Immunomodulatory Effect of Benincasa hispida Extract Fermented by Bacillus subtilis CJH 101 on RAW 264.7 Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Cell Culture and Cell Viability
2.4. Measurements of Nitrite (NO) Production
2.5. Measurement of Cytokines Secretion Analysis
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Effect of HR1901-BS on RAW 264.7 Cell Viability
3.2. Effect of HR1901-BS by Fermentation Time on NO Production in RAW 264.7 Cells
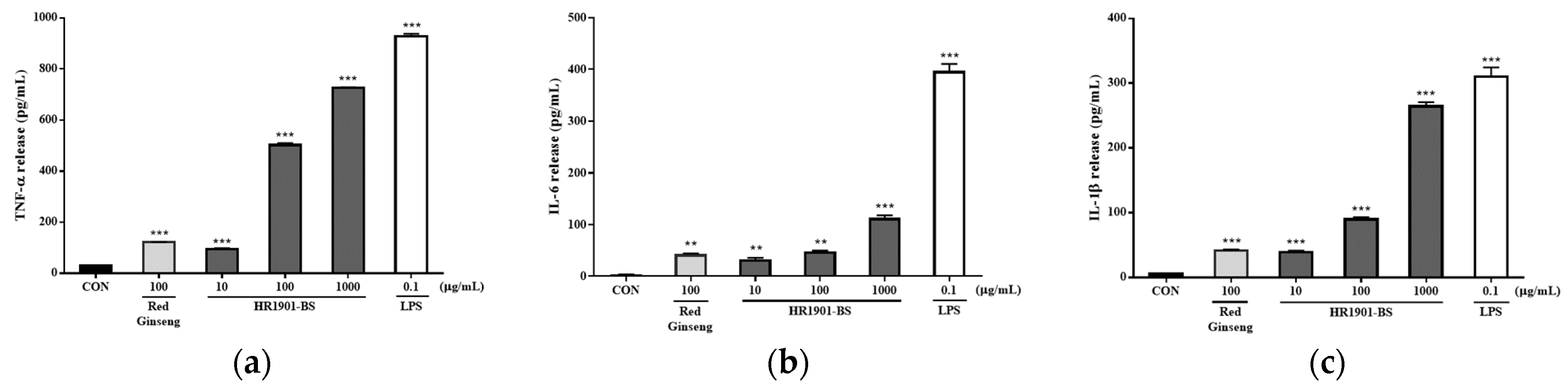
3.3. Effect of HR1901-BS on Cytokine Secretion in RAW 264.7 Cells
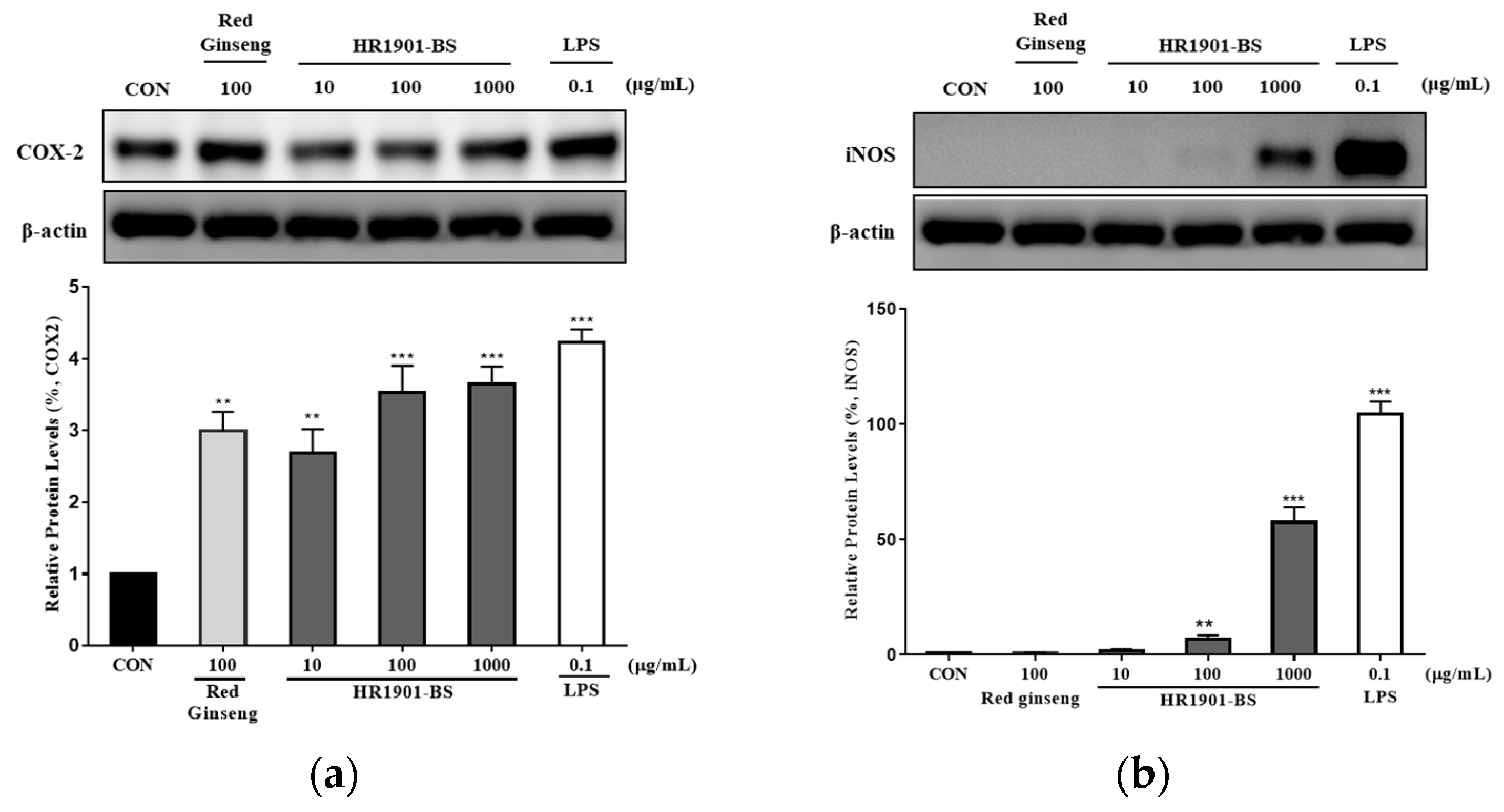
3.4. Effect of HR1901-BS on COX-2 and iNOS Protein Expression in RAW 264.7 Cells
3.5. Effect of HR1901-BS on the MAPK and NF-κB Pathways in RAW 264.7 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Li, H. The impact of aging and COVID-19 on our immune system: A high-resolution map from single cell analysis. Protein Cell 2020, 11, 703–706. [Google Scholar] [CrossRef]
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators inspired by nature: A review on curcumin and echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Schaafsma, G.J. Probiotics. Int. J. Food Microbiol. 1998, 39, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeir, J.; de Vrese, M. Probiotics, prebiotics, and synbiotics—Approaching a definition. Am. J. Clin. Nutr. 2001, 73, 361s–364s. [Google Scholar] [CrossRef]
- Arshad, M.S.; Khan, U.; Sadiq, A.; Khalid, W.; Hussain, M.; Yasmeen, A.; Asghar, Z.; Rehana, H. Coronavirus disease (COVID-19) and immunity booster green foods: A mini review. Food Sci. Nutr. 2020, 8, 3971–3976. [Google Scholar] [CrossRef]
- Farzana, M.; Shahriar, S.; Jeba, F.R.; Tabassum, T.; Araf, Y.; Ullah, A.; Tasnim, J.; Chakraborty, A.; Naima, T.A.; Marma, K.K.S.; et al. Functional food: Complementary to fight against COVID-19. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 33. [Google Scholar] [CrossRef]
- Howell, M.; Shepherd, M. The immune system. Anaesth. Intensive Care Med. 2021, 22, 518–521. [Google Scholar] [CrossRef]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.U. Regulation of the innate immune system by autophagy: Neutrophils, eosinophils, mast cells, NK cells. Cell Death Differ. 2019, 26, 703–714. [Google Scholar] [CrossRef]
- Esche, C.; Stellato, C.; Beck, L.A. Chemokines: Key players in innate and adaptive immunity. J. Investig. Dermatol. 2005, 125, 615–628. [Google Scholar] [CrossRef]
- Cutolo, M. Macrophages as Effectors of the Immunoendocrinologic Interactions in Autoimmune Rheumatic Diseasesa. Ann. N. Y. Acad. Sci. 1999, 876, 32–42. [Google Scholar] [CrossRef]
- Lesourd, B. Nutritional factors and immunological ageing. Proc. Nutr. Soc. 2006, 65, 319–325. [Google Scholar] [CrossRef]
- Mohammad, N.A.; Anwar, F.; Mehmood, T.; Hamid, A.A.; Muhammad, K.; Saari, N. Phenolic compounds, tocochromanols profile and antioxidant properties of winter melon [Benincasa hispida (Thunb.) Cogn.] seed oils. J. Food Meas. Charact. 2019, 13, 940–948. [Google Scholar] [CrossRef]
- Zaini, N.A.M.; Anwar, F.; Hamid, A.A.; Saari, N. Kundur [Benincasa hispida (Thunb.) Cogn.]: A potential source for valuable nutrients and functional foods. Food Res. Int. 2011, 44, 2368–2376. [Google Scholar] [CrossRef]
- Lim, S.J.; Jeong, J.G.; Kim, M.W.; Choi, S.S.; Han, H.K.; Park, J.E. Effects of Benincasa hispida intake on blood glucose and lipid level in streptozotocin induced diabetic rats. Korean J. Nutr. 2003, 36, 335–343. [Google Scholar]
- Du, Q.; Zhang, Q.; Ito, Y. Isolation and identification of phenolic compounds in the fruit of Benincasa hispida by HSCCC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 137–144. [Google Scholar] [CrossRef]
- Kim, M.W. Effects of Benincasa hispida seed supplementation on glycogen status and lipid peroxidation in streptozotocin-induced diabetic rats. Korean J. Nutr. 2004, 37, 865–871. [Google Scholar]
- Grover, J.K.; Adiga, G.; Vats, V.; Rathi, S.S. Extracts of Benincasa hispida prevent development of experimental ulcers. J. Ethnopharmacol. 2001, 78, 159–164. [Google Scholar] [CrossRef]
- Grover, J.K.; Rathi, S.S. Benincasa hispida: An anti-inflammatory agent with cytoprotective activity (abs). Can. J. Physiol. Pharmaol. 1994, 72, 269. [Google Scholar]
- Patil, R.N.; Patil, R.Y.; Ahirwar, B.; Ahirwar, D. Evaluation of antidiabetic and related actions of some Indian medicinal plants in diabetic rats. Asian Pac. J. Trop. Med. 2011, 4, 20–23. [Google Scholar] [CrossRef]
- Roy, C.; Guha, D. Role of Benincasa hispida linn. on brain electrical activity in colchicine induced experimental rat model of alzheimer’s disease: Possible involvements of antioxidants. World J. Pharm. Res 2017, 6, 1439–1443. [Google Scholar]
- Choi, Y.E.; Yang, J.M.; Cho, J.H. Benincasa hispida extract promotes proliferation, differentiation, and mineralization of MC3T3-E1 preosteoblasts and inhibits the differentiation of RAW 246.7 osteoclast precursors. Appl. Sci. 2022, 12, 8849. [Google Scholar] [CrossRef]
- Choi, S.I.; Han, X.; Men, X.; Lee, S.J.; Oh, G.; Choi, Y.E.; Yang, J.M.; Cho, J.H.; Lee, O.H. Benincasa hispida Extract Prevents Ovariectomy-Induced Osteoporosis in Female ICR Mice. Appl. Sci. 2023, 13, 832. [Google Scholar] [CrossRef]
- Thapa, N.; Tamang, J.P. Functionality and therapeutic values of fermented foods. Health Benefits Fermented Foods 2015, 111, 168. [Google Scholar]
- Martinez-Avila, G.C.G.; Aguilera, A.F.; Saucedo, S.; Rojas, R.; Rodriguez, R.; Aguilar, C.N. Fruit wastes fermentation for phenolic antioxidants production and their application in manufacture of edible coatings and films. Crit. Rev. Food Sci. Nutr. 2014, 54, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef]
- Skowron, K.; Budzyńska, A.; Grudlewska-Buda, K.; Wiktorczyk-Kapischke, N.; Andrzejewska, M.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Two Faces of Fermented Foods—The Benefits and Threats of Its Consumption. Front. Microbiol. 2022, 13, 845166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, H.; Li, Z. Study on resistance to stress factors of Bacillus subtilis from swine intestinal juice. J. Anhui Agric. Univ. 2013, 40, 519–522. [Google Scholar]
- Sanders, M.E.; Morelli, L.; Tompkins, T.A. Sporeformers as human probiotics: Bacillus, Sporolactobacillus, and Brevibacillus. Compr. Rev. Food Sci. Food Saf. 2003, 2, 101–110. [Google Scholar] [CrossRef]
- Iebba, V.; Nicoletti, M.; Schippa, S. Gut microbiota and the immune system: An intimate partnership in health and disease. Int. J. Immunopathol. Pharmacol. 2012, 25, 823–833. [Google Scholar] [CrossRef] [PubMed]
- García-Albiach, R.; José, M.; de Felipe, P.; Angulo, S.; Morosini, M.-I.; Bravo, D.; Baquero, F.; del Campo, R. Molecular analysis of yogurt containing Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in human intestinal microbiota. Am. J. Clin. Nutr. 2008, 87, 91–96. [Google Scholar] [CrossRef]
- Guo, M.; Li, M.; Zhang, C.; Zhang, X.; Wu, Y. Dietary administration of the Bacillus subtilis enhances immune responses and disease resistance in chickens. Front. Microbiol. 2020, 11, 1768. [Google Scholar] [CrossRef]
- Hosoi, T.; Hirose, R.; Saegusa, S.; Ametani, A.; Kiuchi, K.; Kaminogawa, S. Cytokine responses of human intestinal epithelial-like Caco-2 cells to the nonpathogenic bacterium Bacillus subtilis (natto). Int. J. Food Microbiol. 2003, 82, 255–264. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell viability assays. Assay Guid. Man. 2016, 1, 1–25. [Google Scholar]
- Csonka, C.; Páli, T.; Bencsik, P.; Görbe, A.; Ferdinandy, P.; Csont, T. Measurement of NO in biological samples. Br. J. Pharmacol. 2015, 172, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jung, K.S.; Jeong, H.G. Suppressive effects of the kahweol and cafestol on cyclooxygenase-2 expression in macrophages. FEBS Lett. 2004, 569, 321–326. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X.; Jin, W.; Guo, Y. Immunomodulatory effects of a low-molecular weight polysaccharide from Enteromorpha prolifera on RAW 264.7 macrophages and cyclophosphamide-induced immunosuppression mouse models. Mar. Drugs 2020, 18, 340. [Google Scholar] [CrossRef]
- Murray, R.Z.; Stow, J.L. Cytokine secretion in macrophages: SNAREs, Rabs, and membrane trafficking. Front. Immunol. 2014, 5, 538. [Google Scholar] [CrossRef]
- Medeiros, A.; Peres-Buzalaf, C.; Verdan, F.F.; Serezani, C.H. Prostaglandin E2 and the suppression of phagocyte innate immune responses in different organs. Mediat. Inflamm. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Rincón, M.; Flavell, R.A.; Davis, R.A. The Jnk and P38 MAP kinase signaling pathways in T cell–mediated immune responses. Free Radic. Biol. Med. 2000, 28, 1328–1337. [Google Scholar] [CrossRef]
- Dong, C.; Davis, R.J.; Flavell, R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 2002, 20, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.J.; Tran, T.H.M.; Park, H.R.; Xu, X.Y.; Subramaniyam, S.; Choi, H.S.; Kim, J.; Koh, S.C.; Kim, Y.J. Immune-enhancing effects of postbiotic produced by Bacillus velezensis Kh2-2 isolated from Korea Foods. Food Res. Int. 2022, 152, 110911. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Ku, J.M.; Kim, H.I.; Ahn, C.W.; Park, S.H.; Seo, H.S.; Shin, Y.C.; Ko, S.G. The immune-enhancing activity of Cervus nippon mantchuricus extract (NGE) in RAW264. 7 macrophage cells and immunosuppressed mice. Food Res. Int. 2017, 99, 623–629. [Google Scholar] [CrossRef]
- Trinh, T.A.; Park, J.; Oh, J.H.; Park, J.S.; Lee, D.; Kim, C.E.; Choi, H.-S.; Kim, S.-B.; Hwang, G.S.; Koo, B.A.; et al. Effect of herbal formulation on immune response enhancement in RAW 264.7 macrophages. Biomolecules 2020, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, J. Modulation of Toll-like receptor signaling in innate immunity by natural products. Int. Immunopharmacol. 2016, 37, 65–70. [Google Scholar] [CrossRef]
- Schultze, J.L.; Schmidt, S.V. Molecular features of macrophage activation. In Semin. Immunol. 2015, 27, 416–423. [Google Scholar] [CrossRef]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative foods: Microbiology, biochemistry, potential human health benefits and public health issues. Foods 2020, 10, 69. [Google Scholar] [CrossRef]
- Tian, W.; Dai, L.; Lu, S.; Luo, Z.; Qiu, Z.; Li, J.; Li, P.; Du, B. Effect of Bacillus sp. DU-106 fermentation on Dendrobium officinale polysaccharide: Structure and immunoregulatory activities. Int. J. Biol. Macromol. 2019, 135, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Pidwill, G.R.; Gibson, J.F.; Cole, J.; Renshaw, S.A.; Foster, S.J. The role of macrophages in Staphylococcus aureus infection. Front. Immunol. 2021, 11, 3506. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Lee, S.H.; Cho, M.L.; Oh, H.J.; Woo, Y.J.; Kim, S.H.; Kim, H.R. IL-17 and Toll-like Receptor 2 or Toll-like Receptor 4 Combined Engagement Upregulates RANKL and IL-6 in Human Rheumatoid Synovial Fibroblasts. J. Korean Rheum. Assoc. 2010, 17, 36–45. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C.A., Jr. Decoding the patterns of self and nonself by the innate immune system. Science 2002, 296, 298–300. [Google Scholar] [CrossRef]
- Horwood, N.J.; Page, T.H.; McDaid, J.P.; Palmer, C.D.; Campbell, J.; Mahon, T.; Brennan, F.M.; Webster, D.; Foxwell, B.M. Bruton’s tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J. Immunol. 2006, 176, 3635–3641. [Google Scholar] [CrossRef]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef]
- Palmieri, E.M.; McGinity, C.; Wink, D.A.; McVicar, D.W. Nitric oxide in macrophage immunometabolism: Hiding in plain sight. Metabolites 2020, 10, 429. [Google Scholar] [CrossRef]
- Booth, J.S.; Nichani, A.K.; Benjamin, P.; Dar, A.; Krieg, A.M.; Babiuk, L.A.; Mutwiri, G.K. Innate immune responses induced by classes of CpG oligodeoxynucleotides in ovine lymph node and blood mononuclear cells. Vet. Immunol. Immunopathol. 2007, 115, 24–34. [Google Scholar] [CrossRef]
- Oyler-Yaniv, J.; Oyler-Yaniv, A.; Maltz, E.; Wollman, R. TNF controls a speed-accuracy tradeoff in the cell death decision to restrict viral spread. Nat. Commun. 2021, 12, 2992. [Google Scholar] [CrossRef]
- Rahman, M.M.; McFadden, G. Modulation of tumor necrosis factor by microbial pathogens. PLoS Pathog. 2006, 2, e4. [Google Scholar] [CrossRef] [PubMed]
- Dienz, O.; Eaton, S.M.; Bond, J.P.; Neveu, W.; Moquin, D.; Noubade, R.; Briso, E.M.; Charland, C.; Leonard, W.J.; Ciliberto, G.; et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 2009, 206, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Anti-inflammatory agents: Present and future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Chang, S.H.; Martinez, G.J.; Yang, X.O.; Nurieva, R.; Kang, H.S.; Ma, L.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 2009, 30, 576–587. [Google Scholar] [CrossRef]
- Kulesza, A.; Paczek, L.; Burdzinska, A. The role of COX-2 and PGE2 in the regulation of immunomodulation and other functions of mesenchymal stromal cells. Biomedicines 2023, 11, 445. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on immune cells and its role in diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Shao, Y.; Liu, J.; Li, J.; Xing, M. Copper or/and arsenic induce oxidative stress-cascaded, nuclear factor kappa B-dependent inflammation and immune imbalance, trigging heat shock response in the kidney of chicken. Oncotarget 2017, 8, 98103. [Google Scholar] [CrossRef][Green Version]
- DiDonato, J.A.; Hayakawa, M.; Rothwarf, D.M.; Zandi, E.; Karin, M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 1997, 388, 548–554. [Google Scholar] [CrossRef]
- Park, J.; Min, J.S.; Kim, B.; Chae, U.B.; Yun, J.W.; Choi, M.S.; Kong, I.K.; Chang, K.T.; Lee, D.S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Chardin, C.; Schenk, S.T.; Hirt, H.; Colcombet, J.; Krapp, A. Mitogen-Activated protein kinases in nutritional signaling in Arabidopsis. Plant Sci. 2017, 260, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.R.; Shen, Q.; Fan, F. TAK1-mediated induction of nitric oxide synthase gene expression in glial cells. J. Neurochem. 2003, 87, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Jana, M.; Dasgupta, S.; Saha, R.N.; Liu, X.; Pahan, K. Induction of tumor necrosis factor-α (TNF-α) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J. Neurochem. 2003, 86, 519–528. [Google Scholar] [CrossRef]
- Lin, A.; Karin, M. NF-kB in cancer: A marked target. Semin. Cancer Biol 2003, 13, 107–114. [Google Scholar] [CrossRef]
- Xie, Q.W.; Whisnant, R.; Nathan, C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J. Exp. Med. 1993, 177, 1779–1784. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. IκB kinases: Key regulators of the NF-κB pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef]
- Silverman, N.; Zhou, R.; Stöven, S.; Pandey, N.; Hultmark, D.; Maniatis, T. A Drosophila IκB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev. 2000, 14, 2461–2471. [Google Scholar] [CrossRef]
- Jeong, J.H.; Jang, S.; Jung, B.J.; Jang, K.S.; Kim, B.G.; Chung, D.K.; Kim, H. Differential immune-stimulatory effects of LTAs from different lactic acid bacteria via MAPK signaling pathway in RAW 264.7 cells. Immunobiology 2015, 220, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, L.; Sun, H.; Wang, Y.; Yang, Z.; Zhang, G.; Jiang, S.; Yang, W. Polysaccharide from alfalfa activates RAW 264.7 macrophages through MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2019, 126, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.N.; Hyun, S.B.; Ahn, K.J.; Hyun, C.G. Immunomodulatory effects of Abelmoschus esculentus water extract through MAPK and NF-κB signaling in RAW 264.7 cells. Biotechnol. Notes 2022, 3, 38–44. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Xie, L.; Xie, J.; Shen, M. Sulfated Chinese yam polysaccharide enhances the immunomodulatory activity of RAW 264.7 cells via the TLR4-MAPK/NF-κB signaling pathway. Food Funct. 2022, 13, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Zhang, Z.; Chen, J.; Song, D.; Liu, B.; Li, J.; Liu, R.; Niu, J.; Wang, D.; Ling, N.; et al. Immune-enhancing effects of a novel glucan from purple sweet potato Ipomoea batatas (L.) lam on RAW264. 7 macrophage cells via TLR2-and TLR4-mediated pathways. J. Agric. Food Chem. 2021, 69, 9313–9325. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.E.; Yang, J.M.; Jeong, C.W.; Hur, H.S.; Cho, J.H. Immunomodulatory Effect of Benincasa hispida Extract Fermented by Bacillus subtilis CJH 101 on RAW 264.7 Macrophages. Fermentation 2023, 9, 701. https://doi.org/10.3390/fermentation9080701
Choi YE, Yang JM, Jeong CW, Hur HS, Cho JH. Immunomodulatory Effect of Benincasa hispida Extract Fermented by Bacillus subtilis CJH 101 on RAW 264.7 Macrophages. Fermentation. 2023; 9(8):701. https://doi.org/10.3390/fermentation9080701
Chicago/Turabian StyleChoi, Ye Eun, Jung Mo Yang, Chae Won Jeong, He Seung Hur, and Ju Hyun Cho. 2023. "Immunomodulatory Effect of Benincasa hispida Extract Fermented by Bacillus subtilis CJH 101 on RAW 264.7 Macrophages" Fermentation 9, no. 8: 701. https://doi.org/10.3390/fermentation9080701
APA StyleChoi, Y. E., Yang, J. M., Jeong, C. W., Hur, H. S., & Cho, J. H. (2023). Immunomodulatory Effect of Benincasa hispida Extract Fermented by Bacillus subtilis CJH 101 on RAW 264.7 Macrophages. Fermentation, 9(8), 701. https://doi.org/10.3390/fermentation9080701




