Abstract
D-glucaric acid is an important bio-based building block of polymers and is a high value-added chemical that can be used in a variety of applications. In the present study, the Udh target genes from Pseudomonas putida and Pseudomonas syringae were used together to construct the expression vector pETDuet-2 × Udh. The transformants of BL21 (DE3) with vector pETDuet-2 × Udh were applied to produce glucaric acid from glucuronic acid. After optimizing the induction conditions, the highest Udh expression was achieved when 0.4 mmol·L−1 isopropyl-β-d–thiogalactoside (IPTG) was added to the cell cultures at an OD600 value of 0.6 followed by culturing at 26 °C for 6 h. The production of glucaric acid substantially reached 5.24 ± 0.015 g·L−1 in fed-batch cultures in a 30 L tank. In the present study, a new system for glucaric acid production was established, which was more economic and friendly to the environment.
1. Introduction
Glucuronic acid is formed by oxidizing the C-6 hydroxyl group of glucose to a carboxylic group [1]. In aqueous solutions, glucuronic acid and glucuronolactone usually convert to each other to reach an equilibrium state, while glucuronic acid is more stable in the form of furan rings. Glucuronic acid exists in many substances, such as chondroitin, heparin, oligosaccharides, etc. The main physiological effect of glucuronic acid is that unconjugated bilirubin (also known as indirect bilirubin) combines with glucuronic acid to produce conjugated bilirubin (also known as direct bilirubin), which has a high solubility in the blood and is generally excreted from the body through the biliary tract, but it can also enter the blood via absorption in the small intestine and excrete toxic substances through the urinary function of the kidney [2]. At the same time, it can be combined with various harmful substances in the liver so as to have a detoxification effect.
D-glucaric acid is a promising glucose derivative, which widely exist in fruits, vegetables and different tissues of mammals. D-glucaric acid is known as one of the “top value-added chemicals from biomass” [3] because it is widely used in different fields; for example, it is used as an important bio-based building block to make nylons, plastics and other synthetic polymers [4]. Researchers believe that glucaric acid can improve the autoimmune mechanism [5], help prevent cancer [6], regulate cholesterol levels in the body [7] and regulate lipoprotein metabolism [8].
In recent years, researchers have focused on the industrial production of gluconic acid. The gluconic acid on the market is produced by the nonselective oxidation of glucose and is used in the food, pharmaceutical and paper industries [9]. This chemically catalyzed process is expensive, nonselective and has a low percent conversion and low yield [10]. Furthermore, this method can produce toxic byproducts during nitric acid oxidation [11]. Therefore, it is necessary to develop an environmentally friendly and efficient way to produce glucuronic acid [12]. D-glucaric acid biosynthesis with recombinant microorganisms is a promising choice compared to the chemical conversion process [13].
D-glucaric acid can be synthesized from glucuronic acid in mammalian and plant cells, which needs more than 10 conversion steps. Consequently, with the increasing market demand for gluconic acid, the efficient conversion of gluconic acid by recombinant E. coli has received more and more attention over the past several years. Moon et al. constructed a novel synthetic pathway for the production of glucuronic acid and glucaric acid from glucose in E. coli coexpressing the genes myo-inositol-1-phosphate synthase (Inol) from Saccharomyces cerevisiae, myo-inositol oxygenase (MIOX) from mouse and uronate dehydrogenase from Pseudomonas syringae to produce glucaric acid from glucose [2]. Liu et al. used inositol as a substrate to successfully construct a glucaric acid synthesis pathway in Pichia pastoris. Qu et al. coexpressed the genes cscB, cscA, cscK, ino1, miox, Udh and suhB in E. coli BL21 (DE3) to produce glucaric acid from sucrose [14]. However, the activity of MIOX was rate limiting, leading to the poor accumulation of myo-inositol, which affects the final glucaric acid content in the respective biosynthesis system [15]. The above researchers indeed succeeded in obtaining glucaric acid, but the metabolic route was too long, and it was difficult to obtain a high glucaric acid production level. Glucuronic acid has been able to be industrialized on a large scale. Fei et al. produced glucuronic acid by microbial fermentation, and the yield reached 106 g·L−1. Therefore, the direct conversion of glucuronic acid to glucuronic acid is a good choice [16]. Using Brenda enzyme databases (https://www.brenda-enzymes.org/ accessed on 14 September 2021), it was found that uronic acid dehydrogenase (Udh, EC 1.1.1.203) catalyzes the dehydrogenation of uronic acid to aldose-lactone [17], which forms glucaric acid when aldose-lactone is hydrolyzed, with NAD+ as a cofactor (Figure 1). Here, we utilized metabolic engineering techniques in Escherichia coli to synthesize gluconic acid using glucuronic acid as the sole precursor. With pETDuet-1 as a vector, the coexpression of Udh from Pseudomonas syringae DC3000 (GenBank: EU377538.1) and Udh from Pseudomonas putida KT2440 (GenBank: LT799039.1) in E. coli BL21 (DE3) can be obtained. A coexpression strategy was applied to improve the biosynthesis level of glucaric acid.
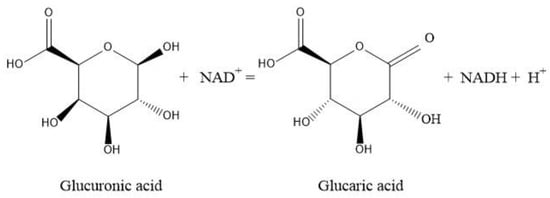
Figure 1.
Biosynthesis of glucaric acid from glucuronic acid catalyzed by uronate dehydrogenases.
In this study, the Udh gene from Pseudomonas putida KT2440 and the Udh gene from Pseudomonas syringae DC3000 were connected to the vector pETDuet-1. The recombinant bacteria BL21/pETDuet-Ppudh, recombinant bacteria BL21/pETDuet-Psudh and recombinant bacteria BL21/pETDuet-2 × Udh were successfully constructed; the recombinant bacteria were purified of protein; and the target product was detected. Moreover, the yield of gluconic acid was increased by optimizing the culture conditions and induction conditions.
The innovative points of this paper are as follows:
- (1)
- We determined the assay method to determine the content of glucosinolates in the fermentation broth and conducted research on the stability of the assay method.
- (2)
- The conditions of the carbon source in the fermentation medium, pH of the culture initiation, inoculum amount and induction intensity were optimized to produce glucosinolates with a high yield. The recombinant bacteria were also supplemented and fermented in a 30 L fermenter according to the optimized conditions in shake flasks.
- (3)
- The permeability of the cell membrane of the high-yielding recombinant bacteria was changed by physical and chemical methods. The physical method used cell freezing and thawing and ultrasonication to change the permeability of the cell membrane and optimize conditions such as the buffer system, buffer system pH and so on. The chemical method used a Cetyltrimethylamine bromide (CTAB) surfactant and toluene organic reagent to change the cell permeability and optimize the concentration of the permeabilizer CTAB and toluene.
2. Materials and Methods
2.1. Plasmids, Strains and the Culture Medium
E. coli DH5α was the cloning host for plasmid preparation and vector construction. E. coli BL21 (DE3) was selected as the expression host. Having the capacity to coexpress two target genes, the expression vector pETDuet-1 (EMD Biosciences, Novagen, Beijing Agent, China) was used to harbor the candidate Udh genes. The LB medium (0.5% yeast extract, 1% tryptone and 1% NaCl) was prepared for bacteria cultivation. The modified basal salts medium (BSM), composed of 2% yeast extract, 2.5% glucose, 0.25% KH2PO4, 0.35% (NH4)2SO4, 0.055% MgSO4·7H2O and 0.000008% FeSO4·6H2O, was adopted for the bacteria fermentation and glucaric acid biosynthesis study. Ampicillin sodium (final concentration: 100 μg·mL−1) was added to the culture broth for the selection of the transformants and plasmid maintenance.
2.2. Reagents and Chemicals
Gluaric acid and glucuronic acid (Sigma-Aldrich, Beijing, China), Affi-Gel boronate media (Bio-Rad, Beijing, China), Ampicillin sodium and isopropyl-β-d-thiogalactoside (IPTG) (TaKaRa, Dalian, China), mercaptoethanol, imidazole, boric acid and other chemicals were bought from Aladdin (Shanghai, China).
2.3. Construction of the Expression Vector pETDuet-2 × Udh
Udh genes from Pseudomonas putida (GenBank: LT799039.1) and Psudh from Pseudomonas syringae (GenBank: EU377538.1) were synthesized by the Shanjing Biotech Corporation (Shanghai, China) and incorporated into the pETDuet-1 vector after codon optimization (http://www.jcat.de/Result.jsp accessed on 20 October 2021) according to the codon preference of E. coli. The synthesized Ppudh and Psudh coding sequences were introduced into the express vector pETDuet-1 at the SalI/NotI sites and NdeI/XholI sites, respectively, to obtain the expression vector pETDuet-2 × Udh. There is a His-tag in the C terminal of the Udh gene that was used for protein separation by using Ni2+-chelating affinity chromatography. The correctness of the recombinant pETDuet-2 × Udh vector was verified by restriction enzyme digestion and agarose electrophoresis. The sequence validity of the Ppudh and Psudh genes was checked by DNA sequencing.
2.4. Expression of the Udh Genes in E. coli BL21
The expression vector pETDuet-2 × Udh was transformed into E. coli BL21 (DE3). The fresh colony was cultured at 37 °C with 1 mmol IPTG in an LB medium supplemented with 100 μg·mL−1 ampicillin when the OD600 value reached 0.6, followed by growth for another 18 h at 32 °C.
The BL21 cells from a 1 mL aliquot culture were collected by centrifugation at 12,000 rpm for 5 min, and we discarded the supernatant. We added 250 μL of 1 × PBS (140 mmol NaCl, 2.7 mmol KCl, 10 mmol Na2HPO4 and 1.8 mmol KH2PO4, pH 7.0) buffer and 250 μL 2 × loading buffer (120 mmol Tris-HCl, pH 6.8, 20% glycerol, 4% SDS, 3% mercaptoethanol and 0.02% bromophenol blue) to the resuspended pellet. The mixed sample was boiled in a water bath (100 °C) for 25 min and centrifuged at 12,000 rpm for 5 min at 4 °C [18]. We added 15 μL of the supernatant from each sample into the SDS-12% polyacrylamide gels and then started electrophoresis in the 1 × Tris-glycine buffer. The polyacrylamide gels were stained with coomassie brilliant blue R250, which visualized the stained protein bands.
2.5. Purification of Recombinant Urinate Dehydrogenase Proteins
His-tagged proteins can be purified by immobilized metal ion affinity chromatography (IMAC) [19]. Cells were harvested by centrifugation at 12,000 rpm for 10 min. The cells resuspended in the PBS buffer were disrupted via 10 cycles of sonication (3 s of sonication and 4 s of cooling in ice) by using an ultrasonic cell disrupter. The mixture was then centrifuged at 12,000 rpm for 10 min at 4 °C, and a stating buffer was added (25 mmol Tris-HCl, 10 mmol imidazole and 100 mmol NaCl, pH 7.5) to the supernatant. The Ni-NTA column was equilibrated with 2 column volumes of ddH2O and the stating buffer. The treated supernatant was loaded into the empty column at a flow rate of 1.0 mL·min−1 and was then washed with two column volumes of different concentrations of the stating buffer. Subsequently, the column was eluted with the 500 mmol imidazole and staling buffer and preserved in ethanol. The fraction solutions were analyzed with SDS-PAGE electrophoresis.
2.6. Optimization of Recombinant Protein Expression Conditions
The effects of temperature, the IPTG concentration, induction time and OD600 on the recombinant E. coli BL21(DE3)-pETDuet-2 × Udh protein expression was evaluated under the assay conditions at various temperature values ranging from 24 °C to 32 °C, different induction times from 6 h to 22 h, IPTG concentrations ranging from 0.1 mmol to 1.0 mmol and OD600 ranging from 0.6 to 2.5.
The induction culture liquid was centrifuged at 12,000 rpm for 10 min, and precooled 25 mmol PBS was used to resuspended precipitation. Then, we crushed the cells by ultrasonic cell crushing (3 s of sonication and 4 s of cooling in ice) until the bacteria turned from white to transparent and were not sticky. The ultrasonic broken bacterial liquid was centrifuged (4 °C, 12,000 rpm and 15 min) and the supernatant and precipitation were collected. Next, we took 1 mL of the supernatant and precipitation solution, added 1 mL of the 2 × loading buffer and boiled it for 10 min. After centrifugation, it was used for the SDS-PAGE analysis.
2.7. Biosynthesis of Glucaric Acid from Glucuronic Acid with Recombinant BL21 Harboring the Expression Vector pETDuet-2 × Udh
A fresh colony of E. coli BL21 was cultured overnight in Luria–Bertani (LB) broth supplemented with ampicillin (100 μg·mL−1) at 37 °C and was oscillated at 200 rpm. Then, the overnight culture was inoculated into 250 mL of a mineral medium in a 1 L flask with 10 g L−1 glucuronic acid (as the substrate) and 100 μg mL−1 ampicillin, then, IPTG was added to the culture at a final concentration of 0.4 mmol at 26 °C and was oscillated at 200 rpm. To confirm glucaric acid production as a product of the glucuronic acid bioconversion, the fermentation liquor was sampled and analyzed via HPLC and MS.
2.8. MS and HPLC Analysis of the Reaction Product
The culture supernatant was pretreated with a boric acid affinity gel to remove the interfering substances. Affi-Gel boronate gel is a boronate-derivatized polyacrylamide gel with an affinity for adjacent cis hydroxyl (cis-diol) groups [20]. It binds to cis-diol molecules on gluconic acid to remove other interfering substances. We then performed fermentation liquor centrifugation at 12,000 rpm for 5 min. Affi-Gel and a 1 mol·L−1 potassium phosphate buffer solution were added to the supernatant, which was washed with 80 mmol·L−1 potassium phosphate and 20 mmol·L−1 boric acid buffer (pH 7.0) to remove the interference substances. Then, the supernatant was eluted with 0.1 mol·L−1 HCl, and we adjusted the pH to neutral with 1 mol·L−1 NaOH. Finally, the supernatant was filtered by using a 0.22 um filter membrane for mass spectrometry and liquid phase analysis. The HPLC separation was performed by using an Aminex HPX-87H column (300 × 7.8 mm, Bio-Rad Laboratories, Hercules, CA USA) [21]. The mobile phase was 5 mmol·L−1 dilute sulfuric acid. The flow rate was 0.5 mL·min−1 and the elution was isogradient. The column temperature was 55 °C. The injection volume was 10 μL. The detector was an ultraviolet detector, and the detection wavelength was 210 nm. MS was performed by scanning the m/z range from 100 to 1000 at 10 s/scan in both the negative and positive ion detection mode. Deionized water was used as the mobile phase at a flow rate of 0.3 mL·min−1 at 30 °C.
2.9. Preliminary Fermentation of Recombinant BL21 for Glucaric Acid Biosynthesis in a 30 L Tank
The seed culture was prepared in 500 mL baffled shake flasks containing 50 mL of the LB medium at 36.5 °C with an oscillation rate of 220 rpm, and the OD600 reached 5.0. A 1% (v/v) inoculum of the seed culture was transferred to a 30 L fermenter (Zhenjiang East Biotech Equipment & Technology Co. Zhenjiang, Jiangsu, China) with an initial 15 L fermentation medium. The modified basal salts medium, which contained 5 g·L−1 yeast extract, 30 g·L−1 glucose, 2.5 g·L−1 KH2PO4, 8 g·L−1 (NH4)2SO4, 0.55 g·L−1 MgSO4·7H2O and 0.08 mg·L−1 FeSO4·6H2O, was used as the fermentation medium, and ampicillin (100 μg·mL−1) was used for cell selection and maintenance. At the beginning of fermentation, the reactor temperature was controlled at 32 °C for rapid cell growth. When the residual glucose concentration was about 10 g·L−1, fed-batch feeding was started at a constant feeding rate of 50 mL·h−1. IPTG was added to the culture at a final concentration of 0.5 mmol when the OD600 reached 30. Glucuronic acid (30 g·L−1) was added at a rate of 50 mL·h−1 when the temperature was reduced to 28 °C. The reaction stopped when the glucose was consumed slowly, and ammonium hydroxide was not needed to maintain the pH concentration. The concentrations of glucuronic acid were assayed with a spectrophotometer every several hours. During the fermentation, the culture pH was controlled at 6.8~7.2 with the automatic addition of 30% ammonium hydroxide while the agitation speed, dissolved oxygen and tank pressure was maintained at 750 rpm, 30% and 0.05 MPa, respectively.
3. Results
3.1. Screening Results of Recombinant Bacteria
Figure 2A is the growth diagram of the blank BL21 bacteria with ampicillin added, and B is the growth diagram of the transformed BL21 recombinant bacteria with ampicillin added. As can be seen from Figure 2, the transformed bacteria grew in the medium containing ampicillin resistance, indicating that the plasmid was successfully transferred into the BL21 bacteria. The next step in the experiment could thus be carried out.
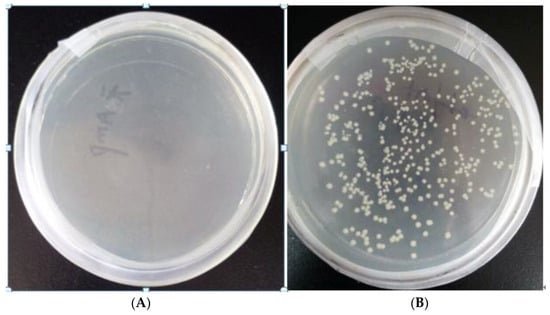
Figure 2.
(A) The growth diagram of blank BL21 bacteria with ampicillin added; (B) the growth diagram of transformed BL21 recombinant bacteria with ampicillin added.
3.2. Growth Curve of Recombinant Escherichia coli
It can be seen from Figure 3 that the light absorption value of the recombinant bacteria at 600 nm reaches the maximum at about 18 h and then slightly decreases and tends to be stable in general. It can be seen that the recombinant bacteria were in a stable growth stage at 18 h, and the concentration of bacterial liquid reached its highest at this time.
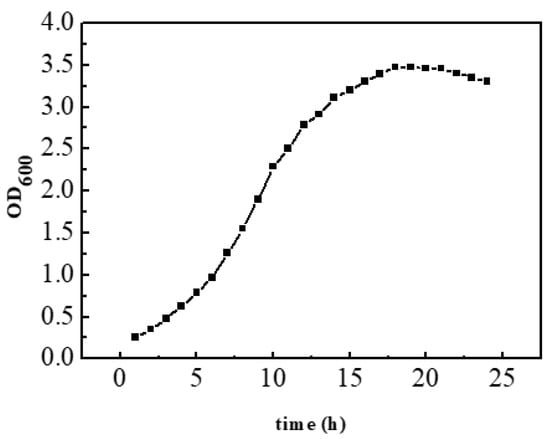
Figure 3.
Growth curve of recombinant Escherichia coli.
3.3. Comparative Analysis of the Conversion Ability of Different Large Intestine Hosts to Produce Glucose Dicarboxylic Acid
As can be seen from Figure 4, gluconic acid can be detected in the fermentation fluid of the recombinant strains BL21/pETDuet-Ppudh, BL21/pETDuet-Psudh and BL21/pETDuet-2 × Udh, indicating that glucuronic acid dehydrogenase is correctly folded and expressed in E. coli BL21. As can be seen from Figure 4, the content of gluconic acid tends to be stable around 20 h after fermentation, and the content of gluconic acid in the fermentation broth of the recombinant bacterium BL21/pETDuet-Ppudh is 0.16 g·L−1. The content of gluconic acid in the fermentation broth of the recombinant bacterium BL21/pETDuet-Psudh was 0.20 g·L−1, and that of the recombinant bacterium BL21/pETDuet-2 × Udh was 0.34 g·L−1. Therefore, the recombinant strain BL21/pETDuet-2 × Udh was selected as the starting strain to produce gluconic acid.
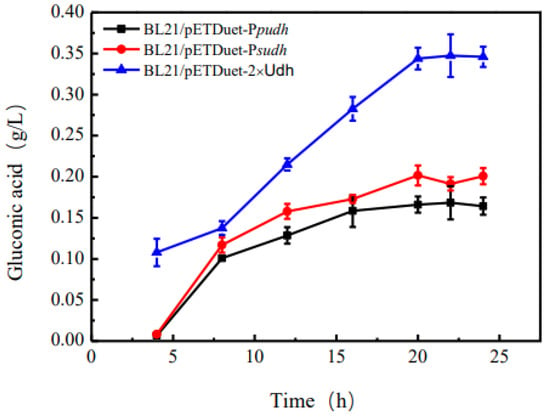
Figure 4.
Relationship between fermentation time and glucosinolate yield in different recombinant strains.
3.4. Analysis of the Expression of Recombinant Protein
To confirm the induced expression of the Udh protein in E. coli, the target proteins were separated by using SDS-PAGE gels (Figure 5). Empty vector Petdute-1 was introduced into Escherichia coli without the Udh gene as a control. The expressed proteins had an MW of 34 kDa with a high expression in the transformed cells, which was concordant with the expected molecular weight of the two expressed proteins with a 6 × His tag (the native Ppudh and Psudh had 268 AA with a speculated MW of 29.3 kDa and 275 AA with a speculated MW of 30.6 kDa, respectively).
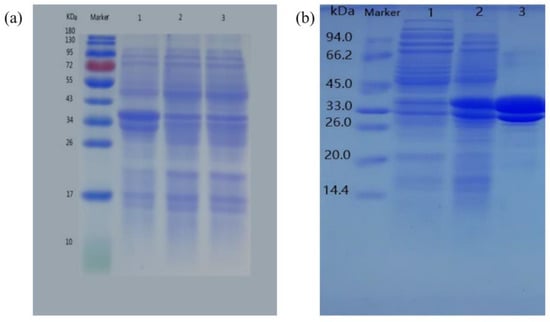
Figure 5.
SDS-PAGE analysis of Udh expression in BL21 strain (a), SDS-PAGE analysis of 2 × Udh samples after affinity chromatography purification (b).
3.5. Purification and Induction Conditions of Udh Expression Construct
After the sample was combined with the resin, different concentrations of the imidazole elution buffer were used to elute Udh from the column. The flow through at different concentrations was collected, and the protein was quantified with coomassie bright blue (G250) to determine the OD595 absorbance; additionally, we selected an eluent with a higher value for SDS-PAGE (Figure 2B). Lane3 was eluted with 100 mmol imidazole, indicating that the target protein was successfully expressed in E. coli BL21.
Protein expression can be divided into extracellular protein and intracellular protein. The soluble expression conditions such as the induction temperature, induction time and IPTG concentration can improve the contents of the extracellular protein and inclusion body protein (Figure 6). As shown in Figure 6b, when the induction temperature was 26 °C, the Udh expression was the highest in both the soluble protein and inclusion body protein. According to Figure 6c, the expression of Udh in both the soluble protein and inclusion body protein was the highest when the induction time was 6 h. When the induction time was OD600 = 0.6, the Udh expression in both the soluble protein and inclusion body protein was the highest. As can be seen from Figure 6c, when the concentration of the IPTG inducer was 0.4 mmol, the Udh expression was the highest in both the soluble protein and inclusion body protein. As can be seen from Figure 6d, the Udh expression was the highest in both the soluble protein and inclusion body protein when the medium OD600 was 0.6 and the IPTG inducer was added. The optimal induction conditions were as follows: 0.4 mmol of IPTG was added into the cell culture medium with an OD600 value of 0.6 and induced at 26 °C for 6 h.
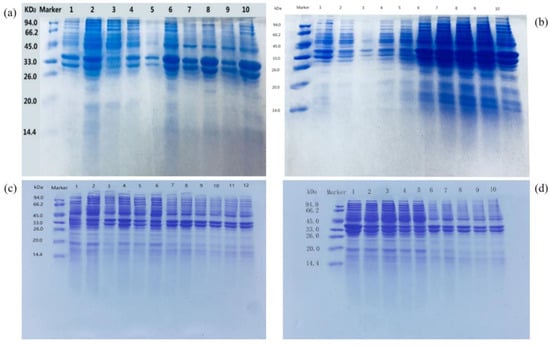
Figure 6.
SDS-PAGE analysis of 2 × Udh samples induced with IPTG at different temperatures (a), induced times (b), concentrations (c) and OD600 eluates (d).
3.6. Production Analysis of Glucaric Acid
MS was performed to analyze the exact molecular weight of the products (Figure 7a,b). The results showed that glucaric acid was characterized by its masses (m/z = 209) and that the peaks of the samples corresponded to masses of the glucaric acid standard. This further demonstrated that the recombinant E. coli BL21(DE3)-pETDuet-2 × Udh strain was successfully constructed to produce glucuronic acid and glucuronic acid as substrates.
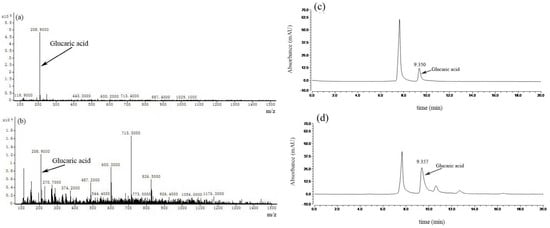
Figure 7.
Mass spectrometric analysis of glucaric acid: (a) sample of glucaric acid standard, (b) sample separated from the enzymatic reaction mixture. High-performance liquid chromatography (HPLC) of glucaric acid: (c) sample of gluconic acid standard, (d) sample separated from the enzymatic reaction mixture.
High-performance liquid chromatography (HPLC) is a common method used to determine glucosinolates and is characterized by simple sample handling, accurate quantification and good results when it comes to the determination of glucosinolates. In our study, HPLC was performed to analyze the production of glucaric acid (Figure 7c,d). The peak time of gluconic acid is 9.077 min, and the peak time of the samples is 9.080 min. The result showed that the peaks of the samples corresponded to the time of the glucaric acid standard. This method can be used for the accurate quantitative analysis of products.
The results of previous studies showed that the peak times of gluconic acid and glucosinolates were similar under the separation conditions of the HPLC method, and they showed one peak on the chromatogram. Therefore, the HPLC method was not able to separate them completely, and thus they could not be quantified and analyzed accurately.
3.7. Production of Glucaric Acid with 30 L Tank Fermentation Strategy
Ion chromatography detection is a new means of detecting glucosinolates. The separation principle is that the detected substances are separated by a column carrying opposite charges and then detected by an electrochemical detector or conductivity detector through the intensity of the electrical signal. This method has the advantages of simplicity, rapidity, high sensitivity and accurate results and has a greater advantage in the detection of organic acids. After analyzing the byproducts of glucose nitrate oxidation by ion chromatography, the separation of some gluconic acid derivatives such as gluconic acid, glucosinolates and glucuronic acid, which are difficult to be separated with the HPLC method, was greatly improved.
In this paper, the linear relationship between the five kinds of different concentrations of the glucaric acid standard solution and the peak area was obtained. The resulting linear equation was found to be Y = 0.0685X + 0.0126 (Y stands for the glucaric acid concentration, unit: mg L−1; X stands for the peak area), and the related coefficient was R2 = 0.999. Based on this, the yield of the BL21(DE3)-pETDuet-2 × Udh strain glucaric acid was determined to be (5.24 ± 0.015) g·L−1 (Figure 8).
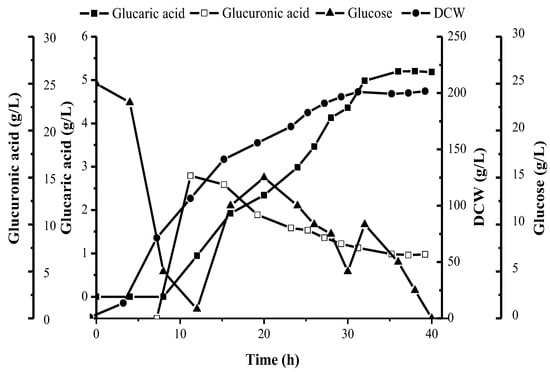
Figure 8.
Fed-batch fermentation of recombinant BL21 in 30 L tank with BSM medium and feeding with glucose and glucuronic acid.
The synthesis of glucosinolates by microbial fermentation has many advantages over traditional chemical methods, such as being more environmentally friendly and having greater potential for low-cost production. The synthesis of glucosinolates by all the biological methods reported so far has been carried out mainly in Escherichia coli and some fungi.
4. Conclusions
In conclusion, the Udh genes from Pseudomonas putida and Pseudomonas syringae were expressed in a recombinant E. coli with an MW of 34 kDa. The systematic optimization of the expression conditions to obtain the maximum expression and qualitative and quantitative detection of the products were carried out by using MS and HPLC. Finally, the production of glucaric acid substantially reached 5.24 ± 0.015 g·L−1 in the fed-batch cultures in a 30 L tank, and the fermentation reaction time of the recombinant E. coli to produce glucaric acid up to 5 g·L−1 was about 40 h, which was shorter than that of other microorganisms. Therefore, this pathway has potential industrial application value for the microbial fermentation of glucaric acid. In further studies, the conditions of fed-batch fermentation need to be optimized for better glucaric acid production.
Based on the results of this paper and those of published papers, future research can be improved in the following aspects:
- (1)
- Replacement of safe strains. In this experiment, glucuronide dehydrogenase was constructed and expressed in E. coli, but E. coli is not regarded as a safe strain by GRAS, which has limited its application in the production of food-grade chemicals. Therefore, safe microbial strains can be considered for glucuronic acid production.
- (2)
- Multifactorial analysis of the method for cell membrane permeabilization. Scholars should combine the permeabilization methods to improve the production of glucosinolates to increase the permeability of cell membranes as much as possible without breaking the integrity of the cells in the conversation so as to further improve the production of glucosinolates.
- (3)
- Addition of nicotinamide to the culture medium. The production of glucosinolate requires NAD+ cofactors, and nicotinamide is a precursor for synthesizing NAD+, which is the most direct way to replenish NAD+. Compared to other precursors, nicotinamide bypasses the nicotinamide phosphoribosyltransferase (NAMPT) restriction enzyme and is able to replenish NAD+ rapidly, and the addition of nicotinamide to the fermentation broth has the potential to increase the content of glucosinolates.
Author Contributions
Conceptualization, X.Y.; methodology, L.N.; project administration, C.Y.; software, Y.W.; validation, Y.L.; formal analysis, F.W.; investigation, N.S.; writing—original draft preparation, L.N.; writing—review and editing, X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: 1. the Doctor’s fund of the University of Jinan, the funding number: XBS2020. 2. Construction of provincial key laboratories of Shandong Provincial Key research and development Program (major scientific and technological innovation projects), the funding number: 2021ZDSYS07.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are unavailable due to privacy or ethical restrictions.
Acknowledgments
This study was financially supported by the following projects: 1. the Shandong postdoctoral innovation project. 2. Construction of provincial key laboratories of Shandong Provincial Key research and development Program (major scientific and technological innovation projects).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smith, T.; Denton, T.; Zhang, J. Synthesis and characterization of higher molecular weight stereorandom poly (d-glucaramides) from 1:1 alkylenediammonium d-glucaric acid. In Proceedings of the 234th ACS National Meeting, Boston, MA, USA, 19–23 August 2007. [Google Scholar]
- Liu, Y.; Gong, X.; Wang, C. Production of glucaric acid from myoinositol in engineered Pichia pastoris. Enzym. Microb. Technol. 2016, 34, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wang, J.; Zhao, Y. Metabolic engineering of Saccharomyces cerevisiae for efficient production of glucaric acid at high titer. Appl. Biochem. Biotechnol. 2018, 17, 67. [Google Scholar]
- Bauer, F.; Coenen, L.; Hansen, T. Technological innovation systems for biorefineries: A review of the literature. Biofuels Bioprod. Biorefining 2017, 11, 534–548. [Google Scholar] [CrossRef]
- Dwivedi, C.; Heck, W.J.; Downie, A.A. Effect of calcium glucarate on beta-glucuronidase activity and glucarate content of certain vegetables and fruits. Biochem. Med. Metab. Biol. 1990, 43, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.J. A large-scale purification of beta-glucuronidase from human liver by immunoaffinity chromatography. Biotechnol. Appl. Biochem. 2011, 14, 296–305. [Google Scholar]
- Moon, T.S.; Yoon, S.H.; Ching, M.J.T.M. Enzymatic assay of d-glucuronate using uronate dehydrogenase. Anal. Biochem. 2009, 392, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Gupta, K.P. Induction of apoptosis by calcium D-glucarate in 7,12-dimethyl benz [a] anthracene-exposed mouse skin. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Su, H.H.; Guo, Z.W.; Wu, X.L. Efficient bioconversion of sucrose to high-value-added glucaric acid by in vitro metabolic engineering. ChemSusChem 2019, 12, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Tae Seok, M.; Sang-Hwal, Y.; Lanza, A.M. Production of glucaric acid from a synthetic pathway in recombinant Escherichia coli. Appl. Environ. Microbiol. 2009, 75, 589. [Google Scholar]
- Shiue, E.; Prather, K.L.J. Improving d-glucaric acid production from myo-inositol in E. coli by increasing MIOX stability and myo-inositol transport. Metab. Eng. 2014, 22, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Yang, J.E.; Ha, J.Y. Bio-based production of monomers and polymers by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2015, 36, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Nielsen, J. Biobased organic acids production by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016, 37, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.N.; Yan, H.J.; Guo, Q. Biosynthesis of d-glucaric acid from sucrose with routed carbon distribution in metabolically engineered Escherichia coli. Metab. Eng. 2018, 47, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Hicks, M.A.; Manchester, S.P. Porting the synthetic D-glucaric acid pathway from Escherichia coli to saccharomyces cerevisiae. Biotechnol. J. 2016, 11, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; You, R.; Hu, M. Production of d-glucuronic acid from myo-inositol using Escherichia coli whole-cell biocatalyst overexpressing a novel myo-inositol oxygenase from thermothelomyces thermophile. Enzym. Microb. Technol. 2019, 127, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xue, Y.; Cao, Z. Characterization of a uronate dehydrogenase from thermobispora bispora for production of glucaric acid from hemicellulose substrate. World J. Microbiol. Biotechnol. 2018, 34, 102. [Google Scholar] [CrossRef] [PubMed]
- Janarthini, R.; Wang, X.; Chen, L. A tobacco-derived thymosin beta4 concatemer promotes cell proliferation and wound healing in mice. Biomed Res. Int. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Rubiyana, Y.; Santoso, A.; Batubara, I. Comparison of immobilized metal affinity chromatography Ni-NTA and Co-TALON for the purification of recombinant human erythropoietin. Makara J. Sci. 2015, 19, 137. [Google Scholar] [CrossRef]
- Yang, Y.; Mitri, K.; Zhang, C. Promiscuity of host cell proteins in the purification of histidine tagged recombinant xylanase a by IMAC procedures: A case study with a Ni2+-tacn-based IMAC system. Protein Expr. Purif. 2019, 162, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.; Villeneuve, D.C.; Chu, I. HPLC determination of d-glucaric acid in human urine. J. Anal. Toxicol. 1993, 17, 146–150. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).