Adu Donyina, G.; Szarvas, A.; Opoku, V.A.; Miko, E.; Tar, M.; Czóbel, S.; Monostori, T. Enhancing sweet potato production: A comprehensive analysis of the role of auxins and cytokinins in micropropagation. Planta 2025, 261, 74. [Google Scholar] [CrossRef]
- Lecona-Guzmán, C.A.; Reyes-Zambrano, S.; Barredo-Pool, F.A.; Abud-Archila, M.; Montes-Molina, J.A.; Rincón-Rosales, R.; Gutierrez-Miceli, F.A. In Vitro Propagation of Agave americana by Indirect Organogenesis. HortScience 2017, 52, 996–999. [Google Scholar] [CrossRef]
- Arzate-Fernandez, A.M.; Velasco, I.M.; Aragón, C.A.; Martinez-Martinez, S.Y.; Norman-Mondragon, T.H. Morphogenetic response of two agave species regenerated In vitro. Trop. Subtrop. Agroecosyst. 2020, 23. [Google Scholar] [CrossRef]
- Alvarez-Aragón, C.; Arzate-Fernández, A.M.; Martínez-Martínez, S.Y.; Martínez-Velasco, I. Regeneration of Agave marmorata Roezl Plants by Somatic Embryogenesis. Trop. Subtrop. Agroecosyst. 2020, 23, 36. [Google Scholar] [CrossRef]
- Correa-Hernández, L.; Baltazar-Bernal, O.; Sánchez-Páez, R.; Bello-Bello, J.J. In vitro multiplication of agave tobala (Agave potatorum Zucc.) using Ebb-and-Flow bioreactor. S. Afr. J. Bot. 2022, 147, 670–677. [Google Scholar] [CrossRef]
- Eucario, M.-Á.; Luis, S.-C.J.; Arturo, M.-M.T.R.; Francisco, P.-P.K.; Jabín, B.-B.J. Temporary immersion bioreactor as an efficient method for in vitro propagation of Agave marmorata. S. Afr. J. Bot. 2024, 169, 6–11. [Google Scholar] [CrossRef]
- Carneiro-dos Santos, F.; de Olivera, D.Q.S.R.; Rodríguez, P.A.; Neves, N.M.; Souza, S.K. Somatic embryogenesis in Agave Sisalana Perrine: Induction, anatomical characterization and regeneration. Pesqui. Agropecuária Trop. 2014, 44, 294–303. (In Spanish) [Google Scholar] [CrossRef]
- Amante, G.; Chimdessa, E. Control of browning in plant tissue culture: A review. J. Sci. Agric. 2021, 5, 67–71. [Google Scholar] [CrossRef]
- Liu, C.; Fan, H.; Zhang, J.; Wu, J.; Zhou, M.; Cao, F.; Tao, G.; Zhou, X. Combating browning: Mechanisms and management strategies in in vitro culture of economic woody plants. For. Res. (Fayettev) 2024, 4, e032. [Google Scholar] [CrossRef]
- Davis, S.D.; Ortiz-Cano, H.G. Lessons from the history of Agave: Ecological and cultural context for valuation of CAM. Ann. Bot. 2023, 132, 819–833. [Google Scholar] [CrossRef]
- Kablan, R.J.-F.; Sylvestre, M.; Onesippe-Potiron, C.; Bilba, K.; Kablan, A.L.C.; Arsène, M.-A.; Rousteau, A.; Cebrian-Torrejon, G. Agave species: A comprehensive review of taxonomy, chemistry, ethnobotany, and ethnopharmacology. In Studies in Natural Products Chemistry; Atta-Ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 80, pp. 187–225. [Google Scholar] [CrossRef]
- Ndzie Bidima, A.-M., II; Efeze, D.N.; Ebanda, F.B.; Enyegue, T.T.O.; Belinga, R.L.N.; Mbang, J.P.E. Modeling of mechanical behavior of Agave sisalana fiber’s yarn using the design of experiments method. Results Mater. 2024, 23, 100605. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Plant 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Alsanie, S.I. Insights on the Mesembryanthemum forsskalii phenotype and study of the effects of several exogenous plant growth regulators via plant tissue culture. BMC Plant Biol. 2025, 25, 15. [Google Scholar] [CrossRef]
- Nowakowska, K.; Pińkowska, A.; Siedlecka, E.; Pacholczak, A. The effect of cytokinins on shoot proliferation, biochemical changes and genetic stability of Rhododendron ‘Kazimierz Odnowiciel’ in the in vitro cultures. Plant Cell Tissue Organ Cult. 2022, 149, 675–684. [Google Scholar] [CrossRef]
- Svolacchia, N.; Sabatini, S. Cytokinins. Curr. Biol. 2023, 33, R10–R13. [Google Scholar] [CrossRef]
- da Cunha Neto, A.R.; Ambrósio, A.S.; Resende, A.J.R.; Santos, B.R.; Nadal, M.C. From Cell Division to Stress Tolerance: The Versatile Roles of Cytokinins in Plants. Phyton Int. J. Exp. Bot. 2025, 94, 539–560. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Steinmacher, D. Plant Growth Regulation in Cell and Tissue Culture In Vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Dobránszki, J.; Teixeira da Silva, J.A. Micropropagation of apple—A review. Biotechnol. Adv. 2010, 28, 462–488. [Google Scholar] [CrossRef] [PubMed]
- Das, T. Micropropagation of Agave sisalana. Plant Cell Tissue Organ Cult. 1992, 31, 253–255. [Google Scholar] [CrossRef]
- Hazra, S.K.; Das, S.; Das, A.K. Sisal plant regeneration via organogenesis. Plant Cell Tissue Organ Cult. 2002, 70, 235–240. [Google Scholar] [CrossRef]
- Nikam, T.D.; Bansude, G.M.; Aneesh, K. Somatic embryogenesis in sisal (Agave sisalana Perr. Ex. Englem). Plant Cell Rpt. 2003, 22, 188–194. [Google Scholar] [CrossRef]
- Martins, J.P.R.; Wawrzyniak, M.K.; Ley-López, J.M.; Kalemba, E.M.; Mendes, M.M.; Chmielarz, P. 6-Benzylaminopurine and kinetin modulations during in vitro propagation of Quercus robur (L.): An assessment of anatomical, biochemical, and physiological profiling of shoots. Plant Cell Tissue Organ Cult. 2022, 151, 149–164. [Google Scholar] [CrossRef]
- El-Mahrouk, M.E.; Dewir, Y.H.; Naidoo, Y. Micropropagation and Genetic Fidelity of the Regenerants of Aglaonema ‘Valentine’ Using Randomly Amplified Polymorphic DNA. HortScience 2016, 51, 398–402. [Google Scholar] [CrossRef]
- Peddaboina, V.; Thamidala, C.; Karampuri, S. In vitro shoot multiplication and plant regeneration in four Capsicum species using thidiazuron. Sci. Hortic. 2006, 107, 117–122. [Google Scholar] [CrossRef]
- Faisal, M.; Ahmad, N.; Anis, M. Shoot multiplication in Rauvolfia tetraphylla L. using thidiazuron. Plant Cell Tissue Organ Cult. 2005, 80, 187–190. [Google Scholar] [CrossRef]
- Parveen, S.; Shahzad, A. TDZ-induced high frequency shoot regeneration in Cassia sophera Linn. via cotyledonary node explants. Physiol. Mol. Biol. Plants 2010, 16, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Bidabadi, S.S.; Jain, S.M. Cellular, Molecular, and Physiological Aspects of In Vitro Plant Regeneration. Plants 2020, 9, 702. [Google Scholar] [CrossRef]
- Yang, W.; Cortijo, S.; Korsbo, N.; Roszak, P.; Schiessl, K.; Gurzadyan, A.; Wightman, R.; Jönsson, H.; Meyerowitz, E. Molecular mechanism of cytokinin-activated cell division in Arabidopsis. Science 2021, 371, 1350–1355. [Google Scholar] [CrossRef]
- van Voorthuizen, M.J.; Nisler, J.; Song, J.; Spíchal, L.; Jameson, P.E. Targeting cytokinin homeostasis in rapid cycling Brassica rapa with plant growth regulators INCYDE and TD-K. Plants 2021, 10, 39. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Pang, Y.; Hu, J.; Kang, X.; Qian, C. Thidiazuron Enhances Strawberry Shoot Multiplication by Regulating Hormone Signal Transduction Pathways. Int. J. Mol. Sci. 2025, 26, 4060. [Google Scholar] [CrossRef]
- Abdalla, N.; El-Ramady, H.; Seliem, M.K.; El-Mahrouk, M.E.; Taha, N.; Bayoumi, Y.; Shalaby, T.A.; Dobránszki, J. An Academic and Technical Overview on Plant Micropropagation Challenges. Horticulturae 2022, 8, 677. [Google Scholar] [CrossRef]
- Mähönen, A.P.; Higuchi, M.; Törmäkangas, K.; Miyawaki, K.; Pischke, M.S.; Sussman, M.R.; Helariutta, Y.; Kakimoto, T. Cytokinins regulate a bidirectional phosphorelay network in Arabidopsis. Curr. Biol. 2006, 16, 1116–1122. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Murthy, H.N.; Ammar, M.H.; Alghamdi, S.S.; Al-Suhaibani, N.A.; Alsadon, A.A.; Paek, K.Y. In vitro Rooting of Leguminous Plants: Difficulties, Alternatives, and Strategies for Improvement. Hortic. Environ. Biotechnol. 2016, 57, 311–322. [Google Scholar] [CrossRef]
- Tahir, M.M.; Fan, L.; Liu, Z.; Raza, H.; Aziz, U.; Shehzaib, A.; Li, S.; He, Y.; Lu, Y.; Ren, X.; et al. Physiological and molecular mechanisms of cytokinin involvement in nitrate-mediated adventitious root formation in apples. J. Integr. Agric. 2024, 23, 4046–4057. [Google Scholar] [CrossRef]
- El Mahrouk, M.E.; Dewir, Y.H.; Omar, A.M.K. In vitro propagation of adult strawberry tree (Arbutus unedo L.) through adventitious shoots and somatic embryogenesis. Propag. Ornam. Plants 2010, 10, 93–98. [Google Scholar]
- Magyar-Tábori, K.; Dobránszki, J.; Jámbor-Benczúr, E.; Bubán, T.; Lazányi, J.; Szalai, J.; Ferenczy, A. Post-effects of cytokinins and auxin levels of proliferation media on rooting ability of in vitro apple shoots (Malus domestica Borkh.) ‘Red Fuji’. Int. J. Hortic. Sci. 2001, 7, 26–29. [Google Scholar] [CrossRef]
- Magyar-Tábori, K.; Dobránszki, J.; Hudák, I. Effect of cytokinin content of the regeneration media on in vitro rooting ability of adventitious apple shoots. Sci. Hortic. 2011, 129, 910–913. [Google Scholar] [CrossRef]
- Luna, M.E.M.; del Valle, J.R.E.; Velasco, V.A.V.; Aparicio, Y.V.; Rodríguez, J.C.C. Benzyladenine concentration, type and dose of carbohydrates in the culture medium for shoot proliferation of Agave americana. Rev. Fac. Cienc. Agrar. Univ. Nac. Cuyo 2014, 46, 97–107. [Google Scholar]
- Van Staden, J.; Zazimalova, E.; George, E.F. Plant Growth Regulators II. Cytokinins, their analogues and antagonists. In Plant Propagation by Tissue Culture, 3rd ed.; George, E.F., Hall, M.A., de Klerk, G.-J., Eds.; The Background; Springer: Dordrecht, The Netherlands, 2008; Volume 1, pp. 205–226. [Google Scholar]
- Mehta, U.J.; Barreto, S.M.; Hazra, S. Effect of Thidiazuron in germinating tamarind seedlings. In Vitro Cell. Dev. Biol. Plant 2004, 40, 279–283. [Google Scholar] [CrossRef]
- Shaik, N.M.; Arha, M.; Nookaraju, A.; Gupta, S.K.; Shrivastava, S.; Yadav, A.K.; Kulkarni, P.S.; Abhilash, O.U.; Rishi, K.; Singh, S.; et al. Improved method of in vitro regeneration in Leucaena leucocephala—A leguminous pulpwood tree species. Physiol. Mol. Biol. Plants 2009, 15, 311–318. [Google Scholar] [CrossRef]
- Ali, H.M.; Khan, T.; Khan, M.A.; Ullah, N. The multipotent thidiazuron: A mechanistic overview of its roles in callogenesis and other plant cultures in vitro. Biotechnol. Appl. Biochem. 2022, 69, 2624–2640. [Google Scholar] [CrossRef]
- Huetteman, C.A.; Preece, J.E. Thidiazuron: A Potent Cytokinin for Woody Plant Tissue Culture. Plant Cell Tissue Organ Cult. 1993, 33, 105–119. [Google Scholar] [CrossRef]
- Abdalla, N.; Dobránszki, J. Meta-Topolin as an Effective Benzyladenine Derivative to Improve the Multiplication Rate and Quality of In Vitro Axillary Shoots of Húsvéti Rozmaring Apple Scion. Plants 2024, 13, 1568. [Google Scholar] [CrossRef]
- Binh, L.T.; Muoi, L.T.; Oanh, H.T.K.; Thang, T.D.; Phong, D.T. Rapid propagation of agave by in vitro tissue culture. Plant Cell Tissue Organ Cult. 1990, 23, 67–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).