1. Introduction
The pitaya, also known as pitahaya or dragon fruit, belongs to a group of climbing hemiepiphytic cacti that produce edible fruits. These species are native to the forests of northern South America, Central America, and Mexico [
1]. In their natural habitat, they grow along the shaded trunks of trees and climb into the forest canopy [
2]. Pitaya is widely cultivated in its original region, and also in Southeast Asia, particularly in Vietnam [
2,
3], and, more recently, in the Mediterranean region, including Spain, Italy, Turkey, and Israel [
4,
5]. The pitaya is part of the family Cactaceae, with
Selenicereus undatus (syn.:
Hylocereus undatus) [
6],
S. megalanthus, S. monacanthus, and
S. costaricensis being the species of greatest agronomic interest [
5,
7,
8].
S. undatus is often referred to as red pitaya due to the common magenta color of its fruit skin at ripening (
Figure 1a). This species is characterized by its spineless fruit and the presence of predominantly green bracts, which contribute to its distinctive and attractive appearance. In contrast,
S. megalanthus, known as yellow pitaya for the external color of its ripe fruit (
Figure 1b), is often preferred for its sweeter taste, but its fruit has spines on its surface [
1,
2].
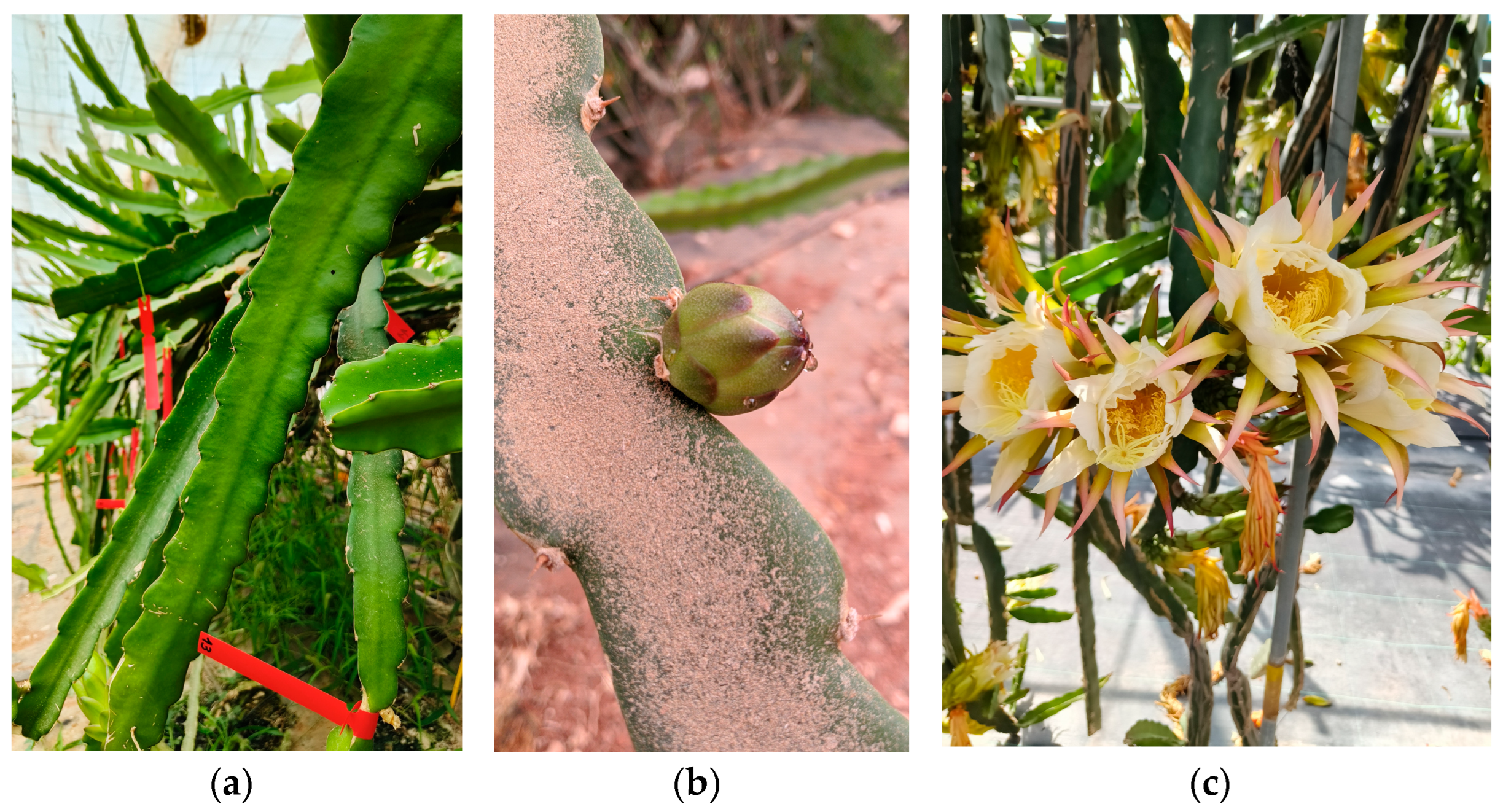
In general, pitayas have long, succulent triangular stems (
Figure 2a), known as cladodes, characterized by their highly developed parenchymal tissue. Cladodes serve as both, the photosynthetic and the fruit-bearing organs. They typically feature three edges or ribs with groups of small spines located on areoles (buds-forming sites) along their margins (
Figure 2b) [
2]. Pitaya flowering is closely linked to the photoperiod and is classified as a long-day plant. This means that floral induction occurs when the photoperiod reaches or exceeds 12 h per day [
9], but only in buds formed early in mature cladodes that have finished their apical growth [
10]. Then, for a cladode to be productive, its buds must mature and acquire what is referred to as “floral competence”, with the inductive photoperiod acting as the switch that initiates the flowering process. In this regard, Hsu [
11] and Jiang et al. [
12] report that floral competence, i.e., the ability of the buds to be induced to flowering once they reach a certain age and stage of maturity, is achieved 13.5–14.5 weeks after their formation in red pitaya. Once the inductive photoperiod has begun, the duration of floral bud emergence in Taiwan lasts for 9 weeks. In Israel, floral bud emergence requires a longer period, i.e., 14 weeks [
13], likely, because the temperature during the period of flower development in Taiwan is higher than in Israel.
Flowering in pitaya is characterized by its occurrence in waves, each lasting approximately one week [
14]. The number of flowering waves varies across pitaya species, ranging from 1 to 8 [
14,
15,
16]. Flowers (
Figure 2c) emerged from the areoles (in fact, compound buds) formed along the edges of 1- and 2-year-old cladodes, although the flowers can also develop on mature shoots less than one-year-old [
1]. Flower opening (anthesis) occurs during the last hours of the day, while wilting (senescence) takes place in the early hours of the following day [
17].
The flower, large and bell-shaped, is hermaphroditic, with an inferior ovary, a single pistil, and numerous stamens. The filaments of the anthers are slender and cream-colored [
7]. The upright flowers tend to orient toward lunar or solar light during the late and early hours of the day, respectively, facilitating the pollination process. In their natural habitat, pitayas are pollinated by frugivorous bats or moths [
17,
18]. In the absence of natural pollinators, hand pollination is required for most varieties due to the pronounced herkogamy of their flowers and their partial self-incompatibility. Bees are not usually considered efficient pollinators [
18], although they are frequent visitors of flowers in the mornings [
5,
19]. With an appropriate choice of pollen, the fertilization rate is remarkably high, resulting in an extraordinary fruit set, with a fruit/flower ratio close to 1 [
14]. The fruit is a multi-seeded berry with an elongated, ovoidal shape. Its epidermis presents, in red pitaya, elongated, waxy, and fleshy-scaled bracts [
20].
Given the climbing nature of pitaya, trellising systems are used to provide adequate support [
21]. Among these systems, the most well-known is the mop top system, widely used in South America and in Southeast Asia. The most common trellising system for greenhouse cultivation is the T-bar. In this case, the plant is guided to the trellis using stakes or strings, and once it reaches the wire (usually a braided wire with two or three strands covered with tubing) [
22], it is allowed to drop on either side of the trellis, forming two productive fruiting walls. Although installation costs are higher for this system, it optimizes the cultivation area, facilitates the cultural practices, and increases the yield [
23].
Regrettably, pitaya management is not yet fully established for many cultivation techniques, including pruning [
24]. Pruning in pitaya promotes the growth of fruitful cladodes and prevents the weight of the canopy from breaking down the supporting structure of the plants [
25]. In addition, pruning diminishes shading [
26], facilitates hand pollination [
22], reduces the incidence of several diseases [
27], and improves the quality of the fruit [
28]. Despite the benefits of pruning in pitaya, most references dealing with it are limited to recommending sanitary pruning, removing dead and broken cladodes and those affected by diseases. Nonetheless, recent research has focused on determining the type and intensity of pruning. In this regard, Arredondo et al. [
29] compared three pruning methods: short pruning, which consisted of eliminating approximately two thirds of each cladode, leaving only four to five basal areoles or compound buds (in each cladode edge); long pruning, which consisted of removing only the terminal 2–3 buds by tipping the cladode; and mixed pruning, where half of the cladodes were trimmed, while the remaining cladodes were heavily cut through short pruning, expecting thus to facilitate cladode renewal. Productive results clearly demonstrated the superiority of long pruning over short pruning, with mixed pruning yielding intermediate results. After establishing long pruning as the most effective procedure, Chiamolera et al. [
28] investigated the optimal pruning intensity by examining its effects on subsequent flowering and fruit production. They compared leaving 6, 9, 12, or 15 cladodes per linear meter in pitayas trained on a trellis system consisting in two productive walls 1.8 m in height. The results indicated that the optimal pruning intensity ranged between 12 and 15 cladodes per linear meter, with the highest flowering levels expected at 14 cladodes per linear meter. After these experiments, determining the optimal pruning date is now considered of highest interest as the next step. In Southeast Spain, pruning of pitaya is commonly performed in March. This timing is chosen due to the end of winter and the onset of spring, which provides longer daylight hours and moderate temperatures compared to earlier months. These conditions favor the reactivation of pitaya growth.
The main objective of this experimentation is to determine the effects of early pruning (in January) on the reproductive cycle of ‘Korean White’ pitaya compared to the development of plants under the traditional pruning date of March.
2. Materials and Methods
2.1. Experimental Sites and Crop Management
The experiment was performed at the UAL Anecoop Foundation, located in Almería (SE Spain) (longitude 2°17′02″ W, latitude 36°51′54″ N), at an altitude of 95 m above sea level and 5 km from the Mediterranean Sea. According to the classification of Papadakis [
30], the climate of the area is semiarid subtropical Mediterranean, with a mean annual temperature of 18.5 °C, December and January being the coldest months and August the warmest. The average accumulated rainfall in the area does not exceed 250 mm per year, while the average annual relative humidity ranges from 67% to 73%. Bright, sunny days are very frequent, and sunlight hours average 3273 h per year. Day length, which determines floral induction in pitaya, varies in this region between 14 h/10 h (day/night) in summer and 10 h/14 h in winter. Since a critical photoperiod of 12 h has been established for red pitaya [
9], the inductive day length in our latitude begins in mid-March and ends in mid-October. The soil in the experimental plot is sandy-clay loam, with 46.4% sand, 20.6% silt, and 33.0% clay, based on samples taken at a depth of 20 cm, as most pitaya roots grow in the top 40 cm of soil [
2,
31].
The pitayas used in these trials belonged to
S. undatus cultivar ‘Korean White’. The experimental plants were cultivated in a parral-type greenhouse with a maximum ridge height of 4.5 m and 4.0 m at the sides, and orientated ESE–WNW. The greenhouse cover was made of 200 μm thick polyethylene with 10 × 20 threads per cm
2 mesh on the sides. The greenhouse was equipped only with natural side ventilation. To avoid excess solar radiation during the summer months, the greenhouse cover was coated with a calcium carbonate suspension, aiming for 30–40% shading [
32,
33,
34]. Reducing sunlight is important for this crop, as excessive radiation causes the yellowing of the cladodes [
32].
The experiments were carried out in two consecutive years (2023 and 2024) on experimental pitayas planted in 2017. The plants were trained in a double-curtain trellis system, forming a hedgerow consisting of two parallel walls of a height of 1.8 m. The walls are the product of two plant rows 50 cm apart from each other. Each production wall is, in turn, formed by a line of plants separated by 1 m of each other, each early trained to develop two main stems. The plants are supported by a welded mesh fence and horizontal wires on which the cladodes rest.
‘Korean White’ is considered partially self-compatible but requires manual pollination due to the absence of natural pollinators and its pronounced herkogamy (spatial separation of anthers and stigma within the same flower). During bloom periods, hand pollination was performed at anthesis during the mornings (from 5:30 to 7:00 a.m.), using fresh pollen collected from open flowers of nearby plants of the same variety. Pollen grains were applied by hand to the stigma of each open flower using a fine paintbrush.
The pitayas were drip-irrigated and fertigated following common recommendations in the area, with 300 units of nitrogen, 600 units of phosphorus, and 900 units of potassium per year, applied proportionally to the volume of irrigation water (between 4200 and 4500 m
3 per hectare, depending on the season), supplied via drip irrigation. Irrigation volume and fertilization rates were adapted to the local climate following the recommendations of Méndez and Coello [
20].
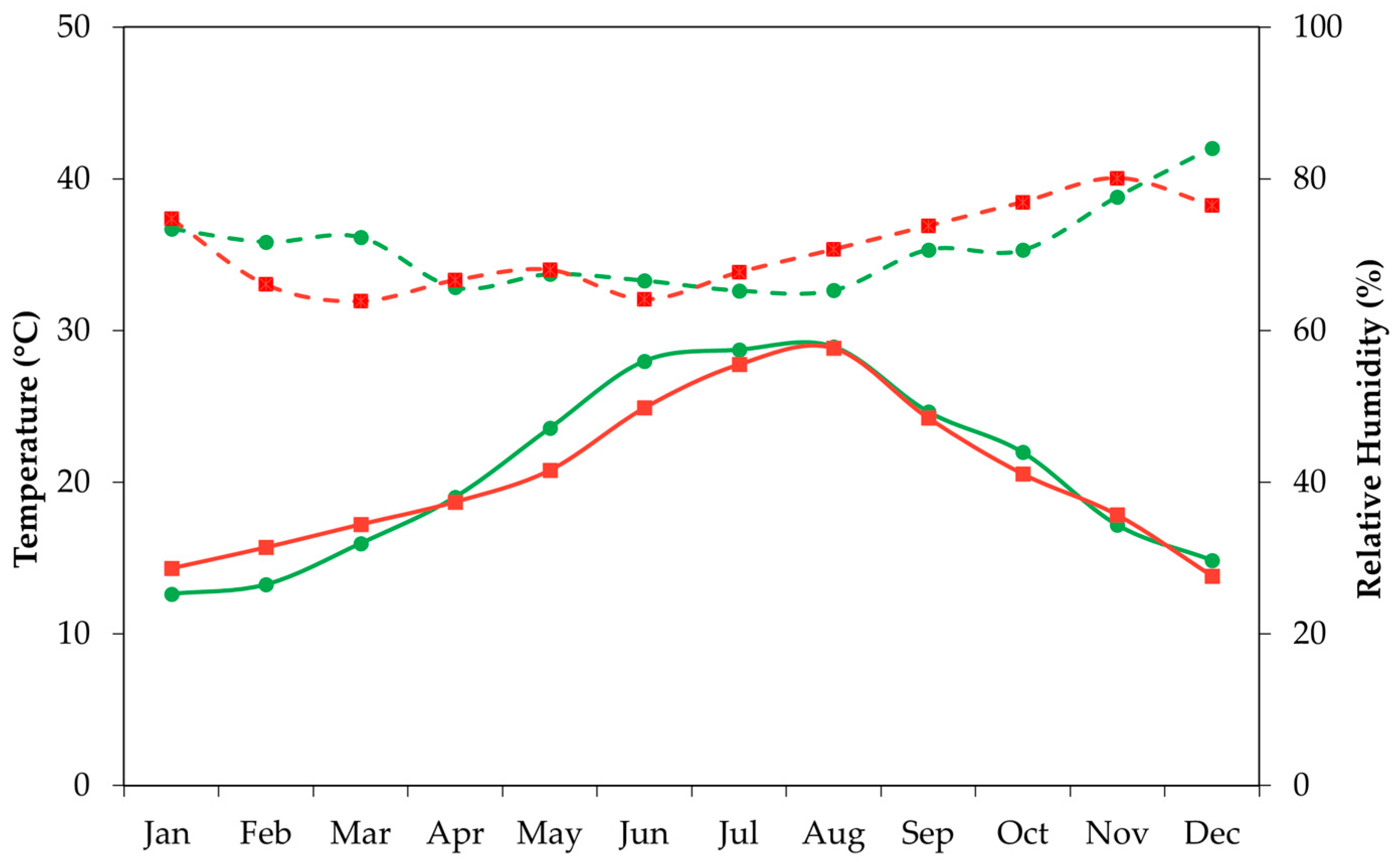
Figure 3 shows the average values for temperature and relative air humidity within the greenhouse. Pest and disease control was conducted following integrated management practices. All cultural practices, except pruning, were carried out in the same manner for all experimental plants.
2.2. Treatments and Experimental Designs
The experimental design was a completely randomized experiment with two pruning dates as treatments (January and March) and eight replicates (four on each side of the hedgerow). Each replicate consisted of 2 m length of the trellis, including two plants per replicate. In both treatments, long pruning (tipping only the last four to five apical nodes of the cladodes) was performed in mid-January or mid-March leaving only 14 well-illuminated cladodes per linear meter of curtain (28 cladodes per replicate), prioritizing the elimination of damaged, diseased, shaded, and clearly unproductive cladodes, cutting them through their insertion point [
27]. Young cladodes formed after pruning in spring, when the vegetative dormant bud sprouted, were left intact.
2.3. Measured Parameters
Reproductive performance (flowering waves and flower production), fruit quality, and fruit size distribution were evaluated. To this end, the number of floral buds per cladode, the number of fruits and the resulting fruit set, and fruit size (weight, equatorial diameter, and length) were recorded and compared between the two treatments. Additionally, fruit skin color and sweetness [total soluble solids (TSS) content] were measured. The distribution of the fruit based on weight intervals was calculated.
Three flowering waves were obtained in each of the two years that the trial was conducted. Floral bud counts were performed on 1 August and 1 September 2023, and on 28 June and 22 August 2024, corresponding to the first and second flowering waves, respectively, for each year. Fruit set, as the percentage of fruits formed after pollination, was determined by counting fruits on 1 September and 10 October 2023, and on 7 August and 7 October 2024. In the case of the third flowering wave, no precise records were obtained because the appearance of the floral buds was low, erratic and scattered in both years.
Given the non-climacteric nature of pitaya, harvesting was carried out at full fruit ripening, assessed by the complete development of magenta skin color. In 2023, the harvest date for the first wave was 13 September. For the second wave, harvesting took place between 9 and 26 October. Finally, the third wave was harvested on 13 December, once the fruits reached an acceptable total soluble solid content. In 2024, harvesting was carried out on 7 August and 7 October for the first and second waves, respectively, and between 11 and 18 November for the third wave. Fruit number and average weight in each wave allowed us to estimate total yield.
The size of the fruit, its TSS content, and fruit skin color were measured in random samples of eight mature fruits per replicate from waves with sufficient fruit production (first and second harvest). The size of each fruit was estimated by measuring its weight, length, and equatorial diameter. Fruit weight was measured using a digital scale (Model EK1200i, A&D, Seoul, Republic of Korea). The diameter and length were recorded at the equatorial zone of each fruit by using a digital caliper (Powerfix Profi, OWIN GmbH & Co. KG, Neckarsulm, Germany). Total soluble solids (TSS) content was measured using a digital refractometer (Model PAL-1, Atago Co., Tokyo, Japan) from the juice of one-quarter of each fruit; data are expressed in °Brix. Skin color was measured at the center of both sides (more and less exposed to sunlight) of each fruit using a portable colorimeter (Model CR200, Konica-Minolta Co., Osaka, Japan). Color measurements of ripe fruits included color lightness (
L*), the red–green component (
a*), the yellow–blue component (
b*), Chroma (C*), and hue angle (
H). In the absence of European and national legislation, fruit size grading was performed according to the “Codex Standard for Pitahaya” (“CODEX STAN 237-2003”) of the Food and Agriculture Organization of the United Nations [
35]. With the values obtained for the hue angle and color saturation, we calculated the Color Index for Red Table Grapes (CIRG) proposed by Carreño et al. [
36] to make a clear distinction among different degrees of red (including magenta), blue, and violet. The CIRG was calculated using the formula CIRG = (180 − arctangent
b*/a*)/(L* + C*).
Finally, given the lack of response to the date of pruning in most of the parameters under measurement, an additional test was conducted to determine if early pruning significantly promoted more vegetative growth. Then, after harvesting the last 2024 season fruit, plants were subjected to early pruning and control pruning in January 2025 and March 2025, respectively, and pruned cladodes were weighed using a field scale with an accuracy of ±0.05 kg. All parameters were compared by Student’s t-test using the Statistix v. 8.0 software package (Analytical Software, Tallahassee, FL, USA).
3. Results
Pruning dates did not affect flowering levels or fruit set, although slightly higher, non-significant values were observed for the control (pruning in March) (
Table 1). Fruit set during the first flowering wave was very high in both treatments, and most flowers set fruit after hand pollination. In contrast, the second wave of bloom produced a substantially lower fruit set (
Table 1), perhaps because the number of flowers was much higher. Last (third) flowering wave was erratic and mostly produced on current-year growth in both treatments and seasons. It was also asynchronous, blooming even before the second harvest took place. Given the low number of flowers in 1-year-old cladodes, many replicates lacked flowers and fruit. Nonetheless, the number of fruit produced in this wave on non-tagged cladodes—that is, in the new growth—was similar in both treatments (
p = 0.62). An average of 15.8 fruit per replicate were produced in the last harvest in plants pruned in January 2023, compared to 13.9 when pruning was performed in March 2023. In the next season, the production was much lower but again similar between treatments (3.3 vs. 3.6 fruit per replicate for January versus March pruning, respectively;
p = 0.78). Consequently, the results indicate that pruning date (January vs. March) did not have a statistically significant effect on the yield per wave or on the total yield in either of the two years (
Table 2).
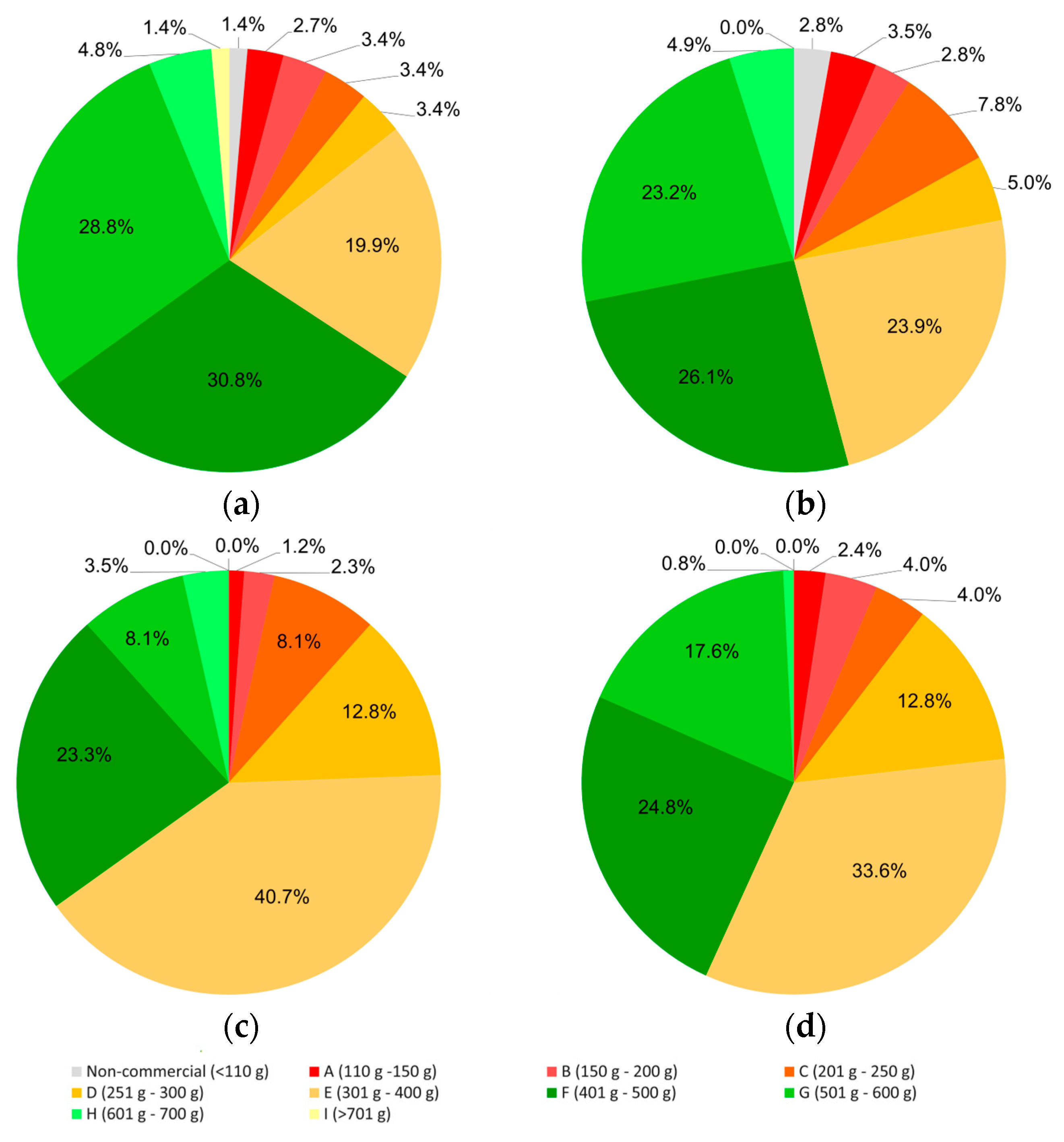
Fruit size distribution following the “CODEX STAN 237-2003” norm [
35] showed that in 2023, more fruit fell in higher categories for pruning performed in January. Thus, 66% of the fruit obtained after early pruning weighed 400 g or more, whereas this percentage was only 54% in the plants pruned in March. Similarly, the discard percentage, although always low, was reduced when pruning was performed in January compared to pruning in March (1.4% vs. 2.8%) (
Figure 4). However, the situation was somehow reversed in 2024, when only 35% of the fruit harvested from plants pruned in January weighed more than 400 g, compared to 43% in plants pruned in March. Nonetheless, a higher proportion of small fruit, below 200 g, was also harvested in late-pruned plants.
Fruit quality was seldom improved by early pruning. Nonetheless, and although significant differences were rarely found, early pruning seemed to promote fruit growth, with occasional increases in fruit weight and length (
Table 3,
Table 4 and
Table 5). On the contrary, fruit diameter was not significantly better when pruning was performed in January. No significant effects of early pruning were observed in any parameter of the CIELAB color space of the skin, whether measured on the shiny side or duller side of the fruit (
Tables S1 and S2). In this regard, no effects were noted on lightness (
L*), the green–red axis (
a*), or the blue–yellow axis (
b*), nor on Chroma (
C*) or hue angle (
H) (
Tables S1 and S2). However, the slightly higher skin color values observed in fruit harvested from plants pruned in January caused occasional improvements in the CIRG index, meaning that pinker fruit were produced under early pruning. The differences reached statistical significance only in the second harvest of 2023 (
Table 4). Similarly, soluble solid content did not significantly vary between treatments in any case, although a trend for higher values was observed in fruit harvested for plants pruned in January.
Finally, a heavier weight of the pruned cladodes was found in 2025, corresponding to the 2024 season growth, in the treatment of early pruning (January), but again, the differences between treatments were small and not significant (p = 0.82) (71.95 vs. 68.48 kg of old cladodes removed per replicate for January and March pruning treatments, respectively).
4. Discussion
The date of pruning did not consistently modify pitaya blooming or yield in our environmental conditions. No advancement in flowering dates was achieved by anticipating pruning dates from March to January. On the contrary, according to Jiang [
37], early pruning in Taiwan, performed between November and December, resulted in a higher proportion of the buds transforming into flowers. However, the flowering dates during the initial flowering waves remained unchanged compared to the control in his experiment, as they were in our case for the three flowering waves produced in 2023 and in 2024. The different response between our experimentation and that carried out in Taiwan for the noted increased flowering could be due to the differential temperatures between the experimental sites (subtropical SE Spain versus tropical Taiwan). Consistent with the results obtained in Taiwan by Jiang [
37], Le Bellec et al. [
8], and Crane and Balerdi [
38] also recommend performing pruning immediately after the last harvest to promote new growth that may become the fruitful organs the following season in the warmer climates of Reunion Island and Florida, respectively. However, despite our experiment being conducted in a greenhouse, winter temperatures at our site remained moderate (
Figure 3), with cold weather prevailing during the 2023 season. Weather improved slightly in winter 2024, but not enough to modify substantially the reproductive cycle of ‘Korean White’ in our location.
According to our hypothesis, the last flowering wave was produced on young but mature enough cladodes, supporting the idea that sooner sprouting due to early pruning could result in higher late-season flowering on young cladodes [
37]. Jiang et al. [
39] state that in white-fleshed pitayas, early pruning accelerates shoot growth, allowing new buds to mature and become responsive to environmental cues earlier. For this reason, they recommend retaining some cladodes sprouting in spring after pruning to produce late-season fruit. Tipping and removal of growing vegetative buds are recommended to achieve early maturation and floral competence in early pruning practice [
37,
39]. While we had removed in spring some new vegetative shoots, we did not tip the remaining new cladodes, as recommended by these authors.
The procedure recommended by Jiang and colleagues [
37,
39], still to be proved successful in our environmental conditions, suggests, on the contrary, the utility of using heating during winter months in our Mediterranean greenhouses in order to obtain the desired results. In this context, Trivelini et al. [
4] have observed in Italy two main sprouting events; the major peak, accounting for 83% of the yearly sprouting, occurs between March and July, whereas a second lower peak occurred between October and November. Therefore, it seems that advancing pruning operations may have little effect in the Mediterranean countries, unless we advance the season by heating the greenhouse. In this regard, temperature and photoperiod are the most relevant exogenous factors controlling pitaya growth and flowering [
9,
40]. The use of artificial lighting, still scarcely used in our region, may ensure that the new growth attains maturity when the photoperiod is still long enough. Hsu [
11] and Jiang et al. [
12] stated that floral competence in red pitaya buds is reached 13.5 and 14.5 weeks after their formation. After this, prevalent temperatures assume a key role in flower development. Our records show that greenhouse winter temperatures were not high enough to promote bud sprouting and shoot growth (
Figure 3). Results from Chu and Chang [
41] suggest that 29/19 °C may be the minimum required temperature for reproductive bud emergence in white-fleshed pitaya, temperatures that were not reached during our winters, neither in 2023 nor in 2024 (
Figure 3). Previous successful experiences with heating a greenhouse for the cultivation of papaya in our experimental site have to be carefully assessed [
42]. The implementation of active climate control for papaya cultivation, including winter heating and fogging in summer, represented a cost of around 7 EUR/m
2, and although the improvements in fruit quality, yield and seasonality compensated for the higher cost, the current price of fuel and the higher base temperature of pitaya compel us to be cautious. Ongoing experiments try to determine the precise base temperature for ‘Korean White’ in order to assess the profitability of implementing greenhouse heating. An aspect that merits further discussion is the clear reduction in fruit weight during the second, more important harvest (
Table 4). Differences in average fruit weight in this heavier harvest were accompanied by a reduction in TSSs (
Table 4), suggesting resource competition was operating during the more intense second flowering wave in ‘Korean White’. Fruit set was also markedly reduced in both treatments during the second, more abundant flowering wave. This was partially due to the higher flower bud drop that takes place in pitaya when the number of flowers per cladode increases as observed by Trivelini et al. [
4] during the most intense flowering wave of pitaya in Italy. In this regard, we have also found a positive relationship between flowering intensity in the cladode and the rate of flower bud drop, suggesting that resource competition plays a role [
27]. Nonetheless, bud abortion in cladodes with few flowers suggests that other factors, such as high ambient temperatures and excess of irrigation, also contribute to bud drop [
4,
40,
41].
Partial self-incompatibility of ‘Korean White’ and reduced pollen viability during summer surely also played a role in reducing average fruit weight, since we found a substantial amount of small-sized fruit in the second, more intense flowering wave (
Figure 4). Conversely, the slight but significant increase in fruit weight observed in the third harvest during the first season in response to early pruning, though not in the second year (
Table 3,
Table 4 and
Table 5), led to better fruit grading (
Figure 4). A trend for producing sweeter fruit after early pruning was observed, although the differences were never significant (
Table 3,
Table 4 and
Table 5). This occasional improvement in fruit quality could be due to the fact that earlier bud sprouting produced a larger canopy, with a greater capacity to produce and export photo-assimilates, leading to slightly heavier, sweeter fruit. In fact, according to the weight of the pruned cladodes, early pruned plants produced more cladodes or longer cladodes, although the differences were again not significant. Furthermore, an excess of cladodes implies more time for pruning execution in January and a higher cost. Pruning during mid-winter may also delay scarring and facilitate bacterial diseases.