Abstract
In this study, we compared the digestibility levels and in vitro fermentation parameters of total mixed rations (TMRs) containing 20% and 40% defatted black soldier fly (Hermetia illucens) larvae (BSF) as a substitute for soybean meal (SBM) in the basal ration (60% roughage/40% concentrated feed) of dairy cows. We evaluated the volatile fatty acid (VFA), total gas production, methane emission, ammonia, pH, carbon dioxide, in vitro dry matter digestibility (IVDMD), and neutral detergent fiber digestibility (IVNDFD) of the TMR0 (basal), TMR20 (20% BSF included), and TMR40 (40% BSF included) diets at the end of 24 and 48 h of incubation. Significantly lower levels of ammonia formation were found in the TMR20 and TMR40 groups at 24 and 48 h (p < 0.001). An increase in total VFA levels was observed in the TMR0 group at 24 h (p < 0.001). The highest IVDMD was determined in TMR20 and TMR40 at 24 h. The highest IVNDFD value was observed in TMR20 at 24 h and in TMR40 at 48 h. The substitution of 20% and 40% of SBM with BSF positively affected IVDMD and IVNDFD (p < 0.001). TMR20 and TMR40 had the highest cumulative gas production at 48 h of incubation (p < 0.05). In conclusion, the use of BSF had a positive impact on digestibility and in vitro rumen fermentation. Therefore, we recommend the use of BSF in formulating dairy cow rations.
1. Introduction
It is predicted that the rapidly increasing human population will lead to a 70% rise in the demand for animal-origin food, such as eggs, meat, and milk by 2050 [1]. This is expected to further drive the demand for livestock feed [2]. Soybean meal (SBM) is a widely used protein source in animal nutrition. However, concerns regarding sustainable nutrition in soybean production have been raised [3]. In recent years, poultry production has shown the highest growth rate, followed by significant increases in pig production. The growth in milk and meat production from ruminant animals has been relatively slower. Although not as much as pigs and poultry, the high demand for high-quality feeds like wheat and soybean in ruminant feeding, which can also be consumed directly by humans, coupled with the growing human population, has resulted in deforestation and reduced forest areas for agriculture [4].
While ruminants primarily rely on legumes, grass, and by-products as feed, they often require protein sources to optimize the production levels. SBM is commonly used as a protein source for ruminants due to its richness in essential amino acids such as tryptophan, threonine, and lysine [5,6]. Additionally, there is a high demand for oilseed by-products, particularly for monogastric animal species. Therefore, the quest for sustainable alternative feed sources for ruminant feeding, other than SBM, is gaining momentum.
The potential applications of insects in industry and biotechnology are vast and exciting. From agriculture to waste management, from sustainable feed production to circular economy practices, insects are paving the way for innovative and environmentally friendly solutions. Insects have emerged as potential alternative feed sources. Due to their high protein and fat content, insects can serve as protein and energy sources in animal diets [7]. Nevertheless, their consumption as an alternative source of nutrients for ruminants is not so widespread and the European Union restriction on ruminants is probably due to the concern of mad cow disease [8]. In the USA, only the black soldier fly (Hermetia illucens) is included in aquaculture. In Canada, Hermetia illucens larvae are authorized in aquaculture and poultry. Brazil has not developed specific legislation in this respect, and insects are only allowed in non-ruminant animals [9]. In countries such as China or South Korea, there are no limitations [10]. Also, there may be insect-related concerns over food safety. But insect contamination with undesirable organic substances such as PCBs, OCPs, BFRs, PFRs, and dioxin, as well as As, Cd, Co, Cr, Cu, Ni, Pb, Sn, and Zn, is lower than meat products [11]. In addition to their nutritional quality, insects offer advantages such as low feed conversion rates, minimal water requirements, and the efficient conversion of organic waste into body mass within a short timeframe [12]. Several studies showed that local waste, such as from restaurants, agriculture/crop production, wholesale markets, or chicken manure, serve as a substrate for insect production [10]. Another positive aspect of insects is that they can feed on the decomposed substrate and even on difficult-to-degrade substances. Also, some species utilize plastic waste materials by eating and digesting. Even though plastics are durable and resistant to damage and biodegradation, insects can use them as food [13]. Through efficient digestion involving micro-organisms, the insect mass can be utilized for feed and fertilizer purposes [14]. Various insect species, including black soldier fly (BSF) larvae, have been evaluated in in vitro studies as potential alternative feeds for ruminants [2]. However, research on the use of insects in the diet of ruminant animals is still in its early stages, with only a limited number of articles published to date. The existing studies primarily focus on evaluating in vitro fermentation parameters of different insect species [2,15]. The nutritional composition of each insect varies depending on the species. The review of Makkar et al. [16] describes that the crude protein (CP) content of insect meals is high, varying from 42% to 63%, similar to that of soymeal, common in ruminant rations.
Black soldier fly (Hermetia illucens) larvae (BSF) are widely utilized in animal feed research due to their nutrient composition and potential for reducing feed costs. They have been reported to contain a protein content of 49–59% and an in vitro protein digestibility of 66–68% [17]. A chemical analysis of the insects showed that they were rich in fat (14–26%) with a high proportion of unsaturated fatty acids [18]. When the dietary fat content is >10%, the rumen fermentation of dairy cows is reduced, especially when the dietary unsaturated fatty acid is more apparent [19]. Unsaturated fatty acids also lead to reduced dry matter intake, milk yield, and milk fat [20]. For these reasons, defatted BSF larvae meal was used in this study. While there has been one in vitro fermentation study using BSF as a substitute for SBM, it was conducted with only one roughage feed source as the basal diet [2]. However, no study has been conducted on incorporating BSF into standard ruminant total mixed rations (TMRs). Therefore, the objective of this study was to evaluate the effects of including 20% and 40% defatted BSF larvae meal instead of SBM in TMRs on in vitro fermentation parameters (total gas production, ammonia, volatile fatty acids, methane emission, and CO2 production) and TMR digestibility.
2. Materials and Methods
2.1. Rumen Fluid Collection and Donor Cows
Two ruminally cannulated non-lactating Holstein cows (approximately 3 years old, with average body weight of 530 kg) were used as donors. Approximately 1.5 L of rumen fluid was collected from different areas in the rumen after 3 h of morning feeding. The cows were fed TMRs containing 13% crude protein (CP), 93% organic matter (OM), 45% neutral detergent fiber (NDF), 28% acid detergent fiber (ADF), and 6% acid detergent lignin, and had free access to drinking water. Rumen fluid was collected using a catheter, filtered through two layers of cheesecloth, placed in a pre-warmed thermos flask at 39 °C, and transferred to the laboratory within 15 min.
2.2. Basal Diet and Black Soldier Fly Larvae
The basal TMR used in the study consisted of corn silage, wheat straw, alfalfa hay, barley paste, and SBM, with a roughage-concentrated feed ratio of 60:40. BSF larvae were added as a substitute for SBM in different proportions (0%, 20%, and 40% on dry matter basis) to the dairy cattle TMR. The TMR was prepared for a 50-month-old Holstein dairy cow weighing 550 kg, with a body condition score of 3.25 and milk yield of 24 L/day, in the 9th week of lactation. Defatted black soldier fly larvae meal was obtained from a commercial company (Hibiotek Biotechnology, İzmir, Turkey, https://en.hibiotek.com; Accessed on 9 June 2023).
2.3. Determination of Chemical Compositions
The basal and experimental TMRs were dried at 55 °C for 48 h (VWR, Venti-line, Portland, OR, USA). After drying, they were ground in a mill (Retsch SM100, Düsseldorf, Germany) using a 1 mm diameter sieve. The chemical and nutrient compositions of basal (TMR0) and experimental (TMR20 and TMR40) TMRs are given in Table 1. Ash, crude protein (CP), and ether extract (EE) contents of the three TMR diets were determined based on the method reported by AOAC [21]. The NDF and ADF contents were analyzed according to procedure explained by Van-Soest et al. [22]. The soluble nitrogen in borate phosphate solution, which represents the non-protein nitrogen fraction of CP, was determined according to Krishnamoorth et al. [23]. The level of non-fibrous carbohydrate (NFC) was calculated using the following equation given in NRC [24]:
NFC = 100 − (NDF% + CP% + EE% + Ash%)

Table 1.
Feed ingredients and chemical composition of experimental total mix rations.
2.4. In Vitro Experimental Design and Incubation Procedure
In order to determine the effects of BSF inclusion on pH, total gas production, volatile fatty acids (VFAs), ammonia-nitrogen (NH3-N), metabolic energy, net energy lactation, methane emission, and carbon dioxide levels of TMR diets, in vitro experiments were conducted. Three experimental groups were used as substrate (TMR0, TMR20, and TMR40) and each group had fifteen replicates. This experimental design was repeated in two runs conducted on different days. Five blanks without substrate (buffered rumen fluid) were included in each run. Each 120 mL fermentation glass bottle contained 460 mg of the substrate and was supplemented with 40 mL of fresh buffer mixture at pH 6.8 and 20 mL of rumen fluid under continuous CO2 flushing [25]. The buffer mixture contained distilled water, macro-mineral solution (0.6 g MgSO4, 5.7 g Na2HPO4, and 6.2 g KH2PO4, in 1 L of bi-distilled water), buffer solution (4 g NH4HCO3 and 35 g NaHCO3 in 1 L of bi-distilled water), trace mineral solution (10 g MnCI2∙4H2O, 13.2 g CaCl2∙2H2O, 0.8 g FeCl3∙6H2O, and 1 g CoCl2∙6H2O in 100 mL of distilled water), resazurin solution (0.1 g resazurin in 100 mL of distilled water), and reducing solution (285 mg Na2S∙7H2O and 4 mL of 1 N NaOH in 96 mL distilled water) [26]. All bottles were incubated for 48 h at 39 °C, and manual shaking was performed at specific time intervals during the fermentation period (1 h, 3 h, 5 h, 7 h, 15 h, 23 h, 33 h, and 47 h).
2.5. Total Gas Production and Fermentation Parameters
Total gas production and fermentation parameters were measured using an automated modular in vitro gas system. The system included sensors in the caps of the bottles that provided gas pressure and bottle temperature data at 5 min intervals. A computer software displayed the information, performed the calculations, and reported the results. Data on gas pressure and bottle temperature were simultaneously obtained from 50 in vitro gas system modules at 5 min intervals. The fermentation kinetics of TMR diet materials were calculated using the logistic model, specifically the “double-pool logistic equation” model and “curve subtraction” technique [27,28]. Gas volume were recorded at 6, 12, 24, and 48 h of incubation. The lag time was computed using the model described by Tunkala et al. [29]:
wherein A indicates the y-intercept, B = rate of gas production (mL/h), C = maximum gas produced (mL/g DM), X = total time (h) of incubation, and M = the time (h) at which the maximum rate of gas production was reached.
y = A + Cexp{−exp [−B(X − M)]}
Metabolic energy (ME) and net energy lactation (NEL) of incubated TMR diets were calculated using equations reported by Menke and Steingass (1988) [30]:
wherein GP indicates 24 h net gas production (mL/200 mg DM), EE is ether extraction, and CP is crude protein.
ME (MJ/kg DM) = 0.157 × GP + 0.0084 × CP + 0.022 × EE − 0.0081 × ash + 1.06
NEL (MJ/kg DM) = 0.115 × GP + 0.0054 × CP + 0.014 × EE − 0.0054 × ash − 0.36
Microbial biomass production at 24 and 48 h (MBP; mg/g DM) were estimated according to Blümmel et al. [31]:
where GP is the 24 and 48 h net gas production (mL/g DM), and IVDMD is the in vitro digestibility of dry matter.
MBP (mg/g DM) = IVDMD − (GP24,48 × 2.2)
2.6. Determination of In Vitro Digestibility of Dry Matter and Neutral Detergent Fiber
In vitro digestibility levels were determined using the ANKOM DaisyII incubator (ANKOM Technology Corporation, Macedon, NY, USA). TMR samples weighing approximately 0.50 g were placed in ANKOM F57 filter bags and heat-sealed. The bags were then placed in glass jars containing 1800 mL of a buffer solution mixture (A and B) as described by ANKOM Technology. Each jar received 400 mL of rumen fluid. Digestibility measurements were conducted using one jar for 24 h incubation and another jar for 48 h incubation. In vitro dry matter digestibility (IVDMD) and in vitro NDF digestibility (IVNDFD) after 24 and 48 h of incubation were calculated as follows:
where W1 indicates the weight of the filter bag, W2 is the weight of the sample, W3 is the final weight (filter bag + sample), NDFFeed is % of NDF of feed (%DM), DMFeed is % of dry matter contained in the feed, and C1 is the correction factor (blank filter bag NDF value).
IVDMD (%DM) = 100 − [(W3 − (W1 × C1)) × 100] (W2 × %DMFeed)
IVNDFD (% DM) = 100 × [(W2 × %NDFFeed) − (W3 − (W1 × C1))]/(W2 × %DMFeed)
2.7. Determination of Volatile Fatty Acids (VFAs) and Ammonia-Nitrogen (NH3-N)
To determine VFAs and NH3-N, fermentation liquid samples were taken at the 24 and 48 h of incubation. Three in vitro bottles were opened and fermentation liquid samples were taken for volatile fatty acids (VFAs) and ammonia-nitrogen (NH3-N), 1 mL sample for each opened bottle was mixed with the eppendorfs which contained 0.2 mL of %25 meta-phosphoric acid [32], and another 1 mL sample was mixed with 20 µL of sulfuric acid containing eppendorf tubes for NH3-N analysis. Samples were kept in a freezer (–20 °C) until analysis. VFA analysis was performed by gas chromatography (GC-6890N, Shimadzu®, Tokyo, Japan) equipped with split injector, flame ionization detector, and capillary column (30 m × 320 µm × 1.00 µm; Agilent J&W GC column). Short-chain fatty acid (acetic, propionic, isobutyric, butyric, isovaleric, n-heptanoic, isovaleric, and valeric acid) mixture (99.5% purity, Chem service, West Chester, USA) was used as a quantitative external standard. The oven temperature program was 80 °C for 1 min, 80–120 °C increasing by 20 °C/min, and 230 °C for 3 min. Injection port temperature was 250 °C, injection volume 1 µL, and split ratio 10:1. Detector temperature was 250 °C, carrier gas H2 (purity ≥ 99.998%) flow 30 mL/min, and air flow 300 mL/min. The samples were thawed at room temperature and centrifuged at 14.500× g for 10 min. The supernatant was transferred to dry and clean vials before gas chromatography procedure. The NH3-N levels were elucidated by the spectro-photometric method developed by Weatherburn [33].
2.8. Detemination of pH, Methane Emission, and Carbon Dioxide
The fermentation bottle caps were removed and the pH was immediately determined at 24 and 48 h. (LAQUA F-72, HORIBA Scientific, Kyoto, Japan). Stoichiometrical models used for estimating methane [34] and carbon dioxide [35] from VFA composition as follows:
Methane (CH4), mmol/L = 0.45 × acetate − 0.275 × propionate + 0.40 × butyrate
Carbon dioxide (CO2), mmol/L = acetate/2 + propionate/4 + 1.5 × butyrate
2.9. Statistical Analysis
Before any statistical analyses were conducted, Kolmogorov–Smirnov test was applied to check the normality of all data. Levene’s test was used to test the homogeneity of variance. When normal distribution and homogeneity of variances were met, the experimental data were analyzed by one-way ANOVA (analysis of variance) of SPSS (SPSS v 22.0, Armonk, NY, USA. SPSS, Inc.) software. Polynomial contrasts were used to determine the significance of linear or quadratic models describing the response in the variables to the increasing levels of insects in the diet. Data were analyzed based on the following model:
Yij = µij + Si + ei, where Yij—overall mean common for each parameter studied, Si—the effect of black soldier fly larvae on the variables studied, and ei—standard error. The values are represented as means with standard errors. Tukey’s test was used to estimate mean differences between experimental groups. At p < 0.05, differences were considered statistically significant (SPSS 22.0 software, IBM Corp., Armonk, NY, USA).
3. Results
The effects using black soldier fly larvae instead of soybean meal in TMRs on the 24 h in vitro fermentation and digestibility levels determined from a Daisyıı incubator are given in Table 2. TMR20 was found to be higher in terms of in vitro neutral detergent fiber digestibility (IVNDFD) (p < 0.05). According to the total VFA results, the group without BSF (TMR0) was found to be higher compared to TMR20 and TMR40 (p < 0.05). The TMR20 group exhibited lower NH3-N levels compared to TMR0 and TMR40 (p < 0.05).

Table 2.
Effect of different inclusion levels of insects in the basal diet on rumen fermentation (n = 30) and digestibility (n = 24) parameters from 24 h in vitro incubation (Mean ± SEM).
In Table 3, TMR20 and TMR40 were found to be higher in terms of IVDMD (p < 0.001). TMR40 exhibited the highest IVNDFD level (p < 0.001). The TMR20 and TMR40 groups produced lower NH3-N than TMR0 after the 48 h incubation (p < 0.05).

Table 3.
Effect of different inclusion levels of insect in the basal diet on rumen fermentation (n = 30) and digestibility (n = 16) parameters from 48 h in vitro incubation (Mean ± SEM).
The cumulative in vitro gas production from 6 to 48 h was also evaluated, and only the total gas production at 48 h was higher in the TMR20 and TMR40 diets compared to the others (p < 0.05) (Table 4). This is further illustrated in Figure 1, where the use of 20% and 40% BSF instead of SBM in TMR resulted in higher gas production after 48 h of incubation.

Table 4.
Effect of different inclusion levels of black soldier fly larvae meal in the basal diet on in vitro profile (Mean ± SEM).
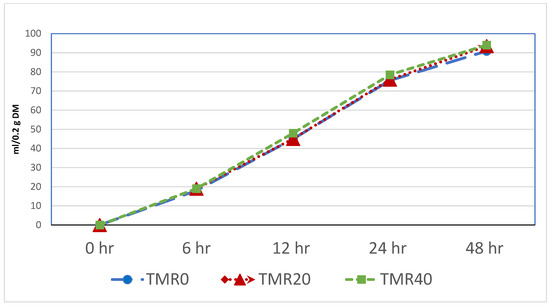
Figure 1.
In vitro cumulative total gas production of TMRs.
4. Discussion
In the study, we prepared TMRs with similar nutrient levels by replacing SBM with increasing levels of BSF larvae. The observed difference in NH3-N production between TMRs containing BSF and SBM can be attributed to several factors related to the protein fractions and degradability. SBM is known to have a high level of the rapidly degrading protein fraction, which leads to increased ammonia production in the rumen [36]. Previous in vitro studies have shown that reducing the SBM level in substrates resulted in lower NH3-N production [37]. Although the TMRs with BSF larvae and the TMR containing only SBM had similar CP levels, the lower NH3-N in the TMR20 and TMR40 diets can be explained by the balance between degradable (RDP) and non-degradable protein (RUP) fractions. The balance of RDP and RUP is a crucial factor influencing protein utilization in ruminants [38]. Similar to this study, Jayanegara et al. [2] increased the CP levels of TMRs by adding 50% BSF larvae instead of SBM and observed a decrease in NH3-N levels. They attributed this to the protein fractions of SBM, which have high levels of B1 (rapidly degraded protein) and B2 (intermediately degraded protein).
Increasing the carbohydrate level in the diets can lead to a decrease in both ammonia and microbial protein, but the efficiency of microbial protein synthesis depends on the level of carbohydrate fermentation [37]. In this study, the A fraction of TMRs prepared using BSF larvae was lower, and the NFC content was similar to the TMR without BSF larvae. Protein utilization is known to be proportional to NFC content [39]. A relatively lower ammonia concentration was observed in an in vitro study where Gryllus bimaculatus was used at 25% inclusion instead of SBM [18]. However, different insect species can exhibit variable fermentation patterns in the rumen, as highlighted in a study with Blatta lateralis beetles, which produced more NH3-N [40]. This variation is attributed to differences in protein, fat, and chitin levels among insect species. Another study on in vitro fermentation of SBM and different insect meals reported lower NH3-N, and that the intestinal degradability of insects may be an important advantage [41]. Decreased ammonia in insect-added TMRs can also be attributed to protein fractions of BSF that are not degraded in the rumen, such as neutral detergent insoluble crude protein (NDICP) and acid detergent insoluble crude protein (ADICP); however, these parameters were not evaluated in this study. This result can also be attributed to rumen-bacteria-stimulating substances, protein-producing ability, or changes in the number of micro-organisms with proteolytic activity [42].
Differences in rumen fermentation parameters can be explained by chitin, crude protein, crude fat contents, and fatty acid profiles present in BSF larvae [41]. Previous studies evaluating the addition of insects or their oils to ruminant diets have shown that insects generally have lower nutritional value due to reductions in IVDMD caused by their chitin and high fat content [2,43]. Interestingly, in this study, the TMR20 and TMR40 groups using BSF larvae exhibited higher IVDMD and IVNDFD levels. Chitin, present in insects, is considered a hard-to-degrade fiber that can reduce nutrient digestibility and absorption [44]. Therefore, removing chitin content from insect products has been suggested as a way to improve nutrient availability and digestibility [45]. However, a study with Jamaican cricket beetles found no difference in in vitro OM digestibility when the chitin content was manually or chemically removed [2]. Similarly, Phesatcha et al. [46] reported an increase in the IVDMD rate when cricket beetles were used instead of SBM. These findings suggest that the negative effect of chitin on in vitro digestibility may not be consistent and may depend on other factors, such as the overall diet composition and ratio of roughage to concentrate feeds, rather than the direct inclusion of insects. In some studies, the decreased IVDMD level with the addition of insect meal was attributed to the increased crude fat level in the ration caused by insects. The fat can coat the surface of fiber cells in the ration and impede the microbial breakdown of cellulose particles in the rumen. In this study, the use of defatted BSF larvae meal instead of soybean meal did not significantly increase the ether extraction level of the TMRs. The increased IVDMD and IVNDFD levels observed in the TMR20 and TMR40 groups may be attributed to the levels of NFCs and starch in the rations. Pinho et al. [47] found that increasing NFC levels influenced in vitro NDF digestibility, and a high NFC/NDF ratio was associated with reduced NDF digestibility. In this study, the NFC/NDF ratios of the TMR0, TMR20, and TMR40 rations were 0.76, 0.72, and 0.73, respectively. Therefore, it is expected that the TMR20 and TMR40 rations containing BSF larvae had higher IVNDFD rates. A high NFC/NDF ratio can lead to a decrease in pH, which may negatively affect the growth of microbial populations responsible for fiber digestion [48]. The higher total VFA level observed in the TMR0 group at the 24 h measurement also supports this finding. It has been shown in other studies that high VFA levels can negatively affect NDF digestibility by causing a decrease in pH [49]. Similarly, Jayanegara et al. [2] reported that a ration consisting of 60% forage, 20% soybean meal, and 20% BSF larvae produced higher total VFA than the ration containing 60% forage and 40% BSF larvae after a 24 h incubation. In another in vitro study, it was found that the addition of BSF larvae to the ration at a level replacing 30% of the concentrated feed resulted in a lower total VFA production than the ration without BSF substitution [25]. Although the protein and fat contents of the tested TMRs were similar, this decrease in VFA production may be related to the chitin and crude protein present in insects, which can affect rumen fermentation. Previous in vitro studies have emphasized the buffering capacity of proteins, the suppressive effect of fats on cellulolytic bacteria, and the difficulty of digesting chitin [41,50,51].
The total gas production measured at 48 h was found to be higher in the TMR20 and TMR40 rations containing BSF larvae than in the TMR0 group without BSF larvae. This increase in gas production may be attributed to the higher starch and carbohydrate levels in the TMRs containing BSF larvae [2]. The starch level of soybean meal used in TMRs was determined to be 5.5% and that of BSF larvae meal 6.19%. Glycogen is a form of starch found in animal tissue and is hence called animal starch. In the polarimetric method that is used for starch determination, the starch is released from the sample by boiling in dilute hydrochloric acid. This procedure effectively gelatinizes the starch granules and simultaneously hydrolyzes the starch to glucose in a single step. Fischer et al. [52] determined almost 8% glycogen level in BSF larvae. Thus, the polarimetric method might determine glycogen as starch in the TMRs of this study. Nutrients such as NDF, ADF, cellulose, and lignin, which are structural components of the feed, tend to decrease gas production, whereas soluble carbohydrates, starch, and protein increase it [53]. The TMR20 and TMR40 diets had higher levels of starch and, consequently, produced more gas during the 48 h incubation period. In a previous in vitro study, the total gas production at 24 and 48 h of incubation were lower in the ratio using 50% BSF larvae instead of SBM [2] and this was associated with the fat content of BSF larvae. Lipids in the ration are converted into glycerol and various fatty acids in the rumen. The resulting unsaturated fatty acids undergo biohydrogenation and are converted into the saturated form, which may not be fully metabolized by rumen micro-organisms [50]. In this study, the fact that the insect-added TMRs produced more gas could be attributed to the relatively lower fat content, as the study by Jayanegara et al. [2] reported an increased fat level from 2.3% to 8.7% when 50% BSF larvae were added instead of SBM. Based on the total gas production results, it is appropriate to use 20% or 40% BSF larvae as a replacement for SBM. Other studies have also emphasized that higher inclusion levels of insects can result in a decrease in in vitro total gas formation. Ahmed and Nishida [25] reported that, when Gryllus bimaculatus and Bombyx mori insects were added at levels exceeding 20% in ruminant diets, in vitro total gas production was reduced, which was attributed to the high fat content of these insects. Similarly, in another study, the total gas production at 24 h was found to be similar to SBM when Brachytrupes portentosus, Acheta domesticus, Gryllus bimaculatus, and Bombyx mori insects were used as a replacement for SBM [18]. These variations in results across different species of insects highlight the importance of considering the nutritional profile, growing environment, and life stage of insects to better understand their potential as feed ingredients in ruminant nutrition [54].
5. Conclusions
Regardless of the species, insects appear to be a promising alternative to commonly used plant protein sources in ruminant nutrition. Although previous studies have suggested that insects are less digestible due to their high fat content, chitin, and fatty acids, this study found that defatted BSF larvae meal increased the in vitro rumen digestibility of TMRs and decreased NH3-N formation when used instead of SBM. Therefore, the defatted BSF larvae meal can be used as a sustainable source to replace 20% and 40% of the soybean meal without any negative effects. In the current geopolitical and environmental moment, in which ruminant farming is complicated, the introduction of insects in their ration can be a viable and reasonable solution. However, due to the lack of available data on rumen degradation and intestinal digestibility of insects and the inconsistent results across studies, further research is needed for a more comprehensive assessment. Future studies should investigate the potential use of insects in ruminant nutrition, with a focus on their effects on the rumen microbial population. This will contribute to a better understanding of how insects affect the microbiota and help determine the most suitable insect species to be used in combination with plant sources.
Author Contributions
Conceptualization, O.K., N.G. and İ.A.; methodology, O.K., N.G., F.İ. and M.S.A.; software, N.G. and Z.S.İ.; validation, N.G. and Z.S.İ.; formal analysis, D.Ş. and A.E.K.; investigation, O.K., Z.S.İ., İ.A., D.Ş. and A.E.K.; data curation, N.G., F.İ. and M.S.A.; writing—original draft preparation, O.K.; writing—review and editing, O.K. and N.G.; visualization, O.K., Z.S.İ., D.Ş. and A.E.K.; supervision, N.G. and F.İ. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Selçuk University Faculty of Veterinary Medicine’s Animal Care and Ethics Committee approved this study (approval number 2023/057). The animals involved in the experiments were housed and cared for at the Selçuk University Faculty of Veterinary Medicine Application and Research farm.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Makkar, H. Feed demand landscape and implications of food-not feed strategy for food security and climate change. Animal 2018, 12, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Novandri, B.; Yantina, N.; Ridla, M. Use of black soldier fly larvae (Hermetia illucens) to substitute soybean meal in ruminant diet: An in vitro rumen fermentation study. Vet. World 2017, 10, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Spiller, M.; Muys, M.; Papini, G.; Sakarika, M.; Buyle, M.; Vlaeminck, S.E. Environmental impact of microbial protein from potato wastewater as feed ingredient: Comparative consequential life cycle assessment of three production systems and soybean meal. Water Res. 2020, 171, 115406. [Google Scholar] [CrossRef] [PubMed]
- Hellstrand, S. Animal production in a sustainable agriculture. Environ. Dev. Sustain. 2013, 15, 999–1036. [Google Scholar] [CrossRef]
- Campos, A.; Pereira, O.; Ribeiro, K.; Santos, S.; Valadares Filho, S. Impact of replacing soybean meal in beef cattle diets with inactive dry yeast, a sugarcane by-product of ethanol distilleries and sugar mills. Anim. Feed Sci. Technol. 2014, 190, 38–46. [Google Scholar] [CrossRef]
- Yildiz, E.; Todorov, N. The comparison of the main protein sources for dairy cows: A review. Bulg. J. Agric. Sci. 2014, 20, 428–446. [Google Scholar]
- Hawkey, K.J.; Lopez-Viso, C.; Brameld, J.M.; Parr, T.; Salter, A.M. Insects: A Potential Source of Protein and Other Nutrients for Feed and Food. Annu. Rev. Anim. Biosci. 2021, 9, 333–354. [Google Scholar] [CrossRef]
- Castillo, C.; Hernández, J. Insects in ruminant nutrition as an urgent measure in the light of the scarcity of raw feedstock. Res. Vet. Sci. 2023, 155, 124–125. [Google Scholar] [CrossRef]
- Domingues, C.H.D.F.; Borges, J.A.R.; Ruviaro, C.F.; Gomes Freire Guidolin, D.; Rosa Mauad Carrijo, J. Understanding the factors influencing consumer willingness to accept the use of insects to feed poultry, cattle, pigs and fish in Brazil. PLoS ONE 2020, 15, e0224059. [Google Scholar] [CrossRef]
- Gasco, L.; Biancarosa, I.; Liland, N.S. From waste to feed: A review of recent knowledge on insects as producers of protein and fat for animal feeds. Curr. Opin. Green Sustain. Chem. 2020, 23, 67–79. [Google Scholar] [CrossRef]
- Poma, G.; Cuykx, M.; Amato, E.; Calaprice, C.; Focan, J.F.; Covacia, A. Evaluation of hazardous chemicals inedible insects and insect-based food intended for human consumption. Food Chem. Toxicol. 2017, 100, 70–79. [Google Scholar] [CrossRef] [PubMed]
- van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.W.; Gulraize; Ali, U.; Ur Rehman, F.; Najeeb, H.; Sohail, M.; Irsa, B.; Muzaffar, Z.; Chaudhry, M.S. Evaluation of standard loose plastic packaging for the management of Rhyzopertha dominica (F.)(Coleoptera: Bostrichidae) and Tribolium castaneum (Herbst)(Coleoptera: Tenebriondiae). J. Insect Sci. 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Przemieniecki, S.W.; Kosewska, A.; Kosewska, O.; Purwin, C.; Lipiński, K.; Ciesielski, S. Polyethylene, polystyrene and lignocellulose wastes as mealworm (Tenebrio molitor L.) diets and their impact on the breeding condition, biometric parameters, metabolism, and digestive microbiome. Sci. Total Environ. 2022, 832, 154758. [Google Scholar] [CrossRef] [PubMed]
- Toral, P.G.; Hervás, G.; González-Rosales, M.G.; Mendoza, A.G.; Robles-Jiménez, L.E.; Frutos, P. Insects as alternative feed for ruminants: Comparison of protein evaluation methods. J. Anim. Sci. Biotechnol. 2022, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Marono, S.; Piccolo, G.; Loponte, R.; Di Meo, C.; Attia, Y.A.; Nizza, A.; Bovera, F. In vitro crude protein digestibility of Tenebrio molitor and Hermetia illucens insect meals and its correlation with chemical composition traits. Ital. J. Anim. Sci. 2015, 14, 3889. [Google Scholar] [CrossRef]
- Ahmed, E.; Fukuma, N.; Hanada, M.; Nishida, T. Insects as Novel Ruminant Feed and a Potential Mitigation Strategy for Methane Emissions. Animals 2021, 11, 2648. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, S.; Xie, T.; Wang, Q.; Wang, Z.; Yang, H.; Li, S.; Wang, W. Effect of Unsaturated Fatty Acid Ratio In Vitro on Rumen Fermentation, Methane Concentration, and Microbial Profile. Fermentation 2022, 8, 540. [Google Scholar] [CrossRef]
- Onetti, S.G.; Shaver, R.D.; McGuire, M.A.; Grummer, R.R. Effect of type and level of dietary fat on rumen fermentation and performance of dairy cows fed corn silage-based diets. J. Dairy Sci. 2001, 84, 2751–2759. [Google Scholar] [CrossRef]
- Association of offical Analytic chemists (AOAC). Official Method of Analytic, 17th ed.; AOAC: Arilington, VA, USA, 2002; Volume 1, pp. 120–155. [Google Scholar]
- Van Soest, P.v.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Krishnamoorthy, U.; Muscato, T.; Sniffen, C.; Van Soest, P. Nitrogen fractions in selected feedstuffs. J. Dairy Sci. 1982, 65, 217–225. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, WA, USA, 2001. [Google Scholar]
- Ahmed, E.; Nishida, T. Optimal Inclusion Levels of Cricket and Silkworm as Alternative Ruminant Feed: A Study on Their Impacts on Rumen Fermentation and Gas Production. Sustainability 2023, 15, 1415. [Google Scholar] [CrossRef]
- Kara, K.; Ozkaya, S.; Guclu, B.K.; Aktug, E.; Demir, S.; Yılmaz, S.; Pirci, G.; Yılmaz, K.; Baytok, E. In vitro ruminal fermentation and nutrient compositions of potato starch by-products. J. Anim. Feed Sci. 2023, 32, 306–315. [Google Scholar] [CrossRef]
- Schofield, P.; Pitt, R.; Pell, A. Kinetics of fiber digestion from in vitro gas production. J. Anim. Sci. 1994, 72, 2980–2991. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.; Tricarico, J. Practical implications of fiber in dairy rations: Making use of forage fiber. In Proceedings of the 22nd Annual Southwest Nutrition & Management Conference, Tempe, AZ, USA, 22–23 February 2007; pp. 22–23. [Google Scholar]
- Tunkala, B.Z.; DiGiacomo, K.; Alvarez Hess, P.S.; Dunshea, F.R.; Leury, B.J. Impact of rumen fluid storage on in vitro feed fermentation characteristics. Fermentation 2023, 9, 392. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7255. [Google Scholar]
- Blümmel, M.; Steingaβ, H.; Becker, K. The relationship between in vitro gas production, in vitro microbial biomass yield and 15N incorporation and its implications for the prediction of voluntary feed intake of roughages. Br. J. Nutr. 1997, 77, 911–921. [Google Scholar] [CrossRef]
- Cobellis, G.; Acuti, G.; Forte, C.; Menghini, L.; De Vincenzi, S.; Orrù, M.; Valiani, A.; Pacetti, D.; Trabalza-Marinucci, M. Use of Rosmarinus officinalis in sheep diet formulations: Effects on ruminal fermentation, microbial numbers and in situ degradability. Small Rumin. Res. 2015, 126, 10–18. [Google Scholar] [CrossRef]
- Weatherburn, M. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.-P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. In Proceedings of the Annales De Zootechnie; Institut National de la Recherche Agronomique: Paris, France, 2000; pp. 231–253. [Google Scholar]
- Blümmel, M.; Aiple, K.P.; Steingaβ, H.; Becker, K. A note on the stoichiometrical relationship of short chain fatty acid production and gas formation in vitro in feedstuffs of widely differing quality. Anim. Physiol. Anim. Nutr. 1999, 81, 157–167. [Google Scholar] [CrossRef]
- Maxin, G.; Ouellet, D.; Lapierre, H. Ruminal degradability of dry matter, crude protein, and amino acids in soybean meal, canola meal, corn, and wheat dried distillers grains. J. Dairy Sci. 2013, 96, 5151–5160. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.-D.; Mamuad, L.L.; Kim, S.-H.; Choi, Y.J.; Soriano, A.P.; Cho, K.K.; Jeon, C.-O.; Lee, S.S.; Lee, S.-S. Effect of soybean meal and soluble starch on biogenic amine production and microbial diversity using in vitro rumen fermentation. Asian-Australas. J. Anim. Sci. 2015, 28, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Das, L.K.; Kundu, S.; Kumar, D.; Datt, C. Metabolizable protein systems in ruminant nutrition: A review. Vet. World. 2014, 7, 622–629. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, B.; Liu, J. Effects of the dietary nonfiber carbohydrate content on lactation performance, rumen fermentation, and nitrogen utilization in mid-lactation dairy cows receiving corn stover. J. Anim. Sci. Biotechnol. 2018, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.; Coppa, M.; Lussiana, C.; Le Morvan, A.; Gasco, L.; Maxin, G. Full-fat insect meals in ruminant nutrition: In vitro rumen fermentation characteristics and lipid biohydrogenation. J. Anim. Sci. Biotechnol. 2022, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen metabolism in the rumen. J. Dairy Sci. 2005, 88 (Suppl. 1), E9–E21. [Google Scholar] [CrossRef] [PubMed]
- Astuti, D.; Anggraeny, A.; Khotijah, L.; Suharti, S.; Jayanegara, A. Performance, physiological status, and rumen fermentation profiles of pre-and post-weaning goat kids fed cricket meal as a protein source. Trop. J. Anim. Sci. 2019, 42, 145–151. [Google Scholar] [CrossRef]
- Chaudhari, S.S.; Arakane, Y.; Specht, C.A.; Moussian, B.; Boyle, D.L.; Park, Y.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S. Knickkopf protein protects and organizes chitin in the newly synthesized insect exoskeleton. Proc. Natl. Acad. Sci. USA 2011, 108, 17028–17033. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, M.; Barroso, F.G.; Fabrikov, D.; Sánchez-Muros, M.J. In vitro crude protein digestibility of insects: A review. Insects 2022, 13, 682. [Google Scholar] [CrossRef]
- Phesatcha, B.; Phesatcha, K.; Viennaxay, B.; Matra, M.; Totakul, P.; Wanapat, M. Cricket Meal (Gryllus bimaculatus) as a Protein Supplement on In Vitro Fermentation Characteristics and Methane Mitigation. Insects 2022, 13, 129. [Google Scholar] [CrossRef]
- Pinho, R.; Santos, E.M.; de Oliveira, J.S.; de Carvalho, G.G.; Alves, J.P.; Macêdo, A.J.d.S.; Pereira, G.A.; Pereira, D.M.; Perazzo, A.F.; Zanine, A.d.M. Relationship between forage neutral detergent fiber and non-fibrous carbohydrates on ruminal fermentation products and neutral detergent fiber digestibility in goats. Rev. Colomb. Cienc. Pecu. 2019, 32, 126–138. [Google Scholar] [CrossRef]
- Homem Junior, A.C.; Ezequiel, J.M.B.; Perez, H.L.; Almeida, M.T.C.; Paschoaloto, J.R.; Carvalho, V.B.d.; Cremasco, L.F.; da Costa, M.B. In vitro fermentation of corn silage using rumen fluid buffered or not and different sample amounts. Cienc. Rural. 2015, 45, 2229–2232. [Google Scholar] [CrossRef]
- Sung, H.-G.; Kobayashi, Y.; Chang, J.; Ha, A.; Hwang, I.-H.; Ha, J. Low Ruminal pH Reduces Dietary Fiber Digestion via Reduced Microbial Attachment. Asian-Australas. J. Anim. Sci. 2007, 20, 200–207. [Google Scholar] [CrossRef]
- Buccioni, A.; Decandia, M.; Minieri, S.; Molle, G.; Cabiddu, A. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim. Feed Sci. Technol. 2012, 174, 1–25. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Jenkins, T.C. A 100-Year Review: Fat feeding of dairy cows. J. Dairy Sci. 2017, 100, 10061–10077. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Romano, N.; Sinha, A.K. Conversion of spent coffee and donuts by black soldier fly (Hermetia illucens) larvae into potential resources for animal and plant farming. Insects 2021, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- De Boever, J.; Aerts, J.; Vanacker, J.; De Brabander, D. Evaluation of the nutritive value of maize silages using a gas production technique. Anim. Feed Sci. Technol. 2005, 123, 255–265. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Pasini, G.; Cullere, M.; Vegro, M.; Simonato, B.; Dalle Zotte, A. Potentiality of protein fractions from the house cricket (Acheta domesticus) and yellow mealworm (Tenebrio molitor) for pasta formulation. LWT-Food Sci. Technol. 2022, 164, 113638. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).