Control of Neopestalotiopsis zimbabwana Using Origanum vulgare L. Essential Oil: Combined In Vitro, In Vivo and In Silico Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological and Molecular Identification
2.2. Plant Material and Hydrodistillation: OEO/HOV Co-Collection, Separation
2.3. GC–MS Analysis of OEO
2.4. In Vitro Assay (PDA), MIC and MFC Definitions
2.5. Leaf Phytotoxicity Screen and Formulations
2.6. In Vivo Assay in Strawberry Plants, Treatments, Inoculation and Disease Assessment
2.7. In Silico Docking Against Cytochrome b (PDB: 5TL8)
3. Results
3.1. Morphological and Molecular Identification
3.2. Chemical Composition of O. vulgare Essential Oil (GC–MS)
3.3. In Vitro Antifungal Activity of Origanum vulgare Essential Oil
3.4. Leaf Phytotoxicity Screen and Formulations
3.5. In Vivo Efficacy of the OEO-Based Formulation in Strawberry Plants
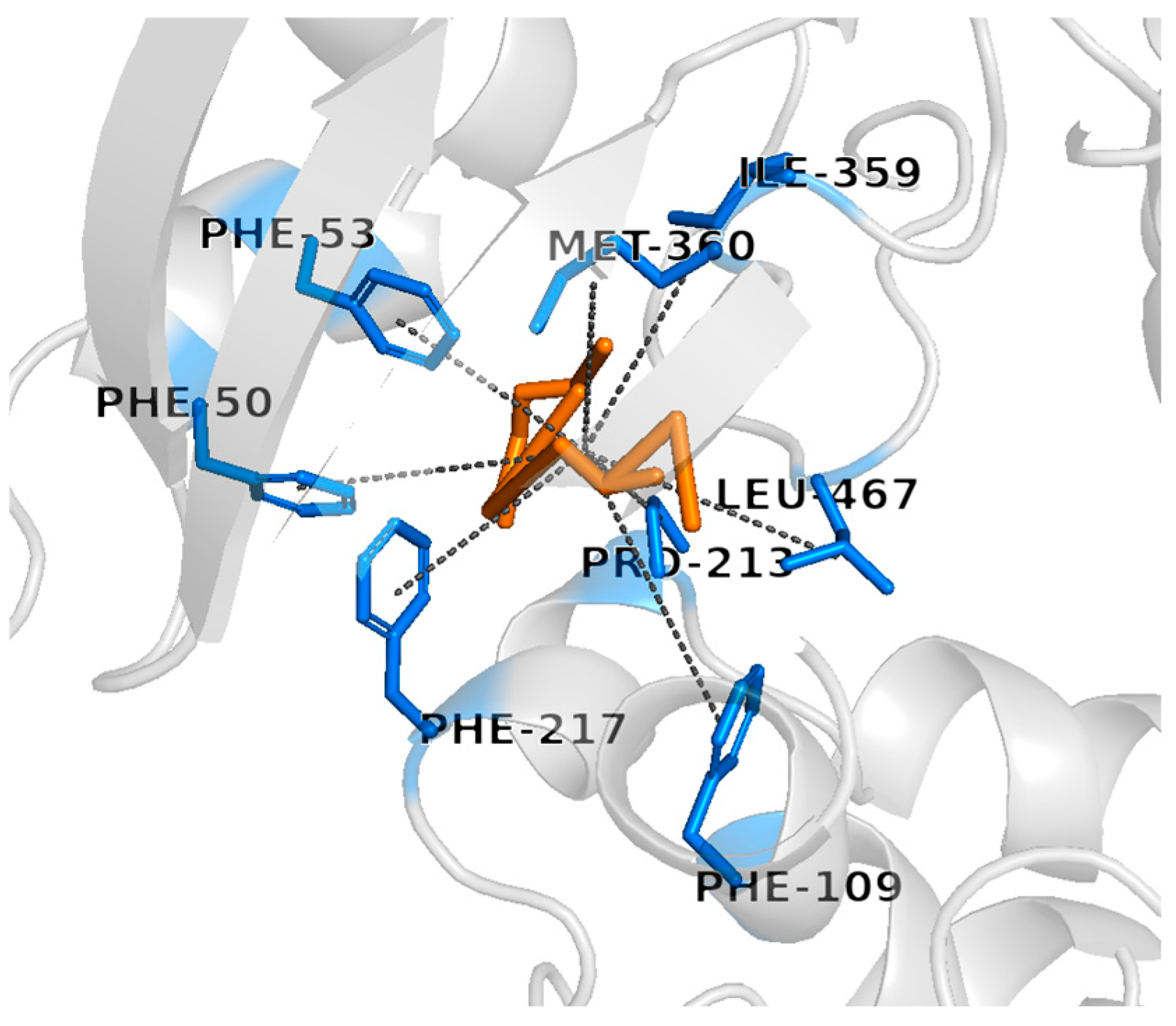
3.6. In Silico Docking Analysis Against Cytochrome b
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dowling, M.; Peres, N.; Villani, S.; Schnabel, G. Managing Colletotrichum on Fruit Crops: A “Complex” Challenge. Plant Dis. 2020, 104, 2301–2316. [Google Scholar] [CrossRef] [PubMed]
- OuYang, Q.; Liu, Y.; Oketch, O.R.; Zhang, M.; Shao, X.; Tao, N. Citronellal Exerts its Antifungal Activity by Targeting Ergosterol Biosynthesis in Penicillium digitatum. J. Fungi 2021, 7, 432. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Hernández, J.G.; León-Ramírez, C.G.; Abraham-Juárez, M.D.R.; Tlapal-Bolaños, B.; Olalde-Portugal, V.; Délano-Frier, J.P.; Martínez-Antonio, A.; Aguilar-Zárate, P. Neopestalotiopsis spp.: A Threat to Strawberry Production and Management. Horticulturae 2025, 11, 288. [Google Scholar] [CrossRef]
- Dardani, G.; Martino, I.; Aloi, F.; Carli, C.; Giordano, R.; Spadaro, D.; Guarnaccia, V. Characterization of Neopestalotiopsis Species Associated with Strawberry Crown Rot in Italy. Agronomy 2025, 15, 422. [Google Scholar] [CrossRef]
- Obregón, V.G.; Meneguzzi, N.G.; Ibañez, J.M.; Lattar, T.E.; Kirschbaum, D.S. First Report of Neopestalotiopsis clavispora Causing Root and Crown Rot on Strawberry Plants in Argentina. Plant Dis. 2018, 102, 1856. [Google Scholar] [CrossRef]
- Sánchez Cortez, K. Actividad Antifúngica de Extractos de Plantas Aromáticas y Medicinales. Master’s Thesis, Colegio de Postgraduados, Programa de Posgrado en Botánica, Montecillo, Mexico, 2024. [Google Scholar]
- Solarte, F.; Muñoz, C.G.; Maharachchikumbura, S.S.N.; Álvarez, E. Diversity of Neopestalotiopsis and Pestalotiopsis spp., Causal Agents of Guava Scab in Colombia. Plant Dis. 2018, 102, 49–59. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Brittain, G.D.; Aglave, B.; Sances, F.V. First Report of Neopestalotiopsis rosae Causing Crown and Root Rot of Strawberry in California. Plant Dis. 2023, 107, 566. [Google Scholar] [CrossRef]
- MacKenzie, S.J.; Mertely, J.C.; Peres, N.A. Curative and Protectant Activity of Fungicides for Control of Crown Rot of Strawberry Caused by Colletotrichum gloeosporioides. Plant Dis. 2009, 93, 815–820. [Google Scholar] [CrossRef]
- Wang, H.; Sun, J.; Liu, Q.; Li, C.; Luan, Y. Dissipation and Dietary Risk Assessment of Prochloraz in Strawberries under Greenhouse Conditions. Molecules 2023, 28, 7498. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, M.; Zhou, Q.; Jiao, Y.; Sun, H.; Cheng, X.; Xue, X. Study on Effects of Different Concentration Adjuvants on the Properties of Prochloraz Emulsion in Water Solution Droplets and Deposition. Agronomy 2023, 13, 2635. [Google Scholar] [CrossRef]
- Massi, F.; Torriani, S.F.F.; Borghi, L.; Toffolatti, S.L. Fungicide Resistance Evolution and Detection in Plant Pathogens: Plasmopara viticola as a Case Study. Microorganisms 2021, 9, 119. [Google Scholar] [CrossRef]
- Suryanarayanan, S. Pesticides and pollinators: A context-sensitive policy approach. Curr. Opin. Insect Sci. 2015, 10, 149–155. [Google Scholar] [CrossRef]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, distribution pathways and effects on human health—a review. Toxicol. Rep. 2021, 8, 1179–1192. [Google Scholar] [CrossRef]
- Jimenez Madrid, A. Neopestalotiopsis Leaf Spot and Fruit Rot Disease. 2025. Available online: https://smallfruits.org/2025/01/survey-of-neopestalotiopsis-leaf-spot-and-fruit-rot-disease-in-the-southeastern-us/ (accessed on 27 August 2025).
- Brannen, P. Dramatic Neopestalotiopsis Disease in Strawberry Tips and Plug Plant Production Nurseries. 2024. Available online: https://site.extension.uga.edu/strawberry/2024/08/dramatic-neopestalotiopsis-disease-in-strawberry-tips-and-plug-plant-production-nurseries/ (accessed on 27 August 2025).
- University of California Statewide Integrated Pest Management Program (UC IPM). What Is Integrated Pest Management (IPM)? 2025. Available online: https://ipm.ucanr.edu/what-is-ipm/ (accessed on 27 August 2025).
- Achar, P.N.; Quyen, P.; Adukwu, E.C.; Sharma, A.; Msimanga, H.Z.; Nagaraja, H.; Sreenivasa, M.Y. Investigation of the Antifungal and Anti-Aflatoxigenic Potential of Plant-Based Essential Oils against Aspergillus flavus in Peanuts. J. Fungi 2020, 6, 383. [Google Scholar] [CrossRef]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Abd Rashed, A.; Rathi, D.-N.G.; Ahmad Nasir, N.A.H.; Abd Rahman, A.Z. Antifungal Properties of Essential Oils and Their Compounds for Application in Skin Fungal Infections: Conventional and Nonconventional Approaches. Molecules 2021, 26, 1093. [Google Scholar] [CrossRef]
- Jan, S.; Rashid, M.; Abd_Allah, E.F.; Ahmad, P. Biological Efficacy of Essential Oils and Plant Extracts of Cultivated and Wild Ecotypes of Origanum vulgare L. BioMed Res. Int. 2020, 2020, 8751718. [Google Scholar] [CrossRef]
- Walasek-Janusz, M.; Grzegorczyk, A.; Malm, A.; Nurzyńska-Wierdak, R.; Zalewski, D. Chemical Composition, and Antioxidant and Antimicrobial Activity of Oregano Essential Oil. Molecules 2024, 29, 435. [Google Scholar] [CrossRef] [PubMed]
- Rasiukevičiūtė, N.; Morkeliūnė, A.; Mažeikienė, I.; Lanauskas, J.; Valiuškaitė, A. Alternative Plant Protection Strategies Using Bacteria and Thyme to Improve Strawberry (cv. Elsanta) Yield and Quality. Plants 2025, 14, 1827. [Google Scholar] [CrossRef] [PubMed]
- Werrie, P.-Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.-L. Phytotoxicity of Essential Oils: Opportunities and Constraints for the Development of Biopesticides. A Review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef]
- Brockman, R.; Kuesel, R.; Archer, K.; O’Hearn, K.; Wilson, N.; Scott, D.; Williams, M.; Bessin, R.; Gonthier, D. The Impact of Plant Essential Oils and Fine Mesh Row Covers on Flea Beetle (Chrysomelidae) Management in Brassicaceous Greens Production. Insects 2020, 11, 714. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. The Extracts of Cinnamon and Clove as Potential Biofungicides against Strawberry Grey Mould. Plants 2020, 9, 613. [Google Scholar] [CrossRef]
- Bairamis, A.; Sotiropoulou, N.-S.D.; Tsadila, C.; Tarantilis, P.; Mossialos, D. Chemical Composition and Antimicrobial Activity of Essential Oils and Hydrosols from Oregano, Sage and Pennyroyal against Oral Pathogens. Appl. Sci. 2024, 14, 3238. [Google Scholar] [CrossRef]
- Hidrobo-Chavez, J.; Ramírez-Villacís, D.X.; Barriga-Medina, N.; Herrera, K.; León-Reyes, A. First Report of Neopestalotiopsis mesopotamica Causing Root and Crown Rot on Strawberry in Ecuador. Plant Dis. 2022, 106, 1066. [Google Scholar] [CrossRef]
- Yao, J.; Huang, S.; He, L.; Wei, S.; Yang, W.; Zhang, Q.; Wang, W.; Yang, X.; Xie, S.; Li, Y.; et al. Antifungal Polyacetylenic Deoxyglycosides Isolated from Endophytic Fungus Xylaria sp. VDL4 Associated with Vaccinium dunalianum. J. Fungi 2025, 11, 209. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Tsai, C.-Y.; Wu, Y.-M.; Ariyawansa, H.-A.; Chung, C.-L.; Chung, P.-C. First Report of Neopestalotiopsis rosae Causing Leaf Blight and Crown Rot on Strawberry in Taiwan. Plant Dis. 2021, 105, 487. [Google Scholar] [CrossRef]
- He, Y.-K.; Yang, Q.; Sun, Y.-R.; Zeng, X.-Y.; Jayawardena, R.; Hyde, K.; Wang, Y. Additions to Neopestalotiopsis (Amphisphaeriales, Sporocadaceae) Fungi: Two New Species and One New Host Record from China. Biodivers. Data J. 2022, 10, e90709. [Google Scholar] [CrossRef] [PubMed]
- Macías Sánchez, K.L.; González Martínez, H.D.R.; Carrera Cerritos, R.; Martínez Espinosa, J.C. In Vitro Evaluation of the Antifungal Effect of AgNPs on Fusarium oxysporum f. sp. lycopersici. Nanomaterials 2023, 13, 1274. [Google Scholar] [CrossRef] [PubMed]
- Heng, Z.-A.; Mu, T.-C.; Keyhani, N.O.; Yang, L.-X.; Zheng, M.-H.; Lv, H.-J.; Zhao, Z.-Y.; Mao, Y.-C.; Shang, J.-Y.; Yang, J.; et al. Three new species of Neopestalotiopsis and Pseudopestalotiopsis (Sporocadaceae, Amphisphaeriales) associated with shrub leaf diseases from Fujian, China. MycoKeys 2025, 119, 1–28. [Google Scholar] [CrossRef]
- Ventura-Medina, P.I.; Gutiérrez-Espinosa, M.A.; Febres, V.; Mora-Aguilera, G.; Robledo-Paz, A. Identification and cloning of three endogenous genes that may confer resistance to pathogens in citrus including CLas and CTV. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2019, 37, 399–420. [Google Scholar] [CrossRef]
- QIAGEN. QIAquick PCR Purification Kit. 2023. Available online: https://www.qiagen.com/us/products/discovery-and-translational-research/dna-rna-purification/dna-purification/dna-clean-up/qiaquick-pcr-purification-kit (accessed on 16 September 2023).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Karaca Öner, E.; Sonkaya, M. Identification of ontogenetic and diurnal variability in oregano (Origanum onites L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1185–1193. [Google Scholar] [CrossRef]
- Baranauskienė, R.; Venskutonis, P.R.; Dambrauskienė, E.; Viškelis, P. Harvesting time influences the yield and oil composition of Origanum vulgare L. ssp. vulgare and ssp. hirtum. Ind. Crops Prod. 2013, 49, 43–51. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Marques, S.D.P.P.M.; Pinheiro, R.O.; Nascimento, R.A.D.; Andrade, E.H.D.A.; Faria, L.J.G.D. Effects of Harvest Time and Hydrodistillation Time on Yield, Composition, and Antioxidant Activity of Mint Essential Oil. Molecules 2023, 28, 7583. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarikhah, G.; Ebadi, M.-T.; Ayyari, M. Qualitative changes of spearmint essential oil as affected by drying methods. Ind. Crops Prod. 2020, 153, 112492. [Google Scholar] [CrossRef]
- Rosińska, A. Evaluation of the Use of Oregano and Coconut Hydrolates to Improve Onion Seed Quality. Agronomy 2022, 12, 2478. [Google Scholar] [CrossRef]
- Schuch, J.M.; Mendes, C.R.; Cardoso, G.L.; André Da Veiga Lima Rosa Costamilan, C.; Matos Lopes, P.R.; Montagnolli, R.N.; Dilarri, G.; Bidoia, E.D. Neem Essential Oil as an Antifungal Agent against Phyllosticta citricarpa. Int. J. Microbiol. 2024, 2024, 6251407. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, X.; Liu, X.; Chen, Z.; Tang, H.; Hu, D.; Li, H. GC-MS Analysis of the Essential Oil from Seseli mairei H. Wolff (Apiaceae) Roots and Their Nematicidal Activity. Molecules 2023, 28, 2205. [Google Scholar] [CrossRef]
- Bhattarai, G.; Feng, C.; Dhillon, B.; Shi, A.; Villarroel-Zeballos, M.; Klosterman, S.J.; Correll, J.C. Detached Leaf Inoculation Assay for Evaluating Resistance to the Spinach Downy Mildew Pathogen. Eur. J. Plant Pathol. 2020, 158, 511–520. [Google Scholar] [CrossRef]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef] [PubMed]
- Baggio, J.S.; Rebello, C.S.; de Morais, M.B.; Marin, M.V.; Gama, A.B.; Forcelini, B.B.; Mertely, J.C.; Peres, N.A. Efficacy of Single- and Multi-Site Fungicides Against Neopestalotiopsis spp. of Strawberry. Plant Dis. 2023, 107, 2177–2184. [Google Scholar] [CrossRef]
- Olivares-Rodriguez, G.; Angeles-Núñez, J.G.; Mondragón-Rojas, F.; Rivas-Valencia, P.; Zárate-Castrejón, J.L.; Mariscal-Amaro, L.A.; Díaz-Espino, L.F.; Martínez-Martínez, T.O. Control in vitro of Neopestalotiopsis sp. isolated from strawberry by Trichoderma and commercial fungicides. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2024, 42, 52. [Google Scholar] [CrossRef]
- Beasley, D.R.; Joyce, D.C.; Coates, L.M.; Wearing, A.H. Saprophytic microorganisms with potential for biological control of Botrytis cinerea on Geraldton waxflower flowers. Aust. J. Exp. Agric. 2001, 41, 697. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- TeamFresh. TeamFresh Nursery. 2023. Available online: https://www.teamfresh.mx/teamfreshnursery (accessed on 13 February 2023).
- Campos-García, T.; Sánchez-García, P.; Alcántar-González, G.; Calderón-Zavala, G. Agronomic and nutritional strawberry response to nutrient solutions with different ratio NH4+/NO3− in the vegetative stage. Rev. Mex. Cienc. Agríc. 2016, 7, 599–606. [Google Scholar] [CrossRef][Green Version]
- Preciado-Rangel, P.; Troyo-Diéguez, E.; Valdez-Aguilar, L.A.; García-Hernández, J.L.; Luna-Ortega, J.G. Interactive Effects of the Potassium and Nitrogen Relationship on Yield and Quality of Strawberry Grown Under Soilless Conditions. Plants 2020, 9, 441. [Google Scholar] [CrossRef]
- Protein Data Bank (PDB). Naegleria fowleri CYP51-Posaconazole Complex (PDB ID: 5TL8). 2023. Available online: https://www.rcsb.org/structure/5TL8 (accessed on 9 January 2024).
- Sun, Z.; Su, X.; Lin, Y.; Long, C.; Zhang, Y.; Zhao, T. Chemical Composition, and Antioxidant and Cholinesterase Inhibitory Activities of Lindera glauca Fruit Essential Oil and Molecular Docking Studies of Six Selected Compounds. Horticulturae 2023, 9, 289. [Google Scholar] [CrossRef]
- PubChem. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 20 January 2024).
- National Center for Biotechnology Information (NCBI). Neopestalotiopsis zimbabwana Strain CBS 111495 Translation Elongation Factor 1-alpha (tef1) Gene, Partial cds; GenBank: KM199545.1. 2015. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KM199545 (accessed on 2 September 2023).
- National Center for Biotechnology Information (NCBI). Neopestalotiopsis rosae Strain CBS 101057 Translation Elongation Factor 1-alpha (tef1) Gene, Partial cds; GenBank: KM199523.1. 2015. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KM199523 (accessed on 2 September 2023).
- Hou, H.; Zhang, X.; Zhao, T.; Zhou, L. Effects of Origanum vulgare essential oil and its two main components, carvacrol and thymol, on the plant pathogen Botrytis cinerea. PeerJ 2020, 8, e9626. [Google Scholar] [CrossRef]
- Darapanit, A.; Boonyuen, N.; Leesutthiphonchai, W.; Nuankaew, S.; Piasai, O. Identification, pathogenicity and effects of plant extracts on Neopestalotiopsis and Pseudopestalotiopsis causing fruit diseases. Sci. Rep. 2021, 11, 22606. [Google Scholar] [CrossRef] [PubMed]
- Cid-Pérez, T.S.; Ávila-Sosa, R.; Ochoa-Velasco, C.E.; Rivera-Chavira, B.E.; Nevárez-Moorillón, G.V. Antioxidant and Antimicrobial Activity of Mexican Oregano (Poliomintha longiflora) Essential Oil, Hydrosol and Extracts from Waste Solid Residues. Plants 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Shuai, L.; Luo, D.; Ba, L. Inhibitory Activity and Mechanism of Action with Thymol against the Blueberry Pathogenic Fungi Caused by Neopestalotiopsis clavispora. Horticulturae 2023, 9, 983. [Google Scholar] [CrossRef]
- Remolif, G.; Buonsenso, F.; Schiavon, G.; Garello, M.; Spadaro, D. Efficacy of Essential Oil Vapours in Reducing Postharvest Rots and Effect on the Fruit Mycobiome of Nectarines. J. Fungi 2024, 10, 341. [Google Scholar] [CrossRef]
- Daniel, N.; Ferdinand, F.; Aditya, P.A. In silico targeting CYP51 of Naegleria fowleri using bioactive compounds from Indonesian plants. J. Pharm. Pharmacogn. Res. 2023, 11, 841–862. [Google Scholar] [CrossRef]
- Haque, E.; Irfan, S.; Kamil, M.; Sheikh, S.; Hasan, A.; Ahmad, A.; Lakshmi, V.; Nazir, A.; Mir, S.S. Terpenoids with antifungal activity trigger mitochondrial dysfunction in Saccharomyces cerevisiae. Microbiology 2016, 85, 436–443. [Google Scholar] [CrossRef]



| # | Compound | Formula | Retention Time (min) | Score Match (%) | Percentages (%) |
|---|---|---|---|---|---|
| 1 | p-cymene | C10H14 | 6.35 | 87 | 4.2 |
| 2 | trans-β-ocimene | C10H16 | 6.62 | 77 | 0.4 |
| 3 | (Z)-sabinene hydrate | C10H18O | 7.35 | 86 | 8.17 |
| 4 | isoterpinolene | C10H16 | 7.87 | 86 | 2.56 |
| 5 | (E)-trans-2-menthenol | C10H18O | 8.71 | 75 | 0.46 |
| 6 | octanoic acid | C8H16O2 | 9.66 | 80 | 3.16 |
| 7 | (−)-terpinen-4-ol | C10H18O | 10.18 | 84 | 11.61 |
| 8 | α-terpineol | C10H18O | 10.5 | 83 | 1.05 |
| 9 | thymol methyl ether | C11H16O | 11.89 | 86 | 3.14 |
| 10 | linalyl propionate | C13H22O2 | 12.18 | 75 | 0.5 |
| 11 | nonanoic acid | C9H18O2 | 12.79 | 83 | 9.36 |
| 12 | thymol | C10H14O | 13.39 | 82 | 7.65 |
| 13 | decanoic acid | C10H20O2 | 15.21 | 80 | 5.21 |
| 14 | α-farnesene | C15H24 | 16.47 | 80 | 2.11 |
| 15 | cis-muurola-4(14),5-diene | C15H24 | 17.99 | 78 | 0.84 |
| 16 | dodecanoic acid | C12H24O2 | 20.13 | 83 | 15.74 |
| 17 | tetradecanoic acid | C14H28O2 | 24.4 | 79 | 7.87 |
| 18 | hexadecanoic acid | C16H32O2 | 28.47 | 82 | 15.98 |
| # | Compound | Affinity (kcal·mol−1) | Docking Energy Comments |
|---|---|---|---|
| 1 | dichloromethane | −2.723 | Moderate-low potential |
| 2 | octanoic acid | −4.946 | Moderate-low potential |
| 3 | decanoic acid | −5.267 | Moderate-low potential |
| 4 | nonanoic acid | −5.277 | Moderate-low potential |
| 5 | thymol methyl ether | −5.500 | Medium-low potential |
| 6 | tetradecanoic acid | −5.767 | Medium-low potential |
| 7 | dodecanoic acid | −5.789 | Modest binding affinity, medium-low potential |
| 8 | linalyl propionate | −5.834 | Medium-low potential |
| 9 | trans-β-ocimene | −5.958 | Medium-low potential |
| 10 | (−)-terpinen-4-ol | −6.053 | Moderate potential |
| 11 | hexadecanoic acid | −6.082 | Moderate potential |
| 12 | (Z)-sabinene hydrate | −6.116 | Moderate potential |
| 13 | (E)-trans-2-menthenol | −6.158 | Moderate potential |
| 14 | thymol | −6.509 | Moderate potential |
| 15 | p-cymene | −6.788 | Moderate potential |
| 16 | cis-muurola-4(14),5-diene | −6.900 | High-moderate potential, noteworthy |
| 17 | α-terpineol | −6.918 | High-moderate potential, noteworthy |
| 18 | isoterpinolene | −6.944 | High-moderate potential, noteworthy |
| 19 | Captan | −7.254 | Synthetic reference, robust standard |
| 20 | α-farnesene | −7.638 | Promising metabolite, high binding affinity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Yáñez, H.; Soto-Hernández, R.M.; Ruiz-Posadas, L.d.M.; Valdovinos-Ponce, G.; Ruiz-López, I.I.; Peña-Valdivia, C.B.; Mora-Báez, G. Control of Neopestalotiopsis zimbabwana Using Origanum vulgare L. Essential Oil: Combined In Vitro, In Vivo and In Silico Approaches. Horticulturae 2025, 11, 1232. https://doi.org/10.3390/horticulturae11101232
Gómez-Yáñez H, Soto-Hernández RM, Ruiz-Posadas LdM, Valdovinos-Ponce G, Ruiz-López II, Peña-Valdivia CB, Mora-Báez G. Control of Neopestalotiopsis zimbabwana Using Origanum vulgare L. Essential Oil: Combined In Vitro, In Vivo and In Silico Approaches. Horticulturae. 2025; 11(10):1232. https://doi.org/10.3390/horticulturae11101232
Chicago/Turabian StyleGómez-Yáñez, Héctor, Ramón Marcos Soto-Hernández, Lucero del Mar Ruiz-Posadas, Guadalupe Valdovinos-Ponce, Irving Israel Ruiz-López, Cecilia Beatriz Peña-Valdivia, and Guadalupe Mora-Báez. 2025. "Control of Neopestalotiopsis zimbabwana Using Origanum vulgare L. Essential Oil: Combined In Vitro, In Vivo and In Silico Approaches" Horticulturae 11, no. 10: 1232. https://doi.org/10.3390/horticulturae11101232
APA StyleGómez-Yáñez, H., Soto-Hernández, R. M., Ruiz-Posadas, L. d. M., Valdovinos-Ponce, G., Ruiz-López, I. I., Peña-Valdivia, C. B., & Mora-Báez, G. (2025). Control of Neopestalotiopsis zimbabwana Using Origanum vulgare L. Essential Oil: Combined In Vitro, In Vivo and In Silico Approaches. Horticulturae, 11(10), 1232. https://doi.org/10.3390/horticulturae11101232







