Abstract
Arbuscular mycorrhizal fungi (AMF) are beneficial microorganisms ubiquitous in soil that form symbiotic mycorrhizal structures with plant roots. When the host plant is exposed to temperature stress, arbuscular mycorrhizal fungi can improve the host plant’s resistance by helping regulate the growth of underground and aboveground parts. In recent years, due to climate change, extremely high and low temperatures have occurred more frequently and for longer durations, significantly impacting plant growth, antioxidant systems, osmotic balance, photosynthesis, and related gene expression. Consequently, numerous scholars have used arbuscular mycorrhizal fungi to aid plants, confirming that arbuscular mycorrhizal fungi can help host plants improve their ability to resist temperature stress. In this paper, the quantitative research method of Meta-analysis was used to collate and build a database of 129 relevant works to evaluate the effects of AMF on plant resistance to temperature stress and explore the response mechanism of AMF to host plants subjected to temperature stress, providing a theoretical basis for further exploring arbuscular mycorrhizal fungi in improving plant resistance to temperature stress.
1. Introduction
Arbuscular mycorrhizae fungi (AMF) are ubiquitous microorganisms in soil, widely distributed in mountainous farmland and various stress environments [1]. Mycorrhizae are symbionts formed by mycorrhizal fungi and plant roots, divided into three types: ectomycorrhizae, endomycorrhizae, and endophytic mycorrhizae. AMF can form mycorrhizae with more than 90% of plants in nature and are difficult to form only with a few families and genera such as stalagmites and quinoa [2]. After AMF symbiotize with host roots, they form a hyphal network, increasing the plant’s contact area with water, mineral elements, and nutrients. This enhances the plant’s absorption and utilization of these substances, thereby improving its nutritional status [3,4,5]. AMF are obligate symbionts that rely on the host plant as their only carbon source [6]. AMF and host plants mutually benefit and complement each other [7]. AMF can increase the sustainability of agriculture and also improve the quantity and quality of crops [8]. There have been research reports demonstrating that yield and plant nutrition were improved by inoculation in 84 and 92% of 81 and 112 experiments, respectively; AMF inoculation has appeared to be highly positive for plant development [9]. Mycorrhizal symbiosis is capable of reducing the use of chemical fertilizer by 25–90%, particularly NPK—depending on crop species, soil type, and management practice—while increasing productivity in the range of 16–78% [10]. Mycorrhizal inoculation could increase the yields of onions by up to 22% [11]. Affokpon et al. [12] reported that field applications of a mixture of 20 AMF strains could increase the yields of carrots by up to 300% and tomatoes by even up to 26%. Bagyaraj et al. [13] report that it is possible to reduce the application of phosphatic fertilizers by 50 and increase pepper production through inoculation with an efficient strain of mycorrhizal fungus. The application of arbuscular mycorrhizal fungi has become an important part of sustainable agriculture research. Sery et al. [14]. discovered cassava production increased by 16.6% and 19.4% after inoculation with AMF. AMF have been determined to increase total potato and cotton crop yields by 9.5% and 28.54% [15,16]. The application of arbuscular mycorrhizal fungi has become an important part of sustainable agriculture research.
Temperature is an essential environmental factor for plant growth and development, participating in all physiological activities and biochemical reactions. Each plant has an optimal growth temperature; deviations from this temperature, either too high or too low, can affect plant growth and development and, in severe cases, cause harm. This phenomenon, caused by the impact of temperature on plants, is called temperature stress. Temperature stress can be divided into two types: high-temperature stress and low-temperature stress [17]. It can inhibit plant growth, physiology and biochemistry, photosynthesis, and related gene expression to varying degrees, and in severe cases, it can directly cause plant death.
To reduce the harm of temperature stress to plants, many scholars have actively explored and found that AMF can effectively help plants improve their ability to resist temperature stress [18]. In this paper, the mechanism of AMF to improve plant resistance to temperature stress was reviewed, which can provide a reference for enhancing plant resistance, improving plant growth potential and the quality of horticultural crops, and further research on the ecological environment and biological resources protection.
On Google Scholar, the Web of Science, and other databases, 288 articles were retrieved using the keywords AMF, temperature stress, and plant resistance, and 129 works related to the topic of this paper were selected for careful reading. They were all used in the review to avoid selectivity.
2. Effects of AMF on Plant Growth under Temperature Stress
Generally, the optimal growth temperature for higher plants is 20~30 °C. Below 0 °C, plants cannot grow, and between 0 and 20 °C, the growth rate accelerates with increasing temperature. Above 30 °C, the growth rate slows down or even ceases. This indicates that temperature stress can inhibit plant growth and significantly impact plant development [19]. It has been observed that temperature stress affects plant growth by influencing the development of roots, stems, leaves, flowers, fruits, seeds, and other organs. The effects of temperature stress on these plant organs are weakened or even eliminated after AMF inoculation. Therefore, AMF can ensure the normal growth and development of various organs in the host plant to some extent (Table 1).
Roots, stems, and leaves are the vegetative organs of plants, responsible for the absorption of water and mineral nutrients, nutrient transport, overall support, and photosynthesis. Temperature stress can lead to slowed root growth, stem overgrowth or softening, and leaf wilting. For example, high-temperature stress in buckwheat primarily damages membrane integrity, increases membrane lipid peroxidation, and severely inhibits root growth [20]. In chrysanthemums, high-temperature stress negatively affects plant height, internode length, and flowering time [21]. Hahn et al. [22] found that young chrysanthemum leaves become narrow and fragile at 30 °C. Low-temperature stress decreases the growth rates of root dry weight, surface area, and length [23]; softens and bends cassava stems [24]; and causes tomato leaves to yellow, droop, and wilt [25]. Under high-temperature stress, compared to the control group, tomato plants inoculated with AMF showed increased numbers of leaves and roots, larger leaf areas, wider crowns, and higher leaf relative water content [26,27]. Plant growth decreased under high-temperature stress, but shoot dry weight remained significantly greater in mycorrhizal plants compared to control plants [28]. Under low-temperature stress, AMF-inoculated plants had significantly increased root surface area and total root length compared to the control group [29]. The fresh and dry mass of shoots and roots in cucumbers improved with AMF inoculation under low-temperature stress [30]. Additionally, the leaf length of Green Ash inoculated with AMF was significantly increased compared to noninoculated plants [31].
Studies have found that temperature stress can lead to flower and fruit drop, a decline in fruit quality, and a decrease in seed germination rates. For example, high-temperature stress reduces maize grain yield [32], causes heat injury to loquat fruit affecting its quality [33], and significantly decreases or even halts the germination rate of chickpea seeds [34]. Low-temperature stress can reduce the quality and yield of lilacs [35], cause pear trees to drop flowers and fruit [36], and prolong corn seed germination time while decreasing the germination rate [37]. Research indicates that AMF can assist the host plant in better nutrient absorption. As shown in Figure 1a, AMF can help the host plant absorb and accumulate nutrients under temperature stress through the mycorrhizal network formed with the host plant, ensuring the yield and quality of the host fruit at later stages. Under high-temperature stress, the ears of AMF-inoculated wheat were significantly higher than those of uninoculated wheat [38]. And, inoculation with AMF can increase soybean yield [39]. Under low-temperature stress, AMF help eggplants accumulate carbohydrates [40].
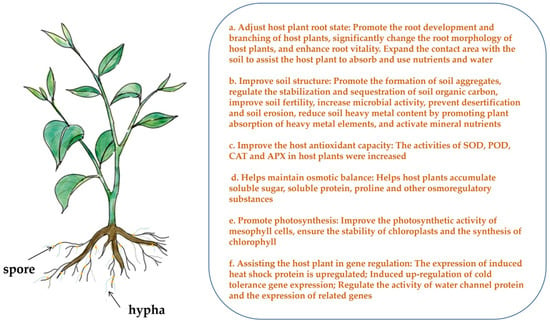
Figure 1.
The effect of arbuscular mycorrhizal fungi on plant growth under temperature stress.
AMF have a symbiotic relationship with the host plant, and while promoting plant growth, their mycelia can connect the roots of neighboring plants to form a common mycorrhizal network. Through microscopic observation, Giovannetti et al. [41] found that mycelium fusion, mycelium wall dissolution, nuclear migration, and protoplast flow occurred in different fungi of Sphaeroides after they formed mycorrhiza networks, which promoted material exchange between strains. On a macro level, AMF can increase the root length, diameter, and root branches of host plants, which is more conducive to root expansion and extension and nutrient absorption, thus promoting the growth of host plants [42]. At the microscopic level, AMF can penetrate the epidermis, outer cortex, and cortical cell layer of roots; change the rate of root cell division and differentiation; accelerate the lignification of cell walls; increase the number of cell layers at the root tip; and thicken the epidermis, thus promoting vigorous root growth and affecting the resistance of the whole plant to temperature stress [43]. In addition to directly aiding host plant growth, AMF can also indirectly support plant development by improving the soil environment and forming an extensive mycelium network symbiotic with plant roots, promoting the absorption and utilization of water and mineral elements from the soil [44]. Soil is the primary medium for plant survival, providing water and mineral elements essential for growth and development. Soil permeability, water content, fertility, salinity, and heavy metal content significantly impact plant growth.
As shown in Figure 1b, AMF inoculation can promote the formation of soil aggregates, regulate the stabilization and sequestration of soil organic carbon, improve soil fertility, increase microbial activity [45], prevent desertification and soil erosion [46], reduce soil heavy metal content by promoting the absorption of heavy metal elements [47], activate mineral nutrients [48], and improve plant salinity tolerance, a key measure for managing saline–alkali land [49]. During the symbiotic growth of AMF and host roots, AMF secrete special substances that create a porous structure, improving water permeability and improving soil stability [50]. In various soil environments, AMF can significantly improve plant growth, and robust plants are better able to withstand the damage caused by temperature stress.

Table 1.
Effect of AMF on host under temperature stress.
Table 1.
Effect of AMF on host under temperature stress.
| Host | Stress Mode | Mycorrhizal Species | Reaction after Inoculation | Reference |
|---|---|---|---|---|
| Zea mays L. | Low-temperature stress | Glomus etunicatum | Root dry weight and shoot fresh weight increased by 95.45% and 26.85%. | [29] |
| High-temperature stress | G. Etunicatum; F. geosporum | Root SOD and CAT activities increased by 30% and 79%; Pn, Gs, and Tr increased by 31.47%, 37.65%, and 11.91%; Ci decreased by 92.01% | [51,52] | |
| Cucumis sativus L. | Low-temperature stress | Funneliformis mosseae | Fresh mass, dry mass of root, root–shoot ratio of fresh mass increased by 51.33%, 31.03%, and 26.06%; phenols, flavonoids, and lignin increased by 73.11%, 50.64%, and 26.96%; the expression of ATPase gene increased and the activity of H+-ATPase was enhanced. | [30,53] |
| Glycine max (L.) Merr. | High-temperature stress | Rhizophagus irregularis, Funneliformis mosseae, Funneliformis geosporum | Pn and Tr increased by 32% and 27%, respectively, and the yield was increased. | [48] |
| Solanum melongena L. | Low-temperature stress | C. etunicatum | Shoot and root dry weight increased by 93.62% and 53.85%. | [49] |
| Tectona grandis L. f. | Low-temperature stress | Glomus versiforme | SOD activity increased by 10.6%. | [51] |
| Lilium brownii var. viridulum Baker | High-temperature stress | Funneliformis mosseae, Glomus versiforme | The contents of proline and soluble protein were increased by 14% and 15%. | [54] |
| Lactuca sativa var. ramosa Hort. | High-temperature stress | Funneliformis mosseae | Pn and Tr increased by 7.7% and 5.9%. | [55] |
| Citrus reticulata Blanco | Low-temperature stress | Glomus etunicatum | Pn, Tr, and Gs increased by 22.59%, 52.08%, and 46.94%. | [56] |
| Triticum aestivum L. | High-temperature stress | Rhizophagus intraradices, Funneliformis mosseae, F. geosporum | Chlorophyll content increased by 26%. | [57] |
| Camellia sinensis (L.) O. Kuntze | Low-temperature stress | Clariodeoglomus etunicatum | Caffeine content increased by 89.8%. | [58] |
3. Effects of AMF on Plant Physiology and Biochemistry under Temperature Stress
3.1. Effects of AMF on Plant Oxidation Systems under Temperature Stress
Under temperature stress, both the membrane proteins and membrane lipids of plant cell membranes are oxidized due to the disruption of the dynamic balance between the production and elimination of reactive oxygen species (ROS), thereby leading to cell damage [59]. To combat this, the plant’s antioxidant system is activated, involving enzymes such as peroxidase (POD), catalase (CAT), superoxide dismutase (SOD), and ascorbate peroxidase (APX), which work to mitigate the harmful effects of temperature stress. High-temperature stress can damage the function of chloroplasts and mitochondria in plant cells, accelerating the production and accumulation of ROS like hydrogen peroxide (H2O2), superoxide radicals (O2−), and hydroxyl radicals (–OH) [60]. Excessive ROS can lead to enzyme protein degeneration, respiratory metabolism weakening, oxidative damage, and in severe cases, programmed cell death [61]. To counteract this, CAT, SOD, POD, and APX remove ROS, thereby preventing membrane lipid peroxidation, delaying aging, and reducing membrane damage [62]. Zou et al. [63] found that under high-temperature stress, SOD, POD, and CAT in plant cells work together to protect the cells, with CAT responding the fastest and being more sensitive to damage. Shu et al. [64] confirmed through the physiological data of wild lilies under high-temperature stress that SOD and POD activities increase, thence reducing or eliminating the damage from high-temperature stress by enhancing the plant’s ability to remove toxic substances, thereby improving heat resistance. Some scholars have found that the APX activity of annual plants increases under high-temperature stress [65,66]. This conclusion was also confirmed in studies on wheat [67]. Under low-temperature stress, prolonged exposure can lead to crystallization in plants, causing mechanical damage to cell membranes and leading to lipid peroxidation. This results in the accumulation of reactive oxygen species (ROS), prompting an increase in antioxidant enzyme activity. However, temperature influences enzyme activity, with low temperatures initially increasing and then reducing the activities of these enzymes [68,69,70,71]. Whenever plants experience temperature stress, their antioxidant enzymes maintain certain activity levels to remove excess ROS, ensuring normal physiological activities. As shown in Figure 1c, AMF can improve the antioxidant capacity of host plants under temperature stress by increasing the activities of SOD, POD, CAT, and APX. Zhu et al. [51] found that AM maize plants exhibited higher SOD and CAT activities than non-AM plants under high-temperature stress. Xing et al. [72] observed significantly improved POD and APX activities in lavender inoculated with AMF under high-temperature stress. Abdel Latef and Chaoxing [27] reported that AM tomato (Lycopersicon esculentum) plants had higher SOD, POD, and APX activities than non-AM plants under low-temperature stress. Zhou et al. [73] showed that AM colonization increased POD and SOD activity in teak seedlings under low-temperature stress.
3.2. Effects of AMF on Plant Osmotic Balance under Temperature Stress
When plants are subjected to temperature stress, cell membranes are damaged by oxidation, thereby affecting osmotic regulation. To maintain intracellular stability, plants accumulate a large amount of osmoregulatory substances such as soluble sugar, soluble protein, and proline [74]. Erman et al. [75] showed that the contents of soluble sugar and proline in Paeonia ostii increased under high-temperature stress. Zhang et al. [76] demonstrated that rhododendrons could increase soluble protein contents under high-temperature stress. Under low-temperature stress, water within the plant can form ice crystals. Osmoregulatory substances not only stabilize osmotic pressure but also lower the freezing point, improving cold resistance [77]. For instance, alfalfa significantly increases the contents of soluble sugar and proline under low-temperature stress [78]. Similarly, tobacco improves cold tolerance by increasing free proline [79]. Chae B found that proline content in tomatoes increases under low-temperature stress [80]. Numerous studies have shown that increasing the content of proline, soluble protein, and soluble sugar can improve cold tolerance in plants. As shown in Figure 1d, AMF can help the host plant maintain osmotic balance by aiding the accumulation of soluble sugar, soluble protein, proline, and other osmoregulatory substances. The amount of these substances is positively correlated with the plant’s stress resistance. In lilies, AMF inoculation increased the contents of proline and soluble protein in leaves under high-temperature stress [54]. Under low-temperature stress, mycorrhizal plants exhibited higher root proline and soluble sugar contents compared to non-mycorrhizal plants [51]. Sorghum research indicates that AMF can reduce temperature stress damage, enhance root vitality, and increase proline and soluble sugar contents [81]. Additionally, soluble sugar content in maize increased following AMF inoculation under low-temperature stress [82].
4. Effects of AMF on Plant Photosynthesis under Temperature Stress
4.1. Effects of AMF on Plant Photosynthetic Characteristics under Temperature Stress
Photosynthesis is crucial for the material transformation and energy metabolism of plants, reflecting their overall health. The strength of photosynthesis significantly influences plant stress resistance. Temperature stress affects plant photosynthesis through two primary factors. First, stomatal factors lead to stomatal closure, reducing stomatal conductance (Gs) and transpiration rate (Tr), thus limiting CO2 absorption. Second, non-stomatal factors directly inhibit the photosynthetic activity of mesophyll cells, increasing intercellular CO2 concentration (Ci) and disrupting the photosynthetic mechanism. Temperature stress decreases the net photosynthetic rate (Pn), Gs, and Tr while increasing Ci. Studies have shown that the Pn of Nerium oleander under high-temperature stress is primarily affected by stomatal factors [83]. The reduced photosynthetic efficiency in wheat and corn was attributed to non-stomatal factors [84,85]. Under low-temperature stress, research on maize [29], grapes [86], and cucumbers [87] has indicated that non-stomatal factors were the main contributors to decreasing photosynthesis. Low temperatures reduce the activity of photosynthetic enzymes and the photosynthetic activity of mesophyll cells. As shown in Figure 1e, AMF can improve plant photosynthesis under temperature stress by increasing the photosynthetic activity of mesophyll cells. The Pn and Tr of lettuce and soybeans inoculated with AMF under high-temperature stress were significantly higher than those without AMF [39,55]. AMF inoculation also increased the Pn of maize under high-temperature stress [52]. Under low-temperature stress, AMF increased the Pn, Gs, and Tr of maize leaves while decreasing Ci [56]. The presence of AMF positively affected the Pn, Tr, and Gs of citrus seedlings under low temperatures [88]. Cucumber seedlings inoculated with AMF showed an increased Pn, alleviated stomatal restriction, and improved seedling quality and resistance to low temperatures [89]. Numerous experimental results demonstrate that AMF can enhance the photosynthetic capacity of plants under temperature stress.
4.2. Effects of AMF on Plant Chlorophyll under Temperature Stress
Chloroplasts are the sites of plant photosynthesis, with chlorophyll playing a crucial role. The amount of chlorophyll directly affects a plant’s photosynthetic ability [90]. Temperature stress can destabilize plant chloroplasts and inhibit chlorophyll synthesis. High-temperature stress can damage the membranes of winter wheat cells, thereby disrupting chloroplast integrity, causing expansion and disintegration, reducing chlorophyll content, and significantly impacting photosynthesis [91]. Similarly, chlorophyll synthesis is hindered under low-temperature stress. For instance, under low-temperature stress, the chlorophyll content in betel nuts [92] and coffee [93] is significantly lower compared to under normal temperatures. Research indicates that AMF can maintain the stability of plant chloroplasts and ensure normal chlorophyll synthesis under temperature stress [29,94]. Under high-temperature stress, the chlorophyll content in AMF-inoculated maize [52] and strawberries [18] was higher than in uninoculated plants. Similarly, under low-temperature stress, the chlorophyll contents in AMF-inoculated wheat [57] and tomatoes [95] were higher than in those plants without inoculation. Therefore, AMF can increase chlorophyll content in plants under temperature stress.
5. Effects of AMF on Plant Secondary Metabolism under Temperature Stress
5.1. Effects of AMF on Plant Terpenoids under Temperature Stress
Terpenoids, also named isoprenoids, are the largest group of plant secondary metabolites and contain all kinds of structural types including monoterpenes, sesquiterpenes, diterpenes, and triterpenes [96]. It has been reported that the symbiosis between AMF and host plants can change the concentrations of gibberellin and jasmonic acid, while jasmonic acid and gibberellic acid can enhance the concentrations of terpenoids by increasing the formation of glandular trichoids and the expression of sesquiterpenoid biosynthesis genes. Therefore, AMF–plant symbiosis can indirectly increase the contents of terpenoids under temperature stress [6,97,98]. Under low-temperature stress, secondary metabolites, such as strigolactones and flavonoids, can promote protein biosynthesis, strengthen the plant immune system, and play a crucial role in the spore germination and hyphal branching of AM fungi. On the other hand, AM fungi can also enhance secondary metabolite content, including triterpenoids, in high plants [27,99,100,101]. Therefore, there was an inter-regulation between secondary metabolites and AMF to modulate the low-temperature tolerance of plants [102].
5.2. Effects of AMF on Plant Phenolic Compounds under Temperature Stress
Phenolic compounds (phenolic) are compounds in which the hydrogen atoms in the benzene ring are replaced by hydroxyl groups in the aromatic hydrocarbons, and general phenols include flavonoids, simple phenols, and quinones. Dehne et al. [103] first noted that AMF are related to the accumulation of plant phenolic substances, and they found that an inoculation with the fungi increased the amount of lignin and soluble phenol in tomatoes. Plant and AMF symbiosis can resist low-temperature stress by increasing the content of plant secondary metabolites and affecting secondary metabolic pathways. Inoculating cucumbers with AMF can raise phenols, flavonoids, and lignin by 73.11%, 50.64%, and 26.96%, respectively, under low-temperature stress [30]. Hajiboland et al. [104] found that AMF had no effect on the concentration of phenols and the activity of metabolic enzymes in barley at room temperature, but they both increased under the combined treatment of low temperatures and AMF. Torres et al. [105] reported that AMF inoculation increased parameters related to phenolic maturity such as anthocyanin content in grapes. Pasbani et al. [40] suggest that an enhanced phenolic metabolism by AMF inoculation might play a crucial role in the AMF-mediated alleviation of cold stress in eggplants.
5.3. Effects of AMF on Nitrogen-Containing Compounds in Plants under Temperature Stress
Nitrogen-containing compounds are a class of plant secondary metabolites containing nitrogen atoms in their molecular structure, including alkaloids, cyanosides, non-protein amino acids, etc. Alkaloids are a class of nitrogen-containing alkaline natural products, and also the largest class of secondary metabolites in nitrogen-containing compounds. The existence and diversification of alkaloids provide a certain material basis for the therapeutic value of drugs [106]. Wei and Wang [107] first observed that Datura + AMF can increase the total content of scopolamine and hyoscyamine in Datura. Abu-Zeyad et al. found that AMF colonization can enhance castanospermine content in the leaves of Castanospermum australe [108]. Many studies have reported the positive role of AMF in alkaloid accumulation. Lei et al. discovered that caffeine content in the leaves of tea seedlings inoculated with AMF was significantly increased in low temperatures [58]. At present, there are relatively few studies on the changes in nitrogen compounds in plants after inoculation with AMF in extreme temperature environments, and the specific contents need to be further studied.
5.4. Effect of AMF on Plant Volatile Organic Compounds under Temperature Stress
Plants can synthesize and release large amounts of volatile organic compounds (VOCs), which are important protective and signaling molecules [109]. Abiotic stresses will induce plant volatile emissions [110]. The study found that AMF can promote the synthesis of VOCs to enhance the defense ability of plants [111]. Maya et al. [28] confirmed that the VOC content of cyclamens inoculated with AMF increased under high-temperature stress. Israel et al. [112]. demonstrated that AMF increased the VOC content of medicinal plants and enhanced the tolerance of medicinal plants to temperature stress by improving the water and nutrient access of medicinal plants under temperature stress. There are relatively few reports on plant VOC content under temperature stress regulated by AMF. However, there are many reports on the regulation of VOC content by AMF under other abiotic stress conditions, and the results of these reports can provide a good reference value for future research on the regulation of VOC content by AMF under temperature stress.
5.5. Effect of AMF on Plant Polyamines under Temperature Stress
Polyamines (PAs) are a type of polycationic, low-molecular, and aliphatic metabolite that is ubiquitous in all organisms and participates in a series of physiological reactions in organisms [113]. PAs are considered to be a plant hormone that plays an important role in plant growth and development and stress resistance [114]. AMF can alter the metabolism of endogenous PAs in plants. Wu et al. [115] revealed that AMF can promote the greater synthesis of Pas, thus stimulating root growth. AMF promote the biosynthesis of PA, leading to the formation of more γ-aminobutyric acid, which acts as a signaling molecule and increases the activity of the enzyme Rubisco, thus promoting the formation of chlorophyll [116]. AMF can induce the increase in γ-aminobutyric acid by regulating Pas, thus promoting the formation of organic permeates such as proline and trehalose and maintaining the osmotic regulation of cells under abiotic stress [117,118,119]. Ouledali et al. [120] found that the regulation of AMF on host stomatal conductance is affected by PA concentration because PA can change the content of abscisic acid in plants; so, it can regulate stomatal conductance and related physiological activities. In addition, AMF also produce small amounts of PAs used to aid their own growth and host growth while improving host stress resistance [114]. AMF can alleviate temperature stress by improving plant growth and helping plants accumulate and utilize PAs [121].
6. Effects of AMF on Plant Related Protein and Gene Expression under Temperature Stress
Temperature stress induces gene transcription and protein synthesis in plants. When the external temperature rises, plants sense the high-temperature signal and increase the content of heat shock proteins (HSPs). HSPs are widely distributed in chloroplasts, mitochondria, cytoplasm, and endoplasmic reticulum, protecting plants from stress. Under high-temperature stress, HSPs are involved in the synthesis and renaturation of new proteins in cells, acting as crucial “molecular chaperones” that help maintain cell survival and repair damaged proteins [122]. High-temperature stress activates the expression of HSPs, and heat shock transcription factors (HSFs) rapidly bind to the heat shock elements (HSEs) of HSP promoters, activating the expression of downstream genes. This process improves plant tolerance to high-temperature stress [123,124]. When the external temperature decreases, plants experience low-temperature stress, causing changes in numerous genes and the synthesis of new proteins. Genes expressed under low-temperature stress are called cold-regulatory genes (COR genes), and the proteins they encode are known as cold-acclimated proteins. It has been found that CBF1-3 is rapidly induced upon exposure to low temperatures, leading to the expression of CBF target genes and improved plant frost resistance [125].
Under temperature stress, AMF can enhance the temperature stress tolerance of plants by inducing gene expression levels. Tian et al. [126] found that AMF could up-regulate the expression of CsPIPs and CsHsp70s at high temperatures. Under low-temperature stress, it was found that AMF-treated cucumber plants showed increased P-type H+-ATPase, V-type H+-ATPase, total ATPase activity, ATP concentration, and plasma membrane protein concentration. Real-time quantitative PCR analysis revealed that the relative expression levels of H+-ATPase-related genes (CsHA2, CsHA3, CsHA4, CsHA8, CsHA9, and CsHA10) were significantly induced after AMF inoculation. These results indicated that AMF could increase the expression of ATPase genes and enhance H+-ATPase activity [53]. Additionally, under temperature stress, AMF can regulate the activity of aquaporins (AQPs) and the expression of related aquaporin genes, thence improving water transport in the host plant and thereby reducing the impact of temperature stress [127]. In a previous study, Ma et al. [128] identified that AMF significantly induced the transcription level of CsPT2-1, CsMPT3, CsPHO1-H1, and CsPT1-11 in cucumbers, thereby enhancing the absorption of phosphorus to improve the ability of response to temperature stress in plants. Moreover, Liu et al. [129] reported that there were relatively higher expression levels of OsTPS1, OsTPS2, and OsTPP1 in rice inoculated with AMF under cold stress, and with the increase in trehalose concentration, this resulted in enhanced cold tolerance in rice. On the other hand, AMF could also induce the expression of genes related to secondary metabolism to promote the capacity of plants to respond to cold stress. Chen et al. [30] reported that the expression levels of WRKY30, PR-1, C4H, CCOMT, CAD, G6PDH, PAL, and LPO were up-regulated by 246%, 290%, 184%, 247%, 196%, 252%, 189%, and 193%, respectively, in plants co-existing with AMF under cold stress. Numerous studies have shown that AMF inoculation can increase the expression of temperature-resistant genes in plants.
7. Conclusions
The role of AMF in promoting the absorption of plant nutrients, the accumulation of metabolites, and the improvement of environmental stress resistance has been relatively clear. Clarifying the mechanism of AMF in improving the resistance of host plants to temperature stress has important theoretical and practical significance for the development of microbial fertilizer, the implementation of ecological planting, the development of green agriculture, and the improvement of agricultural product quality.
However, studies on the molecular mechanism of AMF improving host plant resistance to temperature stress are relatively weak, and the relationship between AMF, environmental temperature, and plants needs to be further studied. In natural settings, temperature stress is often accompanied by other stressors, such as drought. It remains to be verified whether AMF can have the same beneficial effects under combined-stress conditions as it does under single-stress environments. Additionally, the exact efficacy of AMF in these scenarios needs further investigation. Moreover, the symbiotic mechanisms between AMF and host plants require deeper exploration. How do AMF regulate the construction of plant defense systems in the face of temperature stress? What are the signaling substances of the defense system? What are the key regulatory genes in the defense system? Which proteins interact in what way to affect AMF resistance to temperature stress in host plants? And so on with the questions. Future studies should pay more attention to the effects of the mycorrhizal network and interspecies material exchange on the temperature stress resistance of host plants, especially in mycelium fusion, mycelium wall lysis, nuclear migration, protoplast flow, root cell division, cell wall lignification, the apical cell layer, and epidermal development. With the development of metabolomics, genomics, and proteomics combined analysis technologies, it is possible to elucidate the above problems.
Author Contributions
Conceptualization, P.J.; Formal Analysis, Q.Z.; Funding Acquisition, D.Z.; Investigation, X.H. and C.T.; Project Administration, D.Z.; Supervision, D.Z.; Writing, P.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 32001984) and a grant from the Shanghai Key Laboratory of Protected Horticultural Technology.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chandrasekeran, A.; Mahalingam, P.U. Diversity of Arbuscular Mycorrhizae Fungi from Orchard Ecosystem. J. Plant Pathol. Microbiol. 2014, 4, 230. [Google Scholar] [CrossRef]
- Mills, M.W. Bringing Light to below Ground Patterns: Arbuscular Mycorrhizae Fungi Diversity Along an Elevation Gradient in Southern California; California State University: Long Beach, CA, USA, 2015. [Google Scholar]
- Liu, C.-Y.; Wang, P.; Zhang, D.-J.; Zou, Y.-N.; Kuča, K.; Wu, Q.-S. Mycorrhiza-induced change in root hair growth is associated with IAA accumulation and expression of EXPs in trifoliate orange under two P levels. Sci. Hortic. 2018, 234, 227–235. [Google Scholar] [CrossRef]
- Sun, M.; Yuan, D.; Hu, X.; Zhang, D.J. Effects of mycorrhizal fungi on plant growth, nutrient absorption and phytohormones levels in tea under shading condition. Not. Bot. Horti Agrobot. 2020, 48, 2006–2020. [Google Scholar] [CrossRef]
- Aliasgharzad, N.; Bolandnazar, S.A.; Neyshabouri, M.R.; Chaparzadeh, N. Impact of soil sterilization and irrigation intervals on P and K acquisition by mycorrhizal onion (Allium cepa). Biologia 2009, 64, 512–515. [Google Scholar] [CrossRef]
- Liao, D.; Wang, S.; Cui, M.; Liu, J.; Chen, A.; Xu, G. Phytohormones Regulate the Development of Arbuscular Mycorrhizal Symbiosis. Int. J. Mol. Sci. 2018, 19, 3146. [Google Scholar] [CrossRef] [PubMed]
- Limonard, T. Book Reviews. Acta Bot. Neerl. 1985, 34, 443–444. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. The Application of Arbuscular Mycorrhizal Fungi as Microbial Biostimulant, Sustainable Approaches in Modern Agriculture. Plants 2023, 12, 3101. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let's Benefit from Past Successes. Front. Microbiol. 2016, 19, 1559. [Google Scholar] [CrossRef] [PubMed]
- Ebbisa, A. Arbuscular Mycorrhizal Fungi (AMF) in Optimizing Nutrient Bioavailability and Reducing Agrochemicals for Maintaining Sustainable Agroecosystems; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Torres-Barragán, A.; Zavaleta-Mejía, E.; González-Chávez, C.; Ferrera-Cerrato, R. The use of arbuscular mycorrhizae to control onion white rot (Sclerotium cepivorum Berk.) under field conditions. Mycorrhiza 1996, 6, 253–257. [Google Scholar] [CrossRef]
- Affokpon, A.; Coyne, D.L.; Lawouin, L.; Tossou, C.; Agbèdè, R.D.; Coosemans, J. Effectiveness of native West African arbuscular mycorrhizal fungi in protecting vegetable crops against root-knot nematodes. Biol. Fertil. Soils 2011, 47, 207–217. [Google Scholar] [CrossRef]
- Bagyaraj, D.J.; Sreeramulu, K.R. Preinoculation with VA mycorrhiza improves growth and yield of chilli transplanted in the field and saves phosphatic fertilizer. Plant Soil 1982, 69, 375–381. [Google Scholar] [CrossRef]
- Séry, D.J.M.; Kouadjo, Z.G.C.; Voko, B.R.R.; Zeze, A. Selecting Native Arbuscular Mycorrhizal Fungi to Promote Cassava Growth and Increase Yield under Field Conditions. Front. Microbiol. 2016, 22, 2063. [Google Scholar] [CrossRef] [PubMed]
- Hijri, M. Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza 2016, 26, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, H.; Zhang, Q.; Guo, H.; Zhang, L.; Zhang, C.; Gou, Z.; Liu, Y.; Wei, J.; Chen, A.; et al. Arbuscular mycorrhizal fungi (AMF) enhanced the growth, yield, fiber quality and phosphorus regulation in upland cotton (Gossypium hirsutum L.). Sci. Rep. 2020, 10, 2084. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Hirano, I.; Sassa, D.; Koshikawa, K. Alleviation of High Temperature Stress in Strawberry Plants Infected with Arbuscular Mycorrhizal Fungi. Environ. Control Biol. 2004, 2, 105–111. [Google Scholar] [CrossRef]
- Gavito, M.E.; Olsson, P.A.; Rouhier, H.; Medina-Peñafiel, A.; Jakobsen, I.; Bago, A.; Azcón-Aguilar, C. Temperature constraints on the growth and functioning of root organ cultures with arbuscular mycorrhizal fungi. New Phytol. 2005, 168, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Tian, X. Effect of High Temperature Stress on Some Physiological Characteristics of Buckwheat. J. Anhui Agric. Sci. 2008, 36, 13519–13520. [Google Scholar] [CrossRef]
- Khattak, A.M.; Pearson, S. Spectral Filters and Temperature Effects on the Growth and Development of Chrysanthemums under Low Light Integral. Plant Growth Regul. 2006, 49, 61–68. [Google Scholar] [CrossRef]
- Eun-Joo, H.; Young-Ryul, C.; Yong-Beom, L. Air Temperature and Relative Humidity Affect the Growth of Chrysanthemum Plantlets in the Microponic System. Hortic. Environ. Biotechnol. 1998, 39, 625–628. [Google Scholar]
- Luo, N.; Wei, S.; Li, J.; Gu, W.; He, D.; Qu, T.; Qiao, T.; Yang, Z. Effects of low-temperature stress on root system characteristics and electric conductivity of maize seedlings. Chin. J. Ecol. 2014, 33, 2694–2699. [Google Scholar]
- An, D.; Yang, J.; Zhang, P. Transcriptome profiling of low temperature-treated cassava apical shoots showed dynamic responses of tropical plant to cold stress. BMC Genom. 2012, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Zwieniecki, M.A.; Passioura, J.B.; Randall, L.B.; Holbrook, N.M.; St. Clair, D.A. Water relations under root chilling in a sensitive and tolerant tomato species. Plant Cell Environ. 2004, 27, 971–979. [Google Scholar] [CrossRef]
- Li, Y.; Matsubara, Y.; Miyawaki, C.; Liu, Y. Temperature stress tolerance and increase in antioxidative enzyme activities in mycorrhizal strawberry plants. Acta Hortic. 2008, 774, 391–396. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; He, C. Arbuscular mycorrhizal influence on growth, photosynthetic pigments, osmotic adjustment and oxidative stress in tomato plants subjected to low temperature stress. Acta Physiol. Plant. 2011, 33, 1217–1225. [Google Scholar] [CrossRef]
- Maya, M.A.; Matsubara, Y.I. Influence of arbuscular mycorrhiza on the growth and antioxidative activity in cyclamen under heat stress. Mycorrhiza 2013, 23, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.C.; Song, F.B.; Xu, H.W. Arbuscular mycorrhizae improves low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil 2010, 331, 129–137. [Google Scholar] [CrossRef]
- Chen, S.; Jin, W.; Liu, A.; Zhang, S.; Liu, D.; Wang, F.; Lin, X.; He, C. Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperature stress. Sci. Hortic. 2013, 160, 222–229. [Google Scholar] [CrossRef]
- Andersen, C.P.; Sucoff, E.I.; Dixon, R.K. The influence of low soil temperature on the growth of vesicular–arbuscular mycorrhizal Fraxinus pennsylvanica. Can. J. For. Res. 1987, 17, 951–956. [Google Scholar] [CrossRef]
- Lizaso, J.I.; Ruiz-Ramos, M.; Rodríguez, L.; Gabaldon-Leal, C.; Oliveira, J.A.; Lorite, I.J.; Sánchez, D.; García, E.; Rodríguez, A. Impact of high temperatures in maize: Phenology and yield components. Field Crops Res. 2018, 216, 129–140. [Google Scholar] [CrossRef]
- Felicetti, D.A.; Schrader, L.E. Changes in pigment concentrations associated with sunburn browning of five apple cultivars. I. Phenolics. Plant Sci. 2009, 176, 78–83. [Google Scholar] [CrossRef]
- Cara, J.; Richard, T.; Brent, K. Chickpea tolerance to temperature stress: Status and opportunity for improvement. J. Plant Physiol. 2021, 267, 153555. [Google Scholar] [CrossRef]
- Drzejuk, J.; Lukaszewska, A.; Rabiza-Świder, J.; Skutnik, E. Low temperature forcing reduces oxidative stress in lilac flowers. Hortic. Environ. Biotechnol. 2016, 57, 625–632. [Google Scholar] [CrossRef]
- Ballard, J.K. Frost control in pear orchards. Childers Ordering Address 1982, 11, 290–301. [Google Scholar]
- Segeťa, V. Physiology of the cold-resistance of maize during Germination. the reaction of maize (Zea mays L.) to low temperature during germination and its cold-resistance. Biol. Plant. 1964, 6, 189–197. [Google Scholar] [CrossRef]
- Bhantana, P.; Malla, R.; Vista, S.P.; Rana, S.; Mohamed, G.M.; Das Joshi, B.; Shah, S.; Khadka, D.; Timsina, G.P.; Poudel, K.; et al. Use of Arbuscular Mycorrhizal Fungi (AMF) and Zinc Fertilizers in an Adaptation of Plant from Drought and Heat Stress. Biomed. J. Sci. Tech. Res. 2021, 38, 30357–30373. [Google Scholar] [CrossRef]
- Kanchan, J.; Singh, V.B.; Sunita, K.; Alamri, S.A.; Siddiqui, M.H.; Rastogi, A. Inoculation with arbuscular mycorrhizal fungi alleviates the adverse effects of high temperature in soybean. Plants 2022, 11, 2210. [Google Scholar] [CrossRef] [PubMed]
- Pasbani, B.; Salimi, A.; Aliasgharzad, N.; Hajiboland, R. Colonization with arbuscular mycorrhizal fungi mitigates cold stress through improvement of antioxidant defense and accumulation of protecting molecules in eggplants. Sci. Hortic. 2020, 272, 109575. [Google Scholar] [CrossRef]
- Giovannetti, M.; Azzolini, D.; Citernesi, A.S. Anastomosis formation and nuclear and protoplasmic exchange in arbuscular mycorrhizal fungi. Appl. Environ. Microbiol. 1999, 65, 5571–5575. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Hu, J.; Wu, F.; Lin, X. The function and potential application of disease suppression by arbuscular mycorrhizal fungi. Chin. J. Appl. Environ. Biol. 2018, 24, 941–951. [Google Scholar] [CrossRef]
- Deng, J. Mechanisms of the Effects of Arbuscular Mycorrhizal Fungus and Grass Endophyte on Leaf Spot of Perennial Ryegrass; Lanzhou University: Lanzhou, China, 2021. (In Chinese) [Google Scholar]
- Zhu, X.; Song, F.; Liu, S.; Liu, T.; Zhou, X. Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. Under drought stress. Plant Soil Environ. 2012, 58, 186–191. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L.). Protoplasma 2017, 254, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Neagoe, A.; Iordache, V.; Bergmann, H.; Kothe, E. Patterns of effects of arbuscular mycorrhizal fungi on plants grown in contaminated soil. J. Plant Nutr. Soil Sci. 2013, 176, 273–286. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Yano-Melo, A.M.; Orivaldo, J.S., Jr.; Maia, L.C. Tolerance of mycorrhized banana (Musa sp. cv. Pacovan) plantlets to saline stress. Agric. Ecosyst. Environ. 2003, 95, 343–348. [Google Scholar] [CrossRef]
- Kapulnik, Y.; Douds, D.D. Mycorrhizal Fungi Influence Soil Structure. In Arbuscular Mycorrhizas: Physiology and Function; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Xu, H. Influence of arbuscular mycorrhiza on lipid peroxidation and antioxidant enzyme activity of maize plants under temperature stress. Mycorrhiza 2010, 20, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Sharma, M.P.; Jajoo, A. Improved photosynthetic efficacy of maize (Zea mays) plants with arbuscular mycorrhizal fungi (AMF) under high temperature stress. J. Photochem. Photobiol. B Biol. 2018, 180, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Chen, S.; Chang, R.; Liu, D.; Chen, H.; Ahammed, G.J.; Lin, X.; He, C. Arbuscular mycorrhizae improve low temperature tolerance in cucumber via alterations in H2O2 accumulation and ATPase activity. J. Plant Res. 2014, 127, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.S.; Zhang, R.; Guo, S.X. Arbuscular mycorrhizal fungi under high temperature stress on the influence of the heat resistant lily. J. Qingdao Agric. Univ. Nat. Sci. Ed. 2018, 35, 7. (In Chinese) [Google Scholar]
- Yan, Z.; Ma, T.; Guo, S.; Liu, R.; Li, M. Leaf anatomy, photosynthesis and chlorophyll fluorescence of lettuce as influenced by arbuscular mycorrhizal fungi under high temperature stress. Sci. Hortic. 2021, 280, 109933. [Google Scholar] [CrossRef]
- Xian, C.Z.; Feng, B.S.; Hong, W.X. Effects of arbuscular mycorrhizal fungi on photosynthetic characteristics of maize under low temperature stress. Chin. J. Appl. Ecol. 2010, 21, 470. [Google Scholar] [CrossRef]
- Paradis, R.Y.; Dalpé, Y.; Charest, C. The combined effect of arbuscular mycorrhizas and short-term cold exposure on wheat. New Phytol. 2010, 129, 637–642. [Google Scholar] [CrossRef]
- Lei, A.Q.; Zhou, J.H.; Rong, Z.Y.; Alqahtani, M.D.; Gao, X.B.; Wu, Q.S. Mycorrhiza-triggered changes in leaf food quality and secondary metabolite profile in tea at low temperatures. Rhizosphere 2024, 29, 100840. [Google Scholar] [CrossRef]
- Hossain, M.; Bhattacharjee, S.; Armin, S.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Robson, C.A.; Zhao, D.; Vanlerberghe, G.C. Interactions between mitochondrial electron transport, reactive oxygen species, and the susceptibility of Nicotiana tabacum cells to programmed cell death. Botany 2008, 86, 278–290. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Zou, M.Q.; Yuan, L.Y.; Zhu, S.D.; Liu, S.; Ge, J.; Wang, C. Effects of heat stress on photosynthetic characteristics and chloroplast ultrastructure of a heat-sensitive and heat-tolerant cultivar of wucai (Brassica campestris L.). Acta Physiol. Plant. 2017, 39, 30. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, W.B.; Huang, J.Z.; Chen, Q.B. Effects of high temperature stress on physiological indexes of wild Hubei lily. J. Northwest For. Coll. 2019, 34, 62–68. (In Chinese) [Google Scholar]
- Chaitanya, K.V.; Sundar, D.; Masilamani, S.; Reddy, A.R. Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul. 2002, 36, 175–180. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.; Sairam, R.; Kushwaha, S.; Singh, T. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006, 171, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Mohanty, N. Response of seedlings to heat-stress in cultivars of wheat: Growth temperature-dependent differential modulation of photosystem 1 and 2 activity, and foliar antioxidant defense capacity. J. Plant Physiol. 2002, 159, 49–59. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, K.; Gu, Y.; Zhang, L.; Li, W.; Li, Z. Effects of Low-Temperature Stress and Brassinolide Application on the Photosynthesis and Leaf Structure of Tung Tree Seedlings. Front. Plant Sci. 2020, 10, 1767. [Google Scholar] [CrossRef]
- Feng, Z.; Guo, A.; Feng, Z. Amelioration of chilling stress by triadimefon in cucumber seedlings. Plant Growth Regul. 2003, 39, 277–283. [Google Scholar] [CrossRef]
- Li, M.A.; Guo, T.; Chen, X.; Zang, D.; Gong, X.; Sun, J.; Qiu, Y.; Wang, Y. Physiological Responses of Actinidia arguta (Seib.et.Zucc.) to Low Temperature Stress. Agric. Sci. Technol. 2017, 18, 767–770, 776. [Google Scholar]
- Baek, K.H.; Skinner, D.Z. Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci. 2003, 165, 1221–1227. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Liu, F. Arbuscular Mycorrhizal Fungi and Tolerance of Temperature Stress in Plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Wu, Q.S., Ed.; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, H.; Liang, K.; Huang, G.; Pinyopusarerk, K. Improved Tolerance of Teak (Tectona grandis L.f.) Seedlings to Low-Temperature Stress by the Combined Effect of Arbuscular Mycorrhiza and Paclobutrazol. J. Plant Growth Regul. 2012, 31, 427–435. [Google Scholar] [CrossRef]
- Abou-Elwafa, S.F.; Amein, K.A. Genetic Diversity and Potential High Temperature Tolerance in Barley (Hordeum vulgare). World J. Agric. Res. 2016, 4, 1–8. [Google Scholar]
- Hong, E.; Xia, X.; Ji, W.; Li, T.; Xu, X.; Chen, J.; Chen, X.; Zhu, X. Effects of High Temperature Stress on the Physiological and Biochemical Characteristics of Paeonia ostii. Int. J. Mol. Sci. 2023, 24, 11180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Zhou, G.; Sun, B.T.; Li, X.H.; Wang, S.S.; Shan, W. Physiological and Biochemical Effects of High Temperature Stress on the Seedlings of Two Rhododendron Species of Subgenus Hymenanthes. J. Plant Sci. 2011, 29, 362–369. [Google Scholar]
- Li, Z.G.; Yuan, L.X.; Wang, Q.L.; Ding, Z.L.; Dong, C.Y. Combined action of antioxidant defense system and osmolytes in chilling shock-induced chilling tolerance in Jatropha curcas seedlings. Acta Physiol. Plant. 2013, 35, 2127–2136. [Google Scholar] [CrossRef]
- Haagenson, D.M.; Cunningham, S.M.; Volenec, J.J. Root Physiology of Less Fall Dormant, Winter Hardy Alfalfa Selections. Crop Sci. 2003, 43, 1441–1447. [Google Scholar] [CrossRef]
- Bornman, C.H.; Jansson, E.; Nicotiana tabacum callus studies. X. ABA increases resistance to cold damage. Physiol. Plant. 2010, 48, 491–493. [Google Scholar] [CrossRef]
- Chae, W.B. Factors Affecting Tolerance to Low Night Temperature Differ by Fruit Types in Tomato. Agriculture 2021, 11, 681. [Google Scholar] [CrossRef]
- Ali, Z.S.; Sandhya, V.; Grover, M.; Kishore, N.; Rao, L.V.; Venkateswarlu, B. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol. Fertil. Soils 2009, 46, 45–55. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Liu, F.; Liu, S.; Tian, C. Carbon and nitrogen metabolism in arbuscular mycorrhizal maize plants under low-temperature stress. Crop Pasture Sci. 2015, 66, 62–70. [Google Scholar] [CrossRef]
- Berry, J.A.; Bjorkman, O. Photosynthetic Response and Adaptation to Temperature in Higher Plants. Annu. Rev. Plant Physiol. 2003, 31, 491–543. [Google Scholar] [CrossRef]
- Xu, Q.; Paulsen, A.Q.; Guikema, J.A.; Paulsen, G.M. Functional and ultrastructural injury to photosynthesis in wheat by high temperature during maturation. Environ. Exp. Bot. 1995, 35, 43–54. [Google Scholar] [CrossRef]
- AL-Khatib, K.; Paulsen, G. High-temperature effects on photosynthetic processes in temperate and tropical cereals. Crop Sci. 1999, 39, 119–125. [Google Scholar] [CrossRef]
- Hendrickson, L.; Ball, M.C.; Wood, J.T.; Chow, W.S.; Furbank, R.T. Low temperature effects on photosynthesis and growth of grapevine. Plant Cell Environ. 2010, 27, 795–809. [Google Scholar] [CrossRef]
- Chugunova, N.G.; Chermnykh, L.N.; Kosobryukhov, A.A.; Karpilova, I.F.; Chermnykh, R.M. Interrelationship of growth processes and photosynthesis during cucumber leaf ontogeny under low night temperature. Fiziol. Rastenij 1980, 9, 127–141. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Beneficial roles of arbuscular mycorrhizas in citrus seedlings at temperature stress. Sci. Hortic. 2010, 125, 289–293. [Google Scholar] [CrossRef]
- Ma, J.; Janoušková, M.; Yan, Y.; Yu, X.; Zou, Z.; Li, Y.; He, C. The photoprotective role of arbuscular mycorrhizal fungi (AMF) in cucumber seedlings under cold stress. Acta Hortic. 2018, 305–318. [Google Scholar] [CrossRef]
- Buttery, B.R.; Buzzell, R.I. The relationship between chlorophyll content and rate of photosynthesis in soybeans. Can. J. Plant Sci. 1977, 57, 1. [Google Scholar] [CrossRef]
- Ristic, Z.; Bukovnik, U.; Prasad, P.V.V. Correlation between Heat Stability of Thylakoid Membranes and Loss of Chlorophyll in Winter Wheat under Heat Stress. Crop Sci. 2007, 47, 2067–2073. [Google Scholar] [CrossRef]
- Kumar, N.; Gupta, S.; Tripathi, A.N. Gender-specific responses of Piper betle L. to low temperature stress: Changes in chlorophyllase activity. Biol. Plant. 2006, 50, 705–708. [Google Scholar] [CrossRef]
- de Oliveira, J.G.; da Costa Aguiar Alves, P.L.; Vitória, A.P. Alterations in chlorophyll a fluorescence, pigment concentrations and lipid peroxidation to chilling temperature in coffee seedlings. Environ. Exp. Bot. 2009, 67, 71–76. [Google Scholar] [CrossRef]
- Ma, J.; Janouková, M.; Yan, Y.; Yu, X.; Li, Y.; He, C. Arbuscular mycorrhizal fungi (AMF) increase carbohydrate content in cucumber subjected to low temperature stress. In Proceedings of the ISHS Acta Horticulturae, International Horticultural Congress IHC2018: III International Symposium on Innovation and New Technologies in Protected Cultivation, Istanbul, Turkey, 20 March 2020; pp. 359–364. [Google Scholar] [CrossRef]
- Caradonia, F.; Francia, E.; Morcia, C.; Ghizzoni, R.; Moulin, L.; Terzi, V.; Ronga, D. Arbuscular Mycorrhizal Fungi and Plant Growth Promoting Rhizobacteria Avoid Processing Tomato Leaf Damage during Chilling Stress. Agronomy 2019, 9, 299. [Google Scholar] [CrossRef]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Machiani, M.A.; Javanmard, A.; Machiani, R.H.; Sadeghpour, A. Arbuscular mycorrhizal Fungi and Changes in Primary and Secondary Metabolites. Plants 2022, 11, 2183. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K. Chemical identification and functional analysis of apocarotenoids involved in the development of arbuscular mycorrhizal symbiosis. Biosci. Biotechnol. Biochem. 2007, 71, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Heikham, E.; Rupam, K.; Bhoopander, G. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Chen, X.Y.; Song, F.B.; Liu, F.L.; Tian, C.J.; Liu, S.Q.; Xu, H.W.; Zhu, X.C. Effect of different arbuscular mycorrhizal fungi on growth and physiology of maize at ambient and low temperature regimes. Sci. World J. 2014, 2014, 956141. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Mahawar, S.; Jain, D.; Udpadhay, S.K.; Mohanty, S.R.; Singh, A.; Maharjan, E. Boosting Sustainable Agriculture by Arbuscular Mycorrhiza under Stress Condition: Mechanism and Future Prospective. Biomed. Res. Int. 2022, 2022, 5275449. [Google Scholar] [CrossRef] [PubMed]
- Dehne, H.W.; Schonbeck, F. Investigations on the influence of endotrophic mycorrhiza on plant diseaseas. II. Phenol metabolism and lignification. Phytopathology 1979, 86, 210–216. [Google Scholar] [CrossRef]
- Hajiboland, R.; Joudmand, A.; Aliasgharzad, N.; Tolrá, R.; Poschenrieder, C. Arbuscular mycorrhizal fungi alleviate low-temperature stress and increase freezing resistance as a substitute for acclimation treatment in barley. Crop Pasture Sci. 2019, 70, 218–233. [Google Scholar] [CrossRef]
- Torres, N.; Goicoechea, N.; Morales, F.; Antolín, M.C. Berry quality and antioxidant properties in Vitis vinifera cv. Tempranillo as affected by clonal variability, mycorrhizal inoculation and temperature. Crop Past 2016, 67, 961–977. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine; Builders, P.F., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Wei, G.T.; Wang, H.G. Effects of VA mycorrhizal fungi on growth, nutrient uptake and effective compounds in Chinese medicinal herb Datura stramonium L. Sci. Agric. Sin. 1989, 25, 56–61. [Google Scholar]
- Abu-Zeyad, R.; Khan, A.; Khoo, C. Occurrence of arbuscular mycorrhiza in Castanospermum austral A. Cunn. & C. Fraser and effects on growth and production of castanospermine. Mycorrhiza 1999, 9, 111–117. [Google Scholar]
- Blande, J.D.; Glinwood, R. Deciphering Chemical Language of Plant Communication. Springer: Berlin/Heidelberg, Germany, 2016.
- Dong, F.; Fu, X.; Watanabe, N.; Su, X.; Yang, Z. Recent advances in the emission and functions of plant vegetative volatiles. Molecules 2016, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, A.; Valenzuela, M.; Carvajal, M.; Fiaschi, G.; Avio, L.; Giovannetti, M.; D'Onofrio, C.; Seeger, M. The arbuscular mycorrhizal fungus Funneliformis mosseae induces changes and increases the concentration of volatile organic compounds in Vitis vinifera cv. Sangiovese leaf tissue. Plant Physiol. Biochem. 2020, 155, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Langrand, J.; Fontaine, J.; Sahraoui, A.L.-H. Significance of arbuscular mycorrhizal fungi in mitigating abiotic environmental stress in medicinal and aromatic plants: A review. Foods 2022, 11, 2591. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ma, W.; Li, X.; Miao, W.; Zheng, L.; Cheng, B. Polyamines stimulate hyphal branching and infection in the early stage of Glomus etunicatum colonization. World J. Microb. Biotechnol. 2012, 28, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.M.; Zheng, F.L.; Wu, Q.S. Elucidating the dialogue between arbuscular mycorrhizal fungi and polyamines in plants. World J. Microbiol. Biotechnol. 2022, 38, 159. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; He, X.H.; Zou, Y.N.; Liu, C.Y.; Xiao, J.; Li, Y. Arbuscular mycorrhizas alter root system architecture of Citrus tangerine through regulating metabolism of endogenous polyamines. Plant Growth Regul. 2012, 68, 27–35. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Aldubise, A.; Egamberdieva, D. Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J. Plant Interact. 2015, 10, 230–242. [Google Scholar] [CrossRef]
- Priya, M.; Sharma, L.; Kaur, R.; Bindumadhava, H.; Nair, R.M.; Siddique, K.H.M.; Nayyar, H. GABA (γ-aminobutyric acid), as a thermo-protectant, to improve the reproductive function of heat-stressed mungbean plants. Sci. Rep. 2019, 9, 7788. [Google Scholar] [CrossRef] [PubMed]
- Evelin, H.; Giri, B.; Kapoor, R. Ultrastructural evidence for AMF mediated salt stress mitigation in Trigonella foenum-graecum. Mycorrhiza 2013, 23, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Ramazan, S.; Nazir, I.; Yousuf, W.; John, R. Environmental stress tolerance in maize (Zea mays): Role of polyamine metabolism. Funct Plant Biol. 2022, 50, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Ouledali, S.; Ennajeh, M.; Ferrandino, A.; Khemira, H.; Schubert, A.; Secchi, F. Influence of arbuscular mycorrhizal fungi inoculation on the control of stomata functioning by ABA in drought-stressed olive plants. S. Afr. J. Bot. 2019, 121, 152–158. [Google Scholar] [CrossRef]
- Plouznikoff, K.; Declerck, S.; Calonne-Salmon, M. Mitigating abiotic stresses in crop plants by arbuscular mycorrhizal fungi. In Belowground Defence Strategies in Plants. Signaling and Communication in Plants; Vos, C.M.F., Kazan, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 341–400. [Google Scholar] [CrossRef]
- Reddy, P.S.; Mallikarjuna, G.; Kaul, T.; Chakradhar, T.; Mishra, R.N.; Sopory, S.K.; Reddy, M.K. Molecular cloning and characterization of gene encoding for cytoplasmic Hsc70 from Pennisetum glaucum may play a protective role against abiotic stresses. Mol. Genet. Genom. 2010, 283, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Scharf, K.D.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim. Biophys. Acta 2012, 1819, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, B.; Subrahmanyam, D.; Neelamraju, S.; Vishnukiran, T.; Rao, Y.V.; Vijayalakshmi, P.; Voleti, S.R.; Bhadana, V.P.; Mangrauthia, S.K. Integrated Physiological, Biochemical, and Molecular Analysis Identifies Important Traits and Mechanisms Associated with Differential Response of Rice Genotypes to Elevated Temperature. Front. Plant Sci. 2015, 6, 1044. [Google Scholar] [CrossRef] [PubMed]
- Zha, Q.; Xi, X.; He, Y.; Jiang, A. Transcriptomic analysis of the leaves of two grapevine cultivars under high-temperature stress. Sci. Hortic. 2020, 265, 109265. [Google Scholar] [CrossRef]
- Xiao, T.; Liu, X.-Q.; Liu, X.-R.; Li, Q.-S.; Abd_Allah, E.F.; Wu, Q.-S. Mycorrhizal cucumber with Diversispora versiformis has active heat stress tolerance by up-regulating expression of both CsHsp70s and CsPIPs genes. Sci. Hortic. 2023, 319, 112194. [Google Scholar] [CrossRef]
- Zheng, G.; Tian, B.; Zhang, F.; Tao, F.; Li, W. Plant adaptation to frequent alterations between high and low temperatures: Remodelling of membrane lipids and maintenance of unsaturation levels. Plant Cell Env. 2011, 34, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Janoušková, M.; Li, Y.; Yu, X.; Yan, Y.; Zou, Z.; He, C. Impact of arbuscular mycorrhizal fungi (AMF) on cucumber growth and phosphorus uptake under cold stress. Funct. Plant Biol. 2015, 42, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Ma, L.N.; He, X.Y.; Tian, C.J. Water strategy of mycorrhizal rice at low temperature through the regulation of PIP aquaporins with the involvement of trehalose. Appl. Soil Ecol. 2014, 84, 185–191. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).