Abstract
Barley (Hordeum vulgare L.) is one of the first cereals that humans began to cultivate. This study aimed to investigate the possibility of enriching fermented dairy products, using fermented milk as an example, with young barley leaves powder (YBLP) preparation including different starter cultures of lactic acid bacteria (LAB). The addition of YBLP did not affect the maximum rate of acidification and the time at which the maximum acidification rate was achieved. However, it did impact the time required to reach the desired pH level (4.6) for specific starter cultures. Over a 28-day storage period, gradual acidification of the fermented milk was observed. The addition of YBLP has a limited effect on the pH of the fermented milk, with the pH value primarily dependent on the type of starter culture and storage time. The addition of YBLP may have a positive effect on the survival of bacterial cells during the storage of the fermented milk; however, a gradual decrease in the number of LAB cells was observed during refrigerated storage. Furthermore, the addition of YBLP had a significant effect on the hardness, adhesion, and water-holding capacity of some fermented milk immediately after fermentation, depending on the specific starter culture used.
1. Introduction
Barley (Hordeum vulgare L.) is one of the earliest cultivated cereals. It is known for its high vitamin, mineral, and strong antioxidant content (such as superoxide dismutase, vitamin C, vitamin E, β-carotene, and flavones) [1,2,3,4,5,6,7]. Of particular interest are the beneficial properties of barley in the form of a preparation derived from young seedlings of the plant. The production process begins with the germination of the seeds. After two weeks, the young leaves were harvested and juiced, with the solid tissues separated from the liquid. The resulting liquid is then subjected to a spray drying [8]. Young barley leaves are widely consumed for their higher nutritional value and are considered beneficial for human health [9]. Incorporating barley leaf into bread and tea has been shown to enhance their nutritional quality [10,11]. Barley leaves have demonstrated various pharmacological activities, particularly in relation to metabolic syndromes such as depression, diabetes, and atherosclerosis [1,12,13]. Studies indicate that consuming green barley, along with vitamin C and E supplementation, can lower LDL levels and inhibit LDL oxidation, thus reducing the risk of atherosclerosis [13]. These effects are largely attributed to the significant dietary fiber content of barley leaves, especially polysaccharides. Han et al. [14] reported that young barley leaves contain polysaccharides primarily composed of neutral sugars and uronic acid, with small amounts of 2-keto-3-deoxymannooctanoic acid (KDO)-like materials and exhibit immunological activity. No data were found on studies of the antimicrobial activity of young barley leaves.
In recent years, there has been a trend toward consuming functional foods, which are expected to have positive health effects with regular consumption. As a result, there is increasing research focused on enriching products with components derived from herbal preparations or algae, known for their health-promoting properties, and often associated with naturalness by consumers. In the production of fermented milk, the survival of the technical microflora in the product is an important consideration. During cold storage, after the fermentation period and bacterial population multiplication, the number of viable bacterial cells can gradually decrease. However, a certain number of viable bacterial cells is necessary to achieve the desired beneficial effects on the human body [15,16]. Exemplary studies with the addition of Spirulina platensis have demonstrated a positive effect on the survival of bacteria in yogurt during storage. Even with a concentration of 0.5 percent, the bacterial count remained at the level required by the IDF [15]. To date, there are no scientific reports describing the effect of young barley preparations on the texture of fermented milk.
The objective of this study was to investigate the feasibility of enriching fermented dairy products, specifically fermented milk, with young barley leaves powder (YBLP) preparation. Various starter cultures of mesophilic lactic acid bacteria (LAB) were incorporated into the investigation. We hypothesized that YBLP might positively modulate the growth rate of LAB and stimulate the quality parameters of fermented milk. Our findings hold significant implications for the development of novel food products aimed at enhancing human health.
2. Materials and Methods
Young barley leaves powder is derived from barley leaves that are approximately seven days old and is commercially available as a commercial food supplement (Agnex, Białystok, Poland). The powder is obtained by extracting the juice from young sprouting barley leaves, which are dried and finally ground to preserve their health benefits. According to the manufacturer’s specification, the nutritional composition per 100 g is as follows: energy value 1151 kJ/275 kcal, fat 2.5 g (including saturated fatty acids 2.2 g), carbohydrates 25.1 g (including sugars 1.4 g and fiber 23.7 g), protein 21.4 g, and salt 0.17 g.
Under laboratory conditions, fermented milk samples were prepared using milk (1.5% fat, SM Łowicz, Łowicz, Poland) that was previously UHT-treated, then cooled to 27 °C and supplemented with 0.5% by weight of young barley leaves preparation (YBLP) and eight specific mesophilic LAB starter cultures (at the dose of 0.1%), commonly employed in the industrial production of sour milk-type fermented milk. Before addition to the milk, starter cultures were prepared by dissolving them in sterile drinking water. Fermented milk samples without the addition of YBLP were prepared in the same way (control tests). The microbiological composition of the starter cultures used is provided in Table 1.

Table 1.
Microbiological composition of the starter cultures used.
The fermentation process (for obtaining fermented milk) was carried out at a temperature of 27 °C for a duration of 14 h in sterile glass jars of approximately 160 mL each. After fermentation, the fermented milk samples were cooled in a refrigerator set at 6 °C and stored at the same temperature for 28 days. During fermentation, acidification curves were determined by measuring the pH value every two hours. Immediately after preparation, the samples were subjected to microbiological analysis. Additionally, at the completion of fermentation and every seven days of refrigerated storage, the samples were examined for the following: survival of lactic fermentation bacteria, acidity (the pH value), water-holding capacity (WHC), and texture (hardness and adhesiveness). The entire experiment was repeated five times to ensure the reliability and accuracy of the results.
For the pH test, a mixed fermented milk sample was prepared, and a pH meter electrode and a temperature sensor were inserted into the sample [17]. The pH value was recorded once it stabilized on the instrument’s display. The measurements were carried out in duplicates to ensure accuracy. The maximum acidification rate (Vmax) was determined using Equation (1) proposed by Bezerra et al. [18]:
The time at which the highest acidification rate (Tmax) was reached and the time to reach pH 4.6 (Te) were considered indicators to access the kinetics of the fermentation process [19].
To determine the number of mesophilic LAB cells, the traditional plate method was employed following the ISO 6887-5:2020 and ISO 15214:1998 standards [20,21]. M17 agar medium (Merck, Darmstadt, Germany) was used for this purpose. Petri plates containing the samples were incubated aerobically at 30 °C for 72 h. Following incubation, the number of grown colonies was counted. The measurements were carried out in duplicates to ensure accuracy. The results are presented as mean values of colony-forming unit per milliliter of sample (CFU/mL) and subsequently converted to decadic logarithm form.
At the same time as measuring the number of mesophilic LAB cells, the presence of contaminating microflora was measured. The total numbers of molds and yeasts were determined in YGC medium (Merck, Darmstadt, Germany) and the plates were incubated aerobically at 25 °C for 5 days. The total number of Enterobacteriaceae was determined in VRBG medium (Merck, Darmstadt, Germany) overlaid on the same medium and incubated anaerobically at 37 °C for 24 h. The results are presented as mean values of colony-forming units per milliliter of sample (CFU/mL) and subsequently converted to decadic logarithm form.
Hardness and adhesion testing was conducted following the procedure outlined by Ziarno and Zaręba [17]. A Texture Analyzer CT3 10 K (Brookfield Engineering Laboratories Inc., Middleborough, MA, USA), using TexturePro CT V 1.4 Build 17 software (Brookfield Engineering Laboratories Inc., Middleborough, MA, USA), was used for the measurements. The used test type was TPA (Texture Profile Analysis). The used probe was TA4/1000 (cylindrical, 38.1 mm in diameter, and 20 mm in height). During the tests, a force of 0.04 N was applied. The probe moved toward the sample at a speed of 2 mm/s, whereas in the opposite direction, it moved at a speed of 4.5 mm/s. A total of 10 measurements per second were carried out. Each type of fermented milk was subjected to one cycle of measurements at a temperature of 6 °C. The measurements were carried out in duplicates to ensure consistency.
The water-holding capacity of fermented milk was determined following the method described by Ziarno and Zaręba [17]. Briefly, 40.00 g of beverage sample was centrifuged at 3250× g at 4 °C for 20 min. The whey expelled was removed and weighed. The measurements were carried out in duplicates to ensure consistency. WHC (%) was calculated according to the following Formula (2):
In addition, the determination of the antimicrobial activity of YBLP was carried out using the agar diffusion method [22]. To determine antimicrobial activity, YBLP was dissolved in dimethyl sulfoxide (DMSO) or 70% ethanol to yield solutions containing 2.0 mg of YBLP per mL. Mesophilic LAB from lyophilized starter cultures was revived in M17 broth (Merck, Darmstadt, Germany) at 30 °C for 18 h. Petri dishes were loaded with 0.5 mL of each culture broth cultured to a concentration of about 6 log(CFU/mL), which was then covered with M17 agar medium at 30 °C. After solidification, the wells were cut to a diameter of 6 mm, and each was loaded with 20 μL of YBLP solutions. The Petri dishes were incubated at 30 °C under aerobic conditions for 72 h and the respective zone of inhibition was measured. The inhibition tests were conducted in quadruplicate. The wells containing DMSO or 70% ethanol, but no YBLP solutions were used as a negative control.
A two-factor analysis of variance (MANOVA) was used for the statistical analysis, which is applicable in determining the influence of significant factors in a multivariate model. The MANOVA analysis was used to assess the influence of the two factors, as well as the interaction between them. The MANOVA analysis was conducted using the Statgraphics Centurion XVII program (Statgraphics Technologies, Inc., The Plains, VA, USA). Turkey’s test and a significance coefficient α of 0.05 were used in conjunction with the MANOVA analysis.
3. Results and Discussion
3.1. Acidification Curve
The acidification curves are graphical representations of the pH variations that occur during the process of lactic acid fermentation as shown in Figure 1. These curves visually demonstrate the evolution of pH throughout the fermentation, starting from the initial state and concluding with the final pH value. By analyzing these curves, we can determine the duration required to achieve the desired level of acidity, which is vital for ensuring consistent product quality. Additionally, these curves also exhibit unique characteristics depending on the culture starter used. These curves provide valuable information regarding the production rate of acid, the time needed to reach specific pH levels, and the overall efficiency of the fermentation process. In no case were differences observed between the pH values of fermenting milk with and without the addition of YBLP. The initial pH of the milk averaged 6.67 ± 0.2, regardless of the milk samples. During the 14 h fermentation with mesophilic starter cultures of lactic acid bacteria, the acidity of the milk increased until the final pH measurement averaged 4.50 ± 0.3, regardless of whether the samples contained 0.5% YBLP supplementation or not.
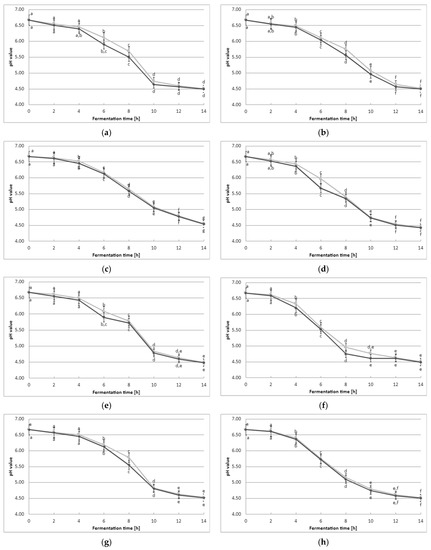
Figure 1.
Effect of YBLP preparate on lactic acid fermentation (average values and standard errors of five replicates of the experiment): (a) Flora Danica, (b) CHN-19, (c) XPL-1, (d) CHOOZIT TP 05, (e) CHOOZIT MTM 2, (f) Probat 222, (g) Kefirferment, and (h) Dickmilch bioferment. a,b,c,d,e,f,g means with different letters within the same culture are significantly different (p < 0.05). Legend: gray—control samples, black lines—samples with 0.5% YBLP preparation.
The calculated Vmax value (Table 2) was determined solely by the type of starter culture that was used to make the fermented milk. However, it was not dependent on the addition of YBLP. Similar trends were observed for Tmax (time at which the maximum rate of acidification was reached). A significant effect of adding YBLP could be observed only in the calculation of Te (time when a pH of 4.5 was reached) for beverages made using the following starter cultures: CHN-19, CHOOZIT MTM 2, and Dickmilch bioferment.

Table 2.
Kinetics parameters of acidification curves (mean values and standard deviation).
Oh et al. [23] observed that Cudrania tricuspidata and Morus alba L. leaf extracts, which are potential prebiotic substrates, significantly accelerated yogurt acidification. The addition of these extracts increased Vmax by 57% and 75%, respectively. The addition of herbs during fermentation also increases the metabolic activity of the bacteria in yogurt [24]. For example, yogurt enriched with peppermint (Mentha piperita), dill (Anethum graveolens), and basil (Ocimum basilicum) showed a decrease in pH levels, which could be attributed to the heightened production of organic acids by LAB starter culture (containing Lactobacillus bulgaricus, Lactobacillus rhamnosus, Bifidobacterium infantis, and Bulgaricus longum). To date, prebiotics are known to accelerate the acidification of yogurt and shorten the fermentation time [25,26]. They also promote an increase in the population of live LAB cells. In our study, we determined the population of living LAB cells in finished beverage samples that were stored in a cool place (the results are presented and discussed below). However, at this point, we demonstrated a lack of negative effect of YBLP supplementation on acidification, albeit depending on the starter culture used.
3.2. Change in pH during Refrigerated Storage of Fermented Milks
The pH value of the fermented milk ranged between 4.44 and 4.55 at the end of the fermentation process, regardless of the type of starter culture used and the addition of YBLP (Table 3). During storage, gradual acidification of the fermented milk samples is noted. Statistical analysis of the results showed that the pH values of the fermented milk samples depended significantly on the storage time. During the days of refrigerated storage of the fermented milk samples, the pH value was determined more by the type of starter culture used than by the addition of YBLP. The addition of YBLP significantly affected the pH of the milk samples fermented with the Flora Danica or Dickmilch bioferment starter cultures (on day 28 of the experiments, samples with the addition of YBLP had a higher pH than control samples). For the other fermented milks, the samples with YBLP did not differ significantly from the control samples in terms of the pH values measured after 28 days of storage. Importantly, we found that the YBLP supplement did not inhibit the acidification activity of the starter cultures tested.

Table 3.
Change in pH during the refrigerated storage of fermented milks (mean values and standard deviation).
Many researchers have observed changes in the pH of fermented milk beverages during cold storage [27,28,29,30]. This is because the LAB in the beverages continue to metabolize lactose and produce lactic acid, even at cold temperatures. The lactic bacteria present in the starter cultures convert lactose into lactic acid, resulting in a reduction in the pH of the milk. The rate of pH decrease is slower than at optimal fermentation temperatures, but it is still noticeable. The gradual changes described above, however, can lead to a further reduction in the pH value of the fermented milk samples. In addition, the fermentation activity and lactose metabolism rate of LAB can vary among different cultures, especially under refrigeration conditions. Certain cultures of LAB may be more efficient at metabolizing lactose and producing lactic acid, resulting in a more rapid decline in pH in refrigerated fermented milk, whereas other LAB may exhibit slower activity, leading to smaller pH changes during storage. This phenomenon is supported by the findings of this study. Unfortunately, there is a lack of available research on the effect of YBLP on the acidity of fermented beverages obtained with this additive. However, Molnár [31] observed that the addition of Spirulina led to increased production by LAB, suggesting a negative correlation between the addition and the lower pH value of the sample. Other studies [32] demonstrated that the addition of A. platensis biomass significantly stimulated the acid production of mesophilic dairy starter cultures (such as Lactococcus lactis subsp. lactis and L. lactis subsp. cremoris), although to varying degrees. The researchers justified their results by highlighting the alkaline character of Spirulina biomass, which influenced acid formation and promoted bacterial viability. Similarly, Cichońska et al. [33] showed that the addition of flaxseed influenced the active acidity of yogurt samples during storage at low temperatures. Previous research [34] investigated the impact of plant extracts on the activity and growth of LAB in milk. The results indicated that the addition of the tested herbal extracts did not inhibit the growth of LAB in fermented milks, such as yogurts.
3.3. Change in Bacterial Cell Population during Refrigerated Storage of Fermented Milks
The initial count of viable LAB cells immediately after the fermentation process was 7.8 log(CFU/mL) and 7.7 log(CFU/mL) in the fermented milk samples obtained without and with the addition of YBLP, respectively (Figure 2). The initial count of viable LAB cells immediately after fermentation did not depend on the type of starter culture used or the addition of YBLP. However, a gradual decrease in the cell population of LAB was observed during refrigerated storage of fermented milk samples. The samples made with CHOOZIT TP05 and Kefirferment starter cultures exhibited the highest survival rate of LAB cells. In contrast, other fermented milk samples demonstrated a reduction in the population of LAB cells. Statistical analysis of the results revealed that the population of LAB cells during the storage of fermented milk samples depended on the storage time, the addition of YBLP, and the type of starter culture used. On days 7, 14, or 21 of storage, the addition of YBLP had a positive effect on the survival of LAB cells. On the other hand, on the final day of the experiments, this positive effect of YBLP supplementation on the survival of LAB cells was observed in samples obtained with the following starter cultures: Kefirferment and Dickmilch bioferment.
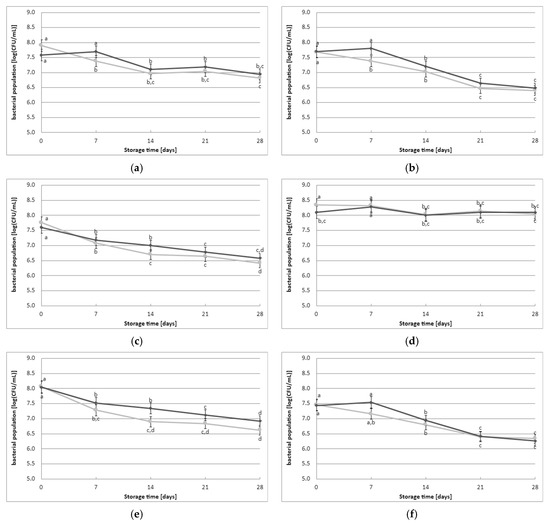
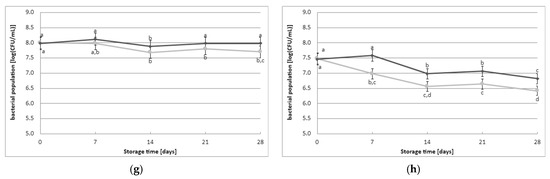
Figure 2.
Effect of YBLP preparate on lactic acid bacteria population (average values and standard errors of five replicates of the experiment): (a) Flora Danica, (b) CHN-19, (c) XPL-1, (d) CHOOZIT TP 05, (e) CHOOZIT MTM 2, (f) Probat 222, (g) Kefirferment, and (h) Dickmilch bioferment. a,b,c,d means with different letters within the same culture are significantly different (p < 0.05). Legend: gray lines—control samples, black lines—samples with 0.5% YBLP preparation.
The results obtained in this study indicated that molds, yeast, and Enterobacteriaceae were found to be absent in all samples during fermentation and refrigerated storage of fermented milk samples.
The storage of lactic acid fermentation products often leads to a decrease in the number of bacterial cells. Considering the beneficial effects of these bacteria on our bodies, this presents a significant issue [15]. However, the statistical analysis conducted in this study indicates the favorable survival of the microflora in the selected starter cultures under refrigeration. Throughout the 28-day storage period, all strains maintained frequencies greater than 6 log(CFU/mL). The results from the study demonstrate the lack of a negative effect of adding the YBLP preparation on the microflora of specific starter cultures and during cold storage of fermented milk. Although data lack in the scientific literature on the effect of adding YBLP on the LAB population, it is possible that other scientists have found evidence supporting the beneficial effect of adding microalgae supplements and other plant supplements on the survival of LAB. For instance, a study on the effect of S. platensis powder on the viability of the starter culture in probiotic yogurt with spinach demonstrated that as little as 0.5% of this additive improved bacterial cell survival to levels required by the IDF, i.e., greater than 6 log(CFU/mL) [15]. Similar studies by other researchers have also confirmed the beneficial effects of plant-based additives on the survival of LAB cells during storage [27,31,35,36]. A study by researchers [16] analyzing the impact of a vegetable green juice powder on the viability of yogurt bacteria, including lactobacilli, throughout the shelf life of the beverages, revealed a positive effect on bacterial survival. This may be due to substances such as adenine, hypoxanthine, and free amino acids, which are responsible for stimulating bacterial cell survival [37,38]. YBLP supplement contains 21.4 g of protein per 100 g. This protein content likely provides a significant amount of free amino nitrogen. In order to understand the effect of YBLP on LAB growth, it would be interesting and relevant to perform free amino nitrogen analyzes of YBLP. It can help determine if there are sufficient nutrients to support the growth of LAB and how the protein content in YBLP contributes to this process.
3.4. Change in Hardness and Adhesion during Refrigerated Storage of Fermented Milks
The hardness of the fermented milk, immediately after the fermentation process, was in the range of 26.0 to 34.7 g for the control samples and from 23.9 to 33.5 g for the samples with the addition of YBLP (Table 4). The addition of YBLP significantly determined the hardness of certain fermented milk samples immediately after fermentation. This was observed for samples fermented by the following starter cultures: Flora Danica, XPL-1, and CHOOZIT MTM 2. Meanwhile, for other fermented milk samples, the addition of YBLP had no effect on beverage values immediately after fermentation. This was observed for samples fermented by the following starter cultures: CHN-19, CHOOZIT TP 05, Probat 222, Kefirferment, and Dickmilch bioferment.

Table 4.
Change in hardness [g] during the refrigerated storage of fermented milks (mean values and standard deviation).
During the storage of fermented milk samples, changes in hardness were observed. The trend of these changes depended on several factors, including the addition of YBLP, storage time, and the type of starter culture used to obtain the beverages. After the first week of storage of fermented milk samples, the hardness values decreased for certain samples fermented by CHN-19 starter culture, regardless of YBLP addition; CHOOZIT TP 05 starter culture with YBLP addition; Probat 222 starter culture, regardless of YBLP addition; Kefirferment starter culture, regardless of YBLP addition. However, for other fermented milk samples, no statistically significant changes in hardness were observed during the first week of storage, as indicated in Table 4.
Further changes in the hardness values of the fermented milk samples were observed over subsequent weeks of storage. On the last day of the experiments, milk samples fermented by Flora Danica, XPL-1, CHOOZIT MTM 2, or Dickmilch bioferment showed a significant increase in the hardness values of the beverages. However, the addition of YBLP did not significantly improve this parameter compared with beverage samples without this additive. In contrast, milk samples fermented by CHN-19, Probat 222, or Kefirferment on the last day of the experiments exhibited a significant reduction in the hardness value of the beverages, whereas the addition of YBLP improved the hardness parameter for milk samples fermented by CHN-19 or Probat 222 compared with beverage samples without this additive. Notably, the best stability of hardness was characterized by samples of milk fermented with the CHOOZIT TP 05 culture demonstrated the best stability in terms of hardness (Table 4).
The adherence value of fermented milk samples was found to be influenced by multiple factors, including the addition of YBLP, the type of starter culture used, and the storage time (Table 5). The addition of YBLP reduced the adhesion value of the samples compared with the control samples. Considering the specific starter culture used, there was an observed increase in the value of the tested parameter in samples obtained with the following starter cultures: CHN-19, CHOOZIT TP05, Probat 222, or Dickmilch bioferment. However, for the remaining samples, no significant changes in the value of the parameter under study were recorded.

Table 5.
Change in adhesion force [g] during the refrigerated storage of fermented milk (mean values and standard deviation).
The examination of the rheological properties of the fermented milk revealed that the addition of YBLP at a level of 0.5% had a negative impact on the adhesion and hardness parameters. However, this effect only slightly worsened the results compared with the control samples. There are no similar studies available in the literature regarding the use of YBLP as an additive for fermented milks. Therefore, the discussion of the results was based on other plant-based additives used in fermented milk samples. A study conducted by Mocanu et al. [27] showed that the addition of S. platensis did not lead to significant rheological changes in the fermented product. However, the authors do not explain the reasons for the observations found. In another study [39], samples containing more than 1% roasted barley powder recorded higher values of plastic viscosity, yield stress, consistency coefficient, and apparent viscosity compared with a control sample of milk fermented by a starter culture containing Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. The aim of the cited research was to investigate the effect of yogurt drink supplementation by different amounts (0, 0.5, 1, 1.5, 2, 2.5, and 3%) of roasted barley powder on the physico–chemical properties, rheological parameters, color attributes, sensory evaluation, and production cost of the formulated yogurt drink. It is worth noting that only dosages greater than 1% of the baked barley powder (i.e., at least double the dosages used in these studies) resulted in higher parameters of the rheological properties. Similarly, Kaur and Riar [40] have found that β-glucan extract (isolated from barley) was particularly effective in set-type products like yogurt because β-glucan’s ability to entrap water in a three-dimensional product network, helping to maintain the structure of a firm consistency of the yogurt. Researchers cited have observed an increase in the hardness of yogurt samples with the addition of β-glucan and a decrease in the adhesiveness values of yogurt samples with the addition of β-glucan. Even the addition of 0.5% of the β-glucan preparation led to significant changes in the tested parameters of the yogurt. However, it should be noted that in this study the YBLP preparation was characterized by a significantly different chemical composition than the β-glucan preparation used in the cited reference. Han et al. [14] reported that young barley leaves contain polysaccharides but in a much lower amount than a purified β-glucan preparation. Studies suggest that barley β-glucan may be involved in hydrophobic interactions between protein molecules, thereby stabilizing the yogurt gel [41,42]. This effect was not observed in the present study. Although Qu et al. [42] demonstrated that the excessive addition of oat β-glucan (>0.3%) destroys the three-dimensional network structure of yogurt. The cited authors hypothesized that the addition of oat β-glucan disrupted the interaction between casein micelles and therefore three-dimensional aggregates cannot form during fermentation. Perhaps some of the results of these studies should be explained by such phenomena.
3.5. Change in Water-Holding Capacity during Refrigerated Storage of Fermented Milks
Each of the tested curds produced by the lactic starter cultures exhibited varying water-holding capacities, both immediately after obtaining the fermented milk and during cold storage in the refrigerator (Figure 3). The milk samples fermented with the CHN-19 culture (irrespective of YBLP content) and those fermented with CHOOZIT TP 05 culture without YBLP addition had the lowest initial average WHC values (WHC of 51.2–51.7%). On the other hand, milk samples fermented with Kefirferment culture without YBLP had the highest initial mean WHC value (mean WHC of 66.3% ± 2.5%). These findings indicate that the type of starter culture used in the milk fermentation process influenced the initial average WHC value of the fermented milk samples. Furthermore, the addition of YBLP was found to have a significant effect on the measured initial mean WHC value of the fermented milk samples, but only for certain starter cultures, including Flora Danica, CHOOZIT TP 05, Kefirferment, and Dickmilch bioferment.
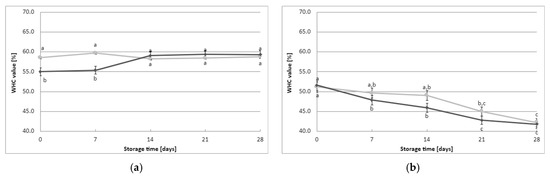
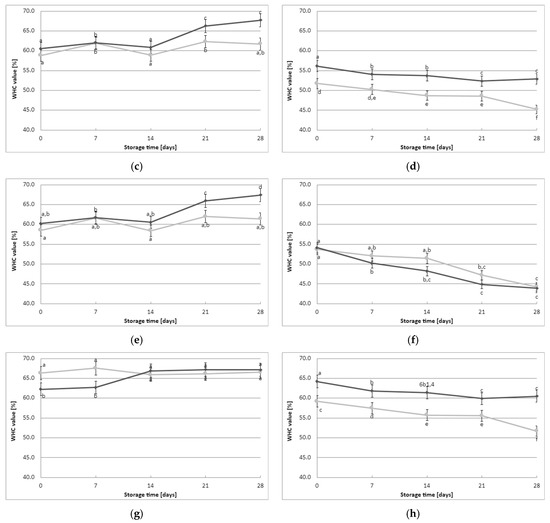
Figure 3.
Effect of young barley leaves powder preparate on water-holding capacity (average values and standard errors of five replicates of the experiment): (a) Flora Danica, (b) CHN-19, (c) XPL-1, (d) CHOOZIT TP 05, (e) CHOOZIT MTM 2, (f) Probat 222, (g) Kefirferment, and (h) Dickmilch bioferment. a,b,c,d,e,f means with different letters within the same culture are significantly different (p < 0.05). Legend: gray lines—control samples, black lines—samples with 0.5% YBLP preparation.
The WHC score of the fermented milk also exhibited changes during the storage of the samples. Notably, differences in WHC were observed in the fermented milk samples for each culture separately (Figure 3). The curd formed by milk fermentation with XPL-1 or CHOOZIT MTM 2 cultures increased its water retention capacity with time, and these changes became particularly significant from day 21 of data recording onward. It is worth noting that both starter cultures were mesophilic-thermophilic cultures, as they contained active mesophilic LAB and the addition of a thermophilic species of S. thermophilus. This may explain the different results obtained for samples fermented with these cultures. When Flora Danica or Kefirferment cultures were used, an increase in WHC values with time was observed only for the YBLP-fermented milk samples, demonstrating the significant positive effect of the additive on water-holding capacity. On the other hand, for milk samples fermented with the other starter cultures, changes over time generally followed a downward trend; however, the magnitude of these changes also depended significantly on the addition of YBLP. In summary, water retention capacity was influenced by both the addition of the formulation, and, to an even greater extent, the starter culture used. The studies conducted in this work demonstrate that the WHC for the samples fermented with the addition of the young barley preparation is higher than that of the control samples.
Water-holding capacity refers to the amount of water retained by a yogurt sample after the application of external force. The results obtained in this study are consistent with existing literature data on the interaction between curd and various texturizing components, such as plant oligosaccharides. However, it is important to note that there is a lack of scientific data available regarding the effect of young barley preparation on the water retention capacity of fermented milk. Nonetheless, the results obtained in this study can be related to the previous study by Mocanu et al. [27], which investigated the effect of the addition of S. platensis. Their findings demonstrated that the treated samples had higher water retention capacity compared with the control samples. The gel system formed in fermented milk, facilitated by the interaction between milk proteins (casein micelles especially), and created a three-dimensional network structure that exhibits good moisture retention capabilities. The results obtained in our study confirmed that YBLP could enhance the WHC of fermented milk by interacting with proteins and micelles. Barley is reportedly rich in soluble fibers, especially β-glucan, although their content significantly differs at different growth stages [43]. The literature data confirm the addition of various texturizing components, such as roasted barley powder [39], edible rose extract [44], green tea powder [45], argel leaf extract [46], pineapple peel powder [47], chia seed extract [48], date palm [49], Moringa [50], and apple pomace [51], among others, can increase the WHC of yogurts during cold storage. Similar phenomena were observed when EPS was added to the yogurts [52,53,54]. Thermal treatment of milk could also contribute to the denaturation of whey proteins, and its interaction with casein, resulting in an increase in WHC [55]. However, over time, the pores within the gel structure tend to become relatively loose, resulting in a decrease in WHC. In the present study, the greatest loss of WHC values over time was observed in samples fermented with mesophilic LAB compared with samples fermented with a mixture of mesophilic and thermophilic LAB, suggesting a different characteristic of the resulting protein curd depending on the physiological activity and biochemical properties of the LAB present in the starter culture.
3.6. Antimicrobial Activity
The YBLP solutions in DMSO or 70% ethanol did not exhibit any inhibitory effect towards mesophilic LAB from lyophilized starter cultures studied. There were no inhibition zones around each well.
Many researchers are studying the effects of various plant additives (including extracts and essential oils) on the growth or activity of LAB [34,56,57,58,59,60,61,62]. For example, Verluyten et al. [63] studied the effect of different spices (i.e., pepper, nutmeg, rosemary, garlic, and paprika) on the growth of L. curvatus LTH 1174 in fermented sausage. They found that only paprika stimulated the growth rate of L. curvatus, while the other additives had an inhibitory effect on the lactic acid bacteria tested. Our results show that the addition of young barley leaves powder preparation at the level of 0.5% does not negatively affect the activity of mesophilic LAB either in model systems (antibacterial activity determined by the agar diffusion method) or under the fermentation conditions of a real dairy product. The positive result can be attributed to several reasons. Some substances contained in young barley leaf powder may promote the growth of beneficial bacteria or may not affect their metabolism, resulting in no adverse effects. In addition, the powder of young barley leaves could contain nutrients and compounds beneficial to the mesophilic LAB. The added nutrients can potentially improve their activity and fermentation processes without hampering their growth. Another explanation is that the young barley leaves powder may not contain substances that inhibit the growth or activity of mesophilic LAB. In addition, the use of 0.5% young barley leaves powder preparation could be in a suitable range that does not overwhelm or disturb the LAB. It is important to note that these are hypotheses only and further research and analysis would be required to determine the exact mechanisms behind the lack of adverse effects.
4. Conclusions
The results of this study unequivocally highlight the need for further exploration of the use of the additive YBLP in fermented milk. In the future, consider studying the effect of YBLP addition on the organoleptic properties of fermented milk. By further studying the organoleptic properties, scientists, and food manufacturers can ensure that adding YBLP to fermented milk does not adversely affect its taste or texture. Understanding how YBLP affects the flavor and overall sensory experience of fermented milk is critical to consumer acceptance and marketability. Research and development in this area could lead to the formulation of YBLP-enriched fermented milk products with potential health benefits and improved consumer acceptance.
We have clearly shown that YBLP has no antibacterial effect on mesophilic lactic acid bacteria cells. The scarcity of reports on the effects of these additives on quality characteristics such as rheology, pH, or WHC suggests the importance of conducting such studies. The addition of YBLP did not affect the maximum acidification rate (Vmax) or the time to reach the maximum acidification rate (Tmax), but it did influence the time to reach the desired pH level (Te) for specific starter cultures. The impact of YBLP on the pH of fermented milk was limited, with the pH primarily dependent on the type of starter culture and storage time. During refrigerated storage, the pH value was more influenced by the starter culture rather than the addition of YBLP. The initial count of viable LAB cells was not affected by the addition of YBLP or the type of starter culture. However, a gradual decrease in the number of LAB cells was observed during refrigerated storage of the fermented milk. YBLP supplementation may have a positive effect on the survival of bacterial cells during storage. Regarding the hardness of fermented milk samples, the addition of YBLP had a significant effect immediately after fermentation for certain samples fermented by starter cultures such as Flora Danica, XPL-1, and CHOOZIT MTM 2. However, for other fermented samples, the addition of YBLP had no impact on hardness values. Changes in hardness were observed during storage, and the trend of these changes depended on the addition of YBLP, storage time, and the type of starter culture. The addition of YBLP also influenced the initial WHC of fermented milk samples, specifically for certain starter cultures. The WHC of fermented milk samples changed during storage, with the variations influenced by both the starter culture and the addition of YBLP. Overall, the research conducted in this study demonstrated that the addition of YBLP resulted in higher WHC compared with the control samples.
It is important to note that in addition to the beneficial effects of LAB, the YBLP formula itself contributes essential nutrients for the human body. It is rich in vitamins, minerals, and essential amino acids, supporting the immune system and showing proven anti-cancer effects [64]. The effectiveness of any substance, including YBLP, depends on various factors, such as the intended effect, the target group, the method of administration, and the desired result. It is worth investigating in the future whether a portion of 0.5% YBLP can have a sufficient health-promoting effect. In this study, it was only shown that 0.5% of YBLP failed to inhibit LAB activity. As for the effectiveness of 0.5% of YBLP, it is difficult to determine its effect without the specific context of the study and its objectives.
Author Contributions
Conceptualization, M.K., M.Z., I.Ś. and D.Z.; methodology, M.K., M.Z., I.Ś. and D.Z.; investigation, M.K., M.Z. and I.Ś.; data curation, M.K., M.Z., I.Ś. and D.Z.; writing—M.K., M.Z., I.Ś. and D.Z.; writing—review and editing, M.K. and M.Z.; project administration, M.K. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Polish Ministry of Science and Higher Education with funds from the Warsaw University of Life Sciences WULS–SGGW (Poland).
Institutional Review Board Statement
This study did not involve humans or animals.
Informed Consent Statement
This study did not involve humans.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors (M.K. and M.Z.) upon reasonable request.
Acknowledgments
The authors gratefully acknowledge the Institute of Food Sciences of Warsaw. University of Life Sciences WULS–SGGW for supporting and providing necessary infrastructure and research stuff).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yamaura, K.; Nakayama, N.; Shimada, M.; Bi, Y.; Fukata, H.; Ueno, K. Antidepressant-like Effects of Young Green Barley Leaf (Hordeum vulgare L.) in the Mouse Forced Swimming Test. Pharmacogn. Res. 2012, 4, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.A.; McClure, J.W. The Occurrence and Photoregulation of Flavonoids in Barley Plastids. Phytochemistry 1976, 15, 805–807. [Google Scholar] [CrossRef]
- Choe, J.-H.; Jang, A.; Choi, J.-H.; Choi, Y.-S.; Han, D.-J.; Kim, H.-Y.; Lee, M.-A.; Kim, H.-W.; Kim, C.-J. Antioxidant Activities of Lotus Leaves (Nelumbo nucifera) and Barley Leaves (Hordeum vulgare) Extracts. Food Sci. Biotechnol. 2010, 19, 831–836. [Google Scholar] [CrossRef]
- Choe, J.-H.; Jang, A.; Lee, E.-S.; Choi, J.-H.; Choi, Y.-S.; Han, D.-J.; Kim, H.-Y.; Lee, M.-A.; Shim, S.-Y.; Kim, C.-J. Oxidative and Color Stability of Cooked Ground Pork Containing Lotus Leaf (Nelumbo nucifera) and Barley Leaf (Hordeum vulgare) Powder during Refrigerated Storage. Meat Sci. 2011, 87, 12–18. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Antioxidative and Antiproliferative Properties of Selected Barley (Hordeum vulgarae L.) Cultivars and Their Potential for Inhibition of Low-Density Lipoprotein (LDL) Cholesterol Oxidation. J. Agric. Food Chem. 2007, 55, 5018–5024. [Google Scholar] [CrossRef]
- Osawa, T.; Katsuzaki, H.; Hagiwara, Y.; Hagiwara, H.; Shibamoto, T. A Novel Antioxidant Isolated from Young Green Barley Leaves. J. Agric. Food Chem. 1992, 40, 1135–1138. [Google Scholar] [CrossRef]
- Benedet, J.A.; Umeda, H.; Shibamoto, T. Antioxidant Activity of Flavonoids Isolated from Young Green Barley Leaves toward Biological Lipid Samples. J. Agric. Food Chem. 2007, 55, 5499–5504. [Google Scholar] [CrossRef]
- Ohkawa, M.; Kinjo, J.; Hagiwara, Y.; Hagiwara, H.; Ueyama, H.; Nakamura, K.; Ishikawa, R.; Ono, M.; Nohara, T. Three New Anti-Oxidative Saponarin Analogs from Young Green Barley Leaves. Chem. Pharm. Bull. 1998, 46, 1887–1890. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Tsubata, M.; Takano, A.; Kamiya, T.; Takagaki, K.; Ito, H.; Sugawa-Katayama, Y.; Tsuji, H. Effects of Young Barley Leaf Powder on Gastrointestinal Functions in Rats and Its Efficacy-Related Physicochemical Properties. Evid. Based Complement. Alternat. Med. 2014, 2014, e974840. [Google Scholar] [CrossRef]
- Škrbić, B.; Milovac, S.; Dodig, D.; Filipčev, B. Effects of Hull-Less Barley Flour and Flakes on Bread Nutritional Composition and Sensory Properties. Food Chem. 2009, 115, 982–988. [Google Scholar] [CrossRef]
- Kim, D.-C.; In, M.-J.; Chae, H.-J. Preparation of Mulberry Leaves Tea and Its Quality Characteristics. J. Appl. Biol. Chem. 2010, 53, 56–59. [Google Scholar] [CrossRef]
- Yu, Y.-M.; Wu, C.-H.; Tseng, Y.-H.; Tsai, C.E.; Chang, W.-C. Antioxidative and Hypolipidemic Effects of Barley Leaf Essence in a Rabbit Model of Atherosclerosis. Jpn. J. Pharmacol. 2002, 89, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-M.; Chang, W.-C.; Chang, C.-T.; Hsieh, C.-L.; Tsai, C.E. Effects of Young Barley Leaf Extract and Antioxidative Vitamins on LDL Oxidation and Free Radical Scavenging Activities in Type 2 Diabetes. Diabetes Metab. 2002, 28, 107–114. [Google Scholar] [PubMed]
- Han, H.-S.; Shin, J.-S.; Song, Y.-R.; Rhee, Y.K.; Cho, C.-W.; Ryu, J.H.; Inn, K.-S.; Hong, H.-D.; Lee, K.-T. Immunostimulatory Effects of Polysaccharides Isolated from Young Barley Leaves (Hordeum vulgare L.) with Dual Activation of Th1 and Th2 in Splenic T Cells and Cyclophosphamide-Induced Immunosuppressed Mice. Int. J. Biol. Macromol. 2020, 147, 954–964. [Google Scholar] [CrossRef]
- Fadaei, V.; Mohamadi-Alasti, F.; Khosravi-Darani, K. Influence of Spirulina Platensis Powder on the Starter Culture Viabilityin Probiotic Yoghurt Containing Spinach during Cold Storage. Eur. J. Exp. Biol. 2013, 3, 389–393. [Google Scholar]
- Yangmei, Q.; Jiang, W.; Hou, H.; Zhang, H. Application of Plant Green Juice Powder in Maintaining Stability of Viable Count of Yoghourt in Shelf Life. Patent No. CN 102106386 B, 22 March 1991. [Google Scholar]
- Ziarno, M.; Zaręba, D. The Effect of the Addition of Microbial Transglutaminase before the Fermentation Process on the Quality Characteristics of Three Types of Yogurt. Food Sci. Biotechnol. 2020, 29, 109–119. [Google Scholar] [CrossRef]
- Bezerra, M.F.; Souza, D.F.S.; Correia, R.T.P. Acidification Kinetics, Physicochemical Properties and Sensory Attributes of Yoghurts Prepared from Mixtures of Goat and Buffalo Milks. Int. J. Dairy Technol. 2012, 65, 437–443. [Google Scholar] [CrossRef]
- Varghese, K.S.; Mishra, H.N. Modelling of Acidification Kinetics and Textural Properties in Dahi (Indian Yogurt) Made from Buffalo Milk Using Response Surface Methodology. Int. J. Dairy Technol. 2008, 61, 284–289. [Google Scholar] [CrossRef]
- ISO 6887-5:2020; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 5: Specific Rules for the Preparation of Milk and Milk Products. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony–Count Technique at 30 Degrees. International Organization for Standardization: Geneva, Switzerland, 1998.
- Bonev, B.; Hooper, J.; Parisot, J. Principles of Assessing Bacterial Susceptibility to Antibiotics Using the Agar Diffusion Method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef]
- Oh, N.S.; Lee, J.Y.; Joung, J.Y.; Kim, K.S.; Shin, Y.K.; Lee, K.-W.; Kim, S.H.; Oh, S.; Kim, Y. Microbiological Characterization and Functionality of Set-Type Yogurt Fermented with Potential Prebiotic Substrates Cudrania Tricuspidata and Morus alba L. Leaf Extracts. J. Dairy Sci. 2016, 99, 6014–6025. [Google Scholar] [CrossRef]
- Amirdivani, S.; Baba, A.S. Changes in Yogurt Fermentation Characteristics, and Antioxidant Potential and in Vitro Inhibition of Angiotensin-1 Converting Enzyme upon the Inclusion of Peppermint, Dill and Basil. LWT—Food Sci. Technol. 2011, 44, 1458–1464. [Google Scholar] [CrossRef]
- Oliveira, R.P.S.; Florence, A.C.R.; Silva, R.C.; Perego, P.; Converti, A.; Gioielli, L.A.; Oliveira, M.N. Effect of Different Prebiotics on the Fermentation Kinetics, Probiotic Survival and Fatty Acids Profiles in Nonfat Symbiotic Fermented Milk. Int. J. Food Microbiol. 2009, 128, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Jaya, S.; Das, H. Effect of Maltodextrin, Glycerol Monostearate and Tricalcium Phosphate on Vacuum Dried Mango Powder Properties. J. Food Eng. 2004, 63, 125–134. [Google Scholar] [CrossRef]
- Mocanu, G.; Botez, E.; Nistor, O.; Georgeta, D.; Andronoiu, D.G.; Vlăsceanu, G. Influence of Spirulina Platensis Biomass over Some Starter Culture of Lactic Bacteria. J. Agroalim. Proc. Technol. 2013, 19, 474–479. [Google Scholar]
- Ziarno, M.; Zaręba, D.; Dryzek, W.; Hassaliu, R.; Florowski, T. Effect of the Addition of Soy Beverage and Propionic Bacteria on Selected Quality Characteristics of Cow’s Milk Yoghurt Products. Appl. Sci. 2022, 12, 12603. [Google Scholar] [CrossRef]
- Ścibisz, I.; Ziarno, M.; Mitek, M. Color Stability of Fruit Yogurt during Storage. J. Food Sci. Technol. 2019, 56, 1997–2009. [Google Scholar] [CrossRef]
- Derewiaka, D.; Stepnowska, N.; Bryś, J.; Ziarno, M.; Ciecierska, M.; Kowalska, J. Chia Seed Oil as an Additive to Yogurt. Grasas Aceites 2019, 70, e302. [Google Scholar] [CrossRef]
- Ásványi-Molnár, N.; Sipos-Kozma, Z.; Tóth, Á.; Ásványi, B.; Varga, L. Development of functional dairy food enriched in spirulina (Arthrospira platensis). Tejgazdaság 2009, 69, 15–22. [Google Scholar]
- Molnár, N.; Gyenis, B.; Varga, L. Influence of a Powdered Spirulina Platensis Biomass on Acid Production of Lactococci in Milk. Milchwissenschaft 2005, 60, 380–382. [Google Scholar]
- Cichońska, P.; Pudło, E.; Wojtczak, A.; Ziarno, M. Effect of the Addition of Whole and Milled Flaxseed on the Quality Characteristics of Yogurt. Foods 2021, 10, 2140. [Google Scholar] [CrossRef]
- Ziarno, M.; Kozłowska, M.; Ścibisz, I.; Kowalczyk, M.; Pawelec, S.; Stochmal, A.; Szleszyński, B. The Effect of Selected Herbal Extracts on Lactic Acid Bacteria Activity. Appl. Sci. 2021, 11, 3898. [Google Scholar] [CrossRef]
- Varga, L.; Szigeti, J.; Kovács, R.; Földes, T.; Buti, S. Influence of a Spirulina Platensis Biomass on the Microflora of Fermented ABT Milks During Storage (R1). J. Dairy Sci. 2002, 85, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Darani, K.K. Effects of Chlorella Vulgaris and Arthrospira Platensis Addition on Viability of Probiotic Bacteria in Yogurt and Its Biochemical Properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- Zielke, H.; Kneifel, H.; Webb, L.E.; Soeder, C.J. Stimulation of Lactobacilli by an Aqueous Extract of the Green Alga Scenedesmus Acutus 276-3a. Eur. J. Appl. Microbiol. Biotechnol. 1978, 6, 79–86. [Google Scholar] [CrossRef]
- Webb, L.E. Detection by Warburg Manometry of Compounds Stimulatory to Lactic Acid Bacteria. J. Dairy Res. 1982, 49, 479–486. [Google Scholar] [CrossRef]
- Abdeldaiem, A.M.; Ali, A.H.; Shah, N.; Ayyash, M.; Mousa, A.H. Physicochemical Analysis, Rheological Properties, and Sensory Evaluation of Yogurt Drink Supplemented with Roasted Barley Powder. LWT 2023, 173, 114319. [Google Scholar] [CrossRef]
- Kaur, R.; Riar, C.S. Sensory, Rheological and Chemical Characteristics during Storage of Set Type Full Fat Yoghurt Fortified with Barley β-Glucan. J. Food Sci. Technol. 2020, 57, 41–51. [Google Scholar] [CrossRef]
- Havrlentová, M.; Petruláková, Z.; Burgárová, A.; Gago, F.; Hlinková, A.; Šturdík, E. β-Glucans and Their Significance for the Preparation of Functional Foods—A Review. Czech J. Food Sci. 2011, 29, 1–14. [Google Scholar] [CrossRef]
- Qu, X.; Nazarenko, Y.; Yang, W.; Nie, Y.; Zhang, Y.; Li, B. Effect of Oat β-Glucan on the Rheological Characteristics and Microstructure of Set-Type Yogurt. Molecules 2021, 26, 4752. [Google Scholar] [CrossRef]
- Yan, J.-K.; Chen, T.-T.; Wang, Z.-W.; Wang, C.; Liu, C.; Li, L. Comparison of Physicochemical Characteristics and Biological Activities of Polysaccharides from Barley (Hordeum vulgare L.) Grass at Different Growth Stages. Food Chem. 2022, 389, 133083. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Mujumdar, A.S.; Chang, L. Effect of Edible Rose (Rosa Rugosa Cv. Plena) Flower Extract Addition on the Physicochemical, Rheological, Functional and Sensory Properties of Set-Type Yogurt. Food Biosci. 2021, 43, 101249. [Google Scholar] [CrossRef]
- Dönmez, Ö.; Mogol, B.A.; Gökmen, V. Syneresis and Rheological Behaviors of Set Yogurt Containing Green Tea and Green Coffee Powders. J. Dairy Sci. 2017, 100, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Ahmed, I.A.; Alqah, H.A.S.; Saleh, A.; Al-Juhaimi, F.Y.; Babiker, E.E.; Ghafoor, K.; Hassan, A.B.; Osman, M.A.; Fickak, A. Physicochemical Quality Attributes and Antioxidant Properties of Set-Type Yogurt Fortified with Argel (Solenostemma argel Hayne) Leaf Extract. LWT 2021, 137, 110389. [Google Scholar] [CrossRef]
- Ardabilchi Marand, M.; Amjadi, S.; Ardabilchi Marand, M.; Roufegarinejad, L.; Jafari, S.M. Fortification of Yogurt with Flaxseed Powder and Evaluation of Its Fatty Acid Profile, Physicochemical, Antioxidant, and Sensory Properties. Powder Technol. 2020, 359, 76–84. [Google Scholar] [CrossRef]
- Kwon, H.C.; Bae, H.; Seo, H.G.; Han, S.G. Short Communication: Chia Seed Extract Enhances Physiochemical and Antioxidant Properties of Yogurt. J. Dairy Sci. 2019, 102, 4870–4876. [Google Scholar] [CrossRef]
- Almusallam, I.A.; Mohamed Ahmed, I.A.; Babiker, E.E.; Al-Juhaimi, F.Y.; Saleh, A.; Qasem, A.A.; Al Maiman, S.; Osman, M.A.; Ghafoor, K.; Hajji, H.A.; et al. Effect of Date Palm (Phoenix dactylifera L.) Spikelets Extract on the Physicochemical and Microbial Properties of Set-Type Yogurt during Cold Storage. LWT 2021, 148, 111762. [Google Scholar] [CrossRef]
- Zhang, T.; Jeong, C.H.; Cheng, W.N.; Bae, H.; Seo, H.G.; Petriello, M.C.; Han, S.G. Moringa Extract Enhances the Fermentative, Textural, and Bioactive Properties of Yogurt. LWT 2019, 101, 276–284. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. Adding Apple Pomace as a Functional Ingredient in Stirred-Type Yogurt and Yogurt Drinks. Food Hydrocoll. 2020, 100, 105453. [Google Scholar] [CrossRef]
- Duboc, P.; Mollet, B. Applications of Exopolysaccharides in the Dairy Industry. Int. Dairy J. 2001, 11, 759–768. [Google Scholar] [CrossRef]
- Hassan, A.N. ADSA Foundation Scholar Award: Possibilities and Challenges of Exopolysaccharide-Producing Lactic Cultures in Dairy Foods. J. Dairy Sci. 2008, 91, 1282–1298. [Google Scholar] [CrossRef]
- Prasanna, P.H.P.; Grandison, A.S.; Charalampopoulos, D. Screening Human Intestinal Bifidobacterium Strains for Growth, Acidification, EPS Production and Viscosity Potential in Low-Fat Milk. Int. Dairy J. 2012, 23, 36–44. [Google Scholar] [CrossRef]
- Corrieu, G.; Béal, C. Yogurt: The Product and Its Manufacture; Academic Press: Cambridge, MA, USA, 2016; Volume 5, p. 617. [Google Scholar]
- Bakirci, I. The Effects of Some Herbs on the Activities of Thermophilic Dairy Cultures. Food Nahr. 1999, 43, 333–335. [Google Scholar] [CrossRef]
- Kivanç, M.; Akgül, A.; Doǧan, A. Inhibitory and Stimulatory Effects of Cumin, Oregano and Their Essential Oils on Growth and Acid Production of Lactobacillus Plantarum and Leuconostoc Mesenteroides. Int. J. Food Microbiol. 1991, 13, 81–85. [Google Scholar] [CrossRef]
- Dunn, L.L.; Davidson, P.M.; Critzer, F.J. Antimicrobial Efficacy of an Array of Essential Oils Against Lactic Acid Bacteria. J. Food Sci. 2016, 81, M438–M444. [Google Scholar] [CrossRef]
- Zaika, L.L.; Kissinger, J.C.; Wasserman, A.E. Inhibition of Lactic Acid Bacteria by Herbs. J. Food Sci. 1983, 48, 1455–1459. [Google Scholar] [CrossRef]
- Kozłowska, M.; Ścibisz, I.; Zaręba, D.; Ziarno, M. Antioxidant Properties and Effect on Lactic Acid Bacterial Growth of Spice Extracts. CyTA—J. Food 2015, 13, 573–577. [Google Scholar] [CrossRef]
- Houle, J.-F.; Lafrance, M.; Julien, J.-P.; Brochu, E.; Champagne, C.P. Selection of Mixed Cultures for Meat Fermentation. J. Food Sci. 1989, 54, 839–842. [Google Scholar] [CrossRef]
- Lachowicz; Jones; Briggs; Bienvenu; Wan; Wilcock; Coventry. The Synergistic Preservative Effects of the Essential Oils of Sweet Basil (Ocimum basilicum L.) against Acid-tolerant Food Microflora. Lett. Appl. Microbiol. 1998, 26, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Verluyten, J.; Leroy, F.; de Vuyst, L. Effects of Different Spices Used in Production of Fermented Sausages on Growth of and Curvacin A Production by Lactobacillus Curvatus LTH 1174. Appl. Environ. Microbiol. 2004, 70, 4807–4813. [Google Scholar] [CrossRef]
- Robles-Escajeda, E.; Lerma, D.; Nyakeriga, A.M.; Ross, J.A.; Kirken, R.A.; Aguilera, R.J.; Varela-Ramirez, A. Searching in Mother Nature for Anti-Cancer Activity: Anti-Proliferative and Pro-Apoptotic Effect Elicited by Green Barley on Leukemia/Lymphoma Cells. PLoS ONE 2013, 8, e73508. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).