Valorisation of Waste Bread for the Production of Yeast Biomass by Yarrowia lipolytica Bioreactor Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Microorganism
2.1.2. Preparation of Waste Bread Powder
2.1.3. Enzymes and Antifoam
2.2. Hydrolysis of Waste Bread Powder
Lyophilisation
2.3. Culture Conditions
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
3.1. Hydrolysis of Waste Bread
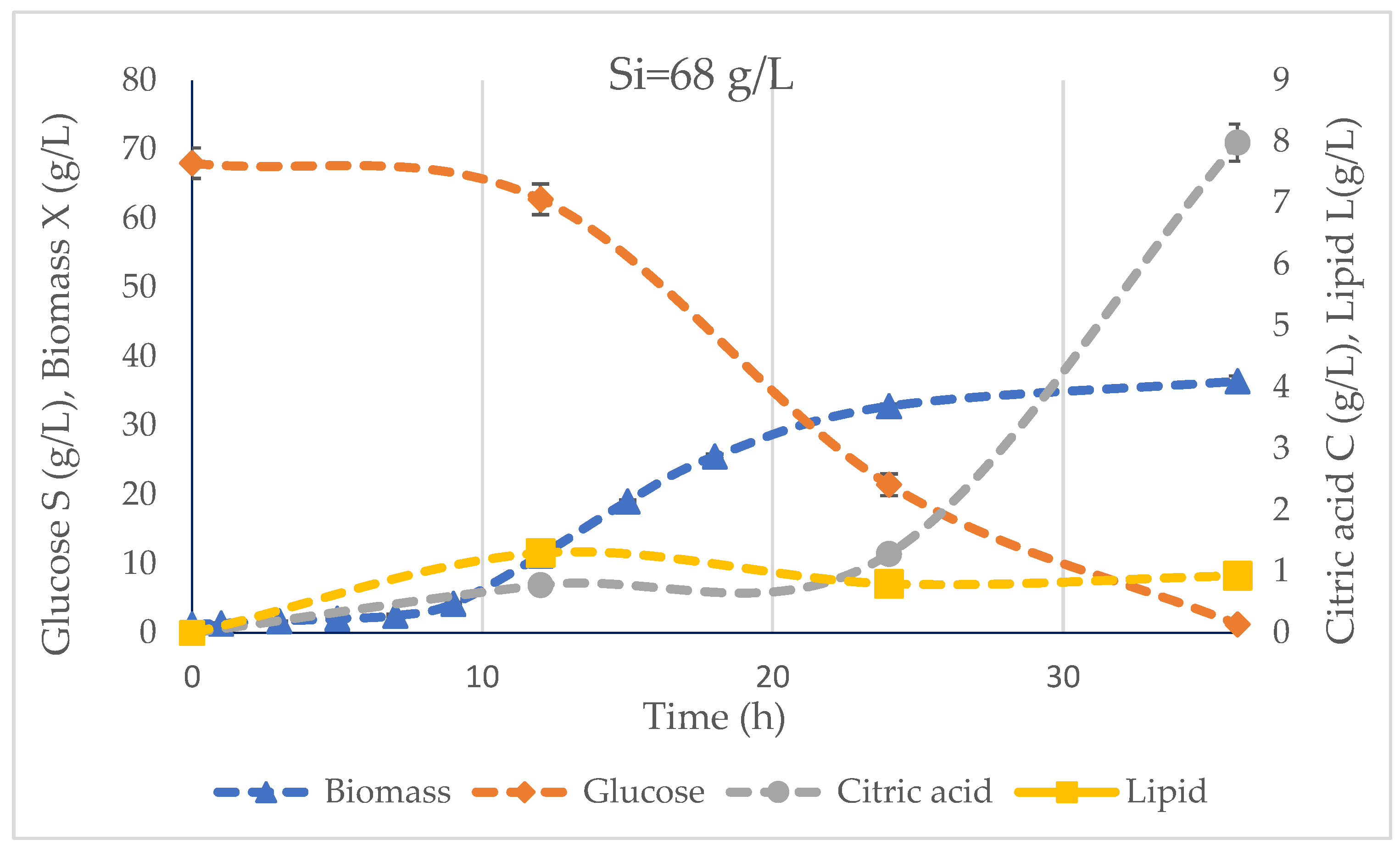
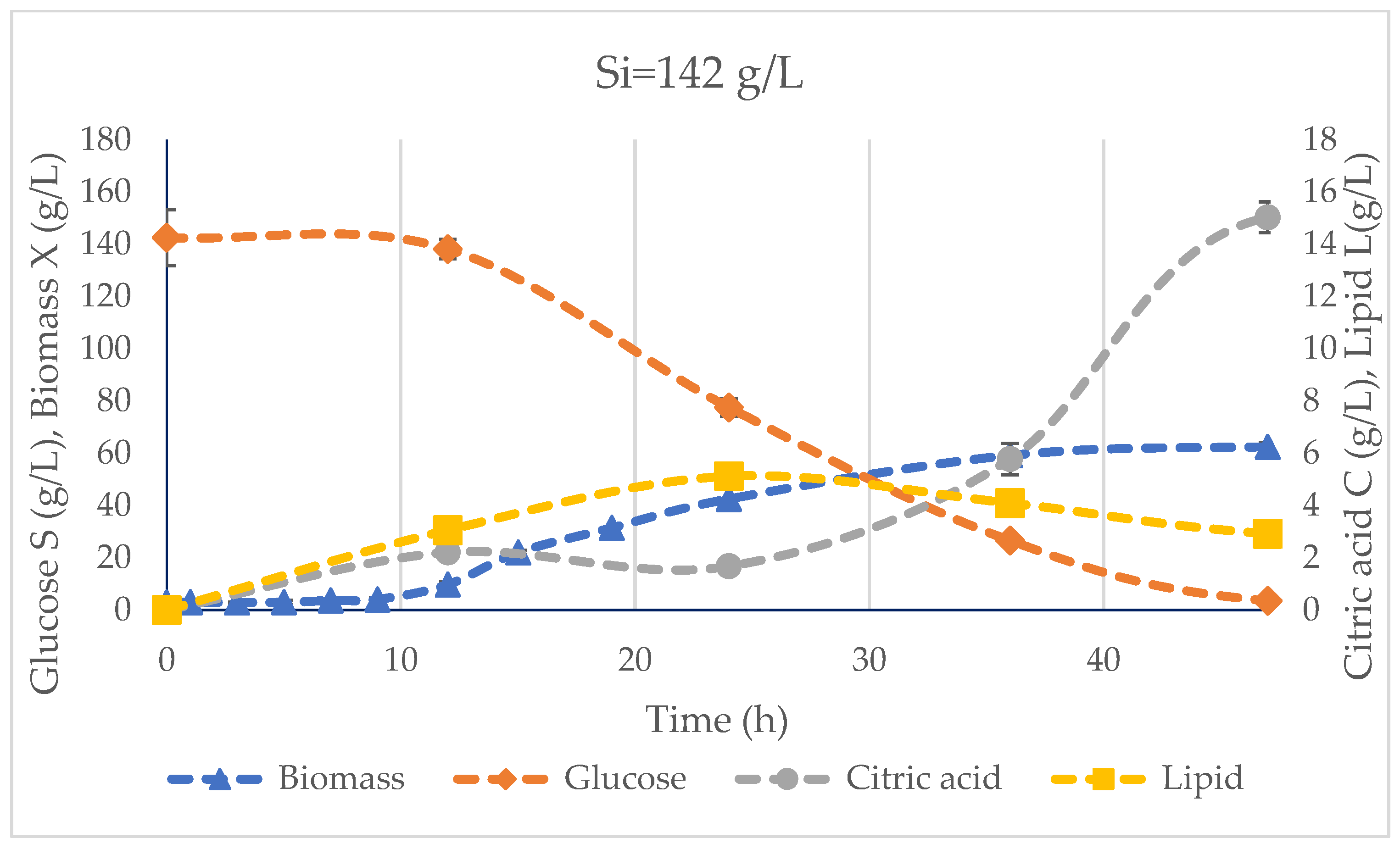
3.2. Bioreactor Fermentation
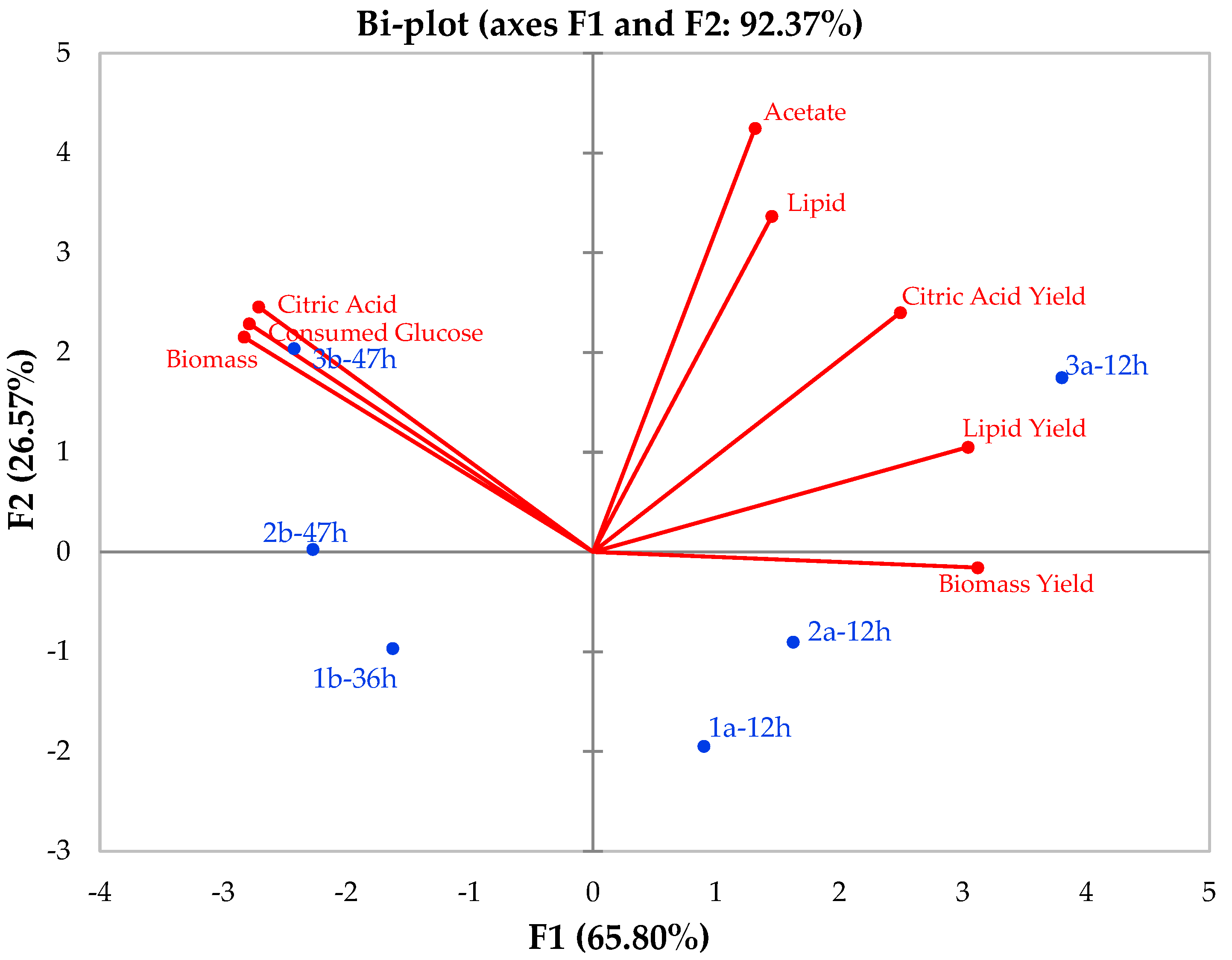
3.3. Principle Component Analysis (PCA) Results of Kinetics Parameters for Y. lipolytica K57 on a Waste Bread Hydrolysate Media in a Reactor Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yegin, S.; Saha, B.C.; Kennedy, G.J.; Berhow, M.A.; Vermillion, K. Efficient bioconversion of waste bread into 2-keto-D-gluconic acid by Pseudomonas reptilivora NRRL B-6. Biomass Conv. Bioref. 2020, 10, 545–553. [Google Scholar] [CrossRef]
- Ünal, M.Ü.; Chowdhury, G.; Şener, A. Effect of temperature and nitrogen supplementation on bioethanol production from waste bread, watermelon and muskmelon by Saccharomyces cerevisiae. Biofuels 2022, 13, 395–399. [Google Scholar] [CrossRef]
- Verni, M.; Minisci, A.; Convertino, S.; Nionelli, L.; Rizzello, C.G. Wasted bread as substrate for the cultivation of starters for the food industry. Front. Microbiol. 2020, 11, 293. [Google Scholar] [CrossRef]
- Ben Rejeb, I.; Charfi, I.; Baraketi, S.; Hached, H.; Gargouri, M. Bread Surplus: A cumulative waste or a staple material for high-value products? Molecules 2022, 27, 8410. [Google Scholar] [CrossRef]
- Melikoglu, M.; Webb, C. Use of waste bread to produce fermentation products. In Food Industry Wastes: Assesment and Recuperation of Commodities; Kosseva, M., Webb, C., Eds.; Elsevier BV: San Diego, CA, USA, 2013; pp. 63–76. [Google Scholar]
- Narisetty, V.; Cox, R.; Bommareddy, R.; Agrawal, D.; Ahmad, E.; Pant, K.K.; Chandel, A.K.; Bhatia, S.K.; Kumar, D.; Binod, P.; et al. Valorisation of xylose to renewable fuels and chemicals, an essential step in augmenting the commercial viability of lignocellulosic biorefineries. Sustain. Energy Fuels 2022, 6, 29–65. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Brancoli, P.; Narisetty, V.; Wallace, S.; Charalampopoulos, D.; Dubey, B.K.; Kumar, G.; Bhatnagar, A.; Bhatia, S.K.; Taherzadeh, M.J. Bread waste–A potential feedstock for sustainable circular biorefineries. Bioresour. Technol. 2022, 369, 128449. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, W.; Kawa-Rygielska, J. Ethanol fermentation of waste bread using granular starch hydrolyzing enzyme: Effect of raw material pretreatment. Fuel 2014, 134, 250–256. [Google Scholar] [CrossRef]
- Doi, T.; Matsumoto, H.; Abe, J.; Morita, S. Feasibility study on the application of rhizosphere microflora of rice for the biohydrogen production from wasted bread. Int. J. Hydrogen Energy 2009, 34, 1735–1743. [Google Scholar] [CrossRef]
- Mihajlovski, K.; Buntić, A.; Milić, M.; Rajilić-Stojanović, M.; Dimitrijević-Braković, S. From agricultural waste to biofuel: Enzymatic potential of a bacterial isolate Streptomyces fulvissimus CKS7 for bioethanol production. Waste Biomass Valor. 2021, 12, 165–174. [Google Scholar] [CrossRef]
- Han, W.; Huang, J.; Zhao, H.; Li, Y. Continuous biohydrogen production from waste bread by anaerobic sludge. Bioresour. Technol. 2016, 212, 1–5. [Google Scholar] [CrossRef]
- Han, W.; Liu, W.X.; Yu, C.M.; Huang, J.G.; Tang, J.H.; Li, Y.F. BioH2 production from waste bread using a two-stage process of enzymatic hydrolysis and dark fermentation. Int. J. Hydrogen Energy 2017, 42, 29929–29934. [Google Scholar] [CrossRef]
- Benabda, O.; Kasmi, M.; Kachouri, F.; Hamdi, M. Valorization of the powdered bread waste hydrolysate as growth medium for baker yeast. Food Bioprod. Process. 2018, 109, 1–8. [Google Scholar] [CrossRef]
- Asghar, M.; Umbreen, A.; Rafiq, S.; Sheikh, M.; Asad, M. Production of α-amylase by Arachniotus sp. using waste bread medium. Int. J. Agri. Biol. 2002, 4, 26–28. [Google Scholar]
- Cerda, A.; El-Bakry, M.; Gea, T.; Sanchez, A. Long term enhanced solid-state fermentation: Inoculation strategies for amylase production from soy and bread wastes by Thermomyces sp. in a sequential batch operation. J. Environ. Chem. Eng. 2016, 4, 2394–2401. [Google Scholar] [CrossRef]
- Leung, C.C.J.; Cheung, A.S.Y.; Zhang, A.Y.Z.; Lam, K.F.; Lin, C.S.K. Utilisation of waste bread for fermentative succinic acid production. Biochem. Eng. C. 2012, 65, 10–15. [Google Scholar] [CrossRef]
- Immonen, M.; Maina, N.H.; Wang, Y.; Coda, R.; Katina, K. Waste bread recycling as a baking ingredient by tailored lactic acid fermentation. Int. J. Food. Microbiol. 2020, 327, 108652. [Google Scholar] [CrossRef]
- Oda, Y.; Park, B.S.; Moon, K.H.; Tonomura, K. Recycling of bakery wastes using an amylolytic lactic acid bacterium. Bioresour. Technol. 1997, 60, 101–106. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Erten, H. Screening various Yarrowia lipoliytica strains for citric acid production. Yeast 2019, 36, 319–327. [Google Scholar] [CrossRef]
- Otto, C.; Holz, M.; Barth, G. Production of organic acids by Yarrowia lipoliytica. In Yarrowia lipoliytica: Biotechnological Applications; Barth, G., Ed.; Springer: Berlin, Germany, 2013; pp. 137–149. [Google Scholar]
- Zainuddin, M.F.; Kar Fai, C.; Mohamed, M.S.; Abdul Rahman, N.; Halim, M. Production of single cell oil by Yarrowia lipolytica JCM 2320 using detoxified desiccated coconut residue hydrolysate. Peer J. 2022, 10, e12833. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Erten, H. Lipids by Yarrowia lipoliytica strains cultivated on glucose in batch cultures. Microorganisms 2020, 8, 1054. [Google Scholar] [CrossRef]
- Paulino, B.N.; Molina, G.; Pastore, G.M.; Bicas, J.L. Current perspectives in the biotechnological production of sweetening syrups and polyols. Curr. Opin. Food Sci. 2021, 41, 36–43. [Google Scholar] [CrossRef]
- da Costa, A.M.; de Oliveira Lopes, V.R.; Vidal, L.; Nicaud, J.M.; de Castro, A.M.; Coelho, M.A.Z. Poly (ethylene terephthalate) (PET) degradation by Yarrowia lipolytica: Investigations on cell growth, enzyme production and monomers consumption. Process Biochem. 2020, 95, 81–90. [Google Scholar] [CrossRef]
- Juszczyk, P.; Rymowicz, W.; Kita, A.; Rywinska, A. Biomass production by Yarrowia lipolytica yeast using waste derived from the production of ethyl esters of polyunsaturated fatty acids of flaxseed oil. Ind. Crops Prod. 2019, 138, 111590. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, N.; Greisen, P.; Li, J.; Qiao, K.; Huang, S.; Stephanopoulos, G. Removal of lycopene substrate inhibition enables high carotenoid productivity in Yarrowia lipolytica. Nat. Commun. 2022, 13, 572. [Google Scholar] [CrossRef] [PubMed]
- Do, D.T.H.; Fickers, P. Engineering Yarrowia lipolytica for the Synthesis of Glutathione from Organic By-Products. Microorganisms 2020, 8, 611. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, Z.; Hu, P.; Zhang, S.; Luo, G. Two-stage fermentation enhanced single-cell protein production by Yarrowia lipolytica from food waste. Bioresour. Technol. 2022, 361, 127677. [Google Scholar] [CrossRef] [PubMed]
- Drzymała, K.; Mirończuk, A.M.; Pietrzak, W.; Dobrowolski, A. Rye and oat agricultural wastes as substrate candidates for biomass production of the non-conventional yeast Yarrowia lipolytica. Sustainability 2020, 12, 7704. [Google Scholar] [CrossRef]
- Jach, M.E.; Sajnaga, E.; Janeczko, M.; Juda, M.; Kochanowicz, E.; Baj, T.; Malm, A. Production of enriched in B vitamins biomass of Yarrowia lipolytica grown in biofuel waste. Saudi J. Biol. Sci. 2021, 28, 2925–2932. [Google Scholar] [CrossRef]
- Van Dien, S. From the first drop to the first truckload: Commercialization of microbial processes for renewable chemicals. Curr. Opin. Biotechnol. 2013, 24, 1061–1068. [Google Scholar] [CrossRef]
- Park, Y.K.; Ledesma-Amaro, R. What makes Yarrowia lipolytica well suited for industry? Trends Biotechnol. 2022, 41, 242–254. [Google Scholar] [CrossRef]
- Rakicka-Pustułka, M.; Mirończuk, A.M.; Celińska, E.; Białas, W.; Rymowicz, W. Scale-up of the erythritol production technology–process simulation and techno-economic analysis. J. Clean. Prod. 2020, 257, 120533. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Muniglia, L.; Chevalot, I.; Aggelis, G.; Marc, I. Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J. Appl. Microbiol. 2002, 92, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Lie, S. The EBC-ninhydrin method for the determination of free alpha amino nitrogen. J. Inst. Brew. 1973, 79, 37–41. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Fresenius J. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012.
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Tsakona, S.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Formulation of fermentation media from flour-rich waste streams for microbial lipid production by Lipomyces starkeyi. J. Biotechnol. 2014, 189, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Demirci, A.S.; Palabıyık, I.; Gümüş, T.; Özalp, S. Waste bread as a biomass source: Optimization of enzymatic hydrolysis and relation between rheological behavior and glucose yield. Waste Biomass Valor. 2017, 8, 775–782. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Erten, H. Production of oils and fats by oleaginous microorganisms with an emphasis given to the potential of the nonconventional yeast Yarrowia lipolytica. Crit. Rev. Biotechnol. 2018, 38, 1230–1243. [Google Scholar] [CrossRef]
- Dlangamandla, N.; Ntwampe, S.K.; Angadam, J.O.; Chidi, B.S.; Mewa-Ngongang, M. Kinetic parameters of Saccharomyces cerevisiae alcohols production using Nepenthes mirabilis pod digestive fluids-mixed agro-waste hydrolysates. Fermentation 2019, 5, 10. [Google Scholar] [CrossRef]
- Dorado, M.P.; Lin, S.K.; Koutinas, A.; Du, C.; Wang, R.; Webb, C. Cereal-based biorefinery development: Utilisation of wheat milling by-products for the production of succinic acid. J. Biotechnol. 2009, 143, 51–59. [Google Scholar] [CrossRef]
- Tsakona, S.; Skiadaresis, A.G.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Valorisation of side streams from wheat milling and confectionery industries for consolidated production and extraction of microbial lipids. Food Chem. 2016, 198, 85–92. [Google Scholar] [CrossRef]
- Karasu Yalcin, S.; Bozdemir, M.T.; Ozbas, Z.Y. Utilization of whey and grape must for citric acid production by two Yarrowia lipolytica strains. Food Biotechnol. 2009, 23, 266–283. [Google Scholar] [CrossRef]
- Wang, L.F.; Wang, Z.P.; Liu, X.Y.; Chi, Z.M. Citric acid production from extract of Jerusalem artichoke tubers by the genetically engineered yeast Yarrowia lipolytica strain 30 and purification of citric acid. Bioprocess Biosyst. Eng. 2013, 36, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Vieira, É.D.; Andrietta, M.D.G.S.; Andrietta, S.R. Yeast biomass production: A new approach in glucose-limited feeding strategy. Braz. J. Microbiol. 2013, 44, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zheng, Y.; Dorgan, K.M.; Chen, S. Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour. Technol. 2011, 102, 6134–6140. [Google Scholar] [CrossRef] [PubMed]
- Tsigie, Y.A.; Wang, C.Y.; Truong, C.T.; Ju, Y.H. Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate. Bioresour. Technol. 2011, 102, 9216–9222. [Google Scholar] [CrossRef]
- Tsigie, Y.A.; Wang, C.Y.; Kasim, N.S.; Diem, Q.D.; Huynh, L.H.; Ho, Q.P.; Truong, C.T.; Ju, Y.H. Oil production from Yarrowia lipolytica Po1g using rice bran hydrolysate. J. Biomed. Biotechnol. 2012, 2012, 378–384. [Google Scholar] [CrossRef]
- Ratledge, C.; Wynn, J.P. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–51. [Google Scholar]



| Composition | % |
|---|---|
| Ash | 1.30 ± 0.03 * |
| Crude Protein | 11.99 ± 0.18 * |
| Crude Fat | 0.71 ± 0.38 * |
| Total Carbohydrate | 82.01 ± 0.19 * |
| Moisture | 3.99 ± 0.04 |
| Starch to Glucose CY (%), Glucose Yield (g G/ g Substrate) | TKN to FAN CY (%), FAN Yield (mg FAN/g Substrate) | Glucose (g/L) | FAN (mg/L) | |
|---|---|---|---|---|
| A | 63.60%, 0.49 g/g | 3.39%, 0.64 mg/g | 82.37 ± 3.30 | 108.73 ± 8.39 |
| B | 97.10%, 0.75 g/g | 3.97%, 0.76 mg/g | 125.75 ± 4.35 | 127.88 ± 5.27 |
| Glucose (g/L) | FAN (mg/L) | |
|---|---|---|
| 1 | 49.42 ± 1.98 | 65.23 ± 5.03 |
| 2 | 96.82 ± 3.35 | 98.47 ± 4.06 |
| 3 | 146.00 ± 5.95 | 134.53 ± 5.47 |
| Si = 68 g/L | Si = 101 g/L | Si = 142 g/L | ||
|---|---|---|---|---|
| Parameters | Value | Value | Value | Unit |
| Glucose consumption (S) | 66.78 ± 0.03 | 101.23 ± 0.00 | 138.95 ± 0.00 | g/L |
| Biomass (X) | 36.44 ± 0.75 | 47.23 ± 0.83 | 62.48 ± 1.43 | g/L |
| Acetic acid production | 1.35 ± 0.06 | 1.49 ± 0.09 | 3.30 ± 0.29 | g/L |
| CA production (C) | 7.99 ± 0.30 | 12.69 ± 0.08 | 15.03 ± 0.59 | g/L |
| Yield biomass (YX/S) | 0.55 ± 0.01 | 0.47 ± 0.01 | 0.45 ± 0.01 | g/g |
| Yield citric acid (YC/S) | 0.12 | 0.13 | 0.11 | g/g |
| Consumption rate (RS) | 1.29 | 3.00 | 2.46 | g/L/h |
| CA production rate (QC) | 0.19 | 0.34 | 0.30 | g/L/h |
| Max. consumption rate (RSmax) | 3.44 | 5.30 | 5.06 | g/L/h |
| Max. production rate (QCmax) | 0.56 | 0.80 | 0.84 | g/L/h |
| Max. growth rate (qmax) | 2.47 | 2.20 | 2.49 | g/L/h |
| Specific growth rate (µ) | 0.30 | 0.30 | 0.37 | 1/h |
| Doubling time (td) | 2.28 | 2.30 | 1.87 | h |
| Max. lipid production (Lmax) | 1.30 ± 0.03 | 2.95 ± 0.29 | 5.12 ± 0.11 | g/L |
| Max. lipid yield (Y(L/S)max) | 0.12 | 0.22 | 0.32 | g/g |
| C/N molar ratio | 29 | 40 | 49 | mol/mol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carsanba, E.; Agirman, B.; Papanikolaou, S.; Fickers, P.; Erten, H. Valorisation of Waste Bread for the Production of Yeast Biomass by Yarrowia lipolytica Bioreactor Fermentation. Fermentation 2023, 9, 687. https://doi.org/10.3390/fermentation9070687
Carsanba E, Agirman B, Papanikolaou S, Fickers P, Erten H. Valorisation of Waste Bread for the Production of Yeast Biomass by Yarrowia lipolytica Bioreactor Fermentation. Fermentation. 2023; 9(7):687. https://doi.org/10.3390/fermentation9070687
Chicago/Turabian StyleCarsanba, Erdem, Bilal Agirman, Seraphim Papanikolaou, Patrick Fickers, and Huseyin Erten. 2023. "Valorisation of Waste Bread for the Production of Yeast Biomass by Yarrowia lipolytica Bioreactor Fermentation" Fermentation 9, no. 7: 687. https://doi.org/10.3390/fermentation9070687
APA StyleCarsanba, E., Agirman, B., Papanikolaou, S., Fickers, P., & Erten, H. (2023). Valorisation of Waste Bread for the Production of Yeast Biomass by Yarrowia lipolytica Bioreactor Fermentation. Fermentation, 9(7), 687. https://doi.org/10.3390/fermentation9070687








