Abstract
Sichuan black tea (SCBT) is well known for its pleasant sweet and citrus-like aroma. However, the origin of this distinctive aroma remains unknown. Herein, the aroma characteristics of SCBT during processing were comprehensively investigated by sensory evaluation, gas chromatography–mass spectrometry, and odor activity value (OAV). A total of 764 volatile compounds were identified and grouped into 16 categories. Notably, terpenoids, heterocyclic compounds, and esters comprised 19.35%, 16.34%, and 16.08% of total volatile compounds produced during processing, respectively. Moreover, the fermentation and second drying stages exhibited the most striking variations, with 99 and 123 volatile compounds being significantly altered. In addition, the OAV analysis led to the identification of 17 volatile compounds as key differential volatile compounds (DVCs): these included citronellol, linalool, p-cymene, (E)-linalool oxide (furanoid), etc. Among them, (3Z)-3,7-dimethylocta-1,3,6-triene and D-limonene that exhibited a grassy aroma decreased during processing, while linalool and p-cymene that had a sweet and citrus aroma increased. Thus, based on a correlation between characteristic aroma data and descriptive sensory analysis data, linalool and p-cymene were identified as the primary volatiles responsible for the sweet and citrus-like aroma. In conclusion, this study improves our understanding of the components and formation mechanism of the sweet and citrus-like aroma of SCBT.
1. Introduction
Owing to its health benefits, flavor, and aroma, tea is a globally popular beverage [1]. Based on the differences in manufacturing processes, tea can be classified into six types, viz. green, yellow, dark, white, black, and oolong tea; each of these teas possess a unique metabolic profile [2]. As a type of fully fermented tea, black tea is processed by picking fresh leaves, withering, rolling, fermenting, and drying [3]. It has the sweetest aroma and is the most consumed tea, accounting for ~80% of global tea sales [4]. Previous studies have found that the aroma of black tea is also significantly influenced by differences in tea cultivars, processing techniques, and environmental conditions [3,5]. For example, the Xinyang black tea produced in Xinyang, Henan province has a honey sugar-like aroma [3], Keemun black tea grown in the southeastern region of China has a distinct rose aroma/Keemun aroma [1,6,7], and the Dianhong tea produced in baoshan, lincang, Yunnan province has a caramel-like aroma [8]. Sichuan black tea (SCBT), produced in Sichuan, China, is one of three highly aromatic black teas that are popular on the market and with consumers [9]. The infusion typically has a pale red or yellowish-red color, a velvety texture, and a distinct sweet and citrus-like aroma that distinguishes it from other black teas [9]. Very few reports on the aroma characteristics of SCBT currently exist. In this regard, Luo et al. and Mao et al. found that geraniol, linalool and its oxides, phenylethyl alcohol, benzyl alcohol, nerolidol, methyl salicylate, benzene acetaldehyde and citral positively contributed to the SCBT aroma profile, while β-cyclocitral, safranal, α-farnesene and dihydroactinidiolide were negative aroma contributors [9,10]. However, the volatile compositions or odor characteristics and their changes during overall processing have rarely been reported, which is the greatest barrier to the scientific elucidation of the aroma quality of SCBT.
It is widely acknowledged that tea aroma is a significant sensory characteristic that reflects the quality of tea [4,11]. Volatile compounds are easily liberated or degraded during tea processing, which can have a significant impact on the sensory characteristics of tea samples in the subsequent stage and the quality of the final tea product, as well as being a key factor in the consumption of high-end tea [4,11,12]. Thus, recognizing the knowledge of tea aroma compositions, aroma properties and their variations throughout the processing would aid tea manufacturers in either enhancing the aroma of tea [11]. In recent years, diverse aroma substances in black teas processing have been identified in tea science [13,14]. The aromatic compounds phenylpropanoids/benzenoids and carotenoid-derived volatiles, which contribute to the honey-like and rose-like fragrances of Danxia2 tea, increasing during processing, whereas 3-hexan-1-ol_acetate, indole, 2-hexenal and [(Z)-hex-3-enyl] hexanoate with a grassy aroma decreased during processing [15]. In addition, the relative concentrations of 20 key aroma active compounds, such as phenylacetaldehyde, β-ionone and methyl salicylate, which differ significantly among the Xinyang black teas during the various fermentation processes [3]. In addition, during the processing of Congou black tea, a large number of alcohols and aldehydes with a grassy aroma are produced, as well as esters with floral and fruity aromas [13]. Correspondingly, a sustained increase in ester production, including (E)-2-hexenyl acetate, hexyl acetate, and hexyl hexoate, would be advantageous for the accumulation of the fruity aroma in Congou black tea [13]. Conversely, linalool and geraniol were responsible for the increased floral and fruity aromas in Keemun and Jinmudan black teas during the sun withering and fermentation processes [6,14]. Other teas, such as green tea, dark tea, white tea, and oolong tea, have also been studied similarly [5,11,16,17,18].
Recently, the widely targeted volatilomics method with higher sensitivity has been shown to be an effective method for detecting and quantifying key aroma compounds in spring-picked and autumn-picked white tea [3,19]. Accordingly, they were used to identify the key volatile components in black tea, such as phenylacetaldehyde, dihydroactinidiolide, β-ionone, and methyl salicylate, which are positively correlated with the flavor of Xinyang black tea [3]. In addition, headspace solid-phase microextraction gas chromatography–mass spectrometry (HS–SPME-GC–MS) has been widely used in food chemistry due to its high resolution, sensitivity, and mass accuracy [3,20]. In fact, it is also a useful method for characterizing the principal aroma-active compounds in premium Dianhong tea [8], and Baihaoyinzhen white tea [21]. However, few systematic studies have been conducted so far to verify the active-aroma compounds in SCBT.
Zaobaijian is bred from the Sichuan medium and small leaf population varieties originating from Junlian County, Sichuan province, China, and it was a national superior varieties. Large areas in Sichuan province have been planted with Zaobaijian in the past ten years. This cultivar exhibits abundant compounds, including tea polyphenols, free amino acids, soluble sugar and water extract. The black tea produced by this cultivar possesses a high and long-lasting aroma of a sweet and citrus-like, mellow and smooth taste. In the current study, using Zaobaijian as a model, we employed volatilomics in conjunction with the HS–SPME-GC–MS technique to examine the dynamic changes in aromatic compounds and to identify the key aromatic compounds involved in the formation of the sweet and citrus-like aroma during SCBT processing. This research advances our understanding of aroma formation during SCBT processing and provides a theoretical foundation and technical guidance for the precise and directional processing of SCBT.
2. Materials and Methods
2.1. Black Tea Samples
The major tea plant cultivars, which are suitable for producing SCBT, are planted and managed in Yibin city (Sichuan, China). Young shoots with one bud and one leaf of Zaobaijian [Camellia. sinensis (L) O. Kountze var. Zaobaijian] were obtained from Sichuan Tea Industry Group (Yibin, China). The manufacturing procedures were as follows: leaves were plucked from the tender shoots and then laid to wilt on an indoor withering trough at 25 °C, and relative humidity of approximately 75% for 12 h; tea leaves were turned over every 3.0 h during the withering stage. The withered tea leaves were then rolled for 60 min using a rolling machine before being placed in a fermentation box to ferment for 5 h at 28 °C and a relative humidity >90%. The color of the tea leaves changed from reddish-yellow to yellow-red as a result of fermentation. The tea leaves were dried at 100 °C for 60 min, followed by 1.5 h of drying at 75 °C. At each stage, three replicates of tea leaves were collected, and the samples were separated into two parts. The first part was subjected to a sensory evaluation, while the second part was stored at −80 °C for volatile compounds analysis.
2.2. Sensory Evaluation
Before the sensory evaluation, six tea samples were freeze-dried for 42 h using a freeze dryer (FD-1A-50, Shanghai, China). Tea samples were evaluated and scored by a panel of seven trained assessors (four males and three females, 26–55 years old), all assessors had more than five years of descriptive sensory analysis experience in teas, and panelists were trained according to the national professional standards for tea sensory evaluation (profession code: 6–02-06–11, China). Tea infusions were prepared according to Chinese standard (GB/T 23776, 2018). In brief, tea infusions were prepared by adding 150 mL of boiling water to 3 g of tea samples from each processing stage in a teacup with a lid. After 5 min of brewing, three-digit numbers were used to code samples, and they were randomly offered to panelists after brewing, the intensity values and aroma descriptors of samples were recorded by panelists. The panelists began by evaluating the aroma profile characteristics of the six samples and agreed that the aroma of tea samples could be described using eight common quality descriptors (floral, fruity, green and grassy, honey, sour, sweet, citrus, and roast) that best represented the six samples. Additionally, then, eight attributes were defined as the following aroma references: linalool as floral odor, hexyl acetate as fruity odor, E-2-hexenal as green-like odor, E,E-2,4-hexadienal as grassy odor, benzeneacetaldehyde as honey odor, acetic acid as sour odor, benzaldehyde as sweet odor, (Z)-citral as citrus odor, and 2-ethylfuran as roast odor. Furthermore, the intensities of the aroma attributes were scored on a scale ranging from 0 to 5; the higher the score, the stronger the intensity, where 0 = none, 1 = very weak, 2 = considerably weak, 3 = considerably strong, 4 = strong, and 5 = very strong. Each sample was evaluated three times by each panelist and the average values of the data were represented on a spider plot [2,3].
2.3. Extraction and Analysis of Volatile Compounds
The volatile compounds were extracted using the headspace solid-phase microextraction (HS–SPME) method, with the following parameters: tea powders were collected after filtration through the screen cloth of an aperture of 425 µm, and 0.5 g of the tea powder was transferred immediately to a 20 mL head-space vial (Agilent, Palo Alto, CA, USA) containing a saturated NaCl solution to inhibit any enzyme reaction. The vials were then sealed with crimp-top caps containing TFE-silicone headspace septa (Agilent). At the time of SPME analysis, each vial was heated to 100 °C for 5 min, after which a 120 µm DVB/CWR/PDMS fiber (Agilent) was exposed to the headspace of the sample for 15 min at 100 °C. The SPME fiber coating was then removed immediately from the headspace vial and inserted into the GC injector (Model 8890; Agilent) for desorption at 250 °C for 5 min in the splitless mode. The identification and quantification of volatile components was carried out using an Agilent Model 8890 GC and a 7000 D mass spectrometer (Agilent), equipped with a 30 m × 0.25 mm × 0.25 μm DB-5MS (5% phenyl-polymethylsiloxane) capillary column. Helium was used as the carrier gas at a linear velocity of 1.2 mL/min. In addition, the injector temperature was maintained at 250 °C, while the detector was maintained at 280 °C. The oven temperature was programmed to rise from 40 °C (3.5 min), at a rate of 10 °C/min to 100 °C, at a rate of 7 °C/min to 180 °C, and at a rate of 25 °C/min to 280 °C, with a hold period of 5 min. The mass spectra were recorded in the electron impact (EI) ionization mode at 70 eV. The quadrupole mass detector, ion source, and transfer line temperatures were set to 150, 230, and 280 °C, respectively. The mode of selected ion monitoring (SIM) was utilized for the identification and quantification of analyses, as also mentioned in previous study [3,22,23,24].
2.4. Quantification and Odor Activity Values (OAVs) Calculation
Volatile compound contents were detected based on the peak areas of the internal standard compound. The internal standard used was 3-hexanone-2,2,4,4-d4 (10 μL, 50 μg/mL), and the relative content of each volatile compound was calculated using the following formula:
where Ci is the mass concentration of each component (µg/g), mis is the mass of the internal standard (µg), Ai and Ais are the chromatographic peak area of each component and internal standard, respectively, and mi is the mass of the sample powder (g).
Correspondingly, OAV was determined by dividing the calculated concentration of volatile compound by the odor threshold in water of each volatile compound. The formula for calculating the OAV value is as follows:
where Ci (µg/g) is the VOC content, and OTi (µg/g) is the aroma threshold of the volatile components in water [11,13,25].
2.5. Statistical Analysis
All results from three replicates were presented as the mean value ± standard deviation (SD). The significant differences between means were analyzed by a one-way analysis of variance (ANOVA) using SPSS (version 22, Chicago, IL, USA), and all comparisons were considered statistically significant if p-value < 0.05. Principal component analysis (PCA), hierarchical cluster analysis (HCA), and orthogonal projections to latent structures-discriminant analysis (OPLS-DA) were conducted to cluster samples based on the concentration of identified volatile compounds using SIMCA-P software (version 14.1, Umetrics AB, Umea, Sweden) [3]. In addition, the variable importance in the projection (VIP) values were extracted from the OPLS-DA result. The significantly different volatile compounds (DVCs) were determined based on VIP > 1 and p < 0.05 values for each pairwise comparison between stages fT–DT2 [3,15]. Images of the data were visualized using GraphPad Prism (version 9) and TBtools. In addition, the flavor of DVCs was annotation for significantly distinct metabolites utilized the TGSC website (http://www.thegoodscentscompany.com/, accessed on 18 March 2023) and FEMA website (https://www.femaflavor.org/, accessed on 18 March 2023) databases [3].
3. Results and Discussion
3.1. Sensory Evaluation of SCBT during Processing
Sensory evaluation was performed to evaluate the sensory properties of all samples of SCBT, including the following six samples: fresh tea leaf (fT), withering tea leaf (WT), rolling tea leaf (RT), fermentation tea leaf (FT), first drying tea leaf (DT1), and second drying tea leaf (DT2) (Figure 1). This analysis utilized eight primary aromas, including floral, fruity, green and grassy, honey, sour, sweet, citrus, and roast. The sensory evaluation scores of each samples were analyzed using ANOVA (Figure 2). The results showed that attributes of green and grassy odor scores decreased significantly (p < 0.05), whereas floral, fruity, sweet, roast, and citrus odor scores increased significantly with the processing (p < 0.05). However, the processing of the tea samples did not result in any discernible changes to other aroma factors. In terms of the aroma profile, fT and RT samples were primarily green and grassy, WT and FT samples were primarily floral and fruity, DT1 sample was a mixture of odors, and DT2 sample had a notable sweet and citrus-like aroma (Table 1). Based on the results, we concluded that the sweet, fruity, and citrus characteristics depicted in Figure 2 were related to the sweet, citrus-like aroma of the DT2 sample. In addition, we investigated the components and formation of the sweet, citrus-like aroma in the following results.

Figure 1.
Flow diagram and process parameters for processing SCBT. Fresh tea leaves (fT), withering (WT), rolloing (RT), fermenting (FT), first drying (DT1), and second drying (DT2).

Figure 2.
Sensory profiles of tea samples during SCBT processing. Note: fresh tea leaves (fT), withering (WT), rolloing (RT), fermenting (FT), first drying (DT1), and second drying (DT2); the asterisk (*) indicates a significant change (p < 0.05) during the processing.

Table 1.
Sensory evaluation of samples in different processing stages.
3.2. Volatile Category in SCBT during Processing
To investigate the unique flavor of SCBT and its relation to processing, we performed the SPME extraction for volatile compounds, analyzed these extracts by GC–MS. The coefficient of variation for the total ion chromatogram and distribution map is depicted in Figure S1. A total of 764 volatile compounds were detected, and relatively detailed information on the volatile compounds was obtained for the following six samples: fT, WT, RT, FT, DT1, and DT2 (Figure 1 and Table S1). A comparative analysis revealed that all samples contained 753 volatile compounds in common, and ten of the 764 volatile compounds detected were newly produced during processing including furan-3-carbaldehyde, benzaldehyde, N-heptan-4-ylidenehydroxylamine, phenol, 2-butan-2-ylphenol, [(Z)-hex-3-enyl] hexanoate, 2-phenoxyacetic acid, [(Z)-hex-3-enyl] (Z)-hex-3-enoate, (E)-2-phenylbut-2-enal, methyl myrtenate and resorcinol monoacetate; other volatile compounds showed dynamic changes during processing (Table S1). The detected volatile compounds were clustered into 16 categories named terpenoids, heterocyclic compounds, esters, hydrocarbons, ketones, alcohols, aldehydes, aromatics, phenols, acids, amines, halogenated hydrocarbons, nitrogen compounds, sulfur compounds, ethers and others (Figure S2). Among these compounds, terpenoids demonstrated the largest number (148) and accounted for 19.35% of the total volatile numbers, followed by heterocyclic compounds (124), esters (123), hydrocarbons (70) and ketones (64) and alcohols (58), with proportions of 16.34%, 16.08%, 9.15%, 8.37%, and 7.58%, respectively. The compounds accounted for more than 75% of the volatile compound profile, which was almost in accordance with other black tea processing studies [3,26,27]. The remaining 180 volatile compounds comprised aldehydes (50), aromatics (48), acids (18), phenols (17), amines (15), nitrogen compounds (10), sulfur compounds (6), ethers (5), halogenated hydrocarbons (4), and others (4) with proportions of 6.54%, 6.27%, 2.35%, 2.22%, 1.96%, 1.31%, 0.78%, 0.65%, 0.52%, and 0.52%, respectively (Figure S2). Additionally, the contents of terpenoids, heterocyclic compounds, and esters made up more than half of the total volatile component content, which was primarily contributed by the content of compounds obtained from the hydrolysis of glucoside (geraniol, linalool, methyl salicylate, benzenemethanol, linalool oxides, etc.) [6].
To further describe in detail, 754, 764, 764, 764, 761, and 763 volatile compounds were found in fT, WT, RT, FT, DT1, and DT2, respectively (Table S1). As depicted in Figure 3A, the concentration of 3-hexanone-2,2,4,4-d4 was measured in order to determine the amount of aroma. The total quantities of volatile compounds in the processing samples were 737.96 ± 8.65, 1099.93 ± 10.23, 951.80 ± 9.28, 915.90 ± 11.04, 457.03 ± 8.96, and 440.26 ± 9.73 μg/g, respectively. The content of these chemical categories varied in processing samples, initially increasing and then decreasing from fT to DT2 (Figure 3A), and the volatiles in different categories were either reduced or newly generated as a result of the release of volatile compounds from hydrolysis, oxidation, modification, or degradation of phenolic compounds, Maillard reaction, glycoside hydrolysis, carotenoid or lipids degradation during processing [11,12,15].
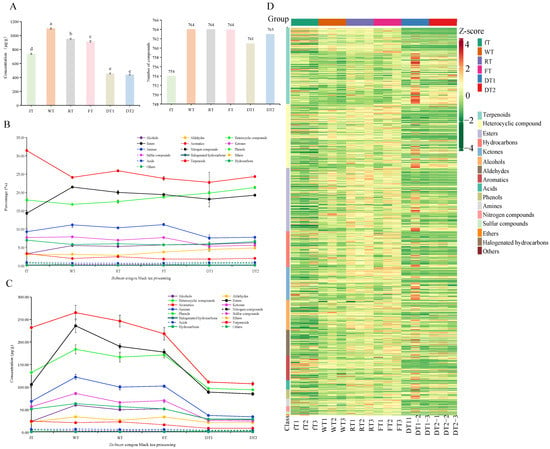
Figure 3.
The profiles of volatile metabolites from SCBT samples during different producing process. Overall aroma concentration (µg/g relative to internal standard) and the number of volatile compounds identified in the SCBT (A). Dynamics change on the proportion (B) and content (C) of volatiles during SCBT processing. (D) Heat map of violatiles for SCBT processing stage. The various superscripts show significant differences (p < 0.05).
3.3. Dynamic Changes in Volatile Compounds in Processing Steps
Previous research has demonstrated that volatile terpenoids are produced during withering and rolling [12], which is advantageous for the formation of tea aroma quality. During processing, volatile terpenoids comprised the major fraction of volatiles in SCBT, and their proportion was greatest in WT samples. Correspondingly, linalool, 3-methyl-6-propan-2-yl-7-oxabicyclo [4.1.0]heptan-2-one, 1-methyl-4-propan-2-ylidenecyclohexene, 3-(4-methylpent-3-enyl)furan, 2-methyl-5-prop-1-en-2-ylcyclohexa-1,3-diene, linalyl acetate, 2,6-dimethyloct-7-en-2-ol, 2,7,7-trimethyl-3-oxatricyclo[4.1.1.02,4]octane, (3E)-3,7-dimethylocta-1,3,6-triene, (1R,2R,5S)-2-methyl-5-propan-2-ylbicyclo[3.1.0]hexan-2-ol, fenchone, and (4S)-2-methyl-6-methylideneoct-7-en-4-ol were of high abundance and their total contents accounted for more than 65% of the total amount of terpenoids in tea samples DT2 to fT; accordingly, they ranged from 65.01% to 79.29%, being the lowest in DT2 and the highest in fT (Figure 3B and Table 2). However, the highest concentration of terpenoids was found in WT, which reached 265.24 μg/g. Since withering is also the first step in the production of black tea, this technological step can improve the flavor quality of black tea infusion by increasing volatile flavor components, the majority of which are terpenoids [28]. Additionally, DT2 had the lowest amount and DT1 had the lowest proportion of terpenoids, which were 107.12 μg/g and 22.74%, respectively, indicating that the majority of the volatile terpenoid changes were completed prior to drying and were not involved in the drying process. Studies have shown that linalool is the primary aroma component in black tea, with floral and citrus odor characteristics that contribute to the aroma quality of black tea [29], In addition, p-cymene and α-phellandrene contribute to the citrus odor [30], while (3E)-3,7-dimethylocta-1,3,6-triene, (2E,4E)-hexa-2,4-dienal and D-limonene impart citrus, sweet odor characteristics [11]. All of them had a high background concentration in fT and a high content in DT2, reaching 42.60 μg/g, 1.46 μg/g, 0.43 μg/g, 2.23 μg/g, 0.27μg/g, and 0.54 μg/g, respectively (Figure 3C,D and Table 2). Thus, the odor of linalool, p-cymene, β-ocimene, and D-limonene may contribute to the citrus-like aroma that is characteristic of SCBT.

Table 2.
Comparison of volatile compounds identified in SCBT from six different processing stages by SPME-GC–MS data displaying the top 10 contents of each category.
Volatile esters are primarily contributing pleasant aromas such as fruit, sweet, and floral in black tea [3]. Both the proportion and concentration of esters initially increased and then decreased from fT to DT2. The proportion of esters was lowest in fT (14.28%), and then gradually increased to its highest value in WT (21.46%) (Figure 3B). Interestingly, WT had the highest concentration of esters, at 236.09 μg/g followed by a dramatic decrease to 84.66 μg/g in DT2, which was nearly one-third of WT (Figure 3C). Methyl salicylate is recognized as an important compound for the overall aroma in teas with at least medium-degree fermentation because it gives tea its sweet and floral aroma, which is the result of glycoside hydrolysis in green, black, and oolong tea [31,32], This, however, cannot be detected in unfermented and lightly fermented teas [33]. It had a high background concentration in fT, increased after fermentation to a maximum content of 119.67 μg/g in WT, and then decreased dramatically to 32.44 μg/g in DT2 (Table 2), possibly due to the breaking of a glycosidically related bond during high-intensity drying [11]. [(E)-hex-3-enyl] butanoate and [(Z)-hex-3-enyl] hexanoate are said to emit a grassy odor [31]. The levels of these compounds decreased gradually in the dried tea leaves of this study. Surprisingly, of butanoic acid_3-hexenyl ester almost completely disappeared in DT2 (Table 2 and Table S1). Notably, [(Z)-hex-3-enyl] 2-hydroxybenzoate and methyl anthranilate with the fruity odor had a significantly higher concentration in DT2 than in other samples, which had concentrations of 0.02 μg/g and 0.18 μg/g, respectively. Furthermore, ethyl dec-9-enoate, [(Z)-hex-3-enyl] hexanoate, [(Z)-hex-3-enyl] (Z)-hex-3-enoate, methyl myrtenate, and resorcinol monoacetate volatiles were newly identified during processing and were highest in WT (Table 2 and Table S1).
Heterocyclic compounds produce fatty, mushroom, herbaceous, and caramel-like aromas [3]. As shown in Table 2 and Table S1, a large number of heterocyclic compounds with pyrazine, pyrrole, and pyran structures formed during the processing of SCBT; the content and variation of heterocyclic compounds in DT2 was comparable to that of terpenoids. Interestingly, the proportion and variation of heterocyclic compound content changed significantly during processing (Figure 3B,C). Large quantities of heterocyclic compounds such as furan, pyrrole, thiophene, and pyrazine, as well as their derivatives, are also produced by the Maillard reaction during the tea manufacturing process [12], while high temperature also acts as a catalyst for the Maillard reaction [34]. Compared to fresh leaves, the proportion of heterocyclic compounds in DT2 was significantly higher, reaching a maximum of 21.32% (Figure 3B). In addition, there was a change in odor type, which was predominantly grassy or floral in RT and earlier samples, but citrus-like and sweet after fully drying. It is worth noting that (E)-linalool oxide was reported to increase the floral note of instant white tea infusion and decrease the roasted note [7,18], and that the content of WT (40.10 μg/g) was significantly higher than that of the other samples (Table 2). During the drying process, the temperature can influence the transformation and decomposition of the original compounds, aroma, and flavor of the final tea and infusion [28]. Previous research has demonstrated that pyrazine flavor compounds can be formed during the drying and roasting process [35], and that methylpyrazine is the aroma active compound of the four most popular high-aromatic black teas worldwide [36]. In this study, several derivatives of methylpyrazine were found, including 2-methoxy-3-propan-2-ylpyrazine, pyrazine-tetramethyl, 2-ethoxy-3-methylpyrazine, 2-methyl-5-propan-2-ylpyrazine, 2-ethyl-5-methylpyrazine, 7-methyl-5H-pyrrolo[2,3-b]pyrazine, and 5H-5-methyl-6,7-dihydrocyclopentapyrazin; among them, the concentration of 2-ethoxy-3-methylpyrazine with the nutty odor [11], was significantly higher in DT2 than those found in other samples (Table S1), indicating their potential roles in the formation of SCBT tea aroma.
Alcohols were the primary volatiles in SCBT during processing. Similar to the cases of terpenoids and esters, the alcohol content increased gradually to a maximum of 59.70 μg/g in WT, and then decreased dramatically to 27.27 μg/g in DT2. However, DT2 contained the highest concentration of alcohols, at 6.19%. The fT had the smallest amount and percentage of alcohols, which were 23.40 μg/g and 3.17%, respectively (Figure 3B,C). Additionally, compared to fresh leaves, the contents of 1-hexanol, 1-heptanol, (Z)-pent-2-en-1-ol, (Z)-hex-3-en-1-ol, (E)-2-pentyn-1-ol and (Z)-hex-3-en-1-ol, were significantly decreased in DT2 (Table 2 and Table S1), and there were have been reported to produce a green odor [11], and it was hypothesized that the volatiles were simultaneously evaporated or transformed into other volatile compounds during the drying period, which led to a decrease in the contents of these compounds [37]. 1-octen-3-ol possesses a predominant earthy and mushroom aroma, which is prevalent in aged dark tea [18]. The concentration of 1-octen-3-ol increased to a maximum of 0.13 g/g in RT, and then decreased dramatically to 0.04 μg/g in DT2. According to a previous study, volatile substances with a soil odor can mask the floral odor [7,18]. Furthermore, the majority of alcohols exhibited a predominantly floral and fruity aroma [2,6], which may contribute to the citrus-like odor that is characteristic of SCBT.
Similar to the cases of alcohols, the proportion of aldehydes gradually increased to the highest value of 33.71 μg/g in WT and then decreased to 20.93 μg/g in DT2 (Figure 3C). The highest proportion of alcohols was in DT2, reaching 4.76% (Figure 3C), in which (E)-2-hexenal, (Z)-3-hexenal, and hexanal, which were produced by lipid oxidation to easily liberate in green tea and black tea [2,12], contributed to the green odor in tea [11], However, all of them were reduced after fully dried treatment in DT2, especially (Z-)3-hexenal, which was significantly decreased by 5.84 fold from WT to DT2, and reduced to 0.46 μg/g (Table 2 and Table S1). honey-sugar aroma [26], while benzaldehyde has been recognized as an important compound for the overall aroma, as it imparts the honey flavor and floral aroma to tea [7,33]. Nonanal has been shown to play an important role in the aroma quality of Keemun black tea and may contribute to the floral and fruity aromas of tea [37,38]. In addition, the other two volatiles, excluding benzeneacetaldehyde, increased following fully dried treatment, with benzaldehyde increasing significantly by 5.68 fold from WT to DT2 to reach 4.35 μg/g (Table 2 and Table S1). Notably, benzaldehyde is liberated from prunasin to mandelonitrile as an intermediate, which isomerizes from acid to aldehyde and commonly occurs as their glycosidically bound form in tea [12], indicating that fully dried processing was advantageous for the production of benzaldehyde [11].
Volatile sulfur compounds are low in concentration and have a low threshold, and they emit complex aromas [3,39]. Very few reports exist on volatile sulfur compounds in black tea aroma, possibly due to the limitations of previous detection methods [3,40]. Thus, using the denoise reduction ability of the wide-target method, 6 volatile sulfur compounds with aromas were identified. These include diallyl disulfide and dipropyl trisulfide which impart an alliaceous aroma, and methyl phenyl sulfide and methyl furfuryl disulfide which impart a roasted coffee aroma. The odor of the other two sulfur compounds, however, cannot be described. Previous research revealed that dimethyl trisulfide has a putrid flavor and dimethyl disulfide has a garlic-like flavor in black tea [12]; however, neither of these compounds have been identified in our study. Therefore, the role of volatile sulfur compounds in the development of black tea aroma must be investigated further.
Ketone compounds are important volatile compounds in black tea with a floral and woody aroma [7]. The highest concentration of ketones was found in WT (85.87 μg/g), which then decreased significantly to 24.26 μg/g in DT2, and the proportion of ketones exhibited dynamic changes during processing. Some common ketone compounds in tea, such as β-damascenone, α-ionone, and β-ionone, have low thresholds and a positive effect on the aroma [7]. In this study, (E)-alpha-ionone was found in low concentrations, with a peak concentration of 0.007 μg/g in DT2. The proportion and concentration of hydrocarbons mirrored those of ketones, respectively. The concentrations of aromatics, amines, ethers, and acids remained low throughout the processing of black tea, falling to 8.48 μg/g, 33.87 μg/g, 0.14 μg/g, and 3.54 μg/g, respectively, between fT and DT2 (Figure 3C and Table S1). Forms of phenol oxidase with a high molecular weight and catechol oxidation activity are typically produced during the withering stage. Thus, the oxidation of phenol begins with the withering step [28]. Thus, the oxidation of phenol starts at the withering step [28,41]. In this study, we observed that the content of phenols was significantly decreased from fT to DT2 With the combined action of hundreds of volatile compounds, the aroma of black tea is remarkably complex. Consequently, it is necessary to conduct additional research into the formation mechanism and their contribution to SCBT processing.
3.4. Multivariate Statistical Analysis Based on Volatile Compounds
To further distinguish the differences and similarities of SCBT processing, unsupervised PCA was utilized to examine the volatile compound profiles of the six tea samples based on peak area (Figure 4A). The contribution rate of the first principal component (PC1) and the second principal component (PC2) were 48.7% and 25.5%, respectively, and the total contribution rate was 74.2%. Remarkably, the six samples of tea were clearly separated according to the processes, particularly between fT and RT, RT and WT, and WT and FT. These results indicated that alterations in metabolites occurred continuously during the first four stages, but to a lesser degree between DT1 and DT2 (Figure 4A). During processing, the sampling points of volatile substances of fresh leaves (fT) are distributed separately in the fourth quadrant, far away from the other processing stage samples, indicating that endogenous volatile components of the fresh leaves are significantly generated after processing [42]. Correspondingly, the heatmap of volatile profiles revealed a clear distinction between fT and the other samples (Figure 3D). The samples with RTs are spatially distributed at the intersection of the first and fourth quadrants; the samples with WTs and FTs are concentrated in the first quadrant; and the samples with DT1 and DT2 are spatially distributed at the intersection of the second and third quadrants, far away from the samples from the other processing stages. All tea samples were within the 95% confidence intervals, and the model was well suited for differential analysis.

Figure 4.
Multivariate statistical analysis of the metabolites presents in SCBT after each processing step, fresh tea leaves (fT), withering (WT), rolling (RT), fermentation (FT), first drying (DT1), and second drying (DT2). (A) Principal component analysis (PCA); (B) hierarchical clustering analysis (HCA) of GC–MS data.
The volatiles were subjected to a hierarchical clustering analysis (HCA) based on Euclidean distance in order to further distinguish the differences between SCBT processing [43]. As can be seen in Figure 4B, each of the six processing stage samples could be divided into clusters I and II. DT1 and DT2 were assigned to cluster I, whereas fT, RT, WT, and FT were assigned to cluster II. The HCA tree structure indicated that the samples possessed distinctive characteristics and that the experimental manipulations were reproducible, and the HCA tree structure was consistent with PCA and sensory analysis data.
3.5. Screening for Key Differential Volatile Compounds of Different Processing Stage
The PCA and HCA results indicated that the volatile compounds differed between processing stages. Orthogonal partial least square-discriminant analysis (OPLS-DA) was used for pairwise comparisons between consecutive steps (fT vs. WT, WT vs. RT, RT vs. FT, FT vs. DT1, and DT1 vs. DT2) to determine the key components (Figure S3). The results of OPLS-DA score plots demonstrated that each group can be distinguished with precision (Figure S3). In these five analyses, the R2Y and Q2 scores were greater than 0.9, indicating that the model adequately explained variance and was highly predictive, and the Y-intercepts of Q2 were all less than 0, indicating that the models were reliable and free of overfitting.
Based on the OPLS-DA model, differential metabolites were screened by their variable importance values (VIP) between the two steps. Correspondingly, metabolites with VIP ≥ 1 were selected as essential volatile compounds. The S-plots (Figure 5A–E) depict the differences in relative aromatic volatile compound content between each successive processing sample. A histogram of the number of differentially regulated volatile compounds reveals that, with the exception of the comparison between the WT and fT samples, the number of significantly down-regulated volatile compounds exceeded the number of significantly up-regulated volatile compounds in four of the pairwise comparisons. Starting with the fresh SCBT leaves, 96 differential volatile compounds were found after the withering process, of which 72 volatile compounds were up-regulated, and 24 volatile compounds were down-regulated. Moreover, there were 93 differential volatile compounds between the RT and WT samples (24 volatile compounds up-regulated, 69 volatile compounds down-regulated), 99 differential volatile compounds between the FT and RT samples (37 volatile compounds up-regulated, 62 volatile compounds down-regulated), and 81 differential volatile compounds between the DT1 and FT samples (3 volatile compounds up-regulated, 78 volatile compounds down-regulated). During the drying process, the final concentrations of 123 volatile compounds changed significantly (51 volatile compounds were up-regulated and 72 volatile compounds were down-regulated) (Figure 5F).
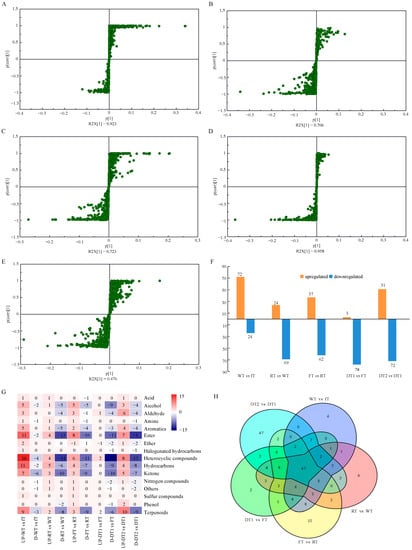
Figure 5.
Metabolites differed between consecutive processing samples. (A–E) S-plots of the differentiated metabolites generated by OPLS-DA of the pair-wise GC–MS data showed distinct metabolome changes between fresh tea leaves (fT) and withering (WT), rolling (RT) and withering (WT), fermentation (FT) and rolling (RT), first drying (DT1) and fermentation (FT), and second drying (DT2) and first drying (DT1); (F) number of differential metabolites of each pairwise comparison of SCBT processing samples; (G) chemical classification of differential metabolites within the five pairwise comparisons, “-”, number of down-regulated; (H) Venn diagram of the differential metabolites of the five pairwise comparisons.
Figure 5G depicts the chemical classification of volatile compounds that differed between each of the five pairwise comparisons. During processing, the number of up-regulated alcohol, aromatics, ester, ether, heterocyclic compound, hydrocarbons, and ketone decreased, whereas the number of up-regulated aldehyde, phenol, and terpenoids increased. Except for the groups of amine, nitrogen compounds, phenol, and others, eleven groups demonstrated that the number of up-regulated volatile compounds was greater than that of down-regulated volatile compounds after withering. Withering is also the first step in black tea preparation. This technological step can improve the flavor quality of black tea infusion by increasing volatile flavor components, the majority of which are terpenoids [28], nine of which were up-regulated in WT relative to fT. Fermentation has been regarded as the most important step in the production of black tea, and it was believed that fermentation contributed to the formation of the characteristic aroma of congou black tea [27,28]. Notably, our results revealed that fermentation causes the greatest change in volatile compounds during the entire SCBT manufacturing process. After the fermentation process, a large number of differential volatile compounds were regulated, mainly including alcohol, ester, heterocyclic compound, hydrocarbons, ketone, and terpenoids. Drying and roasting step is usually the last unit operation of tea processing. It has been discovered that heterocyclic compounds can be formed during the roasting step [35], In contrast to previous research [6,11,35], the number of up-regulated heterocyclic compounds in DT2 was less than the number of down-regulated heterocyclic compounds in DT1 in the present study. This may be attributable to the distinct technical and tea tree varieties of SCBT black tea. Figure 5H is a Venn diagram depicting the common and unique volatile compounds between five pairwise comparisons with VIP > 1. Ultimately, 47 overlapping key differential volatile compounds were selected, including 1 alcohol, 3 aromatics, 8 esters, 1 ether, 12 heterocyclic compounds, 3 hydrocarbons, 4 ketones, 2 nitrogen compound and 1 others, twelve compounds, including 1-methyl-4-propan-2-ylidenecyclohexene, (3Z)-3,7-dimethylocta-1,3,6-triene, 2,7,7-trimethyl-3-oxatricyclo[4.1.1.02,4]octane, fenchone, 4-methyl-2-(2-methylprop-1-enyl)-3,6-dihydro-2H-pyran, linalool, p-cymene D-limonene, citral, linalyl acetate, citronellal and citronellol were identified as volatile terpenoids (Figure 6). Figure 6 depicts a heatmap of the relative abundance of key differential volatile compounds, which were separated into 4 groups based on hierarchical clustering with Euclidean distance and complete linkage. In group 1, the concentration of the majority of compounds was greatest in DT1 and DT2 samples, whereas the opposite was true for all other groups. Except for dicyclopentadiene and methyl cyclohexanecarboxylate, the highest concentrations of volatile compounds were found in group 2 fT, group 2 WT, and group 2 FT, with the exception of dicyclopentadiene and cyclohexanecarboxylate. It has been demonstrated that the majority of these volatile compounds promote tea aroma formation. Accordingly, it is believed that these compounds have a positive impact on the formation of aroma. The majority of the remaining 720 volatile compounds were modified specifically during one processing step, as detailed in Table S1. Consequently, altered aroma components are believed to be primarily responsible for the differences in aroma characteristics resulting from SCBT processing.
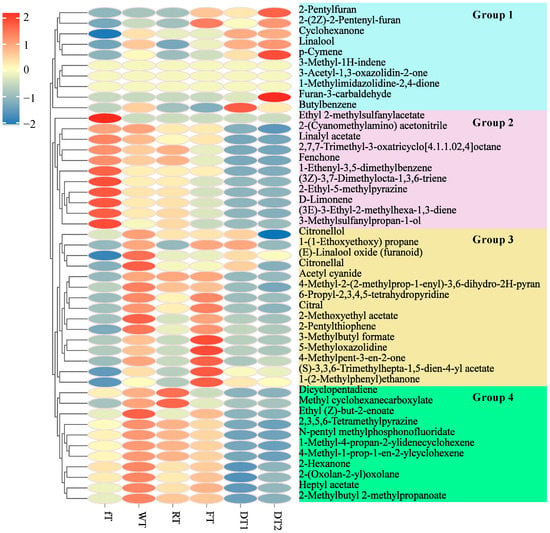
Figure 6.
Heatmap of key differential volatile compounds during processing stages (VIP > 1). Each column in the figure is a treatment condition, and each row represents a volatile component. The depth of the color represents the abundance of information on the corresponding volatile components in the corresponding SCBT samples. Blue is down-regulation, and red is up-regulation. The depth of the color indicates the degree. Blank indicates a lack of enrichment.
3.6. The Formation of the Sweet and Citrus-like Aroma during SCBT Processing
The odor activity value (OAV) is employed to assess the contribution of volatile compounds to the aroma of tea [44]. OAV > 1 is generally regarded as a necessary condition for determining whether volatile compounds contribute to the aroma [45]. Based on this information, we identified a total of 17 volatile compounds and key markers as odor-active compounds (OAV > 1) (Table 2). The majority of these compounds had floral, fruity, herbal, citrus, and nutty odors, indicating that they contributed significantly to the overall aroma profile of SCBT. A total of 7 volatile compounds had OAV values greater than 100, including citronellol, methyl cyclohexanecarboxylate, linalool, p-cymene, (E)-linalool oxide (furanoid), (3Z)-3,7-dimethylocta-1,3,6-triene, and 1-methyl-4-propan-2-ylidenecyclohexene. Moreover, 7 volatile compounds had OAV values ranging from 10 to 100, including D-limonene, 2-pentylfuran, fenchone, citral, linalyl acetate, 2-(2z)-2-pentenyl-furan, and citronellal. The OAV values for 2,3,5,6-tetramethylpyrazine, 4-methylpent-3-en-2-one, and 1-(2-methylphenyl)ethanone ranged between 1 and 10. However, 4-methylpent-3-en-2-one and 1-(2-methylphenyl)ethanone only had an OAV > 1 in FT, which may have been due to the lower relative concentrations in certain samples. These 17 volatile compounds contributed more to the formation of aroma differences between SCBT processing stages. Descriptions of the primary odors of these aroma-active compounds were obtained from an online database (http://www.thegoodscentscompany.com/, accessed on 26 March 2023). Notably, we discovered that as tea processing was prolonged, the total OAV value of key tea volatile compounds increased at first, then decreased, and was highest in WT (Table 3). In addition, citronellol (floral), cyclohexanecarboxylic acid methyl ester (fruity), (E)-linalool oxide (furanoid) (floral), and 1-methyl-4-propan-2-ylidenecyclohexene (herbal) had the highest OAV and contents in WT (Table 3 and Figure 7). Withering is a crucial physical and biochemical preconditioning step in the production of black tea. During the withering process, fresh tea leaves not only lose moisture and become flexible, but the activity of various enzymes that catalyze the conversion of volatile and nonvolatile components increases [39,46], and synergistically promotes the formation of tea flavor quality [47]. Moreover, OAVs of citronellol, methyl cyclohexanecarboxylate, linalool, p-cymene, and (E)-linalool oxide (furanoid) are the top five in all samples, indicating its essential role in aroma formation. It is known that linalool and p-cymene contribute to the sweet, citrus-like aroma of black teas and can be identified as the primary odorants in black teas; they also decrease during the fermentation process, as other studies have indicated [3,48]. However, no literature was found on the changes in citronellol and cyclohexanecarboxylic acid methyl ester during the processing of black tea; however, based on their odor, it is believed that these compounds have a positive effect on aroma formation. In addition to the volatiles mentioned above, the OAVs of fenchone and linalyl acetate were high, and they belonged to aroma active compounds with a green-like and herbal-like odor, which were thought to be associated with the greenness of the tea aroma profile [3]. Therefore, we believed that it has a positive effect on the reduction in green in the SCBT aroma. However, the content changes in citral, citronellal, 2-(2z)-2-pentenyl-furan, and 4-methylpent-3-en-2-one showed different trends in other studies [3,6,7,27], and their effects on aroma formation require further investigation due to the interactions between the aroma compounds.

Table 3.
The selected volatiles with OAVs above one in SCBT processing.

Figure 7.
Key aroma-compounds identified of SCBT in different processing stages by method of GC–MS analysis combined with OAVs (VIP > 1, OAV ≥ 1).
Figure 8 is a scatter plot of scores derived from the PCA of GC–MS, OAV, and descriptive sensory analysis data [4,26]. The two validation metrics, R2Y = 0.859 and Q2 = 0.695, indicate that the model has a high interpretative variance and a high predictive accuracy [4]. As depicted in the figure, the data points of the six samples can be distinguished without difficulty, and the correlation between 17 volatile compounds and sensory attributes can be observed; the size of the green circle represents OAV. We discovered that linalool, p-cymene, furan 2-pentylfuran, and cis-2-(2-Pentenyl) furan were associated with fruity, sweet, and citrus attributes. Similarly, floral attributes were closely related to 1-(2-methylphenyl)ethanone. In addition, (3Z)-3,7-dimethylocta-1,3,6-triene and D-limonene were associated with green and grassy characteristics. Moreover, linalool and p-cymene had the highest area of green circles and were closest to sweet and citrus characteristics, as evidenced by the high correlation between DT2 data and sweet and citrus aromas. Hence, we hypothesized that linalool and p-cymene are the primary SCBT responsible for the sweet and citrus-like aroma. In addition, in the vicinity of sweet and citrus-like characteristics, 2-pentylfuran and 2-(2Z)-2-pentenyl-furan contributed to the aroma only after linalool and p-cymene, consistent with their odor descriptions in Table 3. Based on the above, they were believed to have a moderating effect on the sweet and citrus-like aroma. The PCA results further validate the findings of the qualitative and quantitative analyses as well as the sensory analysis performed on the aroma active compounds in SCBT.

Figure 8.
Correlation ship analysis of odor attributes and aroma-active compounds in different processing stages of SCBT by principal components analysis (PCA), where V1–V17 represent the odor-active compounds identified in Table 3.
4. Conclusions
Changes in volatile compositions and odor profiles during SCBT processing were investigated in the present study. Sensory evaluation and GC–MS results demonstrated that volatiles changed dramatically during SCBT processing, while aroma properties varied at different processing stages. As observed, the fT samples had a green and grassy aroma, WT and FT samples had a floral and fruity aroma, and the DT1 and DT2 samples exhibited a unique sweet and citrus-like aroma, which confirms that the formation of black tea aroma is a dynamic process. In addition, we investigated the components and characteristics of the sweet and citrus-like aroma of SCBT. First, we identified 764 volatile compounds, such as linalool, linalyl acetate, methyl salicylate, trans-linalool oxide (furanoid), (Z)-hex-3-en-1-ol, (2R)-hexan-2-ol, (Z)-dec-2-enal, benzeneacetic acid, and p-cymene. Then, OPLS-DA analysis was employed to eliminate 47 critical differential volatile compounds, 17 of which had a OAV ≥ 1 and were identified as the differential aroma-active compounds that influenced the aroma formation of SCBT. Principal components analysis correlation reveals that linalool and p-cymene are the most significant aroma compounds, whereas the other 15 volatile compounds, such as 2-pentylfuran and 2-(2Z)-2-pentenyl-furan, may have a modified effect on the typical aroma of SCBT. In part, these results provided a clearer outline for the interpretation of the sweet and citrus-like aroma of SCBT, thereby providing a theoretical foundation for the enhancement of the SCBT manufacturing process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9070686/s1, Table S1: Relative contents of the volatile compounds in SCBT samples during the manufacture based on SPME-GC–MS; Figure S1: Volatile metabolite profiling of SCBT from the six processing stages using GC–MS. (A) Overview of the total ion chromatograms of six tea leaf samples form the plucked. (B) Overview of the coefficient of variations of six tea leaf samples form the plucked; Figure S2: Ring diagram of metabolite category composition of SCBT from the six processing stages using GC–MS. Figure S3: OPLS-DA score plots and validation model of 200 random permutation test for the OPLS-DA models generated from GC–MS data. (A) OPLS-DA score plots and (B) validation model for fT and WT groups; (C) OPLS-DA score plots and (D) validation model for WT and RT groups; (E) OPLS-DA score plots and (F) validation model for RT and FT groups; (G) OPLS-DA score plots and (H) validation model for FT and DT1 groups; (I) OPLS-DA score plots and (J) validation model for DT1 and DT2 groups. fresh tea leaves (fT), withering (WT), rolling (RT), fermentation (FT), first drying (DT1), and second drying (DT2).
Author Contributions
Conceptualization, B.J., L.Y. and K.L.; methodology, B.J. and L.Y.; validation, B.J., L.Y. and X.L. formal analysis, B.J. and R.H.; visualization, B.J.; writing—original draft preparation, B.J. and L.Y.; writing—review and editing, L.Y., X.L. and K.L.; supervision, X.L., X.Z. and M.L.; project administration, L.Y., X.L. and K.L.; funding acquisition, L.L., Q.W. and W.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by scientific and technological projects of Sichuan Province of China, (No. 2021YFN0091); Scientific Research Project of Yibin Vocational and Technical College (No. ZRKY21YB-06, ZRKY21YB-07); Science and Technology Innovation Team Project of Yibin Vocational and Technical College (No. ybzy21cxtd-03, ybzy20cxtd06).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Chen, X.; Chen, D.; Jiang, H.; Sun, H.; Zhang, C.; Zhao, H.; Li, X.; Yan, F.; Chen, C.; Xu, Z. Aroma characterization of Hanzhong black tea (Camellia sinensis) using solid phase extraction coupled with gas chromatography–mass spectrometry and olfactometry and sensory analysis. Food Chem. 2019, 274, 130–136. [Google Scholar] [CrossRef]
- Yao, H.; Su, H.; Ma, J.; Zheng, J.; He, W.; Wu, C.; Hou, Z.; Zhao, R.; Zhou, Q. Widely targeted volatileomics analysis reveals the typical aroma formation of Xinyang black tea during fermentation. Food Res. Technol. 2023, 164, 112387. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Y.; Wen, J.; An, K.; Wu, J.; Yu, Y.; Zou, B.; Guo, M. A comparative study of aromatic characterization of Yingde black tea infusions in different steeping temperatures. LWT 2021, 143, 110860. [Google Scholar] [CrossRef]
- Wang, C.; Lv, S.; Wu, Y.; Gao, X.; Li, J.; Zhang, W.; Meng, Q. Oolong tea made from tea plants from different locations in Yunnan and Fujian, China showed similar aroma but different taste characteristics. SpringerPlus 2016, 5, 576. [Google Scholar] [CrossRef]
- Huang, W.; Fang, S.; Wang, J.; Zhuo, C.; Luo, Y.; Yu, Y.; Li, L.; Wang, Y.; Deng, W.; Ning, J. Sensomics analysis of the effect of the withering method on the aroma components of Keemun black tea. Food Chem. 2022, 395, 133549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, P.; Le, M.; Qi, Y.; Yang, Z.; Hu, F.; Ling, T.; Bao, G. Improving flavor of summer Keemun black tea by solid–state fermentation using cordyceps militaris revealed by LC/MS-based metabolomics and GC/MS analysis. Food Chem. 2023, 407, 135172. [Google Scholar] [CrossRef]
- Ma, L.; Gao, M.; Zhang, L.; Qiao, Y.; Li, J.; Du, L.; Zhang, H.; Wang, H. Characterization of the key aroma-active compounds in high–grade Dianhong tea using GC-MS and GC-O combined with sensory–directed flavor analysis. Food Chem. 2022, 378, 132058. [Google Scholar] [CrossRef]
- Luo, X.; Li, L.; Ma, C.; Zhao, X. SPME-GC-MS Analysis of aroma components in black tea prepared with main tea cultivars in Sichuan. Food Sci. 2016, 37, 173–178. [Google Scholar]
- Mao, S.; Lu, C.; Li, M.; Ye, Y.; Wei, X.; Tong, H. Identification of key aromatic compounds in Congou black tea by PLSR with variable importance of projection scores and gas chromatography-mass spectrometry/gas chromatography-olfactometry. J. Sci. Food Agric. 2018, 98, 9066. [Google Scholar] [CrossRef]
- Guo, X.; Ho, C.; Wan, X.; Zhu, H.; Liu, Q.; Wen, Z. Changes of volatile compounds and odor profiles in Wuyi rock tea during processing. Food Chem. 2021, 341, 128230. [Google Scholar] [CrossRef]
- Ho, C.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Liu, D.; Yang, L.; Hu, W.; Kuang, L.; Huang, Y.; Teng, J.; Liu, Y. Multi–omics and enzyme activity analysis of flavour substances formation major metabolic pathways alteration during Congou black tea processing. Food Chem. 2023, 403, 134263. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, Z.; Zhang, Y.; Huang, H.; Ou, X.; Sun, Y. Identification of key components responsible for the aromatic quality of Jinmudan black tea by means of molecular sensory science. Foods 2023, 12, 1794. [Google Scholar] [CrossRef]
- Wu, H.; Huang, W.; Chen, Z.; Chen, Z.; Shi, J.; Kong, Q.; Sun, S.; Jiang, X.; Chen, D.; Yan, S. GC–MS-based metabolomic study reveals dynamic changes of chemical compositions during black tea processing. Food Res. Int. 2019, 120, 330–338. [Google Scholar] [CrossRef]
- Liu, N.; Shen, S.; Huang, L.; Deng, G.; Wei, Y.; Ning, J.; Wang, Y. Revelation of volatile contributions in green teas with different aroma types by GC–MS and GC–IMS. Food Res. Int. 2023, 169, 112845. [Google Scholar] [CrossRef]
- Hu, C.; Li, D.; Ma, Y.; Zhang, W.; Lin, C.; Zheng, X.; Liang, Y.; Lu, J. Formation mechanism of the oolong tea characteristic aroma during bruising and withering treatment. Food Chem. 2018, 269, 202–211. [Google Scholar] [CrossRef]
- Nie, C.; Zhong, X.; He, L.; Gao, Y.; Zhang, X.; Wang, C.; Du, X. Comparison of different aroma-active compounds of Sichuan dark brick tea (Camellia sinensis) and Sichuan fuzhuan brick tea using gas chromatography–mass spectrometry (GC-MS) and aroma descriptive profile tests. Eur. Food Res. Technol. 2019, 245, 1963–1979. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, C.; Xu, K.; Tian, C.; Zhang, M.; Lu, L.; Zhu, C.; Lai, Z.; Guo, Y. A Comprehensive investigation of macro-composition and volatile compounds in spring-picked and autumn-picked white tea. Foods 2022, 11, 3628. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Adelina, N.M.; Hu, J.; Zhang, L.; Zhao, Y. Comparative analysis of volatile profiles in four pine-mushrooms using HS-SPME/GC-MS and E-nose. Food Control 2022, 134, 108711. [Google Scholar] [CrossRef]
- Huang, B.; Wu, J.; Wu, X.; Shi, X.; Lin, H.; Chen, H. Analysis of volatile components in baihaoyinzhen white tea co-fermented with vanilla and citri grandis exocarpium by HS-SPME-GC-MS. IOP conference series. Earth Environ. Sci. 2021, 651, 42011. [Google Scholar] [CrossRef]
- Yue, C.; Cao, H.; Zhang, S.; Hao, Z.; Wu, Z.; Luo, L.; Zeng, L. Aroma characteristics of Wuyi rock tea prepared from 16 different tea plant varieties. Food Chem. 2023, 17, 100586. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Chen, S.; Yang, L.; Chen, X.; Jiang, H. Widely targeted metabolomic deciphers the vertical spatial distribution of flavor substances in Houttuynia cordata Thunb. J. Food Compos. Anal. 2023, 117, 105102. [Google Scholar] [CrossRef]
- Han, C.; Zheng, Y.; Wang, L.; Zhou, C.; Wang, J.; He, J.; Sun, Y.; Cao, J.; Pang, D.; Xia, Q. Contribution of process-induced molten-globule state formation in duck liver protein to the enhanced binding ability of (E,E)-2,4-heptadienal. J. Sci. Food Agric. 2023, 103, 3334–3345. [Google Scholar] [CrossRef]
- Xue, J.; Liu, P.; Yin, J.; Wang, W.; Zhang, J.; Wang, W.; Le, T.; Ni, D.; Jiang, H. Dynamic changes in volatile compounds of shaken black tea during its manufacture by GC × GC–TOFMS and multivariate data analysis. Foods 2022, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Y.; Wu, J.; Wen, J.; Yu, Y.; An, K.; Zou, B. GC-IMS and olfactometry analysis on the tea aroma of Yingde black teas harvested in different seasons. Food Res. Int. 2021, 150, 110784. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, T.; Li, W.; Jiang, Z.; Zhou, T.; Zhang, L.; Yu, Y. Widely targeted metabolomics using UPLC-QTRAP-MS/MS reveals chemical changes during the processing of black tea from the cultivar Camellia sinensis (L.) O. Kuntze cv. Huangjinya. Food Res. Int. 2022, 162, 112169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ho, C.T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [PubMed]
- Kraujalytė, V.; Pelvan, E.; Alasalvar, C. Volatile compounds and sensory characteristics of various instant teas produced from black tea. Food Chem. 2015, 194, 864–872. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Shi, J.; Yan, H.; Wang, M.; Ma, W.; Zhang, Y.; Peng, Q.; Chen, Y.; Lin, Z. Discrimination and identification of aroma profiles and characterized odorants in citrus blend black tea with different citrus species. Molecules 2020, 25, 4208. [Google Scholar] [CrossRef]
- Sharma, P.; Tudu, B.; Bhuyan, L.P.; Tamuly, P.; Bhattacharyya, N.; Bandyopadhyay, R. Detection of methyl salicylate in black tea using a quartz crystal microbalance sensor. IEEE Sens. J. 2016, 16, 5160–5166. [Google Scholar] [CrossRef]
- Wang, K.; Liu, F.; Liu, Z.; Huang, J.; Xu, Z.; Li, Y.; Chen, J.; Gong, Y.; Yang, X. Comparison of catechins and volatile compounds among different types of tea using high performance liquid chromatograph and gas chromatograph mass spectrometer. Int. J. Food Sci. Technol. 2011, 46, 1406–1412. [Google Scholar] [CrossRef]
- Wang, L.; Lee, J.; Chung, J.; Baik, J.; So, S.; Park, S. Discrimination of teas with different degrees of fermentation by SPME–GC analysis of the characteristic volatile flavour compounds. Food Chem. 2008, 109, 196–206. [Google Scholar] [CrossRef]
- Rokugawa, H.; Fujikawa, H. Evaluation of a new Maillard reaction type time-temperature integrator at various temperatures. Food Control 2015, 57, 355–361. [Google Scholar] [CrossRef]
- Guo, X.; Song, C.; Ho, C.; Wan, X. Contribution of L–theanine to the formation of 2,5-dimethylpyrazine, a key roasted peanutty flavor in Oolong tea during manufacturing processes. Food Chem. 2018, 263, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.; Zhang, Y.; Dai, W.; Guo, L.; Tan, J.; Peng, Q.; et al. Identification and quantification of key odorants in the world’s four most famous black teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, Y.; Dai, W.; Lv, H.; Mu, B.; Li, P.; Tan, J.; Ni, D.; Lin, Z. Aroma formation and dynamic changes during white tea processing. Food Chem. 2019, 274, 915–924. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, Y.; Xu, S.; Wei, Y.; Bao, G.; Dai, Q.; Deng, W.; Ning, J. Effects of dynamic and static withering technology on volatile and nonvolatile components of Keemun black tea using GC-MS and HPLC combined with chemometrics. LWT 2020, 130, 109547. [Google Scholar] [CrossRef]
- Du, X.; Song, M.; Baldwin, E.; Rouseff, R. Identification of sulphur volatiles and GC-olfactometry aroma profiling in two fresh tomato cultivars. Food Chem. 2015, 171, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.J.; Ho, C. Volatile sulfur compounds in tropical fruits. J. Food Drug Anal. 2018, 26, 445–468. [Google Scholar] [CrossRef] [PubMed]
- Omiadze, N.T.; Mchedlishvili, N.I.; Rodrigez-Lopes, K.; Abutidze, M.O.; Sadunishvili, T.A.; Pruidze, N.G. Biochemical processes at the stage of withering during black tea production. Appl. Biochem. Microbiol. 2014, 50, 437–441. [Google Scholar] [CrossRef]
- Li, Y.; He, C.; Yu, X.; Zhou, J.; Ntezimana, B.; Yu, Z.; Chen, Y.; Ni, D. Study on improving aroma quality of summer–autumn black tea by red–light irradiation during withering. LWT 2022, 154, 112597. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, L.; Wang, Q.; Yang, L.; Zheng, J.; Zhou, S.; He, C.; Jiao, W.; Xu, B.; Liu, K. The microbial communities in Zaopeis, free amino acids in raw liquor, and their correlations for Wuliangye-flavor raw liquor production. Food Sci. Nutr. 2022, 10, 2681–2693. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Xiao, Z. Evaluation of the synergism among volatile compounds in Oolong tea infusion by odour threshold with sensory analysis and E-nose. Food Chem. 2017, 221, 1484–1490. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef]
- Ghodake, H.M.; Goswami, T.K.; Chakraverty, A. Mathematical modeling of withering characteristics of tea leaves. Dry. Technol. 2006, 24, 159–164. [Google Scholar] [CrossRef]
- Tang, M.G.; Zhang, S.; Xiong, L.G.; Zhou, J.H.; Huang, J.A.; Zhao, A.Q.; Liu, Z.H.; Liu, A.L. A comprehensive review of polyphenol oxidase in tea (Camellia sinensis): Physiological characteristics, oxidation manufacturing, and biosynthesis of functional constituents. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2267–2291. [Google Scholar] [CrossRef]
- Wang, H.; Shen, S.; Wang, J.; Jiang, Y.; Li, J.; Yang, Y.; Hua, J.; Yuan, H. Novel insight into the effect of fermentation time on quality of Yunnan Congou black tea. LWT 2022, 155, 112939. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).