Advanced Fermentation Techniques for Lactic Acid Production from Agricultural Waste
Abstract
1. Introduction
2. Lactic Acid Production from Agricultural Waste
2.1. Fermentable Sugar-Rich Waste
2.2. Starchy Waste
2.3. Dairy Waste
2.4. Lignocellulosic Waste
3. Conventional Fermentation Approaches
3.1. Batch Fermentation
3.2. Continuous Fermentation
3.3. Fed-Batch Fermentation
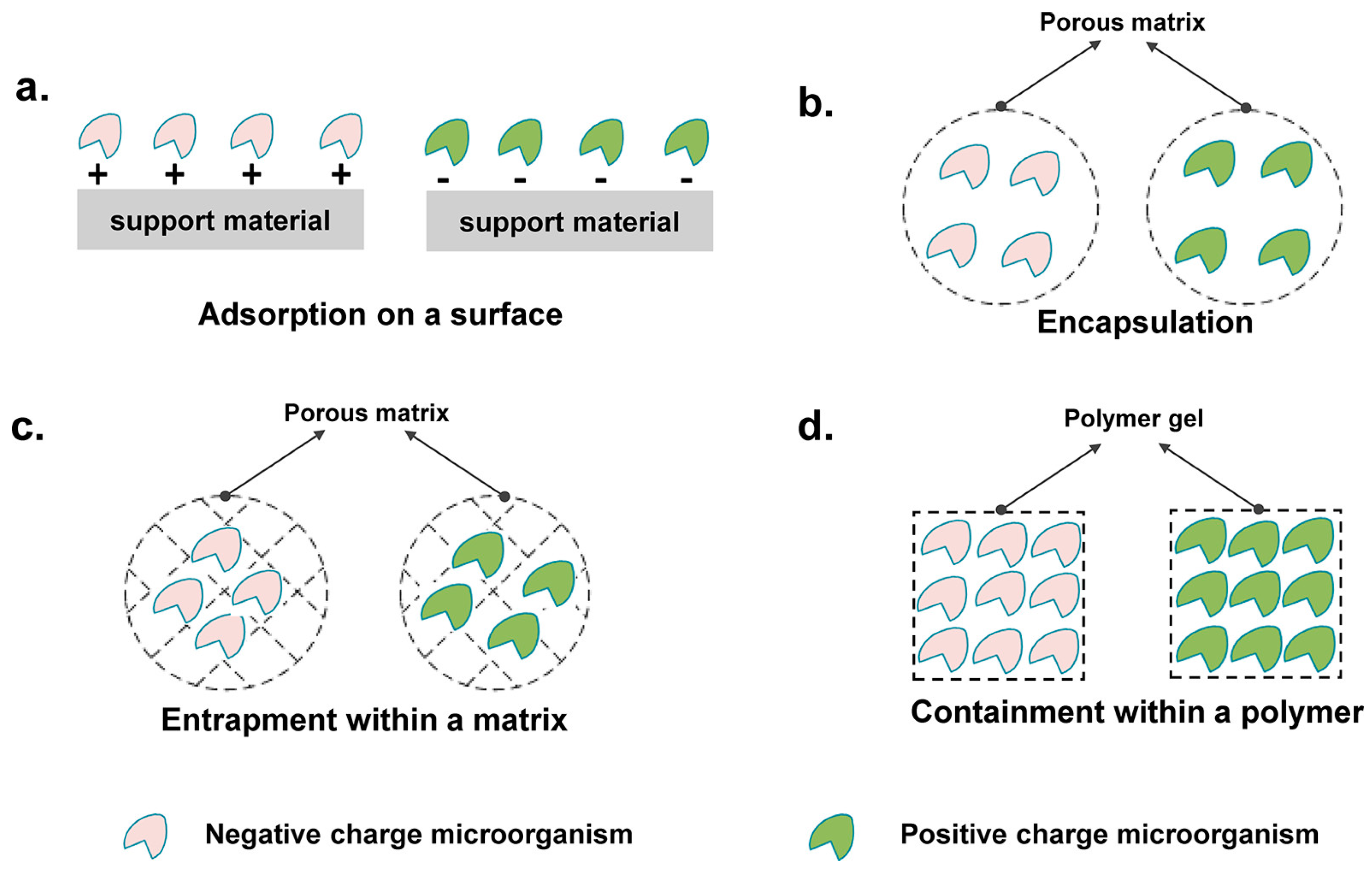
4. Cell Immobilization
5. Membrane-Based Cell Retention
6. Simultaneous Saccharification and Co-Fermentation
7. Co-Culture
8. Genetic and Metabolic Engineering
9. Conclusions and Prospectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current Applications of Poly(Lactic Acid) Composites in Tissue Engineering and Drug Delivery. Compos. B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Komesu, A.; Vaz Rossell, C.E.; Maciel Filho, R. Challenges and Opportunities in Lactic Acid Bioprocess Design—From Economic to Production Aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Vidmantiene, D.; Basinskiene, L.; Cernauskas, D.; Bartkiene, E.; Cizeikiene, D. Green Metrics for Sustainability of Biobased Lactic Acid from Starchy Biomass vs Chemical Synthesis. Catal. Today 2015, 239, 11–16. [Google Scholar] [CrossRef]
- Abedi, E.; Hashemi, S.M.B. Lactic Acid Production—Producing Microorganisms and Substrates Sources-State of Art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Banat, F.; Taher, H. A Review on the Lactic Acid Fermentation from Low-Cost Renewable Materials: Recent Developments and Challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Corrado, I.; Varriale, S.; Pezzella, C. Microbial Processes for Upcycling Food Wastes into Sustainable Bioplastics. In Sustainable Food Science—A Comprehensive Approach; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural Waste: Review of the Evolution, Approaches and Perspectives on Alternative Uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- He, N.; Jia, J.; Qiu, Z.; Fang, C.; Lidén, G.; Liu, X.; Bao, J. Cyclic L-Lactide Synthesis from Lignocellulose Biomass by Biorefining with Complete Inhibitor Removal and Highly Simultaneous Sugars Assimilation. Biotechnol. Bioeng. 2022, 119, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Biomaterial Products|TripleW. Available online: https://www.triplew.co/biomaterials (accessed on 9 August 2023).
- WASTE2FUNC—Bio Base Europe Pilot Plant. Available online: https://www.bbeu.org/projects/waste2func/ (accessed on 9 August 2023).
- Abdel-Rahman, M.A.; Hassan, S.E.D.; Alrefaey, H.M.A.; El-Belely, E.F.; Elsakhawy, T.; Fouda, A.; Desouky, S.G.; Khattab, S.M.R. Subsequent Improvement of Lactic Acid Production from Beet Molasses by Enterococcus Hirae Ds10 Using Different Fermentation Strategies. Bioresour. Technol. Rep. 2021, 13, 100617. [Google Scholar] [CrossRef]
- Mladenović, D.D.; Djukić-Vuković, A.P.; Kocić-Tanackov, S.D.; Pejin, J.D.; Mojović, L.V. Lactic Acid Production on a Combined Distillery Stillage and Sugar Beet Molasses Substrate. J. Chem. Technol. Biotechnol. 2015, 91, 2474–2479. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, Z.; Zheng, Y.; Zhou, J.; Xiu, Z. Efficient Production of Lactic Acid from Sugarcane Molasses by a Newly Microbial Consortium CEE-DL15. Process. Biochem. 2019, 81, 132–138. [Google Scholar] [CrossRef]
- Kawai, M.; Harada, R.; Yoda, N.; Yamasaki-Yashiki, S.; Fukusaki, E.; Katakura, Y. Suppression of Lactate Production by Using Sucrose as a Carbon Source in Lactic Acid Bacteria. J. Biosci. Bioeng. 2020, 129, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Salvañal, L.; Clementz, A.; Guerra, L.; Yori, J.C.; Romanini, D. L-Lactic Acid Production Using the Syrup Obtained in Biorefinery of Carrot Discards. Food Bioprod. Process. 2021, 127, 465–471. [Google Scholar] [CrossRef]
- Nancib, A.; Nancib, N.; Boubendir, A.; Boudrant, J. The Use of Date Waste for Lactic Acid Production by a Fed-Batch Culture Using Lactobacillus Casei Subsp. Rhamnosus. Braz. J. Microbiol. 2015, 46, 893–902. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carpinelli Macedo, J.V.; de Barros Ranke, F.F.; Escaramboni, B.; Campioni, T.S.; Fernández Núñez, E.G.; de Oliva Neto, P. Cost-Effective Lactic Acid Production by Fermentation of Agro-Industrial Residues. Biocatal. Agric. Biotechnol. 2020, 27, 101706. [Google Scholar] [CrossRef]
- Chen, H.; Chen, B.; Su, Z.; Wang, K.; Wang, B.; Wang, Y.; Si, Z.; Wu, Y.; Cai, D.; Qin, P. Efficient Lactic Acid Production from Cassava Bagasse by Mixed Culture of Bacillus Coagulans and Lactobacillus Rhamnosus Using Stepwise PH Controlled Simultaneous Saccharification and Co-Fermentation. Ind. Crops Prod. 2020, 146, 112175. [Google Scholar] [CrossRef]
- Gali, K.K.; Murugesan, M.; Tadi, S.R.R.; Mohan, N.; Swaminathan, N.; Katiyar, V.; Sivaprakasam, S. Bioprospecting of Cassava Fibrous Waste as a Precursor for Stereospecific Lactic Acid Production: Inhibition Insights for Value Addition and Sustainable Utilization. Biomass Convers. Biorefinery 2021, 13, 2255–2265. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, W.; Luo, J.; Qi, B.; Wan, Y. One Step Open Fermentation for Lactic Acid Production from Inedible Starchy Biomass by Thermophilic Bacillus Coagulans IPE22. Bioresour. Technol. 2019, 272, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, D.; He, M.; Wang, Z.; Qin, P.; Tan, T. Open Fermentative Production of L-Lactic Acid Using White Rice Bran by Simultaneous Saccharification and Fermentation. Bioresour. Technol. 2015, 198, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Tosungnoen, S.; Chookietwattana, K.; Dararat, S. Lactic Acid Production from Repeated-Batch and Simultaneous Saccharification and Fermentation of Cassava Starch Wastewater by Amylolytic Lactobacillus Plantarum MSUL 702. APCBEE Procedia 2014, 8, 204–209. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, H.; Yang, Y.; Zhou, X.; Xiu, Z. High-Efficient l-Lactic Acid Production from Inedible Starchy Biomass by One-Step Open Fermentation Using Thermotolerant Lactobacillus Rhamnosus DUT1908. Bioprocess Biosyst. Eng. 2021, 44, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, Y.; Liu, H.; Wei, C.; Qi, W.; Xiu, Z. Simultaneous Liquefaction, Saccharification, and Fermentation of l-Lactic Acid Using Aging Paddy Rice with Hull by an Isolated Thermotolerant Enterococcus Faecalis DUT1805. Bioprocess Biosyst. Eng. 2020, 43, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Smerilli, M.; Neureiter, M.; Wurz, S.; Haas, C.; Frühauf, S.; Fuchs, W. Direct Fermentation of Potato Starch and Potato Residues to Lactic Acid by Geobacillus Stearothermophilus under Non-Sterile Conditions. J. Chem. Technol. Biotechnol. 2015, 90, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Kunasundari, B.; Zulkeple, M.F.; Teoh, Y.P. Screening for Direct Production of Lactic Acid from Rice Starch Waste by Geobacillus Stearothermophilus. In Proceedings of the MATEC Web of Conferences, Wuhan, China, 1 February 2017; Volume 97. [Google Scholar]
- Verma, S.K.; Iram, D.; Sansi, M.S.; Pandey, K.K.; Vij, S.; Sood, S.K. Sustainable Utilization of Dairy Waste Paneer Whey by Pediococcus Pentosaceus NCDC 273 for Lactic Acid Production. Biocatal. Agric. Biotechnol. 2023, 47, 102588. [Google Scholar] [CrossRef]
- Luongo, V.; Policastro, G.; Ghimire, A.; Pirozzi, F.; Fabbricino, M. Repeated-Batch Fermentation of Cheese Whey for Semi-Continuous Lactic Acid Production Using Mixed Cultures at Uncontrolled PH. Sustainability 2019, 11, 3330. [Google Scholar] [CrossRef]
- Catone, M.V.; Palomino, M.M.; Legisa, D.M.; Martin, J.F.; García, V.M.; Ruzal, S.M.; Allievi, M.C. Lactic acid production using cheese whey based medium in a stirred tank reactor by a ccpA mutant of Lacticaseibacillus casei. World J. Microbiol. Biotechnol. 2021, 37, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mukherjee, S.; Reddy Tadi, S.R.; Ramesh, A.; Sivaprakasam, S. Kinetics of Growth, Plantaricin and Lactic Acid Production in Whey Permeate Based Medium by Probiotic Lactobacillus Plantarum CRA52. LWT 2021, 139, 110744. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Zadeike, D.; Bartkiene, E.; Klupsaite, D. Application of Acid Tolerant Pedioccocus Strains for Increasing the Sustainability of Lactic Acid Production from Cheese Whey. LWT 2016, 72, 399–406. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, Z.; Xu, Q.; Qian, Z.; Liu, J.; Ouyang, J. Valorization of Dairy Waste for Enhanced D-Lactic Acid Production at Low Cost. Process. Biochem. 2018, 71, 18–22. [Google Scholar] [CrossRef]
- Moradi, S.; Zeraatpisheh, F.; Tabatabaee-Yazdi, F. Investigation of lactic acid production in optimized dairy wastewater culture medium. Biomass-Convers. Biorefinery 2022, 1, 3. [Google Scholar] [CrossRef]
- Montero-Zamora, J.; Fernández-Fernández, S.; Redondo-Solano, M.; Mazón-Villegas, B.; Mora-Villalobos, J.A.; Barboza, N. Assessment of Different Lactic Acid Bacteria Isolated from Agro-Industrial Residues: First Report of the Potential Role of Weissella Soli for Lactic Acid Production from Milk Whey. Appl. Microbiol. 2022, 12, 48. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of Lignocellulosic Biomass: A Review on Recent Advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Dutta, N.; Usman, M.; Ashraf, M.A.; Luo, G.; Gamal El-Din, M.; Zhang, S. Methods to Convert Lignocellulosic Waste into Biohydrogen, Biogas, Bioethanol, Biodiesel and Value-Added Chemicals: A Review. Environ. Chem. Lett. 2023, 21, 803–820. [Google Scholar] [CrossRef]
- Hu, J.; Lin, Y.; Zhang, Z.; Xiang, T.; Mei, Y.; Zhao, S.; Liang, Y.; Peng, N. High-Titer Lactic Acid Production by Lactobacillus Pentosus FL0421 from Corn Stover Using Fed-Batch Simultaneous Saccharification and Fermentation. Bioresour. Technol. 2016, 214, 74–80. [Google Scholar] [CrossRef]
- Ahring, B.K.; Traverso, J.J.; Murali, N.; Srinivas, K. Continuous Fermentation of Clarified Corn Stover Hydrolysate for the Production of Lactic Acid at High Yield and Productivity. Biochem. Eng. J. 2016, 109, 162–169. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Lu, X.; Zheng, X.; Yan, D.; Xin, J.; El-Tantawy El-Sayed, I.; Kang, Y.; Yang, J. Highly Efficient Enzymolysis and Fermentation of Corn Stalk into L-Lactic Acid by Enzyme-Bacteria Friendly Ionic Liquid Pretreatment. Green Chem. Eng. 2022, 3, 321–327. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Jiang, H.; Liang, C.; Zhang, Z.C. Enhanced lactic acid production from P2O5-pretreated biomass by domesticated Pediococcus pentosaceus without detoxification. Bioprocess Biosyst. Eng. 2021, 44, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Alrumman, S.A. Enzymatic Saccharification and Fermentation of Cellulosic Date Palm Wastes to Glucose and Lactic Acid. Braz. J. Microbiol. 2016, 47, 110–119. [Google Scholar] [CrossRef]
- Kunasundari, B.; Arai, T.; Sudesh, K.; Hashim, R.; Sulaiman, O.; Stalin, N.J.; Kosugi, A. Detoxification of Sap from Felled Oil Palm Trunks for the Efficient Production of Lactic Acid. Appl. Biochem. Biotechnol. 2017, 183, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Saelee, N. Lactic Acid Production from Old Oil Palm Trunk Sap in the Open Batch, Open Repeated Batch, Fed-Batch, and Repeated Fed-Batch Fermentation by Lactobacillus Rhamnosus ATCC 10863. Fermentation 2022, 8, 430. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Production of High Concentration of L-Lactic Acid from Oil Palm Empty Fruit Bunch by Thermophilic Bacillus Coagulans JI12. Biotechnol. Appl. Biochem. 2018, 65, 145–149. [Google Scholar] [CrossRef]
- de la Torre, I.; Acedos, M.G.; Ladero, M.; Santos, V.E. On the Use of Resting L. Delbrueckii Spp. Delbrueckii Cells for D-Lactic Acid Production from Orange Peel Wastes Hydrolysates. Biochem. Eng. J. 2019, 145, 162–169. [Google Scholar] [CrossRef]
- Chen, H.; Huo, W.; Wang, B.; Wang, Y.; Wen, H.; Cai, D.; Zhang, C.; Wu, Y.; Qin, P. L-Lactic Acid Production by Simultaneous Saccharification and Fermentation of Dilute Ethylediamine Pre-Treated Rice Straw. Ind. Crops Prod. 2019, 141, 111749. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Raj, T.; Chauhan, P.S.; Kumari, P.; Satlewal, A.; Gupta, R.P.; Kumar, R. High-Titer Lactic Acid Production from Pilot-Scale Pretreated Non-Detoxified Rice Straw Hydrolysate at High-Solid Loading. Biochem. Eng. J. 2022, 187, 108668. [Google Scholar] [CrossRef]
- Yadav, N.; Nain, L.; Khare, S.K. One-Pot Production of Lactic Acid from Rice Straw Pretreated with Ionic Liquid. Bioresour. Technol. 2021, 323, 124563. [Google Scholar] [CrossRef]
- Ouyang, S.; Zou, L.; Qiao, H.; Shi, J.; Zheng, Z.; Ouyang, J. One-Pot Process for Lactic Acid Production from Wheat Straw by an Adapted Bacillus Coagulans and Identification of Genes Related to Hydrolysate-Tolerance. Bioresour. Technol. 2020, 315, 123855. [Google Scholar] [CrossRef]
- Chawla, S.K.; Goyal, D. Optimization of Pretreatment of Wheat Straw Using Response Surface Methodology for Production of Lactic Acid Using Bacillus Sonorenesis Strain DGS15. Bioenergy Res. 2023, 16, 967–978. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernández, C.; Ballesteros, M.; Tomás-Pejó, E. Lactobacillus Pentosus CECT 4023 T Co-Utilizes Glucose and Xylose to Produce Lactic Acid from Wheat Straw Hydrolysate: Anaerobiosis as a Key Factor. Biotechnol. Prog. 2019, 35, e2739. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, S.; Huang, J.; Guo, H.; Bi, X.; Hou, M.; Chen, X.; Hou, S.; Lin, H.; Lu, Y.; et al. Optimization of Initial Cation Concentrations for L-Lactic Acid Production from Fructose by Lactobacillus Pentosus Cells. Appl. Biochem. Biotechnol. 2021, 193, 1496–1512. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Jiang, S.; Zhang, J.; Zhang, Q.; Ning, Y.; Fang, M.; Liu, S. Parametric Optimization and Kinetic Study of L-Lactic Acid Production by Homologous Batch Fermentation of Lactobacillus Pentosus Cells. Biotechnol. Appl. Biochem. 2021, 68, 809–822. [Google Scholar] [CrossRef]
- Reddy, L.V.; Park, J.H.; Wee, Y.J. Homofermentative Production of Optically Pure L-Lactic Acid from Sucrose and Mixed Sugars by Batch Fermentation of Enterococcus Faecalis RKY1. Biotechnol. Bioprocess Eng. 2015, 20, 1099–1105. [Google Scholar] [CrossRef]
- Anagnostopoulou, C.; Kontogiannopoulos, K.N.; Gaspari, M.; Morlino, M.S.; Assimopoulou, A.N.; Kougias, P.G. Valorization of Household Food Wastes to Lactic Acid Production: A Response Surface Methodology Approach to Optimize Fermentation Process. Chemosphere 2022, 296, 133871. [Google Scholar] [CrossRef]
- Gordeeva, Y.L.; Rudakovskaya, E.G.; Gordeeva, E.L.; Borodkin, A.G. Mathematical Modeling of Biotechnological Process of Lactic Acid Production by Batch Fermentation: A Review. Theor. Found. Chem. Eng. 2017, 51, 282–298. [Google Scholar] [CrossRef]
- Garcia, B.E.; Rodriguez, E.; Salazar, Y.; Valle, P.A.; Flores-Gallegos, A.C.; Miriam Rutiaga-Quiñones, O.; Rodriguez-Herrera, R. Primary Model for Biomass Growth Prediction in Batch Fermentation. Symmetry 2021, 13, 1468. [Google Scholar] [CrossRef]
- Veeravalli, S.S.; Mathews, A.P. Continuous Fermentation of Xylose to Short Chain Fatty Acids by Lactobacillus Buchneri under Low PH Conditions. Chem. Eng. J. 2018, 337, 764–771. [Google Scholar] [CrossRef]
- Choi, G.; Kim, J.; Lee, C. Effect of Low PH Start-up on Continuous Mixed-Culture Lactic Acid Fermentation of Dairy Effluent. Appl. Microbiol. Biotechnol. 2016, 100, 10179–10191. [Google Scholar] [CrossRef]
- Peinemann, J.C.; Demichelis, F.; Fiore, S.; Pleissner, D. Techno-Economic Assessment of Non-Sterile Batch and Continuous Production of Lactic Acid from Food Waste. Bioresour. Technol. 2019, 289, 121631. [Google Scholar] [CrossRef]
- Pejin, J.; Radosavljević, M.; Kocić-Tanackov, S.; Mladenović, D.; Djukić-Vuković, A.; Mojović, L. Fed-Batch l-(+)-Lactic Acid Fermentation of Brewer’s Spent Grain Hydrolysate. J. Inst. Brew. 2017, 123, 537–543. [Google Scholar] [CrossRef]
- de Oliveira, P.M.; Santos, L.P.; Coelho, L.F.; Avila Neto, P.M.; Sass, D.C.; Contiero, J. Production of l (+) Lactic Acid by Lactobacillus Casei Ke11: Fed Batch Fermentation Strategies. Fermentation 2021, 7, 151. [Google Scholar] [CrossRef]
- Lobeda, K.; Jin, Q.; Wu, J.; Zhang, W.; Huang, H. Lactic Acid Production from Food Waste Hydrolysate by Lactobacillus Pentosus: Focus on Nitrogen Supplementation, Initial Sugar Concentration, PH, and Fed-Batch Fermentation. J. Food Sci. 2022, 87, 3071–3083. [Google Scholar] [CrossRef]
- Campos, J.; Tejada, L.G.; Bao, J.; Lidén, G. Fed-Batch Strategies for Biodetoxification in Production of Optically Pure Lactic Acid from Softwood Hydrolysate Using Pediococcus Acidilactici. Process. Biochem. 2023, 125, 162–170. [Google Scholar] [CrossRef]
- Lu, J.; Peng, W.; Lv, Y.; Jiang, Y.; Xu, B.; Zhang, W.; Zhou, J.; Dong, W.; Xin, F.; Jiang, M. Application of Cell Immobilization Technology in Microbial Cocultivation Systems for Biochemicals Production. Ind. Eng. Chem. Res. 2020, 59, 17026–17034. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Guo, H.; Jiang, S.; Zhang, J.; Ning, Y.; Fang, M.; Liu, S. Optimization of Immobilization Conditions for Lactobacillus Pentosus Cells. Bioprocess Biosyst. Eng. 2020, 43, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Laffend, H.; Jiang, S.; Zhang, J.; Ning, Y.; Fang, M.; Liu, S. Optimization of Immobilized Lactobacillus Pentosus Cell Fermentation for Lactic Acid Production. Bioresour. Bioprocess. 2020, 7, 15. [Google Scholar] [CrossRef]
- Wang, J.; Guo, H.; Huang, J.; Jiang, S.; Hou, S.; Chen, X.; Lv, H.; Bi, X.; Hou, M.; Lin, H.; et al. L-Lactic Acid Production from Fructose by Chitosan Film-Coated Sodium Alginate-Polyvinyl Alcohol Immobilized Lactobacillus Pentosus Cells and Its Kinetic Analysis. Bioresour. Bioprocess. 2021, 8, 27. [Google Scholar] [CrossRef]
- Radosavljević, M.; Lević, S.; Belović, M.; Pejin, J.; Djukić-Vuković, A.; Mojović, L.; Nedović, V. Encapsulation of Lactobacillus Rhamnosus in Polyvinyl Alcohol for the Production of L-(+)-Lactic Acid. Process. Biochem. 2021, 100, 149–160. [Google Scholar] [CrossRef]
- Shahri, S.Z.; Vahabzadeh, F.; Mogharei, A. Lactic Acid Production by Loofah-Immobilized Rhizopus Oryzae through One-Step Fermentation Process Using Starch Substrate. Bioprocess Biosyst. Eng. 2020, 43, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Mladenović, D.; Pejin, J.; Kocić-Tanackov, S.; Radovanović, Ž.; Djukić-Vuković, A.; Mojović, L. Lactic Acid Production on Molasses Enriched Potato Stillage by Lactobacillus Paracasei Immobilized onto Agro-Industrial Waste Supports. Ind. Crops Prod. 2018, 124, 142–148. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Venus, J. A Review on the Current Developments in Continuous Lactic Acid Fermentations and Case Studies Utilising Inexpensive Raw Materials. Process. Biochem. 2019, 79, 1–10. [Google Scholar] [CrossRef]
- Ma, K.; Cui, Y.; Zhao, K.; Yang, Y.; Wang, Y.; Hu, G.; He, M. D-Lactic Acid Production from Agricultural Residues by Membrane Integrated Continuous Fermentation Coupled with B Vitamin Supplementation. Biotechnol. Biofuels Bioprod. 2022, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, M.; Blanco-Catalá, J.; Schneider, R.; Turon, X.; Venus, J. High L(+)-Lactic Acid Productivity in Continuous Fermentations Using Bakery Waste and Lucerne Green Juice as Renewable Substrates. Bioresour. Technol. 2020, 316, 123949. [Google Scholar] [CrossRef]
- Ma, K.; Hu, G.; Pan, L.; Wang, Z.; Zhou, Y.; Wang, Y.; Ruan, Z.; He, M. Highly Efficient Production of Optically Pure L-Lactic Acid from Corn Stover Hydrolysate by Thermophilic Bacillus Coagulans. Bioresour. Technol. 2016, 219, 114–122. [Google Scholar] [CrossRef]
- Olszewska-Widdrat, A.; Alexandri, M.; López-Gómez, J.P.; Schneider, R.; Venus, J. Batch and Continuous Lactic Acid Fermentation Based on a Multi-Substrate Approach. Microorganisms 2020, 8, 1084. [Google Scholar] [CrossRef]
- Gupta, V.; Odaneth, A.A.; Lali, A.M. High Cell Density Continuous Fermentation for L-Lactic Acid Production from Cane Molasses. Prep. Biochem. Biotechnol. 2023, 1–15. [Google Scholar] [CrossRef]
- Zhou, J.; Ouyang, J.; Xu, Q.; Zheng, Z. Cost-Effective Simultaneous Saccharification and Fermentation of L-Lactic Acid from Bagasse Sulfite Pulp by Bacillus Coagulans CC17. Bioresour. Technol. 2016, 222, 431–438. [Google Scholar] [CrossRef]
- Tian, X.; Liu, X.; Zhang, Y.; Chen, Y.; Hang, H.; Chu, J.; Zhuang, Y. Metabolic Engineering Coupled with Adaptive Evolution Strategies for the Efficient Production of High-Quality L-Lactic Acid by Lactobacillus Paracasei. Bioresour. Technol. 2021, 323, 124549. [Google Scholar] [CrossRef]
- Li, J.; Shi, S.; Wang, Y.; Jiang, Z. Integrated Production of Optically Pure L-Lactic Acid from Paper Mill Sludge by Simultaneous Saccharification and Co-Fermentation (SSCF). Waste Manag. 2021, 129, 35–46. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Liu, X.; Bao, J. Continuous Simultaneous Saccharification and Co-Fermentation (SSCF) for Cellulosic L-Lactic Acid Production. Ind. Crops Prod. 2022, 187, 115527. [Google Scholar] [CrossRef]
- Klongklaew, A.; Unban, K.; Kalaimurugan, D.; Kanpiengjai, A.; Azaizeh, H.; Schroedter, L.; Schneider, R.; Venus, J.; Khanongnuch, C. Bioconversion of Dilute Acid Pretreated Corn Stover to L-Lactic Acid Using Co-Culture of Furfural Tolerant Enterococcus Mundtii WX1 and Lactobacillus Rhamnosus SCJ9. Fermentation 2023, 9, 112. [Google Scholar] [CrossRef]
- Ricci, A.; Diaz, A.B.; Caro, I.; Bernini, V.; Galaverna, G.; Lazzi, C.; Blandino, A. Orange Peels: From by-Product to Resource through Lactic Acid Fermentation. J. Sci. Food Agric. 2019, 99, 6761–6767. [Google Scholar] [CrossRef]
- Zhang, Y.; Vadlani, P.V. Lactic Acid Production from Biomass-Derived Sugars via Co-Fermentation of Lactobacillus Brevis and Lactobacillus Plantarum. J. Biosci. Bioeng. 2015, 119, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.Y.; Qian, H.; Zhang, W.G. Improvement of L-Lactic Acid Production from Jerusalem Artichoke Tubers by Mixed Culture of Aspergillus niger and Lactobacillus Sp. Bioresour. Technol. 2009, 100, 1872–1874. [Google Scholar] [CrossRef]
- Sahoo, T.K.; Jayaraman, G. Co-Culture of Lactobacillus Delbrueckii and Engineered Lactococcus Lactis Enhances Stoichiometric Yield of d-Lactic Acid from Whey Permeate. Appl. Microbiol. Biotechnol. 2019, 103, 5653–5662. [Google Scholar] [CrossRef]
- Ou, M.S.; Awasthi, D.; Nieves, I.; Wang, L.; Erickson, J.; Vermerris, W.; Ingram, L.O.; Shanmugam, K.T. Sweet Sorghum Juice and Bagasse as Feedstocks for the Production of Optically Pure Lactic Acid by Native and Engineered Bacillus Coagulans Strains. BioEnergy Res. 2016, 9, 123–131. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, C.; He, Q.; Zheng, Z.; Ouyang, J. Metabolic Engineering of Escherichia Coli K12 for Homofermentative Production of L-Lactate from Xylose. Appl. Biochem. Biotechnol. 2018, 184, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Aso, Y.; Hashimoto, A.; Ohara, H. Engineering Lactococcus Lactis for D-Lactic Acid Production from Starch. Curr. Microbiol. 2019, 76, 1186–1192. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Yuan, S.F.; Wang, C.A.; Huang, Y.J.; Guo, G.L.; Hwang, W.S. Production of Optically Pure L-Lactic Acid from Lignocellulosic Hydrolysate by Using a Newly Isolated and d-Lactate Dehydrogenase Gene-Deficient Lactobacillus Paracasei Strain. Bioresour. Technol. 2015, 198, 651–657. [Google Scholar] [CrossRef]
- Okano, K.; Uematsu, G.; Hama, S.; Tanaka, T.; Noda, H.; Kondo, A.; Honda, K. Metabolic Engineering of Lactobacillus Plantarum for Direct L-Lactic Acid Production From Raw Corn Starch. Biotechnol. J. 2018, 13, e1700517. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernández, C.; Tomás-Pejó, E. Evolutionary Engineering of Lactobacillus Pentosus Improves Lactic Acid Productivity from Xylose-Rich Media at Low PH. Bioresour. Technol. 2019, 288, 121540. [Google Scholar] [CrossRef]
- Qiu, Z.; Gao, Q.; Bao, J. Engineering Pediococcus Acidilactici with Xylose Assimilation Pathway for High Titer Cellulosic L-Lactic Acid Fermentation. Bioresour. Technol. 2018, 249, 9–15. [Google Scholar] [CrossRef] [PubMed]

| Lignocellulosic Waste | Pretreatment | Microorganism | LA, g/L | Yield, g/g | Productivity, g/(L·h) | Reference |
|---|---|---|---|---|---|---|
| Corn stover | Alkaline, hot water | Lactobacillus pentosus | 92.3 | 0.66 | 1.92 | [38] |
| Grinding, wet explosion, cellulase | Bacillus coagulans | - | 0.95 | 3.69 | [39] | |
| Cornstalk | Ionic liquid, cellulase | Bacillus sp. | - | 0.963 | - | [40] |
| Steam explosion, ball milling with P2O5 | Pediococcus pentosaceus | 29.8 | 0.82 | 3.4 | [41] | |
| Date palm waste | Grinding, alkaline | Lactobacillus delbrueckii | 27.8 | 0.76 | 0.386 | [42] |
| Oil palm | Extruding, alkaline | Bacillus coagulans | 63.3 | 0.92 | 2.64 | [43] |
| Extruding | Lactobacillus rhamnosus | 91.30 | 0.87 | 3.88 | [44] | |
| Oil palm empty fruit bunch | Acid | Bacillus coagulans | 105.4 | - | 9.3 | [45] |
| Cellulase | Bacillus coagulans | 114.0 | - | 5.7 | [45] | |
| Orange peel | Milling, cellulase | Lactobacillus delbrueckii | - | 0.94 | 6.72 | [46] |
| Rice straw | Ethylenediamine | Bacillus coagulans | 92.5 | - | 2.01 | [47] |
| Dilute acid, steam explosion, cellulase | Lactobacillus lactis | 82.2 | 0.872 | 0.61 | [48] | |
| Ionic liquid, hot water, cellulase | Lactobacillus plantarum | 36.75 | - | 0.51 | [49] | |
| Wheat straw | Milling, cellulase | Bacillus coagulans | 26.3 | 0.709 | 0.253 | [50] |
| Grinding, acid | Bacillus sonorenesis | 55.9 | 0.97 | 0.77 | [51] | |
| Milling, steam explosion | Lactobacillus pentosus | 17.7 | 0.82 | 0.37 | [52] |
| Microorganism | Modifications | Substrate | Optical Purity | LA, g/L | Yield, g/g | Productivity, g/(L·h) | Reference |
|---|---|---|---|---|---|---|---|
| Bacillus coagulans | Deletion of l-lactate dehydrogenase (ldh) gene and acetolactate synthase (alsS) gene, mutation of a growth-based suppressor, introduction of d-lactate dehydrogenase (D-LDH) gene | Sweet sorghum juice | d-LA >99% | 125 | - | 5 | [88] |
| Escherichia coli | Six chromosomal deletions (pflB, ldhA, ackA, pta, frdA, adhE), over-expression of l-lactate dehydrogenase (ldhL) gene, intensification of xylose catabolism | Xylose medium | l-LA >99% | 8.12 | 0.91 | - | [89] |
| Lacticaseibacillus casei | Mutation of catabolite control protein A (ccpA) gene | Cheese whey | l-LA 94.2% | 44.23 | 0.8 | - | [29] |
| Lactococcus lactis | Replacement of l-lactate dehydrogenase (L-Ldh) gene with d-lactate dehydrogenase (D-Ldh) gene, intergration of α-amylase (amyA) gene | Starch | d-LA 93.8% | 15.0 | - | 0.63 | [90] |
| Lactobacillus paracasei | Disruption of d-lactate dehydrogenase (ldhD) gene | Wood hydrolysate | l-LA | 94.86 | 0.96 | 3.23 | [91] |
| Rice straw hydrolysate | l-LA | 66.67 | 0.97 | 5.27 | [91] | ||
| Lactobacillus paracasei | Replacement of d-lactate dehydrogenase (ldhD) gene with l-lactate dehydrogenase 1 (ldhL1) gene, adaptive evolution at 45 °C | High glucose medium | l-LA 99.1% | 221.0 | 0.96 | 7.5 | [80] |
| Lactobacillus plantarum | Deletion of d-lactate dehydrogenase (ldhA) gene, mutation of (ΔldhD) gene, disruption of lactate racemase operon (larA-E) | Raw corn starch | l-LA 98.6% | 53 | 0.91 | - | [92] |
| Lactobacillus pentosus | Adaptive evolution at high xylose concentration and low pH | Wheat straw hydrolysate | - | 13.5 | 0.86 | 0.74 | [93] |
| Pediococcus acidilactici | Disruption of phosphoketolase (pkt) gene, integration of transketolase (tkt), transaldolase (tal), xylose isomerase (xylA) and xylulokinase (xylB) genes, long-term adaptive evolution | Wheat straw | l-LA | 130.8 | 0.95 | 1.82 | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Wang, J.; Liu, S. Advanced Fermentation Techniques for Lactic Acid Production from Agricultural Waste. Fermentation 2023, 9, 765. https://doi.org/10.3390/fermentation9080765
Huang J, Wang J, Liu S. Advanced Fermentation Techniques for Lactic Acid Production from Agricultural Waste. Fermentation. 2023; 9(8):765. https://doi.org/10.3390/fermentation9080765
Chicago/Turabian StyleHuang, Jiaqi, Jianfei Wang, and Shijie Liu. 2023. "Advanced Fermentation Techniques for Lactic Acid Production from Agricultural Waste" Fermentation 9, no. 8: 765. https://doi.org/10.3390/fermentation9080765
APA StyleHuang, J., Wang, J., & Liu, S. (2023). Advanced Fermentation Techniques for Lactic Acid Production from Agricultural Waste. Fermentation, 9(8), 765. https://doi.org/10.3390/fermentation9080765







