Abstract
Impatiens walleriana is of great ornamental value, but it may suffer growth inhibition when it is exposed to sub-low temperatures for a long time. Although there are many studies on the positive effect of arbuscular mycorrhizal fungi (AMF) on cold tolerance, whether AMF could alleviate the sub-low temperature injury of Impatiens walleriana is unknown. In this experiment, two kinds of Impatiens walleriana were inoculated with AMF and treated with sub-low temperature to evaluate the physiological and biochemical characteristics of Impatiens walleriana seedlings. The results showed that the plant height of ‘Super Elf (Rose red)’ under stress and inoculated with 50 g and 100 g AMF compared to sub-low temperature treatment increased by 4.94% and 19.01%, and the plant height of ‘Super Elf (red)’ under stress and inoculated with 50 g and 100 g AMF increased by 3.11% and 17.03%, respectively. Compared to sub-low temperature treatment, the stem diameter of ‘Super Elf (Rose red)’ under stress and inoculated with 50 g and 100g AMF increased by 47.17% and 50.94%, respectively. The same mitigation effect was observed in ‘Super Elf (red)’. Compared with sub-low temperature treatment, Fv’/Fm’, Y(II) and qP of ‘Super Elf (Rose red)’ inoculated with 50 g AMF significantly increased by 75.76%, 52.17%, and 43.48%, while NPQ significantly decreased by 2.96 times, whereas the corresponding values for ‘Super Elf (Rose red)’ inoculated with 100 g AMF increased by 87.88%, 82.61%, and 65.22%, while NPQ significantly increased by 1.47 times. Compared with sub-low temperature treatment, Fv’/Fm’, Y(II) and qP of ‘Super Elf (red)’ inoculated with 50 g AMF significantly increased by 53.49%, 28.95%, and 29.31%, while NPQ significantly decreased by 0.84 times, whereas the corresponding values for ‘Super Elf (red)’ inoculated with 100 g AMF increased by 53.49%, 23.68%, and 22.41%, while NPQ significantly increased by 3.48 times. Meanwhile, ‘Super Elf (Rose red)’ and ‘Super Elf (red)’ vaccination with AMF reduced the levels of O2− and H2O2 accumulation in leaves at sub-low temperatures and mitigated the extent of damage to cell membrane lipid peroxidation. Therefore, AMF inoculation can improve the tolerance of plants to sub-low temperatures.
1. Introduction
African impatiens (Impatiens walleriana), also known as Waller impatiens and Ocean impatiens, are native to the tropical regions of eastern Africa. They are annual herbaceous plants of Impatiens in Impatiens. They have rich colors, such as bright red, rose red, pink, purple red and so on. They have the characteristics of strong negative resistance, strong adaptability, easy survival and rapid growth [1]. In addition, Impatiens walleriana also has high ornamental value and economic value, and is widely loved by people [2]. Medically, Impatiens walleriana showed good antioxidant activity [3]. In production, Impatiens walleriana can be used as a dye and food preservative [4].
The influence of temperature on plant growth and development is mainly achieved through its influence on various physiological activities in plants, such as active oxygen species, and other indicators can reflect the physiological state of plants to a certain extent [5,6]. Impatiens walleriana is native to tropical Africa and does not tolerate low temperatures. Most of its physiological processes are highly sensitive to temperature, and the suitable temperature range for growth is 17~25 °C [7,8], and excessively high temperature easily results in damage from pests and diseases. In winter, it is not suitable to cultivate Impatiens walleriana in an environment below 10 °C. If the temperature is too low, it will inhibit the plant’s growth and easily cause it to lose its leaves [9].
Sub-low temperature is a common occurrence in the solar greenhouse in winter and spring, that is, it is often lower than 20 °C during the day and 5~12 °C at night, severely restricting the growth of plants [10]. At sub-low temperature, physiological activities of plants are disordered, and yield and quality are seriously decreased [11,12]. The experiment showed that sub-low temperature treatment reduced the plant growth and leaf physiological characteristics of tomato (Solanum lycopersicum L.) [13], cucumber (Cucumis sativus L.) [14], etc. Therefore, it is of great significance to study the physiological response mechanism of Impatiens walleriana to sub-low temperature and seek appropriate regulatory measures to enhance cold tolerance, so as to promote its wide application in horticulture and improve its economic benefits.
Arbuscular mycorrhizal fungi (AMF) are beneficial fungi widely present in soil [15]. They are able to form mutualistic symbiosis with 80% of plants on land, making them one of the most widely distributed mutualistic symbionts in terrestrial ecosystems [16,17]. They can also improve the absorption capacity of water and nutrients by improving the soil structure and expanding the root absorption area of host plants, thus improving the growth state of plants and enhancing plant stress tolerance [18,19]. Recent studies have found that AMF inoculation can protect cell membrane structure and improve physiological metabolism by promoting plant growth, improving antioxidant enzyme activity and osmotic regulation ability, and maintaining normal electron transfer of photosystem II [20,21]. Among them, some scholars have found that under the stress of heavy metal cadmium, the interaction between Impatiens walleriana and AMF fungi can improve antioxidant enzyme activity, reduce membrane lipid peroxidation, and promote the absorption of heavy metal cadmium by Impatiens walleriana [22]. For further low-temperature treatment, AMF was found to improve cucumber [23], barley [24] and antirrhinum majus [25]. Therefore, inoculation with AMF may also enhance the sub-low temperature tolerance of Impatiens walleriana, which needs to be proved.
In this experiment, we selected two cultivars of Impatiens walleriana. By comparing the effects of different AMF inoculation concentrations on the growth and physiological characteristics of Impatiens walleriana at sub-low temperature, we hope to provide a theoretical basis for the application of AMF to the production practices for Impatiens walleriana.
2. Materials and Methods
2.1. Plant Materials
In this study, two different cultivars of Impatiens walleriana were screened: namely ‘Super Elf (Rose red)’ and ‘Super Elf (red)’ provided by Ball Hort Company, United States of America. These two cultivars of Impatiens walleriana have similar tolerance to cold stress.
2.2. Treatment Design
2.2.1. Hole Tray and Strain Preparation
Huai’an chaimihe agricultural technology company China was entrusted with microbial agent preparation. Two-thirds of a cultivation bowl was filled with sterilized sand, then spore liquid containing Glomus mosseae was mixed into the sand. The ratio of fungi liquid to sand in weight was 3%, spore liquid mixed with another 13 species of AMF (Acaulospora foveate, Acaulospora morrowiae, Acaulospora spinosa, Gigaspora albida, Gigaspora gigantean, Gigaspora margarita, Glomus claroideum, Glomus clarum, Glomus diaphanum, Glomus etunicatum, Glomus intraradices, Glomus versiforme, and Scutellospora erythropa) mixed into the sand at a 3% ratio of fungi liquid to sand in weight. Host seeds (clover) were sown in the sand after disinfection by soaking in 5% potassium permanganate for 1 h. Then, the cultivation bowl was transferred to an artificial climate chamber for cultivation. The bows were irrigated with Hogland nutrient solution once weekly. During harvesting, the aboveground stems and leaves of the host plant were cut off and placed in a pot at room temperature of 25 °C and humidity of 50% to dry. All the culture materials (including plant roots, hyphae, spores, and substrate) in the pot were collected and stored at room temperature at 25 °C for later treatments.
In the artificial climate chamber of Sichuan Agricultural University, the seeds of Impatiens walleriana were sown in 72-hole cavity trays filled with substrate and AMF (spore concentration of 120 spores/g). The components of the substrate were 60% peat, 20% vermiculite, and 20% perlite. The tray was disinfected with 75% alcohol before placing the substrates. The ratios of AMF to substrate were 50 g/500 g and 100 g/500 g, respectively.
2.2.2. Seed Pretreatment
Firstly, the neat and plump seeds of Impatiens walleriana were selected for soaking in warm soup. The soaking temperature was 55 °C, and the soaking time was 6 h. The aim was to promote seed germination and sterilization. Secondly, they were sown into the hole tray and placed in an artificial climate box for seedling cultivation. Seedling conditions were 25 °C/18 °C (day/night), light intensity of 250 μmol·m−2·s−1, photoperiod of 12 h/12 h (day/night), and relative humidity of 70%. On the 50th day after plant emergence, the quality of arbuscular mycorrhizal infection was observed in order to carry out the next experiment, and the plants were prepared for treatment. The mycorrhizal infection rate (%) = (length of root segments infected by mycorrhizal fungi/total length of inspected root segments) × 100.
2.2.3. Treatment Details
The fixed seedlings were put into the artificial climate box and subjected to sub-low temperature treatment. The stress treatment temperature was 12 °C/8 °C (day/night), and 25 °C/18 °C (day/night) was used as the control (CK). Conditions such as light and humidity were the same as pre-treatment.
Under normal temperature and sub-low temperature, three treatments were carried out on two Impatiens walleriana varieties. The treatment methods were non-inoculation treatment, inoculation with 50 g AMF treatment and inoculation with 100 g AMF treatment. There were six treatments for these two varieties. The two varieties had 10 pots of plants under each treatment condition, 3 replicates, and a total of 180 pots of plants. The six treatments were as follows:
- (1)
- The control treatment (CK): normal temperature treatment without AMF inoculation;
- (2)
- AMF-50 treatment: 50 g AMF inoculated at normal temperature;
- (3)
- AMF-100 treatment: 100 g AMF inoculated at normal temperature;
- (4)
- CK+L treatment: sub-low temperature treatment without AMF inoculation (12 °C/8 °C (day/night));
- (5)
- AMF-50+L treatment: 50 g AMF inoculated with sub-low temperature treatment (12 °C/8 °C (day/night));
- (6)
- AMF-100+L treatment: 100 g AMF inoculated with sub-low temperature treatment (12 °C/8 °C (day/night)).
After 10 days of sub-low temperature treatment, plant height, stem diameter and chlorophyll fluorescence parameters of each treatment were measured. Meanwhile, leaf and root samples of treated plants in each group were collected to test the plasma membrane permeability, O2− content, H2O2 content and damage degree of membrane lipid peroxidation.
2.3. The Effect of Mycorrhizal Infection
When the seedling length reached about 10 cm, the roots of Impatiens walleriana were taken to observe the mycorrhizal infection effect. This method was based on the method of Liu et al. [11]. The roots of seedlings were first stained with Quick black ink, and then the mycorrhizal infection effect was observed under a microscope and photographed.
2.4. Determination of Growth Indicators
After sub-low temperature stress treatment (10 d), the plant height and stem diameter of each treatment group were measured by ruler and vernier caliper. Each treatment had 5 biological replicates, that is, 6 treatments had a total of 30 biological replicates.
2.5. Determination of Chlorophyll Fluorescence
The chlorophyll fluorescence was measured with a portable modulated chlorophyll fluorescence analyzer (PAM-2500, Walz, Effeltrich, Germany) by referring to the method of Baker [26].
2.6. Determination of Plasma Membrane Permeability of Leaves and Roots
Plasma membrane permeability was measured with a DDS-200 conductance meter (Chengdu Century Ark Technology Company Limited, Chengdu, China), slightly modified by referring to the method of Wang et al. [27].
2.7. Determination of O2− and H2O2 Content in Leaves
The accumulation of O2− and H2O2 in plants was qualitatively analyzed by histochemical staining. The method of Xu et al. [28] was applied to histochemical staining and photographic recording of the radicle of different cultivars and different treatments of Impatiens walleriana.
2.8. Determination of Damage Degree of Leaf Membrane Lipid Peroxidation
The damage degree of membrane lipid peroxidation was detected by Schiff’s reagent staining method and photographed [29].
2.9. Data Statistics and Analysis
Microsoft Excel 2019 and SPSS 27.0 software were used for statistical analysis of experimental data. Univariate analysis of variance (p < 0.05) was used to analyze the significant difference. Origin 2021 was used to perform the principal component analysis (PCA) and correlation analysis.
3. Results
3.1. Effects of AMF on Growth Indices of Impatiens walleriana under Sub-Low Temperature Stress
We observed the degree of infestation of clumping mycorrhizae by different treatments. As shown in Figure S1, no arbuscular mycorrhizal structures were found in the root system without AMF vaccination. After vaccination with AMF, clear arbuscular mycorrhizal structures such as vesicles and hyphae could be seen. The mycorrhizal colonization ratio for the 50 g AMF treatment and the 100 g AMF treatment was 18.28% and 24.63%, respectively (Table S1). Compared with 50 g AMF treatment, the number of arbuscular vesicles in root after vaccination with 100 g AMF treatment increased by 41.67% and 50%, respectively (Table S1). According to Figure 1, the height and stem diameter of Impatiens walleriana showed differences among treatments. Compared with CK treatment, the plant height of ‘Super Elf (Rose red)’ under sub-low temperature stress decreased by 19.18% (Figure 1A). Compared to sub-low temperature treatment, the plant height of ‘Super Elf (Rose red)’ under stress and inoculated with 50 g and 100 g AMF increased by 4.94% and 19.01%, respectively (Figure 1A). Meanwhile, in another cultivar, compared to sub-low temperature treatment, the plant height of ‘Super Elf (red)’ inoculated with 50 g AMF and 100 g AMF under stress increased by 3.11% and 17.03%, respectively (Figure 1A).
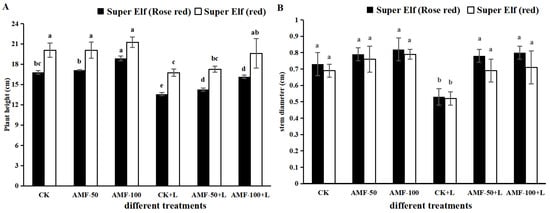
Figure 1.
Effects of AMF on (A) plant height and (B) stem diameter of two Impatiens walleriana cultivars under sub-low temperature stress. Values are means ± standard error. The different lowercase letters indicate significant differences among different treatments according to the Tukey’s HSD test (p < 0.05).
Compared with CK treatment, the stem diameter of ‘Super Elf (Rose red)’ under sub-low temperature stress decreased by 27.40% (Figure 1B). Compared to sub-low temperature treatment, the stem diameter of ‘Super Elf (Rose red)’ under stress and inoculated with 50 g and 100g AMF increased by 47.17% and 50.94%, respectively (Figure 1B). Additionally, the same mitigation effect was observed in the other cultivar. However, there were no changes in plant height and stem diameter of ‘Super Elf (Rose Red)’ inoculated with 50 g AMF and 100 g AMF as compared to CK treatment. The plant height and stem diameter of the two Impatiens walleriana cultivars decreased significantly under sub-low temperature stress. The biomass of the two Impatiens walleriana cultivars was significantly increased by vaccination with AMF, and that of ‘Super Elf (Rose red)’ was more significantly increased by vaccination with 100 g AMF.
3.2. Effect of AMF on Chlorophyll Fluorescence of Impatiens walleriana Leaves under Sub-Low Temperature Stress
According to Figure 2, compared with CK, Fv’/Fm’, Y(II) and qP of ‘Super Elf (Rose red)’ treatment under sub-low temperature stress significantly decreased, by 53.52%, 59.65%, and 39.47%, while NPQ significantly increased by 4.82 times, whereas the corresponding Fv’/Fm’, Y(II) and qP values for ‘Super Elf (red)’ significantly decreased by 41.09%, 29.63%, and 19.44%, while NPQ significantly increased by 14.66 times. Compared with the sub-low temperature treatment, the Fv’/Fm’, Y(II) and qP of ‘Super Elf (Rose red)’ inoculated with 50 g AMF under stress significantly increased by 75.76%, 52.17%, and 43.48%, while NPQ significantly decreased by 2.96 times, the corresponding Fv’/Fm’, Y(II) and qP values for ‘Super Elf (Rose red)’ inoculated with 100 g AMF under stress increased by 87.88%, 82.61%, and 65.22%, while NPQ significantly increased by 1.47 times, respectively (Figure 2). Meanwhile, in the ‘Super Elf (red)’, compared with sub-low temperature treatment, Fv’/Fm’, Y(II) and qP of ‘Super Elf (red)’ inoculated with 50 g AMF under stress significantly increased by 53.49%, 28.95%, and 29.31%, while NPQ significantly decreased by 0.84 times, whereas the corresponding Fv’/Fm’, Y(II) and qP of ‘Super Elf (red)’ inoculated with 100 g AMF under stress increased by 53.49%, 23.68%, and 22.41%, while NPQ significantly increased by 3.48 times (Figure 2), respectively. There were no significant changes in Fv/Fm for the leaves of the two Impatiens walleriana cultivars. The one-way ANOVA suggested that Fv’/Fm’, Y(II) and qP of the two cultivars inoculated with AMF were significantly increased, and NPQ was significantly decreased compared with the uninoculated specimens under the same sub-low temperature condition, and ‘Super Elf (Rose red)’ was more significantly increased by vaccination with 100 g AMF.
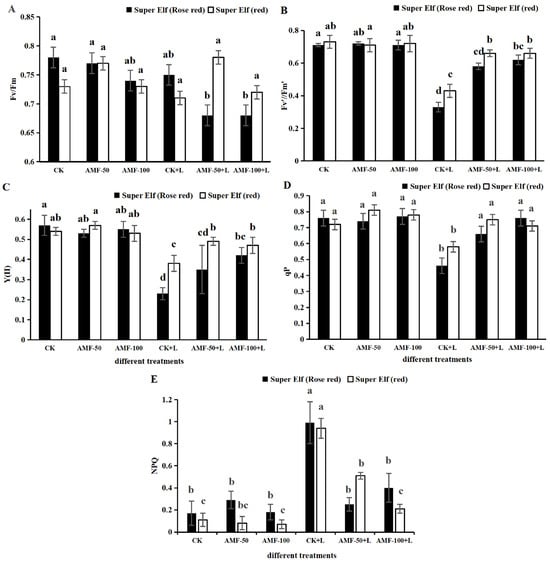
Figure 2.
Effects of AMF on Fv/Fm (A), Fv’/Fm’ (B), Y(II) (C), qP (D), and NPQ (E) of two Impatiens walleriana cultivars under sub-low temperature stress. Values are means ± standard error. The different lowercase letters indicate significant differences among different treatments according to the Tukey’s HSD test (p < 0.05).
3.3. Effects of AMF on Plasma Membrane Permeability of Impatiens walleriana Leaves and Roots under Sub-Low Temperature Stress
As shown in Figure 3, compared with CK, plasma membrane permeability of leaves and roots of ‘Super Elf (Rose red)’ under sub-low temperature stress increased by 36.15% and 59.62%, respectively. Compared with sub-low temperature treatment, plasma membrane permeability of leaves and roots of ‘Super Elf (Rose red)’ inoculated with 50 g AMF significantly decreased by 5.65 times and 43.27%, while that of leaves and roots of ‘Super Elf (Rose red)’ inoculated with 100 g AMF decreased by 4.9 times and 44.49% (Figure 3), respectively. Meanwhile, the plasma membrane permeability of leaves and roots of ‘Super Elf (red)’ showed the same trend. After ‘Super Elf (red)’ was inoculated with 100 g AMF, the plasma membrane permeability of leaves and roots under stress decreased by 69.33% and 63.14%, respectively.
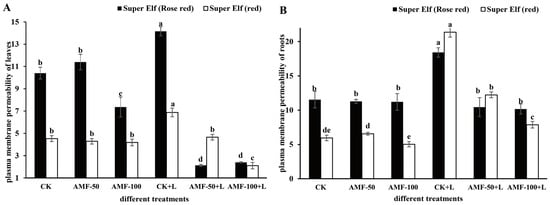
Figure 3.
Effects of AMF on plasma membrane permeability of Impatiens walleriana leaves (A) and roots (B) of two Impatiens walleriana cultivars under sub-low temperature stress. Values are means ± standard error. The different lowercase letters indicate significant differences among different treatments according to the Tukey’s HSD test (p < 0.05).
The relative electrolyte permeability of the leaves and roots of the ‘Super Elf (Rose red)’ and ‘Super Elf (red)’ inoculated with AMF were significantly lower than that of the uninoculated strains, indicating that AMF inoculation could alleviate the cell membrane damage to a certain extent. After analyzing the relative electrolyte permeability values of the leaves of two cultivars under six treatments, we found that when dealing with AMF-50+L and AMF-100+L, the values were the smallest, indicating that the AMF under these two treatments had the best protection effect on Impatiens walleriana. In the same way, the relative electrolyte permeability value of roots were analyzed, and the optimal treatment for the two cultivars was different. ‘Super Elf (Rose red)’ treated with AMF-100+L showed better tolerance than the rest of the treatments under sub-cold temperature.
3.4. Effects of AMF on O2− and H2O2 Content of Impatiens walleriana Leaves under Sub-Low Temperature Stress
As the level of accumulation of O2− content and H2O2 content in the leaves of the plant increased, the area stained by a number of spots of the leaves increased, indicating the presence of damage in the leaves, whereas the opposite indicated a small degree of damage in the leaves. The accumulation of O2− was observed by the number of blue spots. A large number of blue-stained areas appeared in the leaves of ‘Super Elf (Rose red)’ and ‘Super Elf (red)’ under sub-low temperature stress as compared to CK treatment (Figure 4d,j). Under the sub-low temperature treatment, the stained area of leaves of ‘Super Elf (Rose red)’ inoculated with 50 g AMF was reduced, and a few blue areas appeared (Figure 4e), whereas almost no blue areas appeared in the leaves inoculated with 100 g AMF (Figure 4f). ‘Super Elf (red)’ was colored to the same degree as ‘Super Elf (Rose red)’ (Figure 4k,l). In addition, under normal conditions, the degree of staining of the leaves of specimens under AMF treatment was basically the same as that of CK (Figure 4b,c,h,i).
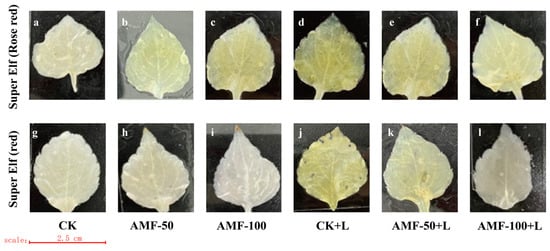
Figure 4.
Effect of AMF on O2− content in ‘Super Elf (Rose red)’ leaves under ck (a), AMF-50 (b), AMF-100 (c), CK+L (d), AMF-50+L (e), AMF-100+L (f); Effect of AMF on O2− content in ‘Super Elf (red)’ leaves under ck (g), AMF-50 (h), AMF-100 (i), CK+L (j), AMF-50+L (k), AMF-100+L (l).
The accumulation of H2O2 was observed by the number of brown spots. Compared with the CK treatment, there were many brown spots on the leaves of ‘Super Elf (Rose red)’ and ‘Super Elf (red)’ under sub-low temperature stress (Figure 5d,j). No brown spots appeared on the leaves of Impatiens walleriana inoculated with AMF under normal conditions (Figure 5b,c,h,i), as in CK. Under the same sub-low temperature treatment, the brown spots on the leaves of ‘Super Elf (Rose red)’ inoculated with 50 g AMF were relatively reduced (Figure 5e), while the leaves of the ‘Super Elf (Rose red)’ inoculated with 100 g AMF were almost free of brown spots (Figure 5f). ‘Super Elf (red)’ was colored to the same degree as ‘Super Elf (Rose red)’ (Figure 5j,l). ‘Super Elf (Rose red)’ inoculated with 100 g AMF extremely significantly reduced the degree of staining and decreased ROS damage.
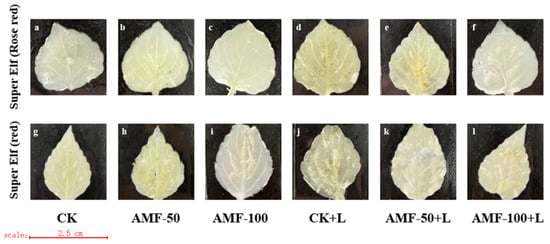
Figure 5.
Effect of AMF on H2O2 content in ‘Super Elf (Rose red)’ leaves under ck (a), AMF-50 (b), AMF-100 (c), CK+L (d), AMF-50+L (e), AMF-100+L (f); Effect of AMF on O2− content in ‘Super Elf (red)’ leaves under ck (g), AMF-50 (h), AMF-100 (i), CK+L (j), AMF-50+L (k), AMF-100+L (l).
3.5. Effect of AMF on the Degree of Membrane Lipid Peroxidation of Impatiens walleriana Leaves under Sub-Low Temperature Stress
Membrane lipid peroxidation causes some damage to plant cells in specific environments. Leaves were injured by membrane lipid peroxidation, and the number of black dots increased. As shown in Figure 6, a large number of small black dots appeared on the leaves of two Impatiens walleriana species under sub-low temperature treatment compared to CK (Figure 6d,j). There were no small black dots on the leaves of ‘Super Elf (Rose red)’ inoculated with AMF under CK (Figure 6b,c). Under sub-low temperature treatment, the leaves of ‘Super Elf (Rose red)’ inoculated with 50 g AMF had reduced black dots (Figure 6e), and the leaves inoculated with 100 g of AMF had almost no black dots (Figure 6f). The black dots on the leaves of ‘Super Elf (Red)’ inoculated with 50 g and 100 g of AMF were all reduced as compared to CK (Figure 6k,l), but it could be noticed that the effects of 100 g AMF treatment (AMF-100+L) in two Impatiens walleriana species were more pronounced compared to the 50 g AMF treatment (AMF-50+L) (Figure 6e,f).

Figure 6.
Effect of AMF on the degree of membrane lipid peroxidation in ‘Super Elf (Rose red)’ leaves under ck (a), AMF-50 (b), AMF-100 (c), CK+L (d), AMF-50+L (e), AMF-100+L (f); Effect of AMF on the degree of membrane lipid peroxidation in ‘Super Elf (red)’ leaves under ck (g), AMF-50 (h), AMF-100 (i), CK+L (j), AMF-50+L (k), AMF-100+L (l).
3.6. Principal Component and Correlation Analysis
The correlation between the six treatments of ‘Super Elf (Rose Red)’ and ‘Super Elf (Red)’ is depicted in Figure 7A. Plant height and stem diameter were positively correlated with Fv’/Fm’, Y(II) and qP. Root plasma membrane permeability was negatively correlated with NPQ, leaf and root plasma membrane permeability.
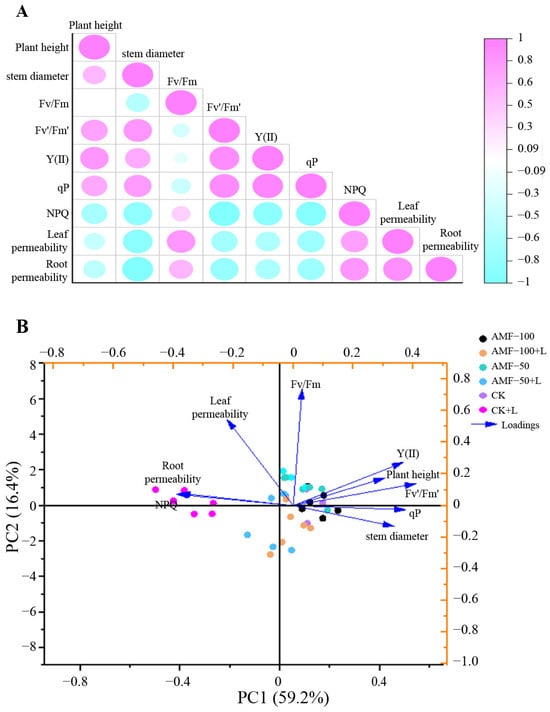
Figure 7.
Correlation (A) and PCA (B) for different traits observed in two Impatiens walleriana cultivars among six treatments.
The PCA of ‘Super Elf (Rose Red)’ and ‘Super Elf (Red)’ across the six treatments is shown in Figure 7B. The PCA showed that the total observed trait variability of the plants was 75.6%, and the measured trait variability was 59.2% and 16.4% in PC1 and PC2, respectively. In PC1, Y(II), plant height, stem diameter and qP were positively correlated, whereas NPQ, leaf and root plasma membrane permeability were negatively correlated.
4. Discussion
4.1. Effects of AMF on Plant Growth under Sub-Low Temperature Stress
Sub-low temperature usually limits the transfer of plant nutrients, leads to reduced carbon assimilation and increased growth energy consumption, and affects various physiological and biochemical processes of plants [10,13]. AMF forms a remarkable symbiotic relationship with plants and spreads throughout the root system through an extended mycelial network [17,30]. These mycelia help the plant root system to move beyond the zone of depletion and enhance the plant’s tolerance to biotic and abiotic stresses, thereby providing more soil mineral nutrients and water to the host plant [31]. Plants treated with AMF showed increased tolerance to low-temperature stress, higher growth potential, less leaf damage, and enhanced plant capacity to absorb soil water and nutrients such as nitrogen and phosphorus, thereby improving crop yield and plant quality [32]. According to Yan et al. [33], the biomass of perennial ryegrass was significantly reduced under low-temperature treatments compared to the control, but inoculation with AMF significantly promoted the growth of perennial ryegrass and mitigated the low-temperature injury. According to Chen et al. [34], it was found that cucumber seedlings inoculated with AMF significantly increased the dry and fresh weight, thus improving their cold tolerance. Meanwhile, AMF and sub-low temperature exhibited additive effects, which caused similar changes in plant metabolism, such as improved osmoregulation and accumulation of protective molecules [34,35]. In this experiment, two cultivars of Impatiens walleriana inoculated with AMF under sub-low temperature increased plant height and stem diameter (Figure 1). The underlying mechanism of AMF-initiated effects is not clear.
4.2. Effects of AMF on Plant Photosynthesis under Sub-Low Temperature Stress
Photosynthesis is a highly sensitive process during plant growth. The plant’s cystoid membrane is severely damaged at low temperatures, while energy distribution, light energy uptake, and electron transfer in photosynthesis are limited, and PSII is disrupted [36]. QP is the capture of raw light energy by the plant, and its increase reflects the share of light energy absorbed by PSII antenna pigments [37], whereas the reduction in NPQ has been suggested as an energy dissipation mechanism to protect photosynthetic organs from excessive light exposure [38]. Feng et al. [39] found that Pn, PSII and Fv/Fm of chrysanthemum decreased under low temperature and low light conditions, which affected the growth and development of chrysanthemum and reduced its ornamental quality. In contrast, inoculation with AMF may help to hinder electron transfer to alleviate the photoinhibition of PS I by low temperature stress and protect the photosynthetic organs, in which the stability of PS I is the key to the rapid recovery of PSII [40]. AMF obtains photosynthetic products by infecting the root system of the host plant, thereby enhancing the uptake of mineral nutrients by the host plant and reducing the effects of abiotic stresses [22]. In this experiment, we found that two cultivars of Impatiens walleriana significantly increased Fv’/Fm’, PSII, and qP and significantly reduced NPQ in CK compared to AMF-inoculated plants (Figure 2), indicating that AMF inoculation can alleviate the effects of sub-low temperature stress on electron transport to some extent and reduce the damage to leaf photosystems and thus improve the photosynthetic capacity of two cultivars of Impatiens walleriana leaves.
4.3. Effects of AMF on Plant Antioxidant System under Sub-Low Temperature Stress
The cold tolerance of plants is closely related to their antioxidant system. Reactive oxygen species (ROS) are by-products of metabolic processes in mitochondria, chloroplasts and peroxisomes [41]. When the growth environment is normal, ROS production and removal in plants are in dynamic equilibrium [42]. Subcooled environments lead to a disruption of plant ROS homeostasis, which can result in a plant ROS burst. High levels of ROS cause severe membrane lipid peroxidation in plants by attacking cell membranes and damaging proteins, lipids, and other cellular components [43]. Cellular osmotic balance and cytoplasmic efflux in plants are disrupted, resulting in reduced cell membrane fluidity [44]. In this experiment, two cultivars of Impatiens walleriana inoculated with AMF under subcooling stress decreased the relative electrolyte osmolality values of the leaves (Figure 3A), suggesting an increase in the cell membrane defense capacity. ‘Super Elf (Rose red)’ significantly increased the relative electrolyte osmolality values of roots inoculated with AMF-100 under normal or subcooled conditions, and ‘Super Elf (red)’ increased the relative electrolyte osmolality values of roots inoculated with both AMF-50 and AMF-100 under subcooled conditions, suggesting that the two cultivars of Impatiens walleriana inoculated with AMF under subcooled conditions increased cell membrane permeability (Figure 3B). Hydrogen peroxide (H2O2), superoxide anion radical (O2−) and hydroxyl radical (OH−) are a few toxic ROS that are overproduced under subcooled conditions [45]. Researchers significantly reduced MDA, H2O2, and O2− levels in the exoplasm and vesicles of tomato root cells inoculated with AMF under cold stress compared to the control without AMF. The study of Pasbani et al. [46] reported reduced root colonization of eggplant by inoculation with AMF to improve photochemical responses, activation of antioxidant defenses, and accumulation of protective molecules to alleviate cold stress in eggplant. Bidabadi and Mehralian [15] reported watermelon inoculation with AMF significantly reduced H2O2 and MDA, and alleviated oxidative stress. In this experiment, inoculation with AMF could enhance the scavenging capacity of two cultivars of Impatiens walleriana for O2− and H2O2, reduce the damage from subfreezing temperature on leaf, root and membrane lipid peroxidation, improve the antioxidant mechanism of the plants under sub-low temperature, and increase the tolerance to sub-low temperature (Figure 4, Figure 5 and Figure 6).
5. Conclusions
Plant height, stem diameter and chlorophyll fluorescence parameters of ‘Super Elf (Rose red)’ and ‘Super Elf (red)’ under sub-low temperature stress were increased by 50 g and 100 g AMF treatments. Under sub-low temperature stress, ‘Super Elf (Rose red)’ and ‘Super Elf (red)’ inoculated with AMF showed a decrease in O2− and H2O2 content, as observed by histochemical staining, which improved osmotic regulation and reduced membrane lipid peroxidation and sub-low temperature stress injury. The results indicate that AMF has potential application in Impatiens walleriana cultivation under sub-low temperature environments for sustainable cultivation of plants. This finding will be helpful for further research to improve the cold tolerance of plants in mildly low temperature areas. The physiological and biochemical changes induced by its potential genetic mechanisms need to be further investigated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10080856/s1, Figure S1: Effects of staining of Impatiens walleriana roots under sub-low temperature treatment was observed by microscopy.; Table S1: Effects of AMF on mycorrhizal development of Impatiens walleriana.
Author Contributions
Conceptualization, X.Z.; Formal analysis, D.Y.; Investigation, X.L.; Methodology, W.W.; Project administration, Z.H.; Software, J.B.; Supervision, Z.H.; Validation, X.Z.; Writing—original draft, X.Z. and D.Y.; Writing—review & editing, X.Z. and D.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Study on Vegetable Science of Farmland System in Qinghai—Tibet Plateau (Grant No. 2019QZKK0303).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, M.H.; Jiang, Y.; He, X.F.; Zhou, S.F. African Impatiens production and cultivation techniques. Agric. Sci. Technol. Inf. 2011, 4, 21–22. [Google Scholar] [CrossRef]
- Li, Y.; Guo, W.C.; Liu, J.H. The chemical constituents and pharmacological effects of Impatiens balsamina and its edible and ornamental. Spec. Econ. Anim. Plants 2015, 9, 33–36. [Google Scholar] [CrossRef]
- Hu, X.L.; Han, Z.X.; Liu, Y.F.; Zhao, H.; Li, Y.L.; Mao, N.W. Study on Antioxidative Activity of Extracts from Flowers of Impatiens balsamina L. Food Sci. 2007, 2, 48–50. [Google Scholar] [CrossRef]
- Shi, W.L. Research Status of Impatiens Germplasm Resources. Nanfang Agric. Mach. 2024, 55, 159–161. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Role of Reactive Oxygen Species and Hormones in Plant Responses to Temperature Changes. Int. J. Mol. Sci. 2021, 22, 8843–8865. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.R.; Gladon, R.J.; Taber, H.G. Effect of excessive calcium applications on growth and postharvest performance of bedding-plant impatiens. J. Plant Nutr. 2007, 30, 1639–1649. [Google Scholar] [CrossRef]
- Nelson, P.V.; Song, C.Y.; Huang, J.S.; Niedziela, C.E.; Swallow, W.H. Relative Effects of Fertilizer Nitrogen Form and Phosphate Level on Control of Bedding Plant Seedling Growth. Hortscience 2012, 47, 249–253. [Google Scholar] [CrossRef]
- Ye, L.F. Cultivation Techniques of African Impatiens. Agric. Inf. China 2013, 11, 81–82. [Google Scholar]
- Li, L.J.; Yang, B.L.; Zhao, X.P.; Wang, P.; Lyu, D.; Qin, S.J. Auxin Participates in the Regulation of the Antioxidant System in Malus baccata Borkh. Roots under Sub-Low Temperature by Exogenous Sucrose Application. Horticulturae 2023, 9, 297–315. [Google Scholar] [CrossRef]
- Liu, L.L.; Song, H.; Shi, K.J.; Liu, B.; Zhang, Y.; Tang, L.; Cao, W.X.; Zhu, Y. Response of wheat grain quality to low temperature during jointing and booting stages-On the importance of considering canopy temperature. Agric. For. Meteorol. 2019, 278, 107658. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ma, K.F.; Li, Q.W. Effects of Low-Temperature Accumulation on Flowering of Prunus mume. Horticulturae 2023, 9, 628–637. [Google Scholar] [CrossRef]
- Gao, H.; Wu, F.Z. Physiological and transcriptomic analysis of tomato in response to sub-optimal temperature stress. Plant Signal. Behav. 2024, 19, e2332018. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Chen, X.J.; Shi, K.; Xia, X.J.; Considine, M.J.; Yu, J.Q.; Zhou, Y.H. The sub/supra-optimal temperature-induced inhibition of photosynthesis and oxidative damage in cucumber leaves are alleviated by grafting onto figleaf gourd/luffa rootstocks. Physiol. Plant 2014, 152, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Bidabadi, S.S.; Mehralian, M. Arbuscular Mycorrhizal Fungi Inoculation to Enhance Chilling Stress Tolerance of Watermelon. Gesunde Pflanz. 2020, 72, 171–179. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, M.M.; Wang, Z.F.; Li, J.R.; Liu, K.; Huang, D. The role of arbuscular mycorrhizal symbiosis in plant abiotic stress. Front. Microbiol. 2024, 14, 1323881. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.S.; Zhang, Q.; Pan, J.B.; Jiang, S.J.; Liu, Y.J.; Bahadur, A.; Peng, Z.L.; Yang, Y.; Feng, H.Y. Effect of arbuscular mycorrhizal fungi on soil enzyme activity is coupled with increased plant biomass. Eur. J. Soil. Sci. 2020, 71, 84–92. [Google Scholar] [CrossRef]
- Dias, T.; Correia, P.; Carvalho, L.; Melo, J.; Varennes, A.; Cruz, C. Arbuscular mycorrhizal fungal species differ in their capacity to overrule the soil’s legacy from maize monocropping. Appl. Soil. Ecol. 2018, 125, 177–183. [Google Scholar] [CrossRef]
- Liu, A.R.; Chen, S.C.; Wang, M.M.; Liu, D.L.; Chang, R.; Wang, Z.H.; Lin, X.M.; Bai, B.; Ahammed, G.J. Arbuscular Mycorrhizal Fungus Alleviates Chilling Stress by Boosting Redox Poise and Antioxidant Potential of Tomato Seedlings. J. Plant Res. 2016, 35, 109–120. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S.; Kataria, S.; Alamri, S.A.; Siddiqui, M.H.; Rastogi, A. Inoculation with Arbuscular Mycorrhizal Fungi Alleviates the Adverse Effects of High Temperature in Soybean. Plants 2022, 11, 2210–2222. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.Q.; Xu, L.L.; Chen, X.Y.; Yang, P.; Han, S.M. Effects of arbuscular mycorrhizal fungi on physiological characteristics of Impatiens balsamina under cadmium stress. Jiangsu Agric. Sci. 2019, 47, 186–188. [Google Scholar] [CrossRef]
- Liu, A.R.; Chen, S.C.; Chang, R.; Liu, D.L.; Chen, H.R.; Ahammed, G.J.; Lin, X.M.; He, C.X. Arbuscular mycorrhizae improve low temperature tolerance in cucumber via alterations in H2O2 accumulation and ATPase activity. J. Plant Res. 2014, 127, 775–785. [Google Scholar] [CrossRef]
- Hajiboland, R.; Joudmand, A.; Aliasgharzad, N.; Tolrá, R.; Poschenrieder, C. Arbuscular mycorrhizal fungi alleviate low-temperature stress and increase freezing resistance as a substitute for acclimation treatment in barley. Crop Pasture Sci. 2019, 70, 218–233. [Google Scholar] [CrossRef]
- Li, W.; Zhai, Y.L.; Xing, H.S.; Xing, L.J.; Guo, S.X. Arbuscular mycorrhizal fungi promote photosynthesis in Antirrhinum majus L. under low-temperature and weak-light conditions. Not. Bot. Horti Agrobot. Cluj. Napoca 2023, 51, 13012. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shao, X.F.; Gong, Y.F.; Zhu, Y.; Wang, H.F.; Zhang, X.L.; Yu, D.D.; Yu, F.; Qiu, Z.Y.; Lu, H. The metabolism of soluble carbohydrates related to chilling injury in peach fruit exposed to cold stress. Postharvest Biol. Technol. 2013, 86, 53–61. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.Y.; Ge, Q.; Li, Y.L.; Sun, J.H.; Zhang, Y.; Liu, X.J. Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol. 2012, 196, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Pompella, A.; Maellaro, E.; Casini, A.F.; Comporti, M. Histochemical detection of lipid peroxidation in the liver of bromobenzene-poisoned mice. Am. J. Pathol. 1987, 129, 295–301. [Google Scholar] [CrossRef]
- Mathur, S.; Sharma, M.P.; Jajoo, A. Improved photosynthetic efficacy of maize (Zea mays) plants with arbuscular mycorrhizal fungi (AMF) under high temperature stress. J. Photochem. Photobiol. B 2018, 180, 149–154. [Google Scholar] [CrossRef]
- Lee, Y.; Krishnamoorthy, R.; Selvakumar, G.; Kim, K.; Sa, T. Alleviation of salt stress in maize plant by co-inoculation of arbuscular mycorrhizal fungi and Methylobacterium oryzae CBMB20. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 533–540. [Google Scholar] [CrossRef]
- Chen, X.Y.; Song, F.B.; Liu, F.L.; Tian, C.J.; Liu, S.Q.; Xu, H.W.; Zhu, X. Effect of Different Arbuscular Mycorrhizal Fungi on Growth and Physiology of Maize at Ambient and Low Temperature Regimes. Sci. World J. 2014, 1, 956141. [Google Scholar] [CrossRef]
- Yan, P.; Li, G.; Sun, H.Q.; Zhang, Z.H.; Yang, R.Y.; Sun, J.N. Can arbuscular mycorrhizal fungi and biochar enhance plant resistance to low-temperature stress? Agron. J. 2021, 113, 1457–1466. [Google Scholar] [CrossRef]
- Chen, S.C.; Jin, W.J.; Liu, A.R.; Zhang, S.J.; Liu, D.L.; Wang, F.H.; Lin, X.M.; He, C.X. Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperature stress. Sci. Hortic. 2013, 160, 222–229. [Google Scholar] [CrossRef]
- Liu, Y.F.; Han, X.R.; Zhan, X.M.; Yang, J.F.; Wang, Y.Z.; Song, Q.B.; Chen, X. Regulation of Calcium on Peanut Photosynthesis Under Low Night Temperature Stress. J. Integr. Agric. 2013, 12, 2172–2178. [Google Scholar] [CrossRef]
- Machino, S.; Nagano, S.; Hikosaka, K. The latitudinal and altitudinal variations in the biochemical mechanisms of temperature dependence of photosynthesis within Fallopia japonica. Environ. Exp. Bot. 2021, 181, 104248. [Google Scholar] [CrossRef]
- He, B.; Chen, Y.; Zhang, H.; Xia, C.Y.; Zhang, Q.; Li, W. The effect of colored plastic films on the photosynthetic characteristics and content of active ingredients of Dysosma versipellis. Hortic. Environ. Biotechnol. 2018, 59, 519–528. [Google Scholar] [CrossRef]
- Ghosh, D.; Mohapatra, S.; Dogra, V. Improving photosynthetic efficiency by modulating non-photochemical quenching. Trends Plant Sci. 2023, 28, 264–266. [Google Scholar] [CrossRef]
- Feng, Z.; Liang, F.; Zheng, C.S.; Shu, H.R.; Sun, X.Z.; Yong-kweon, Y. Effects of Acetylsalicylic Acid and Calcium Chloride on Photosynthetic Apparatus and Reactive Oxygen-Scavenging Enzymes in Chrysanthemum Under Low Temperature Stress with Low Light. Agric. Sci. China 2010, 9, 1777–1786. [Google Scholar] [CrossRef]
- Li, S.X.; Yang, W.Y.; Guo, J.H.; Li, X.N.; Lin, J.X.; Zhu, X.C. Changes in photosynthesis and respiratory metabolism of maize seedlings growing under low temperature stress may be regulated by arbuscular mycorrhizal fungi. Plant Physiol. Biochem. 2020, 154, 1–10. [Google Scholar] [CrossRef]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef]
- Cerqueira, J.A.; Andrade, M.T.; Rafael, D.D.; Zhu, F.; Martins, S.V.C.; Nunes-Nesi, A.; Benedito, V.; Fernie, A.R.; Zsögön, A. Anthocyanins and reactive oxygen species: A team of rivals regulating plant development? Plant Mol. Biol. 2023, 112, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Zhang, P.; Zhang, L.L.; Wang, H.; Ho, C.T.; Li, S.M.; Shahidi, F.; Zhao, H. Detection of cellular redox reactions and antioxidant activity assays. J. Funct. Foods 2017, 37, 467–479. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Yuan, L.Q.; Ma, Y.B.; Zhou, F.; Gao, Y.; Yang, S.C.; Li, T.L.; Hu, X.H. Exogenous 5-aminolevulinic acid alleviates low-temperature damage by modulating the xanthophyll cycle and nutrient uptake in tomato seedlings. Plant Physiol. Biochem. 2022, 189, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Li, Z.; Suo, H.; Ou, L.; Miao, W.; Deng, W. Study on the Mechanism of Grafting to Improve the Tolerance of Pepper to Low Temperature. Agronomy 2023, 13, 1347–1362. [Google Scholar] [CrossRef]
- Pasbani, B.; Salimi, A.; Aliasgharzad, N.; Hajiboland, R. Colonization with arbuscular mycorrhizal fungi mitigates cold stress through improvement of antioxidant defense and accumulation of protecting molecules in eggplants. Sci. Hortic. 2020, 272, 109575. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).